Functionalized ZnO-Based Nanocomposites for Diverse Biological Applications: Current Trends and Future Perspectives
Abstract
1. Introduction
2. Methodology
3. Synthetic Approaches for ZnO Nanoparticles’ Fabrication
3.1. Mechanochemical Synthetic Approach
3.1.1. Laser Ablation Approach
3.1.2. High-Energy Ball Milling Approach
3.2. Hydro-/Solvothermal Synthetic Approach
3.3. Sol–Gel Synthetic Approach
3.4. Emulsion/Microemulsion Precipitation Synthetic Approach
3.5. Controlled Precipitation Synthetic Approach
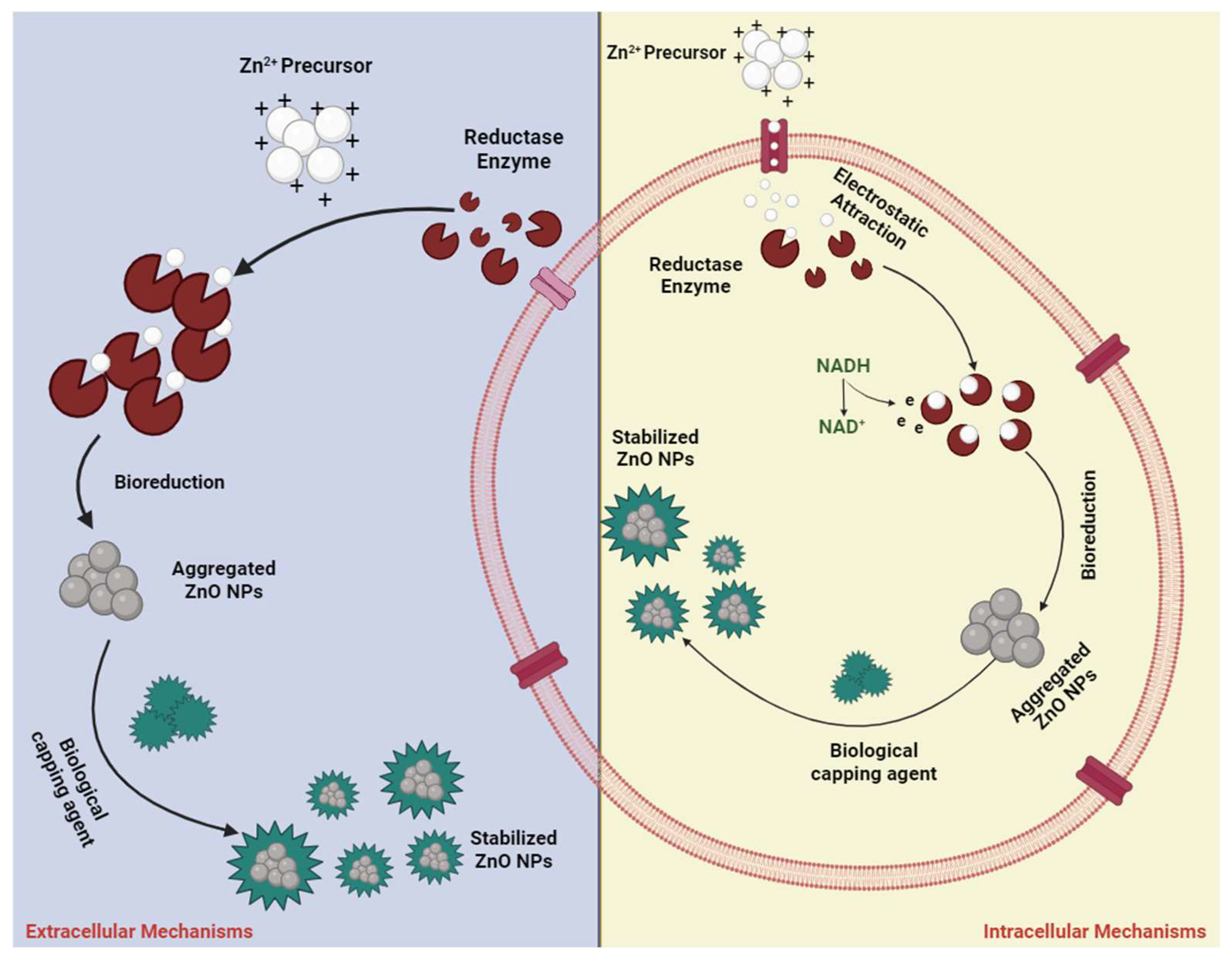
3.6. Green Synthesis/Biosynthesis Approach
3.7. Other Synthetic Approaches
4. Modification Approaches of ZnO Nanoparticles
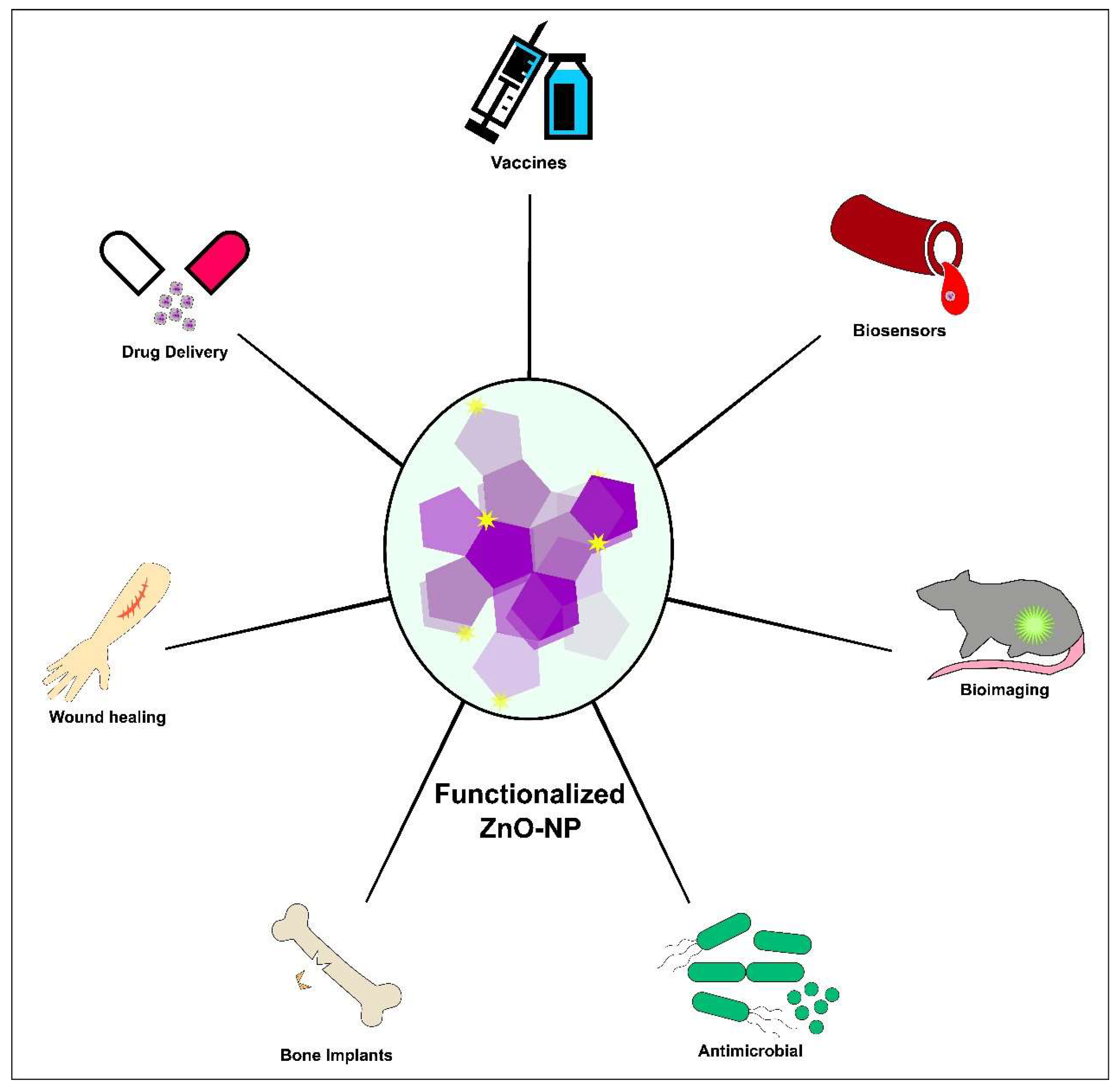
5. Biological Applications of Nanostructured ZnO
5.1. ZnO-Based Biosensors
5.1.1. Non-Enzymatic Biosensors
5.1.2. Glucose Biosensors
5.1.3. Enzymatic Biosensors
5.2. Bioimaging
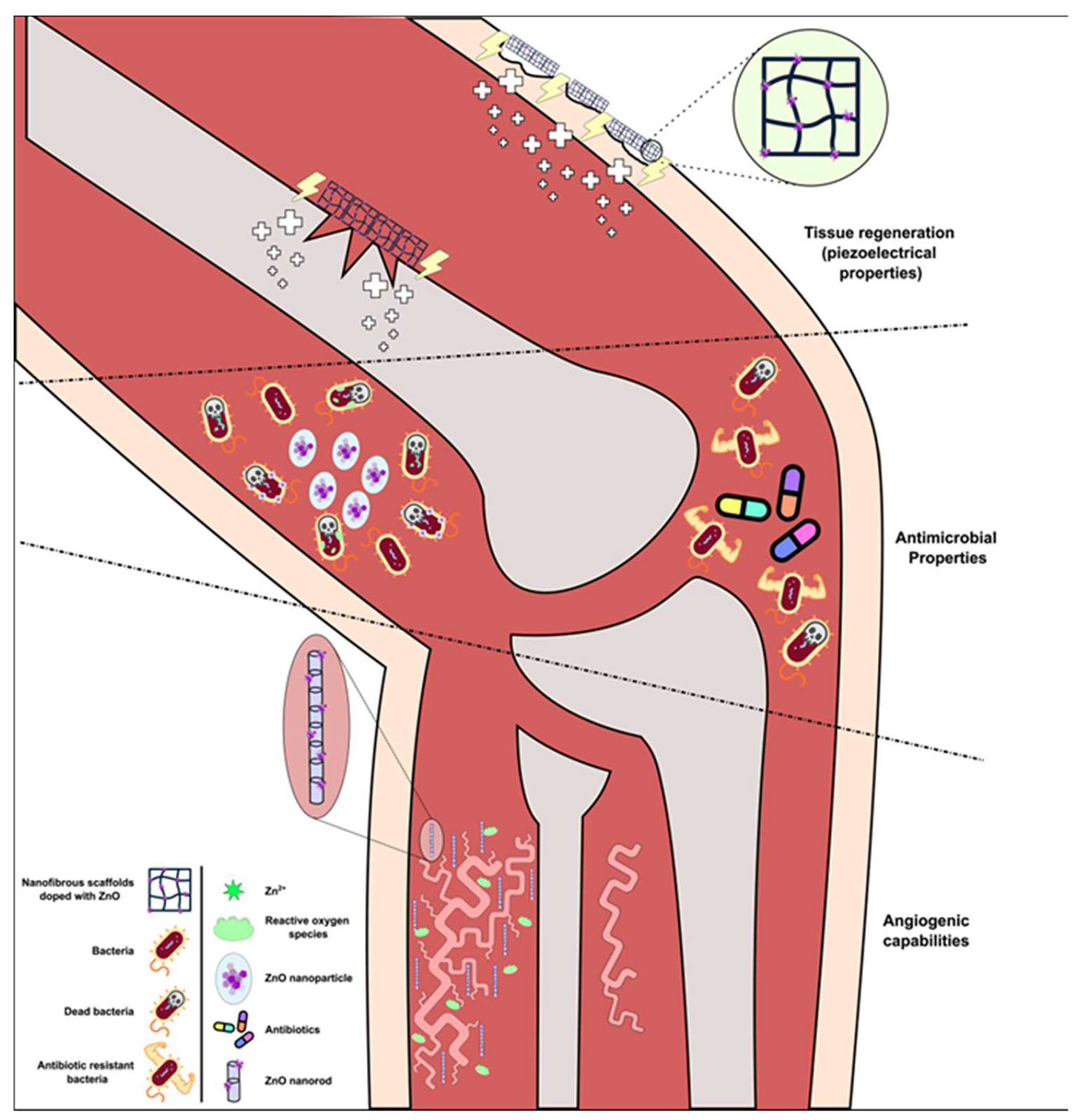
5.3. ZnO Nanostructures for Tissue Regeneration
5.3.1. Antimicrobial Properties
5.3.2. ZnO-Based Nanostructures for Wound Healing and Bone Implants
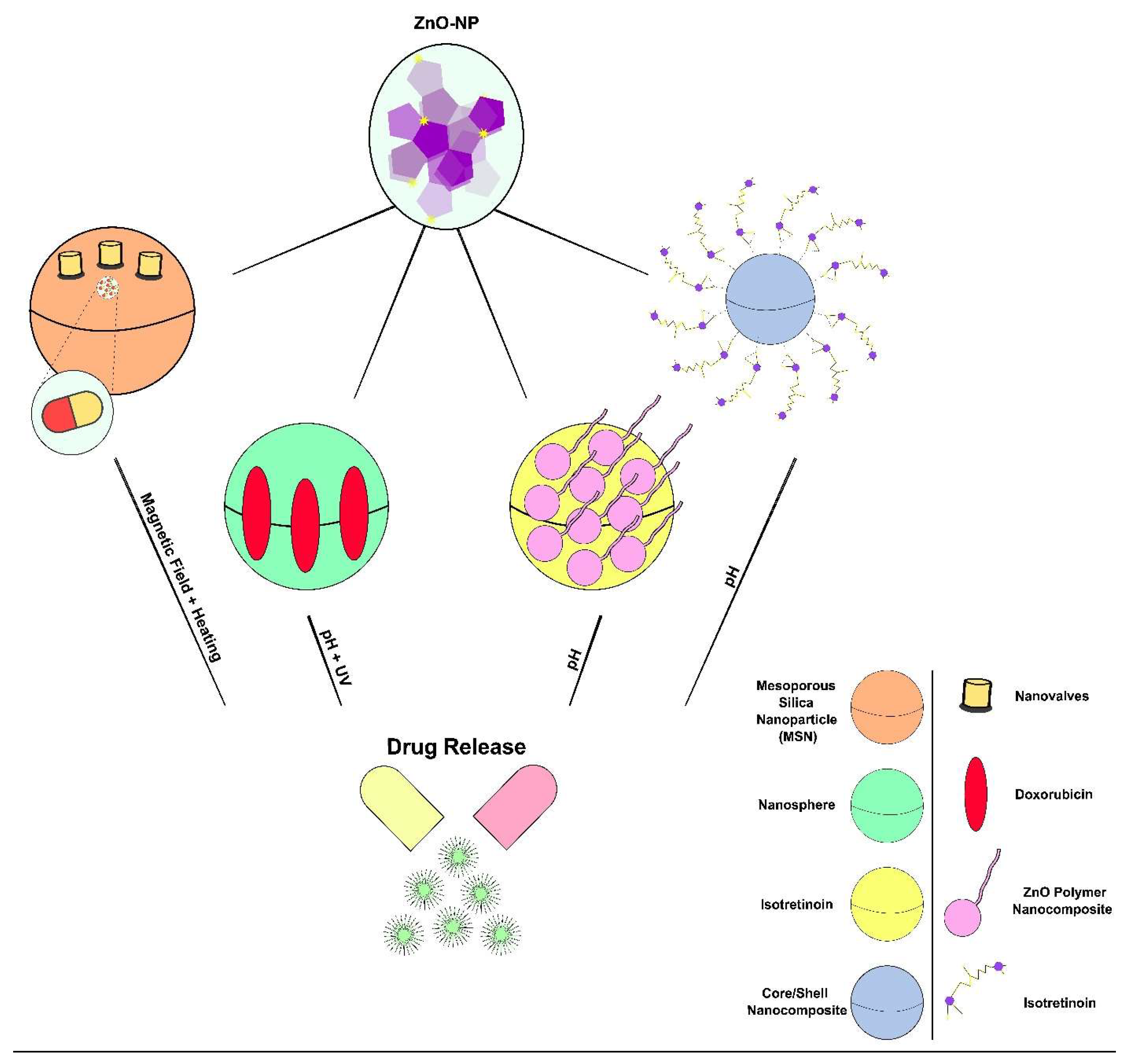
5.4. ZnO Nanostructures for Drug Delivery
5.5. Applications of ZnO-Based Nanocomposites for Vaccines and Immunotherapy
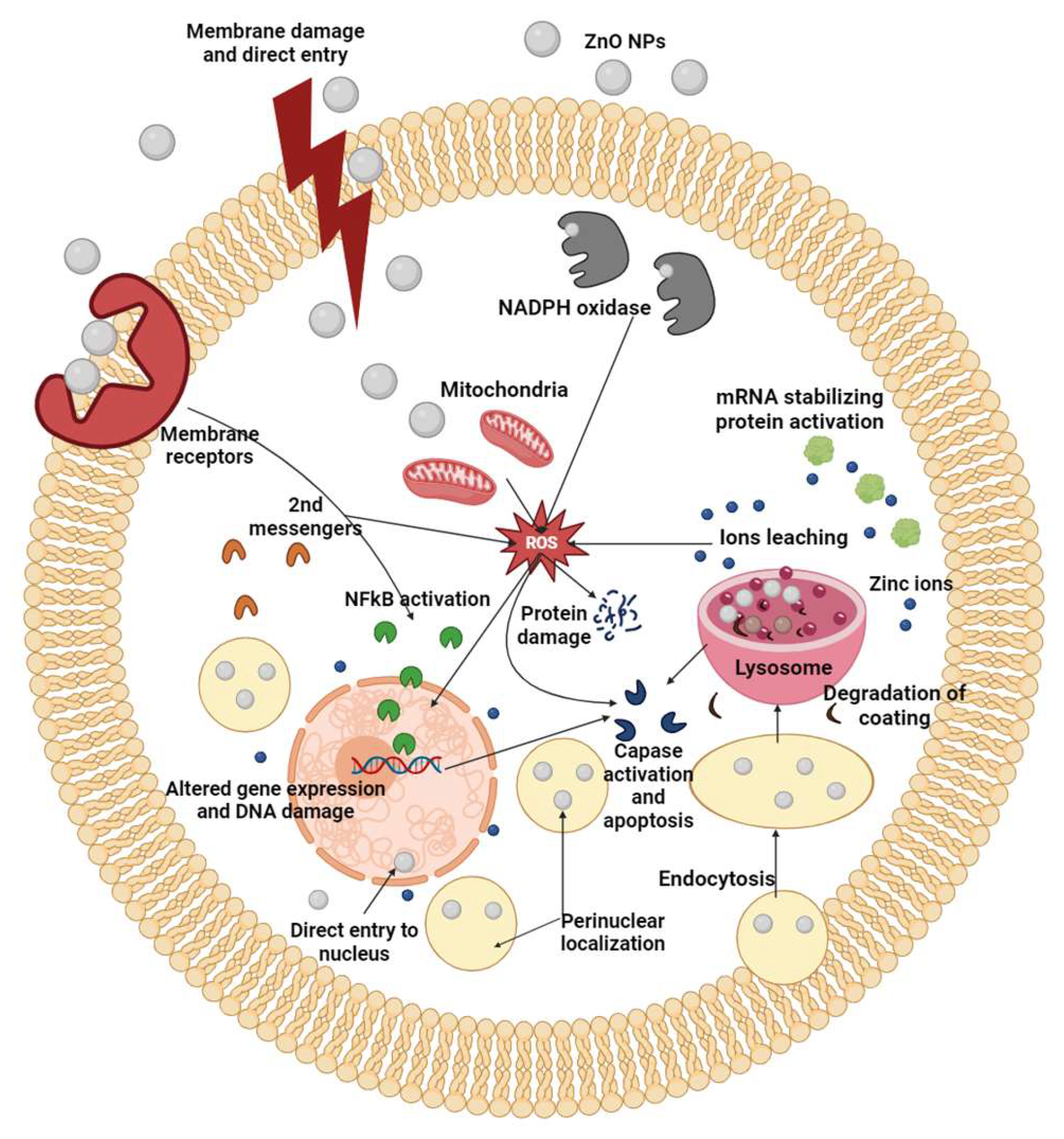
6. Toxicity of ZnO Nanoparticles
6.1. Neurotoxicity
6.2. Glial Cell Toxicity
6.3. Reactive Oxygen Species (ROS) Generation
6.4. Oxidative Stress
6.5. Toxicity-Involved Principal Pathways
7. Discussion-Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sahoo, S.K.; Parveen, S.; Panda, J.J. The present and future of nanotechnology in human health care. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 20–31. [Google Scholar] [CrossRef]
- Sanna, V.; Sechi, M. Nanoparticle therapeutics for prostate cancer treatment. Maturitas 2012, 73, 27–32. [Google Scholar] [CrossRef]
- Lagopati, N.; Evangelou, K.; Falaras, P.; Tsilibary, E.P.C.; Vasileiou, P.V.; Havaki, S.; Angelopoulou, A.; Pavlatou, E.A.; Gorgoulis, V.G. Nanomedicine: Photo-activated nanostructured titanium dioxide, as a promising anticancer agent. Pharmacol. Ther. 2021, 222, 107795. [Google Scholar] [CrossRef]
- Katifelis, H.; Nikou, M.-P.; Mukha, I.; Vityuk, N.; Lagopati, N.; Piperi, C.; Farooqi, A.A.; Pippa, N.; Efstathopoulos, E.P.; Gazouli, M. Ag/Au bimetallic nanoparticles trigger different cell death pathways and affect damage associated molecular pattern release in human cell lines. Cancers 2022, 14, 1546. [Google Scholar] [CrossRef]
- Lagopati, N.; Kotsinas, A.; Veroutis, D.; Evangelou, K.; Papaspyropoulos, A.; Arfanis, M.; Falaras, P.; Kitsiou, P.V.; Pateras, I.; Bergonzini, A.; et al. Biological effect of silver-modified nanostructured titanium dioxide in cancer. Cancer Genom. Proteom. 2021, 18, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Goyal, A.K.; Rath, G. Recent advances in metal nanoparticles in cancer therapy. J. Drug Target. 2017, 26, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Zavar, S. A novel three component synthesis of 2-amino-4H-chromenes derivatives using nano ZnO catalyst. Arab. J. Chem. 2017, 10, S67–S70. [Google Scholar] [CrossRef]
- He, C.X.; Lei, B.X.; Wang, Y.F.; Su, C.Y.; Fang, Y.P.; Kuang, D.B. Sonochemical preparation of hierarchical ZnO hollow spheres for efficient dye-sensitized solar cells. Chem. Eur. J. 2010, 16, 8757–8761. [Google Scholar] [CrossRef] [PubMed]
- Jagadale, S.B.; Patil, V.L.; Vanalakar, S.A.; Patil, P.S.; Deshmukh, H.P. Preparation, characterization of 1D ZnO nanorods and their gas sensing properties. Ceram. Int. 2018, 44, 3333–3340. [Google Scholar] [CrossRef]
- Mao, Z.; Shi, Q.; Zhang, L.; Cao, H. The formation and UV-blocking property of needle-shaped ZnO nanorod on cotton fabric. Thin Solid Films 2009, 517, 2681–2686. [Google Scholar] [CrossRef]
- Lupan, O.; Guerin, V.M.; Ghimpu, L.; Tiginyanu, I.M.; Pauporté, T. Nanofibrous-like ZnO layers deposited by magnetron sputtering and their integration in dye-sensitized solar cells. Chem. Phys. Lett. 2012, 550, 125–129. [Google Scholar] [CrossRef]
- Xing, Y.J.; Xi, Z.H.; Xue, Z.Q.; Zhang, X.D.; Song, J.H.; Wang, R.M.; Xu, J.; Song, Y.; Zhang, S.L.; Yu, D.P. Optical properties of the ZnO nanotubes synthesized via vapor phase growth. Appl. Phys. Lett. 2003, 83, 1689–1691. [Google Scholar] [CrossRef]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, characterization, and applications of ZnO nanowires. J. Nanomater. 2012, 2012, 624520. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Khan, M.A.; Jeon, M.S.; Yang, O.B. Controlled synthesis of various ZnO nanostructured materials by capping agents-assisted hydrothermal method for dye-sensitized solar cells. Electrochim. Acta 2008, 53, 7869–7874. [Google Scholar] [CrossRef]
- Huang, X.H.; Guo, R.Q.; Wu, J.B.; Zhang, P. Mesoporous ZnO nanosheets for lithium ion batteries. Mater. Lett. 2014, 122, 82–85. [Google Scholar] [CrossRef]
- Wang, F.; Liu, R.; Pan, A.; Xie, S.; Zou, B. A simple and cheap way to produce porous ZnO ribbons and their photovoltaic response. Mater. Lett. 2007, 61, 4459–4462. [Google Scholar] [CrossRef]
- Wang, Z.L. ZnO nanowire and nanobelt platform for nanotechnology. Mater. Sci. Eng. R Rep. 2009, 64, 33–71. [Google Scholar] [CrossRef]
- Lai, Y.; Meng, M.; Yu, Y.; Wang, X.; Ding, T. Photoluminescence and photocatalysis of the flower-like nano-ZnO photocatalysts prepared by a facile hydrothermal method with or without ultrasonic assistance. Appl. Catal. B Environ. 2011, 105, 335–345. [Google Scholar] [CrossRef]
- Xu, Z.; Ben, Y.; Chen, Z.; Qi, F. Facile synthesis of snowflake-like ZnO nanostructures at low temperature and their super catalytic activity for the ozone decomposition. Mater. Res. Bull. 2013, 48, 1725–1727. [Google Scholar] [CrossRef]
- Hou, K.; Gao, Z.; Da, M.; Li, Z.; Sun, H.; Li, J.; Xie, Y.; Kang, T.; Mijit, A. Oriented growth of urchin-like zinc oxide micro/nano-scale structures in aqueous solution. Mater. Res. Bull. 2012, 47, 1010–1015. [Google Scholar] [CrossRef]
- Behzad, F.; Sefidgar, E.; Samadi, A.; Lin, W.; Pouladi, I.; Pi, J. An overview of zinc oxide nanoparticles produced by plant extracts for anti-tuberculosis treatments. Curr. Med. Chem. 2022, 29, 86–98. [Google Scholar] [CrossRef]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J. Hazard. Mater. 2021, 402, 123560. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Zhang, L.; Yuan, J.; Yan, S.; Wang, C. Low-temperature synthesis of ZnO nanoparticles by solid-state pyrolytic reaction. Nanotechnology 2002, 14, 11. [Google Scholar] [CrossRef]
- Hong, H.; Shi, J.; Yang, Y.; Zhang, Y.; Engle, J.W.; Nickles, R.J.; Wang, X.; Cai, W. Cancer-targeted optical imaging with fluorescent zinc oxide nanowires. Nano Lett. 2011, 11, 3744–3750. [Google Scholar] [CrossRef]
- Sahoo, S.; Maiti, M.; Ganguly, A.; George, J.J.; Bhowmick, A.K. Effect of zinc oxide nanoparticles as cure activator on the properties of natural rubber and nitrile rubber. J. Appl. Polym. Sci. 2007, 105, 2407–2415. [Google Scholar] [CrossRef]
- Prasanna, A.P.S.; Venkataprasanna, K.S.; Pannerselvam, B.; Asokan, V.; Jeniffer, R.S.; Venkatasubbu, G.D. Multifunctional ZnO/SiO2 core/shell nanoparticles for bioimaging and drug delivery application. J. Fluoresc. 2020, 30, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Design and optimization of process parameters of polyvinyl(alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 2019, 207, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Gatou, M.-A.; Lagopati, N.; Vagena, I.-A.; Gazouli, M.; Pavlatou, E.A. ZnO nanoparticles from different precursors and their photocatalytic potential for biomedical use. Nanomaterials 2023, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Esa, Y.A.M.; Sapawe, N. A short review on zinc metal nanoparticles synthesize by green chemistry via natural plant extracts. Mater. Today Proc. 2020, 31, 386–393. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Shi, L.E.; Li, Z.H.; Zheng, W.; Zhao, Y.F.; Jin, Y.F.; Tang, Z.X. Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: A review. Food Addit. Contam. 2014, 31, 173–186. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles-An eco-friendly approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide-from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Auld, D.S. Zinc coordination sphere in biochemical zinc sites. In Zinc Biochemistry, Physiology, and Homeostasis; Maret, W., Ed.; Springer: Dordrecht, The Netherlands, 2001. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; McGrath, S.P. Heavy metals and soil microbes. Soil Biol. Biochem. 2009, 41, 2031–2037. [Google Scholar] [CrossRef]
- Huang, M.H.; Wu, Y.; Feick, H.; Tran, N.; Weber, E.; Yang, P. Catalytic growth of zinc oxide nanowires by vapor transport. Adv. Mater. 2001, 13, 113–116. [Google Scholar] [CrossRef]
- Kuo, T.J.; Lin, C.N.; Kuo, C.L.; Huang, M.H. Growth of ultralong ZnO nanowires on silicon substrates by vapor transport and their use as recyclable photocatalysts. Chem. Mater. 2007, 19, 5143–5147. [Google Scholar] [CrossRef]
- Kind, H.; Yan, H.; Messer, B.; Law, M.; Yang, P. Nanowire ultraviolet photodetectors and optical switches. Adv. Mater. 2002, 14, 158–160. [Google Scholar] [CrossRef]
- Luo, Q.P.; Lei, B.X.; Yu, X.Y.; Kuang, D.B.; Su, C.Y. Hiearchical ZnO rod-in-tube nano-architecture arrays produced via a two-step hydrothermal and ultrasonication process. J. Mater. Chem. 2011, 21, 8709–8714. [Google Scholar] [CrossRef]
- Chen, Y.H.; Shen, Y.M.; Wang, S.C.; Huang, J.L. Fabrication of one-dimensional ZnO nanotube and nanowire arrays with an anodic alumina oxide template via electrochemical deposition. Thin Solid Films 2014, 570, 303–309. [Google Scholar] [CrossRef]
- Hezam, A.; Namratha, K.; Ponnamma, D.; Drmosh, Q.A.; Saeed, A.M.N.; Cheng, C.; Byrappa, K. Direct Z-scheme Cs2O-Bi2O3-ZnO heterostructures as efficient sunlight-driven photocatalysts. ACS Omega 2018, 3, 12260–12269. [Google Scholar] [CrossRef]
- Toe, M.Z.; Pung, S.Y.; Le, A.T.; Yaccob, K.A.B.; Matsuda, A.; Tan, W.K.; Han, S.S. Morphology and optical properties of ZnO nanorods coupled with metal oxides of various bandgaps by photo-oxidation. J. Lumin. 2021, 229, 117649. [Google Scholar] [CrossRef]
- Zaidi, Z.; Vaghasiya, K.; Vijay, A.; Sharma, M.; Verma, R.K.; Vaidya, S. Hollow ZnO from assembly of nanoparticles: Photocatalytic and antibacterial activity. J. Mater. Sci. 2018, 53, 14964–14974. [Google Scholar] [CrossRef]
- Raja, A.; Ashokkumar, S.; Marthandam, R.P.; Jayachandiran, J.; Khatiwada, C.P.; Kaviyarasu, K.; Raman, R.G.; Swaminathan, M. Eco-friendly preparation of zinc oxide nanoparticles using Tabernaemontana divaricata and its photocatalytic and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2018, 181, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Maruthupandy, M.; Rajivgandhi, G.; Muneeswaran, T.; Song, J.M.; Manoharan, N. Biologically synthesized zinc oxide nanoparticles as nanoantibiotics against ESBLs producing gram negative bacteria. Microb. Pathog. 2018, 121, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Gunalan, S.; Sivaraj, R.; Rajendran, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int. 2012, 22, 693–700. [Google Scholar] [CrossRef]
- Xie, J.; Li, H.; Zhang, T.; Song, B.; Wang, X.; Gu, Z. Recent advances in ZnO nanomaterial-mediated biological applications and action mechanisms. Nanomaterials 2023, 13, 1500. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Shanmugam, V. A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: Mechanism-based approach. Bioorg. Chem. 2020, 94, 103423. [Google Scholar] [CrossRef]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. A multifuntional nanoplatform based on responsive fluorescent plasmonic ZnO-Au@ PEG hybrid nanogels. Adv. Funct. Mater. 2011, 21, 2830–2839. [Google Scholar] [CrossRef]
- Osmond-McLeod, M.J.; Oytam, Y.; Kirby, J.K.; Gomez-Fernandez, L.; Baxter, B.; McCall, M.J. Dermal absorption and short-term biological impact in hairless mice from sunscreens containing zinc oxide nano- or larger particles. Nanotoxicology 2014, 8, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of zinc in bone formation and bone resorption. J. Trace Elem. Exp. Med. 1998, 11, 119–135. [Google Scholar] [CrossRef]
- Kumar, P.T.S.; Abhilash, S.; Manzoor, K.; Nair, S.V.; Tamura, H.; Jayakumar, R. Preparation and characterization of novel β-chitin/nanosilver composite scaffolds for wound dressing applications. Carbohydr. Polym. 2010, 80, 761–767. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to Escherichia coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef]
- De Berardis, B.; Civitelli, G.; Condello, M.; Lista, P.; Pozzi, R.; Arancia, G.; Meschini, S. Exposure to ZnO nanoparticles induces oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol. Appl. Pharmacol. 2010, 246, 116–127. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Mai, T.Y.; Nguyen, T.X.M.; Huynh, K.P.H.; Le, M.V.; Nguyen, T.A.N. Mechanochemical synthesis of zinc oxide nanoparticles and their antibacterial activity against Escherichia coli. Mater. Sci. Forum 2020, 1007, 59–64. [Google Scholar] [CrossRef]
- Aghababazadeh, R.; Mazinani, B.; Mirhabibi, A.; Tamizifar, M. ZnO nanoparticles synthesised by mechanochemical processing. J. Phys. Conf. Ser. 2006, 26, 312. [Google Scholar] [CrossRef]
- Moballegh, A.; Shahverdi, H.R.; Aghababazadeh, R.; Mirhabibi, A.R. ZnO nanoparticles obtained by mechanochemical technique and the optical properties. Surf. Sci. 2007, 601, 2850–2854. [Google Scholar] [CrossRef]
- Cavalu, S.; Kamel, E.; Laslo, V.; Fritea, L.; Costea, T.; Antoniac, I.V.; Vasile, E.; Antoniac, A.; Semenescu, A.; Mohan, A.; et al. Eco-friendly, facile and rapid way for synthesis of selenium nanoparticles production, structural and morphological characterization. Rev. Chim. 2017, 68, 2963–2966. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for synthesis of nanoparticles and fabrication of nanocomposites. In Micro and Nano Technologies-Synthesis of Inorganic Nanomaterials; Bhagyaraj, S.M., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 121–139. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Mintcheva, N.; Aljulaih, A.A.; Wunderlich, W.; Kulinich, S.A.; Iwamori, S. Laser-ablated ZnO nanoparticles and their photocatalytic activity toward organic pollutants. Materials 2018, 11, 1127. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef]
- Al-Dahash, G.; Mubder Khilkala, W.; Abd Alwahid, S.N. Preparation and characterization of ZnO nanoparticles by laser ablation in NaOH aqueous solution. Iran. J. Chem. Chem. Eng. 2018, 37, 11–16. [Google Scholar] [CrossRef]
- Farahani, S.V.; Mahmoodi, A.; Goranneviss, M. The effect of laser environment on the characteristics of ZnO nanoparticles by laser ablation. Int. Nano Lett. 2016, 6, 45–49. [Google Scholar] [CrossRef]
- Thareja, R.K.; Shukla, S. Synthesis and characterization of zinc oxide nanoparticles by laser ablation of zinc in liquid. Appl. Surf. Sci. 2007, 253, 8889–8895. [Google Scholar] [CrossRef]
- Camarda, P.; Vaccaro, L.; Sciortino, A.; Messina, F.; Buscarino, G.; Agnello, S.; Gelardi, F.M.; Popescu, R.; Schneider, R.; Gerthsen, D.; et al. Synthesis of multi-color luminescent ZnO nanoparticles by ultra-short pulsed laser ablation. Appl. Surf. Sci. 2020, 506, 144954. [Google Scholar] [CrossRef]
- Gavrilenko, E.A.; Goncharova, D.A.; Lapin, I.N.; Nemoykina, A.L.; Svetlichnyi, V.A.; Aljulaih, A.A.; Mintcheva, N.; Kulinich, S.A. Comparative study of physicochemical and antibacterial properties of ZnO nanoparticles prepared by laser ablation of Zn target in water and air. Materials 2019, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Hodaei, A.; Ataie, A.; Mostafavi, E. Intermediate milling energy optimization to enhance the characteristics of barium hexaferrite magnetic nanoparticles. J. Alloys Compd. 2015, 640, 162–168. [Google Scholar] [CrossRef]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.E. Ball milling: A green technology for the preparation and functionalization of nanocellulose derivatives. Nanoscale Adv. 2019, 1, 937–947. [Google Scholar] [CrossRef]
- Prommalikit, C.; Mekprasart, W.; Pecharapa, W. Effect of milling speed and time on ultrafine ZnO powder by high energy ball milling technique. J. Phys. Conf. Ser. 2019, 1259, 012023. [Google Scholar] [CrossRef]
- Mohammadi, N.; Mirhosseini, M.; Shirzad, M.; Dehghan Hamdan, A.; Yazdani, N. Synthesizing ZnO nanoparticles by high-energy milling and investigating their antimicrobial effect. JSSU 2015, 23, 2070–2082. Available online: http://jssu.ssu.ac.ir/article-1-2933-en.html (accessed on 15 January 2024).
- Yang, G.; Park, S.-J. Conventional and microwave hydrothermal synthesis and application of functional materials: A review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef]
- Komarneni, S. Nanophase materials by hydrothermal, microwave-hydrothermal and microwave-solvothermal methods. Curr. Sci. 2003, 85, 1730–1734. Available online: http://www.jstor.org/stable/24109979 (accessed on 15 January 2024).
- Cavalu, S.; Antoniac, J.V.; Fritea, L.; Mates, I.M.; Milea, C.; Laslo, V.; Vicas, S.; Mohan, A. Surface modifications of the titanium mesh for cranioplasty using selenium nanoparticles coating. J. Adhes. Sci. Technol. 2018, 32, 2509–2522. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Bao, Y.; Zhu, Z.; Wang, X.; Zhang, J. Synthesis of large-scale uniform mulberry-like ZnO particles with microwave hydrothermal method and its antibacterial property. Ceram. Int. 2013, 39, 2803–2810. [Google Scholar] [CrossRef]
- Aremu, O.H.; Akintayo, C.O.; Naidoo, E.B.; Nelana, S.M.; Ayanda, O.S. Synthesis and applications of nano-sized zinc oxide in wastewater treatment: A review. Int. J. Environ. Sci. Technol. 2021, 18, 3237–3256. [Google Scholar] [CrossRef]
- Laila, I.K.; Mufti, N.; Maryam, S.; Fuad, A.; Taufiq, A. Synthesis and characterization of ZnO nanorods by hydrothermal methods and its application on perovskite solar cells. J. Phys. Conf. Ser. 2018, 1093, 012012. [Google Scholar] [CrossRef]
- Parhi, P.; Kramer, J.; Manivannan, V. Microwave initiated hydrothermal synthesis of nano-sized complex fluorides, KMF 3 (K = Zn, Mn, Co, and Fe). J. Mater. Sci. 2008, 43, 5540–5545. [Google Scholar] [CrossRef]
- Sonawane, G.H.; Patil, S.P.; Sonawane, S.H. Nanocomposites and Its Applications. In Micro and Nano Technologies-Applications of Nanomaterials; Bhagyaraj, S.M., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 1–22. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Maboudian, R.; Carraro, C.; Han, C.; Liu, W.; Wei, D. Facile synthesis of ZnO-SnO2 hetero-structured nanowires for high-performance NO2 sensing application. Sens. Actuators B Chem. 2021, 333, 129613. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, C.; He, L.; Zhang, K.; Zhang, J.; Jin, L.; Asiri, A.M.; Alamry, K.A.; Chu, X. ZnFe2O4/ZnO nanosheets assembled microspheres for high performance trimethylamine gas sensing. J. Alloys Compd. 2020, 849, 156461. [Google Scholar] [CrossRef]
- Bharti, D.B.; Bharati, A.V. Synthesis of ZnO nanoparticles using a hydrothermal method and a study its optical activity. Luminescence 2017, 32, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Wirunmongkol, T.; Narongchai, O.; Pavasupree, S. Simple hydrothermal preparation of zinc oxide powders using Thai autoclave unit. Energy Procedia 2013, 34, 801–807. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the alcohols on the ZnO synthesis and its properties: The photocatalytic and antimicrobial activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef]
- Motelica, L.; Oprea, O.-C.; Vasile, B.-S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial activity of solvothermal obtained ZnO nanoparticles with different morphology and photocatalytic activity against a dye mixture: Methylene blue, rhodamine b and methyl orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef]
- Al Abdullah, K.; Awad, S.; Zaraket, J.; Salame, C. Synthesis of ZnO nanopowders by using sol-gel and studying their structural and electrical properties at different temperature. Energy Procedia 2017, 119, 565–570. [Google Scholar] [CrossRef]
- Arya, S.; Mahajan, P.; Mahajan, S.; Khosla, A.; Datt, R.; Gupta, V.; Young, S.-J.; Oruganti, S.K. Influence of processing parameters to control morphology and optical properties of sol-gel synthesized ZnO nanoparticles. ECS J. Solid State Sci. Technol. 2021, 10, 023002. [Google Scholar] [CrossRef]
- Simon, V.; Cavalu, S.; Simon, S.; Mocuta, H.; Vanea, E.; Prinz, M.; Neumann, M. Surface functionalization of sol-gel derived aluminosilicates in simulated body fluids. Solid State Ion. 2009, 180, 764–769. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, M.R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Emran, T.B.; Cavalu, S. Exploring the journey of zinc oxide nanoparticles (ZnO-NPs) toward biomedical applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.; Kumar, V.; Kumar, P. On Mn doped ZnO nano particles reinforced in PVDF matrix for fused filament fabrication: Mechanical, thermal, morphological and 4D properties. J. Manuf. Process. 2021, 62, 817–832. [Google Scholar] [CrossRef]
- Kumar, A. Sol gel synthesis of zinc oxide nanoparticles and their application as nano-composite electrode material for supercapacitor. J. Mol. Struct. 2020, 1220, 128654. [Google Scholar] [CrossRef]
- Hasnidawani, J.N.; Azlina, H.N.; Norita, H.; Bonnia, N.N.; Ratim, S.; Ali, E.S. Synthesis of ZnO nanostructures using sol-gel method. Procedia Chem. 2016, 19, 211–216. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials: 1st Nano Update. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef]
- Pineda-Reyes, A.M.; Olvera, M.D.L.L. Synthesis of ZnO nanoparticles from water-in-oil (w/o) microemulsions. Mater. Chem. Phys. 2018, 203, 141–147. [Google Scholar] [CrossRef]
- Ali, H.S.; Alghamdi, A.S.; Murtaza, G.; Arif, H.S.; Naeem, W.; Farid, G.; Sharif, S.; Ashiq, M.G.B.; Shabbir, S.A. Facile microemulsion synthesis of vanadium-doped ZnO nanoparticles to analyze the compositional, optical, and electronic properties. Materials 2019, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Emsaki, M.; Hassanzadeh-Tabrizi, S.A.; Saffar-Teluri, A. Microemulsion synthesis of ZnO-ZnWO4 nanoparticles for superior photodegradation of organic dyes in water. J. Mater. Sci. Mater. Electron. 2018, 29, 2384–2391. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Wang, A.; Li, X.; Wang, G.; Zhao, L. Synthesis of ZnO nanoparticles from microemulsions in a flow type microreactor. Chem. Eng. J. 2014, 235, 191–197. [Google Scholar] [CrossRef]
- Li, X.; He, G.; Xiao, G.; Liu, H.; Wang, M. Synthesis and morphology control of ZnO nanostructures in microemulsions. J. Colloid Interface Sci. 2009, 333, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Jeyasingh, E.; Charles, K.A.; Thangaraj, P.; Chandrasekaran, K.; Viswanathan, M.R. Effect of Mn doping on the structural, optical, magnetic properties, and antibacterial activity of ZnO nanospheres. J. Sol-Gel Sci. Technol. 2022, 102, 357–371. [Google Scholar] [CrossRef]
- Jeyachitra, R.; Kalpana, S.; Senthil, T.S.; Kang, M. Electrical behavior and enhanced photocatalytic activity of (Ag, Ni) co-doped ZnO nanoparticles synthesized from co-precipitation technique. Water Sci. Technol. 2020, 81, 1296–1307. [Google Scholar] [CrossRef]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 864–870. [Google Scholar] [CrossRef]
- Vanathi, P.; Rajiv, P.; Narendhran, S.; Rajeshwari, S.; Rahman, P.K.; Venckatesh, R. Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: A green chemistry approach. Mater. Lett. 2014, 134, 13–15. [Google Scholar] [CrossRef]
- Qu, J.; Yuan, X.; Wang, X.; Shao, P. Zinc accumulation and synthesis of ZnO nanoparticles using Physalis alkekengi L. Environ. Pollut. 2011, 159, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.D.; Thompson, P.D. Phytosterols and vascular disease. Atherosclerosis 2006, 186, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulou, M.A.; Kefalas, P.; Papageorgiou, V.P.; Assimopoulou, A.N.; Boskou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 2006, 94, 19–25. [Google Scholar] [CrossRef]
- Mohamad, N.A.N.; Arham, N.A.; Jai, J.; Hadi, A. Plant extract as reducing agent in synthesis of metallic nanoparticles: A review. Adv. Mater. Res. 2014, 832, 350–355. [Google Scholar] [CrossRef]
- Vilchis-Nestor, A.R.; Sánchez-Mendieta, V.; Camacho-López, M.A.; Gómez-Espinosa, R.M.; Camacho-López, M.A.; Arenas-Alatorre, J.A. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater. Lett. 2008, 62, 3103–3105. [Google Scholar] [CrossRef]
- Song, J.Y.; Jang, H.K.; Kim, B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009, 44, 1133–1138. [Google Scholar] [CrossRef]
- Amin, M.; Anwar, F.; Janjua, M.R.S.A.; Iqbal, M.A.; Rashid, U. Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: Characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. Int. J. Mol. Sci. 2012, 13, 9923–9941. [Google Scholar] [CrossRef]
- Umamaheswari, A.; Prabu, S.L.; John, S.A.; Puratchikody, A. Green synthesis of zinc oxide nanoparticles using leaf extracts of Raphanus sativus var. Longipinnatus and evaluation of their anticancer property in A549 cell lines. Biotechnol. Rep. 2021, 29, e00595. [Google Scholar] [CrossRef] [PubMed]
- Alrajhi, A.H.; Ahmed, N.M.; Al Shafouri, M.; Almessiere, M.A.; Mohammed Al-Ghamdi, A. Green synthesis of zinc oxide nanoparticles using salvia officials extract. Mater. Sci. Semicond. Process. 2021, 125, 105641. [Google Scholar] [CrossRef]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; Abd El-Azim, M.H.M. Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.A.; Owais, M.; Rajpoot, R.; Ahmad, F.; Khan, N.; Zubair, S. Biomimetically synthesized ZnO nanoparticles attain potent antibacterial activity against less susceptible S. aureus skin infection in experimental animals. RSC Adv. 2017, 7, 36361–36373. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N.A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.G.; Ballester, A. Mechanism and applications of metal nanoparticles prepared by bio-mediated process. Remedial Spec. Educ. 2014, 3, 199–216. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Kirthi, A.V.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Rao, K.B. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta Part A 2012, 90, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Balraj, B.; Senthilkumar, N.; Siva, C.; Krithikadevi, R.; Julie, A.; Potheher, I.V.; Arulmozhi, M. Synthesis and characterization of zinc oxide nanoparticles using marine Streptomyces sp. with its investigations on anticancer and antibacterial activity. Res. Chem. Intermed. 2017, 43, 2367–2376. [Google Scholar] [CrossRef]
- Mashrai, A.; Khanam, H.; Aljawfi, R.N. Biological synthesis of ZnO nanoparticles using C. albicans and studying their catalytic performance in the synthesis of steroidal pyrazolines. Arab. J. Chem. 2017, 10, S1530–S1536. [Google Scholar] [CrossRef]
- Zikalala, N.E.; Azizi, S.; Zikalala, S.A.; Kamika, I.; Maaza, M.; Zinatizadeh, A.A.; Mokrani, T.; Kaviyarasu, K. An evaluation of the biocatalyst for the synthesis and application of zinc oxide nanoparticles for water remediation-a review. Catalysts 2022, 12, 1442. [Google Scholar] [CrossRef]
- El-Belely, E.F.; Farag, M.M.S.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Arthrospira platensis (Class: Cyanophyceae) and evaluation of their biomedical activities. Nanomaterials 2021, 11, 95. [Google Scholar] [CrossRef]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and enhanced photocatalytic activity of zinc oxide nanoparticles toward organosulfur pollutants. Sci. Rep. 2019, 9, 6866. [Google Scholar] [CrossRef]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nano 2018, 9, 3. [Google Scholar] [CrossRef]
- Abinaya, M.; Vaseeharan, B.; Divya, M.; Sharmili, A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Bacterial exopolysaccharide (EPS)-coated ZnO nanoparticles showed high antibiofilm activity and larvicidal toxicity against malaria and Zika virus vectors. J. Trace Elem. Med. Biol. 2018, 45, 93–103. [Google Scholar] [CrossRef]
- Saravanan, M.; Gopinath, V.; Chaurasia, M.K.; Syed, A.; Ameen, F.; Purushothaman, N. Green synthesis of anisotropic zinc oxide nanoparticles with antibacterial and cytofriendly properties. Microb. Pathogen. 2018, 115, 57–63. [Google Scholar] [CrossRef]
- Król, A.; Railean-Plugaru, V.; Pomastowski, P.; Złoch, M.; Buszewski, B. Mechanism study of intracellular zinc oxide nanocomposites formation. Colloids Surf. A 2018, 553, 349–358. [Google Scholar] [CrossRef]
- Singh, B.N.; Rawat, A.K.S.; Khan, W.; Naqvi, A.H.; Singh, B.R. Biosynthesis of stable antioxidant ZnO nanoparticles by Pseudomonas aeruginosa rhamnolipids. PLoS ONE 2014, 9, e106937. [Google Scholar] [CrossRef]
- Sumanth, B.; Lakshmeesha, T.R.; Ansari, M.A.; Alzohairy, M.A.; Udayashankar, A.C.; Shobha, B.; Niranjana, S.R.; Srinivas, C.; Almatroudi, A. Mycogenic synthesis of extracellular zinc oxide nanoparticles from Xylaria acuta and its nanoantibiotic potential. Int. J. Nanomed. 2020, 15, 8519–8536. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Abu-Elghait, M.; Ahmed, N.E.; Salem, S.S. Eco-friendly mycogenic synthesis of ZnO and CuO nanoparticles for in vitro antibacterial, antibiofilm, and antifungal applications. Biol. Trace Elem. Res. 2021, 199, 2788–2799. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Fouda, A.; Abdel-Rahman, M.A.; Hassan, S.E.D.; El-Gamal, M.S.; Salem, S.S.; Shaheen, T.I. Fungal strain impacts the shape, bioactivity and multifunctional properties of green synthesized zinc oxide nanoparticles. Biocatal. Agric. Biotechnol. 2019, 19, 101103. [Google Scholar] [CrossRef]
- Bird, S.M.; El-Zubir, O.; Rawlings, A.E.; Leggett, G.J.; Staniland, S.S. A novel design strategy for nanoparticles on nanopatterns: Interferometric lithographic patterning of Mms6 biotemplated magnetic nanoparticles. J. Mater. Chem. C 2016, 4, 3948–3955. [Google Scholar] [CrossRef]
- Soto-Robles, C.A.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Nava, O.; Vilchis-Nestor, A.R.; Lugo-Medina, E.; Ranjithkumar, R.; Castro-Beltrán, A. Study on the effect of the concentration of Hibiscus sabdariffa extract on the green synthesis of ZnO nanoparticles. Results Phys. 2019, 15, 102807. [Google Scholar] [CrossRef]
- Anand, G.T.; Renuka, D.; Ramesh, R.; Anandaraj, L.; Sundaram, S.J.; Ramalingam, G.; Magdalane, C.M.; Bashir, A.K.H.; Maaza, M.; Kaviyarasu, K. Green synthesis of ZnO nanoparticle using Prunus dulcis (Almond Gum) for antimicrobial and supercapacitor applications. Surf. Interfaces 2019, 17, 100376. [Google Scholar] [CrossRef]
- Ganesan, V.; Hariram, M.; Vivekanandhan, S.; Muthuramkumar, S. Periconium sp.(endophytic fungi) extract mediated sol-gel synthesis of ZnO nanoparticles for antimicrobial and antioxidant applications. Mater. Sci. Semicond. Process. 2020, 105, 104739. [Google Scholar] [CrossRef]
- Deng, L.; Wang, M.; Ji, X.; Cheng, S. Target synthesis of dense C-coated ZnO for advanced lithium storage via a facile and cost-effective approach. Ionics 2021, 27, 423–428. [Google Scholar] [CrossRef]
- Choi, S.C.; Lee, D.K.; Sohn, S.H. Effects of experimental configuration on the morphology of two-dimensional ZnO nanostructures synthesized by thermal chemical-vapor deposition. Crystals 2020, 10, 517. [Google Scholar] [CrossRef]
- Manoharan, C.; Pavithra, G.; Bououdina, M.; Dhanapandian, S.; Dhamodharan, P. Characterization and study of antibacterial activity of spray pyrolysed ZnO: Al thin films. Appl. Nanosci. 2016, 6, 815–825. [Google Scholar] [CrossRef]
- Srinivasulu, T.; Saritha, K.; Reddy, K.R. Synthesis and characterization of Fe-doped ZnO thin films deposited by chemical spray pyrolysis. Mod. Electron. Mater. 2017, 3, 76–85. [Google Scholar] [CrossRef]
- Salah, N.; Al-Shawafi, W.M.; Alshahrie, A.; Baghdadi, N.; Soliman, Y.M.; Memic, A. Size controlled, antimicrobial ZnO nanostructures produced by the microwave assisted route. Mater. Sci. Eng. C 2019, 99, 1164–1173. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Kundu, M.; Rana, S.; Kumar, R.; Das, J.; Sil, P.C. Microwave induced synthesis of ZnO nanorods and their efficacy as a drug carrier with profound anticancer and antibacterial properties. Toxicol. Rep. 2019, 6, 176–185. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A review of microwave synthesis of zinc oxide nanomaterials: Reactants, process parameters and morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.R.; Sarvi, M.N. Recent achievements in the microbial synthesis of semiconductor metal sulfide nanoparticles. Mater. Sci. Semicond. Process. 2015, 40, 293–301. [Google Scholar] [CrossRef]
- Sun, W.; Zhai, Z.; Wang, D.; Liu, S.; Jiao, K. Electrochemistry of hemoglobin entrapped in a Nafion/nano-ZnO film on carbon ionic liquid electrode. Bioelectrochemistry 2009, 74, 295–300. [Google Scholar] [CrossRef]
- Panigrahi, U.K.; Sahu, B.; Behuria, H.G.; Sahu, S.K.; Dhal, S.P.; Hussain, S.; Mallick, P. Synthesis, characterization and bioactivity of thio-acetamide modified ZnO nanoparticles embedded in zinc acetate matrix. Nano Express 2021, 2, 010012. [Google Scholar] [CrossRef]
- Khurana, N.; Arora, P.; Pente, A.S.; Pancholi, K.C.; Kumar, V.; Kaushik, C.P.; Rattan, S. Surface modification of zinc oxide nanoparticles by vinyltriethoxy silane (VTES). Inorg. Chem. Commun. 2021, 124, 108347. [Google Scholar] [CrossRef]
- Kulkarni, D.R.; Malode, S.J.; Prabhu, K.K.; Ayachit, N.H.; Kulkarni, R.M.; Shetti, N.P. Development of a novel nanosensor using Ca-doped ZnO for antihistamine drug. Mater. Chem. Phys. 2020, 246, 122791. [Google Scholar] [CrossRef]
- Ilager, D.; Shetti, N.P.; Malladi, R.S.; Shetty, N.S.; Reddy, K.R.; Aminabhavi, T.M. Synthesis of Ca-doped ZnO nanoparticles and its application as highly efficient electrochemical sensor for the determination of anti-viral drug, acyclovir. J. Mol. Liq. 2021, 322, 114552. [Google Scholar] [CrossRef]
- Shome, S.; Talukdar, A.D.; Tewari, S.; Choudhury, S.; Bhattacharya, M.K.; Upadhyaya, H. Conjugation of micro/nanocurcumin particles to ZnO nanoparticles changes the surface charge and hydrodynamic size thereby enhancing its antibacterial activity against Escherichia coli and Staphylococcus aureus. Biotechnol. Appl. Biochem. 2021, 68, 603–615. [Google Scholar] [CrossRef]
- Mallakpour, S.; Lormahdiabadi, M. Production of the ZnO-folic acid nanoparticles and poly (vinyl alcohol) nanocomposites: Investigation of morphology, wettability, thermal, and antibacterial properties. J. Polym. Res. 2020, 27, 259. [Google Scholar] [CrossRef]
- Bisht, G.; Rayamajhi, S.; Kc, B.; Paudel, S.N.; Karna, D.; Shrestha, B.G. Synthesis, characterization, and study of in vitro cytotoxicity of ZnO-Fe3O4 magnetic composite nanoparticles in human breast cancer cell line (MDA-MB-231) and mouse fibroblast (NIH 3T3). Nanoscale Res. Lett. 2016, 11, 537. [Google Scholar] [CrossRef]
- Alavi-Tabari, S.A.; Khalilzadeh, M.A.; Karimi-Maleh, H. Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle. J. Electroanal. Chem. 2018, 811, 84–88. [Google Scholar] [CrossRef]
- Naser, S.S.; Ghosh, B.; Simnani, F.Z.; Singh, D.; Choudhury, A.; Nandi, A.; Sinha, A.; Jha, E.; Panda, P.K.; Suar, M.; et al. Emerging trends in the application of green synthesized biocompatible ZnO nanoparticles for translational paradigm in cancer therapy. J. Nanotheranostics 2023, 4, 248–279. [Google Scholar] [CrossRef]
- Zhu, P.; Weng, Z.; Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Biomedical applications of functionalized ZnO nanomaterials: From biosensors to bioimaging. Adv. Mater. Interfaces 2015, 3, 1500494. [Google Scholar] [CrossRef]
- Deshpande, M.V.; Amalnerkar, D.P. Biosensors prepared from electrochemically synthesized conducting polymers. Prog. Polym. Sci. 1993, 18, 623–649. [Google Scholar] [CrossRef]
- Lagopati, N.; Valamvanos, T.-F.; Proutsou, V.; Karachalios, K.; Pippa, N.; Gatou, M.-A.; Vagena, I.-A.; Cela, S.; Pavlatou, E.A.; Gazouli, M.; et al. The role of nano-sensors in breath analysis for early and non-invasive disease diagnosis. Chemosensors 2023, 11, 317. [Google Scholar] [CrossRef]
- Liu, H.; Ge, J.; Ma, E.; Yang, L. Advanced biomaterials for biosensor and theranostics. In Biomaterials in Translational Medicine; Woodhead Publishing: Sawston, UK, 2019; pp. 213–255. [Google Scholar] [CrossRef]
- Ahmad, R.; Mahmoudi, T.; Ahn, M.-S.; Hahn, Y.-B. Recent advances in nanowires-based field-effect transistors for biological sensor applications. Biosens. Bioelectron. 2018, 100, 312–325. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Jang, N.K.; Khang, G.; Hahn, Y.-B. Fabrication of highly sensitive uric acid biosensor based on directly grown ZnO nanosheets on electrode surface. Sens. Actuators B 2015, 206, 146–151. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Ahn, M.-S.; Hahn, Y.-B. Solution process synthesis of high aspect ratio ZnO nanorods on electrode surface for sensitive electrochemical detection of uric acid. Sci. Rep. 2017, 7, 46475. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Zhao, R.; Chen, G. A highly sensitive H2O2 sensor based on zinc oxide nanorod arrays film sensing interface. Analyst 2010, 135, 1992–1996. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, L.; Song, J.; Xing, R.; Xu, S.; Liu, D.; Song, H. Ultrasensitive non-enzymatic glucose sensor based on three-dimensional network of ZnO-CuO hierarchical nanocomposites by electrospinning. Sci. Rep. 2014, 4, 7382–7391. [Google Scholar] [CrossRef]
- Palanisamy, S.; Chen, S.; Sarawathi, R. A novel nonenzymatic hydrogen peroxide sensor based on reduced graphene oxide/ZnO composite modified electrode. Sens. Actuators B 2012, 166–167, 372–377. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Glucose Biosensors: 40 Years of Advances and Challenges. Electroanalysis 2001, 13, 983–988. [Google Scholar] [CrossRef]
- Wu, J.; Yin, F. Easy fabrication of a sensitive non-enzymatic glucose sensor based on electrospinning CuO-ZnO nanocomposites. Integr. Ferroelectr. 2013, 147, 47–58. [Google Scholar] [CrossRef]
- SoYoon, S.; Ramadoss, A.; Saravanakumar, B.; Kim, S.J. Novel Cu/CuO/ZnO hybrid hierarchical nanostructures for non-enzymatic glucose sensor application. J. Electroanal. Chem. 2014, 717–718, 90–95. [Google Scholar] [CrossRef]
- Marie, M.; Manoharan, A.; Kuchuk, A.; Ang, S.; Manasreh, M.O. Vertically grown zinc oxide nanorods functionalized with ferric oxide for in vivo and non-enzymatic glucose detection. Nanotechnology 2018, 29, 115501–115510. [Google Scholar] [CrossRef]
- Jung, D.-U.-J.; Ahmad, R.; Hahn, Y.B. Nonenzymatic flexible field-effect transistor-based glucose sensor fabricated using NiO quantum dots modified ZnO nanorods. J. Colloid Interface Sci. 2018, 512, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Yang, Y.H.; Yang, H.F.; Liu, Z.-M.; Shen, G.-L.; Yu, R.-Q. Nanosized flower-like ZnO synthesized by a simple hydrothermal method and applied as matrix for horseradish peroxidase immobilization for electro-biosensing. J. Inorg. Biochem. 2005, 99, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Ahn, M.S.; Yoo, J.Y.; Hahn, Y.B. Preparation of a highly conductive seed layer for calcium sensor fabrication with enhanced sensing performance. ACS Sens. 2018, 3, 772–778. [Google Scholar] [CrossRef]
- Wanas, W.; Abd El-Kaream, S.A.; Ebrahim, S.; Soliman, M.; Karim, M. Cancer bioimaging using dual mode luminescence of graphene/FA-ZnO nanocomposite based on novel green technique. Sci Rep. 2023, 13, 27. [Google Scholar] [CrossRef]
- Xiong, H.-M.; Xu, Y.; Ren, Q.-G.; Xia, Y.-Y. Stable aqueous ZnO@polymer core-shell nanoparticles with tunable photoluminescence and their application in cell imaging. J. Am. Chem. Soc. 2008, 130, 7522–7523. [Google Scholar] [CrossRef]
- Lv, Y.; Xiao, W.; Li, W.; Xue, J.; Ding, J. Controllable synthesis of ZnO nanoparticles with high intensity visible photoemission and investigation of its mechanism. Nanotechnology 2013, 24, 175702. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter Jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef]
- Janaki, A.C.; Sailatha, E.; Gunasekaran, S. Synthesis, characteristics and antimicrobial activity of ZnO nanoparticles. Spectrochim. Acta Part A 2015, 144, 17–22. [Google Scholar] [CrossRef]
- Cierech, M.; Wojnarowicz, J.; Szmigiel, D.; Bączkowski, B.; Grudniak, A.M.; Wolska, K.I.; Łojkowski, W.; Mierzwińska-Nastalska, E. Preparation and characterization of ZnO-PMMA resin nanocomposites for denture bases. Acta Bioeng. Biomech. 2016, 18, 31–41. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Yin, H.; Casey, P.S.; McCall, M.J.; Fenech, M. Effects of surface chemistry on cytotoxicity, genotoxicity, and the generation of reactive oxygen species induced by ZnO Nanoparticles. Langmuir 2010, 26, 15399–15408. [Google Scholar] [CrossRef] [PubMed]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced Bioactivity of ZnO nanoparticles-an antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef]
- Careta, O.; Fornell, J.; Pellicer, E.; Ibañez, E.; Blanquer, A.; Esteve, J.; Sort, J.; Murillo, G.; Nogués, C. ZnO nanosheet-coated TiZrPdSiNb alloy as a piezoelectric hybrid material for self-stimulating orthopedic implants. Biomedicines 2021, 9, 352. [Google Scholar] [CrossRef]
- Murillo, G.; Blanquer, A.; Vargas-Estevez, C.; Barrios, L.; Ibáñez, E.; Nogués, C.; Esteve, J. Electromechanical nanogenerator-cell interaction modulates cell activity. Adv. Mater. 2017, 29, 1605048. [Google Scholar] [CrossRef] [PubMed]
- Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Bogdał, D.; Matýsek, D. Hybrid bilayer PLA/chitosan nanofibrous scaffolds doped with ZnO, Fe3O4, and Au nanoparticles with bioactive properties for skin tissue engineering. Polymers 2020, 12, 159. [Google Scholar] [CrossRef]
- Blinov, A.V.; Kachanov, M.D.; Gvozdenko, A.A.; Nagdalian, A.A.; Blinova, A.A.; Rekhman, Z.A.; Golik, A.B.; Vakalov, D.S.; Maglakelidze, D.G.; Nagapetova, A.G.; et al. Synthesis and characterization of zinc oxide nanoparticles stabilized with biopolymers for application in wound-healing mixed gels. Gels 2023, 9, 57. [Google Scholar] [CrossRef]
- Raguvaran, R.; Manuja, B.K.; Chopra, M.; Thakur, R.; Anand, T.; Kalia, A.; Manuja, A. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int. J. Biol. Macromol. 2017, 96, 185–191. [Google Scholar] [CrossRef]
- Balaure, P.C.; Holban, A.M.; Grumezescu, A.M.; Mogoşanu, G.D.; Bălşeanu, T.A.; Stan, M.S.; Dinischiotu, A.; Volceanov, A.; Mogoantă, L. In vitro and in vivo studies of novel fabricated bioactive dressings based on collagen and zinc oxide 3D scaffolds. Int. J. Pharm. 2019, 557, 199–207. [Google Scholar] [CrossRef]
- Rakhshaei, R.; Namazi, H. A potential bioactive wound dressing based on carboxymethyl cellulose/ZnO impregnated MCM-41 nanocomposite hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 1, 456–464. [Google Scholar] [CrossRef]
- Nekounam, H.; Samani, S.; Samadian, H.; Shokrgozar, M.A.; Majidi, R.-F. Zinc oxide-carbon nanofiber (ZnO-CNF) nanocomposite for bone tissue engineering: An inquiry into structural, physical and biological properties. Mater. Chem. Phys. 2023, 295, 127052. [Google Scholar] [CrossRef]
- Amna, T.; Shamshi Hassan, M.; Khil, M.S.; Lee, H.K.; Hwang, I.H. Electrospun nano-fibers of ZnO-TiO2 hybrid: Characterization and potential as an extracellular scaffold for supporting myoblasts. Surf. Interface Anal. 2014, 46, 72–76. [Google Scholar] [CrossRef]
- Dias, H.B.; Bernardi, M.I.B.; Marangoni, V.S.; de Abreu Bernardi, A.C.; de Souza Rastelli, A.N.; Hernandes, A.C. Synthesis, characterization and application of Ag doped ZnO nanoparticles in a composite resin. Mater. Sci. Eng. C 2019, 96, 391–401. [Google Scholar] [CrossRef]
- Hassan, A.; Elebeedy, D.; Matar, E.R.; Fahmy Mohamed Elsayed, A.; Abd El Maksoud, A.I. Investigation of angiogenesis and wound healing potential mechanisms of zinc oxide nanorods. Front. Pharmacol. 2021, 12, 661217. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, H.; Guo, M.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species in vascular formation and development. Oxidative Med. Cell. Longev. 2013, 2013, e374963. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Xiong, H.-M. Biological applications of ZnO nanoparticles. Curr. Mol. Imaging 2013, 2, 177–192. [Google Scholar] [CrossRef]
- Peng, H.; Cui, B.; Li, G.; Wang, Y.; Li, N.; Chang, Z.; Wang, Y. A Multifunctional β-CD-modified Fe3O4@ZnO:Er(3+),Yb(3+) nanocarrier for antitumor drug delivery and microwave-triggered drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 253–263. [Google Scholar] [CrossRef]
- Xiong, H.-M. ZnO nanoparticles applied to bioimaging and drug delivery. Adv. Mater. 2013, 25, 5329–5335. [Google Scholar] [CrossRef]
- Thomas, C.R.; Ferris, D.P.; Lee, J.-H.; Choi, E.; Cho, M.H.; Kim, E.S.; Stoddart, J.F.; Shin, J.-S.; Cheon, J.; Zink, J.I. Noninvasive remote-controlled release of drug molecules in vitro using magnetic actuation of mechanized nanoparticles. J. Am. Chem. Soc. 2010, 132, 10623–10625. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, J.-S.; Zhang, P.; Chen, J.; Kong, J.-L.; Sun, L.-H.; Xiong, H.-M.; Möhwald, H. Self-assembled ZnO nanoparticle capsules for carrying and delivering isotretinoin to cancer cells. ACS Appl. Mater. Interfaces 2017, 9, 18474–18481. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.-B.; Sarrafzadeh, M.-H.; Fakhroueian, Z.; Shahriari, S.; Khorramizadeh, M.R. Functionalization of ZnO nanoparticles by 3-mercaptopropionic acid for aqueous curcumin delivery: Synthesis, characterization, and anticancer assessment. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Puvvada, N.; Rajput, S.; Kumar, B.N.P.; Sarkar, S.; Konar, S.; Brunt, K.R.; Rao, R.R.; Mazumdar, A.; Das, S.K.; Basu, R.; et al. Novel ZnO hollow nanocarriers containing Paclitaxel targeting folate-receptors in a malignant pH-microenvironment for effective monitoring and promoting breast tumor regression. Sci. Rep. 2015, 5, 11760. [Google Scholar] [CrossRef] [PubMed]
- Perera, W.P.T.D.; Dissanayake, D.M.R.K.; Unagolla, J.M.; De Silva, R.T.; Bathige, S.D.N.K.; Pahalagedara, L.R. Albumin grafted coaxial electrosparyed polycaprolactone-zinc oxide nanoparticle for sustained release and activity enhanced antibacterial drug delivery. RSC Adv. 2022, 12, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, R.; Senthilkumar, S.; Suganthi, A.; Rajarajan, M. Synthesis and characterization of Doxorubicin modified ZnO/PEG nanomaterials and its photodynamic action. J. Photochem. Photobiol. B 2012, 116, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, H. The synergistic effect and mechanism of Doxorubicin-ZnO nano-complexes as a multimodal agent integrating diverse anticancer therapeutics. Int. J. Nanomed. 2013, 8, 1835–1841. [Google Scholar] [CrossRef]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Yan, H.; Du, D.; Lin, Y. pH-responsive ZnO nanocluster for lung cancer chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 5739–5747. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, C.; Li, A.; Wang, J.; Cai, X. Red fluorescent ZnO nanoparticle grafted with polyglycerol and conjugated RGD peptide as drug delivery vehicles for efficient target cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 104–113. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef]
- Torres-Sangiao, E.; Holban, A.M.; Gestal, M.C. Advanced nanobiomaterials: Vaccines, diagnosis and treatment of infectious diseases. Molecules 2016, 21, 867. [Google Scholar] [CrossRef]
- Cho, N.H.; Cheong, T.C.; Min, J.H.; Wu, J.; Lee, S.J.; Kim, D.; Yang, J.S.; Kim, S.; Kim, Y.K.; Seong, S.Y. A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat. Nanotechnol. 2011, 6, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Ramani, M.; Mudge, M.C.; Morris, R.T.; Zhang, Y.; Warcholek, S.A.; Hurst, M.N.; Riviere, J.E.; DeLong, R.K. Zinc oxide nanoparticle-poly I:C RNA complexes: Implication as therapeutics against experimental melanoma. Mol. Pharm. 2017, 14, 614–625. [Google Scholar] [CrossRef]
- Sharma, P.; Shin, J.B.; Park, B.C.; Lee, J.W.; Byun, S.W.; Jang, N.Y.; Kim, Y.J.; Kim, Y.; Kim, Y.K.; Cho, N.H. Application of radially grown ZnO nanowires on poly-l-lactide microfibers complexed with a tumor antigen for cancer immunotherapy. Nanoscale 2019, 11, 4591–4600. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles—A review. Environ. Pollut. 2013, 172, 76–85. [Google Scholar] [CrossRef]
- Brayner, R.; Dahoumane, S.A.; Yéprémian, C.; Djediat, C.; Meyer, M.; Couté, A.; Fiévet, F. ZnO nanoparticles: Synthesis, characterization, and ecotoxicological studies. Langmuir 2010, 26, 6522–6528. [Google Scholar] [CrossRef] [PubMed]
- Blinova, I.; Ivask, A.; Heinlaan, M.; Mortimer, M.; Kahru, A. Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environ. Pollut. 2010, 158, 41–47. [Google Scholar] [CrossRef]
- Khare, P.; Sonane, M.; Pandey, R.; Ali, S.; Gupta, K.C.; Satish, A. Adverse effects of TiO2 and ZnO nanoparticles in soil nematode, Caenorhabditis elegans. J. Biomed. Nanotechnol. 2011, 7, 116–117. [Google Scholar] [CrossRef]
- Manzo, S.; Rocco, A.; Carotenuto, R.; De Luca Picione, F.; Miglietta, M.L.; Rametta, G.; Di Francia, G. Investigation of ZnO nanoparticles’ ecotoxicological effects towards different soil organisms. Environ. Sci. Pollut. Res. 2011, 18, 756–763. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, Z.; Tian, W.; He, X.; Ma, Y.; Zhao, Y.; Chai, Z. Toxicity of zinc oxide nanoparticles to zebrafish embryo: A physicochemical study of toxicity mechanism. J. Nanopart. Res. 2010, 12, 1645–1654. [Google Scholar] [CrossRef]
- Cho, W.S.; Duffin, R.; Howie, S.E.; Scotton, C.J.; Wallace, W.A.; MacNee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part. Fibre Toxicol. 2011, 8, 27. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; De Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef]
- Adamcakova-Dodd, A.; Stebounova, L.V.; Kim, J.S.; Vorrink, S.U.; Ault, A.P.; O’Shaughnessy, P.T.; Grassian, V.H.; Thorne, P.S. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part. Fibre Toxicol. 2014, 11, 15. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Wiench, K.; Landsiedel, R.; Schulte, S.; Inman, A.O.; Riviere, J.E. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in UVB sunburned skin: An in vitro and in vivo study. Toxicol. Sci. 2011, 123, 264–280. [Google Scholar] [CrossRef]
- Sruthi, S.; Valappil Mohanan, P. Engineered zinc oxide nanoparticles; biological interactions at the organ level. Curr. Med. Chem. 2016, 23, 4057–4068. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Lin, B.; Wu, L.; Li, K.; Liu, H.; Yan, J.; Liu, X.; Xi, Z. Neurotoxicity induced by zinc oxide nanoparticles: Age-related differences and interaction. Sci. Rep. 2015, 5, 16117. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, S.; Kleinsasser, N. Dermal toxicity of ZnO nanoparticles: A worrying feature of sunscreen? Nanomedicine 2012, 7, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.Y.; Cheng, T.J.; Yang, D.M.; Wang, C.T.; Chiung, Y.M.; Liu, P.S. Demonstration of an olfactory bulb-brain translocation pathway for ZnO nanoparticles in rodent cells in vitro and in vivo. J. Mol. Neurosci. 2012, 48, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Luan, Q.; Chen, W.; Wang, Y.; Wu, M.; Zhang, H.; Jiao, Z. Nanosized zinc oxide particles induce neural stem cell apoptosis. Nanotechnology 2009, 20, 115101. [Google Scholar] [CrossRef]
- Yin, Y.; Lin, Q.; Sun, H.; Chen, D.; Wu, Q.; Chen, X.; Li, S. Cytotoxic effects of ZnO hierarchical architectures on RSC96 Schwann cells. Nanoscale Res. Lett. 2012, 7, 439. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Costa, C.; Kiliç, G.; Costa, S.; Pásaro, E.; Laffon, B.; Teixeira, J.P. Neuronal cytotoxicity and genotoxicity induced by zinc oxide nanoparticles. Environ. Int. 2013, 55, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Kang, B.C.; Lee, J.K.; Jeong, J.; Che, J.H.; Seok, S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, Y.; Zhang, T.; Ren, G.; Yang, Z. Effects of nanoparticle zinc oxide on spatial cognition and synaptic plasticity in mice with depressive-like behaviors. J. Biomed. Sci. 2012, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Ansar, S.; Abudawood, M.; Hamed, S.S.; Aleem, M.M. Exposure to zinc oxide nanoparticles induces neurotoxicity and proinflammatory response: Amelioration by hesperidin. Biol. Trace Elem. Res. 2017, 175, 360–366. [Google Scholar] [CrossRef]
- Amara, S.; Slama, I.B.; Omri, K.; Ghoul, J.E.; Mir, L.E.; Rhouma, K.B.; Abdelmelek, H.; Sakly, M. Effects of nanoparticle zinc oxide on emotional behavior and trace elements homeostasis in rat brain. Toxicol. Ind. Health 2015, 31, 1202–1209. [Google Scholar] [CrossRef]
- Streit, W.J.; Xue, Q.S. Life and death of microglia. J. Neuroimmune Pharmacol. 2009, 4, 371–379. [Google Scholar] [CrossRef]
- Jessen, K.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef]
- Colton, C.A.; Gilbert, D.L. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 1987, 223, 284–288. [Google Scholar] [CrossRef]
- Block, L.M.; Hong, J.-S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Long, T.C.; Saleh, N.; Tilton, R.D.; Lowry, G.V.; Veronesi, B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2006, 40, 4346–4352. [Google Scholar] [CrossRef] [PubMed]
- Schlegelmilch, T.; Henke, K.; Peri, F. Microglia in the developing brain: From immunity to behaviour. Curr. Opin. Neurobiol. 2011, 21, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, D.; Sargsyan, S.; Monk, P.N.; Shaw, P.J. Astrocyte function and role in motor neuron disease: A future therapeutic target? Glia 2009, 57, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Swanson, R.A. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 2003, 23, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Zheng, H.; Zhang, Z.; Li, M.; Huang, Z.; Schluesener, H.J.; Li, Y.; Xu, S. Glia activation induced by peripheral administration of aluminum oxide nanoparticles in rat brains. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Ramírez, S.G.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Iglesias, G.G.; López-Marure, R. Titanium dioxide nanoparticles inhibit proliferation and induce morphological changes and apoptosis in glial cells. Toxicology 2012, 302, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Ostrovsky, S.; Kazimirsky, G.; Gedanken, A.; Brodie, C. Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res. 2009, 2, 882–890. [Google Scholar] [CrossRef]
- Panyala, N.R.; Peña-Méndez, E.M.; Havel, J. Gold and nano-gold in medicine: Overview, toxicology and perspectives. J. Appl. Biomed. 2009, 7, 75–91. [Google Scholar] [CrossRef]
- Kessler, R. Engineered nanoparticles in consumer products: Understanding a new ingredient. Environ. Health Perspect. 2011, 119, A120–A125. [Google Scholar] [CrossRef]
- Soenen, S.J.; Rivera-Gil, P.; Montenegro, J.-M.; Parak, W.J.; De Smedt, S.C.; Braeckmans, K. Cellular toxicity of inorganic nanoparticles: Common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 2011, 6, 446–465. [Google Scholar] [CrossRef]
- Xia, T.; Zhao, Y.; Sager, T.; George, S.; Pokhrel, S.; Li, N.; Schoenfeld, D.; Meng, H.; Lin, S.; Wang, X.; et al. Decreased dissolution of ZnO by iron doping yields nanoparticles with reduced toxicity in the rodent lung and Zebrafish embryos. ACS Nano 2011, 5, 1223–1235. [Google Scholar] [CrossRef]
- Yamabi, S.; Imai, H. Growth conditions for wurtzite zinc oxide films in aqueous solutions. J. Mater. Chem. 2002, 12, 3773–3778. [Google Scholar] [CrossRef]
- Kumar, S.G.; Koteswara Rao, K.S.R. Zinc oxide based photocatalysis: Tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 2012, 17, 852–870. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Kelleher, S.L. Cellular mechanisms of zinc dysregulation: A perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients 2012, 4, 875–903. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Metal binding affinity and selectivity in metalloproteins: Insights from computational studies. Annu. Rev. Biophys. 2008, 37, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Huang, Y.; Bu, Q.; Lv, L.; Deng, P.; Zhou, J.; Wang, Y.; Yang, Y.; Liu, Q.; Cen, X.; et al. Zinc oxide nanoparticles cause nephrotoxicity and kidney metabolism alterations in rats. J. Environ. Sci. Health Part A 2012, 47, 577–588. [Google Scholar] [CrossRef]
- Wilhelmi, V.; Fischer, U.; Weighardt, H.; Schulze-Osthoff, K.; Nickel, C.; Stahlmecke, B.; Kuhlbusch, T.A.G.; Scherbart, A.M.; Esser, C.; Schins, R.P.F.; et al. Zinc oxide nanoparticles induce necrosis and apoptosis in macrophages in a p47phox- and Nrf2-independent manner. PLoS ONE 2013, 8, e65704. [Google Scholar] [CrossRef]
- Frohlich, E. Cellular targets and mechanisms in the cytotoxic action of non-biodegradable engineered nanoparticles. Curr. Drug Metab. 2013, 14, 976–988. [Google Scholar] [CrossRef]
- Guo, D.; Bi, H.; Wang, D.; Wu, Q. Zinc oxide nanoparticles decrease the expression and activity of plasma membrane calcium ATPase, disrupt the intracellular calcium homeostasis in rat retinal ganglion cells. Int. J. Biochem. Cell Biol. 2013, 45, 1849–1859. [Google Scholar] [CrossRef]
- Luo, M.; Shen, C.; Feltis, B.N.; Martin, L.L.; Hughes, A.E.; Wright, P.F.; Turney, T.W. Reducing ZnO nanoparticle cytotoxicity by surface modification. Nanoscale 2014, 6, 5791–5798. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Prooxidant Mech. Toxicol. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Ng, K.W.; Khoo, S.P.K.; Heng, B.C.; Setyawati, M.I.; Tan, E.C.; Zhao, X.; Xiong, S.; Fang, W.; Leong, D.T.; Loo, J.S.C. The role of the tumor suppressor p53 pathway in the cellular DNA damage response to zinc oxide nanoparticles. Biomaterials 2011, 32, 8218–8225. [Google Scholar] [CrossRef] [PubMed]
- Simón-Vázquez, R.; Lozano-Fernández, T.; Dávila-Grana, A.; González-Fernández, A. Analysis of the activation routes induced by different metal oxide nanoparticles on human lung epithelial cells. Future Sci. OA 2016, 2, FSO118. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Singh, S.K.; Das, M.; Tripathi, A.; Dwivedi, P.D. Toll-like receptor 6 mediated inflammatory and functional responses of zinc oxide nanoparticles primed macrophages. Immunology 2014, 142, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.; Teodoro, T.; Volchuk, A. Endoplasmic reticulum stress: Signaling the unfolded protein response. Physiology 2007, 22, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shao, H.; Liu, W.; Gu, W.; Shu, X.; Mo, Y.; Chen, X.; Zhang, Q.; Jiang, M. Endoplasmic reticulum stress and oxidative stress are involved in ZnO nanoparticle-induced hepatotoxicity. Toxicol. Lett. 2015, 234, 40–49. [Google Scholar] [CrossRef]
- Chen, R.; Huo, L.; Shi, X.; Bai, R.; Zhang, Z.; Zhao, Y.; Chang, Y.; Chen, C. Endoplasmic reticulum stress induced by zinc oxide nanoparticles is an earlier biomarker for nanotoxicological evaluation. ACS Nano 2014, 8, 2562–2574. [Google Scholar] [CrossRef]
- Moos, P.J.; Olszewski, K.; Honeggar, M.; Cassidy, P.; Leachman, S.; Woessner, D.; Shane Cutler, N.; Veranth, J.M. Responses of human cells to ZnO nanoparticles: A gene transcription study. Metallomics 2011, 3, 1199–1211. [Google Scholar] [CrossRef]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wang, J.; Chen, A.; Liu, J.; Feng, X.; Shao, L. Involvement of PINK1/parkin-mediated mitophagy in ZnO nanoparticle-induced toxicity in BV-2 cells. Int. J. Nanomed. 2017, 12, 1891–1903. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, D.; Liu, W.; Yan, Y.; Zhou, F.; Wu, W.; Yan, Z. Reactive oxygen species trigger NF-κB-mediated NLRP3 inflammasome activation induced by zinc oxide nanoparticles in A549 cells. Toxicol. Ind. Health 2017, 33, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Pujalté, I.; Passagne, I.; Daculsi, R.; de Portal, C.; Ohayon-Courtès, C.; L’Azou, B. Cytotoxic effects and cellular oxidative mechanisms of metallic nanoparticles on renal tubular cells: Impact of particle solubility. Toxicol. Res. 2015, 4, 409–422. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Ge, W.; Zhang, P.; Liu, X.; Zhang, W.; Hao, Y.; Yu, S.; Li, L.; Chu, M.; et al. Oocyte exposure to ZnO nanoparticles inhibits early embryonic development through the γ-H2AX and NF-κB signaling pathways. Oncotarget 2017, 8, 42673–42692. [Google Scholar] [CrossRef]

| Nanocomposite | Sensor | Reference |
|---|---|---|
| ZnO NRs/ITO | Non enzymatic | [167] |
| ZnO-CuO/FTO | Non enzymatic | [168] |
| RGO/ZnO film | Non enzymatic | [169] |
| ZnO-CuO nanofibers | Glucose | [172] |
| Cu/CuO/ZnO | Glucose | [173] |
| ZnO/Fe2O3/FTO | Glucose | [174] |
| NiO QDs/ZnO | Glucose | [175] |
| ZnO/chitosan | Enzymatic | [176] |
| ZnO/Ag | Enzymatic | [177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagena, I.-A.; Gatou, M.-A.; Theocharous, G.; Pantelis, P.; Gazouli, M.; Pippa, N.; Gorgoulis, V.G.; Pavlatou, E.A.; Lagopati, N. Functionalized ZnO-Based Nanocomposites for Diverse Biological Applications: Current Trends and Future Perspectives. Nanomaterials 2024, 14, 397. https://doi.org/10.3390/nano14050397
Vagena I-A, Gatou M-A, Theocharous G, Pantelis P, Gazouli M, Pippa N, Gorgoulis VG, Pavlatou EA, Lagopati N. Functionalized ZnO-Based Nanocomposites for Diverse Biological Applications: Current Trends and Future Perspectives. Nanomaterials. 2024; 14(5):397. https://doi.org/10.3390/nano14050397
Chicago/Turabian StyleVagena, Ioanna-Aglaia, Maria-Anna Gatou, Giorgos Theocharous, Pavlos Pantelis, Maria Gazouli, Natassa Pippa, Vassilis G. Gorgoulis, Evangelia A. Pavlatou, and Nefeli Lagopati. 2024. "Functionalized ZnO-Based Nanocomposites for Diverse Biological Applications: Current Trends and Future Perspectives" Nanomaterials 14, no. 5: 397. https://doi.org/10.3390/nano14050397
APA StyleVagena, I.-A., Gatou, M.-A., Theocharous, G., Pantelis, P., Gazouli, M., Pippa, N., Gorgoulis, V. G., Pavlatou, E. A., & Lagopati, N. (2024). Functionalized ZnO-Based Nanocomposites for Diverse Biological Applications: Current Trends and Future Perspectives. Nanomaterials, 14(5), 397. https://doi.org/10.3390/nano14050397











