Solid Electrochemiluminescence Sensor by Immobilization of Emitter Ruthenium(II)tris(bipyridine) in Bipolar Silica Nanochannel Film for Sensitive Detection of Oxalate in Serum and Urine
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Characterizations and Instrumentations
2.3. Preparation of an n-SNA- or bp-SNA-Modified ITO Electrode
2.4. Immobilization of Ru(bpy)32+ on bp-SNA/ITO
2.5. ECL Detection of Oxalate Ions
3. Results and Discussion
3.1. The Strategy for the Construction of Solid-State ECL Sensors
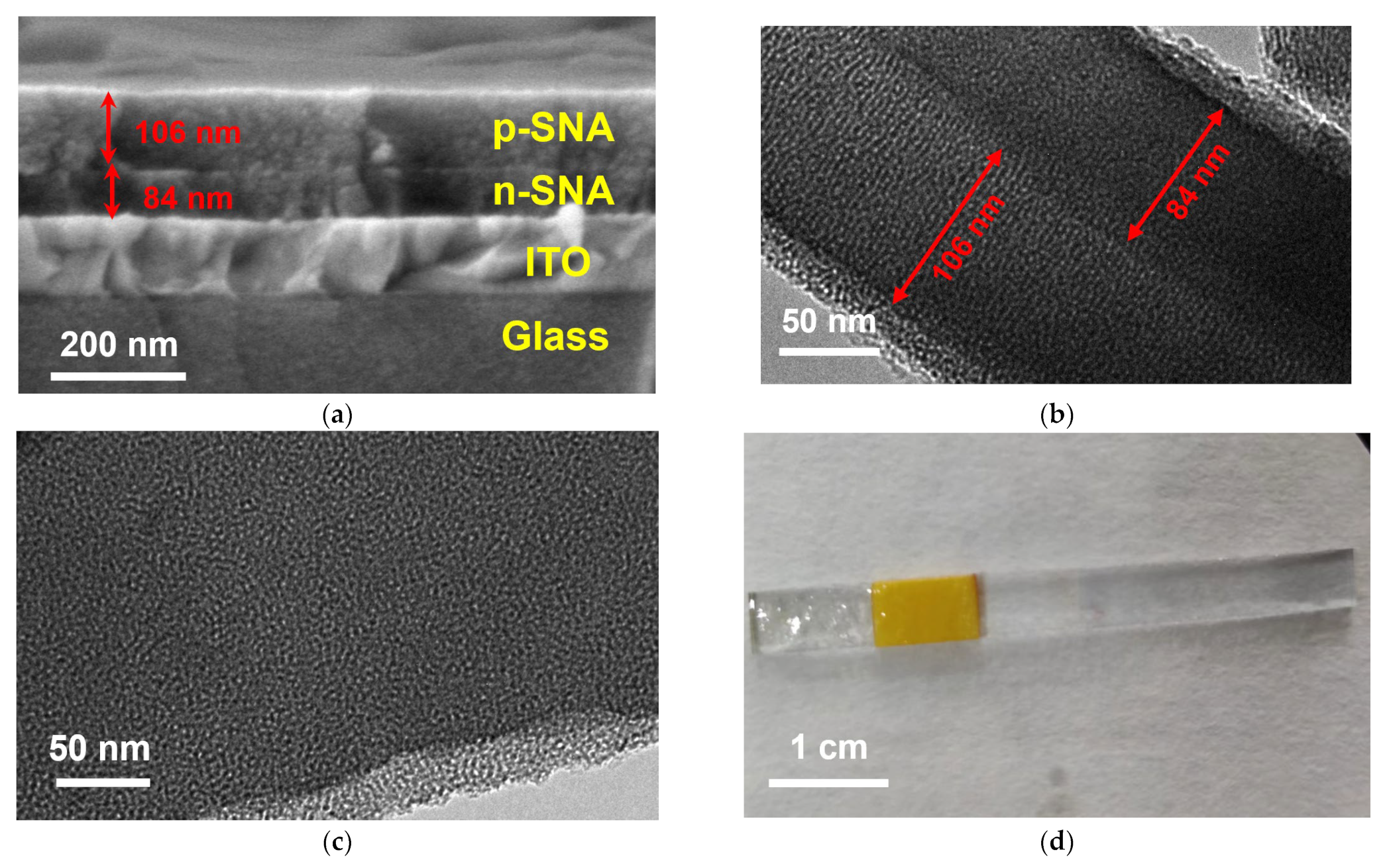
3.2. Characterization of the bp-SNA/ITO Electrode
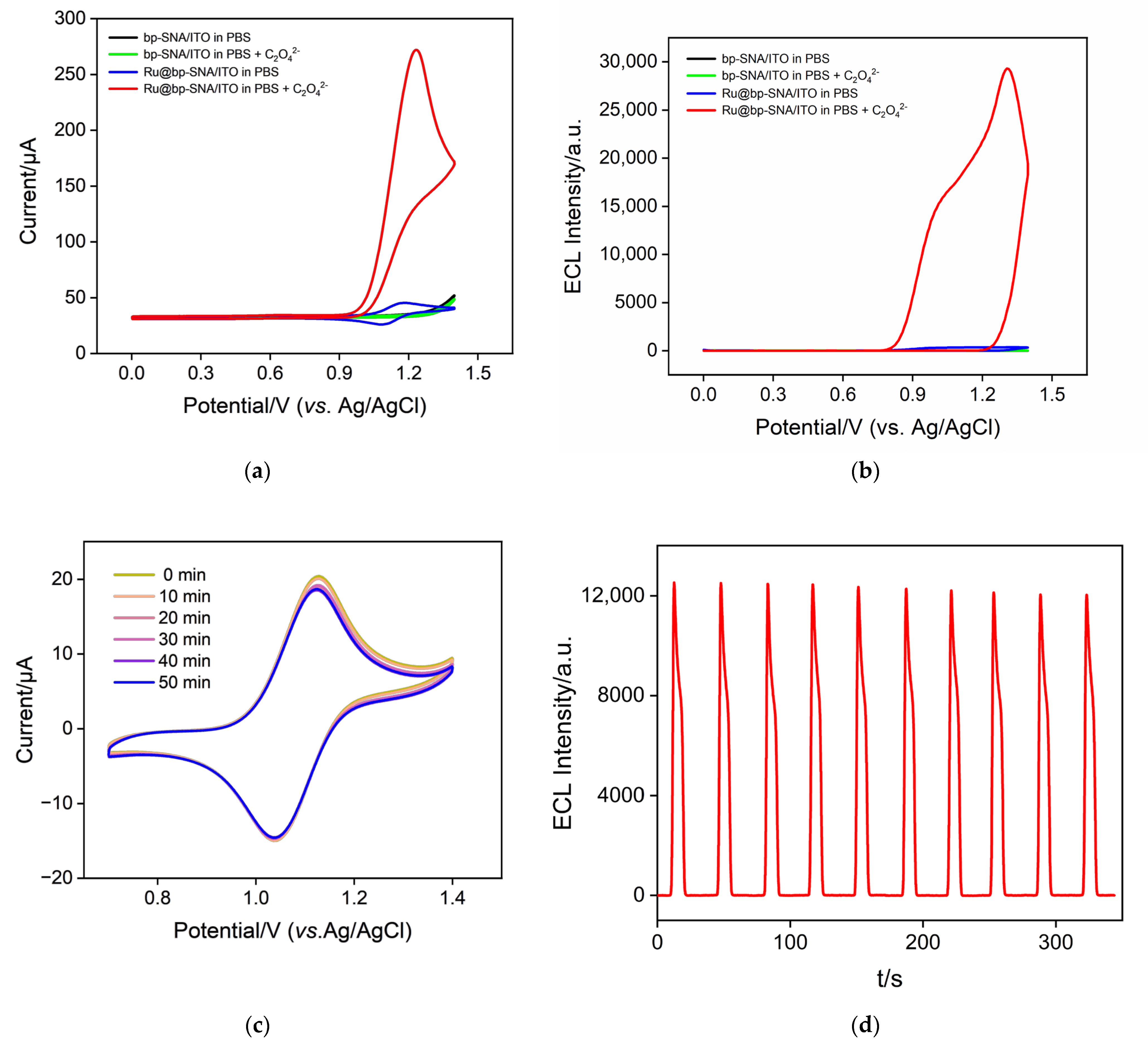
3.3. ECL Process of Ru(bpy)32+ Using Oxalate Ions as Co-Reactants
3.4. Stability of the Immobilized Ru(bpy)32+
3.5. Optimization Conditions for Ru@bp-SNA/ITO Construction and Oxalate Detection
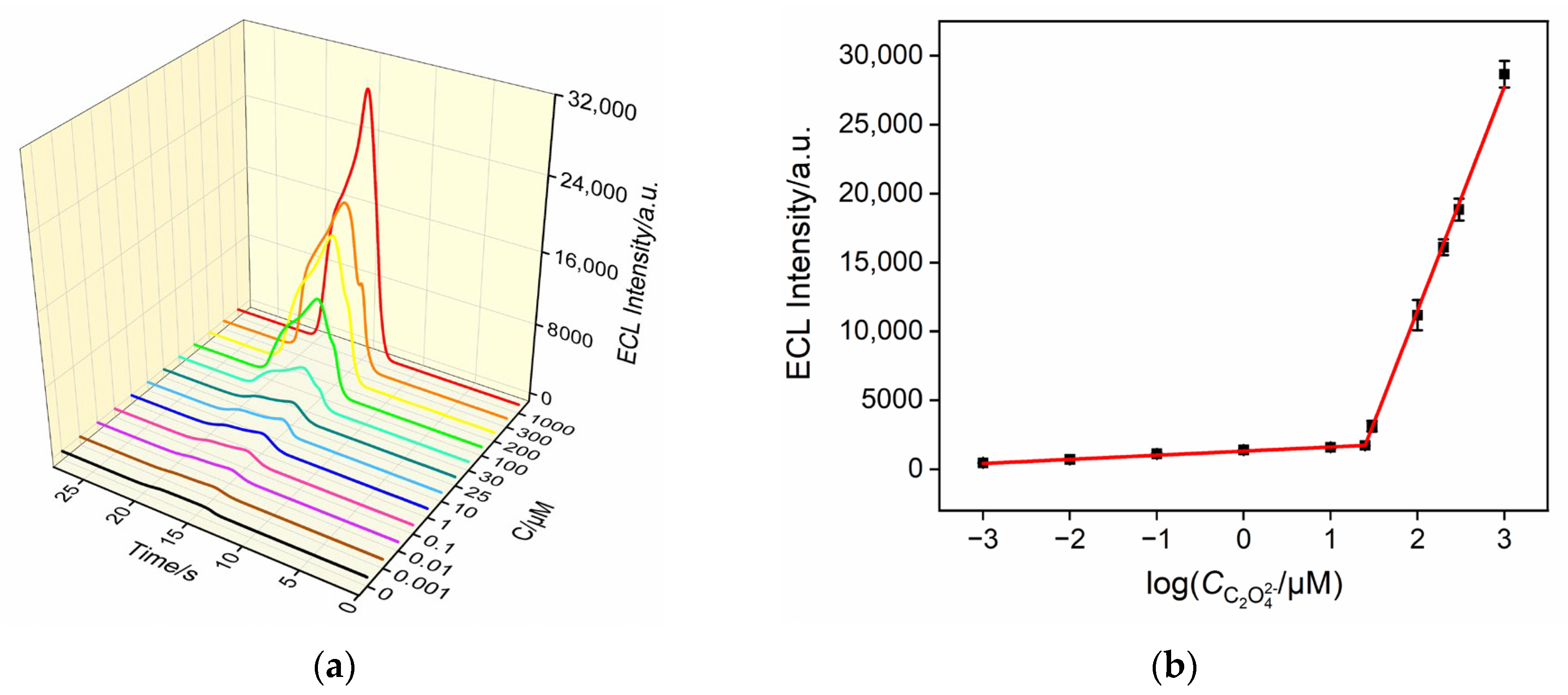
3.6. ECL Detection of Oxalate
3.7. Selectivity, Reproducibility, and Stability of Detection
3.8. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arafa, A.; Eshak, E.S.; Iso, H. Oxalates, urinary stones and risk of cardiovascular diseases. Med. Hypotheses 2020, 137, 109570. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Devlin, J.C.; Hu, J.; Volkova, A.; Battaglia, T.W.; Ho, M.; Asplin, J.R.; Byrd, A.; Loke, P.; Li, H.; et al. Microbial genetic and transcriptional contributions to oxalate degradation by the gut microbiota in health and disease. eLife 2021, 10, e63642. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.W.; Choy, D.; Penniston, K.L.; Lange, D. Inhibition of urinary stone disease by a multi-species bacterial network ensures healthy oxalate homeostasis. Kidney Int. 2019, 96, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Zaguri, D.; Shaham-Niv, S.; Naaman, E.; Mimouni, M.; Magen, D.; Pollack, S.; Kreiser, T.; Leibu, R.; Rencus-Lazar, S.; Adler-Abramovich, L.; et al. Induction of retinopathy by fibrillar oxalate assemblies. Commun. Chem. 2020, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Marickar, Y.M. Calcium oxalate stone and gout. Urol. Res. 2009, 37, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Liu, J.; Yan, X.; Hu, S. Identification of metabolite biomarkers for gout using capillary ion chromatography with mass spectrometry. Anal. Chem. 2017, 89, 11737–11743. [Google Scholar] [CrossRef]
- Mitchell, T.; Kumar, P.; Reddy, T.; Wood, K.D.; Knight, J.; Assimos, D.G.; Holmes, R.P. Dietary oxalate and kidney stone formation. Am. J. Physiol. Renal. Physiol. 2019, 316, F409–F413. [Google Scholar] [CrossRef]
- Song, Q.; Liao, W.; Chen, X.; He, Z.; Li, D.; Li, B.; Liu, J.; Liu, L.; Xiong, Y.; Song, C.; et al. Oxalate activates autophagy to induce ferroptosis of renal tubular epithelial cells and participates in the formation of kidney stones. Oxid Med. Cell Longev. 2021, 2021, 6630343. [Google Scholar] [CrossRef]
- Chen, T.; Qian, B.; Zou, J.; Luo, P.; Zou, J.; Li, W.; Chen, Q.; Zheng, L. Oxalate as a potent promoter of kidney stone formation. Front. Med. 2023, 10, 1159616. [Google Scholar] [CrossRef]
- Eisner, B.H.; Porten, S.P.; Bechis, S.K.; Stoller, M.L. Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. J. Urol. 2010, 183, 2244–2248. [Google Scholar] [CrossRef]
- Pfau, A.; Ermer, T.; Coca, S.G.; Tio, M.C.; Genser, B.; Reichel, M.; Finkelstein, F.O.; Marz, W.; Wanner, C.; Waikar, S.S.; et al. High oxalate concentrations correlate with increased risk for sudden cardiac death in dialysis patients. J. Am. Soc. Nephrol. 2021, 32, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhou, S.; Wang, X.; Liang, T.; Liu, X.; Wang, P.; Wan, H. Enzyme-based color bar-style lateral flow strip for equipment-free and semi-quantitative determination of urinary oxalate. Sens. Actuators B Chem. 2023, 385, 133699. [Google Scholar] [CrossRef]
- Suryavanshi, M.V.; Jadhav, S.D.; Gune, R.P.; Bhatia, M.S.; Shouche, Y.S. HPLC analysis of human urine for oxalate content. Int. J. Pharm. Pharm. Sci. 2016, 8, 54–59. [Google Scholar] [CrossRef][Green Version]
- Vernel-Pauillac, F.; Merien, F. A novel real-time duplex PCR assay for detecting penA and ponA genotypes in Neisseria gonorrhoeae: Comparison with phenotypes determined by the E-test. Clin. Chem. 2006, 52, 2294–2296. [Google Scholar] [CrossRef]
- Marshall, D.J.; Adaway, J.E.; Keevil, B.G. A combined liquid chromatography tandem mass spectrometry assay for the quantification of urinary oxalate and citrate in patients with nephrolithiasis. Ann. Clin. Biochem. 2018, 55, 461–468. [Google Scholar] [CrossRef]
- Arantes, I.V.S.; Crapnell, R.D.; Bernalte, E.; Whittingham, M.J.; Paixao, T.; Banks, C.E. Mixed graphite/carbon black recycled PLA conductive additive manufacturing filament for the electrochemical detection of oxalate. Anal. Chem. 2023, 95, 15086–15093. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Bai, Y.; Liu, Q.; Yan, L.; Long, T.; Li, M.; Huang, J.; Ying, B.; Chen, P. Three-fluorescence sensor for minute-time scale low-cost analysis of urinary oxalate in urolithiasis metabolic assessment. Anal. Chim. Acta 2023, 1237, 340586. [Google Scholar] [CrossRef]
- Patra, A.; Chakraborty, S.; Lohar, S.; Zangrando, E.; Chattopadhyay, P. A phenolato-bridged dinuclear Ni(II) complex for selective fluorescent sensing of oxalate in aqueous medium. Inorg. Chim. Acta 2021, 525, 120493. [Google Scholar] [CrossRef]
- Zhao, S.; Yin, D.; Du, H.; Tian, X.; Chen, Y.; Zhang, W.; Yu, A.; Zhang, S. Determination of oxalate and citrate in urine by capillary electrophoresis using solid-phase extraction and capacitively coupled contactless conductivity based on an improved mini-cell. J. Sep. Sci. 2018, 41, 2623–2631. [Google Scholar] [CrossRef]
- Liu, R.; Xu, H.; Xiao, C.; Liu, H.; Zhong, S.; Zeng, C.-H. Preparation, luminescence and highly sensitive oxalate sensor of porous EuBO3 microwafers. Opt. Mater. 2018, 86, 360–365. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; Qu, h.; Xi, F. Immunosensor with enhanced electrochemiluminescence signal using platinum nanoparticles confined within nanochannels for highly sensitive detection of carcinoembryonic antigen. Molecules 2023, 28, 6559. [Google Scholar] [CrossRef]
- Huang, J.; Xu, S.; Yan, F.; Liu, J. Electrochemiluminescence enzyme biosensors for ultrasensitive determination of glucose using glucose dehydrogenase immobilized on vertical silica nanochannels. Sens. Actuators B Chem. 2024, 402, 135119. [Google Scholar] [CrossRef]
- Chang, Q.; Gu, X.; He, L.; Xi, F. A highly sensitive immunosensor based on nanochannel-confined nano-gold enhanced electrochemiluminescence for procalcitonin detection. Front. Chem. 2023, 11, 1274424. [Google Scholar] [CrossRef]
- Chen, H.; Huang, J.; Zhang, R.; Yan, F. Dual-mode electrochemiluminescence and electrochemical sensor for alpha-fetoprotein detection in human serum based on vertically ordered mesoporous silica films. Front. Chem. 2022, 10, 1023998. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xu, M.; Li, J.; Li, Y.; Hua, D. Visualized electrochemiluminescence iodine sensor based on polymer dots with Co-reactive group for real-time monitoring system. Talanta 2023, 257, 124369. [Google Scholar] [CrossRef]
- Hai, Y.; Yuan, H.; Xiao, D. High electrochemiluminescence intensity of the Ru(bpy)32+/oxalate system on a platinum net electrode. Microchim. Acta 2006, 157, 127–131. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.; Mao, H.; You, T. Multifunctional solid-state electrochemiluminescence sensing platform based on poly(ethylenimine) capped N-doped carbon dots as novel co-reactant. Biosens. Bioelectron. 2017, 89, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xu, J.; Yao, Q.; Xu, X.; Chen, X.; Ni, J.; Wang, Q.; Lin, Z. Electrophoretic deposition of Ru(bpy)(3)(2+) in vertically-ordered silica nanochannels: A solid-state electrochemiluminescence sensor for prolidase assay. Biosens. Bioelectron. 2024, 247, 115967. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Han, Q.; Xi, F. The fabrication of a probe-integrated electrochemiluminescence aptasensor based on double-layered nanochannel array with opposite charges for the sensitive determination of C-reactive protein. Molecules 2023, 28, 7867. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhang, T.; Luo, T.; Luo, X.; Yan, F.; Tang, W.; Liu, J. Bipolar silica nanochannel array confined electrochemiluminescence for ultrasensitive detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2022, 215, 114563. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Ratiometric fluorescent nanohybrid for noninvasive and visual monitoring of sweat glucose. ACS Sens. 2020, 5, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Graphene quantum dot-decorated luminescent porous silicon dressing for theranostics of diabetic wounds. Acta Biomater. 2021, 131, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xuan, L.; Gong, J.; Liu, J.; Wang, X.; Xi, F.; Chen, J. Three-dimensional macroscopic graphene supported vertically-ordered mesoporous silica-nanochannel film for direct and ultrasensitive detection of uric acid in serum. Talanta 2022, 238, 123027. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, S.; Zhou, X.; Yan, F.; Hu, W. Silica nanochannel array on co-electrodeposited graphene-carbon nanotubes 3D composite film for antifouling detection of uric acid in human serum and urine samples. Microchem. J. 2023, 190, 108632. [Google Scholar] [CrossRef]
- Zhao, J.; Duan, W.; Liu, X.; Xi, F.; Wu, J. Microneedle patch integrated with porous silicon confined dual nanozymes for synergistic and hyperthermia-enhanced nanocatalytic ferroptosis treatment of melanoma. Adv. Funct. Mater. 2023, 33, 2308183. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Wang, T.; Jiang, X.; Qu, X.; Duan, W.; Xi, F.; He, Z.; Wu, J. Tissue imprinting on 2D nanoflakes-capped silicon nanowires for lipidomic mass spectrometry imaging and cancer diagnosis. ACS Nano 2022, 16, 6916–6928. [Google Scholar] [CrossRef]
- Duan, W.; Jin, Y.; Cui, Y.; Xi, F.; Liu, X.; Wo, F.; Wu, J. A co-delivery platform for synergistic promotion of angiogenesis based on biodegradable, therapeutic and self-reporting luminescent porous silicon microparticles. Biomaterials 2021, 272, 120772. [Google Scholar] [CrossRef]
- Zhou, H.; Ding, Y.; Su, R.; Lu, D.; Tang, H.; Xi, F. Silica nanochannel array film supported by ß-cyclodextrin-functionalized graphene modified gold film electrode for sensitive and direct electroanalysis of acetaminophen. Front. Chem. 2022, 9, 812086. [Google Scholar] [CrossRef]
- Yan, L.; Xu, S.; Xi, F. Disposal immunosensor for sensitive electrochemical detection of prostate-specific antigen based on amino-rich nanochannels array-modified patterned indium tin oxide electrode. Nanomaterials 2022, 12, 3810. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Tang, H.; Xi, F. Sensitive electrochemical detection of p-nitrophenol by pre-activated glassy carbon electrode integrated with silica nanochannel array film. Front. Chem. 2022, 10, 954748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, L.; Yan, F.; Wang, K. Vertically-ordered mesoporous silica film based electrochemical aptasensor for highly sensitive detection of alpha-fetoprotein in human serum. Biosensors 2023, 13, 628. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, X.; Yan, F.; Zhou, B. Homogeneous electrochemical aptasensor for sensitive detection of zearalenone using nanocomposite probe and silica nanochannel film. Molecules 2023, 28, 7241. [Google Scholar] [CrossRef]
- Huang, L.; Su, R.; Xi, F. Sensitive detection of noradrenaline in human whole blood based on Au nanoparticles embedded vertically-ordered silica nanochannels modified pre-activated glassy carbon electrodes. Front. Chem. 2023, 11, 1126213. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, X.; Xie, L.; Tang, H.; Yan, F. Vertically-ordered mesoporous silica films grown on boron nitride-graphene composite modified electrodes for rapid and sensitive detection of carbendazim in real samples. Front. Chem. 2022, 10, 939510. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, T.; Zhou, H.; Yan, F.; Liu, Y. Silica nanochannels boosting Ru(bpy)32+-mediated electrochemical sensor for the detection of guanine in beer and pharmaceutical samples. Front. Nutr. 2022, 9, 987442. [Google Scholar] [CrossRef]
- Chen, D.; Luo, X.; Xi, F. Probe-integrated electrochemical immunosensor based on electrostatic nanocage array for reagentless and sensitive detection of tumor biomarker. Front. Chem. 2023, 11, 1121450. [Google Scholar] [CrossRef]
- Li, D.; Xu, S.; Jin, H.; Wang, J.; Yan, F. Copper nanoparticles confined in a silica nanochannel film for the electrochemical detection of nitrate ions in water samples. Molecules 2023, 28, 7515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Luo, X.; Yan, F.; Mou, Y. Electrostatic nanocage-confined probe for electrochemical detection of CA19-9 in human serum. ACS Omega 2023, 8, 48491–48498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, X.; Yan, F.; Lin, J. N-doped graphene quantum dots confined within silica nanochannels for enhanced electrochemical detection of doxorubicin. Molecules 2023, 28, 6443. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Guo, W.; Xu, L.; Yang, Q.; Su, B. Two orders-of-magnitude enhancement in the electrochemiluminescence of by vertically ordered silica mesochannels. Anal. Chim. Acta 2015, 886, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Zheng, G.; Dou, Y.; Li, W.; Mou, C.Y.; Zhang, X.; Asiri, A.M.; Zhao, D. Highly ordered mesoporous silica films with perpendicular mesochannels by a simple Stober-solution growth approach. Angew. Chem. Int. Ed. 2012, 51, 2173–2177. [Google Scholar] [CrossRef] [PubMed]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Su, R.; Yu, G.; Liu, L.; Yan, F. Highly sensitive electrochemical detection of paraquat in environmental water samples using a vertically ordered mesoporous silica film and a nanocarbon composite. Nanomaterials 2022, 12, 3632. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Fan, X.; Yan, F.; Wang, Y. Highly sensitive electrochemical immunosensor based on electrodeposited platinum nanostructures confined in silica nanochannels for the detection of the carcinoembryonic antigen. Front. Chem. 2023, 11, 1271556. [Google Scholar] [CrossRef]





| Samples | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Serum a | 0.1000 | 0.1090 | 109.0 | 1.2 |
| 100.0 | 91.05 | 91.05 | 3.2 | |
| 1000 | 1072 | 107.2 | 4.4 | |
| Urine b | 0.0000 | 24.48 | - | 4.3 |
| 100.0 | 115.7 | 91.19 | 1.3 | |
| 200.0 | 235.2 | 105.4 | 3.2 | |
| 1000 | 1090 | 106.6 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, R.; Zhao, Y.; Liu, J. Solid Electrochemiluminescence Sensor by Immobilization of Emitter Ruthenium(II)tris(bipyridine) in Bipolar Silica Nanochannel Film for Sensitive Detection of Oxalate in Serum and Urine. Nanomaterials 2024, 14, 390. https://doi.org/10.3390/nano14050390
Yu R, Zhao Y, Liu J. Solid Electrochemiluminescence Sensor by Immobilization of Emitter Ruthenium(II)tris(bipyridine) in Bipolar Silica Nanochannel Film for Sensitive Detection of Oxalate in Serum and Urine. Nanomaterials. 2024; 14(5):390. https://doi.org/10.3390/nano14050390
Chicago/Turabian StyleYu, Ruliang, Yujiao Zhao, and Jiyang Liu. 2024. "Solid Electrochemiluminescence Sensor by Immobilization of Emitter Ruthenium(II)tris(bipyridine) in Bipolar Silica Nanochannel Film for Sensitive Detection of Oxalate in Serum and Urine" Nanomaterials 14, no. 5: 390. https://doi.org/10.3390/nano14050390
APA StyleYu, R., Zhao, Y., & Liu, J. (2024). Solid Electrochemiluminescence Sensor by Immobilization of Emitter Ruthenium(II)tris(bipyridine) in Bipolar Silica Nanochannel Film for Sensitive Detection of Oxalate in Serum and Urine. Nanomaterials, 14(5), 390. https://doi.org/10.3390/nano14050390






