Advancements and Prospects in Perovskite Solar Cells: From Hybrid to All-Inorganic Materials
Abstract
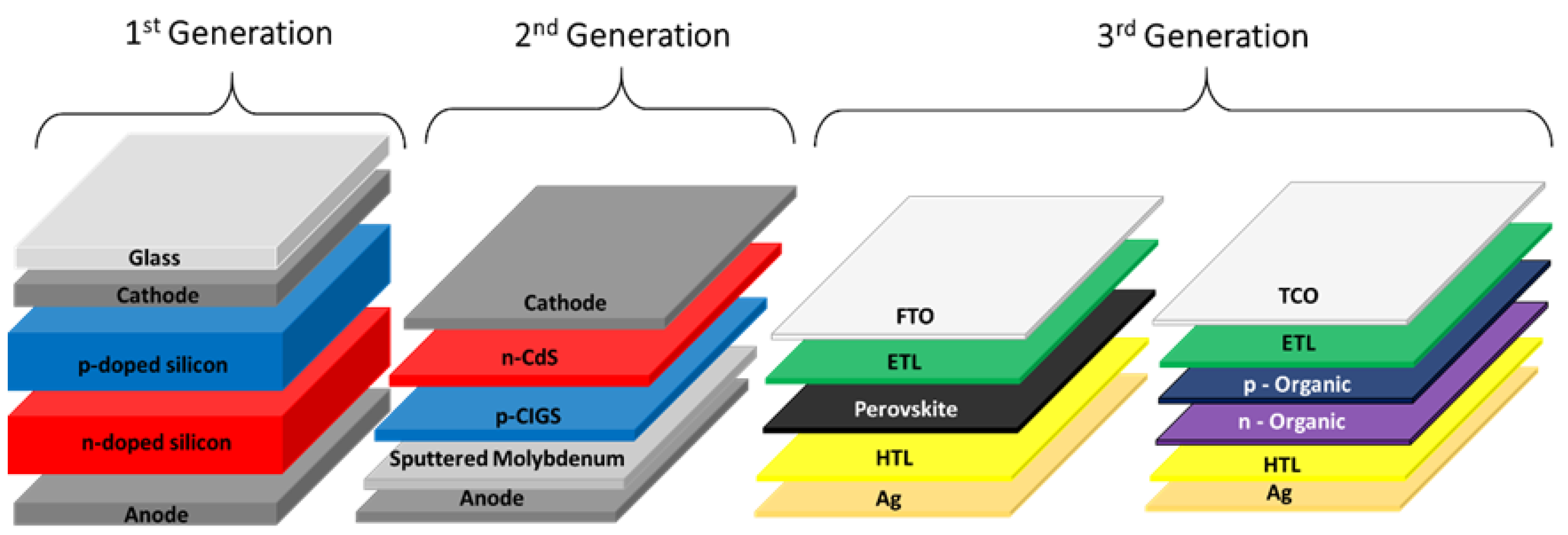
1. Introduction
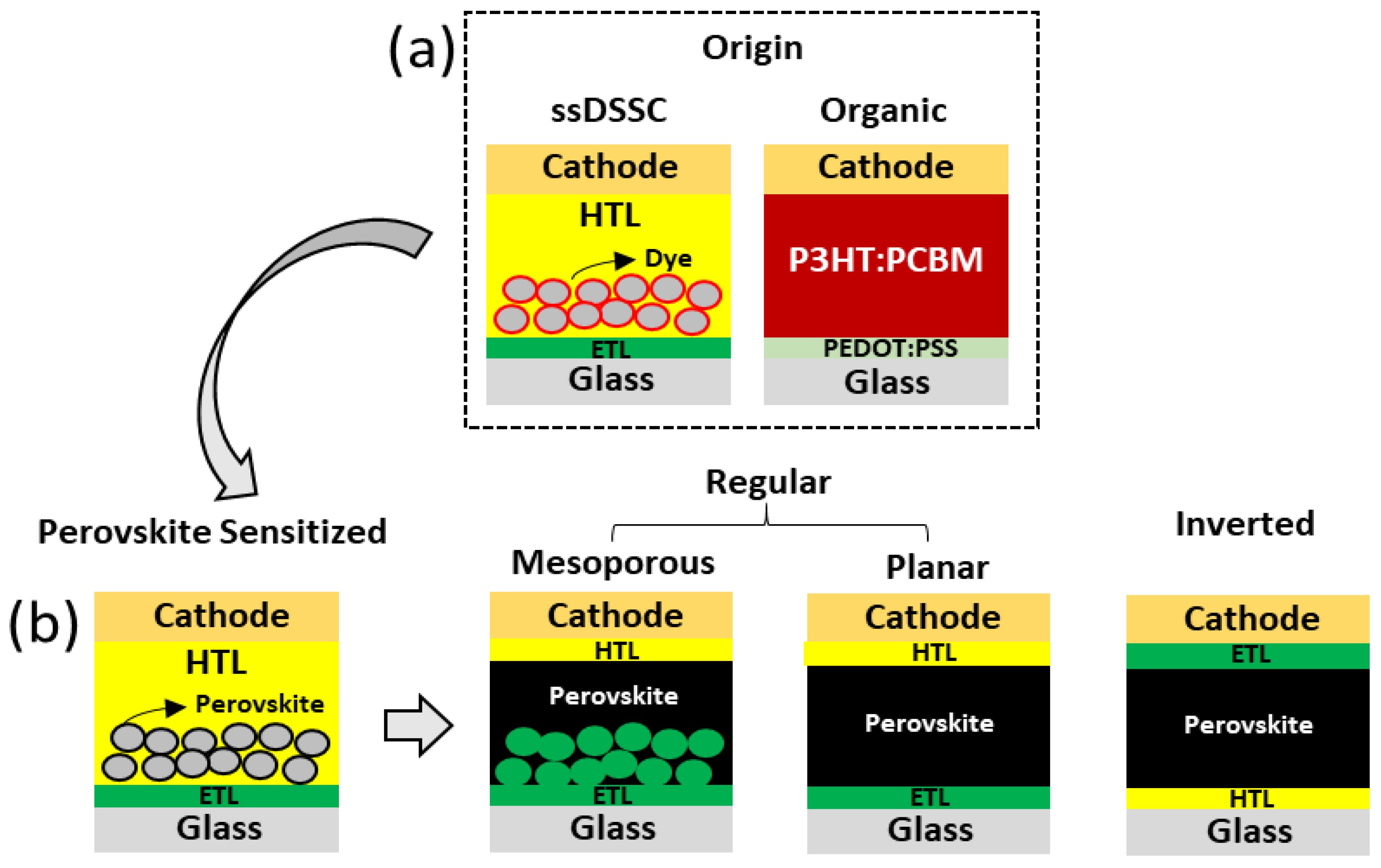
2. Hybrid Perovskite Structure and Its Components
Advances in Lead-Based Hybrid Perovskites
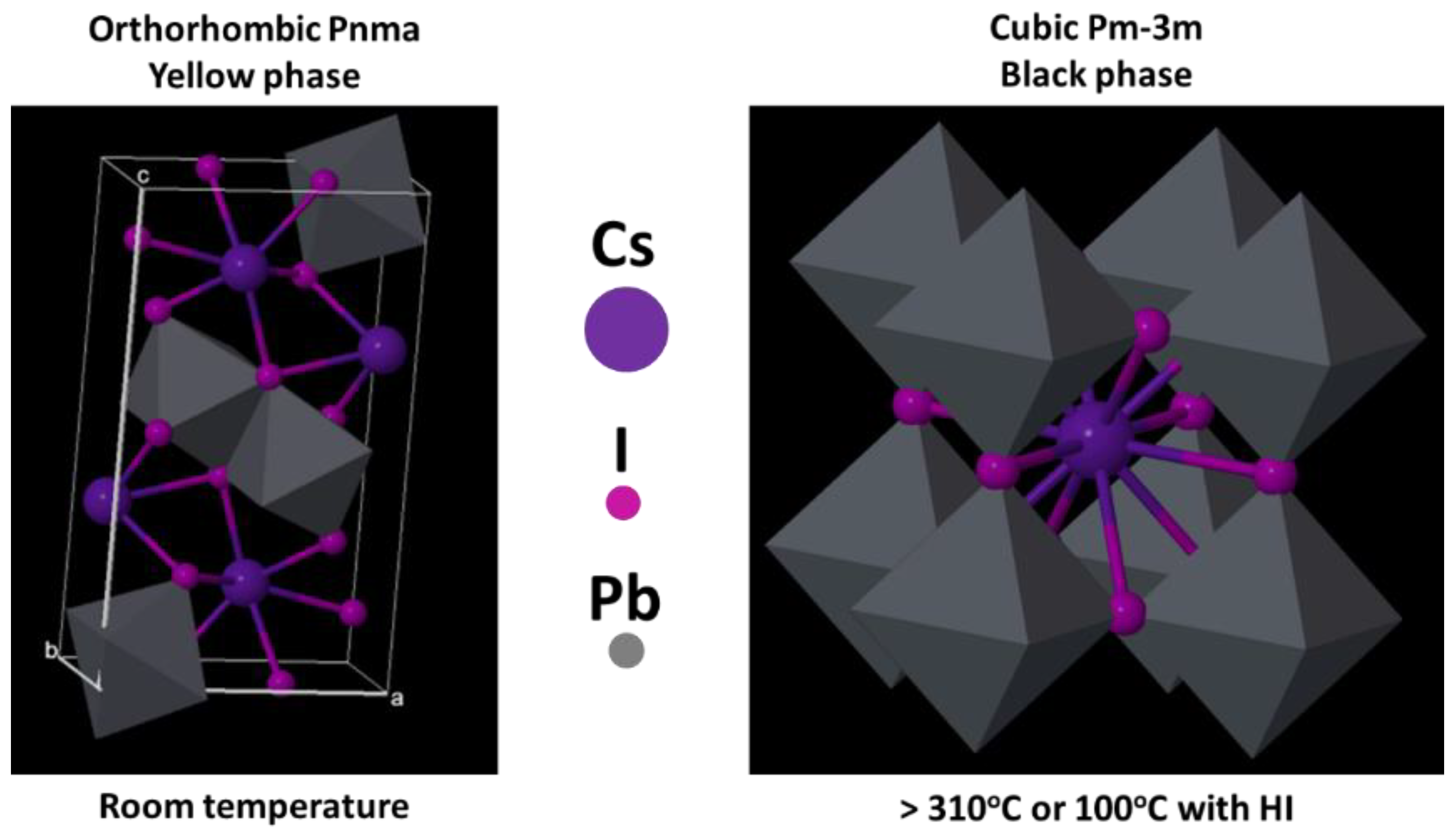
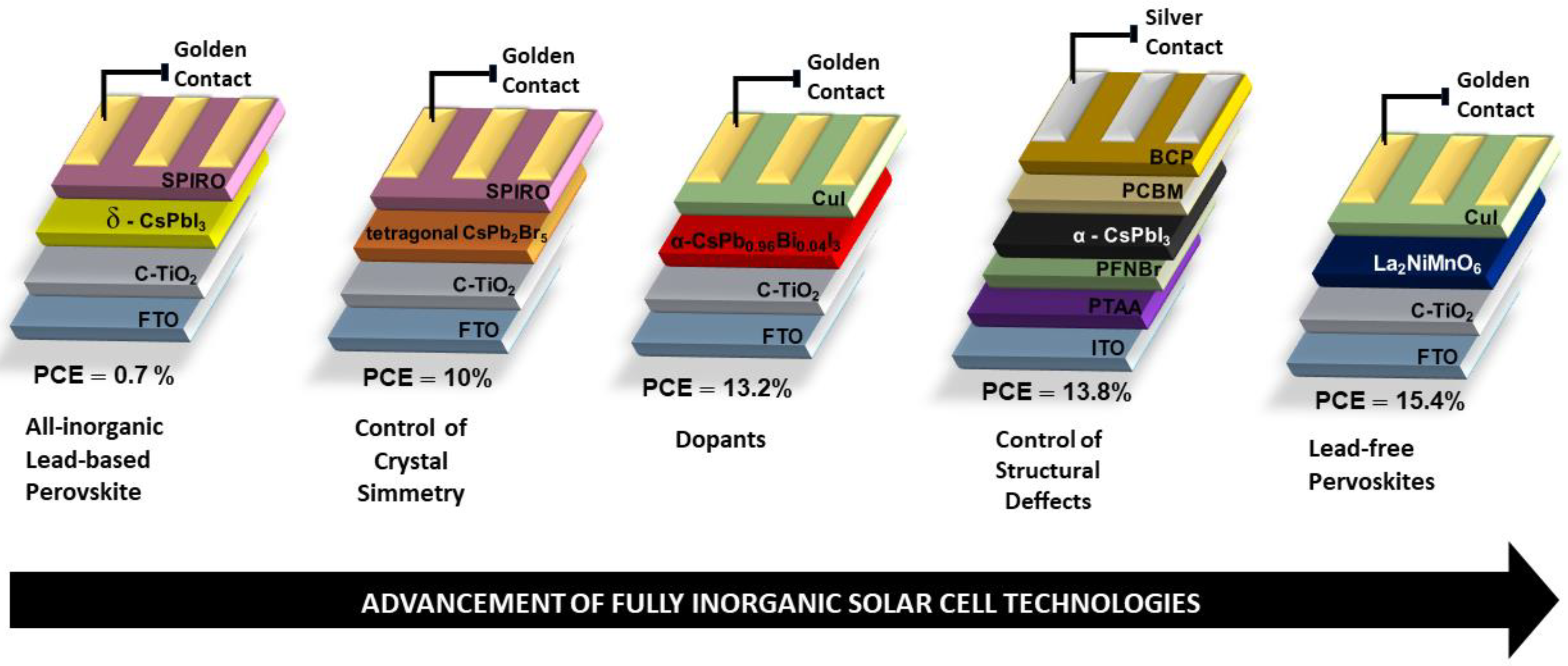
3. Fully Inorganic Perovskites
4. Low-Lead and Lead-Free All-Inorganic Perovskites
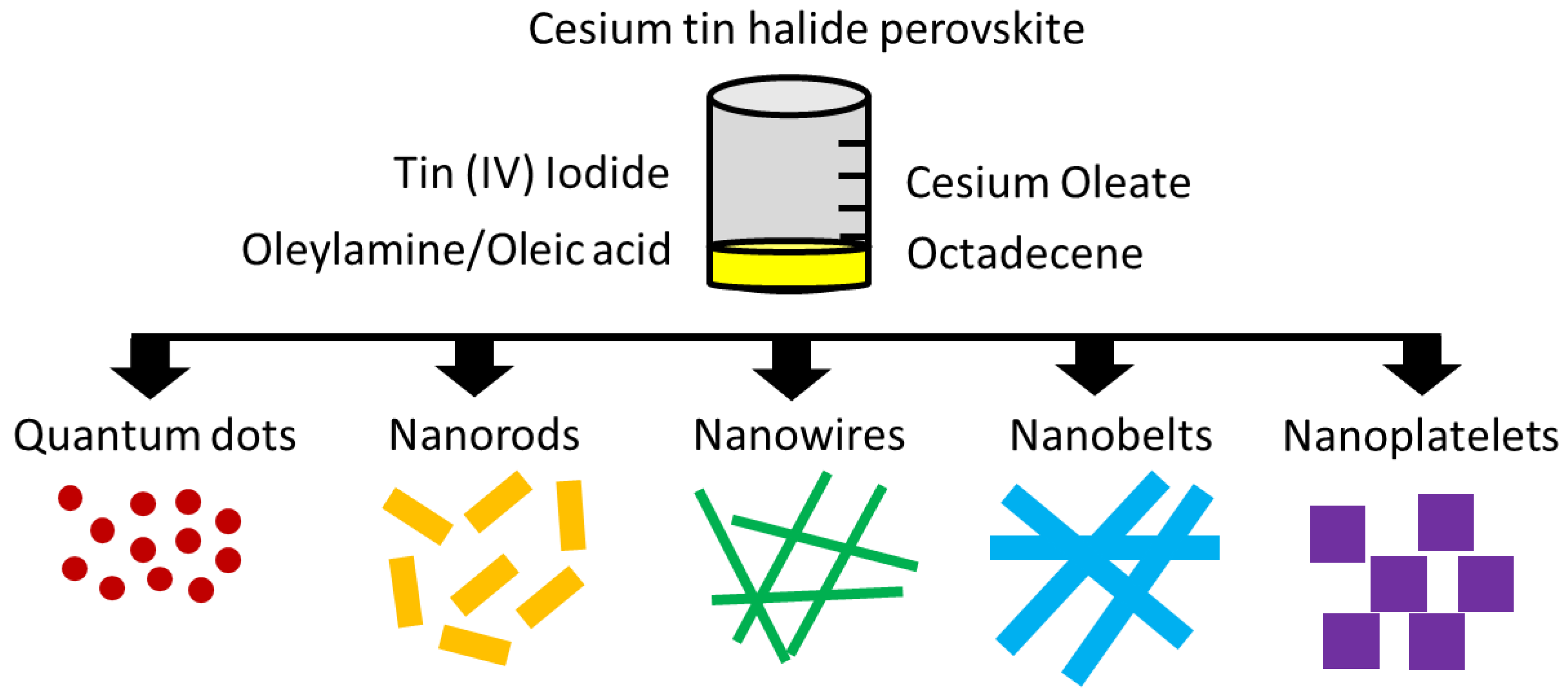
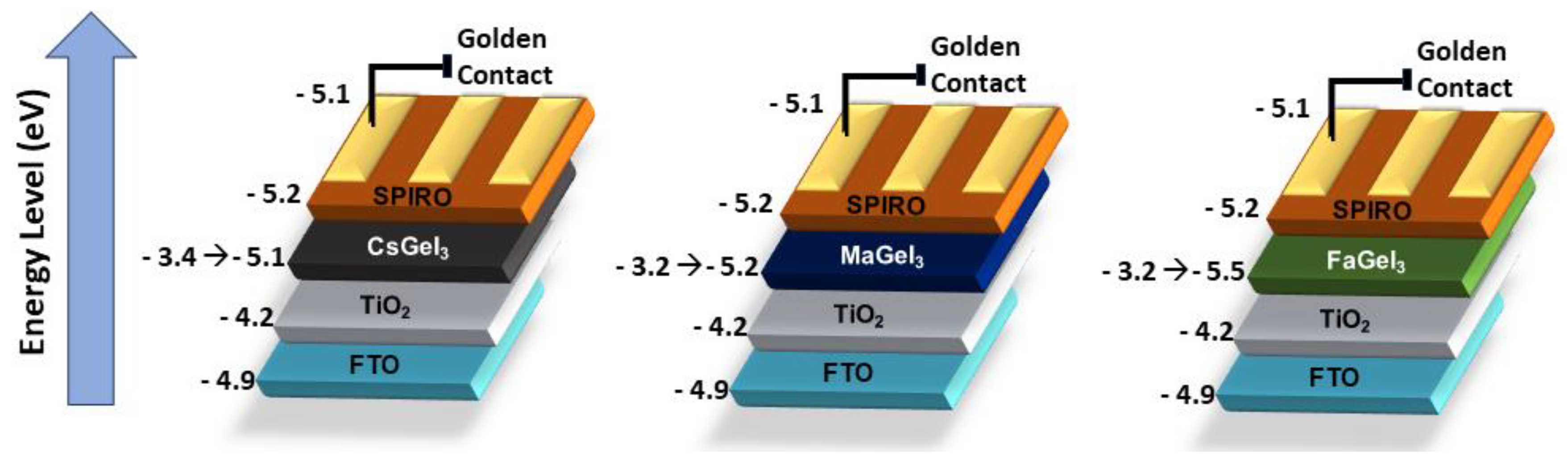
4.1. Tin-Based Perovskites
4.2. Germanium-Based Perovskites
4.3. Bismuth-Based Perovskites
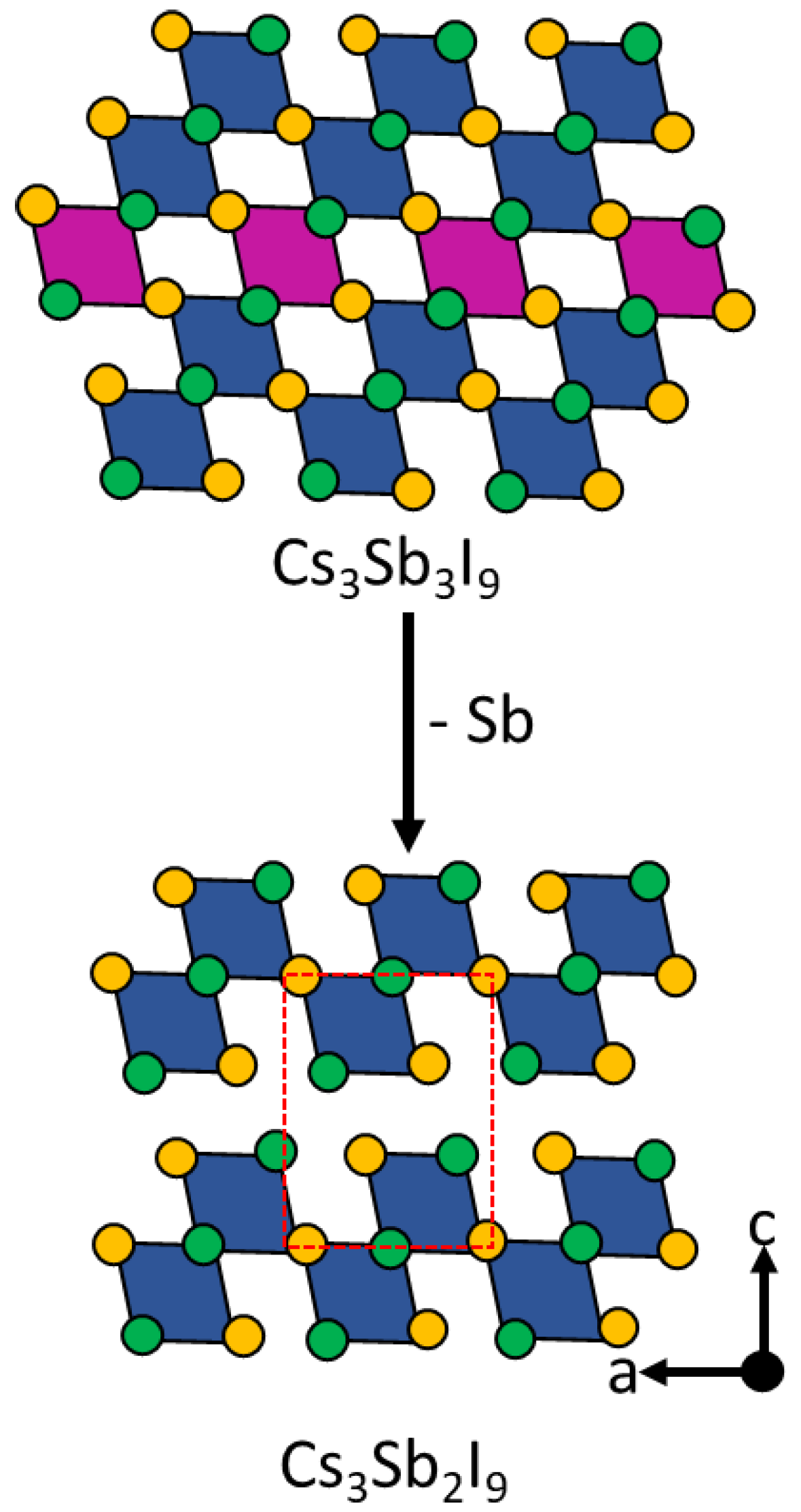
4.4. Antimony-Based Perovskites
4.5. Titanium-Based Perovskites
4.6. Copper-Based Perovskites
4.7. Double Perovskites and Bulk Heterojunction
5. Degradation Mechanisms
6. Encapsulation
7. Final Considerations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, F.; Li, M.; Siffalovic, P.; Cao, G.; Tian, J. From Scalable Solution Fabrication of Perovskite Films towards Commercialization of Solar Cells. Energy Environ. Sci. 2019, 12, 518–549. [Google Scholar] [CrossRef]
- Mitzi, D.B.; Wang, S.; Feild, C.A.; Chess, C.A.; Guloy, A.M. Conducting Layered Organic-Inorganic Halides Containing <110>-Oriented Perovskite Sheets. Science 1995, 267, 1473–1476. [Google Scholar] [CrossRef] [PubMed]
- Snaith, H.J. Perovskites: The Emergence of a New Era for Low-Cost, High-Efficiency Solar Cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Lee, C.-P.; Lin, R.Y.-Y.; Lin, L.-Y.; Li, C.-T.; Chu, T.-C.; Sun, S.-S.; Lin, J.T.; Ho, K.-C. Recent Progress in Organic Sensitizers for Dye-Sensitized Solar Cells. RSC Adv. 2015, 5, 23810–23825. [Google Scholar] [CrossRef]
- Tachibana, Y.; Akiyama, H.Y.; Ohtsuka, Y.; Torimoto, T.; Kuwabata, S. CdS Quantum Dots Sensitized TiO2 Sandwich Type Photoelectrochemical Solar Cells. Chem. Lett. 2006, 36, 88–89. [Google Scholar] [CrossRef]
- Robel, I.; Kuno, M.; Kamat, P.V. Size-Dependent Electron Injection from Excited CdSe Quantum Dots into TiO2 Nanoparticles. J. Am. Chem. Soc. 2007, 129, 4136–4137. [Google Scholar] [CrossRef] [PubMed]
- Plass, R.; Pelet, S.; Krueger, J.; Grätzel, M.; Bach, U. Quantum Dot Sensitization of Organic–Inorganic Hybrid Solar Cells. J. Phys. Chem. B 2002, 106, 7578–7580. [Google Scholar] [CrossRef]
- Zaban, A.; Mićić, O.I.; Gregg, B.A.; Nozik, A.J. Photosensitization of Nanoporous TiO2 Electrodes with InP Quantum Dots. Langmuir 1998, 14, 3153–3156. [Google Scholar] [CrossRef]
- Yu, P.; Zhu, K.; Norman, A.G.; Ferrere, S.; Frank, A.J.; Nozik, A.J. Nanocrystalline TiO2 Solar Cells Sensitized with InAs Quantum Dots. J. Phys. Chem. B 2006, 110, 25451–25454. [Google Scholar] [CrossRef]
- Mora-Seró, I. How Do Perovskite Solar Cells Work? Joule 2018, 2, 585–587. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, J.; Wei, A.; Liu, J.; Zhao, Y.; Xiao, Z. Study of Perovskite Solar Cells Based on Mixed-Organic-Cation FAxMA1−xPbI3 Absorption Layer. Phys. Chem. Chem. Phys. 2019, 21, 11822–11828. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, R.; Bi, Z.; Yu, Y.; Xu, G.; Hou, H.; Wu, Q.; Yu, H.; Xu, X. Multiple-Ring Aromatic Spacer Cation Tailored Interlayer Interaction for Efficient and Air-Stable Ruddlesden–Popper Perovskite Solar Cells. Sol. RRL 2021, 5, 2100495. [Google Scholar] [CrossRef]
- Marimuthu, T.; Yuvakkumar, R.; Kumar, P.S.; Vo, D.-V.N.; Xu, X.; Xu, G. Two-Dimensional Hybrid Perovskite Solar Cells: A Review. Environ. Chem. Lett. 2022, 20, 189–210. [Google Scholar] [CrossRef]
- Liu, P.; Han, N.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. High-quality Ruddlesden–Popper Perovskite Film Formation for High-performance Perovskite Solar Cells. Adv. Mater. 2021, 33, 2002582. [Google Scholar] [CrossRef]
- Assadi, M.K.; Bakhoda, S.; Saidur, R.; Hanaei, H. Recent Progress in Perovskite Solar Cells. Renew. Sustain. Energy Rev. 2018, 81, 2812–2822. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Fan, J.; Mai, Y. A Brief Review on the Lead Element Substitution in Perovskite Solar Cells. J. Energy Chem. 2018, 27, 1054–1066. [Google Scholar] [CrossRef]
- Meng, L.; Wei, Q.; Yang, Z.; Yang, D.; Feng, J.; Ren, X.; Liu, Y.; Liu, S. (Frank) Improved Perovskite Solar Cell Efficiency by Tuning the Colloidal Size and Free Ion Concentration in Precursor Solution Using Formic Acid Additive. J. Energy Chem. 2020, 41, 43–51. [Google Scholar] [CrossRef]
- Torabi, N.; Behjat, A.; Zhou, Y.; Docampo, P.; Stoddard, R.J.; Hillhouse, H.W.; Ameri, T. Progress and Challenges in Perovskite Photovoltaics from Single- to Multi-Junction Cells. Mater. Today Energy 2019, 12, 70–94. [Google Scholar] [CrossRef]
- Xue, C.; Shi, Y.; Zhang, C.; Lv, Y.; Feng, Y.; Tian, W.; Jin, S.; Ma, T. Favorable Growth of Well-Crystallized Layered Hybrid Perovskite by Combination of Thermal and Solvent Assistance. J. Power Sources 2019, 422, 156–162. [Google Scholar] [CrossRef]
- Jean, J.; Brown, P.R.; Jaffe, R.L.; Buonassisi, T.; Bulović, V. Pathways for Solar Photovoltaics. Energy Environ. Sci. 2015, 8, 1200–1219. [Google Scholar] [CrossRef]
- Green, M.A. Silicon Photovoltaic Modules: A Brief History of the First 50 Years. Prog. Photovolt. Res. Appl. 2005, 13, 447–455. [Google Scholar] [CrossRef]
- Yan, J.; Saunders, B.R. Third-Generation Solar Cells: A Review and Comparison of Polymer:Fullerene, Hybrid Polymer and Perovskite Solar Cells. RSC Adv. 2014, 4, 43286–43314. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The Future of Energy Supply: Challenges and Opportunities. Angew. Chem. Int. Ed. 2007, 46, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; De Paoli, M.-A. Dye-Sensitized Solar Cells: A Successful Combination of Materials. J. Braz. Chem. Soc. 2003, 14, 898–901. [Google Scholar] [CrossRef]
- Pan, Z.; Rao, H.; Mora-Seró, I.; Bisquert, J.; Zhong, X. Quantum Dot-Sensitized Solar Cells. Chem. Soc. Rev. 2018, 47, 7659–7702. [Google Scholar] [CrossRef] [PubMed]
- Park, N.-G. Organometal Perovskite Light Absorbers Toward a 20% Efficiency Low-Cost Solid-State Mesoscopic Solar Cell. J. Phys. Chem. Lett. 2013, 4, 2423–2429. [Google Scholar] [CrossRef]
- Kim, H.-S.; Im, S.H.; Park, N.-G. Organolead Halide Perovskite: New Horizons in Solar Cell Research. J. Phys. Chem. C 2014, 118, 5615–5625. [Google Scholar] [CrossRef]
- NREL Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 28 September 2023).
- Raphael, E.; Silva, M.N.; Szostak, R.; Schiavon, M.A.; Nogueira, A.F. Células Solares de Perovskitas: Uma Nova Tecnologia Emergente. Quim. Nova 2018, 41, 61–74. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The Emergence of Perovskite Solar Cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Petrović, M.; Chellappan, V.; Ramakrishna, S. Perovskites: Solar Cells & Engineering Applications—Materials and Device Developments. Sol. Energy 2015, 122, 678–699. [Google Scholar] [CrossRef]
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.D. Solar Cell Efficiency Tables (Version 44). Prog. Photovolt. Res. Appl. 2014, 22, 701–710. [Google Scholar] [CrossRef]
- Fraas, L.; Partain, L. Solar Cells: A Brief History and Introduction. In Solar Cells and Their Applications; John Wiley & Sons, Inc.: New York, NY, USA, 2010; pp. 1–15. ISBN 9780470636886. [Google Scholar]
- Ohnishi, M.; Takeoka, A.; Nakano, S.; Kuwano, Y. Advanced Photovoltaic Technologies and Residential Applications. Renew. Energy 1995, 6, 275–282. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Yu, M.; Long, Y.-Z.; Sun, B.; Fan, Z. Recent Advances in Solar Cells Based on One-Dimensional Nanostructure Arrays. Nanoscale 2012, 4, 2783–2796. [Google Scholar] [CrossRef] [PubMed]
- Sugathan, V.; John, E.; Sudhakar, K. Recent Improvements in Dye Sensitized Solar Cells: A Review. Renew. Sustain. Energy Rev. 2015, 52, 54–64. [Google Scholar] [CrossRef]
- Shalini, S.; Balasundara Prabhu, R.; Prasanna, S.; Mallick, T.K.; Senthilarasu, S. Review on Natural Dye Sensitized Solar Cells: Operation, Materials and Methods. Renew. Sustain. Energy Rev. 2015, 51, 1306–1325. [Google Scholar] [CrossRef]
- Tian, J.; Cao, G. Semiconductor Quantum Dot-Sensitized Solar Cells. Nano Rev. 2013, 4, 22578. [Google Scholar] [CrossRef]
- Fernandes, S.L.; Véron, A.C.; Neto, N.F.A.; Nüesch, F.A.; da Silva, J.H.D.; Zaghete, M.A.; Graeff, C.F.d.O. Nb2O5 Hole Blocking Layer for Hysteresis-Free Perovskite Solar Cells. Mater. Lett. 2016, 181, 103–107. [Google Scholar] [CrossRef]
- Saranin, D.; Komaricheva, T.; Luchnikov, L.; Muratov, D.S.; Le, T.S.; Karpov, Y.; Gostishchev, P.; Yurchuk, S.; Kuznetsov, D.; Didenko, S. Hysteresis-Free Perovskite Solar Cells with Compact and Nanoparticle NiO for Indoor Application. Sol. Energy Mater. Sol. Cells 2021, 227, 111095. [Google Scholar] [CrossRef]
- Khan, S.A.; Zain, Z.M.; Mansoor, M.; Mahfuz, M.M.H.; Rahman, A.; Rashid, M.A.N.; Rais, M.S. Performance Investigation of ZnO/PVA Nanocomposite Film for Organic Solar Cell. Mater. Today Proc. 2021, 47, 2615–2621. [Google Scholar] [CrossRef]
- Kouhnavard, M.; Ikeda, S.; Ludin, N.A.; Ahmad Khairudin, N.B.; Ghaffari, B.V.; Mat-Teridi, M.A.; Ibrahim, M.A.; Sepeai, S.; Sopian, K. A Review of Semiconductor Materials as Sensitizers for Quantum Dot-Sensitized Solar Cells. Renew. Sustain. Energy Rev. 2014, 37, 397–407. [Google Scholar] [CrossRef]
- Yang, M.; Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Fan, L. Hotspots, Frontiers, and Emerging Trends of Tandem Solar Cell Research: A Comprehensive Review. Int. J. Energy Res. 2022, 46, 104–123. [Google Scholar] [CrossRef]
- Jacobsson, T.J.; Hultqvist, A.; Svanström, S.; Riekehr, L.; Cappel, U.B.; Unger, E.; Rensmo, H.; Johansson, E.M.J.; Edoff, M.; Boschloo, G. 2-Terminal CIGS-Perovskite Tandem Cells: A Layer by Layer Exploration. Sol. Energy 2020, 207, 270–288. [Google Scholar] [CrossRef]
- Ren, H.; Yu, S.; Chao, L.; Xia, Y.; Sun, Y.; Zuo, S.; Li, F.; Niu, T.; Yang, Y.; Ju, H. Efficient and Stable Ruddlesden–Popper Perovskite Solar Cell with Tailored Interlayer Molecular Interaction. Nat. Photonics 2020, 14, 154–163. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Ran, R.; Shao, Z. Ruddlesden–Popper Perovskites in Electrocatalysis. Mater. Horiz. 2020, 7, 2519–2565. [Google Scholar] [CrossRef]
- Tsai, H.; Nie, W.; Blancon, J.-C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.J.; Tretiak, S.; et al. High-Efficiency Two-Dimensional Ruddlesden–Popper Perovskite Solar Cells. Nature 2016, 536, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Bati, A.S.R.; Zhong, Y.L.; Burn, P.L.; Nazeeruddin, M.K.; Shaw, P.E.; Batmunkh, M. Next-Generation Applications for Integrated Perovskite Solar Cells. Commun. Mater. 2023, 4, 2. [Google Scholar] [CrossRef]
- Wells, H.L. Über Die Cäsium- Und Kalium-Bleihalogenide. Z. Anorg. Chem. 1893, 3, 195–210. [Google Scholar] [CrossRef]
- Xu, W.-J.; Kopyl, S.; Kholkin, A.; Rocha, J. Hybrid Organic-Inorganic Perovskites: Polar Properties and Applications. Coord. Chem. Rev. 2019, 387, 398–414. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, Z.; Xu, J.; Cao, G. Synergistic Combination of Semiconductor Quantum Dots and Organic-Inorganic Halide Perovskites for Hybrid Solar Cells. Coord. Chem. Rev. 2018, 374, 279–313. [Google Scholar] [CrossRef]
- Ivanov, I.L.; Steparuk, A.S.; Bolyachkina, M.S.; Tsvetkov, D.S.; Safronov, A.P.; Zuev, A.Y. Thermodynamics of Formation of Hybrid Perovskite-Type Methylammonium Lead Halides. J. Chem. Thermodyn. 2018, 116, 253–258. [Google Scholar] [CrossRef]
- Ji, L.-J.; Sun, S.-J.; Qin, Y.; Li, K.; Li, W. Mechanical Properties of Hybrid Organic-Inorganic Perovskites. Coord. Chem. Rev. 2019, 391, 15–29. [Google Scholar] [CrossRef]
- Ptak, M.; Mączka, M.; Gągor, A.; Sieradzki, A.; Stroppa, A.; Di Sante, D.; Perez-Mato, J.M.; Macalik, L. Experimental and Theoretical Studies of Structural Phase Transition in a Novel Polar Perovskite-like [C2H5NH3][Na0.5Fe0.5(HCOO)3] Formate. Dalton Trans. 2016, 45, 2574–2583. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kim, H.J.; Jeong, I.; Lee, J.; Lee, H.; Son, H.J.; Kim, D.-E.; Ko, M.J. Mechanically Recoverable and Highly Efficient Perovskite Solar Cells: Investigation of Intrinsic Flexibility of Organic–Inorganic Perovskite. Adv. Energy Mater. 2015, 5, 1501406. [Google Scholar] [CrossRef]
- Rolston, N.; Watson, B.L.; Bailie, C.D.; McGehee, M.D.; Bastos, J.P.; Gehlhaar, R.; Kim, J.-E.; Vak, D.; Mallajosyula, A.T.; Gupta, G.; et al. Mechanical Integrity of Solution-Processed Perovskite Solar Cells. Extrem. Mech. Lett. 2016, 9, 353–358. [Google Scholar] [CrossRef]
- Berry, J.; Buonassisi, T.; Egger, D.A.; Hodes, G.; Kronik, L.; Loo, Y.-L.; Lubomirsky, I.; Marder, S.R.; Mastai, Y.; Miller, J.S.; et al. Hybrid Organic–Inorganic Perovskites (HOIPs): Opportunities and Challenges. Adv. Mater. 2015, 27, 5102–5112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Deng, Y.; Wei, H.; Zheng, X.; Yu, Z.; Shao, Y.; Shield, J.E.; Huang, J. Strained Hybrid Perovskite Thin Films and Their Impact on the Intrinsic Stability of Perovskite Solar Cells. Sci. Adv. 2017, 3, eaao5616. [Google Scholar] [CrossRef]
- Basu, A.; Kour, P.; Parmar, S.; Naphade, R.; Ogale, S. Flex-Mode Mechatronic Functionality of Lead Iodide Hybrid Perovskite Systems. J. Phys. Chem. C 2018, 122, 4802–4808. [Google Scholar] [CrossRef]
- Hong, K.; Van Le, Q.; Kim, S.Y.; Jang, H.W. Low-Dimensional Halide Perovskites: Review and Issues. J. Mater. Chem. C Mater. 2018, 6, 2189–2209. [Google Scholar] [CrossRef]
- Long, G.; Sabatini, R.; Saidaminov, M.I.; Lakhwani, G.; Rasmita, A.; Liu, X.; Sargent, E.H.; Gao, W. Chiral-Perovskite Optoelectronics. Nat. Rev. Mater. 2020, 5, 423–439. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Mao, L.; Malliakas, C.D.; Kanatzidis, M.G. Structure–Band Gap Relationships in Hexagonal Polytypes and Low-Dimensional Structures of Hybrid Tin Iodide Perovskites. Inorg. Chem. 2017, 56, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Stoumpos, C.C.; Cao, D.H.; Clark, D.J.; Young, J.; Rondinelli, J.M.; Jang, J.I.; Hupp, J.T.; Kanatzidis, M.G. Ruddlesden–Popper Hybrid Lead Iodide Perovskite 2D Homologous Semiconductors. Chem. Mater. 2016, 28, 2852–2867. [Google Scholar] [CrossRef]
- Zhou, C.; Tian, Y.; Wang, M.; Rose, A.; Besara, T.; Doyle, N.K.; Yuan, Z.; Wang, J.C.; Clark, R.; Hu, Y.; et al. Low-Dimensional Organic Tin Bromide Perovskites and Their Photoinduced Structural Transformation. Angew. Chem. Int. Ed. 2017, 56, 9018–9022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lin, H.; He, Q.; Xu, L.; Worku, M.; Chaaban, M.; Lee, S.; Shi, X.; Du, M.-H.; Ma, B. Low Dimensional Metal Halide Perovskites and Hybrids. Mater. Sci. Eng. R Rep. 2019, 137, 38–65. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, M.; Qiao, H.; Yang, H. A One-Pot Method for Controlled Synthesis and Selective Etching of Organic-Inorganic Hybrid Perovskite Crystals. J. Energy Chem. 2019, 33, 149–154. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, L.; Li, W.; Li, F.; Pai, N.K.; Scully, A.D.; Tsai, C.; Bach, U.; Simonov, A.N.; Cheng, Y. Diammonium and Monoammonium Mixed-organic-cation Perovskites for High Performance Solar Cells with Improved Stability. Adv. Energy Mater. 2017, 7, 1700444. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Zhong, Y.; Hao, H.; Yang, M.; Liu, R. Improved Phase Stability of the CsPbI 3 Perovskite via Organic Cation Doping. Phys. Chem. Chem. Phys. 2019, 21, 11175–11180. [Google Scholar] [CrossRef]
- Murugadoss, G.; Thangamuthu, R.; Senthil Kumar, S.M.; Anandhan, N.; Rajesh Kumar, M.; Rathishkumar, A. Synthesis of Ligand-Free, Large Scale with High Quality All-Inorganic CsPbI3 and CsPb2Br5 Nanocrystals and Fabrication of All-Inorganic Perovskite Solar Cells. J. Alloys Compd. 2019, 787, 17–26. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, Q. A Strategic Review on Processing Routes towards Scalable Fabrication of Perovskite Solar Cells. J. Energy Chem. 2022, 64, 538–560. [Google Scholar] [CrossRef]
- Sun, H.; Dai, P.; Li, X.; Ning, J.; Wang, S.; Qi, Y. Strategies and Methods for Fabricating High Quality Metal Halide Perovskite Thin Films for Solar Cells. J. Energy Chem. 2021, 60, 300–333. [Google Scholar] [CrossRef]
- Yao, H.; Shi, S.; Li, Z.; Ci, Z.; Zhu, G.; Ding, L.; Jin, Z. Strategies from Small-Area to Scalable Fabrication for Perovskite Solar Cells. J. Energy Chem. 2021, 57, 567–586. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Peters, J.A.; Liu, Z.; Sebastian, M.; Im, J.; Chasapis, T.C.; Wibowo, A.C.; Chung, D.Y.; Freeman, A.J.; et al. Crystal Growth of the Perovskite Semiconductor CsPbBr3: A New Material for High-Energy Radiation Detection. Cryst. Growth Des. 2013, 13, 2722–2727. [Google Scholar] [CrossRef]
- Kulbak, M.; Cahen, D.; Hodes, G. How Important Is the Organic Part of Lead Halide Perovskite Photovoltaic Cells? Efficient CsPbBr3 Cells. J. Phys. Chem. Lett. 2015, 6, 2452–2456. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, X.; Zhou, Y.; Jiang, Q.; Ye, Q.; Chu, Z.; Li, X.; Yang, X.; Yin, Z.; You, J. Solvent-Controlled Growth of Inorganic Perovskite Films in Dry Environment for Efficient and Stable Solar Cells. Nat. Commun. 2018, 9, 2225. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.E.; Paternò, G.M.; Sutton, R.J.; Zampetti, A.; Haghighirad, A.A.; Cacialli, F.; Snaith, H.J. Inorganic Caesium Lead Iodide Perovskite Solar Cells. J. Mater. Chem. A Mater. 2015, 3, 19688–19695. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Sutton, R.J.; Filip, M.R.; Haghighirad, A.A.; Sakai, N.; Wenger, B.; Giustino, F.; Snaith, H.J. Cubic or Orthorhombic? Revealing the Crystal Structure of Metastable Black-Phase CsPbI3 by Theory and Experiment. ACS Energy Lett. 2018, 3, 1787–1794. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Sanehira, Y.; Ikegami, M.; Miyasaka, T. Stabilization of α-CsPbI3 in Ambient Room Temperature Conditions by Incorporating Eu into CsPbI3. Chem. Mater. 2018, 30, 6668–6674. [Google Scholar] [CrossRef]
- Hu, Y.; Bai, F.; Liu, X.; Ji, Q.; Miao, X.; Qiu, T.; Zhang, S. Bismuth Incorporation Stabilized α-CsPbI3 for Fully Inorganic Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 2219–2227. [Google Scholar] [CrossRef]
- Kumar, M.; Raj, A.; Kumar, A.; Anshul, A. Theoretical Evidence of High Power Conversion Efficiency in Double Perovskite Solar Cell Device. Opt. Mater. 2021, 111, 110565. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Li, E.; Ru, P.; Chen, H.; Chen, Z.; Wu, Y.; Tian, H.; Zhu, W.-H. Electron-Enriched Thione Enables Strong Pb–S Interaction for Stabilizing High Quality CsPbI3 Perovskite Films with Low-Temperature Processing. Chem. Sci. 2020, 11, 3132–3140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, K.; Wan, L.; Tan, Y.; Wang, Z.-S. Black Phase of Inorganic Perovskite Stabilized with Carboxyimidazolium Iodide for Stable and Efficient Inverted Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 6906–6915. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhao, W.; Chen, S.; Jin, Z.; Liu, S.F. Mn Doping of CsPbI3 Film towards High-Efficiency Solar Cell. ACS Appl. Energy Mater. 2020, 3, 5190–5197. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Li, Z.; Zhang, H.; Dang, Y.; Kong, F. Highly Emissive Halide Perovskite Nanocrystals: From Lead to Lead-Free. CrystEngComm 2021, 23, 3619–3630. [Google Scholar] [CrossRef]
- Dave, K.; Fang, M.H.; Bao, Z.; Fu, H.T.; Liu, R.S. Recent Developments in Lead-free Double Perovskites: Structure, Doping, and Applications. Chem.–Asian J. 2020, 15, 242–252. [Google Scholar] [CrossRef]
- Mahajan, P.; Datt, R.; Tsoi, W.C.; Gupta, V.; Tomar, A.; Arya, S. Recent Progress, Fabrication Challenges and Stability Issues of Lead-Free Tin-Based Perovskite Thin Films in the Field of Photovoltaics. Coord. Chem. Rev. 2021, 429, 213633. [Google Scholar] [CrossRef]
- Glück, N.; Bein, T. Prospects of Lead-Free Perovskite-Inspired Materials for Photovoltaic Applications. Energy Environ. Sci. 2020, 13, 4691–4716. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Lou, Y.; Zhao, Y. All-Inorganic Lead-Free Perovskites for Optoelectronic Applications. Mater. Chem. Front. 2019, 3, 365–375. [Google Scholar] [CrossRef]
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G.E.; Pathak, S.K.; Johnston, M.B.; et al. Lead-Free Organic–Inorganic Tin Halide Perovskites for Photovoltaic Applications. Energy Environ. Sci. 2014, 7, 3061–3068. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef]
- Huang, J.; Lai, M.; Lin, J.; Yang, P. Rich Chemistry in Inorganic Halide Perovskite Nanostructures. Adv. Mater. 2018, 30, 1802856. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiang, S.; Li, W.; Liu, H.; Zhu, L.; Yang, S. Inorganic Perovskite Solar Cells: A Rapidly Growing Field. Sol. RRL 2018, 2, 1700188. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Hawash, Z.; Raga, S.R.; Ono, L.K.; Qi, Y. Thermal Degradation of CH3NH3PbI3 Perovskite into NH3 and CH3I Gases Observed by Coupled Thermogravimetry–Mass Spectrometry Analysis. Energy Environ. Sci. 2016, 9, 3406–3410. [Google Scholar] [CrossRef]
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Domanski, K.; Alharbi, E.A.; Hagfeldt, A.; Grätzel, M.; Tress, W. Systematic Investigation of the Impact of Operation Conditions on the Degradation Behaviour of Perovskite Solar Cells. Nat. Energy 2018, 3, 61–67. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Q.; Lu, Y.; Lu, F.; Mu, X.; Wei, S.-H.; Sui, M. Hydrogenated Cs2AgBiBr6 for Significantly Improved Efficiency of Lead-Free Inorganic Double Perovskite Solar Cell. Nat. Commun. 2022, 13, 3397. [Google Scholar] [CrossRef]
- Chung, I.; Song, J.-H.; Im, J.; Androulakis, J.; Malliakas, C.D.; Li, H.; Freeman, A.J.; Kenney, J.T.; Kanatzidis, M.G. CsSnI3: Semiconductor or Metal? High Electrical Conductivity and Strong Near-Infrared Photoluminescence from a Single Material. High Hole Mobility and Phase-Transitions. J. Am. Chem. Soc. 2012, 134, 8579–8587. [Google Scholar] [CrossRef]
- Huang, L.; Lambrecht, W.R.L. Electronic Band Structure, Phonons, and Exciton Binding Energies of Halide Perovskites CsSnCl3, CsSnBr3, and CsSnI3. Phys. Rev. B 2013, 88, 165203. [Google Scholar] [CrossRef]
- Lv, S.; Gao, W.; Liu, Y.; Dong, H.; Sun, N.; Niu, T.; Xia, Y.; Wu, Z.; Song, L.; Ran, C. Stability of Sn-Pb Mixed Organic–Inorganic Halide Perovskite Solar Cells: Progress, Challenges, and Perspectives. J. Energy Chem. 2022, 65, 371–404. [Google Scholar] [CrossRef]
- Parida, B.; Yoon, S.; Jeong, S.M.; Cho, J.S.; Kim, J.-K.; Kang, D.-W. Recent Progress on Cesium Lead/Tin Halide-Based Inorganic Perovskites for Stable and Efficient Solar Cells: A Review. Sol. Energy Mater. Sol. Cells 2020, 204, 110212. [Google Scholar] [CrossRef]
- Qian, F.; Hu, M.; Gong, J.; Ge, C.; Zhou, Y.; Guo, J.; Chen, M.; Ge, Z.; Padture, N.P.; Zhou, Y.; et al. Enhanced Thermoelectric Performance in Lead-Free Inorganic CsSn1–XGexI3 Perovskite Semiconductors. J. Phys. Chem. C 2020, 124, 11749–11753. [Google Scholar] [CrossRef]
- Wang, A.; Yan, X.; Zhang, M.; Sun, S.; Yang, M.; Shen, W.; Pan, X.; Wang, P.; Deng, Z. Controlled Synthesis of Lead-Free and Stable Perovskite Derivative Cs2SnI6 Nanocrystals via a Facile Hot-Injection Process. Chem. Mater. 2016, 28, 8132–8140. [Google Scholar] [CrossRef]
- Song, T.-B.; Yokoyama, T.; Stoumpos, C.C.; Logsdon, J.; Cao, D.H.; Wasielewski, M.R.; Aramaki, S.; Kanatzidis, M.G. Importance of Reducing Vapor Atmosphere in the Fabrication of Tin-Based Perovskite Solar Cells. J. Am. Chem. Soc. 2017, 139, 836–842. [Google Scholar] [CrossRef] [PubMed]
- bin Mohd Yusoff, A.R.; Vasilopoulou, M.; Georgiadou, D.G.; Palilis, L.C.; Abate, A.; Nazeeruddin, M.K. Passivation and Process Engineering Approaches of Halide Perovskite Films for High Efficiency and Stability Perovskite Solar Cells. Energy Environ. Sci. 2021, 14, 2906–2953. [Google Scholar] [CrossRef]
- Fu, L.; Li, H.; Wang, L.; Yin, R.; Li, B.; Yin, L. Defect Passivation Strategies in Perovskites for an Enhanced Photovoltaic Performance. Energy Environ. Sci. 2020, 13, 4017–4056. [Google Scholar] [CrossRef]
- Wang, A.; Guo, Y.; Muhammad, F.; Deng, Z. Controlled Synthesis of Lead-Free Cesium Tin Halide Perovskite Cubic Nanocages with High Stability. Chem. Mater. 2017, 29, 6493–6501. [Google Scholar] [CrossRef]
- Zhang, Z.; Kamarudin, M.A.; Baranwal, A.K.; Kapil, G.; Sahamir, S.R.; Sanehira, Y.; Chen, M.; Wang, L.; Shen, Q.; Hayase, S. Sequential Passivation for Lead-Free Tin Perovskite Solar Cells with High Efficiency. Angew. Chem. 2022, 134, e202210101. [Google Scholar] [CrossRef]
- Marshall, K.P.; Walker, M.; Walton, R.I.; Hatton, R.A. Enhanced Stability and Efficiency in Hole-Transport-Layer-Free CsSnI3 Perovskite Photovoltaics. Nat. Energy 2016, 1, 16178. [Google Scholar] [CrossRef]
- Marshall, K.P.; Walton, R.I.; Hatton, R.A. Tin Perovskite/Fullerene Planar Layer Photovoltaics: Improving the Efficiency and Stability of Lead-Free Devices. J. Mater. Chem. A Mater. 2015, 3, 11631–11640. [Google Scholar] [CrossRef]
- Moghe, D.; Wang, L.; Traverse, C.J.; Redoute, A.; Sponseller, M.; Brown, P.R.; Bulović, V.; Lunt, R.R. All Vapor-Deposited Lead-Free Doped CsSnBr3 Planar Solar Cells. Nano Energy 2016, 28, 469–474. [Google Scholar] [CrossRef]
- Zhu, H.L.; Xiao, J.; Mao, J.; Zhang, H.; Zhao, Y.; Choy, W.C.H. Controllable Crystallization of CH3NH3Sn0.25Pb0.75I3 Perovskites for Hysteresis-Free Solar Cells with Efficiency Reaching 15.2%. Adv. Funct. Mater. 2017, 27, 1605469. [Google Scholar] [CrossRef]
- Luo, J.; He, R.; Lai, H.; Chen, C.; Zhu, J.; Xu, Y.; Yao, F.; Ma, T.; Luo, Y.; Yi, Z. Improved Carrier Management via a Multifunctional Modifier for High-Quality Low-Bandgap Sn–Pb Perovskites and Efficient All-Perovskite Tandem Solar Cells. Adv. Mater. 2023, 35, 2300352. [Google Scholar] [CrossRef] [PubMed]
- Yeom, K.; Lee, D.; Park, N. Hard and Soft Acid and Base (HSAB) Engineering for Efficient and Stable Sn-Pb Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2202496. [Google Scholar] [CrossRef]
- Sun, P.-P.; Li, Q.-S.; Yang, L.-N.; Li, Z.-S. Theoretical Insights into a Potential Lead-Free Hybrid Perovskite: Substituting Pb2+ with Ge2+. Nanoscale 2016, 8, 1503–1512. [Google Scholar] [CrossRef]
- Krishnamoorthy, T.; Ding, H.; Yan, C.; Leong, W.L.; Baikie, T.; Zhang, Z.; Sherburne, M.; Li, S.; Asta, M.; Mathews, N.; et al. Lead-Free Germanium Iodide Perovskite Materials for Photovoltaic Applications. J. Mater. Chem. A Mater. 2015, 3, 23829–23832. [Google Scholar] [CrossRef]
- Chen, L.-J. Synthesis and Optical Properties of Lead-Free Cesium Germanium Halide Perovskite Quantum Rods. RSC Adv. 2018, 8, 18396–18399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, T.; Hu, C.; Fu, Z.; Lin, J.; Cheng, Z.; Wu, J.; Qi, Y.; Ruan, Y.; Huang, L. Investigation of Efficient All-Inorganic HTL-Free CsGeI3 Perovskite Solar Cells by Device Simulation. Mater. Today Commun. 2023, 34, 105347. [Google Scholar] [CrossRef]
- Solanki, S.; Bharathi, K.V.; Bhargava, K. Fundamental Analysis of Lead-Free CsGeI3 Perovskite Solar Cell. Mater. Today Proc. 2022, 67, 180–186. [Google Scholar] [CrossRef]
- Lozhkina, O.A.; Murashkina, A.A.; Shilovskikh, V.V.; Kapitonov, Y.V.; Ryabchuk, V.K.; Emeline, A.V.; Miyasaka, T. Invalidity of Band-Gap Engineering Concept for Bi3+ Heterovalent Doping in CsPbBr3 Halide Perovskite. J. Phys. Chem. Lett. 2018, 9, 5408–5411. [Google Scholar] [CrossRef]
- Park, B.-W.; Philippe, B.; Zhang, X.; Rensmo, H.; Boschloo, G.; Johansson, E.M.J. Bismuth Based Hybrid Perovskites A3Bi2I9 (A: Methylammonium or Cesium) for Solar Cell Application. Adv. Mater. 2015, 27, 6806–6813. [Google Scholar] [CrossRef]
- Korukunda, T.B.; Joshi, D.N.; Meroni, S.; Watson, T.; Dutta, V. Enhanced Infiltration and Morphology of Bismuth Perovskite in Carbon-Stack Solar Cells—A Synergistic Effect of Electric Fields in Modified Spray Technique. Sol. Energy 2022, 241, 386–395. [Google Scholar] [CrossRef]
- Karthick, S.; Hawashin, H.; Parou, N.; Vedraine, S.; Velumani, S.; Bouclé, J. Copper and Bismuth Incorporated Mixed Cation Perovskite Solar Cells by One-Step Solution Process. Sol. Energy 2021, 218, 226–236. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J.; Hong, F.; Mao, X.; Zheng, K.; Yang, S.; Li, Y.; Pullerits, T.; Deng, W.; Han, K. Lead-Free, Air-Stable All-Inorganic Cesium Bismuth Halide Perovskite Nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 12471–12475. [Google Scholar] [CrossRef] [PubMed]
- Leng, M.; Yang, Y.; Zeng, K.; Chen, Z.; Tan, Z.; Li, S.; Li, J.; Xu, B.; Li, D.; Hautzinger, M.P.; et al. All-Inorganic Bismuth-Based Perovskite Quantum Dots with Bright Blue Photoluminescence and Excellent Stability. Adv. Funct. Mater. 2018, 28, 1704446. [Google Scholar] [CrossRef]
- Johansson, M.B.; Zhu, H.; Johansson, E.M.J. Extended Photo-Conversion Spectrum in Low-Toxic Bismuth Halide Perovskite Solar Cells. J. Phys. Chem. Lett. 2016, 7, 3467–3471. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Fang, M.; Chen, J.; Zhao, Y. Formation of Highly Luminescent Cesium Bismuth Halide Perovskite Quantum Dots Tuned by Anion Exchange. Chem. Commun. 2018, 54, 3779–3782. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, B.; Ding, R.; Chen, W.; Kondrotas, R.; Zhao, Y.; Lu, S.; Li, Z.; Tang, J. Reactive Close-Spaced Sublimation Processed CuSbSe2 Thin Films and Their Photovoltaic Application. APL Mater. 2018, 6, 084801. [Google Scholar] [CrossRef]
- Jiang, F.; Yang, D.; Jiang, Y.; Liu, T.; Zhao, X.; Ming, Y.; Luo, B.; Qin, F.; Fan, J.; Han, H.; et al. Chlorine-Incorporation-Induced Formation of the Layered Phase for Antimony-Based Lead-Free Perovskite Solar Cells. J. Am. Chem. Soc. 2018, 140, 1019–1027. [Google Scholar] [CrossRef]
- Ganose, A.M.; Savory, C.N.; Scanlon, D.O. Beyond Methylammonium Lead Iodide: Prospects for the Emergent Field of Ns2 Containing Solar Absorbers. Chem. Commun. 2017, 53, 20–44. [Google Scholar] [CrossRef]
- Zuo, C.; Ding, L. Lead-Free Perovskite Materials (NH4)3Sb2IxBr9−x. Angew. Chem. Int. Ed. 2017, 56, 6528–6532. [Google Scholar] [CrossRef]
- Saparov, B.; Hong, F.; Sun, J.-P.; Duan, H.-S.; Meng, W.; Cameron, S.; Hill, I.G.; Yan, Y.; Mitzi, D.B. Thin-Film Preparation and Characterization of Cs3Sb2I9: A Lead-Free Layered Perovskite Semiconductor. Chem. Mater. 2015, 27, 5622–5632. [Google Scholar] [CrossRef]
- Hebig, J.-C.; Kühn, I.; Flohre, J.; Kirchartz, T. Optoelectronic Properties of (CH3NH3)3Sb2I9 Thin Films for Photovoltaic Applications. ACS Energy Lett. 2016, 1, 309–314. [Google Scholar] [CrossRef]
- Chonamada, T.D.; Dey, A.B.; Santra, P.K. Degradation Studies of Cs3Sb2I9: A Lead-Free Perovskite. ACS Appl. Energy Mater. 2020, 3, 47–55. [Google Scholar] [CrossRef]
- Singh, A.; Boopathi, K.M.; Mohapatra, A.; Chen, Y.F.; Li, G.; Chu, C.W. Photovoltaic Performance of Vapor-Assisted Solution-Processed Layer Polymorph of Cs3Sb2I9. ACS Appl. Mater. Interfaces 2018, 10, 2566–2573. [Google Scholar] [CrossRef]
- Tang, G.; Xiao, Z.; Hosono, H.; Kamiya, T.; Fang, D.; Hong, J. Layered Halide Double Perovskites Cs3+nM(II)NSb2X9+3n (M = Sn, Ge) for Photovoltaic Applications. J. Phys. Chem. Lett. 2018, 9, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Correa-Baena, J.-P.; Nienhaus, L.; Kurchin, R.C.; Shin, S.S.; Wieghold, S.; Putri Hartono, N.T.; Layurova, M.; Klein, N.D.; Poindexter, J.R.; Polizzotti, A.; et al. A-Site Cation in Inorganic A3Sb2I9 Perovskite Influences Structural Dimensionality, Exciton Binding Energy, and Solar Cell Performance. Chem. Mater. 2018, 30, 3734–3742. [Google Scholar] [CrossRef]
- Ahmad, K.; Khan, M.Q.; Kim, H. Simulation and Fabrication of All-Inorganic Antimony Halide Perovskite-like Material Based Pb-Free Perovskite Solar Cells. Opt. Mater. 2022, 128, 112374. [Google Scholar] [CrossRef]
- Liang, J.; Soni, K.; Lou, J. Morphology Evolution of Ultra-Stable and Low-Cost All-Inorganic Lead-Free Perovskite Solar Cells. Mater. Today Energy 2023, 32, 101241. [Google Scholar] [CrossRef]
- Ju, M.-G.; Chen, M.; Zhou, Y.; Garces, H.F.; Dai, J.; Ma, L.; Padture, N.P.; Zeng, X.C. Earth-Abundant Nontoxic Titanium(IV)-Based Vacancy-Ordered Double Perovskite Halides with Tunable 1.0 to 1.8 EV Bandgaps for Photovoltaic Applications. ACS Energy Lett. 2018, 3, 297–304. [Google Scholar] [CrossRef]
- Chen, M.; Ju, M.-G.; Carl, A.D.; Zong, Y.; Grimm, R.L.; Gu, J.; Zeng, X.C.; Zhou, Y.; Padture, N.P. Cesium Titanium(IV) Bromide Thin Films Based Stable Lead-Free Perovskite Solar Cells. Joule 2018, 2, 558–570. [Google Scholar] [CrossRef]
- Yang, P.; Liu, G.; Liu, B.; Liu, X.; Lou, Y.; Chen, J.; Zhao, Y. All-Inorganic Cs2CuX4 (X = Cl, Br, and Br/I) Perovskite Quantum Dots with Blue-Green Luminescence. Chem. Commun. 2018, 54, 11638–11641. [Google Scholar] [CrossRef] [PubMed]
- Volonakis, G.; Haghighirad, A.A.; Milot, R.L.; Sio, W.H.; Filip, M.R.; Wenger, B.; Johnston, M.B.; Herz, L.M.; Snaith, H.J.; Giustino, F. Cs2InAgCl6: A New Lead-Free Halide Double Perovskite with Direct Band Gap. J. Phys. Chem. Lett. 2017, 8, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xia, Z.; Molokeev, M.S.; Zhang, X.; Peng, D.; Liu, Q. Composition Design, Optical Gap and Stability Investigations of Lead-Free Halide Double Perovskite Cs2AgInCl6. J. Mater. Chem. A Mater. 2017, 5, 15031–15037. [Google Scholar] [CrossRef]
- Wei, F.; Deng, Z.; Sun, S.; Zhang, F.; Evans, D.M.; Kieslich, G.; Tominaka, S.; Carpenter, M.A.; Zhang, J.; Bristowe, P.D.; et al. Synthesis and Properties of a Lead-Free Hybrid Double Perovskite: (CH3NH3)2AgBiBr6. Chem. Mater. 2017, 29, 1089–1094. [Google Scholar] [CrossRef]
- Slavney, A.H.; Hu, T.; Lindenberg, A.M.; Karunadasa, H.I. A Bismuth-Halide Double Perovskite with Long Carrier Recombination Lifetime for Photovoltaic Applications. J. Am. Chem. Soc. 2016, 138, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Okamoto, H.; Hamakawa, Y. Amorphous Si/Polycrystalline Si Stacked Solar Cell Having More Than 12% Conversion Efficiency. Jpn. J. Appl. Phys. 1983, 22, L605–L607. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Q.; Liu, Y.; Luo, W.; Guo, X.; Huang, Z.; Ting, H.; Sun, W.; Zhong, X.; Wei, S.; et al. The Dawn of Lead-Free Perovskite Solar Cell: Highly Stable Double Perovskite Cs2AgBiBr6 Film. Adv. Sci. 2017, 5, 1700759. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; He, X.; Fang, Z.; Lian, W.; Shang, Y.; Li, X.; Zhou, W.; Zhang, M.; Chen, T.; Lu, Y. Bulk Heterojunction Gifts Bismuth-Based Lead-Free Perovskite Solar Cells with Record Efficiency. Nano Energy 2020, 68, 104362. [Google Scholar] [CrossRef]
- Fan, Q.; Biesold-McGee, G.V.; Ma, J.; Xu, Q.; Pan, S.; Peng, J.; Lin, Z. Lead-Free Halide Perovskite Nanocrystals: Crystal Structures, Synthesis, Stabilities, and Optical Properties. Angew. Chem. Int. Ed. 2020, 59, 1030–1046. [Google Scholar] [CrossRef]
- Madan, J.; Shivani; Pandey, R.; Sharma, R. Device Simulation of 17.3% Efficient Lead-Free All-Perovskite Tandem Solar Cell. Sol. Energy 2020, 197, 212–221. [Google Scholar] [CrossRef]
- Cho, J.; DuBose, J.T.; Kamat, P.V. Charge Injection from Excited Cs2AgBiBr6 Quantum Dots into Semiconductor Oxides. Chem. Mater. 2020, 32, 510–517. [Google Scholar] [CrossRef]
- Lv, C.; Yang, X.; Shi, Z.; Wang, L.; Sui, L.; Li, Q.; Qin, J.; Liu, K.; Zhang, Z.; Li, X.; et al. Pressure-Induced Ultra-Broad-Band Emission of a Cs2AgBiBr6 Perovskite Thin Film. J. Phys. Chem. C 2020, 124, 1732–1738. [Google Scholar] [CrossRef]
- Kumar Chini, M.; Goverapet Srinivasan, S.; Tailor, N.K.; Yukta; Salahub, D.; Satapathi, S. Lead-Free, Stable Mixed Halide Double Perovskites Cs2AgBiBr6 and Cs2AgBiBr6−xClx—A Detailed Theoretical and Experimental Study. Chem. Phys. 2020, 529, 110547. [Google Scholar] [CrossRef]
- Soni, A.; Bhamu, K.C.; Sahariya, J. Investigating Effect of Strain on Electronic and Optical Properties of Lead Free Double Perovskite Cs2AgInCl6 Solar Cell Compound: A First Principle Calculation. J. Alloys Compd. 2020, 817, 152758. [Google Scholar] [CrossRef]
- Yamamoto, K.; Narita, G.; Yamasaki, J.; Iikubo, S. First-Principles Study of Thermoelectric Properties of Mixed Iodide Perovskite Cs(B,B′)I3 (B, B′ = Ge, Sn, and Pb). J. Phys. Chem. Solids 2020, 140, 109372. [Google Scholar] [CrossRef]
- Kong, D.; Cheng, D.; Wang, X.; Zhang, K.; Wang, H.; Liu, K.; Li, H.; Sheng, X.; Yin, L. Solution Processed Lead-Free Cesium Titanium Halide Perovskites and Their Structural, Thermal and Optical Characteristics. J. Mater. Chem. C Mater. 2020, 8, 1591–1597. [Google Scholar] [CrossRef]
- Li, W.; Zhu, S.; Zhao, Y.; Qiu, Y. Structure, Electronic and Optical Properties of Cs2Ti(Br1−XYx)6 (Y = Cl, I; x = 0, 0.25, 0.5, 0.75, 1) Perovskites: The First Principles Investigations. J. Solid State Chem. 2020, 284, 121213. [Google Scholar] [CrossRef]
- Li, C.; Jiang, K.; Jiang, J.; Hu, Z.; Liu, A.; Hu, G.; Shi, W.; Chu, J. Enhanced Photovoltaic Response of Lead-Free Ferroelectric Solar Cells Based on (K,Bi)(Nb,Yb)O3 Films. Phys. Chem. Chem. Phys. 2020, 22, 3691–3701. [Google Scholar] [CrossRef]
- Ahn, N.; Kwak, K.; Jang, M.S.; Yoon, H.; Lee, B.Y.; Lee, J.-K.; Pikhitsa, P.V.; Byun, J.; Choi, M. Trapped Charge-Driven Degradation of Perovskite Solar Cells. Nat. Commun. 2016, 7, 13422. [Google Scholar] [CrossRef]
- Mahiny, M.; Ahmadi-Kandjani, S.; Olyaeefar, B. Classical Modeling of Extrinsic Degradation in Polycrystalline Perovskite Solar Cells; Defect Induced Degradation. Sol. Energy Mater. Sol. Cells 2023, 261, 112500. [Google Scholar] [CrossRef]
- Aristidou, N.; Eames, C.; Sanchez-Molina, I.; Bu, X.; Kosco, J.; Islam, M.S.; Haque, S.A. Fast Oxygen Diffusion and Iodide Defects Mediate Oxygen-Induced Degradation of Perovskite Solar Cells. Nat. Commun. 2017, 8, 15218. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Song, J.; Dai, X.; Liu, Y.; Rudd, P.N.; Hong, X.; Huang, J. Synergistic Effect of Elevated Device Temperature and Excess Charge Carriers on the Rapid Light-induced Degradation of Perovskite Solar Cells. Adv. Mater. 2019, 31, 1902413. [Google Scholar] [CrossRef] [PubMed]
- Dunfield, S.P.; Bliss, L.; Zhang, F.; Luther, J.M.; Zhu, K.; van Hest, M.F.A.M.; Reese, M.O.; Berry, J.J. From Defects to Degradation: A Mechanistic Understanding of Degradation in Perovskite Solar Cell Devices and Modules. Adv. Energy Mater. 2020, 10, 1904054. [Google Scholar] [CrossRef]
- Misra, R.K.; Aharon, S.; Li, B.; Mogilyansky, D.; Visoly-Fisher, I.; Etgar, L.; Katz, E.A. Temperature-and Component-Dependent Degradation of Perovskite Photovoltaic Materials under Concentrated Sunlight. J. Phys. Chem. Lett. 2015, 6, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xin, G.; Scott, S.M.; Xu, W.; Yao, T.; Gong, B.; Wang, Y.; Li, M.; Lian, J. Deciphering the Degradation Mechanism of the Lead-Free All Inorganic Perovskite Cs2SnI6. NPJ Mater. Degrad. 2019, 3, 7. [Google Scholar] [CrossRef]
- Yao, W.; Ling, Q.; Dai, Q.; Fang, S.; Yang, C.; Huang, L.; Liu, X.; Zhang, H.; Zhang, J.; Zhu, Y. In Situ Microscopic Observation of Humidity-Induced Degradation in All-Inorganic Perovskite Films. ACS Appl. Energy Mater. 2022, 5, 8092–8102. [Google Scholar] [CrossRef]
- Xiang, L.; Gao, F.; Cao, Y.; Li, D.; Liu, Q.; Liu, H.; Li, S. Progress on the Stability and Encapsulation Techniques of Perovskite Solar Cells. Org. Electron. 2022, 106, 106515. [Google Scholar] [CrossRef]
- Raman, R.K.; Gurusamy Thangavelu, S.A.; Venkataraj, S.; Krishnamoorthy, A. Materials, Methods and Strategies for Encapsulation of Perovskite Solar Cells: From Past to Present. Renew. Sustain. Energy Rev. 2021, 151, 111608. [Google Scholar] [CrossRef]
- Aitola, K.; Gava Sonai, G.; Markkanen, M.; Jaqueline Kaschuk, J.; Hou, X.; Miettunen, K.; Lund, P.D. Encapsulation of Commercial and Emerging Solar Cells with Focus on Perovskite Solar Cells. Sol. Energy 2022, 237, 264–283. [Google Scholar] [CrossRef]





| Cations | Ionic Radius (Å) | Electronic Configuration |
|---|---|---|
| Pb2+ | 1.19 | 6s2 |
| Sn2+ | 1.02 | 5s2 |
| Ge2+ | 0.73 | 4s2 |
| Bi3+ | 1.03 | 6s2 |
| Sb3+ | 0.76 | 5s2 |
| Sn4+ | 0.69 | 4d10 |
| Ti4+ | 0.53 | 3p6 |
| Cu2+ | 0.73 | 3d9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maziviero, F.V.; Melo, D.M.A.; Medeiros, R.L.B.A.; Oliveira, Â.A.S.; Macedo, H.P.; Braga, R.M.; Morgado, E., Jr. Advancements and Prospects in Perovskite Solar Cells: From Hybrid to All-Inorganic Materials. Nanomaterials 2024, 14, 332. https://doi.org/10.3390/nano14040332
Maziviero FV, Melo DMA, Medeiros RLBA, Oliveira ÂAS, Macedo HP, Braga RM, Morgado E Jr. Advancements and Prospects in Perovskite Solar Cells: From Hybrid to All-Inorganic Materials. Nanomaterials. 2024; 14(4):332. https://doi.org/10.3390/nano14040332
Chicago/Turabian StyleMaziviero, Fernando Velcic, Dulce M. A. Melo, Rodolfo L. B. A. Medeiros, Ângelo A. S. Oliveira, Heloísa P. Macedo, Renata M. Braga, and Edisson Morgado, Jr. 2024. "Advancements and Prospects in Perovskite Solar Cells: From Hybrid to All-Inorganic Materials" Nanomaterials 14, no. 4: 332. https://doi.org/10.3390/nano14040332
APA StyleMaziviero, F. V., Melo, D. M. A., Medeiros, R. L. B. A., Oliveira, Â. A. S., Macedo, H. P., Braga, R. M., & Morgado, E., Jr. (2024). Advancements and Prospects in Perovskite Solar Cells: From Hybrid to All-Inorganic Materials. Nanomaterials, 14(4), 332. https://doi.org/10.3390/nano14040332







