Light Absorption Analysis and Optimization of Ag@TiO2 Core-Shell Nanospheroid and Nanorod
Abstract
1. Introduction
2. Model and Method
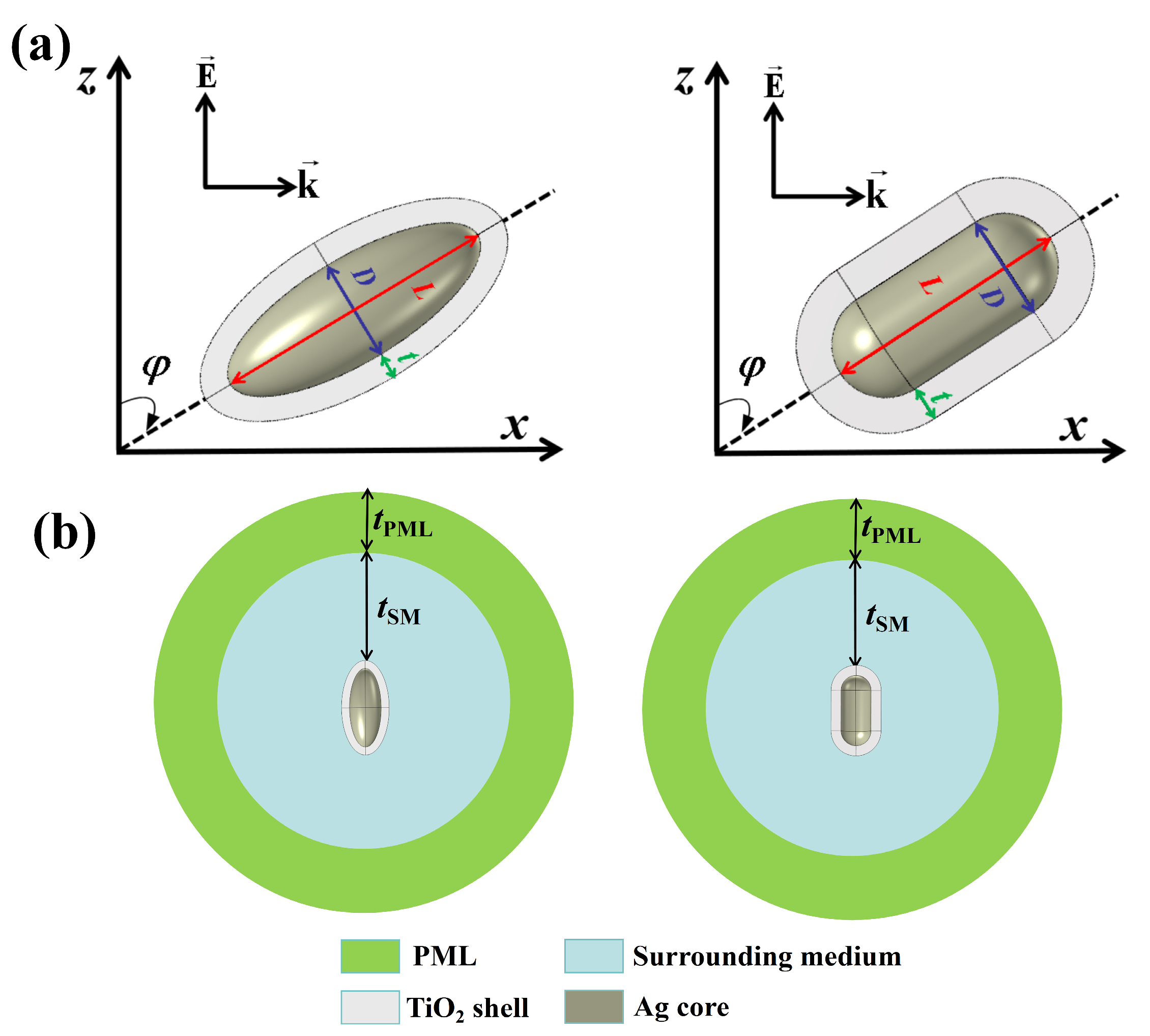
2.1. Simulation Model
2.2. Calculation of Light Absorption
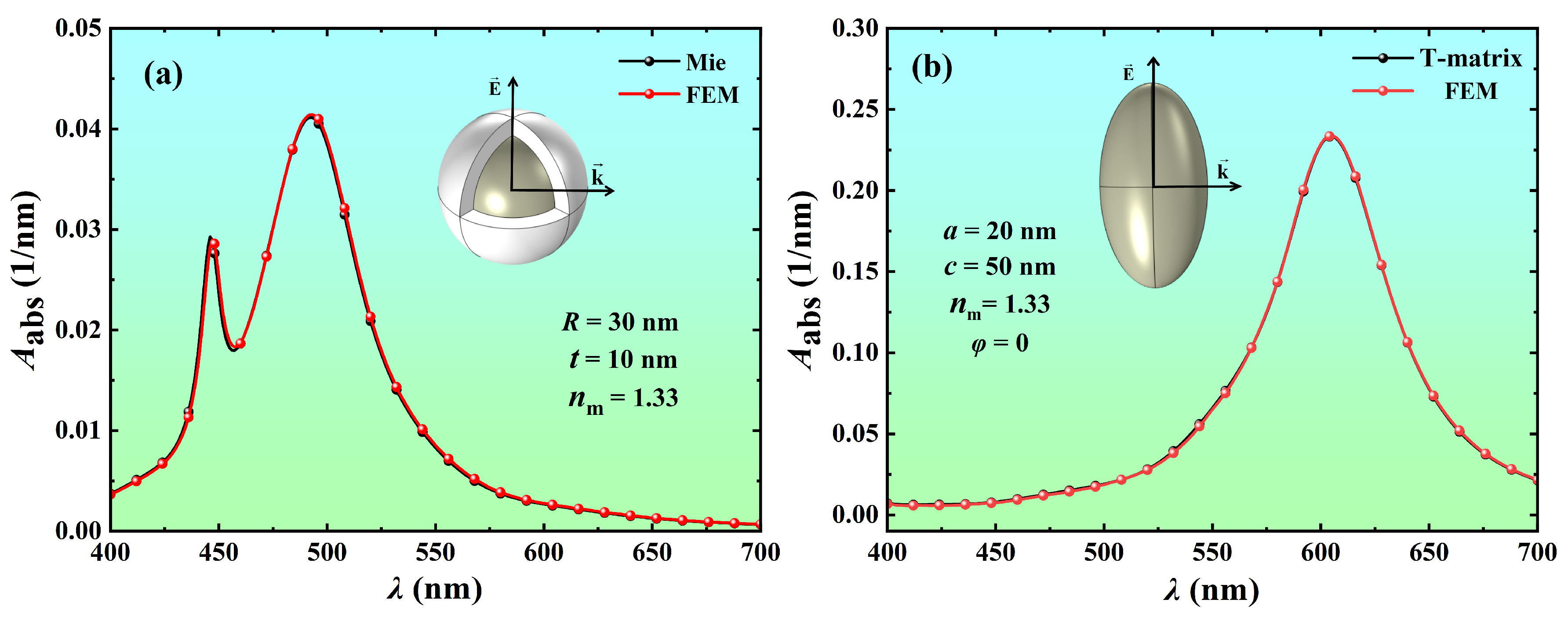
2.3. Numerical Verification of FEM
3. Results and Discussion
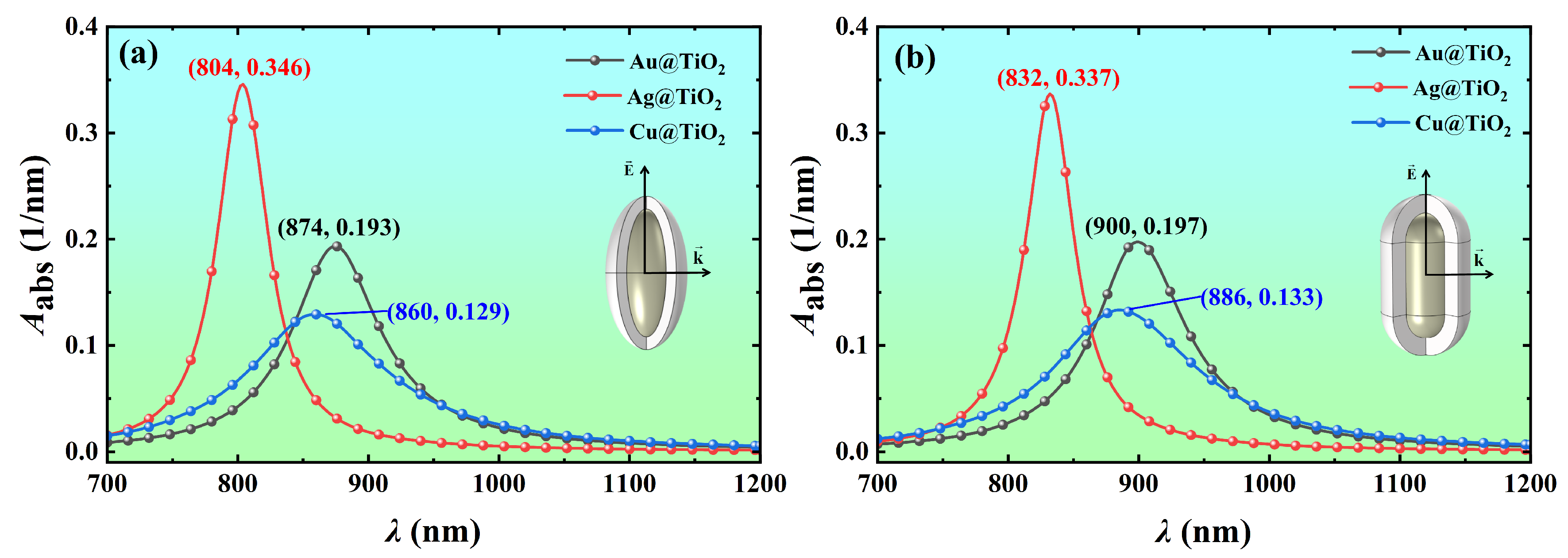
3.1. Effect of Core Material on Light Absorption
3.2. Effect of Particle Size on Light Absorption
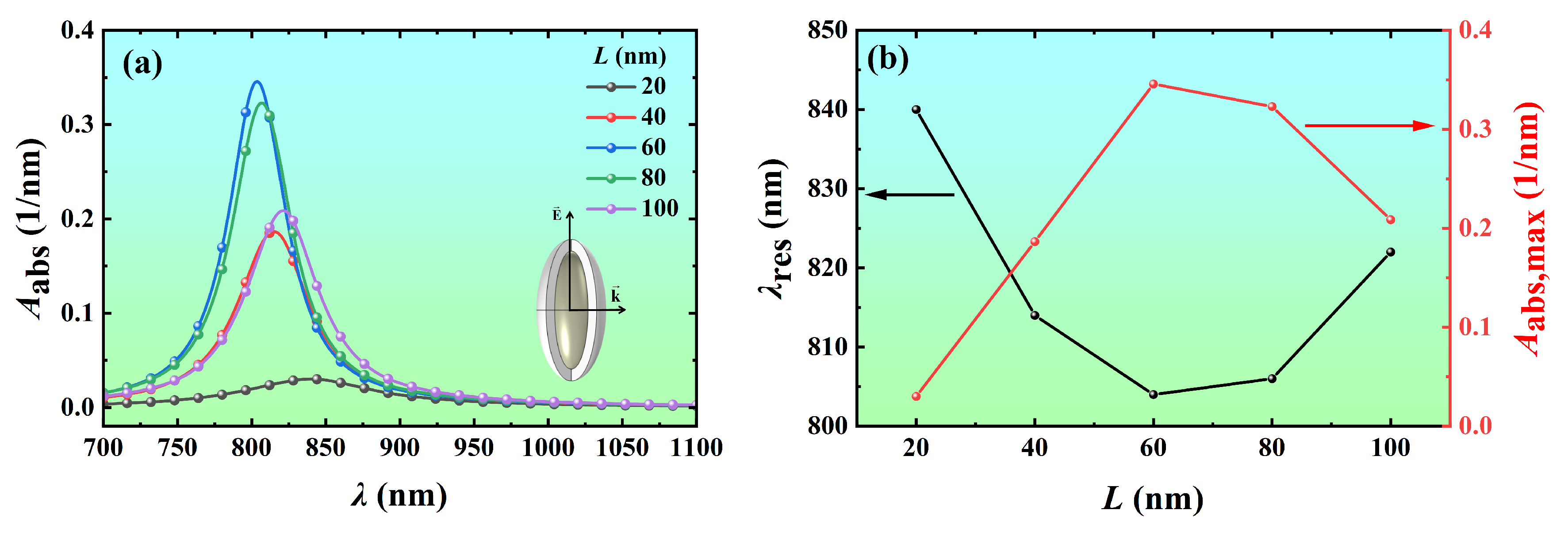
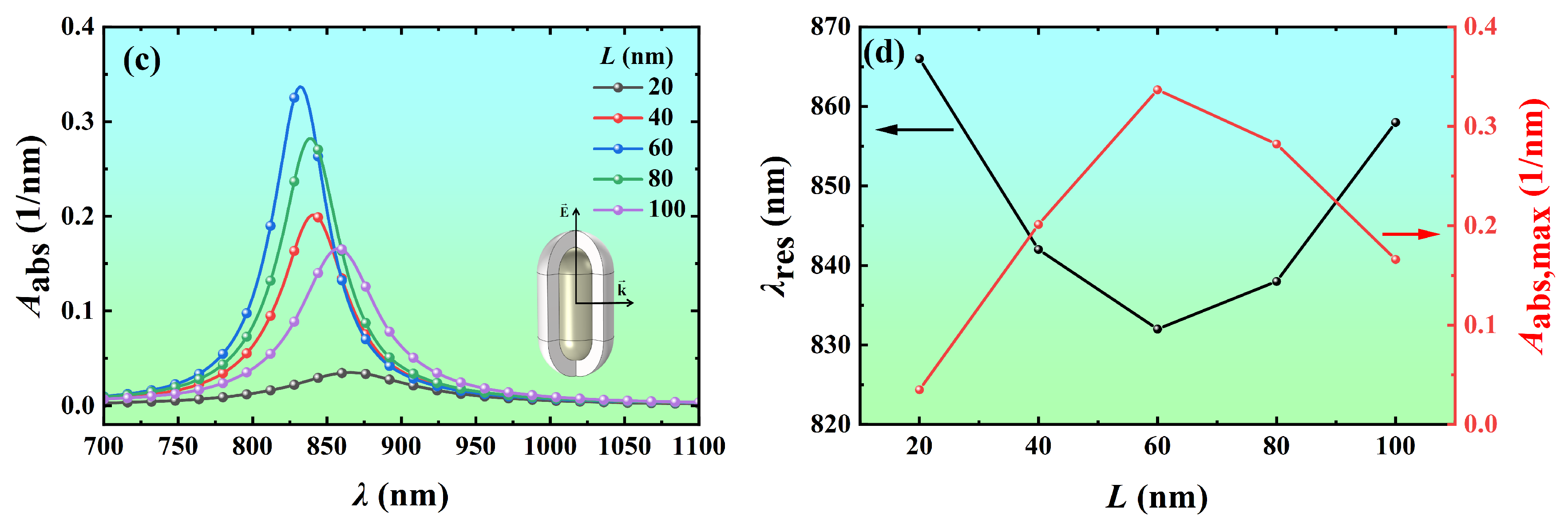
3.2.1. Effect of Core Length
3.2.2. Effect of Core Aspect Ratio
3.2.3. Effect of Shell Thickness
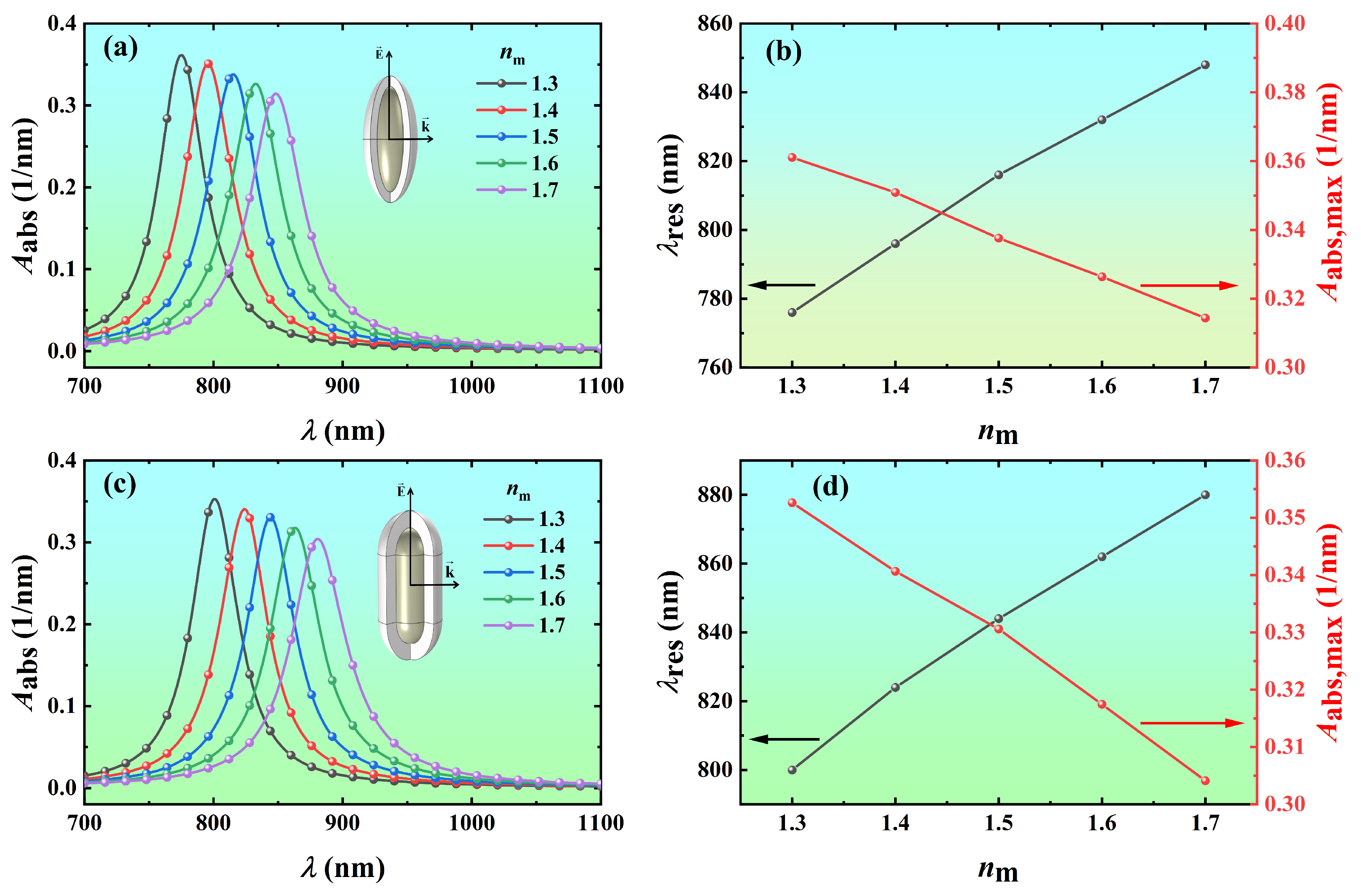
3.3. Effect of Refractive Index of Surrounding Medium on Light Absorption
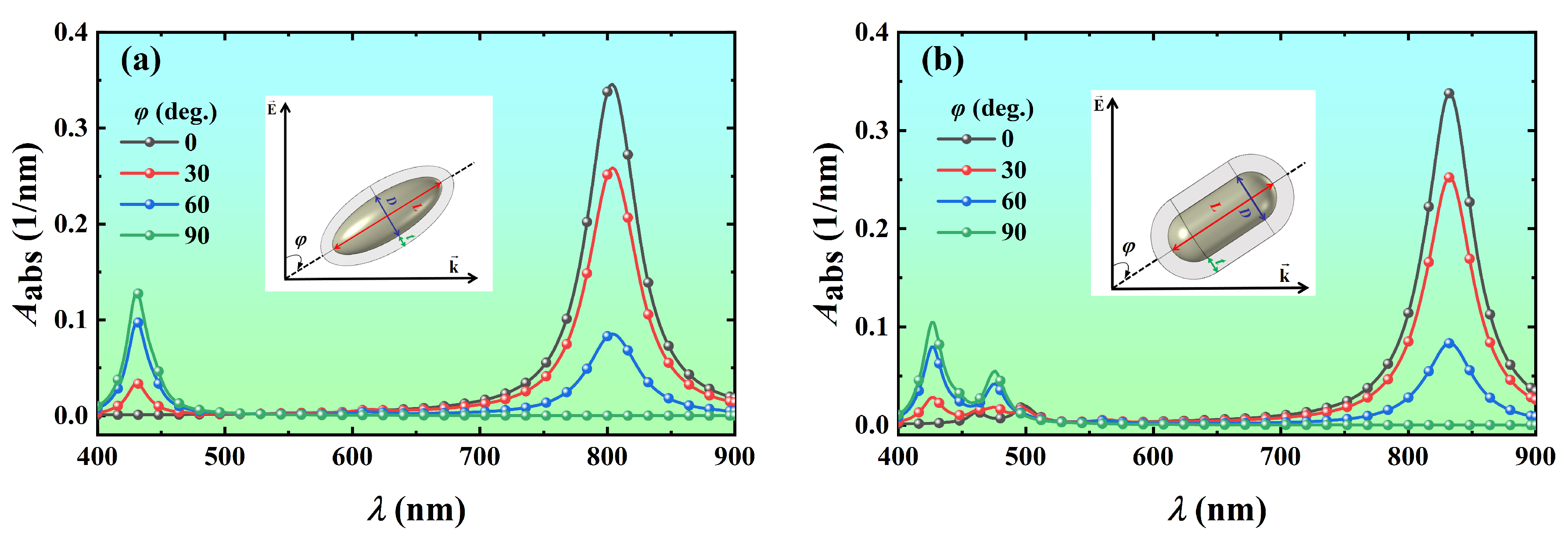
3.4. Effect of Particle Orientation on Light Absorption
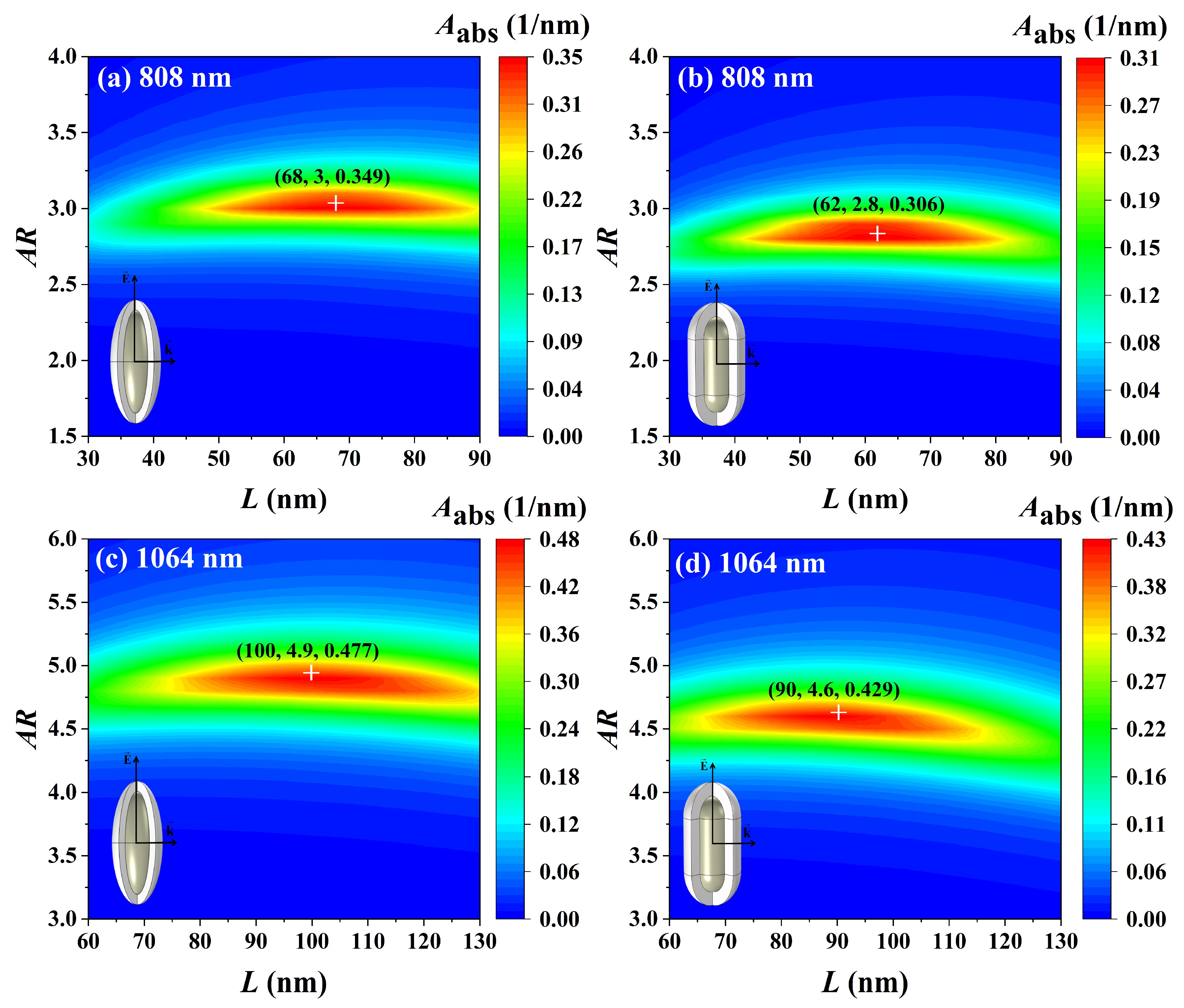
3.5. Optimization of Light Absorption Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, S.; Sharma, N.; Sahi, S.V. Advances in Cancer Therapeutics: Conventional Thermal Therapy to Nanotechnology-Based Photothermal Therapy. Pharmaceutics 2021, 13, 1174. [Google Scholar] [CrossRef] [PubMed]
- Gotwals, P.; Cameron, S.; Cipolletta, D.; Cremasco, V.; Crystal, A.; Hewes, B.; Mueller, B.; Quaratino, S.; Sabatos-Peyton, C.; Petruzzelli, L.; et al. Prospects for Combining Targeted and Conventional Cancer Therapy with Immunotherapy. Nat. Rev. Cancer 2017, 17, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical Development and Potential of Photothermal and Photodynamic Therapies for Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Pan, X.; Liu, H. Two-Dimensional Nanomaterials for Photothermal Therapy. Angew. Chem. Int. Ed. 2020, 59, 5890–5900. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-F.; Chang, S.-H.G.; Cheng, F.-Y.; Shanmugam, V.; Cheng, Y.-S.; Su, C.-H.; Yeh, C.-S. Au Nanorod Design as Light-Absorber in the First and Second Biological Near-Infrared Windows for in vivo Photothermal Therapy. ACS Nano 2013, 7, 5330–5342. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ma, Z.; Wang, H.; Zhou, B.; Zhu, S.; Zhong, Y.; Wang, J.; Wan, H.; Antaris, A.; Ma, R.; et al. Rational Design of Molecular Fluorophores for Biological Imaging in the NIR-II Window. Adv. Mater. 2017, 29, 1605497. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Bao, T.; Zhang, X.; Gao, Q.; Yu, J.; Dong, X.; Yan, L.; Gu, Z.; Zhao, Y. Biodegradable MoOx Nanoparticles with Efficient near-Infrared Photothermal and Photodynamic Synergetic Cancer Therapy at the Second Biological Window. Nanoscale 2018, 10, 1517–1531. [Google Scholar] [CrossRef]
- Du, J.; Wang, X.; Dong, X.; Zhang, C.; Mei, L.; Zang, Y.; Yan, L.; Zhang, H.; Gu, Z. Enhanced Radiosensitization of Ternary Cu3BiSe3 Nanoparticles by Photo-Induced Hyperthermia in the Second near-Infrared Biological Window. Nanoscale 2019, 11, 7157–7165. [Google Scholar] [CrossRef]
- Doughty, A.; Hoover, A.; Layton, E.; Murray, C.; Howard, E.; Chen, W. Nanomaterial Applications in Photothermal Therapy for Cancer. Materials 2019, 12, 779. [Google Scholar] [CrossRef]
- Jaque, D.; Martínez Maestro, L.; Del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodríguez, E.; García Solé, J. Nanoparticles for Photothermal Therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic Photothermal Therapy (PPTT) Using Gold Nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Garcia, M.A. Surface Plasmons in Metallic Nanoparticles: Fundamentals and Applications. J. Phys. D Appl. Phys. 2011, 44, 283001. [Google Scholar] [CrossRef]
- Mendes, P.C.D.; Song, Y.; Ma, W.; Gani, T.Z.H.; Lim, K.H.; Kawi, S.; Kozlov, S.M. Opportunities in the Design of Metal@oxide Core-Shell Nanoparticles. Adv. Phys. X 2023, 8, 2175623. [Google Scholar] [CrossRef]
- Ivanchenko, M.; Carroll, A.L.; Brothers, A.B.; Jing, H. Plasmonic Ag@Cu2O Core–Shell Nanostructures Exhibiting near-Infrared Photothermal Effect. RSC Adv. 2023, 13, 31569–31577. [Google Scholar] [CrossRef]
- Alikhaidarova, E.; Afanasyev, D.; Ibrayev, N.; Nuraje, N. Plasmonic Enhanced Polymer Solar Cell with Inclusion of Ag@SiO2 Core-shell Nanostructures. Polym. Adv. Technol. 2022, 33, 1000–1008. [Google Scholar] [CrossRef]
- Mazhani, M.; Alula, M.T.; Murape, D. Development of a Cysteine Sensor Based on the Peroxidase-like Activity of AgNPs@Fe3O4 Core-Shell Nanostructures. Anal. Chim. Acta 2020, 1107, 193–202. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Duan, X.; Zhang, C.; Zhao, K.; Xu, X. Core-Shell Ag@In2O3 Hollow Hetero-Nanostructures for Selective Ethanol Detection in Air. Sens. Actuators B Chem. 2020, 305, 127450. [Google Scholar] [CrossRef]
- Nie, C.; Du, P.; Zhao, H.; Xie, H.; Li, Y.; Yao, L.; Shi, Y.; Hu, L.; Si, S.; Zhang, M.; et al. Ag@TiO2 Nanoprisms with Highly Efficient Near-Infrared Photothermal Conversion for Melanoma Therapy. Chem.–Asian J. 2020, 15, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Lyu, L.-M.; Koga, K.; Shimoyama, Y.; Kon, Y. Plasmonic Ag@TiO2 Core–Shell Nanoparticles for Enhanced CO2 Photoconversion to CH4. ACS Sustain. Chem. Eng. 2019, 7, 18955–18964. [Google Scholar] [CrossRef]
- Dayakar, T.; Venkateswara Rao, K.; Park, J.; Krishna, P.; Swaroopa, P.; Ji, Y. Biosynthesis of Ag@CuO Core–Shell Nanostructures for Non-Enzymatic Glucose Sensing Using Screen-Printed Electrode. J. Mater. Sci. Mater. Electron. 2019, 30, 9725–9734. [Google Scholar] [CrossRef]
- N’Konou, K.; Chalh, M.; Monnier, V.; Blanchard, N.P.; Chevolot, Y.; Lucas, B.; Vedraine, S.; Torchio, P. Impact of Ag@SiO2 Core-Shell Nanoparticles on the Photoelectric Current of Plasmonic Inverted Organic Solar Cells. Synth. Met. 2018, 239, 22–28. [Google Scholar] [CrossRef]
- Lee, C.; Shin, K.; Lee, Y.J.; Jung, C.; Lee, H.M. Effects of Shell Thickness on Ag-Cu2O Core-Shell Nanoparticles with Bumpy Structures for Enhancing Photocatalytic Activity and Stability. Catal. Today 2018, 303, 313–319. [Google Scholar] [CrossRef]
- Rajbongshi, H.; Bhattacharjee, S.; Datta, P. Photocatalytic Activity of Ag/ZnO Core–Shell Nanoparticles with Shell Thickness as Controlling Parameter under Green Environment. Mater. Res. Express 2017, 4, 025501. [Google Scholar] [CrossRef]
- Shen, W.; Tang, J.; Yang, R.; Cong, H.; Bao, X.; Wang, Y.; Wang, X.; Huang, Z.; Liu, J.; Huang, L.; et al. Enhanced Efficiency of Polymer Solar Cells by Incorporated Ag–SiO2 Core–Shell Nanoparticles in the Active Layer. RSC Adv. 2014, 4, 4379–4386. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.C.; Krishnan, V. Recent Advances in Plasmonic Photocatalysis Based on TiO2 and Noble Metal Nanoparticles for Energy Conversion, Environmental Remediation, and Organic Synthesis. Small 2022, 18, 2101638. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Liu, J.; Zhang, J. Construction of Plasmonic Metal@Semiconductor Core–Shell Photocatalysts: From Epitaxial to Nonepitaxial Strategies. Small Struct. 2022, 3, 2200045. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, A. Recent Advances in the Synthesis and Application of Photocatalytic Metal–Metal Oxide Core–Shell Nanoparticles for Environmental Remediation and Their Recycling Process. RSC Adv. 2016, 6, 83589–83612. [Google Scholar] [CrossRef]
- Amirjani, A.; Sadrnezhaad, S.K. Computational Electromagnetics in Plasmonic Nanostructures. J. Mater. Chem. C 2021, 9, 9791–9819. [Google Scholar] [CrossRef]
- Myroshnychenko, V.; Rodríguez-Fernández, J.; Pastoriza-Santos, I.; Funston, A.M.; Novo, C.; Mulvaney, P.; Liz-Marzán, L.M.; García de Abajo, F.J. Modelling the Optical Response of Gold Nanoparticles. Chem. Soc. Rev. 2008, 37, 1792. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Pinchuk, A.O.; McMahon, J.M.; Li, S.; Ausman, L.K.; Atkinson, A.L.; Schatz, G.C. Methods for Describing the Electromagnetic Properties of Silver and Gold Nanoparticles. Acc. Chem. Res. 2008, 41, 1710–1720. [Google Scholar] [CrossRef]
- Song, X.; Li, J. Electromagnetic Response of Core-Satellite Nanoparticles for Application in Photothermal Conversion. Plasmonics 2023, 18, 661–676. [Google Scholar] [CrossRef]
- Hövel, H.; Fritz, S.; Hilger, A.; Kreibig, U.; Vollmer, M. Width of Cluster Plasmon Resonances: Bulk Dielectric Functions and Chemical Interface Damping. Phys. Rev. B 1993, 48, 18178–18188. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 1995; Volume 25, ISBN 978-3-642-08191-0. [Google Scholar]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Ashcroft, N.W.; Mermin, N.D. Solid State Physics; Holt, Rinehart and Winston: New York, NY, USA, 1976; ISBN 978-0-03-083993-1. [Google Scholar]
- Rakić, A.D.; Djurišić, A.B.; Elazar, J.M.; Majewski, M.L. Optical Properties of Metallic Films for Vertical-Cavity Optoelectronic Devices. Appl. Opt. 1998, 37, 5271. [Google Scholar] [CrossRef] [PubMed]
- Moroz, A. Electron Mean-Free Path in Metal-Coated Nanowires. J. Opt. Soc. Am. B 2011, 28, 1130. [Google Scholar] [CrossRef]
- Sharma, R.; Sarkar, A.; Jha, R.; Kumar Sharma, A.; Sharma, D. Sol-Gel Mediated Synthesis of TiO2 Nanocrystals: Structural, Optical, and Electrochemical Properties. Int. J. Appl. Ceram. Tech. 2020, 17, 1400–1409. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley: New York, NY, USA, 1983; ISBN 978-0-471-05772-7. [Google Scholar]
- Mishchenko, M.I.; Travis, L.D.; Lacis, A.A. Scattering, Absorption, and Emission of Light by Small Particles; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2002; ISBN 978-0-521-78252-4. [Google Scholar]
- Somerville, W.R.C. SMARTIES: User-Friendly Codes for Fast and Accurate Calculations of Light Scattering by Spheroids. J. Quant. Spectrosc. Radiat. Transf. 2016, 174, 39–55. [Google Scholar] [CrossRef]
- Duck, F.A. Physical Properties of Tissue: A Comprehensive Reference Book; Academic Press: London, UK, 1990; ISBN 978-0-12-222800-1. [Google Scholar]
- Trügler, A. Optical Properties of Metallic Nanoparticles; Springer Series in Materials Science; Springer International Publishing: Cham, Switzerland, 2016; Volume 232, ISBN 978-3-319-25072-4. [Google Scholar]
- Guidez, E.B.; Aikens, C.M. Diameter Dependence of the Excitation Spectra of Silver and Gold Nanorods. J. Phys. Chem. C 2013, 117, 12325–12336. [Google Scholar] [CrossRef]
- Verma, S.S.; Sekhon, J.S. Influence of Aspect Ratio and Surrounding Medium on Localized Surface Plasmon Resonance (LSPR) of Gold Nanorod. J. Opt. 2012, 41, 89–93. [Google Scholar] [CrossRef]
- Murphy, C.J.; Gole, A.M.; Hunyadi, S.E.; Stone, J.W.; Sisco, P.N.; Alkilany, A.; Kinard, B.E.; Hankins, P. Chemical Sensing and Imaging with Metallic Nanorods. Chem. Commun. 2008, 5, 544–557. [Google Scholar] [CrossRef]
- Brioude, A.; Jiang, X.C.; Pileni, M.P. Optical Properties of Gold Nanorods: DDA Simulations Supported by Experiments. J. Phys. Chem. B 2005, 109, 13138–13142. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Kou, X.; Yang, Z.; Wang, J. Tailoring Longitudinal Surface Plasmon Wavelengths, Scattering and Absorption Cross Sections of Gold Nanorods. ACS Nano 2008, 2, 677–686. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cheng, X.; Zhang, H.; Bai, X.; Ai, R.; Shao, L.; Wang, J. Gold Nanorods: The Most Versatile Plasmonic Nanoparticles. Chem. Rev. 2021, 121, 13342–13453. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold Nanorods and Their Plasmonic Properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold Nanorods: From Synthesis and Properties to Biological and Biomedical Applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef]
- Sekizawa, Y.; Hasegawa, Y.; Mitomo, H.; Toyokawa, C.; Yonamine, Y.; Ijiro, K. Dynamic Orientation Control of Gold Nanorods in Polymer Brushes by Their Thickness Changes for Plasmon Switching. Adv. Mater. Inter. 2024, 11, 2301066. [Google Scholar] [CrossRef]
- Wang, H.; Qian, H.; Li, W.; Wang, K.; Li, H.; Zheng, X.; Gu, P.; Chen, S.; Yi, M.; Xu, J.; et al. Large-Area Arrays of Polymer-Tethered Gold Nanorods with Controllable Orientation and Their Application in Nano-Floating-Gate Memory Devices. Small 2023, 19, 2208288. [Google Scholar] [CrossRef]
- Korkos, S.; Jantunen, V.; Arstila, K.; Sajavaara, T.; Leino, A.; Nordlund, K.; Djurabekova, F. Nanorod Orientation Control by Swift Heavy Ion Irradiation. Appl. Phys. Lett. 2022, 120, 171602. [Google Scholar] [CrossRef]
- Rong, Y.; Song, L.; Si, P.; Zhang, L.; Lu, X.; Zhang, J.; Nie, Z.; Huang, Y.; Chen, T. Macroscopic Assembly of Gold Nanorods into Superstructures with Controllable Orientations by Anisotropic Affinity Interaction. Langmuir 2017, 33, 13867–13873. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A. Controlling the Orientations of Gold Nanorods inside Highly Packed 2D Arrays. Phys. Chem. Chem. Phys. 2014, 16, 26153–26162. [Google Scholar] [CrossRef] [PubMed]
- Thai, T.; Zheng, Y.; Ng, S.H.; Mudie, S.; Altissimo, M.; Bach, U. Self-Assembly of Vertically Aligned Gold Nanorod Arrays on Patterned Substrates. Angew. Chem. Int. Ed. 2012, 51, 8732–8735. [Google Scholar] [CrossRef] [PubMed]
- Nepal, D.; Onses, M.S.; Park, K.; Jespersen, M.; Thode, C.J.; Nealey, P.F.; Vaia, R.A. Control over Position, Orientation, and Spacing of Arrays of Gold Nanorods Using Chemically Nanopatterned Surfaces and Tailored Particle–Particle–Surface Interactions. ACS Nano 2012, 6, 5693–5701. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wumaier, D.; Tuersun, P.; Li, S.; Li, Y.; Wang, M.; Xu, D. Light Absorption Analysis and Optimization of Ag@TiO2 Core-Shell Nanospheroid and Nanorod. Nanomaterials 2024, 14, 325. https://doi.org/10.3390/nano14040325
Wumaier D, Tuersun P, Li S, Li Y, Wang M, Xu D. Light Absorption Analysis and Optimization of Ag@TiO2 Core-Shell Nanospheroid and Nanorod. Nanomaterials. 2024; 14(4):325. https://doi.org/10.3390/nano14040325
Chicago/Turabian StyleWumaier, Dilishati, Paerhatijiang Tuersun, Shuyuan Li, Yixuan Li, Meng Wang, and Dibo Xu. 2024. "Light Absorption Analysis and Optimization of Ag@TiO2 Core-Shell Nanospheroid and Nanorod" Nanomaterials 14, no. 4: 325. https://doi.org/10.3390/nano14040325
APA StyleWumaier, D., Tuersun, P., Li, S., Li, Y., Wang, M., & Xu, D. (2024). Light Absorption Analysis and Optimization of Ag@TiO2 Core-Shell Nanospheroid and Nanorod. Nanomaterials, 14(4), 325. https://doi.org/10.3390/nano14040325





