Intracranial Gene Delivery Mediated by Albumin-Based Nanobubbles and Low-Frequency Ultrasound
Abstract
1. Introduction
2. Materials and Methods
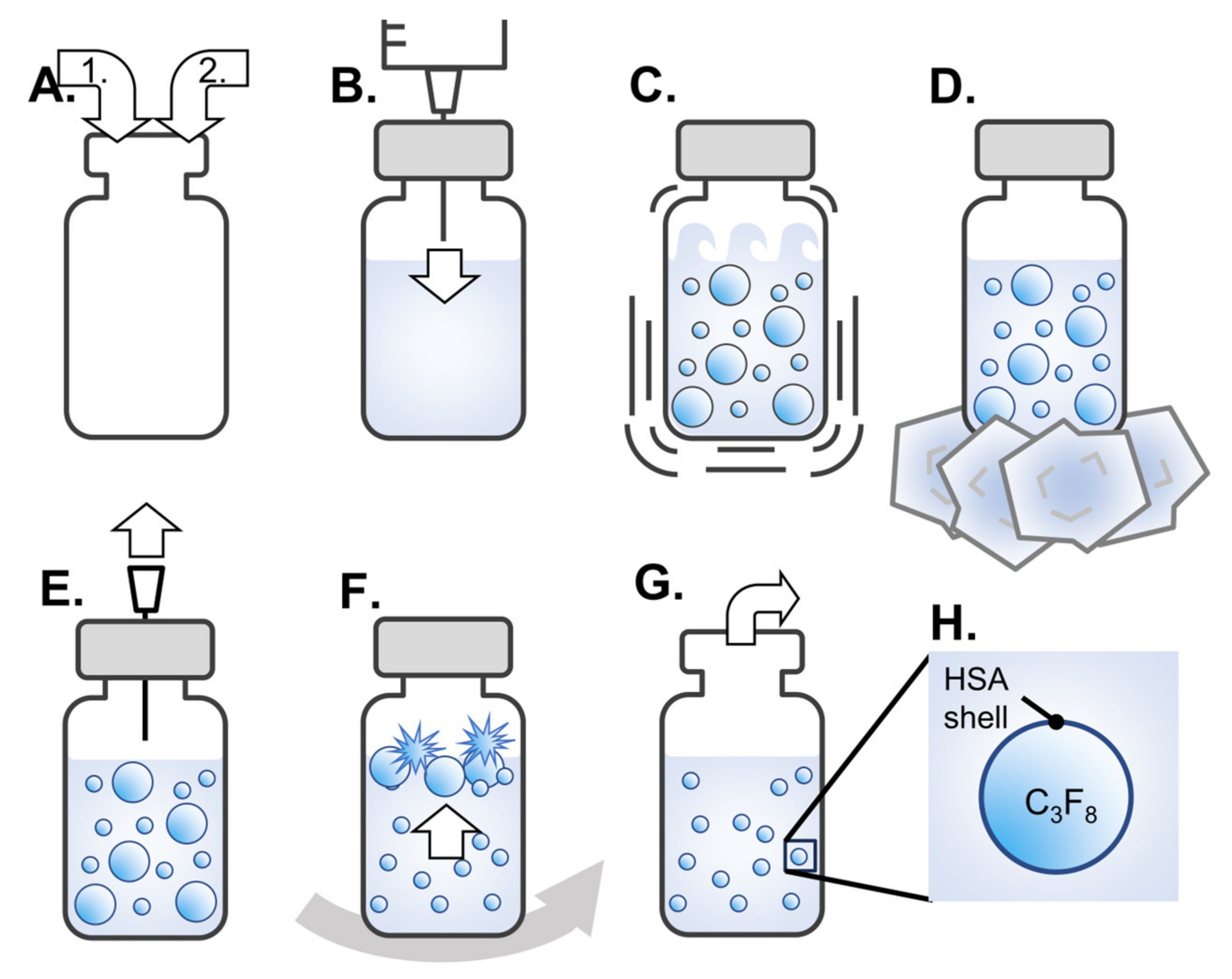
2.1. Preparation of Albumin-Based NBs
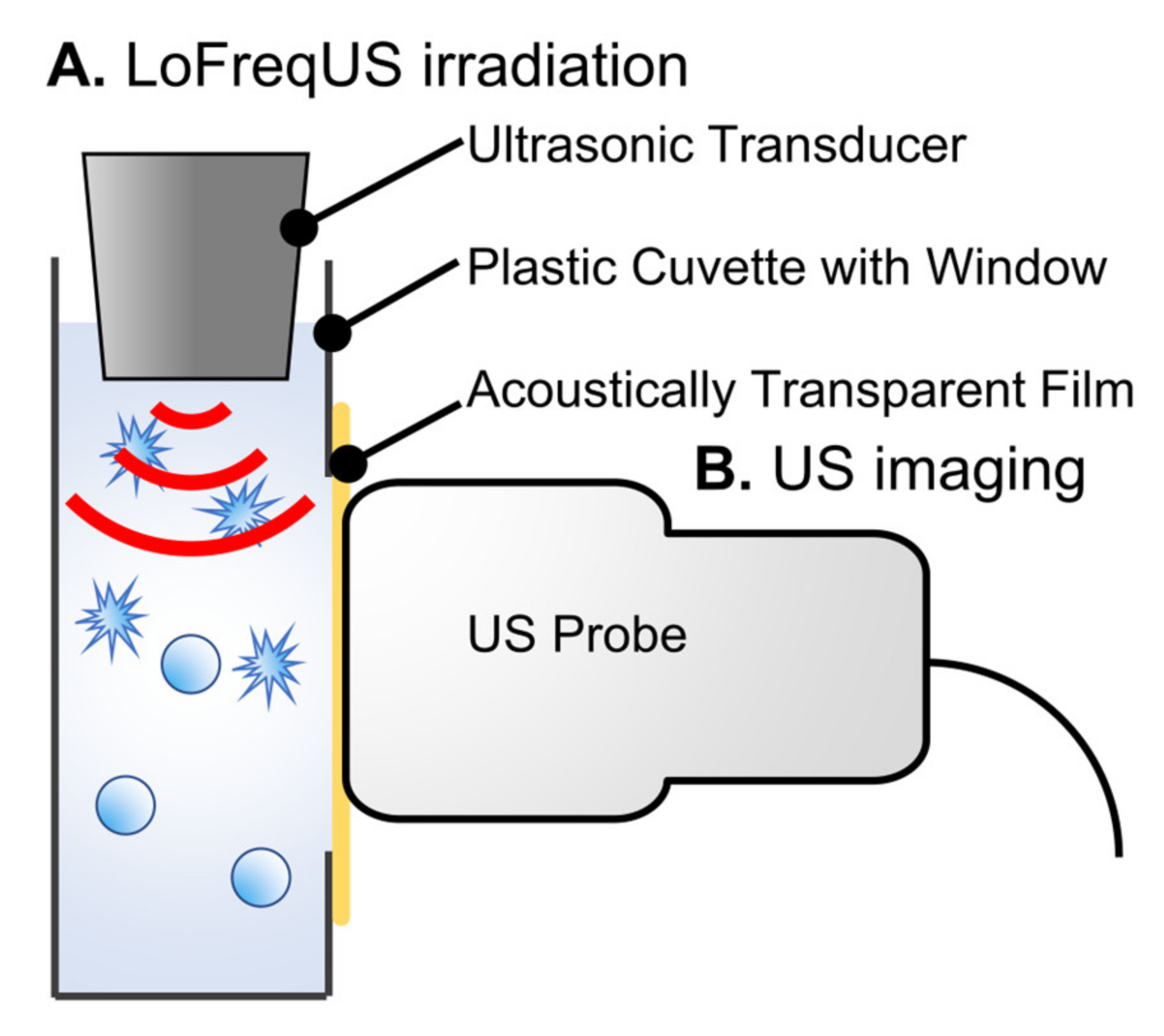
2.2. Low-Frequency Ultrasound Irradiation to Albumin-Based NBs
2.3. Characterization of Albumin-Based NBs
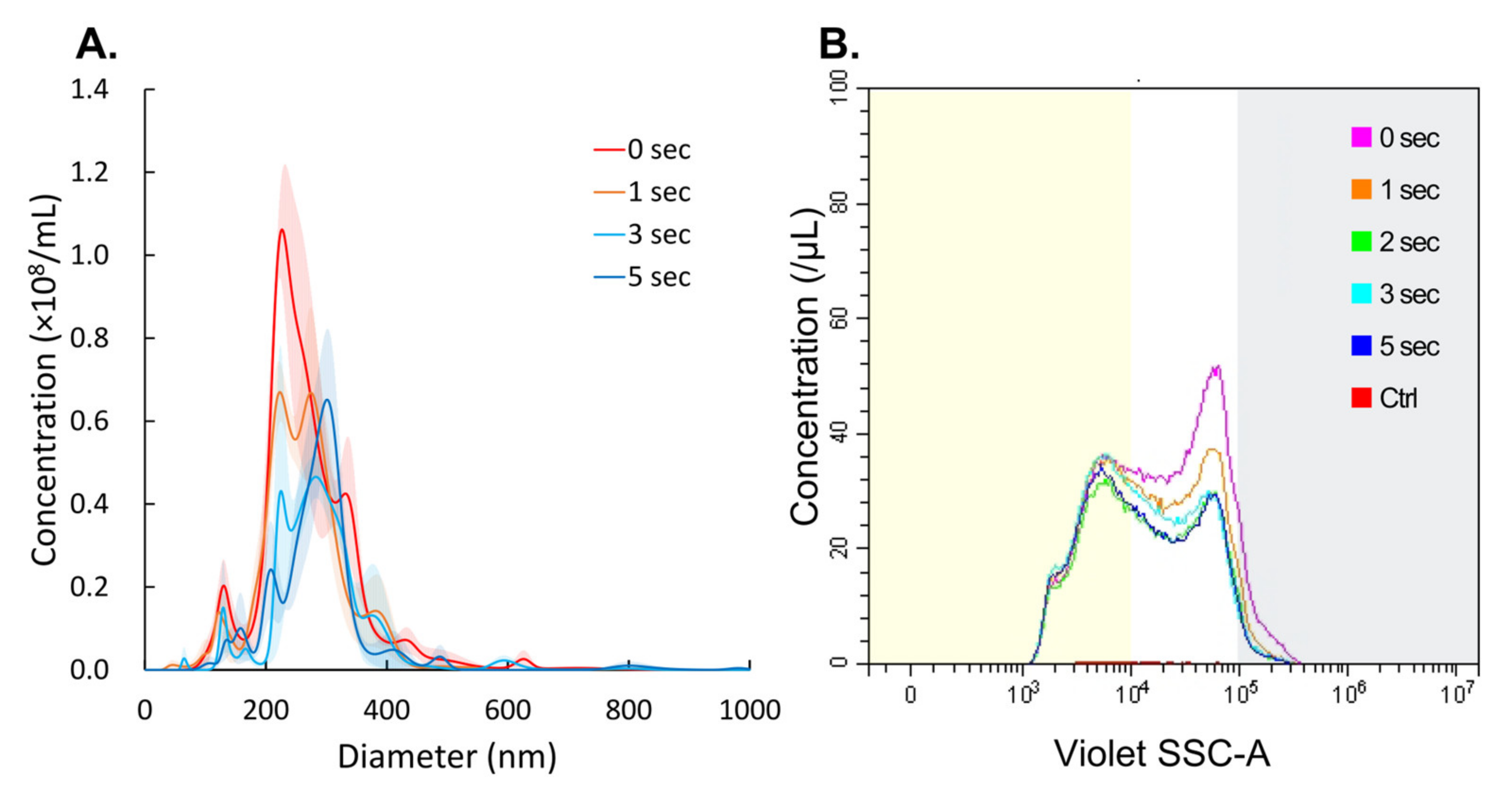
2.3.1. Nanoparticle Tracking Analysis
2.3.2. Flow Cytometric Analysis
2.4. In Vitro Ultrasound Imaging of Albumin-Based NBs
2.5. Preparation of pDNA and mRNA
2.6. Cell Culture
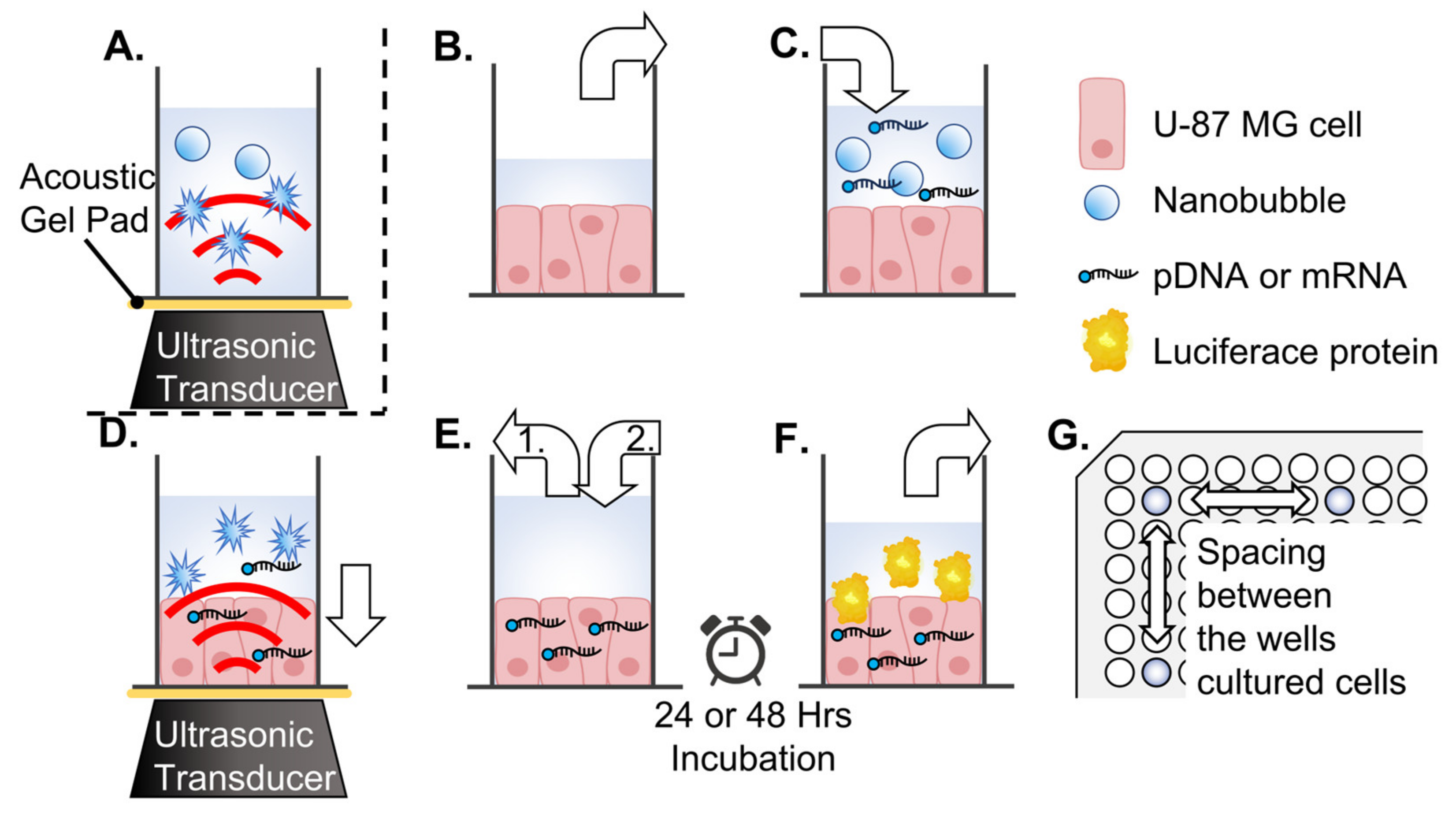
2.7. In Vitro Cell Sonoporation System
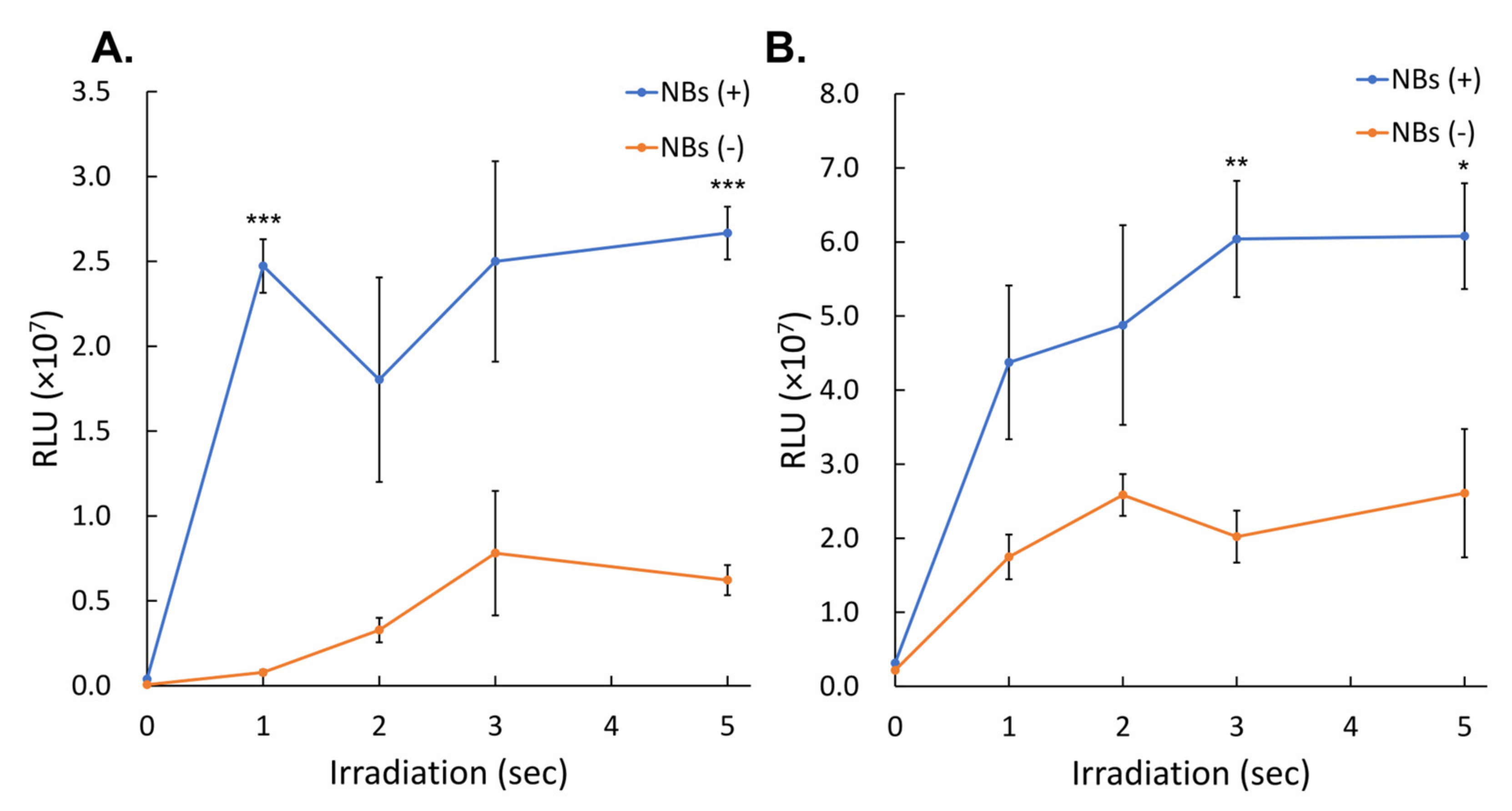
2.8. In Vitro Evaluation of Luciferase Expression
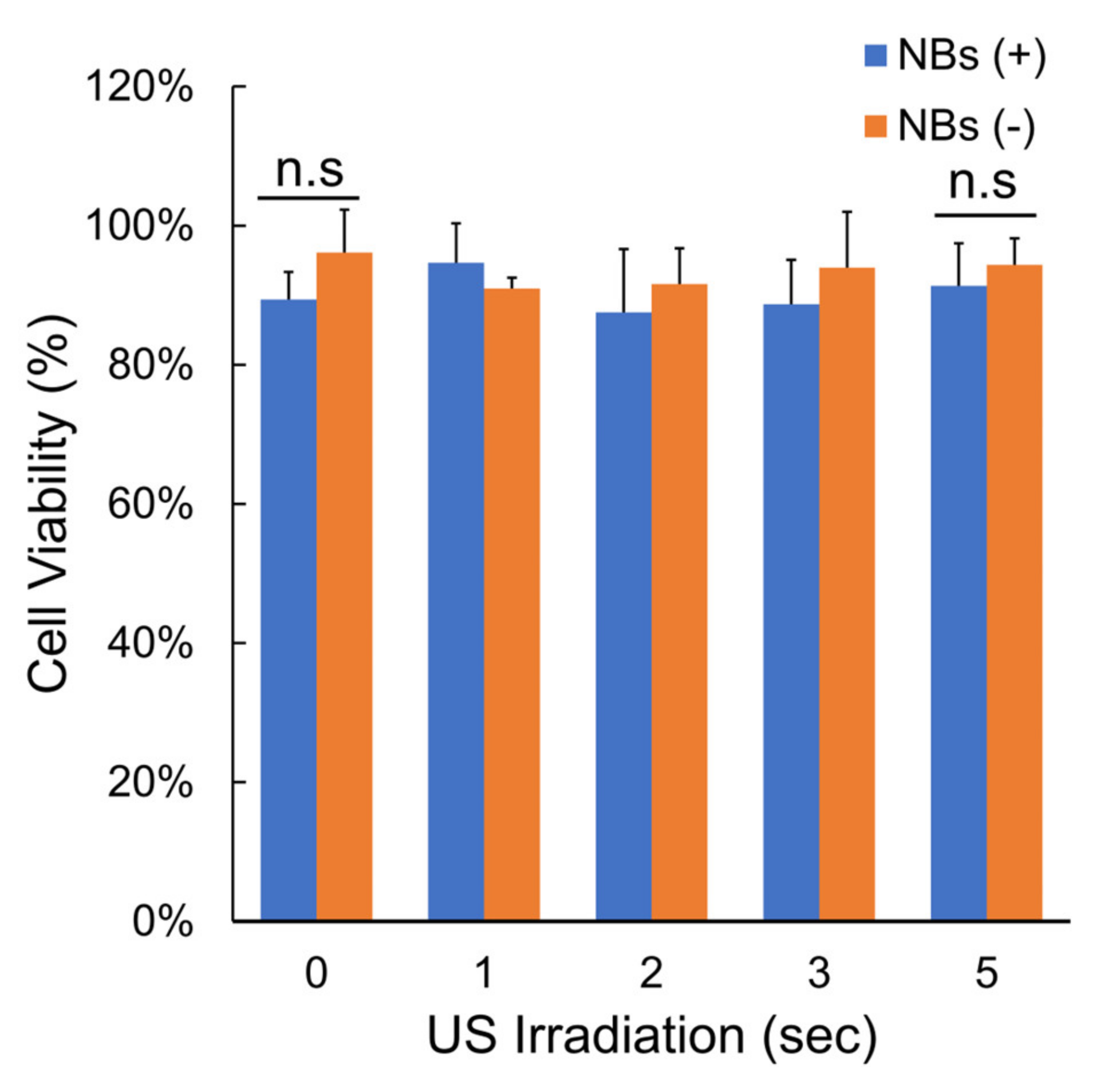
2.9. Cell Viability Assay
2.10. Animals
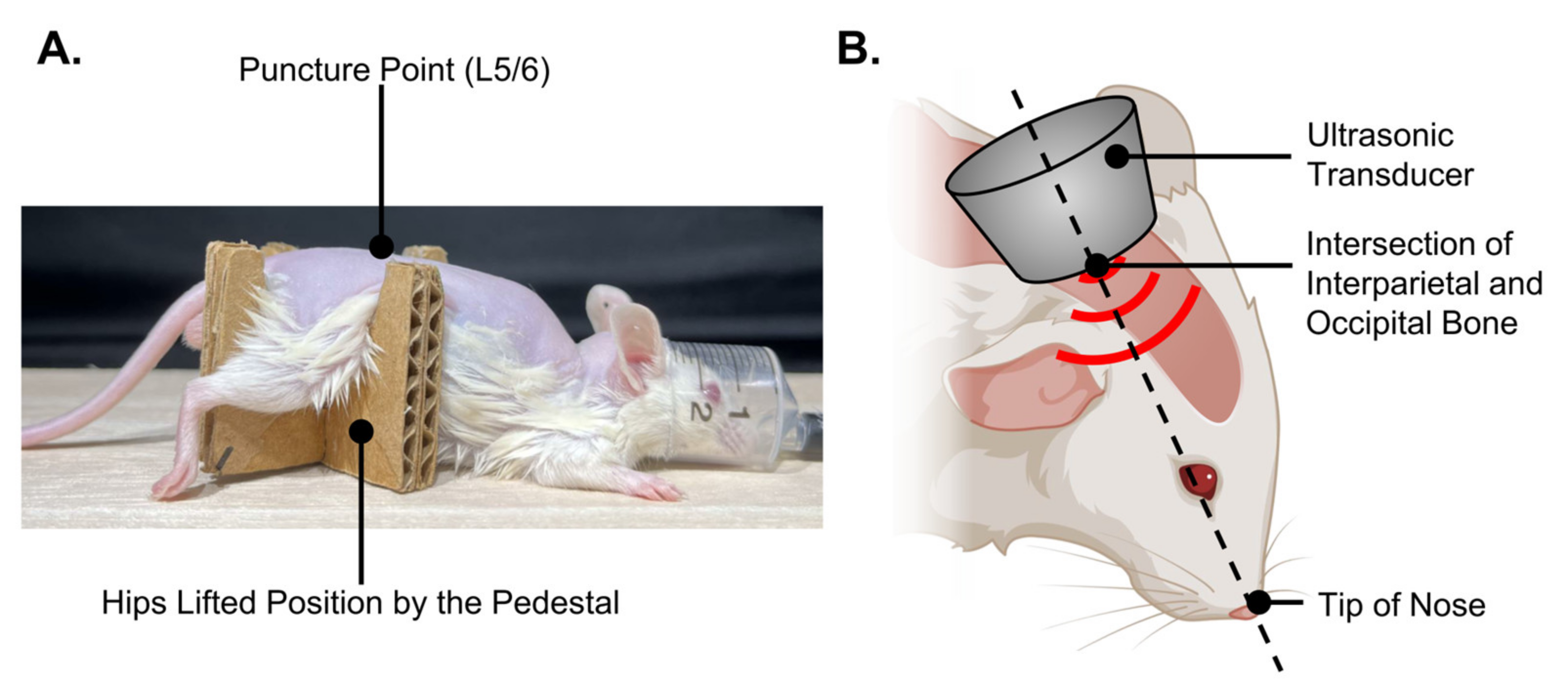
2.11. In Vivo Intrathecal Administration and Ultrasound Irradiation
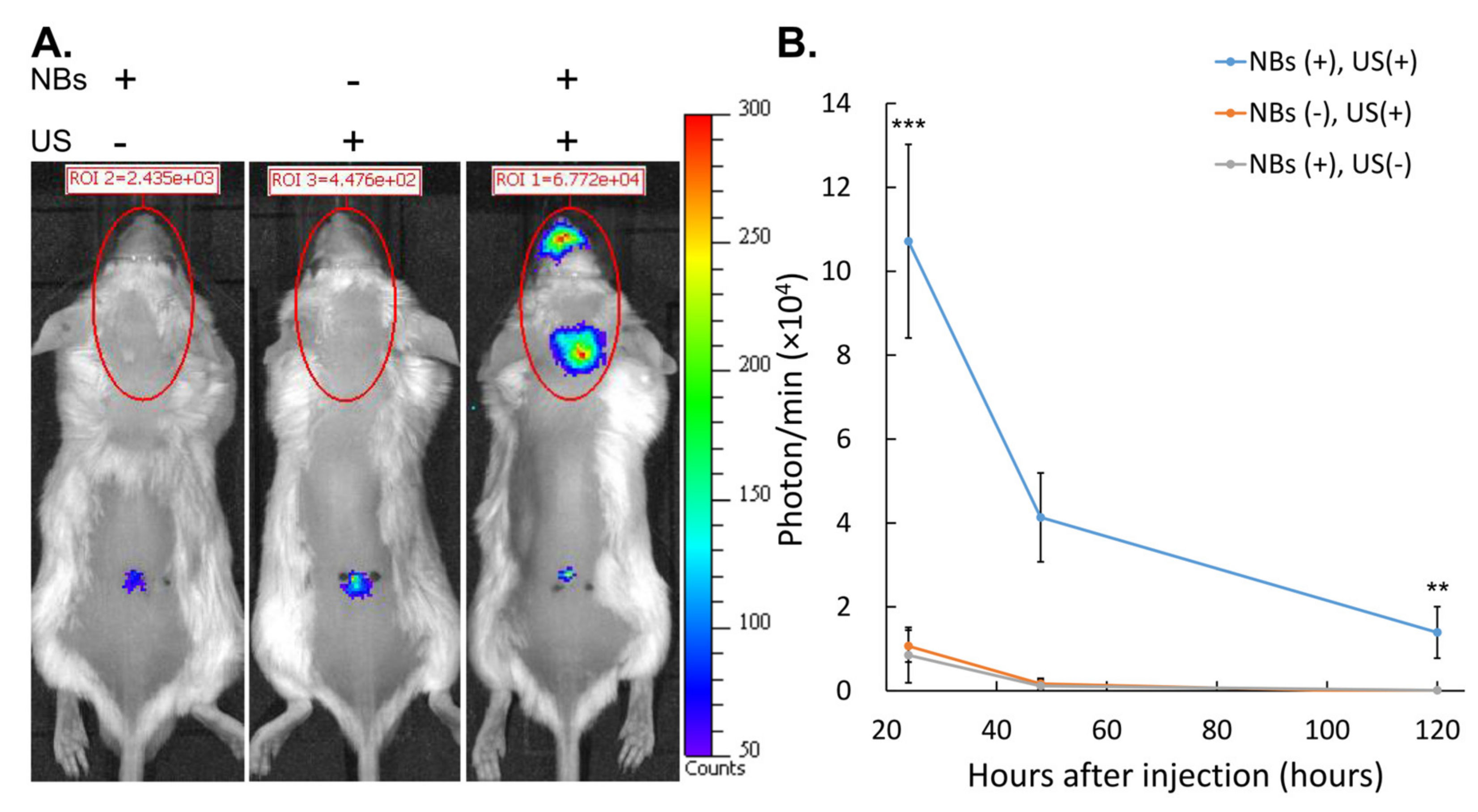
2.12. In Vivo Evaluation of Luciferase Expression
2.13. Statistical Analysis
3. Results
3.1. NBs Collapse by LoFreqUS Irradiation
3.2. Gene Transfer by LoFreqUS
3.3. Intrathecal Administration of Dye by Lumbar Puncture
3.4. Gene Expression after Intrathecal Administration and Intracranial LoFreqUS Irradiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Sonication Duration (s) | All Ranged NBs (/mL) | NBs < 200 nm (/mL) | NBs 200–500 nm (/mL) | NBs > 500 nm (/mL) |
|---|---|---|---|---|
| 0 | 23.0 × 108 | 8.0 × 108 | 13.8 × 108 | 1.4 × 108 |
| 1 | 19.6 × 108 (85.2%) | 8.1 × 108 (101.5%) | 10.9 × 108 (79.4%) | 0.6 × 108 (39.7%) |
| 2 | 16.2 × 108 (70.3%) | 7.1 × 108 (88.9%) | 8.7 × 108 (63.4%) | 0.4 × 108 (24.9%) |
| 3 | 17.9 × 108 (77.9%) | 8.2 × 108 (103.4%) | 9.3 × 108 (67.6%) | 0.4 × 108 (25.6%) |
| 5 | 16.6 × 108 (72.2%) | 7.6 × 108 (95.9%) | 8.6 × 108 (62.7%) | 0.3 × 108 (22.9%) |
References
- Arsiwala, T.A.; Sprowls, S.A.; Blethen, K.E.; Adkins, C.E.; Saralkar, P.A.; Fladeland, R.A.; Pentz, W.; Gabriele, A.; Kielkowski, B.; Mehta, R.I.; et al. Ultrasound-mediated disruption of the blood tumor barrier for improved therapeutic delivery. Neoplasia 2021, 23, 676–691. [Google Scholar] [CrossRef]
- Tachibana, K.; Kida, H. Recent Trends in Application of Encapsulated Ultrafine Bubbles in Medicine, 1st ed.; Jenny Stanford Publishing: New York, NY, USA, 2021. [Google Scholar]
- Kida, H.; Tachibana, K. Application of Ultrasound-Responsive Reagents for Drug Delivery Systems; Wiley: Hoboken, NJ, USA, 2023; pp. 181–215. [Google Scholar] [CrossRef]
- Wasielewska, J.M.; White, A.R. Focused Ultrasound-mediated Drug Delivery in Humans—A Path Towards Translation in Neurodegenerative Diseases. Pharm. Res. 2022, 39, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Zhang, Z. Advances in Biological Application of and Research on Low-Frequency Ultrasound. Ultrasound Med. Biol. 2021, 47, 2839–2852. [Google Scholar] [CrossRef] [PubMed]
- Kida, H.; Feril, L.B.; Irie, Y.; Endo, H.; Itaka, K.; Tachibana, K. Influence of nanobubble size distribution on ultrasound-mediated plasmid DNA and messenger RNA gene delivery. Front. Pharmacol. 2022, 13, 855495. [Google Scholar] [CrossRef] [PubMed]
- Kida, H.; Yamasaki, Y.; Feril, L.B., Jr.; Endo, H.; Itaka, K.; Tachibana, K. Efficient mRNA Delivery with Lyophilized Human Serum Albumin-Based Nanobubbles. Nanomaterials 2023, 13, 1283. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.H.; Lin, K.H.; Chuang, H.C.; Tsai, C.H.; Lin, Y.C.; Wang, C.H.; Shih, C.P.; Liu, H.L. Low-frequency dual-frequency ultrasound-mediated microbubble cavitation for transdermal minoxidil delivery and hair growth enhancement. Sci. Rep. 2020, 10, 4338. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, M.; Hetzel, A.; Krüger, S.; Kretzer, S.; Talazko, J.; Ziyeh, S.; Weber, J.; Els, T. Blood-brain barrier disruption by low-frequency ultrasound. Stroke 2006, 37, 1546–1548. [Google Scholar] [CrossRef] [PubMed]
- ISO 20480-1:2017; Fine Bubble Technology—General Principles for Usage and Measurement of Fine Bubbles—Part 1: Terminology. ISO: Geneva, Switzerland, 2017.
- Watanabe, A.; Sheng, H.; Endo, H.; Feril, L.B.; Irie, Y.; Ogawa, K.; Moosavi-Nejad, S.; Tachibana, K. Echographic and physical characterization of albumin-stabilized nanobubbles. Heliyon 2019, 5, e01907. [Google Scholar] [CrossRef] [PubMed]
- Puhl, D.L.; D’Amato, A.R.; Gilbert, R.J. Challenges of gene delivery to the central nervous system and the growing use of biomaterial vectors. Brain Res. Bull. 2019, 150, 216–230. [Google Scholar] [CrossRef]
- Wang, Y.S.; Kumari, M.; Chen, G.H.; Hong, M.H.; Yuan, J.P.; Tsai, J.L.; Wu, H.C. mRNA-based vaccines and therapeutics: An in-depth survey of current and upcoming clinical applications. J. Biomed. Sci. 2023, 30, 84. [Google Scholar] [CrossRef]
- Ajeeb, R.; Clegg, J.R. Intrathecal delivery of Macromolecules: Clinical status and emerging technologies. Adv. Drug Deliv. Rev. 2023, 199, 114949. [Google Scholar] [CrossRef]
- Kida, H.; Nishimura, K.; Ogawa, K.; Watanabe, A.; Feril, L.B.; Irie, Y.; Endo, H.; Kawakami, S.; Tachibana, K. Nanobubble mediated gene delivery in conjunction with a hand-held ultrasound scanner. Front. Pharmacol. 2020, 11, 363. [Google Scholar] [CrossRef]
- Njoo, C.; Heinl, C.; Kuner, R. In vivo SiRNA transfection and gene knockdown in spinal cord via rapid noninvasive lumbar intrathecal injections in mice. J. Vis. Exp. 2014, 85, e51229. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- McFaline-Figueroa, J.R.; Lee, E.Q. Brain Tumors. Am. J. Med. 2018, 131, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Cooper, M.; Rutter, M. Neurodevelopmental disorders. Lancet Psychiatry 2017, 4, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Kida, H.; Takahashi, T.; Nakamura, Y.; Kinoshita, T.; Hara, M.; Okamoto, M.; Okayama, S.; Nakamura, K.; Kosai, K.I.; Taniwaki, T.; et al. Pathogenesis of Lethal Aspiration Pneumonia in Mecp2-null Mouse Model for Rett Syndrome. Sci. Rep. 2017, 7, 12032. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Miura, S.; Kida, H.; Irie, K.I.; Yamanishi, Y.; Hoshino, T.; Taniwaki, T. MIBG myocardial scintigraphy in progressive supranuclear palsy. J. Neurol. Sci. 2019, 396, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.L. Spinocerebellar degeneration. Expert Opin. Pharmacother. 2003, 4, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Yau, W.Y.; O’Connor, E.; Houlden, H. Spinocerebellar ataxia: An update. J. Neurol. 2019, 266, 533–544. [Google Scholar] [CrossRef]
- Sun, Y.M.; Lu, C.; Wu, Z.Y. Spinocerebellar ataxia: Relationship between phenotype and genotype—A review. Clin. Genet. 2016, 90, 305–314. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Ling, A.L.; Solomon, I.H.; Landivar, A.M.; Nakashima, H.; Woods, J.K.; Santos, A.; Masud, N.; Fell, G.; Mo, X.; Yilmaz, A.S.; et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature 2023, 623, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.G.; Shen, J.; Yang, J.; Wang, J.W.; Zhao, R.C.; Zhang, T.L.; Guo, J.; Zhang, X. Nucleic acid drug vectors for diagnosis and treatment of brain diseases. Signal Transduct. Target. Ther. 2023, 8, 39. [Google Scholar] [CrossRef]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef]
- Tang, X.; Peng, H.; Xu, P.; Zhang, L.; Fu, R.; Tu, H.; Guo, X.; Huang, K.; Lu, J.; Chen, H.; et al. Synthetic mRNA-based gene therapy for glioblastoma: TRAIL-mRNA synergistically enhances PTEN-mRNA-based therapy. Mol. Ther. Oncolyt. 2022, 24, 707–718. [Google Scholar] [CrossRef]
- Lin, C.Y.; Perche, F.; Ikegami, M.; Uchida, S.; Kataoka, K.; Itaka, K. Messenger RNA-based therapeutics for brain diseases: An animal study for augmenting clearance of beta-amyloid by intracerebral administration of neprilysin mRNA loaded in polyplex nanomicelles. J. Control. Release 2016, 235, 268–275. [Google Scholar] [CrossRef]
- Fukushima, Y.; Uchida, S.; Imai, H.; Nakatomi, H.; Kataoka, K.; Saito, N.; Itaka, K. Treatment of ischemic neuronal death by introducing brain-derived neurotrophic factor mRNA using polyplex nanomicelle. Biomaterials 2021, 270, 120681. [Google Scholar] [CrossRef]
- Crowley, S.T.; Fukushima, Y.; Uchida, S.; Kataoka, K.; Itaka, K. Enhancement of Motor Function Recovery after Spinal Cord Injury in Mice by Delivery of Brain-Derived Neurotrophic Factor mRNA. Mol. Ther. Nucleic Acids 2019, 17, 465–476. [Google Scholar] [CrossRef]
- Minnaert, M. XVI. On musical air-bubbles and the sounds of running water. Lond. Edinb. Philos. Mag. J. Sci. 1933, 16, 235–248. [Google Scholar] [CrossRef]
- Helfield, B.; Zou, Y.; Matsuura, N. Acoustically-Stimulated Nanobubbles: Opportunities in Medical Ultrasound Imaging and Therapy. Front. Phys. 2021, 9, 654374. [Google Scholar] [CrossRef]
- Bismuth, M.; Katz, S.; Mano, T.; Aronovich, R.; Hershkovitz, D.; Exner, A.A.; Ilovitsh, T. Low frequency nanobubble-enhanced ultrasound mechanotherapy for noninvasive cancer surgery. Nanoscale 2022, 14, 13614–13627. [Google Scholar] [CrossRef]
- Bismuth, M.; Eck, M.; Ilovitsh, T. Nanobubble-mediated cancer cell sonoporation using low-frequency ultrasound. Nanoscale 2023, 15, 17899–17909. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Qiao, Q.; Han, X.; Jing, H.; Zhang, H.; Liang, H.; Cheng, W. Targeted nanobubbles in low-frequency ultrasound-mediated gene transfection and growth inhibition of hepatocellular carcinoma cells. Tumour Biol. 2016, 37, 12113–12121. [Google Scholar] [CrossRef]
- Yasuda, K.; Matsushima, H.; Asakura, Y. Generation and reduction of bulk nanobubbles by ultrasonic irradiation. Chem. Eng. Sci. 2019, 195, 455–461. [Google Scholar] [CrossRef]
- Keyel, P.A. Dnases in health and disease. Dev. Biol. 2017, 429, 1–11. [Google Scholar] [CrossRef]
- Houseley, J.; Tollervey, D. The many pathways of RNA degradation. Cell 2009, 136, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Bowers, W.J.; Breakefield, X.O.; Sena-Esteves, M. Genetic therapy for the nervous system. Hum. Mol. Genet. 2011, 20, R28–R41. [Google Scholar] [CrossRef] [PubMed]
- Tran, N. Blood-Brain Barrier. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; p. 426. [Google Scholar] [CrossRef]
- Reese, T.S.; Karnovsky, M.J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967, 34, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.R.; Habgood, M.D.; Møllgård, K.; Dziegielewska, K.M. The biological significance of brain barrier mechanisms: Help or hindrance in drug delivery to the central nervous system? F1000Research 2016, 5, 313. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.W.; Powlovich, L.; Sheybani, N.; LeBlang, S. Focused ultrasound for the treatment of glioblastoma. J. Neurooncol. 2022, 157, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Cody, R.; Mohsenifard, M.; Yip, G.; Aldridge, E.; Bridge, F.; Datta, M.; Guy, S.; Jordan, P.; Kyndt, C.; Newnham, E.; et al. Worth the risk? Contemporary indications, yield and complications of lumbar punctures in a metropolitan Australian health service. Intern. Med. J. 2023, 53, 1332–1338. [Google Scholar] [CrossRef]
- Pflepsen, K.R.; Peterson, C.D.; Kitto, K.F.; Vulchanova, L.; Wilcox, G.L.; Fairbanks, C.A. Detailed Method for Intrathecal Delivery of Gene Therapeutics by Direct Lumbar Puncture in Mice. Methods Mol. Biol. 2019, 1937, 305–312. [Google Scholar] [CrossRef]
- Kagiava, A.; Karaiskos, C.; Richter, J.; Tryfonos, C.; Lapathitis, G.; Sargiannidou, I.; Christodoulou, C.; Kleopa, K.A. Intrathecal gene therapy in mouse models expressing CMT1X mutations. Hum. Mol. Genet. 2018, 27, 1460–1473. [Google Scholar] [CrossRef]
- Shi, L.; Tang, G.P.; Gao, S.J.; Ma, Y.X.; Liu, B.H.; Li, Y.; Zeng, J.M.; Ng, Y.K.; Leong, K.W.; Wang, S. Repeated intrathecal administration of plasmid DNA complexed with polyethylene glycol-grafted polyethylenimine led to prolonged transgene expression in the spinal cord. Gene Ther. 2003, 10, 1179–1188. [Google Scholar] [CrossRef]
- Hughes, T.S.; Langer, S.J.; Johnson, K.W.; Chavez, R.A.; Watkins, L.R.; Milligan, E.D.; Leinwand, L.A. Intrathecal injection of naked plasmid DNA provides long-term expression of secreted proteins. Mol. Ther. 2009, 17, 88–94. [Google Scholar] [CrossRef]
- Izmailov, A.A.; Povysheva, T.V.; Bashirov, F.V.; Sokolov, M.E.; Fadeev, F.O.; Garifulin, R.R.; Naroditsky, B.S.; Logunov, D.Y.; Salafutdinov, I.I.; Chelyshev, Y.A.; et al. Spinal Cord Molecular and Cellular Changes Induced by Adenoviral Vector- and Cell-Mediated Triple Gene Therapy after Severe Contusion. Front. Pharmacol. 2017, 8, 813. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E. Nucleases in the cerebrospinal fluid. III. Simultaneous determination of ribo- and desoxyribonuclease in the CSF of patients with poliomyelitis. J. Pediatr. 1955, 46, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E. Nucleases in the cerebrospinal fluid. I. Ribonuclease in normal, neurologically normal, and in pathological CSF’s. Can. J. Med. Sci. 1953, 31, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Blank, A.; Dekker, C.A. Ribonucleases of human serum, urine, cerebrospinal fluid, and leukocytes. Activity staining following electrophoresis in sodium dodecyl sulfate-polyacrylamide gels. Biochemistry 1981, 20, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Schieven, G.L.; Blank, A.; Dekker, C.A. Ribonucleases of human cerebrospinal fluid: Detection of altered glycosylation relative to their serum counterparts. Biochemistry 1982, 21, 5148–5155. [Google Scholar] [CrossRef]
- Hettiarachchi, H.D.; Hsu, Y.; Harris, T.J., Jr.; Penn, R.; Linninger, A.A. The effect of pulsatile flow on intrathecal drug delivery in the spinal canal. Ann. Biomed. Eng. 2011, 39, 2592–2602. [Google Scholar] [CrossRef] [PubMed]
- Coenen, W.; Haughton, V.; Lasheras, J.C.; Lawrence, J.J.; Martínez-Bazán, C.; Pawlak, G.; Sánchez, A.L. On the dispersion of a drug delivered intrathecally in the spinal canal. J. Fluid Mech. 2019, 861, 679–720. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Tran, K.; Burt, D.R.; Zhong, L.; Gao, G. Slow Infusion of Recombinant Adeno-Associated Viruses into the Mouse Cerebrospinal Fluid Space. Hum. Gene Ther. Methods 2018, 29, 75–85. [Google Scholar] [CrossRef]
- Bailey, R.M.; Rozenberg, A.; Gray, S.J. Comparison of high-dose intracisterna magna and lumbar puncture intrathecal delivery of AAV9 in mice to treat neuropathies. Brain Res. 2020, 1739, 146832. [Google Scholar] [CrossRef]
- Wolf, D.A.; Hesterman, J.Y.; Sullivan, J.M.; Orcutt, K.D.; Silva, M.D.; Lobo, M.; Wellman, T.; Hoppin, J.; Verma, A. Dynamic dual-isotope molecular imaging elucidates principles for optimizing intrathecal drug delivery. JCI Insight 2016, 1, e85311. [Google Scholar] [CrossRef]
- Weed, L.H. Studies on Cerebro-Spinal Fluid. No. IV: The dual Source of Cerebro-Spinal Fluid. J. Med. Res. 1914, 31, 93–118.111. [Google Scholar] [PubMed]
- Walter, B.A.; Valera, V.A.; Takahashi, S.; Ushiki, T. The olfactory route for cerebrospinal fluid drainage into the peripheral lymphatic system. Neuropathol. Appl. Neurobiol. 2006, 32, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ni, Z.; Chen, Y.; Wang, D.; Qi, Y.; Zhang, Q.; Wang, S. Olfactory route for cerebrospinal fluid drainage into the cervical lymphatic system in a rabbit experimental model. Neural Regen. Res. 2012, 7, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Maloveská, M.; Humeník, F.; Vikartovská, Z.; Hudáková, N.; Almášiová, V.; Krešáková, L.; Čížková, D. Brain Fluid Channels for Metabolite Removal. Physiol. Res. 2022, 71, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ammi, A.Y.; Mast, T.D.; Huang, I.H.; Abruzzo, T.A.; Coussios, C.C.; Shaw, G.J.; Holland, C.K. Characterization of ultrasound propagation through ex-vivo human temporal bone. Ultrasound Med. Biol. 2008, 34, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Clement, G.T.; Hynynen, K. Longitudinal and shear mode ultrasound propagation in human skull bone. Ultrasound Med. Biol. 2006, 32, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Pinton, G.; Aubry, J.F.; Bossy, E.; Muller, M.; Pernot, M.; Tanter, M. Attenuation, scattering, and absorption of ultrasound in the skull bone. Med. Phys. 2012, 39, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Heiderstadt, K.M.; Blizard, D.A. Increased juvenile and adult body weights in BALB/cByJ mice reared in a communal nest. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 484–487. [Google Scholar]
- Oyama, N.; Kawaguchi, M.; Itaka, K.; Kawakami, S. Efficient Messenger RNA Delivery to the Kidney Using Renal Pelvis Injection in Mice. Pharmaceutics 2021, 13, 1810. [Google Scholar] [CrossRef]
- Wolff, J.A.; Budker, V. The mechanism of naked DNA uptake and expression. Adv. Genet. 2005, 54, 3–20. [Google Scholar] [CrossRef]
- Schoellhammer, C.M.; Lauwers, G.Y.; Goettel, J.A.; Oberli, M.A.; Cleveland, C.; Park, J.Y.; Minahan, D.; Chen, Y.; Anderson, D.G.; Jaklenec, A.; et al. Ultrasound-Mediated Delivery of RNA to Colonic Mucosa of Live Mice. Gastroenterology 2017, 152, 1151–1160. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koga, T.; Kida, H.; Yamasaki, Y.; Feril, L.B., Jr.; Endo, H.; Itaka, K.; Abe, H.; Tachibana, K. Intracranial Gene Delivery Mediated by Albumin-Based Nanobubbles and Low-Frequency Ultrasound. Nanomaterials 2024, 14, 285. https://doi.org/10.3390/nano14030285
Koga T, Kida H, Yamasaki Y, Feril LB Jr., Endo H, Itaka K, Abe H, Tachibana K. Intracranial Gene Delivery Mediated by Albumin-Based Nanobubbles and Low-Frequency Ultrasound. Nanomaterials. 2024; 14(3):285. https://doi.org/10.3390/nano14030285
Chicago/Turabian StyleKoga, Takayuki, Hiroshi Kida, Yutaro Yamasaki, Loreto B. Feril, Jr., Hitomi Endo, Keiji Itaka, Hiroshi Abe, and Katsuro Tachibana. 2024. "Intracranial Gene Delivery Mediated by Albumin-Based Nanobubbles and Low-Frequency Ultrasound" Nanomaterials 14, no. 3: 285. https://doi.org/10.3390/nano14030285
APA StyleKoga, T., Kida, H., Yamasaki, Y., Feril, L. B., Jr., Endo, H., Itaka, K., Abe, H., & Tachibana, K. (2024). Intracranial Gene Delivery Mediated by Albumin-Based Nanobubbles and Low-Frequency Ultrasound. Nanomaterials, 14(3), 285. https://doi.org/10.3390/nano14030285







