Optimization for the Process Parameters of Nickel–Titanium Nitride Composites Fabricated via Jet Pulse Electrodeposition
Abstract
1. Introduction
2. Experiment
2.1. Coatings Preparation
2.2. Statistical Design of Experiment
2.3. Characterization
3. Results and Discussion
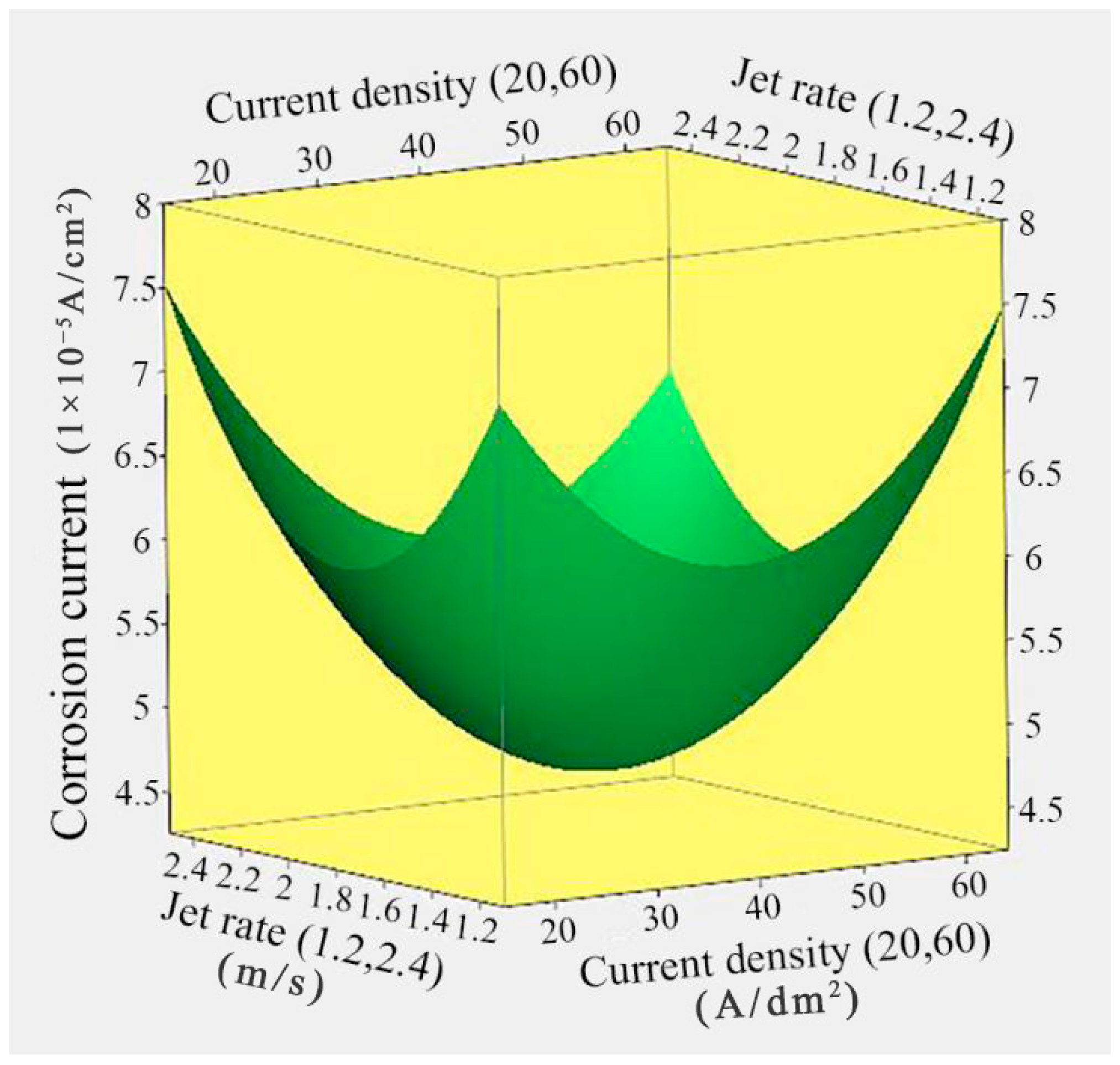
3.1. Effect of Current Density and Jet Rate on the Corrosion Resistance
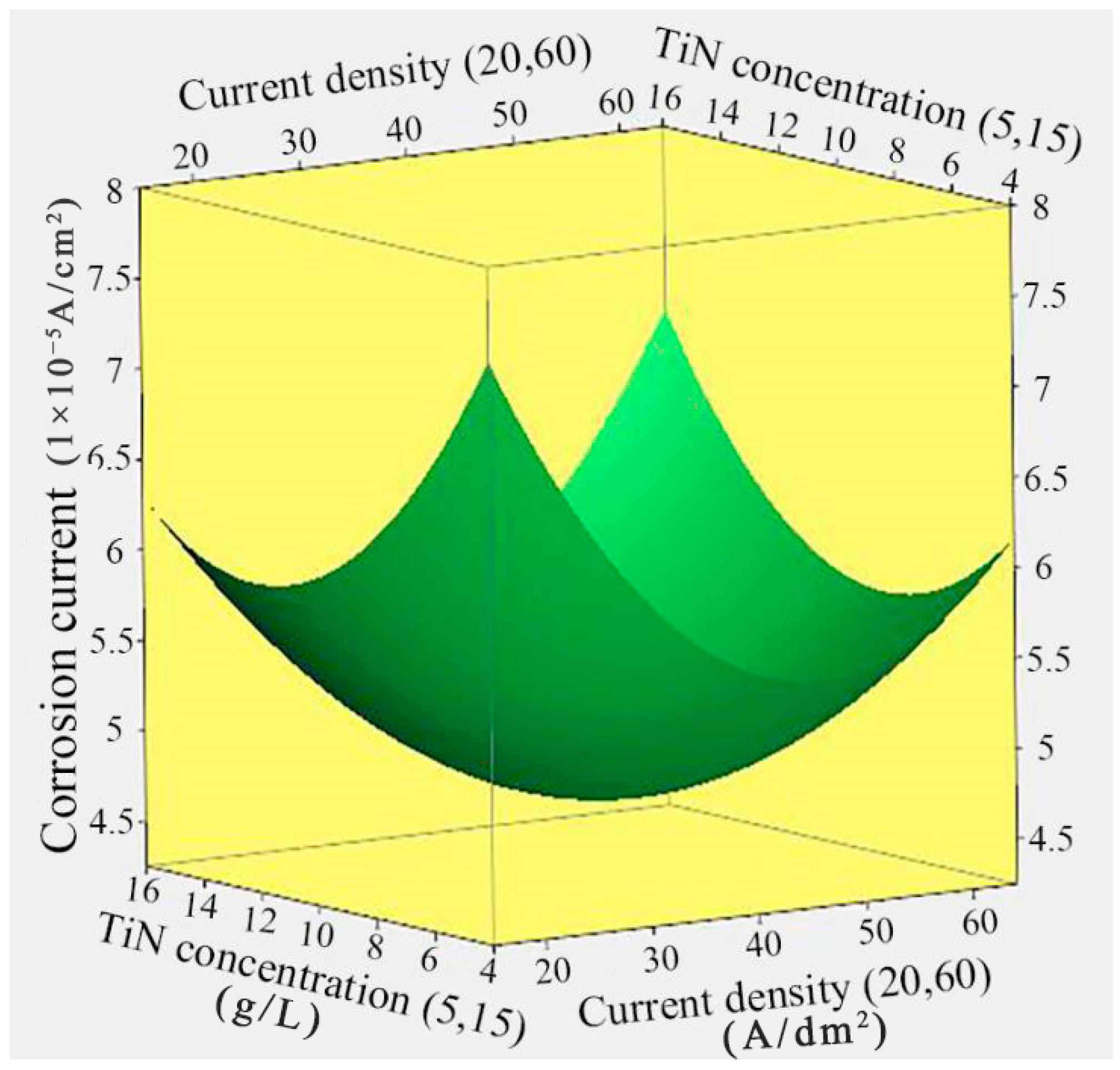
3.2. Effect of Current Density and TiN Concentration on the Corrosion Current
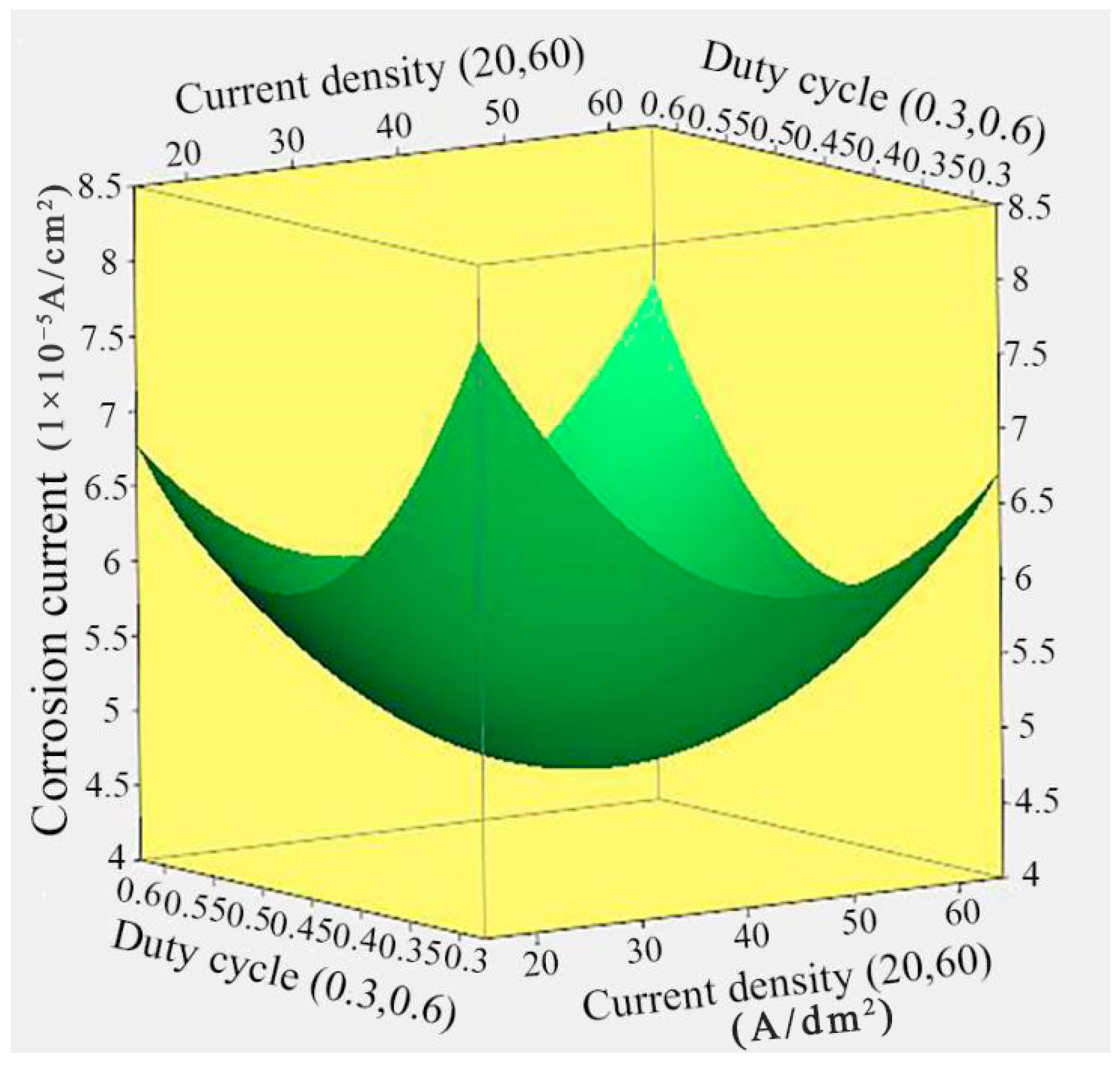
3.3. Effect of Current Density and Duty Cycle on the Corrosion Current
3.4. Effect of Jet Rate and TiN Concentration on the Corrosion Current
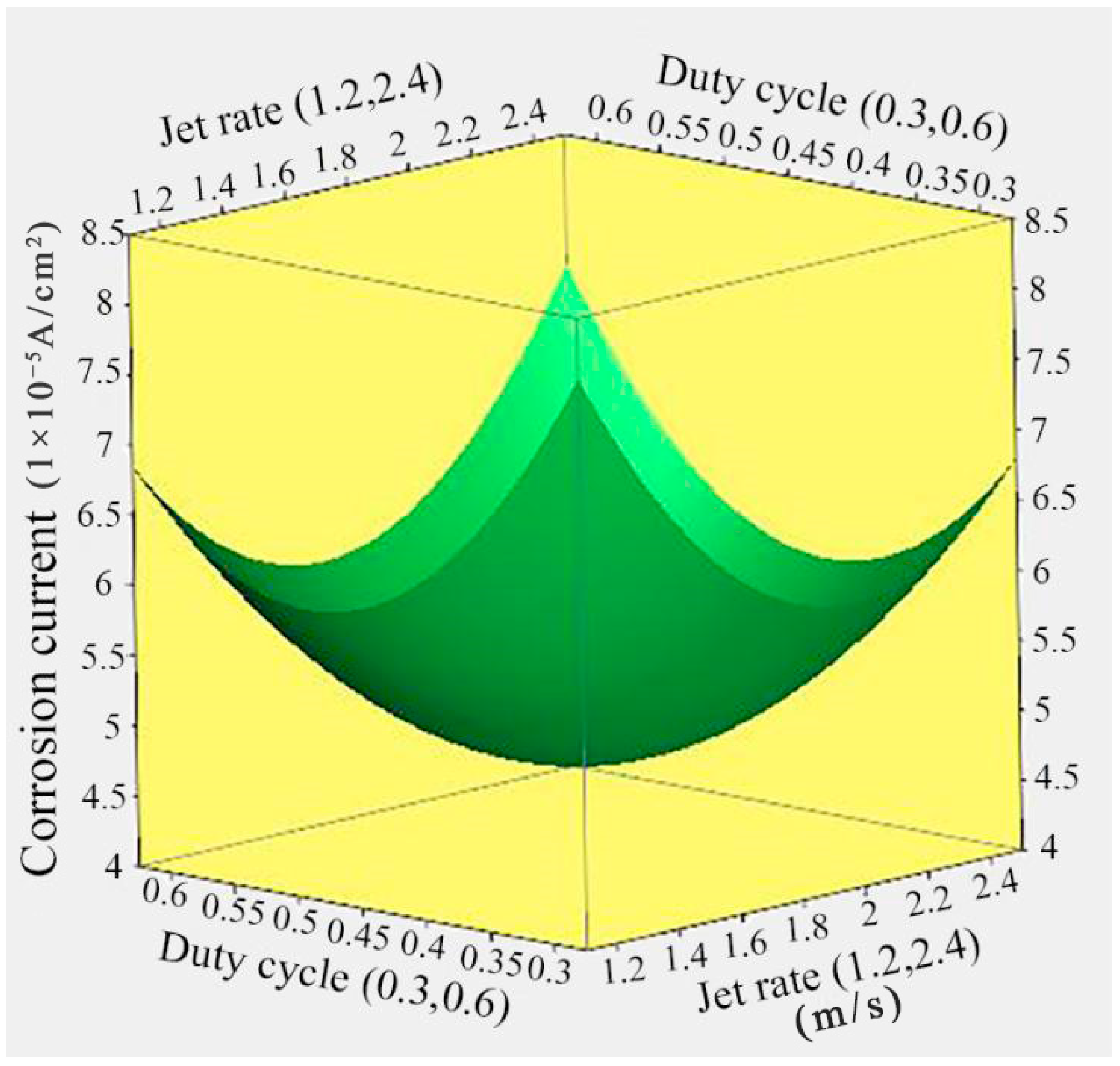
3.5. Effect of Jet Rate and Duty Cycle on the Corrosion Current
3.6. Effect of TiN Concentration and Duty Cycle on the Corrosion Current
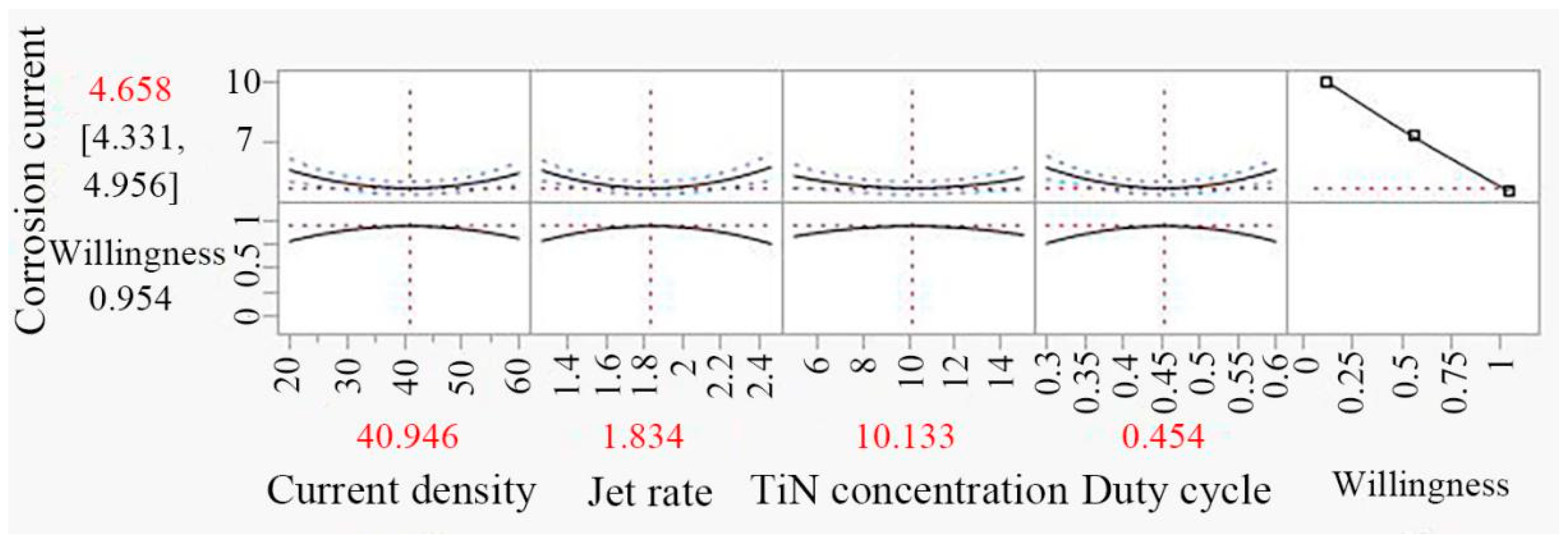
3.7. Optimization of Result and Experimental Verification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lelevic, A.; Walsh, F.C. Electrodeposition of Ni-P alloy coatings: A review. Surf. Coat. Tech. 2019, 369, 198–220. [Google Scholar] [CrossRef]
- Pan, C.; Gong, M.; Feng, S.; Chen, X.; Zhao, X.; He, P. Corrosion and thermal fatigue behaviors of induction-clad Ni-coated TiC particle-reinforced Ni60 coating in molten aluminum alloy. Surf. Coat. Technol. 2021, 419, 127278. [Google Scholar] [CrossRef]
- Wang, H.; Yu, W.; Liu, H.; Li, Q.; Xia, F.; Ma, C. Prediction of wear resistance of ultrasonic electrodeposited Ni-SiC nanocoatings using BP-NN model. Int. J. Electrochem. Sci. 2021, 16, 210423. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, L.X.; Feng, S.F.; Liu, H.J. Prefabrication of two-dimensional Ni-W/TiN films on oil-gas X52 steels by pulse electrodeposition. J. Indian Chem. Soc. 2022, 99, 100621. [Google Scholar] [CrossRef]
- Liang, X.S.; Ouyang, J.H.; Li, Y.F.; Wang, Y.M. Electrodeposition and tribological properties of Ni-SrSO4 composite coatings. Appl. Surf. Sci. 2009, 255, 4316–4321. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, L.; Zhang, H.; Wang, J.; Ma, C.; Xu, F. Study on magnetic-field-assisted electrodeposited Ni-SiC nanocomposites. J. Mater. Eng. Perform. 2022, 31, 602–612. [Google Scholar] [CrossRef]
- Ma, C.; Li, H.; Xia, F.; Xiao, Z. Effect of Ultrasonic Power on Microstructure and Properties of Ultrasonic-Assisted Electrodeposited Ni–Al2O3 Thin Nanocoatings. Trans. Indian Inst. Met. 2022, 75, 1669–1679. [Google Scholar] [CrossRef]
- Ge, W.; He, T.; Wang, M.; Li, J. Nano-grain Ni/ZrO2 functional gradient coating fabricated by double pulses electrodeposition with enhanced high temperature corrosion performance. Coatings 2020, 10, 332. [Google Scholar] [CrossRef]
- Yang, Z.; Yi, S.; Cao, L.; Tang, S.; Li, Q. Process Simulation and Abrasion Behavior of Jet Electrodeposited Ni–TiN Nanocoatings. Coatings 2022, 12, 86. [Google Scholar] [CrossRef]
- Wang, C.; Shen, L.D.; Qiu, M.B.; Tian, Z.J.; Jiang, W. Characterizations of Ni-CeO2 nanocomposite coating by interlaced jet electrodeposition. J. Alloys Compd. 2017, 727, 269–277. [Google Scholar] [CrossRef]
- Tan, J.; Song, H.; Zheng, X.; Zhang, Q.; Wang, M. High performance Co–Cr3C2 composite coating by jet electrodeposition. Surf. Eng. 2018, 34, 861–869. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, Y.; Jiang, J.; Wang, S.; Shan, W.; Ma, R. Pulse jet electrodeposition of nanocrystalline copper and its application as an electrical discharge machining electrode. Int. J. Electrochem. Sci. 2020, 15, 2648–2658. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, H. Effect of Pulse Parameters on the Microstructural Evolution and Properties of Jet Electrodeposited Cu–Al2O3 Nanocomposite Coating. Mater. Trans. 2021, 62, 726–730. [Google Scholar] [CrossRef]
- Ning, D.; Zhang, A.; Murtaza, M.; Wu, H. Effect of surfactants on the electrodeposition of Cu-TiO2 composite coatings prepared by jet electrodeposition. J. Alloys Compd. 2019, 777, 1245–1250. [Google Scholar] [CrossRef]
- Cao, M.; Yue, Y.; Guo, X.; Wang, B. Effects of SiC Particle Concentration on the Ultrasonic- Assisted Jet Electrodeposited Ni-SiC Nanocoatings. Int. J. Electrochem. Sci. 2022, 17, 220113. [Google Scholar] [CrossRef]
- Jiang, W.; Qiu, M.; Shen, L.; Lou, G.; Yang, X.; Eckert, K.; Tian, Z. Effect of deposition current density on the Co–Ni/SiO2 alloy composite coatings using scanning jet electrodeposition. Surf. Topogr. Metrol. Prop. 2021, 9, 015027. [Google Scholar] [CrossRef]
- Li, H.; Kang, M.; Zhang, Y.; Liu, Y.; Jin, M.; Mbugua, N.S.; Zhu, G.; Liu, C. Fabrication of Ni–Co–BN (h) nanocomposite coatings with jet electrodeposition in different pulse parameters. Coatings 2019, 9, 50. [Google Scholar] [CrossRef]
- Xia, F.; Li, C.; Ma, C.; Li, Q.; Xing, H. Effect of pulse current density on microstructure and wear property of Ni-TiN nanocoatings deposited via pulse electrodeposition. Appl. Surf. Sci. 2021, 538, 148139. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, C.; Zhang, Y. Effect of Jet Speed on the Microstructure and Performances of Ni-TiN Nanocomposites Prepared via Jet Pulse Electrodeposition. J. Mater. Eng. Perform. 2024, 33, 6738–6747. [Google Scholar] [CrossRef]
- Hefnawy, A.; Elkhoshkhany, N.; Essam, A. Ni–TiN and Ni-Co-TiN composite coatings for corrosion protection: Fabrication and electrochemical characterization. J. Alloys Compd. 2018, 735, 600–606. [Google Scholar] [CrossRef]
- Li, Z.P.; Lu, D.G.; Gao, X.J. Optimization of mixture proportions by statistical experimental design using response surface method-A review. J. Build Eng. 2021, 36, 102101. [Google Scholar] [CrossRef]
- Kumar, S.; Mandal, A.; Das, A.K.; Dixit, A.R. Parametric study and characterization of AlN-Ni-Ti6Al4V composite cladding on titanium alloy. Surf. Coat. Technol. 2018, 349, 37–49. [Google Scholar] [CrossRef]
- Esmailzadeh, S.; Shahrabi, T.; Yaghoubinezhad, Y.; Darband, G.B. Optimization and characterization of pulse electrodeposited nickel selenide nanostructure as a bifunctional electrocatalyst by response surface methodology. Int. J. Hydrogen Energy 2021, 46, 18898–18912. [Google Scholar]
- Li, B.; Zhang, W.; Huan, Y.; Dong, J. Synthesis and characterization of Ni-B/Al2O3 nanocomposite coating by electrodeposition using trimethylamine borane as boron precursor. Surf. Coat. Technol. 2018, 337, 186–197. [Google Scholar]
- Dheeraj, P.; Patra, A.; Sengupta, S.; Das, S.; Das, K. Synergistic effect of peak current density and nature of surfactant on microstructure, mechanical and electrochemical properties of pulsed electrodeposited Ni-Co-SiC nanocomposites. J. Alloys Compd. 2017, 729, 1093–1107. [Google Scholar]
- Qiao, G.; Jing, T.; Wang, N.; Gao, Y.; Zhao, X.; Zhou, J.; Wang, W. High-speed jet electrodeposition and microstructure of nanocrystalline Ni–Co alloys. Electrochim. Acta 2005, 51, 85–92. [Google Scholar]
- Cui, W.; Song, R.; Zhang, Y.; Wang, P. Preparation and characterization of Ni–TiN nanocomposites prefabricated by jet pulse electrodeposition. Jpn. J. Appl. Phys. 2018, 57, 125001. [Google Scholar] [CrossRef]
- Zhang, Z.; Xian, J.; Wu, H.; Jin, M.; Shen, Z. Influence of particle concentration on the elemental penetration region and properties of Ni-P-SiC composite coatings prepared through sandblasting and scanning electrodeposition on 45 steel surfaces. Coatings 2021, 11, 1237. [Google Scholar] [CrossRef]
- Song, H.; Dan, J.; Li, J.; Du, J.; Xiao, J.; Xu, J. Experimental study on the cutting force during laser-assisted machining of fused silica based on the Taguchi method and response surface methodology. J. Manuf. Process. 2019, 38, 9–20. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Yu, W.; He, Y.; Xia, F.; Ma, C.; Shakoor, A. Effect of TiN concentration on microstructure and properties of Ni/W–TiN composites obtained by pulse current electrodeposition. Ceram. Int. 2021, 47, 24331–24339. [Google Scholar]
- Li, C.; Xia, F.; Yao, L.; Li, H.; Jia, X. Investigation of the mechanical properties and corrosion behaviors of Ni-BN-TiC layers constructed via laser cladding technique. Ceram. Int. 2023, 49, 6671–6677. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, R.; Cui, G.; Li, Z. Influence of pulse parameters on the morphology and corrosion resistance of nickel-graphene composite coating. Int. J. Electrochem. Sci. 2019, 14, 4754–4768. [Google Scholar] [CrossRef]
- Corni, I.; Chater, R.J.; Boccaccini, A.R.; Ryan, M.P. Electro co-deposition of Ni–Al2O3 composite coatings. J. Mater. Sci. 2012, 47, 5361–5373. [Google Scholar] [CrossRef]
- Yu, X.; Ma, Z.; Li, J.; Ma, C. Study of the Novel Ni/Co–SiC Coatings Deposited by Pulse Current Electrodeposition. Influence of the Pulse Frequency and the Duty Cycle. Int. J. Electrochem. Sci. 2021, 16, 21036. [Google Scholar] [CrossRef]
- Jegan, A.; Venkatesan, R. Characterization and optimization of pulse electrodeposition of Ni/nano-Al2O3 composite coatings. Int. J. Miner. Metall. Mater. 2013, 20, 479–485. [Google Scholar] [CrossRef]
- Xia, F.; Jia, W.; Jiang, M.; Cui, W.; Wang, J. Microstructure and corrosion properties of Ni-TiN nanocoatings prepared by jet pulse electrodeposition. Ceram. Int. 2017, 43, 14623–14628. [Google Scholar] [CrossRef]
- Ye, P.; Niu, Q.; Wang, F. Effect of electrolyte composition and deposition voltage on the deposition rate of copper microcolumns jet electrodeposition. Mater. Sci. Eng. B 2023, 298, 116857. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, Y.; Jiang, J.; Wang, S.; Shan, W. Improvement of Microstructure and Wear Resistance of the Ni-La2O3 Nanocomposite Coatings by Jet-Electrodeposition. J. Electron. Mater. 2021, 50, 3429–3437. [Google Scholar] [CrossRef]
- Liu, T.; Li, C.; Li, Q.; Li, L.; Xia, F.; Xing, H.; Ma, C. Synthesis and wear characterization of ultrasonic electrodeposited Ni-TiN thin coatings. Int. J. Electrochem. Sci. 2021, 16, 151028. [Google Scholar] [CrossRef]
- Zhu, X.; Chao, C.; Zheng, G.; Zhang, Z.; Li, J. Electrodeposition and corrosion behavior of nanostructured Ni-TiN composite films. Trans. Nonferrous Met. Soc. China 2011, 21, 2216–2224. [Google Scholar] [CrossRef]
- Li, C.; Xia, F.; Ma, C.; Li, Q. Research on the corrosion behavior of Ni-SiC nanocoating prepared using a jet electrodeposition technique. J. Mater. Eng. Perform. 2021, 30, 6336–6344. [Google Scholar] [CrossRef]
- Li, B.; Mei, T.; Du, S.; Zhang, W. Synthesis of Ni–Fe and Ni–Fe/ZrO2 composite coating and evaluation of its structural and corrosion resistance. Mater. Chem. Phys. 2020, 243, 122595. [Google Scholar] [CrossRef]





| Composition | Specific |
|---|---|
| NiSO4·6H2O | 270 g/L |
| NiCl2·6H2O | 35 g/L |
| H3BO3 | 20 g/L |
| C6H8O7 | 2.5 g/L |
| CTAB | 60 mg/L |
| pH | 4.3 |
| Plating temperature | 50 °C |
| Plating time | 1 h |
| Level | Pilot Factors | |||
|---|---|---|---|---|
| Current Density (A/dm2) | Jet Rate (m/s) | TiN Concentration (g/L) | Duty Cycle | |
| −1 | 20 | 1.2 | 5 | 0.30 |
| 0 | 40 | 1.8 | 10 | 0.45 |
| 1 | 60 | 2.4 | 15 | 0.60 |
| Number | (A/dm2) | (m/s) | (g/L) | Y (1 × 10−5 A/cm2) | |
|---|---|---|---|---|---|
| 1 | 20 | 1.2 | 5 | 0.6 | 7.61 |
| 2 | 20 | 2.4 | 15 | 0.3 | 8.22 |
| 3 | 20 | 1.2 | 15 | 0.6 | 6.86 |
| 4 | 20 | 1.2 | 5 | 0.3 | 9.52 |
| 5 | 20 | 2.4 | 15 | 0.6 | 7.79 |
| 6 | 20 | 1.2 | 15 | 0.3 | 7.91 |
| 7 | 20 | 2.4 | 5 | 0.3 | 8.28 |
| 8 | 20 | 1.8 | 10 | 0.45 | 5.87 |
| 9 | 20 | 2.4 | 5 | 0.6 | 8.33 |
| 10 | 40 | 1.8 | 10 | 0.45 | 4.71 |
| 11 | 40 | 1.8 | 10 | 0.45 | 4.71 |
| 12 | 40 | 1.8 | 10 | 0.6 | 5.37 |
| 13 | 40 | 2.4 | 10 | 0.45 | 5.36 |
| 14 | 40 | 1.2 | 10 | 0.45 | 5.89 |
| 15 | 40 | 1.8 | 5 | 0.45 | 5.58 |
| 16 | 40 | 1.8 | 15 | 0.45 | 4.87 |
| 17 | 40 | 1.8 | 10 | 0.3 | 5.89 |
| 18 | 60 | 1.2 | 5 | 0.6 | 7.89 |
| 19 | 60 | 2.4 | 15 | 0.6 | 8.43 |
| 20 | 60 | 1.2 | 15 | 0.3 | 8.45 |
| 21 | 60 | 1.8 | 10 | 0.45 | 5.08 |
| 22 | 60 | 1.2 | 5 | 0.3 | 8.35 |
| 23 | 60 | 2.4 | 5 | 0.3 | 6.06 |
| 24 | 60 | 2.4 | 15 | 0.3 | 7.92 |
| 25 | 60 | 1.2 | 15 | 0.6 | 8.34 |
| 26 | 60 | 2.4 | 5 | 0.6 | 8.33 |
| Source | Sum of Square | F-Ratio | p-Value |
|---|---|---|---|
| 2.4538 | 17.4111 | 0.0016 | |
| 0.8434 | 5.9841 | 0.0325 | |
| 1.7382 | 12.3337 | 0.0049 | |
| 2.1978 | 15.5945 | 0.0023 |
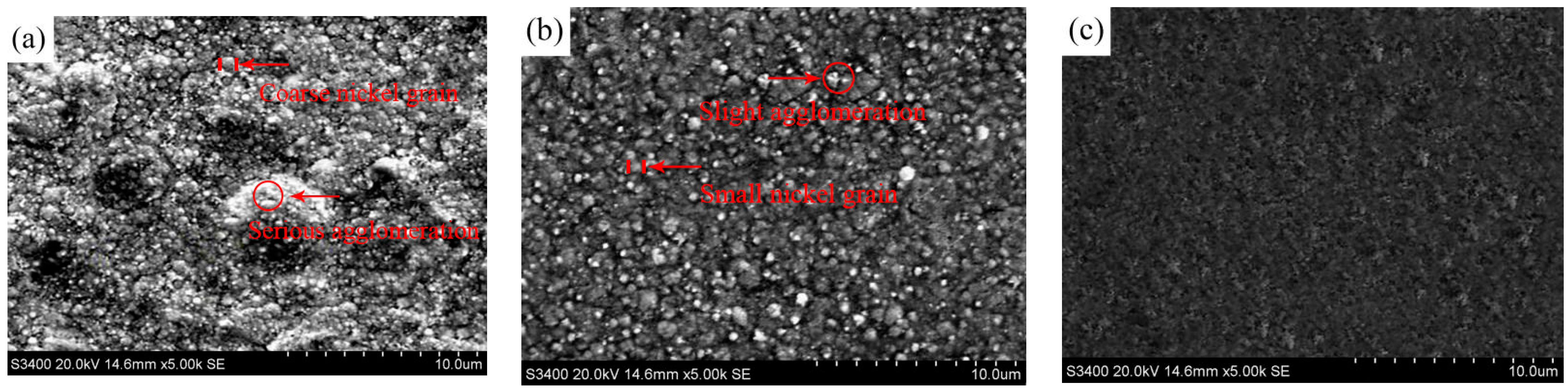
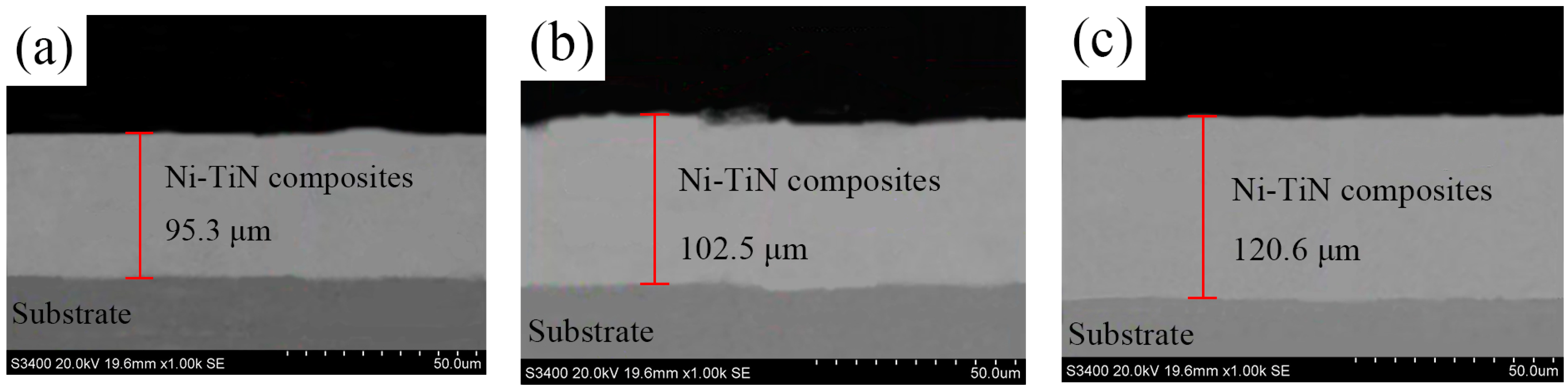
| Ni/TiN Specimens | Ni Content (wt.%) | Ti Content (wt.%) | N Content (wt.%) |
|---|---|---|---|
| JPE-1 | 93.15 | 4.26 | 2.59 |
| JPE-2 | 84.03 | 10.74 | 5.23 |
| JPE-3 | 80.67 | 12.95 | 6.38 |
| Ni/TiN Specimens | Average Diameter of Ni Grain (μm) |
|---|---|
| JPE-1 | 0.64 |
| JPE-2 | 0.35 |
| JPE-3 | 0.12 |
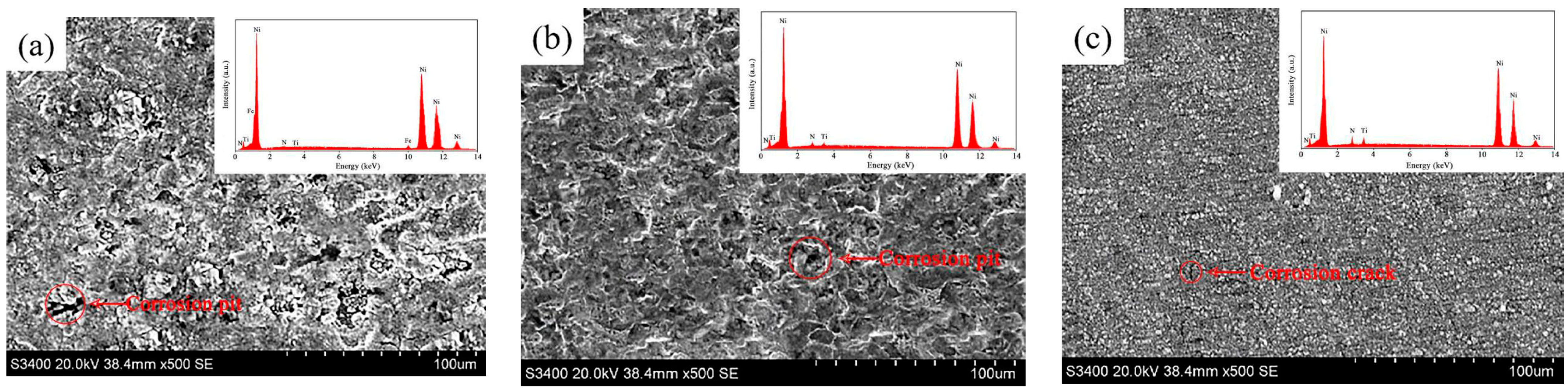
| Ni/TiN Specimens | Ecorr (V) | Icorr (1 × 10−5 A/cm2) |
|---|---|---|
| JPE-1 | 93.15 | 4.26 |
| JPE-2 | 84.03 | 10.74 |
| JPE-3 | 80.67 | 12.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Tian, D.; Li, C.; Li, X.; Li, W.; Cao, M.; Zhang, F.; Wang, B. Optimization for the Process Parameters of Nickel–Titanium Nitride Composites Fabricated via Jet Pulse Electrodeposition. Nanomaterials 2024, 14, 2034. https://doi.org/10.3390/nano14242034
Guo X, Tian D, Li C, Li X, Li W, Cao M, Zhang F, Wang B. Optimization for the Process Parameters of Nickel–Titanium Nitride Composites Fabricated via Jet Pulse Electrodeposition. Nanomaterials. 2024; 14(24):2034. https://doi.org/10.3390/nano14242034
Chicago/Turabian StyleGuo, Xue, Dehao Tian, Chaoyu Li, Xiang Li, Wei Li, Mengyu Cao, Fengwu Zhang, and Baojin Wang. 2024. "Optimization for the Process Parameters of Nickel–Titanium Nitride Composites Fabricated via Jet Pulse Electrodeposition" Nanomaterials 14, no. 24: 2034. https://doi.org/10.3390/nano14242034
APA StyleGuo, X., Tian, D., Li, C., Li, X., Li, W., Cao, M., Zhang, F., & Wang, B. (2024). Optimization for the Process Parameters of Nickel–Titanium Nitride Composites Fabricated via Jet Pulse Electrodeposition. Nanomaterials, 14(24), 2034. https://doi.org/10.3390/nano14242034






