Application of Biochar-Based Materials for Effective Pollutant Removal in Wastewater Treatment
Abstract
1. Introduction
2. Production and Structural Characteristics of Biochar
2.1. Composition of Biochar
2.1.1. Carbon Sources of Biochar
2.1.2. Elemental Composition of Biomass
2.2. Production of Biochar
2.2.1. Pretreatment of Precursors
2.2.2. Activation of Biochar
2.2.3. Synthesis Methods of Biochar
2.3. Structural Characteristics of Biochar
3. Application of Biochar in the Degradation of Wastewater Pollutants
3.1. Application of Biochar in Adsorption System
3.1.1. Native Biochar Adsorbents
3.1.2. Metal-Doped Biochar Adsorbents
3.1.3. Heteroatom-Doped Biochar Adsorbents
3.2. Application of Biochar-Based Catalysts in Fenton-like Systems
3.2.1. Native Biochar-Based Catalysts in Fenton-like Systems
3.2.2. Metal-Based Biochar Catalysts in Fenton-like Systems
Monometallic-Based Biochar Catalysts
Multimetal-Based Biochar Catalysts
3.2.3. Heteroatom-Doped Biochar Catalysts in Fenton-like Systems
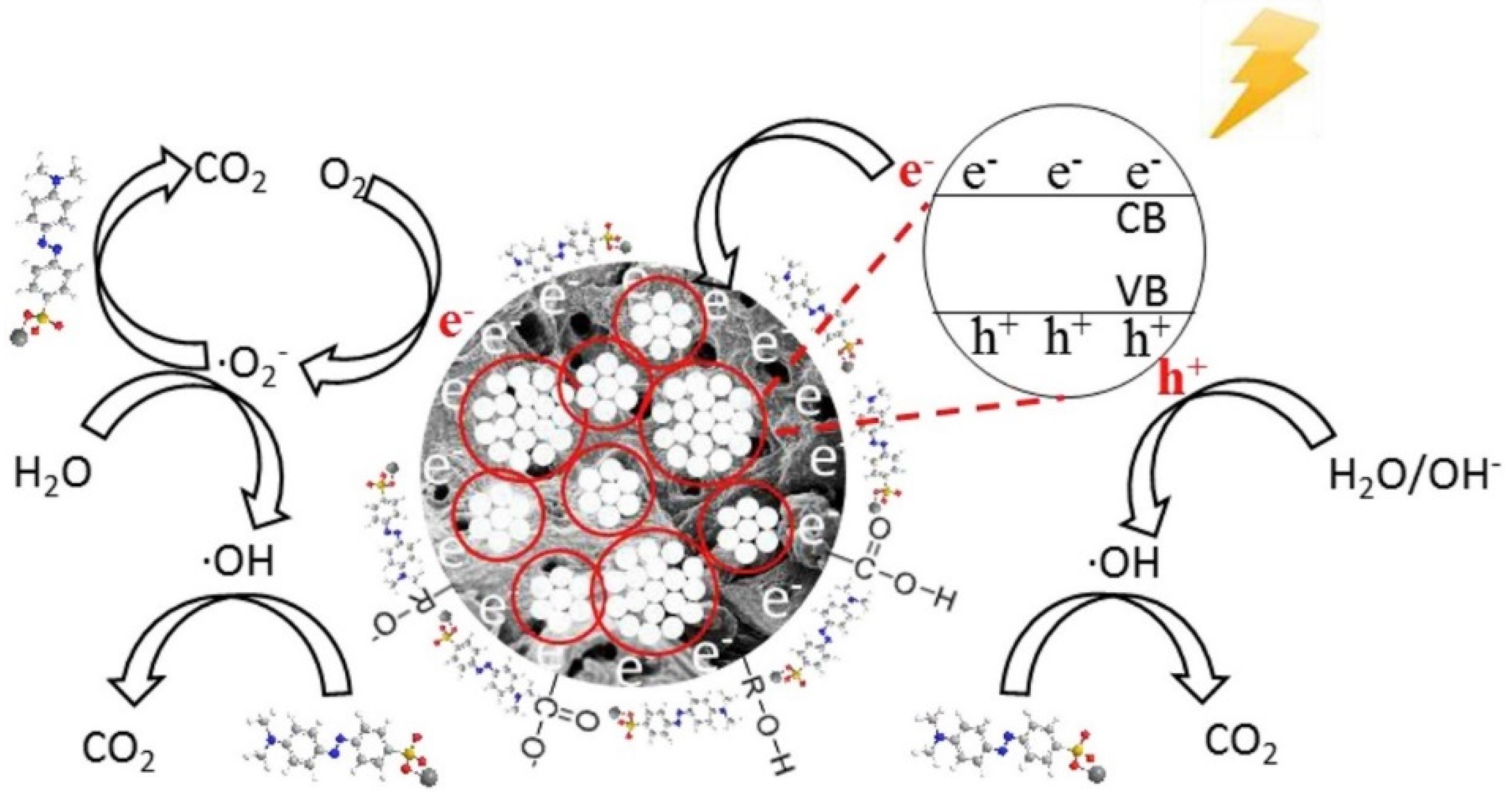
3.3. Application of Biochar-Based Catalysts in Photocatalytic Systems
3.3.1. Native Biochar-Based Catalysts in Photocatalytic Systems
3.3.2. Semiconductor-Doped Biochar-Based Catalysts in Photocatalytic Systems
TiO2/Biochar-Based Catalysts
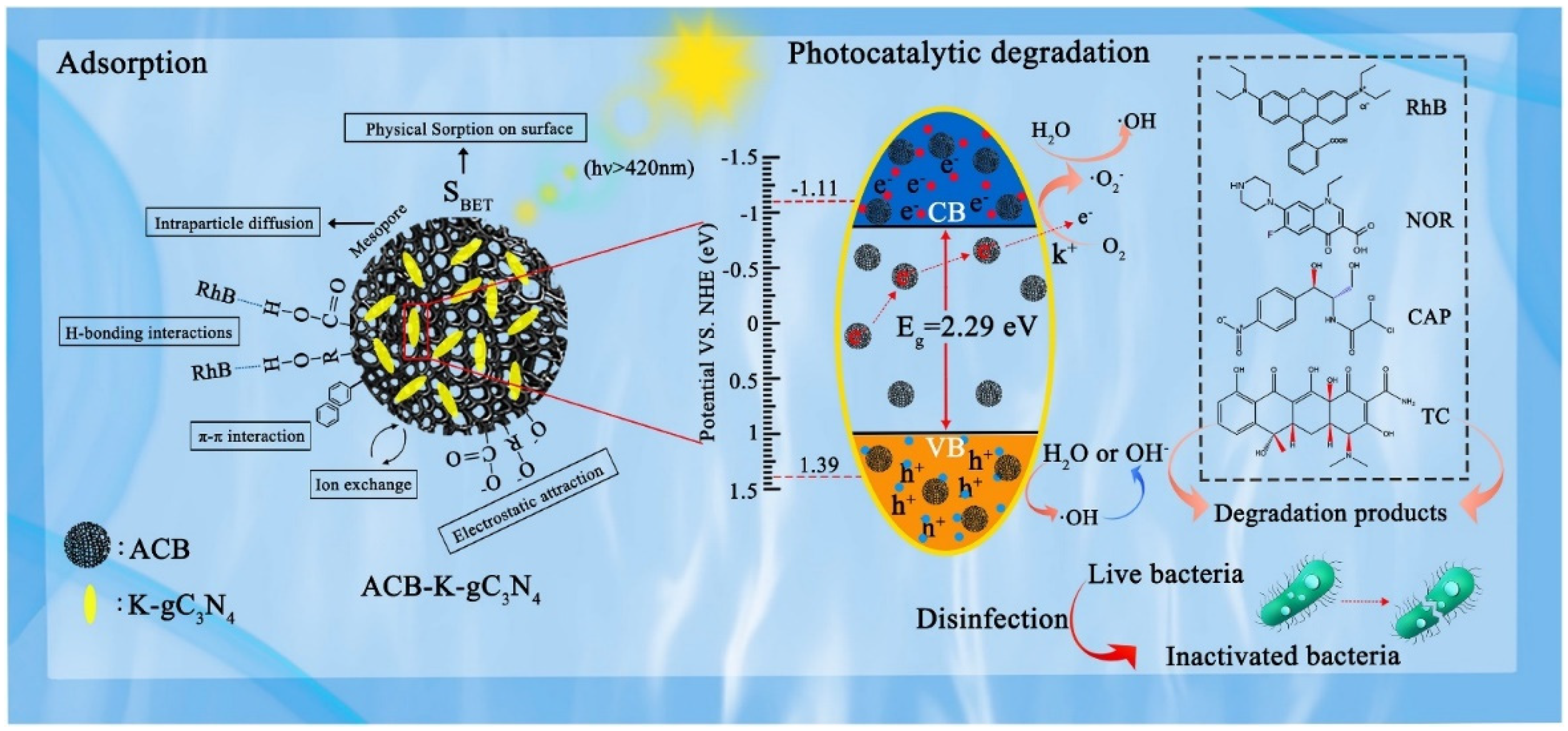
Graphite (g-C3N4)/Biochar-Based Catalysts
3.3.3. Heteroatom-Doped Biochar Catalysts in Photocatalytic Systems
3.3.4. Composite Biochar-Based Catalysts in Photocatalytic Systems
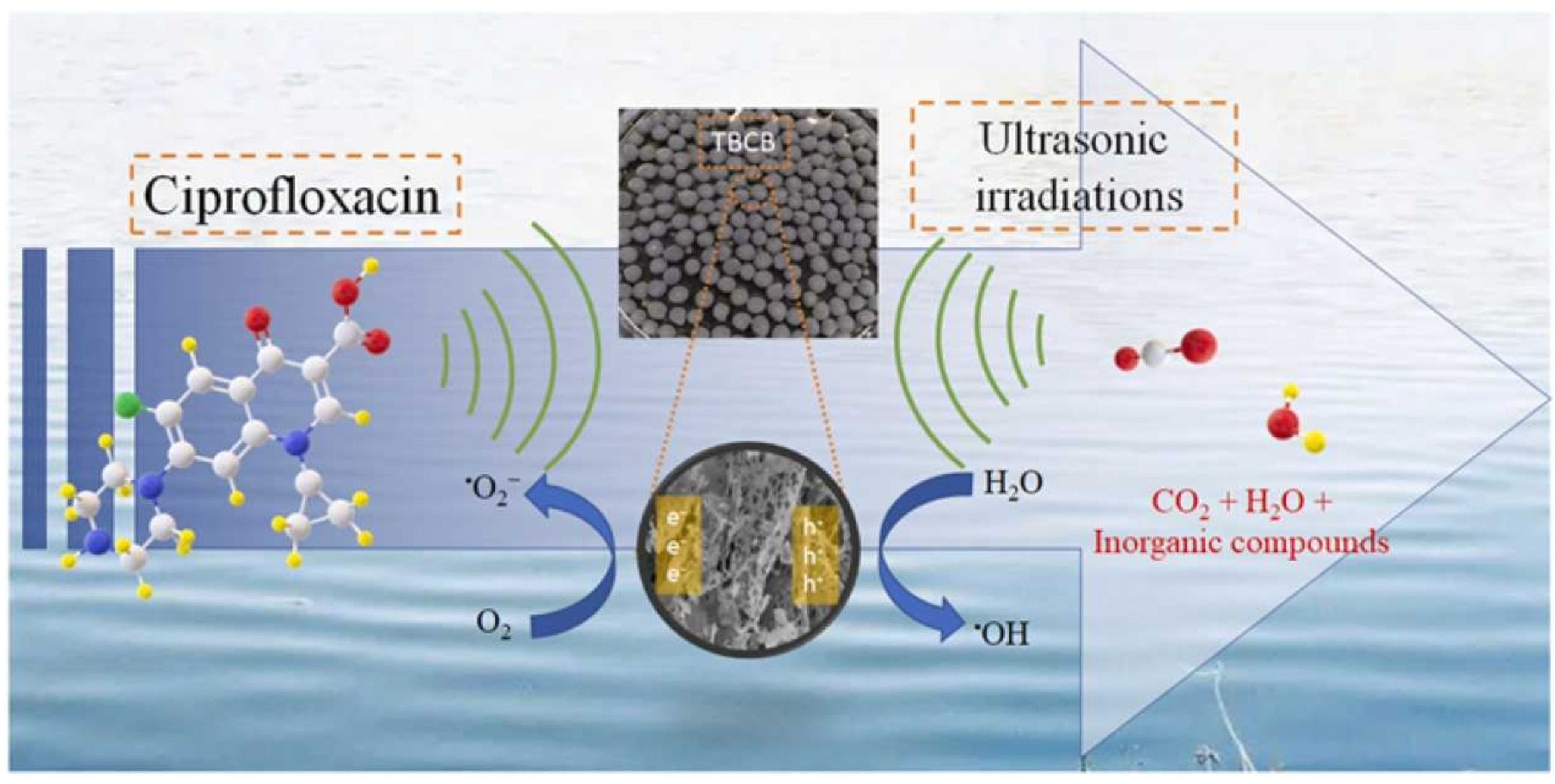
3.4. Application of Biochar-Based Catalysts in Sonocatalytic Systems
3.4.1. Native Biochar-Based Catalysts in Sonocatalytic Systems
3.4.2. Composite-Based Biochar Catalysts in Sonocatalytic Systems
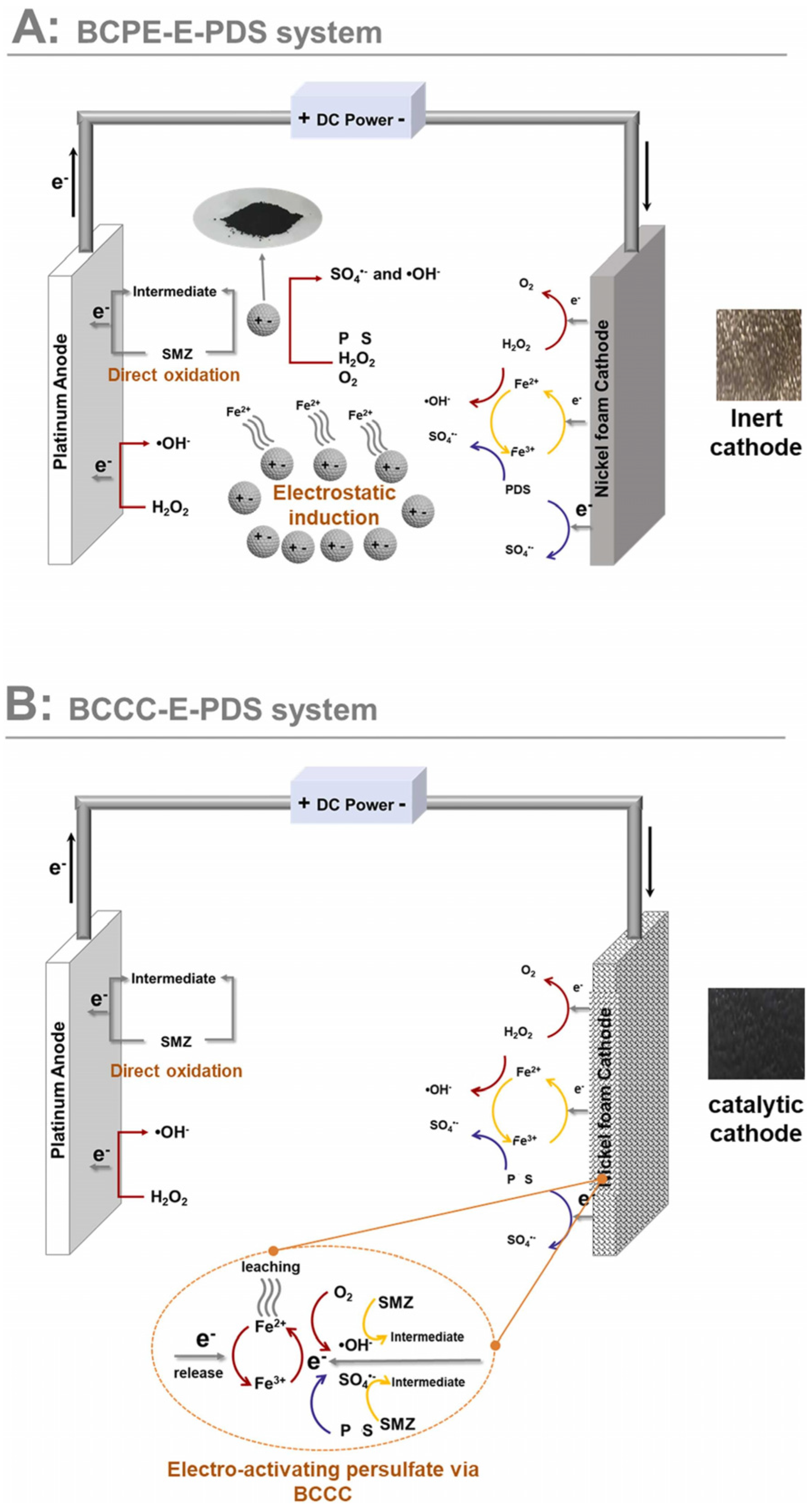
3.5. Application of Biochar-Based Catalysts in Electrocatalytic Systems
3.5.1. Native Biochar Electrode in Electrocatalytic Systems
3.5.2. Metal-Based Biochar Catalysts in Electrocatalytic Systems
3.5.3. Heteroatom-Doped Biochar Catalysts in Electrocatalytic Systems
3.5.4. Metal/Heteroatom-Doped Biochar Catalysts in Electrocatalytic Systems
4. Biochar Regeneration
5. Pilot-Scale Biochar Water Treatment
6. Conclusion and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- du Plessis, A. Persistent Degradation: Global Water Quality Challenges and Required Actions. One Earth 2022, 5, 129–131. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.K.; Gautam, R.K.; Banerjee, S.; Chattopadhyaya, M.C.; Pandey, J.D. Heavy metals in the environment: Fate, transport, toxicity and remediation technologies. Nova 2016, 60, 101–130. [Google Scholar]
- Chen, W.; Meng, J.; Han, X.; Lan, Y.; Zhang, W. Past, Present, and Future of Biochar. Biochar 2019, 1, 75–87. [Google Scholar]
- Chen, S.S.; Cao, Y.; Tsang, D.C.W.; Tessonnier, J.-P.; Shang, J.; Hou, D.; Shen, Z.; Zhang, S.; Ok, Y.S.; Wu, K.C.-W. Effective Dispersion of MgO Nanostructure on Biochar Support as a Basic Catalyst for Glucose Isomerization. ACS Sustain. Chem. Eng. 2020, 8, 6990–7001. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Choi, S.W.; Dissanayake, P.D.; Shang, J.; Wang, C.-H.; Yang, X.; Kim, S.; Tsang, D.C.W.; Lee, K.B.; Ok, Y.S. Gasification Biochar from Biowaste (Food Waste and Wood Waste) for Effective CO2 Adsorption. J. Hazard. Mater. 2020, 391, 121147. [Google Scholar] [CrossRef]
- Ma, Y.; Yao, Y.; Qian, S.; Deng, Z.; Liu, Y.; Ma, J.; Zhang, Z. Ball Milling Boosted Hydrothermal N-Doped Sludge-Derived Biochar towards Efficiently Adsorptive Removal of Sulfamethoxazole from Waters: Behavior, Mechanism and DFT Study. Sep. Purif. Technol. 2024, 338, 126453. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A Comparative Review of Biochar and Hydrochar in Terms of Production, Physico-Chemical Properties and Applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar]
- Aller, M.F. Biochar Properties: Transport, Fate, and Impact. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1183–1296. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Huang, Y.W.; Chen, W.F.; Sun, D.Q.; Guan, X.C.; Zhang, W.M.; Yu, L.; Gao, J.P.; Meng, J. Research on Physical and Chemical Properties of Different Biochars. Adv. Mater. Res. 2012, 518–523, 807–816. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Zhang, Q.; Tian, W.; Khan, E.; Tsang, D.C.W. Leaching Characteristics of Nutrients in Food Waste Digestate-Derived Biochar. Bioresour. Technol. 2024, 399, 130634. [Google Scholar] [CrossRef] [PubMed]
- Ameloot, N.; Graber, E.R.; Verheijen, F.G.A.; De Neve, S. Interactions between Biochar Stability and Soil Organisms: Review and Research Needs. Eur. J. Soil Sci. 2013, 64, 379–390. [Google Scholar] [CrossRef]
- Wu, J.; Yi, Y.; Li, Y.; Fang, Z.; Tsang, E.P. Excellently Reactive Ni/Fe Bimetallic Catalyst Supported by Biochar for the Remediation of Decabromodiphenyl Contaminated Soil: Reactivity, Mechanism, Pathways and Reducing Secondary Risks. J. Hazard. Mater. 2016, 320, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Zeghioud, H.; Fryda, L.; Djelal, H.; Assadi, A.; Kane, A. A Comprehensive Review of Biochar in Removal of Organic Pollutants from Wastewater: Characterization, Toxicity, Activation/Functionalization and Influencing Treatment Factors. J. Water Process Eng. 2022, 47, 102801. [Google Scholar] [CrossRef]
- Tang, C.; Ni, Z.; Xu, C.; Luo, Y.; Cai, X.; Gao, Q.; Fang, Y.; Zhong, G.; Qiu, R.; Zhang, S. Enhanced Adsorption of Organic Pollutants Using N-Doped Porous Carbon Derived from Hemp Stems: Insights into the Mechanism. Sep. Purif. Technol. 2024, 333, 125878. [Google Scholar] [CrossRef]
- Zhou, X.; Lai, C.; Almatrafi, E.; Liu, S.; Yan, H.; Qian, S.; Li, H.; Qin, L.; Yi, H.; Fu, Y.; et al. Unveiling the Roles of Dissolved Organic Matters Derived from Different Biochar in Biochar/Persulfate System: Mechanism and Toxicity. Sci. Total Environ. 2023, 864, 161062. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Wang, J.; Zhao, L.; Jiang, K.; Xu, X. Coupling of KMnO4-Assisted Sludge Dewatering and Pyrolysis to Prepare Mn, Fe-Co doped Biochar Catalysts for Peroxymonosulfate-Induced Elimination of Phenolic Pollutants. Chem. Eng. J. 2021, 411, 128459. [Google Scholar] [CrossRef]
- Zhang, P.; O’Connor, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Tsang, D.C.W.; Ok, Y.S.; Hou, D. A Green Biochar/Iron Oxide Composite for Methylene Blue Removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Sun, Z.; Hao, C.; Toan, S.; Zhang, R.; Li, H.; Wu, Y.; Liu, H.; Sun, Z. Recent Advances in Exsolved Perovskite Oxide Construction: Exsolution Theory, Modulation, Challenges, and Prospects. J. Mater. Chem. A 2023, 11, 17961–17976. [Google Scholar] [CrossRef]
- Gupta, A.D.; Singh, H.; Varjani, S.; Awasthi, M.K.; Giri, B.S.; Pandey, A. A Critical Review on Biochar-Based Catalysts for the Abatement of Toxic Pollutants from Water via Advanced Oxidation Processes (AOPs). Sci. Total Environ. 2022, 849, 157831. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Huang, Y.; Hou, X.; Ai, Z.; Zhang, L. Photochemistry of Hydrochar: Reactive Oxygen Species Generation and Sulfadimidine Degradation. Environ. Sci. Technol. 2017, 51, 11278–11287. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Sun, Y.; Du, W.; Tang, Y.; Liu, Q.; Zhang, Z.; Doherty, W.; Frost, R.L.; Qian, G.; Tsang, D.C.W. Formation, Characteristics, and Applications of Environmentally Persistent Free Radicals in Biochars: A Review. Bioresour. Technol. 2019, 281, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, X.; Xie, R.; Zhang, Y.; Jin, Y.; Jiang, W. A Novel Porous Biochar-Supported Fe-Mn Composite as a Persulfate Activator for the Removal of Acid Red 88. Sep. Purif. Technol. 2020, 250, 117232. [Google Scholar] [CrossRef]
- Liang, F.; Liu, Z.; Jiang, X.; Li, J.; Xiao, K.; Xu, W.; Chen, X.; Liang, J.; Lin, Z.; Li, M.; et al. NaOH-Modified Biochar Supported Fe/Mn Bimetallic Composites as Efficient Peroxymonosulfate Activator for Enhance Tetracycline Removal. Chem. Eng. J. 2023, 454, 139949. [Google Scholar] [CrossRef]
- Qi, G.; Pan, Z.; Zhang, X.; Miao, X.; Xiang, W.; Gao, B. Effect of Ball Milling with Hydrogen Peroxide or Ammonia Hydroxide on Sorption Performance of Volatile Organic Compounds by Biochar from Different Pyrolysis Temperatures. Chem. Eng. J. 2022, 450, 138027. [Google Scholar] [CrossRef]
- Fan, X.; Yu, Y.; Dong, S.; Liu, Y.; Song, C.; Li, Q.; Wang, X. Heteroatoms-Doped Biochar Derived from Deciduous Resource as Persulfate Catalysts for Efficient Degradation of Phenol. J. Water Process Eng. 2022, 48, 102866. [Google Scholar] [CrossRef]
- Welter, N.; Leichtweis, J.; Silvestri, S.; Sánchez, P.I.Z.; Mejía, A.C.C.; Carissimi, E. Preparation of a New Green Composite Based on Chitin Biochar and ZnFe2O4 for Photo-Fenton Degradation of Rhodamine B. J. Alloys Compd. 2022, 901, 163758. [Google Scholar] [CrossRef]
- He, D.; Zhu, K.; Huang, J.; Shen, Y.; Lei, L.; He, H.; Chen, W. N, S Co-Doped Magnetic Mesoporous Carbon Nanosheets for Activating Peroxymonosulfate to Rapidly Degrade Tetracycline: Synergistic Effect and Mechanism. J. Hazard. Mater. 2022, 424, 127569. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Cheng, M.; Lai, C.; Zeng, G.; Qin, L.; Liu, X.; Li, B.; Zhang, W.; Yi, Y.; et al. Improving the Fenton-like Catalytic Performance of MnOx-Fe3O4/Biochar Using Reducing Agents: A Comparative Study. J. Hazard. Mater. 2021, 406, 124333. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, B.; Gao, B.; Cheng, N.; Feng, Q.; Chen, M.; Wang, S. Degradation of Organic Pollutants from Water by Biochar-Assisted Advanced Oxidation Processes: Mechanisms and Applications. J. Hazard. Mater. 2023, 442, 130075. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, F.; He, Y.; Liu, X.; Song, C.; Xu, Y.; Zhang, Y. Heterogeneous Fenton-like Degradation of Ofloxacin over Sludge Derived Carbon as Catalysts: Mechanism and Performance. Sci. Total Environ. 2019, 654, 942–947. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R. Highly Dispersed Iron-Doped Biochar Derived from Sawdust for Fenton-like Degradation of Toxic Dyes. J. Clean. Prod. 2021, 297, 126681. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-Catalyzed Sulfate Radical-Based Advanced Oxidation: A Review on Heterogeneous Catalysts and Applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Ding, D.; Yang, S.; Qian, X.; Chen, L.; Cai, T. Nitrogen-Doping Positively Whilst Sulfur-Doping Negatively Affect the Catalytic Activity of Biochar for the Degradation of Organic Contaminant. Appl. Catal. B Environ. 2020, 263, 118348. [Google Scholar] [CrossRef]
- Canonica, S.; Kohn, T.; Mac, M.; Real, F.J.; Wirz, J.; von Gunten, U. Photosensitizer Method to Determine Rate Constants for the Reaction of Carbonate Radical with Organic Compounds. Environ. Sci. Technol. 2005, 39, 9182–9188. [Google Scholar] [CrossRef]
- Li, M.; Li, P.; Zhang, L.; Chen, M.; Tang, J.; Qin, C.; Ling Jie Lee, S.; Lin, S. Facile Fabrication of ZnO Decorated ZnFe-Layered Double Hydroxides @ Biochar Nanocomposites for Synergistic Photodegradation of Tetracycline under Visible Light. Chem. Eng. J. 2022, 434, 134772. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of Biochar for the Removal of Pollutants from Aqueous Solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Li, D.-C.; Jiang, H. The Thermochemical Conversion of Non-Lignocellulosic Biomass to Form Biochar: A Review on Characterizations and Mechanism Elucidation. Bioresour. Technol. 2017, 246, 57–68. [Google Scholar] [CrossRef]
- Qin, F.; Zhang, C.; Zeng, G.; Huang, D.; Tan, X.; Duan, A. Lignocellulosic Biomass Carbonization for Biochar Production and Characterization of Biochar Reactivity. Renew. Sustain. Energy Rev. 2022, 157, 112056. [Google Scholar] [CrossRef]
- Minakshi, M.; Mujeeb, A.; Whale, J.; Evans, R.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Shrestha, L.K. Synthesis of Porous Carbon Honeycomb Structures Derived from Hemp for Hybrid Supercapacitors with Improved Electrochemistry. ChemPlusChem 2024, e202400408. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, M.; Samayamanthry, A.; Whale, J.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Kumar Shrestha, L. Phosphorous—Containing Activated Carbon Derived from Natural Honeydew Peel Powers Aqueous Supercapacitors. Chem. Asian J. 2024, 19, e202400622. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, Q.; Dou, X.; Zhang, L.; Yang, X. Conversion of Carbonaceous Materials into Solid Acids for Tylosin Mitigation: Effect of Preprocessing Methods on the Reactivity of Sulfonation Reaction. Biochar 2023, 5, 32. [Google Scholar] [CrossRef]
- Mohammad, I.N.; Ongkudon, C.M.; Misson, M. Physicochemical Properties and Lignin Degradation of Thermal-Pretreated Oil Palm Empty Fruit Bunch. Energies 2020, 13, 5966. [Google Scholar] [CrossRef]
- Sitotaw, Y.W.; Habtu, N.G.; Gebreyohannes, A.Y.; Nunes, S.P.; Van Gerven, T. Ball Milling as an Important Pretreatment Technique in Lignocellulose Biorefineries: A Review. Biomass Convers. Biorefinery 2023, 13, 15593–15616. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Yang, C.; Dang, B.; Li, C.; Sun, Q. A Multilevel Gradient Structural Carbon Derived from Naturally Preprocessed Biomass. Carbon 2020, 168, 624–632. [Google Scholar] [CrossRef]
- Cueva, L.L.Z.; Griffin, G.J.; Ward, L.P.; Madapusi, S.; Shah, K.; Parthasarathy, R. A Study of Chemical Pre-Treatment and Pyrolysis Operating Conditions to Enhance Biochar Production from Rice Straw. J. Anal. Appl. Pyrolysis 2022, 163, 105455. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, W.; Mašek, O.; Chen, X.; Gu, B.; Sharma, B.K.; Cao, X. Roles of Phosphoric Acid in Biochar Formation: Synchronously Improving Carbon Retention and Sorption Capacity. J Environ. Qual. 2017, 46, 393–401. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; He, G.; Yilmaz, M.; Yuan, S. KMnO4-Activated Spinach Waste Biochar: An Efficient Adsorbent for Adsorption of Heavy Metal Ions in Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133174. [Google Scholar] [CrossRef]
- Yang, J.; Tang, S.; Mei, W.; Chen, Y.; Yi, W.; Lv, P.; Yang, G. Valorising Lignocellulosic Biomass to High-Performance Electrocatalysts via Anaerobic Digestion Pretreatment. Biochar 2024, 6, 23. [Google Scholar] [CrossRef]
- Prasad, B.R.; Padhi, R.K.; Ghosh, G. A Review on Key Pretreatment Approaches for Lignocellulosic Biomass to Produce Biofuel and Value-Added Products. Int. J. Environ. Sci. Technol. 2023, 20, 6929–6944. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of Activation Conditions on the Physicochemical Properties of Activated Biochar: A Review. Biomass Convers. Biorefinery 2022, 12, 925–947. [Google Scholar] [CrossRef]
- Huang, J.; Zimmerman, A.R.; Chen, H.; Gao, B. Ball Milled Biochar Effectively Removes Sulfamethoxazole and Sulfapyridine Antibiotics from Water and Wastewater. Environ. Pollut. 2020, 258, 113809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Lyu, H.; Zhao, Q.; Jiang, L.; Liu, L. Ball-Milled Biochar for Galaxolide Removal: Sorption Performance and Governing Mechanisms. Sci. Total Environ. 2019, 659, 1537–1545. [Google Scholar] [CrossRef]
- Di Stasi, C.; Greco, G.; Canevesi, R.L.S.; Izquierdo, M.T.; Fierro, V.; Celzard, A.; González, B.; Manyà, J.J. Influence of Activation Conditions on Textural Properties and Performance of Activated Biochars for Pyrolysis Vapors Upgrading. Fuel 2021, 289, 119759. [Google Scholar] [CrossRef]
- Gadore, V.; Mishra, S.R.; Ahmaruzzaman, M. Bio-Inspired Sustainable Synthesis of Novel SnS2/Biochar Nanocomposite for Adsorption Coupled Photodegradation of Amoxicillin and Congo Red: Effects of Reaction Parameters, and Water Matrices. J. Environ. Manag. 2023, 334, 117496. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Li, B.; Fan, S.; Xu, H.; Guan, D.-X. Improved Adsorption Properties of Tetracycline on KOH/KMnO4 Modified Biochar Derived from Wheat Straw. Chemosphere 2022, 296, 133981. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Ren, X.; Dong, W.; Chen, H.; Cai, T.; Zeng, W.; Li, W.; Tang, L. Soybean Residue Based Biochar Prepared by Ball Milling Assisted Alkali Activation to Activate Peroxydisulfate for the Degradation of Tetracycline. J. Colloid Interface Sci. 2021, 599, 631–641. [Google Scholar] [CrossRef]
- Yu, F.; Tian, F.; Zou, H.; Ye, Z.; Peng, C.; Huang, J.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wei, X.; et al. ZnO/Biochar Nanocomposites via Solvent Free Ball Milling for Enhanced Adsorption and Photocatalytic Degradation of Methylene Blue. J. Hazard. Mater. 2021, 415, 125511. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and Utilization of Biochar: A Review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- El Bari, H.; Fanezoune, C.K.; Dorneanu, B.; Arellano-Garcia, H.; Majozi, T.; Elhenawy, Y.; Bayssi, O.; Hirt, A.; Peixinho, J.; Dhahak, A.; et al. Catalytic Fast Pyrolysis of Lignocellulosic Biomass: Recent Advances and Comprehensive Overview. J. Anal. Appl. Pyrolysis 2024, 178, 106390. [Google Scholar] [CrossRef]
- Sekar, M.; Mathimani, T.; Alagumalai, A.; Chi, N.T.L.; Duc, P.A.; Bhatia, S.K.; Brindhadevi, K.; Pugazhendhi, A. A Review on the Pyrolysis of Algal Biomass for Biochar and Bio-Oil—Bottlenecks and Scope. Fuel 2021, 283, 119190. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Palem, R.R.; AL-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Review on Biomass Feedstocks, Pyrolysis Mechanism and Physicochemical Properties of Biochar: State-of-the-Art Framework to Speed up Vision of Circular Bioeconomy. J. Clean. Prod. 2021, 297, 126645. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional Heterogeneity of Different Biochar: Effect of Pyrolysis Temperature and Feedstocks. J. Environ. Manag. 2021, 278, 111501. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S.; Mehala, R.; Sharumitha, T.J.; Surendhar, D. Enhanced Adsorptive Removal of Sulfamethoxazole from Water Using Biochar Derived from Hydrothermal Carbonization of Sugarcane Bagasse. J. Hazard. Mater. 2021, 407, 124825. [Google Scholar] [CrossRef]
- Shao, Y.; Li, C.; Fan, M.; Gao, G.; Jiang, Y.; Sun, K.; Zhang, L.; Zhang, S.; Xu, L.; Hu, X. Torrefaction and Hydrothermal Carbonization Pretreatments of Rice Impact Nature of Biochar from Subsequent Pyrolysis. Ind. Crops Prod. 2023, 205, 117515. [Google Scholar] [CrossRef]
- Xu, S.; Chen, J.; Peng, H.; Leng, S.; Li, H.; Qu, W.; Hu, Y.; Li, H.; Jiang, S.; Zhou, W.; et al. Effect of Biomass Type and Pyrolysis Temperature on Nitrogen in Biochar, and the Comparison with Hydrochar. Fuel 2021, 291, 120128. [Google Scholar] [CrossRef]
- Genli, N.; Kutluay, S.; Baytar, O.; Şahin, Ö. Preparation and Characterization of Activated Carbon from Hydrochar by Hydrothermal Carbonization of Chickpea Stem: An Application in Methylene Blue Removal by RSM Optimization. Int. J. Phytoremediation 2022, 24, 88–100. [Google Scholar] [CrossRef]
- Liu, Y.; Paskevicius, M.; Sofianos, M.V.; Parkinson, G.; Li, C.-Z. In Situ SAXS Studies of the Pore Development in Biochar during Gasification. Carbon 2021, 172, 454–462. [Google Scholar] [CrossRef]
- Del Grosso, M.; Cutz, L.; Tiringer, U.; Tsekos, C.; Taheri, P.; de Jong, W. Influence of Indirectly Heated Steam-Blown Gasification Process Conditions on Biochar Physico-Chemical Properties. Fuel Process. Technol. 2022, 235, 107347. [Google Scholar] [CrossRef]
- Akgül, A.; Akın, S.Ş.; Güleç, F.; Kazanç, F. Biochar Gasification: Insights from Pyrolysis Atmospheres and Gasification Heating Rates. Fuel 2024, 360, 130469. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, W.; Kookana, R.; Katayama, A. Characteristics of Biochar and Its Application in Remediation of Contaminated Soil. J. Biosci. Bioeng. 2013, 116, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Yang, L.; Lei, X.; Zhang, W.; Ai, Z.; Yang, Z.; Zhan, H.; Yang, J.; Yuan, X.; Peng, H.; et al. Machine Learning Predicting and Engineering the Yield, N Content, and Specific Surface Area of Biochar Derived from Pyrolysis of Biomass. Biochar 2022, 4, 63. [Google Scholar] [CrossRef]
- Wystalska, K.; Kwarciak-Kozlowska, A.; Wlodarczyk, R. Influence of Technical Parameters of the Pyrolysis Process on the Surface Area, Porosity, and Hydrophobicity of Biochar from Sunflower Husk Pellet. Sustainability 2023, 15, 394. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, Y.; Yan, J.; Qian, L.; Han, L.; Chen, M. Activation Mechanism of Peroxymonosulfate by Biochar for Catalytic Degradation of 1,4-Dioxane: Important Role of Biochar Defect Structures. Chem. Eng. J. 2019, 370, 614–624. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Y.-X.; Tang, J.-C.; Tang, J.-C.; Zhu, W.-Y. Properties of maize stalk biochar produced under different pyrolysis temperatures and its sorption capability to naphthalene. Huan Jing Ke Xue 2014, 35, 1884–1890. [Google Scholar]
- Lian, F.; Huang, F.; Chen, W.; Xing, B.; Zhu, L. Sorption of Apolar and Polar Organic Contaminants by Waste Tire Rubber and Its Chars in Single- and Bi-Solute Systems. Environ. Pollut. 2011, 159, 850–857. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge, Taylor & Francis Group: London, UK; New York, NY, USA, 2015; ISBN 978-0-415-70415-1. [Google Scholar]
- Deng, J.; Xiong, T.; Wang, H.; Zheng, A.; Wang, Y. Effects of Cellulose, Hemicellulose, and Lignin on the Structure and Morphology of Porous Carbons. ACS Sustain. Chem. Eng. 2016, 4, 3750–3756. [Google Scholar] [CrossRef]
- Daifullah, A.A.M.; Girgis, B.S.; Gad, H.M.H. A Study of the Factors Affecting the Removal of Humic Acid by Activated Carbon Prepared from Biomass Material. Colloids Surf. A Physicochem. Eng. Asp. 2004, 235, 1–10. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Peng, J.; Li, N.; Zhu, X. Preparation of High Surface Area Activated Carbons from Tobacco Stems with K2CO3 Activation Using Microwave Radiation. Ind. Crops Prod. 2008, 27, 341–347. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Preparation of Waste Tea Activated Carbon Using Potassium Acetate as an Activating Agent for Adsorption of Acid Blue 25 Dye. Chem. Eng. J. 2011, 171, 502–509. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar Technology in Wastewater Treatment: A Critical Review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, X.; Dai, Y. Phosphoric Acid Activation of Cow Dung Biochar for Adsorbing Enrofloxacin in Water: Icing on the Cake. Environ. Pollut. 2024, 341, 122887. [Google Scholar] [CrossRef]
- Zhuo, S.-N.; Dai, T.-C.; Ren, H.-Y.; Liu, B.-F. Simultaneous Adsorption of Phosphate and Tetracycline by Calcium Modified Corn Stover Biochar: Performance and Mechanism. Bioresour. Technol. 2022, 359, 127477. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Zhang, Y.; Lin, T.; Zeng, G.; Zhang, S.; Wang, Y.; He, W.; Zhang, M.; Long, H. An Efficient Adsorbent: Simultaneous Activated and Magnetic ZnO Doped Biochar Derived from Camphor Leaves for Ciprofloxacin Adsorption. Bioresour. Technol. 2019, 288, 121511. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Y.; Zhu, Y.; Xue, M.; Zheng, A.; Han, Y.; Yin, Z.; Hong, Z.; Xie, C.; Li, X.; et al. Ball-Milled Bismuth Oxychloride/Biochar Nanocomposites with Rich Oxygen Vacancies for Reactive Red-120 Adsorption in Aqueous Solution. Biochar 2022, 4, 21. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Nguyen, T.-K.-T.; Chen, W.-H.; Chen, C.-W.; Bui, X.-T.; Patel, A.K.; Dong, C.-D. Hydrothermal and Pyrolytic Conversion of Sunflower Seed Husk into Novel Porous Biochar for Efficient Adsorption of Tetracycline. Bioresour. Technol. 2023, 373, 128711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, J.; Tian, Y.; Liu, C.; Zhang, S.; Cao, L.; Zhou, Y.; Zhang, S. Effective Removal of Tetracycline Antibiotics from Water by Magnetic Functionalized Biochar Derived from Rice Waste. Environ. Pollut. 2023, 330, 121681. [Google Scholar] [CrossRef]
- Li, Z.; Tian, W.; Chu, M.; Zou, M.; Zhao, J. Molecular Imprinting Functionalization of Magnetic Biochar to Adsorb Sulfamethoxazole: Mechanism, Regeneration and Targeted Adsorption. Process Saf. Environ. Prot. 2023, 171, 238–249. [Google Scholar] [CrossRef]
- Wu, J.; Wang, T.; Liu, Y.; Tang, W.; Geng, S.; Chen, J. Norfloxacin Adsorption and Subsequent Degradation on Ball-Milling Tailored N-Doped Biochar. Chemosphere 2022, 303, 135264. [Google Scholar] [CrossRef]
- Che, H.; Wei, G.; Fan, Z.; Zhu, Y.; Zhang, L.; Wei, Z.; Huang, X.; Wei, L. Super Facile One-Step Synthesis of Sugarcane Bagasse Derived N-Doped Porous Biochar for Adsorption of Ciprofloxacin. J. Environ. Manag. 2023, 335, 117566. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Lin, Z.; Chen, H.; Yan, S.; Zhu, H.; Zhang, H.; Sun, H.; Zhang, S.; Zhang, S.; Wu, Y. Roles of Graphitization Degree and Surface Functional Groups of N-Doped Activated Biochar for Phenol Adsorption. J. Anal. Appl. Pyrolysis 2022, 167, 105700. [Google Scholar] [CrossRef]
- Khan, N.; Chowdhary, P.; Gnansounou, E.; Chaturvedi, P. Biochar and Environmental Sustainability: Emerging Trends and Techno-Economic Perspectives. Bioresour. Technol. 2021, 332, 125102. [Google Scholar] [CrossRef] [PubMed]
- Prasad Yadav, T.; Manohar Yadav, R.; Pratap Singh, D. Mechanical Milling: A Top Down Approach for the Synthesis of Nanomaterials and Nanocomposites. Nanosci. Nanotechnol. 2012, 2, 22–48. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; He, C.; Xiang, L.; Dou, Q.; Liu, Y.; Wang, M.; Wen, X.; Fu, Y.; Islam, M.U.; Chang, S.X.; et al. Effects of Ball Milling on Biochar Adsorption of Contaminants in Water: A Meta-Analysis. Sci. Total Environ. 2023, 882, 163643. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Gaston, L.A.; Zhou, B.; Li, M.; Xiao, R.; Wang, Q.; Zhang, Z.; Huang, H.; Liang, W.; et al. An Overview of Carbothermal Synthesis of Metal–Biochar Composites for the Removal of Oxyanion Contaminants from Aqueous Solution. Carbon 2018, 129, 674–687. [Google Scholar] [CrossRef]
- Yang, H.; Yu, H.; Wang, J.; Ning, T.; Chen, P.; Yu, J.; Di, S.; Zhu, S. Magnetic Porous Biochar as a Renewable and Highly Effective Adsorbent for the Removal of Tetracycline Hydrochloride in Water. Environ. Sci. Pollut. Res. 2021, 28, 61513–61525. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic Biochar for Environmental Remediation: A Review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef]
- Ding, J.; Li, X.; Shan, Y.; Yu, S.; Yu, W.; Liu, Y.; Zhao, W.; Li, X.; Liu, M.; Ding, Y. Super Facile One-Step Synthesis of Aromatic Amine Waste Residue Derived N-Rich Porous Carbon for Hyper Efficient p-Nitrophenol Adsorption. J. Environ. Chem. Eng. 2021, 9, 105106. [Google Scholar] [CrossRef]
- Fontmorin, J.M.; Burgos Castillo, R.C.; Tang, W.Z.; Sillanpää, M. Stability of 5,5-Dimethyl-1-Pyrroline-N-Oxide as a Spin-Trap for Quantification of Hydroxyl Radicals in Processes Based on Fenton Reaction. Water Res. 2016, 99, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, M.; Liang, S.; Feng, Z.; Zhao, J. Mechanism of Persulfate Activation by Biochar for the Catalytic Degradation of Antibiotics: Synergistic Effects of Environmentally Persistent Free Radicals and the Defective Structure of Biochar. Sci. Total Environ. 2021, 794, 148707. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Yu, J.; Tang, L.; Ren, X.; Pang, Y.; Zhang, H.; Xie, Q.; Liu, Y.; Liu, H.; Luo, T. Analysis of Reaction Pathways and Catalytic Sites on Metal-Free Porous Biochar for Persulfate Activation Process. Chemosphere 2020, 261, 127747. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, D.; Li, S.; Liu, J.; Deng, H.; Xia, D. Novel Sludge-Sugarcane Bagasse Mixed Biochar as an Efficient Activator for Peroxymonosulfate to Degrade Bisphenol AF. Chem. Eng. J. 2023, 462, 142114. [Google Scholar] [CrossRef]
- Wang, J.; Shen, M.; Wang, H.; Du, Y.; Zhou, X.; Liao, Z.; Wang, H.; Chen, Z. Red Mud Modified Sludge Biochar for the Activation of Peroxymonosulfate: Singlet Oxygen Dominated Mechanism and Toxicity Prediction. Sci. Total Environ. 2020, 740, 140388. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, D.; Zhang, R.; Ding, Y.; Ren, Z.; Fu, M.; Cao, X.; Zeng, G. Singlet Oxygen-Dominated Activation of Peroxymonosulfate by Passion Fruit Shell Derived Biochar for Catalytic Degradation of Tetracycline through a Non-Radical Oxidation Pathway. J. Hazard. Mater. 2021, 419, 126495. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Liu, D. Plasma Regulates Active Sites on Biochar to Boost Peroxomonosulfate Activation for Phenol Degradation. J. Environ. Chem. Eng. 2022, 10, 107833. [Google Scholar] [CrossRef]
- Huang, H.; Guo, T.; Wang, K.; Li, Y.; Zhang, G. Efficient Activation of Persulfate by a Magnetic Recyclable Rape Straw Biochar Catalyst for the Degradation of Tetracycline Hydrochloride in Water. Sci. Total Environ. 2021, 758, 143957. [Google Scholar] [CrossRef]
- Li, W.; Liu, B.; Wang, Z.; Wang, K.; Lan, Y.; Zhou, L. Efficient Activation of Peroxydisulfate (PDS) by Rice Straw Biochar Modified by Copper Oxide (RSBC-CuO) for the Degradation of Phenacetin (PNT). Chem. Eng. J. 2020, 395, 125094. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, S.-S.; Geng, Y.; Zhen, J.; Zhan, J.; Cao, C.; Ni, S.-Q. Synergistic Catalysis by Fe3O4-Biochar/Peroxymonosulfate System for the Removal of Bisphenol a. Sep. Purif. Technol. 2021, 276, 119351. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, P.; Xu, S.; Cheng, G.; Li, Z.; Li, Y.; Li, K.; Ma, S. Fabrication of Fe3O4 and Graphitized Porous Biochar Composites for Activating Peroxymonosulfate to Degrade P-Hydroxybenzoic Acid: Insights on the Mechanism. Chem. Eng. J. 2019, 375, 121980. [Google Scholar] [CrossRef]
- Qin, Y.; Li, X.; Wang, L.; Luo, J.; Li, Y.; Yao, C.; Xiao, Z.; Zhai, S.; An, Q. Valuable Cobalt/Biochar with Enriched Surface Oxygen-Containing Groups Prepared from Bio-Waste Shrimp Shell for Efficient Peroxymonosulfate Activation. Sep. Purif. Technol. 2022, 281, 119901. [Google Scholar] [CrossRef]
- Luo, J.; Bo, S.; Qin, Y.; An, Q.; Xiao, Z.; Zhai, S. Transforming Goat Manure into Surface-Loaded Cobalt/Biochar as PMS Activator for Highly Efficient Ciprofloxacin Degradation. Chem. Eng. J. 2020, 395, 125063. [Google Scholar] [CrossRef]
- Song, J.; Zhao, C.; Cao, X.; Cheng, W. Enhanced Catalytic Degradation of Antibiotics by Peanut Shell-Derived Biochar-Co3O4 Activated Peroxymonosulfate: An Experimental and Mechanistic Study. Process Saf. Environ. Prot. 2023, 171, 423–436. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, S.; Xu, Q.; Liu, J.; Zhong, C.; Xie, Z.; Zhao, Y. Efficient Activation of Persulfate by Nickel-Supported Cherry Core Biochar Composite for Removal of Bisphenol A. J. Environ. Manag. 2022, 324, 116305. [Google Scholar] [CrossRef]
- Xu, S.; Wen, L.; Yu, C.; Li, S.; Tang, J. Activation of Peroxymonosulfate by MnFe2O4@BC Composite for Bisphenol A Degradation: The Coexisting of Free-Radical and Non-Radical Pathways. Chem. Eng. J. 2022, 442, 136250. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; Wang, X.; Zhang, Y.; Xia, S. MgFe2O4/MgO Modified Biochar with Oxygen Vacancy and Surface Hydroxyl Groups for Enhanced Peroxymonosulfate Activation to Remove Sulfamethoxazole through Singlet Oxygen-Dominated Nonradical Oxidation Process. Chem. Eng. J. 2023, 477, 146960. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, X.; Yan, Z.; Sun, Z. Activation of Peroxymonosulfate by Biochar In-Situ Enriched with Cobalt Tungstate and Cobalt: Insights into the Role of Rich Oxygen Vacancies and Catalytic Mechanism. Chem. Eng. J. 2023, 475, 146124. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zheng, H.; Niu, J.; Leong, Y.K.; Lee, D.-J.; Chang, J.-S. Biochar Loaded with CoFe2O4 Enhances the Formation of High-Valent Fe (IV) and Co (IV) and Oxygen Vacancy in the Peracetic Acid Activation System for Enhanced Antibiotic Degradation. Bioresour. Technol. 2023, 387, 129536. [Google Scholar] [CrossRef]
- He, J.; Tang, J.; Zhang, Z.; Wang, L.; Liu, Q.; Liu, X. Magnetic Ball-Milled FeS@biochar as Persulfate Activator for Degradation of Tetracycline. Chem. Eng. J. 2021, 404, 126997. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Tan, X.; Wu, H.; Liang, J.; Song, B.; Tang, N.; Zhang, P.; Yang, Y.; Chen, Q.; et al. Nitrogen-Doped Biochar Fiber with Graphitization from Boehmeria Nivea for Promoted Peroxymonosulfate Activation and Non-Radical Degradation Pathways with Enhancing Electron Transfer. Appl. Catal. B Environ. 2020, 269, 118850. [Google Scholar] [CrossRef]
- Zhong, J.; Feng, Y.; Yang, B.; Xiong, Q.; Ying, G.-G. Accelerated Degradation of Sulfadiazine by Nitrogen-Doped Magnetic Biochar-Activated Persulfate: Role of Oxygen Vacancy. Sep. Purif. Technol. 2022, 289, 120735. [Google Scholar] [CrossRef]
- Wang, R.-Z.; Huang, D.-L.; Liu, Y.-G.; Zhang, C.; Lai, C.; Wang, X.; Zeng, G.-M.; Gong, X.-M.; Duan, A.; Zhang, Q.; et al. Recent Advances in Biochar-Based Catalysts: Properties, Applications and Mechanisms for Pollution Remediation. Chem. Eng. J. 2019, 371, 380–403. [Google Scholar] [CrossRef]
- Fang, G.; Gao, J.; Liu, C.; Dionysiou, D.D.; Wang, Y.; Zhou, D. Key Role of Persistent Free Radicals in Hydrogen Peroxide Activation by Biochar: Implications to Organic Contaminant Degradation. Environ. Sci. Technol. 2014, 48, 1902–1910. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, M.; Sun, X.; Zhao, J. Implication for Adsorption and Degradation of Dyes by Humic Acid: Light Driven of Environmentally Persistent Free Radicals to Activate Reactive Oxygen Species. Bioresour. Technol. 2020, 307, 123183. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, L.; Wang, Q.; Li, X.; Guo, Y.; Song, W.; Li, Y. Ball Milling Boosted the Activation of Peroxymonosulfate by Biochar for Tetracycline Removal. J. Environ. Chem. Eng. 2021, 9, 106870. [Google Scholar] [CrossRef]
- Lyu, H.; Tang, J.; Cui, M.; Gao, B.; Shen, B. Biochar/Iron (BC/Fe) Composites for Soil and Groundwater Remediation: Synthesis, Applications, and Mechanisms. Chemosphere 2020, 246, 125609. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Y.; Xu, L.; Gao, T.; Zhong, A.; Song, Z. Recent Advances in Nanoscale Zero-Valent Iron (nZVI)-Based Advanced Oxidation Processes (AOPs): Applications, Mechanisms, and Future Prospects. Nanomaterials 2023, 13, 2830. [Google Scholar] [CrossRef]
- Chen, A.; Wang, H.; Zhan, X.; Gong, K.; Xie, W.; Liang, W.; Zhang, W.; Peng, C. Applications and Synergistic Degradation Mechanisms of nZVI-Modified Biochar for the Remediation of Organic Polluted Soil and Water: A Review. Sci. Total Environ. 2024, 911, 168548. [Google Scholar] [CrossRef]
- Zhu, F.; Wu, Y.; Liang, Y.; Li, H.; Liang, W. Degradation Mechanism of Norfloxacin in Water Using Persulfate Activated by BC@nZVI/Ni. Chem. Eng. J. 2020, 389, 124276. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, L.; Song, C.; Chen, Z.; Meng, F.; Song, M. Selective Degradation of Electron-Rich Organic Pollutants Induced by CuO@Biochar: The Key Role of Outer-Sphere Interaction and Singlet Oxygen. Environ. Sci. Technol. 2022, 56, 10710–10720. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, D.; Huang, W.; Wei, X.; Huang, W. Biochar Supported CuO Composites Used as an Efficient Peroxymonosulfate Activator for Highly Saline Organic Wastewater Treatment. Sci. Total Environ. 2020, 721, 137764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, D.; Li, X.; Feng, W.; Lyu, C.; Zhang, Y. Mechanism and Performance of Singlet Oxygen Dominated Peroxymonosulfate Activation on CoOOH Nanoparticles for 2,4-Dichlorophenol Degradation in Water. J. Hazard. Mater. 2020, 384, 121350. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Pervez, M.N.; Sun, P.; Cao, C.; Li, B.; Naddeo, V.; Jin, W.; Zhao, Y. Highly Efficient Removal of Bisphenol A by a Novel Co-Doped LaFeO3 Perovskite/PMS System in Salinity Water. Sci. Total Environ. 2021, 801, 149490. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, W.; Wu, S.; Yin, R.; Zhu, M. Surface Dual Redox Cycles of Mn (III)/Mn (IV) and Cu(I)/Cu(II) for Heterogeneous Peroxymonosulfate Activation to Degrade Diclofenac: Performance, Mechanism and Toxicity Assessment. J. Hazard. Mater. 2021, 410, 124623. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zheng, N.; Hu, R.; Hu, Z.; Yu, J.C. Hydrothermal and Pyrolytic Conversion of Biomasses into Catalysts for Advanced Oxidation Treatments. Adv. Funct. Mater. 2021, 31, 2006505. [Google Scholar] [CrossRef]
- Chen, L.; Zuo, X.; Zhou, L.; Huang, Y.; Yang, S.; Cai, T.; Ding, D. Efficient Heterogeneous Activation of Peroxymonosulfate by Facilely Prepared Co/Fe Bimetallic Oxides: Kinetics and Mechanism. Chem. Eng. J. 2018, 345, 364–374. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Zuo, X.; Huang, Y.; Cai, T.; Ding, D. Biochar Modification Significantly Promotes the Activity of Co3O4 towards Heterogeneous Activation of Peroxymonosulfate. Chem. Eng. J. 2018, 354, 856–865. [Google Scholar] [CrossRef]
- Feng, Z.; Zhou, B.; Yuan, R.; Li, H.; He, P.; Wang, F.; Chen, Z.; Chen, H. Biochar Derived from Different Crop Straws as Persulfate Activator for the Degradation of Sulfadiazine: Influence of Biomass Types and Systemic Cause Analysis. Chem. Eng. J. 2022, 440, 135669. [Google Scholar] [CrossRef]
- Liang, H.; Sun, H.; Patel, A.; Shukla, P.; Zhu, Z.H.; Wang, S. Excellent Performance of Mesoporous Co3O4/MnO2 Nanoparticles in Heterogeneous Activation of Peroxymonosulfate for Phenol Degradation in Aqueous Solutions. Appl. Catal. B Environ. 2012, 127, 330–335. [Google Scholar] [CrossRef]
- He, L.; Lv, L.; Pillai, S.C.; Wang, H.; Xue, J.; Ma, Y.; Liu, Y.; Chen, Y.; Wu, L.; Zhang, Z.; et al. Efficient Degradation of Diclofenac Sodium by Periodate Activation Using Fe/Cu Bimetallic Modified Sewage Sludge Biochar/UV System. Sci. Total Environ. 2021, 783, 146974. [Google Scholar] [CrossRef]
- Zhang, B.; Mei, M.; Li, K.; Liu, J.; Wang, T.; Chen, S.; Li, J. One-Pot Synthesis of MnFe2O4 Functionalized Magnetic Biochar by the Sol-Gel Pyrolysis Method for Diclofenac Sodium Removal. J. Clean. Prod. 2022, 381, 135210. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, M.; Cao, Q.; Sun, P.; Chen, Y.; Meng, F. The Superoxide Radicals’ Production via Persulfate Activated with CuFe2O4@Biochar Composites to Promote the Redox Pairs Cycling for Efficient Degradation of o-Nitrochlorobenzene in Soil. J. Hazard. Mater. 2020, 400, 122887. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Chen, G.; Zhao, Y.; Wang, S. Synergy between MgFe2O4 and Biochar Derived from Banana Pseudo-Stem Promotes Persulfate Activation for Efficient Tetracycline Degradation. Chem. Eng. J. 2023, 468, 143773. [Google Scholar] [CrossRef]
- Yang, S.; Li, L.; Xiao, T.; Zhang, Y.; Zheng, D. Promoting Effect of Ammonia Modification on Activated Carbon Catalyzed Peroxymonosulfate Oxidation. Sep. Purif. Technol. 2016, 160, 81–88. [Google Scholar] [CrossRef]
- Chen, G.; Sun, M.; Wei, Q.; Zhang, Y.; Zhu, B.; Du, B. Ag3PO4/Graphene-Oxide Composite with Remarkably Enhanced Visible-Light-Driven Photocatalytic Activity toward Dyes in Water. J. Hazard. Mater. 2013, 244–245, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Kan, E. Heterogeneous Photocatalytic Degradation of Sulfamethoxazole in Water Using a Biochar-Supported TiO2 Photocatalyst. J. Environ. Manag. 2016, 180, 94–101. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.; Su, B.; Ma, J. Solid-Phase Fabrication of TiO2/Chitosan-Biochar Composites with Superior UV–Vis Light Driven Photocatalytic Degradation Performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129114. [Google Scholar] [CrossRef]
- Lu, L.; Shan, R.; Shi, Y.; Wang, S.; Yuan, H. A Novel TiO2/Biochar Composite Catalysts for Photocatalytic Degradation of Methyl Orange. Chemosphere 2019, 222, 391–398. [Google Scholar] [CrossRef]
- Xiong, Z.; Chen, H.; Lu, L.; Shan, R.; Zhang, Y.; Yuan, H.; Chen, Y. Nitrogen-Doped TiO2/Nitrogen-Containing Biochar Composite Catalyst as a Photocatalytic Material for the Decontamination of Aqueous Organic Pollutants. ACS Omega 2023, 8, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lyu, H.; Yang, C.; Zhao, B.; Wang, L.; Tang, J. Graphitic Carbon Nitride/Biochar Composite Synthesized by a Facile Ball-Milling Method for the Adsorption and Photocatalytic Degradation of Enrofloxacin. J. Environ. Sci. 2021, 103, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhang, S.; Chen, B.; Wu, P.; Feng, N.; Deng, F.; Wang, Z. Visible-Light Photocatalysis Degradation of Enrofloxacin by Crawfish Shell Biochar Combined with g-C3N4: Effects and Mechanisms. J. Environ. Chem. Eng. 2023, 11, 109693. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Deng, Y.; Xiong, S.; Zheng, J.; Li, L.; Zhou, Z.; Su, L.; Zhao, J. π-π Stacking Derived from Graphene-like Biochar/g-C3N4 with Tunable Band Structure for Photocatalytic Antibiotics Degradation via Peroxymonosulfate Activation. J. Hazard. Mater. 2022, 423, 126944. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lin, M. Synthesis of Biochar-Supported K-Doped g-C3N4 Photocatalyst for Enhancing the Polycyclic Aromatic Hydrocarbon Degradation Activity. Int. J. Environ. Res. Public Health 2020, 17, 2065. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, T.; Wang, D.; Cai, D.; Chen, S.; Wang, H.; Shu, S.; Zhu, Y. Degradation of Tetracycline Using Persulfate Activated by a Honeycomb Structured S-Doped g-C3N4/Biochar under Visible Light. Sep. Purif. Technol. 2022, 300, 121833. [Google Scholar] [CrossRef]
- Gholami, P.; Khataee, A.; Soltani, R.D.C.; Dinpazhoh, L.; Bhatnagar, A. Photocatalytic Degradation of Gemifloxacin Antibiotic Using Zn-Co-LDH@biochar Nanocomposite. J. Hazard. Mater. 2020, 382, 121070. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Yan, M.; Tan, X.; Liang, J.; Zeng, G.; Wu, H.; Song, B.; Zhou, C.; Yang, Y.; Wang, H. Facile Assembled Biochar-Based Nanocomposite with Improved Graphitization for Efficient Photocatalytic Activity Driven by Visible Light. Appl. Catal. B Environ. 2019, 250, 78–88. [Google Scholar] [CrossRef]
- Yang, Q.; Li, X.; Tian, Q.; Pan, A.; Liu, X.; Yin, H.; Shi, Y.; Fang, G. Synergistic Effect of Adsorption and Photocatalysis of BiOBr/Lignin-Biochar Composites with Oxygen Vacancies under Visible Light Irradiation. J. Ind. Eng. Chem. 2023, 117, 117–129. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, A.; Li, J.; Han, Y.; Xue, M.; Zhang, L.; Yin, Z.; Xie, C.; Chen, Z.; Ji, L.; et al. Integrated Adsorption and Photodegradation of Tetracycline by Bismuth Oxycarbonate/Biochar Nanocomposites. Chem. Eng. J. 2023, 457, 141228. [Google Scholar] [CrossRef]
- Fang, G.; Liu, C.; Wang, Y.; Dionysiou, D.D.; Zhou, D. Photogeneration of Reactive Oxygen Species from Biochar Suspension for Diethyl Phthalate Degradation. Appl. Catal. B Environ. 2017, 214, 34–45. [Google Scholar] [CrossRef]
- Wan, D.; Wang, J.; Dionysiou, D.D.; Kong, Y.; Yao, W.; Selvinsimpson, S.; Chen, Y. Photogeneration of Reactive Species from Biochar-Derived Dissolved Black Carbon for the Degradation of Amine and Phenolic Pollutants. Environ. Sci. Technol. 2021, 55, 8866–8876. [Google Scholar] [CrossRef] [PubMed]
- Navidpour, A.H.; Abbasi, S.; Li, D.; Mojiri, A.; Zhou, J.L. Investigation of Advanced Oxidation Process in the Presence of TiO2 Semiconductor as Photocatalyst: Property, Principle, Kinetic Analysis, and Photocatalytic Activity. Catalysts 2023, 13, 232. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Hua, L.; Li, S.; Zhang, X.; Sheng, W.; Cao, S. High Photocatalytic Activity of Hierarchical SiO2@C-Doped TiO2 Hollow Spheres in UV and Visible Light towards Degradation of Rhodamine B. J. Hazard. Mater. 2017, 340, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W.; Li, S.; Liu, Y. Enhanced Photocatalytic Degradation of Organic Dyes by Ultrasonic-Assisted Electrospray TiO2/Graphene Oxide on Polyacrylonitrile/β-Cyclodextrin Nanofibrous Membranes. Ultrason. Sonochemistry 2021, 70, 105343. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Guo, X.; Li, J.; Sun, H.; Zhang, H.; Wang, W. Electrospinning Preparation and Dye Adsorption Capacity of TiO2@Carbon Flexible Fiber. Ceram. Int. 2019, 45, 11856–11860. [Google Scholar] [CrossRef]
- Mancuso, A.; Kiani, A.; Sacco, O.; Lettieri, M.; Fittipaldi, R.; Vaiano, V.; Acocella, M.R.; Venditto, V. Ball–Milled TiO2/Biochar Hybrid System as a Heterogeneous Photocatalyst for Tannery Dyes Removal in Aqueous Solution. J. Mol. Liq. 2024, 399, 124357. [Google Scholar] [CrossRef]
- Tang, R.; Xiong, S.; Gong, D.; Deng, Y.; Wang, Y.; Su, L.; Ding, C.; Yang, L.; Liao, C. Ti3C2 2D MXene: Recent Progress and Perspectives in Photocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 56663–56680. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.; Zhao, W.; Liu, S.; Zhao, Q. Oxygen-Facilitated Dynamic Active-Site Generation on Strained MoS2 during Photo-Catalytic Hydrogen Evolution. Chem. Eng. J. 2021, 405, 127028. [Google Scholar] [CrossRef]
- Jin, C.; Kang, J.; Li, Z.; Wang, M.; Wu, Z.; Xie, Y. Enhanced Visible Light Photocatalytic Degradation of Tetracycline by MoS2/Ag/g-C3N4 Z-Scheme Composites with Peroxymonosulfate. Appl. Surf. Sci. 2020, 514, 146076. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, C.; Tang, L.; Zhou, Y.; Chen, Z.; Feng, H.; Wang, J.; Yu, J.; Liu, Y. Ultrathin Low Dimensional Heterostructure Composites with Superior Photocatalytic Activity: Insight into the Multichannel Charge Transfer Mechanism. Chem. Eng. J. 2020, 393, 124718. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Wang, Z.; Lou, Y.; Pan, C.; Zhu, Y. Unprecedentedly Efficient Mineralization Performance of Photocatalysis-Self-Fenton System towards Organic Pollutants over Oxygen-Doped Porous g-C3N4 Nanosheets. Appl. Catal. B Environ. 2022, 312, 121438. [Google Scholar] [CrossRef]
- Sun, J.; Lin, X.; Xie, J.; Zhang, Y.; Wang, Q.; Ying, Z. Facile Synthesis of Novel Ternary G-C3N4/Ferrite/Biochar Hybrid Photocatalyst for Efficient Degradation of Methylene Blue under Visible-Light Irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125556. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, J.; Cai, J.; Liu, Q.; Zhang, X. Visible-Light-Driven Photocatalytic Degradation of Dye and Antibiotics by Activated Biochar Composited with K+ Doped g-C3N4: Effects, Mechanisms, Actual Wastewater Treatment and Disinfection. Sci. Total Environ. 2022, 839, 155955. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, Y.; Li, Y.; Lin, L.; Zhang, C.; Zhang, W.; Wang, L.; Niu, L. A Novel BC/g-C3N4 Porous Hydrogel Carrier Used in Intimately Coupled Photocatalysis and Biodegradation System for Efficient Removal of Tetracycline Hydrochloride in Water. Chemosphere 2023, 317, 137888. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of Sulfate Radical through Heterogeneous Catalysis for Organic Contaminants Removal: Current Development, Challenges and Prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, B.; Chen, W.; Tao, Z.; Liu, J.; Wang, L. Biochar Doped Carbon Nitride to Enhance the Photocatalytic Hydrogen Evolution through Synergy of Nitrogen Vacancies and Bridging Carbon Structure: Nanoarchitectonics and First-Principles Calculation. Carbon 2023, 209, 117988. [Google Scholar] [CrossRef]
- Wang, T.; Dissanayake, P.D.; Sun, M.; Tao, Z.; Han, W.; An, N.; Gu, Q.; Xia, D.; Tian, B.; Ok, Y.S.; et al. Adsorption and Visible-Light Photocatalytic Degradation of Organic Pollutants by Functionalized Biochar: Role of Iodine Doping and Reactive Species. Environ. Res. 2021, 197, 111026. [Google Scholar] [CrossRef]
- Hosny, M.; Fawzy, M.; Eltaweil, A.S. Green Synthesis of Bimetallic Ag/ZnO@Biohar Nanocomposite for Photocatalytic Degradation of Tetracycline, Antibacterial and Antioxidant Activities. Sci. Rep. 2022, 12, 7316. [Google Scholar] [CrossRef]
- Khataee, A.; Kayan, B.; Gholami, P.; Kalderis, D.; Akay, S.; Dinpazhoh, L. Sonocatalytic Degradation of Reactive Yellow 39 Using Synthesized ZrO2 Nanoparticles on Biochar. Ultrason. Sonochemistry 2017, 39, 540–549. [Google Scholar] [CrossRef]
- Kim, J.; Park, B.; Son, Y.; Khim, J. Peat Moss-Derived Biochar for Sonocatalytic Applications. Ultrason. Sonochemistry 2018, 42, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, S.; Vakros, J.; Diamadopoulos, E.; Mantzavinos, D. Sonochemical Degradation of Propylparaben in the Presence of Agro-Industrial Biochar. J. Environ. Chem. Eng. 2020, 8, 104010. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Zhao, Z.; Musoke, F.S.N.; Wu, X. Ultrasonic Activated Biochar and Its Removal of Harmful Substances in Environment. Microorganisms 2022, 10, 1593. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.L.; Lim, S.; Lee, R.K.L. Enhancement of Sonocatalytic Degradation of Organic Dye by Using Titanium Dioxide (TiO2)/Activated Carbon (AC) Derived from Oil Palm Empty Fruit Bunch. Environ. Sci. Pollut. Res. 2020, 27, 34638–34652. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Zu, P.; Zhang, C.-M.; Guan, J.; Song, C.; Sun, X.-F.; Wang, S.-G. Sonocatalytic Degradation of Ciprofloxacin Using Hydrogel Beads of TiO2 Incorporated Biochar and Chitosan. J. Hazard. Mater. 2022, 434, 128879. [Google Scholar] [CrossRef]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Hassani, A.; Bhatnagar, A. Facile Hydrothermal Synthesis of Novel Fe-Cu Layered Double Hydroxide/Biochar Nanocomposite with Enhanced Sonocatalytic Activity for Degradation of Cefazolin Sodium. J. Hazard. Mater. 2020, 381, 120742. [Google Scholar] [CrossRef]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Orooji, Y. Sonocatalytic Activity of Biochar-Supported ZnO Nanorods in Degradation of Gemifloxacin: Synergy Study, Effect of Parameters and Phytotoxicity Evaluation. Ultrason. Sonochemistry 2019, 55, 44–56. [Google Scholar] [CrossRef]
- Jun, B.-M.; Kim, Y.; Yoon, Y.; Yea, Y.; Park, C.M. Enhanced Sonocatalytic Degradation of Recalcitrant Organic Contaminants Using a Magnetically Recoverable Ag/Fe-Loaded Activated Biochar Composite. Ceram. Int. 2020, 46, 22521–22531. [Google Scholar] [CrossRef]
- Li, G.; Zhang, S.; Yu, P.; Mi, X.; Guo, Y.; Shen, C. Effect of Hexadecyl Trimethyl Ammonium Bromide on Sonocatalytic Degradation of Orange II by Using Bagasse Biochar. Desalin. Water Treat. 2023, 313, 209–215. [Google Scholar] [CrossRef]
- Zhou, J.; Hou, W.; Qi, P.; Gao, X.; Luo, Z.; Cen, K. CeO2–TiO2 Sorbents for the Removal of Elemental Mercury from Syngas. Environ. Sci. Technol. 2013, 47, 10056–10062. [Google Scholar] [CrossRef]
- Khataee, A.; Gholami, P.; Kalderis, D.; Pachatouridou, E.; Konsolakis, M. Preparation of Novel CeO2-Biochar Nanocomposite for Sonocatalytic Degradation of a Textile Dye. Ultrason. Sonochemistry 2018, 41, 503–513. [Google Scholar] [CrossRef]
- Khataee, A.; Kayan, B.; Gholami, P.; Kalderis, D.; Akay, S. Sonocatalytic Degradation of an Anthraquinone Dye Using TiO2-Biochar Nanocomposite. Ultrason. Sonochemistry 2017, 39, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Bensalah, N.; Midassi, S.; Ahmad, M.I.; Bedoui, A. Degradation of Hydroxychloroquine by Electrochemical Advanced Oxidation Processes. Chem. Eng. J. 2020, 402, 126279. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, C.; Rauf, M.; Luo, H.; Sun, X.; Jiang, Y. Gas Diffusion Electrodes for H2O2 Production and Their Applications for Electrochemical Degradation of Organic Pollutants in Water: A Review. Sci. Total Environ. 2021, 759, 143459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Liu, S.; Pan, Q.; Song, Z.; Chen, G.; Zhao, C. Enhanced Degradation of Carbamazepine by Electrochemical Activation of Peroxymonosulfate with PTFE/Black Carbon as Cathode. J. Environ. Chem. Eng. 2023, 11, 109920. [Google Scholar] [CrossRef]
- Mirehbar, K.; Sánchez, J.S.; Pinilla, S.; Oropeza, F.E.; Sirés, I.; de la Peña O’Shea, V.A.; Palma, J.; Lado, J.J. Development of a 3D Ni-Mn Binary Oxide Anode for Energy-Efficient Electro-Oxidation of Organic Pollutants. J. Environ. Chem. Eng. 2024, 12, 112562. [Google Scholar] [CrossRef]
- Stanković, D.M.; Kukuruzar, A.; Savić, S.; Ognjanović, M.; Janković-Častvan, I.M.; Roglić, G.; Antić, B.; Manojlović, D.; Dojčinović, B. Sponge-like Europium Oxide from Hollow Carbon Sphere as a Template for an Anode Material for Reactive Blue 52 Electrochemical Degradation. Mater. Chem. Phys. 2021, 273, 125154. [Google Scholar] [CrossRef]
- Qiu, F.; Wang, L.; Fan, Y.; Pan, Y.; Song, H.; Ye, Z.; Zhang, S. Review on Structural Adjustment Strategies of Titanium-Based Metal Oxide Dimensionally Stable Anodes in Electrochemical Advanced Oxidation Technology. Adv. Eng. Mater. 2024, 26, 2400122. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, Q.; Sun, L.; Guo, H.; Tai, X.; Li, D.; Liu, L.; Ling, C.; Sun, X. Facilitating Active Species by Decorating CeO2 on Ni3S2 Nanosheets for Efficient Water Oxidation Electrocatalysis. Chin. J. Catal. 2021, 42, 482–489. [Google Scholar] [CrossRef]
- Yu, F.; Tao, L.; Yang, Y.; Wang, S. Electrochemical Catalytic Mechanism of N-Doped Electrode for in-Situ Generation of OH in Metal-Free EAOPs to Degrade Organic Pollutants. Sep. Purif. Technol. 2021, 277, 119432. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, M.; Liang, L. Highly Efficient In-Situ Metal-Free Electrochemical Advanced Oxidation Process Using Graphite Felt Modified with N-Doped Graphene. Chem. Eng. J. 2018, 338, 700–708. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S.; Zhang, N.; Chen, H.; Yang, Y.; Tu, Y.; Jiao, C.; Xu, Z.; Xia, Y.; Suo, H.; et al. Sewage Sludge-Derived Catalyst for Extremely Efficient Electrocatalytic Elimination of Organic Pollutants in Water. Chem. Eng. J. 2023, 469, 143777. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Kamali, M.; Zhang, X.; Feijoo, S.; Al-Salem, S.M.; Dewil, R.; Appels, L. Biochar in Hydroxyl Radical-Based Electrochemical Advanced Oxidation Processes (eAOPs)—Mechanisms and Prospects. Chem. Eng. J. 2023, 467, 143291. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Yang, X.; Tan, X.; Wan, C.; Liu, X. Characterization of Electrodes Modified with Sludge-Derived Biochar and Its Performance of Electrocatalytic Oxidation of Azo Dyes. J. Environ. Manag. 2022, 324, 116445. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, J.; Chen, S.; Yang, L.; Shen, S.; Chen, X.; Zhang, Z. Ball Milling and Acetic Acid Co-Modified Sludge Biochar Enhanced by Electrochemistry to Activate Peroxymonosulfate for Sustainable Degradation of Environmental Concentration Neonicotinoids. J. Hazard. Mater. 2023, 444, 130336. [Google Scholar] [CrossRef]
- Zhang, B.; Isobe, T.; Nabae, Y.; Hu, J.; Zhang, Y.; Song, M.; Wu, Y.; Chen, B.; Fujii, M. Kraft Lignin-Derived Multi-Porous Carbon toward Sustainable Electro-Fenton Treatment of Emerging Contaminants. Resour. Conserv. Recycl. 2024, 203, 107413. [Google Scholar] [CrossRef]
- Ansari, M.N.; Sarrouf, S.; Ehsan, M.F.; Manzoor, S.; Ashiq, M.N.; Alshawabkeh, A.N. Polarity Reversal for Enhanced In-Situ Electrochemical Synthesis of H2O2 over Banana-Peel Derived Biochar Cathode for Water Remediation. Electrochim. Acta 2023, 453, 142351. [Google Scholar] [CrossRef]
- Kim, J.-G.; Sarrouf, S.; Ehsan, M.F.; Baek, K.; Alshawabkeh, A.N. In-Situ Hydrogen Peroxide Formation and Persulfate Activation over Banana Peel-Derived Biochar Cathode for Electrochemical Water Treatment in a Flow Reactor. Chemosphere 2023, 331, 138849. [Google Scholar] [CrossRef]
- Ge, Y.; Ke, J.; Li, X.; Wang, J.; Yang, Q.; Liu, Y.; Guo, R.; Chen, J. Electro-Activating Persulfate via Biochar Catalytic Cathode for Sulfamethazine Degradation: Performance and Mechanism Insight. J. Environ. Chem. Eng. 2022, 10, 109020. [Google Scholar] [CrossRef]
- Sun, C.; Chen, T.; Huang, Q.; Duan, X.; Zhan, M.; Ji, L.; Li, X.; Wang, S.; Yan, J. Biochar Cathode: Reinforcing Electro-Fenton Pathway against Four-Electron Reduction by Controlled Carbonization and Surface Chemistry. Sci. Total Environ. 2021, 754, 142136. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Yang, L.; Li, W.; Wang, W.; Liu, P. Ti–Sn–Ce/Bamboo Biochar Particle Electrodes for Enhanced Electrocatalytic Treatment of Coking Wastewater in a Three-Dimensional Electrochemical Reaction System. J. Clean. Prod. 2020, 258, 120273. [Google Scholar] [CrossRef]
- Wang, W.; Li, W.; Li, H.; Xu, C.; Zhao, G.; Ren, Y. Kapok Fiber Derived Biochar as an Efficient Electro-Catalyst for H2O2 in-Situ Generation in an Electro-Fenton System for Sulfamethoxazole Degradation. J. Water Process Eng. 2022, 50, 103311. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, L.; Sun, X.; Dong, H.; Yu, H.; Yu, H. Radical and Non-Radical Cooperative Degradation in Metal-Free Electro-Fenton Based on Nitrogen Self-Doped Biochar. J. Hazard. Mater. 2022, 435, 129063. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhu, J.; Wang, Z.; Liu, Y.; Yang, Z.; Yang, W. Novel Fe/N Co-Doping Biochar Based Electro-Fenton Catalytic Membrane Enabling Enhanced Tetracycline Removal and Self-Cleaning Performance. J. Clean. Prod. 2023, 402, 136731. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Ji, M.; Kong, X. Treatment of Mixed Chemical Wastewater and the Agglomeration Mechanism via an Internal Electrolysis Filter. Chem. Eng. J. 2013, 215–216, 50–56. [Google Scholar] [CrossRef]
- van der Zee, F.P.; Bisschops, I.A.E.; Lettinga, G.; Field, J.A. Activated Carbon as an Electron Acceptor and Redox Mediator during the Anaerobic Biotransformation of Azo Dyes. Environ. Sci. Technol. 2003, 37, 402–408. [Google Scholar] [CrossRef]
- Li, X.; Qin, Y.; Song, H.; Zou, W.; Cao, Z.; Ding, L.; Pan, Y.; Zhou, M. Efficient Removal of Bisphenol A by a Novel Biochar-Based Fe/C Granule via Persulfate Activation: Performance, Mechanism, and Toxicity Assessment. Process Saf. Environ. Prot. 2023, 169, 48–60. [Google Scholar] [CrossRef]
- Yu, F.; Guo, Y.; Li, H.; Yang, J. Activated Persulfate by the Synergistic Electro-Activation and Bimetals Cathode (MBC@CF) Leads to Highly Efficient Degradation of Tetracycline. Sep. Purif. Technol. 2024, 335, 126204. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Singh, S.K.; Takeyasu, K.; Nakamura, J. Active Sites and Mechanism of Oxygen Reduction Reaction Electrocatalysis on Nitrogen-Doped Carbon Materials. Adv. Mater. 2019, 31, 1804297. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, W.; Gao, J.; Sun, F.; Zhao, G. H2O2 Electrogeneration from O2 Electroreduction by N-Doped Carbon Materials: A Mini-Review on Preparation Methods, Selectivity of N Sites, and Prospects. Adv. Mater. Interfaces 2021, 8, 2002091. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, M.; Tang, D.; Xu, Y.; Ran, H.; He, J.; Chen, K.; Sun, J. High H2O2 Selectivity and Enhanced Fe2+ Regeneration toward an Effective Electro-Fenton Process Based on a Self-Doped Porous Biochar Cathode. Appl. Catal. B Environ. 2022, 315, 121523. [Google Scholar] [CrossRef]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A Comprehensive Review on the Chemical Regeneration of Biochar Adsorbent for Sustainable Wastewater Treatment. NPJ Clean Water 2022, 5, 29. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Y.; Fu, J.; Yuan, L.; Li, Z.; Liu, C.; Zhao, D.; Wang, X. A Novel Magnetic Biochar/MgFe-Layered Double Hydroxides Composite Removing Pb2+ from Aqueous Solution: Isotherms, Kinetics and Thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 278–287. [Google Scholar] [CrossRef]
- Pan, J.; Gao, B.; Wang, S.; Guo, K.; Xu, X.; Yue, Q. Waste-to-Resources: Green Preparation of Magnetic Biogas Residues-Based Biochar for Effective Heavy Metal Removals. Sci. Total Environ. 2020, 737, 140283. [Google Scholar] [CrossRef]
- Sayin, F.; Akar, S.T.; Akar, T. From Green Biowaste to Water Treatment Applications: Utilization of Modified New Biochar for the Efficient Removal of Ciprofloxacin. Sustain. Chem. Pharm. 2021, 24, 100522. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Sirajudheen, P.; Karthikeyan, P.; Meenakshi, S. Fabrication of Sulfur-Doped Biochar Derived from Tapioca Peel Waste with Superior Adsorption Performance for the Removal of Malachite Green and Rhodamine B Dyes. Surf. Interfaces 2021, 23, 100920. [Google Scholar] [CrossRef]
- Gao, L.; Li, Z.; Yi, W.; Li, Y.; Zhang, P.; Zhang, A.; Wang, L. Impacts of Pyrolysis Temperature on Lead Adsorption by Cotton Stalk-Derived Biochar and Related Mechanisms. J. Environ. Chem. Eng. 2021, 9, 105602. [Google Scholar] [CrossRef]
- Aktaş, Ö.; Çeçen, F. Bioregeneration of Activated Carbon: A Review. Int. Biodeterior. Biodegrad. 2007, 59, 257–272. [Google Scholar] [CrossRef]
- El Gamal, M.; Mousa, H.A.; El-Naas, M.H.; Zacharia, R.; Judd, S. Bio-Regeneration of Activated Carbon: A Comprehensive Review. Sep. Purif. Technol. 2018, 197, 345–359. [Google Scholar] [CrossRef]
- Ng, S.L.; Seng, C.E.; Lim, P.E. Quantification of Bioregeneration of Activated Carbon and Activated Rice Husk Loaded with Phenolic Compounds. Chemosphere 2009, 75, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Du, E. The Effects of Thermal Regeneration Conditions and Inorganic Compounds on the Characteristics of Activated Carbon Used in Power Plant. Energy Procedia 2012, 17, 444–449. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. A Rapid Regeneration of Methylene Blue Dye-Loaded Activated Carbons with Microwave Heating. J. Anal. Appl. Pyrolysis 2012, 98, 123–128. [Google Scholar] [CrossRef]
- Scamehorn, J.F. Removal of Vinyl Chloride from Gaseous Streams by Adsorption on Activated Carbon. Ind. Eng. Chem. Process Des. Dev. 1979, 18, 210–217. [Google Scholar] [CrossRef]
- Radic, D.B.; Stanojevic, M.M.; Obradovic, M.O.; Jovovic, A.M. Thermal Analysis of Physical and Chemical Changes Occuring During Regeneration of Activated Carbon. Therm. Sci. 2017, 21, 1067–1081. [Google Scholar] [CrossRef]
- Deng, J.; Fang, Y.; Hou, C.; Zhang, Y.; Li, M.; Han, J.; Du, W.; Tang, C.; Hu, X. Ultrasonic Assisted Activation of Persulfate for the Treatment of Spent Porous Biochar: Degradation of Adsorbed PFOA and Adsorbent Regeneration. J. Environ. Chem. Eng. 2023, 11, 111146. [Google Scholar] [CrossRef]
- Fan, Q.; Cui, L.; Quan, G.; Wang, S.; Sun, J.; Han, X.; Wang, J.; Yan, J. Effects of Wet Oxidation Process on Biochar Surface in Acid and Alkaline Soil Environments. Materials 2018, 11, 2362. [Google Scholar] [CrossRef]
- James, A.L.; Perkins, W.T.; Sian, J.; Hammond, D.; Hodgson, E.M. Application of Biochar for Minewater Remediation: Effect of Scaling up Production on Performance under Laboratory and Field Conditions. Bioresour. Technol. 2022, 359, 127439. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E.R.V. The Use of Carbon Adsorbents for the Removal of Perfluoroalkyl Acids from Potable Reuse Systems. Chemosphere 2017, 184, 168–175. [Google Scholar] [CrossRef]
- Lakshmi, D.; Akhil, D.; Kartik, A.; Gopinath, K.P.; Arun, J.; Bhatnagar, A.; Rinklebe, J.; Kim, W.; Muthusamy, G. Artificial Intelligence (AI) Applications in Adsorption of Heavy Metals Using Modified Biochar. Sci. Total Environ. 2021, 801, 149623. [Google Scholar] [CrossRef]
- Bhagat, S.K.; Tung, T.M.; Yaseen, Z.M. Development of Artificial Intelligence for Modeling Wastewater Heavy Metal Removal: State of the Art, Application Assessment and Possible Future Research. J. Clean. Prod. 2020, 250, 119473. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Wang, X. Machine Learning Prediction of Biochar Yield and Carbon Contents in Biochar Based on Biomass Characteristics and Pyrolysis Conditions. Bioresour. Technol. 2019, 288, 121527. [Google Scholar] [CrossRef]
- Jaffari, Z.H.; Jeong, H.; Shin, J.; Kwak, J.; Son, C.; Lee, Y.-G.; Kim, S.; Chon, K.; Hwa Cho, K. Machine-Learning-Based Prediction and Optimization of Emerging Contaminants’ Adsorption Capacity on Biochar Materials. Chem. Eng. J. 2023, 466, 143073. [Google Scholar] [CrossRef]

| Synthesis Methods | Preparation Condition | Ref. | |
|---|---|---|---|
| Pyrolysis | fast pyrolysis | 400–800 °C, seconds | [63,64] |
| slow pyrolysis | 300–900 °C, minutes to days | [65,66] | |
| Hydrothermal carbonization | 180–250 °C, 0.5–48 h | [67,68,69,70] | |
| Gasification | 700–900 °C, 10–20 s | [71,72,73] | |
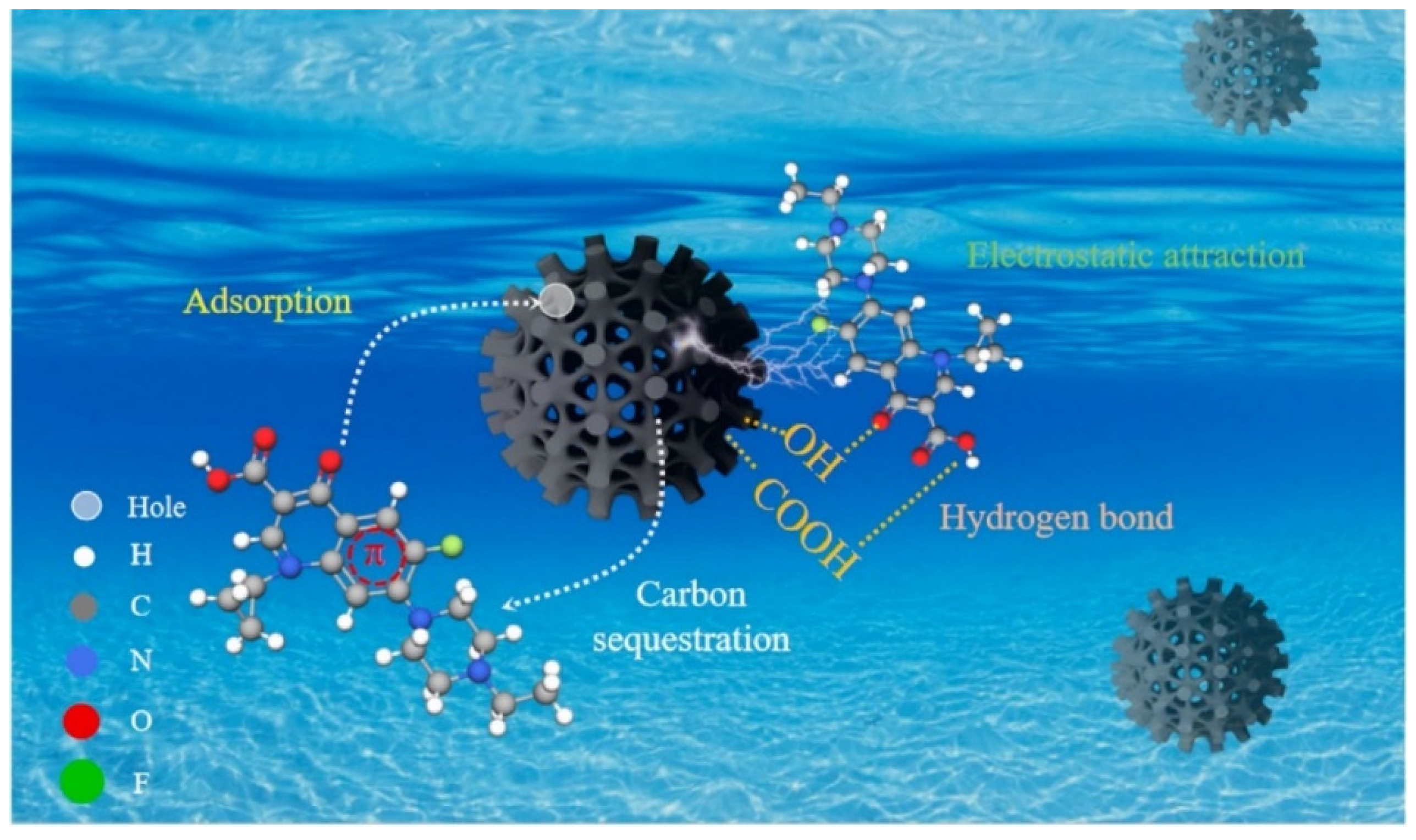
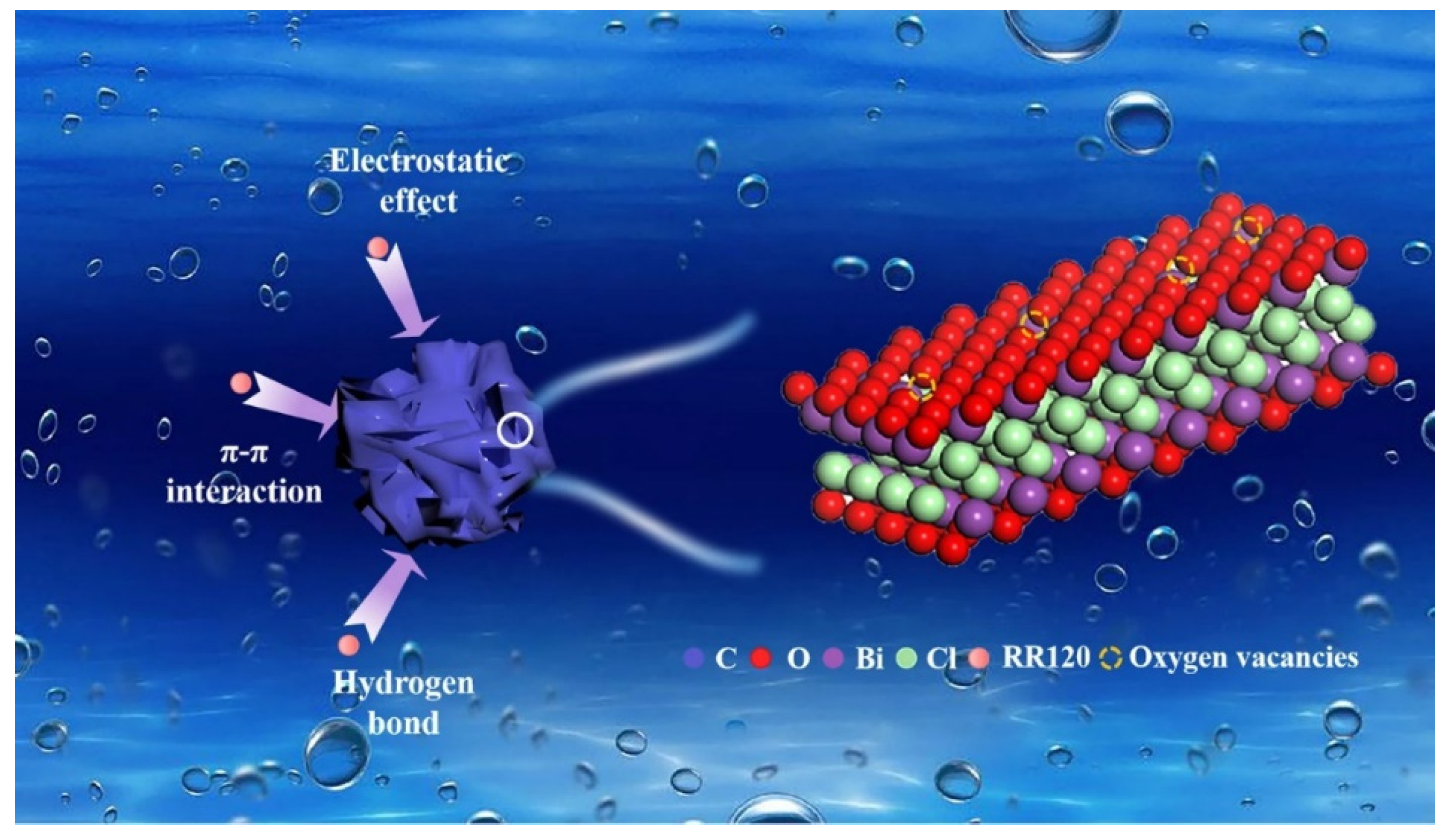
| BC-Based Adsorbents | Targeted Pollutants | Operational Parameters | Qmax (mg/g) | Dominant Mechanism | Ref. |
|---|---|---|---|---|---|
| Phosphoric acid/cow dung BC | Enrofloxacin | ICa = 20 mg/L; dosage = 0.32 g/L; pH = 5; t = 6 h, T = 25 °C | 63.61 | π–π interactions, pore filling, electrostatic interactions, and hydrogen bonding | [86] |
| KMnO4+KOH modified BC | Tetracycline | ICa = 200 mg/L; dosage = 0.25 g/L; t = 24 h; T = 318 K | 584.19 | Pore filling, π–π interactions; hydrogen bonding, and metal complexation | [58] |
| Calcium modified BC | Tetracycline | ICa = 20 mg/L; dosage = 1.0 g/L; pH = 8.0; t = 24 h | 33.53 | π–π interactions, and hydrogen bonding | [87] |
| ZnO/magnetic camphor leaves BC | Ciprofloxacin | ICa = 100 mg/L; dosage = 0.5 g/L; pH = 4; t = 120 h; T = 40 °C | 449.4 | π–π interactions, electrostatic interactions, and cation exchange interactions | [88] |
| Iron oxide/banana-peel BC | Methylene blue | ICa = 500 mg/L; dosage = 0.5 g/L; pH = 6.1; t = 12 h; T = 40 °C | 862 | An electron transfer process driven by electrostatic attraction | [19] |
| BiOCl/BC | Reactive red-120 | ICa = 10 mg/L; dosage = 0.4 g/L; pH = 2, t = 18 h; T = 25 °C | 116.38 | Electrostatic interactions, π–π interactions, and hydrogen bonding | [89] |
| ZnCl2/sunflower seed husk BC | Tetracycline | ICa = 20 mg/L; dosage = 0.025 g/L; t = 24 h; T = 25 °C | 673 | Chemical and electrostatic interactions | [90] |
| Magnetic BC microsphere | Tetracycline | ICa = 100 mg/L; dosage = 0.5 g/L; pH = 5; t = 24 h; T = 25 °C | 94.63 | Pore filling, electrostatic attraction, and π–π interactions | [91] |
| Surface-imprinted magnetic BC | Sulfamethoxazole | ICa = 1000 mg/L; dosage = 1.0 g/L; t = 4 h; T = 35 °C | 25.65 | Hydrogen bonding, electrostatic interactions, and π–π interactions | [92] |
| Urea N-doped BC | Norfloxacin | ICa = 50 mg/L; dosage = 1.0 g/L; pH = 5.0; t = 48 h | 11.48 | Hydrogen bonding, π–π electron donor-acceptor, and pore-filling interactions | [93] |
| N-doped/porous BC | Ciprofloxacin | ICa = 30 mg/L; dosage = 0.125 g/L; pH = 6.58; t = 1 h; T = 30 °C | 212 | Combined filling pore, π–π conjugation, and hydrogen bonding. | [94] |
| N-doped/poplar BC | Phenol | ICa = 100 mg/L; dosage = 0.2 g/L; t = 48 h; T = 25 °C | 126.5–170.7 | π–π stacking interactions | [95] |
| Hydrothermal N-doped sludge-derived BC | Sulfamethoxazole | ICa = 15 mg/L; dosage = 0.0075 g/L; t = 12 h; T = 308 K | 68.6 | Lewis acid-base interactions, π–π conjugation, pore filling, and electrostatic interactions | [7] |
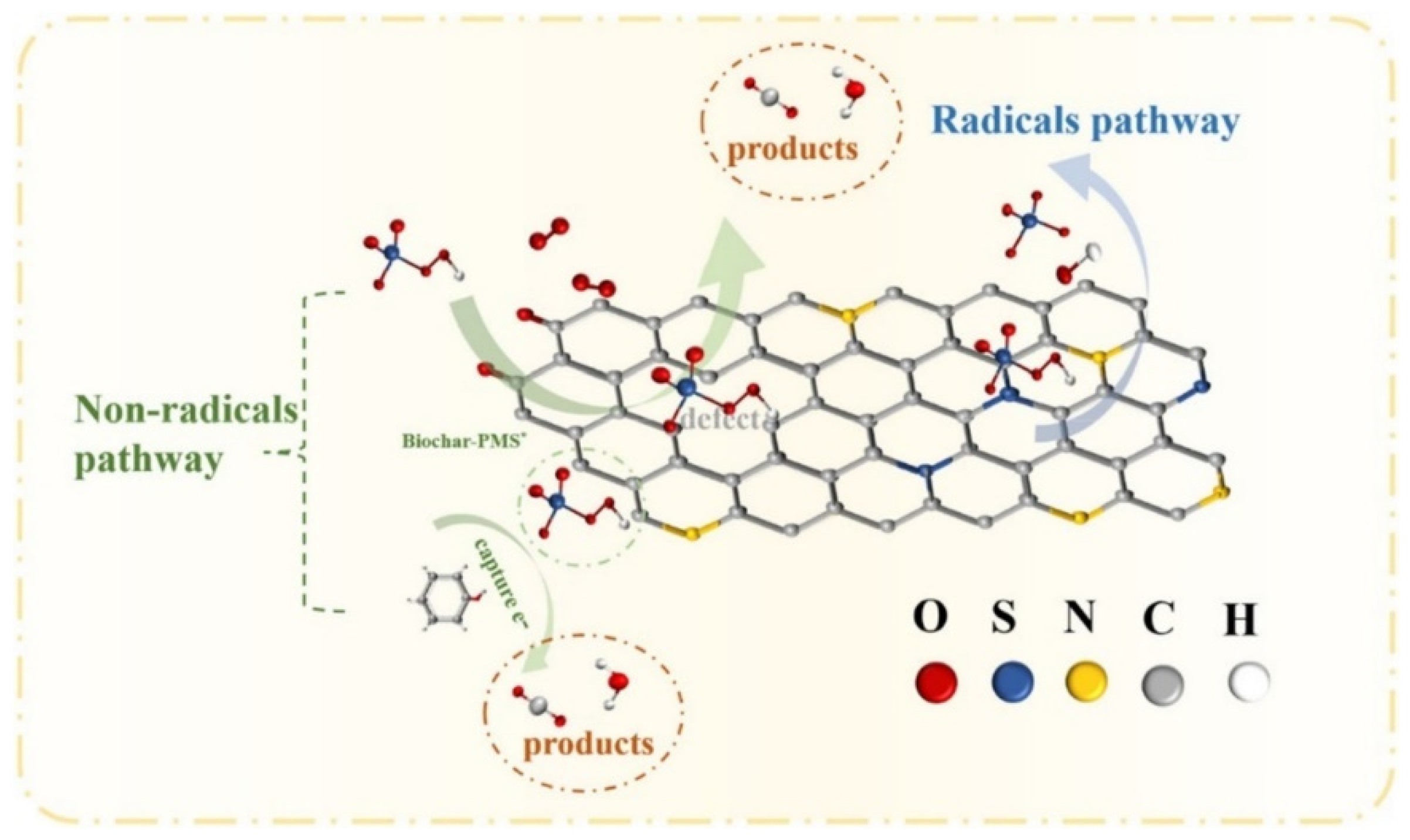
| BC-Based Catalytic Systems | Targeted Pollutants | Operational Parameters | Reduction Efficiency | Dominant Radicals | Ref. |
|---|---|---|---|---|---|
| Pine BC+PMS | Tetracycline | ICa = 20 mg/L; PMS dose = 3 mM; catalyst dosage = 3.0 g/L; pH = 7.0 | 90% | SO4•− and HO• | [104] |
| Pine needle BC+PMS | 1,4-dioxane | ICa = 20 μM; PMS dose = 8 mM; catalyst dosage = 1 g/L; pH = 6.5 | 84.2% | SO4•− and HO• | [77] |
| Corn cob BC+PDS | 2,4-dichlorophenol | ICa = 100 mg/L; PDS dose = 1 g/L; catalyst dosage = 0.2 g/L; pH = 6.0 | 86% | 1O2 | [105] |
| Soybean residue BC+PDS | Tetracycline hydrochloride | ICa = 50 mg/L; PDS dose = 1 mM; catalyst dosage = 0.2 g/L; pH = 7 | 84.1% | 1O2 | [59] |
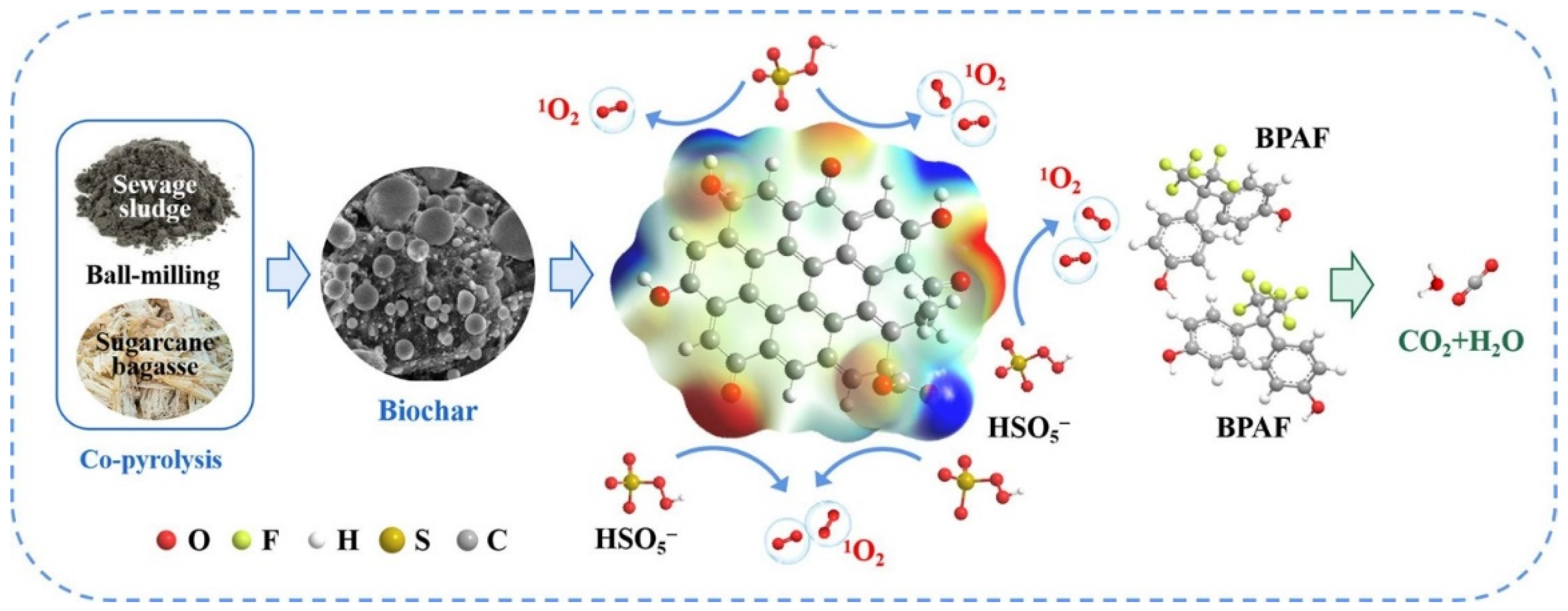
| Sludge-sugarcane bagasse BC+PMS | Bisphenol AF | ICa = 20 mg/L; PMS dose = 50 mg/L; catalyst dosage = 0.2 g/L; pH = 7 | 93.7% | 1O2 | [106] |
| Red mud BC+PMS | Sulfamethoxazole | ICa = 0.02 mM; PMS dose = 0.15 mM; catalyst dosage = 1.5 g/L; pH = 4.12 | 100% | 1O2 | [107] |
| Passion fruit shell BC+PMS | Tetracycline | ICa = 20 mg/L; PMS dose = 0.3 g/L; catalyst dosage = 0.4 g/L; pH = 5.4 | 90.9% | 1O2 | [108] |
| Pine need BC+PMS | Phenol | ICa = 10 mg/L; PMS dose = 3.0 mM; catalyst dosage = 0.2 g/L; pH = 5.2 | 100% | 1O2, HO• and SO4•− | [109] |
| Magnetic rape straw BC+PS | Tetracycline hydrochloride | ICa = 20 mg/L; PMS dose = 8 mM; catalyst dosage = 1 g/L; pH = 5.68 | 98% | SO4•− and 1O2 | [110] |
| CuO/Rice straw BC+PDS | Phenacetin | ICa = 10 mg/L; PDS dose = 50 mg/L; catalyst dosage = 0.3 g/L; pH = 4.26 | 100% | 1O2 and O2•− | [111] |
| nano Fe3O4/Leaves BC+PMS | Bisphenol a | ICa = 20 mg/L; PMS dose = 5 mM; catalyst dosage = 2 g/L; pH = 3 | 100% | SO4•− | [112] |
| Fe3O4/Porous BC+PMS | P-hydroxybenzoic acid | ICa = 10 mg/L; PMS dose = 1 g/L; catalyst dosage = 0.2 g/L; pH = 8.5 | 100% | SO4•− | [113] |
| Co-doped/Shrimp shell BC+PMS | Ciprofloxacin | ICa = 30 mg/L; PMS dose = 0.4 g/L; catalyst dosage = 0.15 g/L; pH = 6.8 | 89.5% | SO4•− and O2•− | [114] |
| Co-doped/Goat manure BC+PMS | Ciprofloxacin | ICa = 20 mg/L; PMS dose = 0.4 g/L; catalyst dosage = 0.1 g/L; pH = 6.3 | 96.5% | SO4•−, HO• and O2•− | [115] |
| Co3O4/Peanut shell BC+PMS | Ofloxacin | ICa = 20 mg/L; PMS dose = 1 mM; catalyst dosage = 0.8 g/L; pH = 7 | 97.3% | 1O2 and SO4•− | [116] |
| Ni-doped/Cherry core BC+PMS | Bisphenol-A | ICa = 20 mg/L; PMS dose = 1 g/L; catalyst dosage = 0.03 g/L; pH = 3 | 100% | SO4•−, HO• and 1O2 | [117] |
| Fe/Mn-doped/Sludge BC+PMS | Phenol | ICa = 0.32 mM; PMS dose = 4 mM; catalyst dosage = 0.5 g/L; pH = 9 | 100% | 1O2 | [18] |
| MnFe2O4/BC+PMS | Bisphenol-A | ICa = 20 mg/L; PMS dose = 0.2 g/L; catalyst dosage = 0.2 g/L; pH = 7.0 | 100% | O2•− and 1O2 | [118] |
| MgFe2O4/MgO/BC+PMS | Sulfamethoxazole | ICa = 20 mg/L; PMS dose = 1 mM; catalyst dosage = 0.4 g/L; pH = 5.6 | 100% | 1O2 | [119] |
| CoWO4/Co-doped/BC+PMS | Chlortetracycline | ICa = 20 mg/L; PMS dose = 0.3 mM; catalyst dosage = 0.03 g/L; pH = 5.2 | 100% | 1O2 | [120] |
| CoFe2O4/BC+PAA | Tetracycline hydrochloride | ICa = 10 mg/L; PDS dose = 0.6 mM; catalyst dosage = 1 g/L; pH = 5 | 96% | 1O2 | [121] |
| FeS/BC+PS | Tetracycline | ICa = 200 mg/L; PMS dose = 10 mM; catalyst dosage = 0.3 g/L; pH = 3.6 | 87.4% | SO4•− and HO• | [122] |
| N-doped/Boehmeria nivea BC+PMS | Tetracycline | ICa = 20 mg/L; PMS dose = 1 mM; catalyst dosage = 0.1 g/L; pH = 7.0 | 96.5% | 1O2 | [123] |
| N-doped/Magnetic BC+PMS | Sulfadiazine | ICa = 10 mg/L; PMS dose = 1 mM; catalyst dosage = 0.25 g/L; pH = 5.5 | 95.2% | SO4•− and HO• | [124] |
| BC-Based Catalytic Systems | Targeted Pollutants | Operational Parameters | Reduction Efficiency | Dominant Radicals | Ref. |
|---|---|---|---|---|---|
| TiO2/Chitosan BC+UV | Rhodamine B | ICa = 80 mg/L; catalyst dose = 0.5 g/L; power = 500 W; λ < 420 nm; t = 270 min | 100% | O2•− and HO• | [150] |
| TiO2/walnut shells BC+UV | Methyl orange | ICa = 20 mg/L; catalyst dose = 0.25 g/L; power = 500 W; λ = 360 nm; t = 150 min | 96.9% | HO• | [151] |
| N-doped/TiO2/BC+UV | Methyl orange | ICa = 20 mg/L; catalyst dose = 0.25 g/L; power = 500 W; λ = 360 nm; t = 90 min | 97.6% | HO• | [152] |
| g-C3N4/BC+UV | Enrofloxacin | ICa = 10 mg/L; catalyst dose = 1 g/L; power = 500 W; pH = 6.6, λ < 420 nm; t = 12 h | 81.1% | O2•− and holes (h+) | [153] |
| g-C3N4/Crawfish shell BC+visible light | Enrofloxacin | ICa = 10 mg/L; catalyst dose = 1 g/L; power = 500 W; pH = 7, λ > 420 nm; t = 8 h | 90% | O2•− | [154] |
| g-C3N4/Graphene-like BC+PMS+visible light | Tetracycline | ICa = 10 mg/L; catalyst dose = 0.2 g/L; PMS dose = 0.2 g/L.; pH = 5.45; t = 60 min | 90% | O2•− and 1O2 | [155] |
| K-doped/g-C3N4/BC+visible light | Naphthalen | ICa = 20 mg/L; catalyst dose = 0.5 g/L; power = 200 W; λ = 400~800 nm; t = 180 min | 82.2% | HO•, O2•− and h+ | [156] |
| S-doped/g-C3N4/BC+visible light | Tetracycline | ICa = 10 mg/L; catalyst dose = 1 g/L; λ > 420 nm; t = 60 min | 81.7% | O2•− and h+ | [157] |
| Fe/Cu/Sludge BC+PI+UV | Diclofenac sodium | ICa = 20 mg/L; PI dose = 5 mM; catalyst dose = 0.1 g/L; power = 60 W; pH = 6.9; λ = 254 nm; t = 60 min | 99.7% | IO3• | [143] |
| Zn-Co-LDH/BC+UV | gemifloxacin Gntibiotic | ICa = 15 mg/L; catalyst dose = 0.75 g/L; power = 10 W; pH = 5.5; t = 130 min | 92.7% | HO• | [158] |
| g-MoS2/Straw BC+visible light | Tetracycline hydrochloride | ICa = 20 mg/L; catalyst dose = 10 mg/L; power = 300 W; pH = 5; λ > 420 nm; t = 60 min | 90% | h+ and HO• | [159] |
| BiOBr/Lignin-BC+visible light | Rhodamine B | ICa = 30 mg/L; catalyst dose = 0.2 g/L; power = 300 W; λ > 420 nm; t = 60 min | 99.2% | O2•− and h+ | [160] |
| Bi2O2CO3/Rice husk BC+visible light | Tetracycline | ICa = 70 mg/L; catalyst dose = 0.6 g/L; power = 300 W, pH = 6.37; t = 60 min | 84.7% | 1O2, O2•− and h+ | [161] |
| SnS2/Tea leaves BC+LED light | Amoxi cillin | ICa = 20 mg/L; catalyst dose = 0.2 g/L; power = 23 W; pH = 5; t = 120 min | 93.7% | HO• | [57] |
| BC-Based Catalytic Systems | Targeted Pollutants | Operational Parameters | Reduction Efficiency | Dominant Radicals | Ref. |
|---|---|---|---|---|---|
| Rice husk BC | Propylparaben | ICa = 1 mg/L; catalyst dose = 125 mg/L; pH = 5.7, t = 45 min; power = 36 W | 78% | HO• | [183] |
| Chitosan/TiO2/Grapefruit BC | Ciprofloxacin | ICa = 10 mg/L; catalyst dose = 1 g/L; pH = 6.0, t = 25 min; power = 150 W | 85.23% | O2•−, h+ and HO• | [186] |
| ZnO nanorods/Wheat husks and paper sludge BC | Gemifloxacin | ICa = 20 mg/L; catalyst dose = 1.5 g/L; pH = 5.5, t = 45 min; power = 300 W | 96.10% | HO• | [187] |
| Fe-Cu-LDH/BC | Cefazolin sodium | ICa = 0.1 mM; catalyst dose = 1 g/L; pH = 6.5, t = 80 min; power = 300 W | 97.60% | HO• | [188] |
| Ag3PO4–Fe3O4/Bamboo BC | Bisphenol A | ICa = 10 mg/L; catalyst dose = 50 mg/L; pH = 7, t = 60 min; power = 177 W | 100% | HO• | [189] |
| BC-Based Catalytic Systems | Targeted Pollutants | Operational Parameters | Current/ Potential | Reduction Efficiency | Dominant Radicals | Ref. |
|---|---|---|---|---|---|---|
| Sludge BC | Methyl orange | ICa = 10 mg/L; t = 240 min; Na2SO4 dose = 0.1 mM; d = 2 cm | 0.05 A | 94.5% | HO• | [205] |
| Sludge BC+PMS | Imidacloprid | ICa = 0.5 mg/L; pH = 3.0, t = 60 min; PMS dose = 1 M, catalyst dose = 0.2 g/L | 25 V | 95.% | HO• and 1O2 | [206] |
| Lignin-derived BC | Dexamethasone | ICa = 25 mg/L; pH = 3, t = 120 min; Na2SO4 dose = 50 mM; d = 2.0 cm | 11.11 mA/cm2 | 100% | HO• | [207] |
| Banana-peel BC | Bromophenol blue | ICa = 10 mg/L; pH = 7, t = 60 min; Na2SO4 dose = 10 mM; d = 3.0 cm | 100 mA | 87.4% | / | [208] |
| Banana-peel BC+PS | Ibuprofen | ICa = 2 mg/L; pH = 3, t = 120 min; Na2SO4 dose = 3 mM; d = 3.0 cm | 250 mA | 100% | HO• and SO4•− | [209] |
| Nickel foam/Sludge BC+PS | Sulfamethazine | ICa = 50 mg/L; pH = 3.0, t = 60 min; PS dose = 10 mM; Na2SO4 dose = 40 mM; d = 2.0 cm | 50 mA | 100% | HO• and SO4•− | [210] |
| ZnCl2/BC | Phenol | ICa = 40 mg/L; pH = 3.0, t = 90 min; Na2SO4 dose = 0.1 M; d = 3 cm | −0.25 V | 100% | HO• | [211] |
| PTFE/Black carbon+PMS | Carbamazepine | ICa = 0.042 mM; pH = 3.0, t = 40 min; PMS dose = 50 mM; Na2SO4 dose = 0.1 M | 0.0286 A/cm2 | 97.6% | HO•, SO4•−, 1O2, and O2•− | [196] |
| Ti-Sn-Ce/Bamboo BC | Coking wastewater | ICa = 2 mg/L; pH = 7.9, t = 150 min; Na2SO4 dose = 3 mM; d = 2.0 cm | 30 mA/cm2 | 100% | HO• | [212] |
| N-doped/Kapok fiber BC | Sulfamethoxazole | ICa = 10 mg/L; pH = 5, t = 60 min; Na2SO4 dose = 50 mM | 11 mA/cm2 | 100% | HO• | [213] |
| N-doped/Coffee residues BC | Tetracycline | ICa = 100 mg/L; pH = 7, t = 120 min; Na2SO4 dose = 5 mM; d = 2.0 cm | 4 mA/cm2 | 70.4% | HO•, 1O2, and O2•− | [214] |
| Fe/N-doped/BC catalytic membrane | Tetracycline | ICa = 5 mg/L; pH = 7, t = 180 min; Na2SO4 dose = 20 mM | 0.01 mA/cm2 | 100% | HO• and O2•- | [215] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Liu, Z.; Huang, S.; Zhang, H.; Yang, H.; Liu, Y.; Zhang, K.; Zeng, Y. Application of Biochar-Based Materials for Effective Pollutant Removal in Wastewater Treatment. Nanomaterials 2024, 14, 1933. https://doi.org/10.3390/nano14231933
Han M, Liu Z, Huang S, Zhang H, Yang H, Liu Y, Zhang K, Zeng Y. Application of Biochar-Based Materials for Effective Pollutant Removal in Wastewater Treatment. Nanomaterials. 2024; 14(23):1933. https://doi.org/10.3390/nano14231933
Chicago/Turabian StyleHan, Meiyao, Ziyang Liu, Shiyue Huang, Huanxing Zhang, Huilin Yang, Yuan Liu, Ke Zhang, and Yusheng Zeng. 2024. "Application of Biochar-Based Materials for Effective Pollutant Removal in Wastewater Treatment" Nanomaterials 14, no. 23: 1933. https://doi.org/10.3390/nano14231933
APA StyleHan, M., Liu, Z., Huang, S., Zhang, H., Yang, H., Liu, Y., Zhang, K., & Zeng, Y. (2024). Application of Biochar-Based Materials for Effective Pollutant Removal in Wastewater Treatment. Nanomaterials, 14(23), 1933. https://doi.org/10.3390/nano14231933







