Liquid CO2-Capture Technologies: A Review
Abstract
:1. Introduction
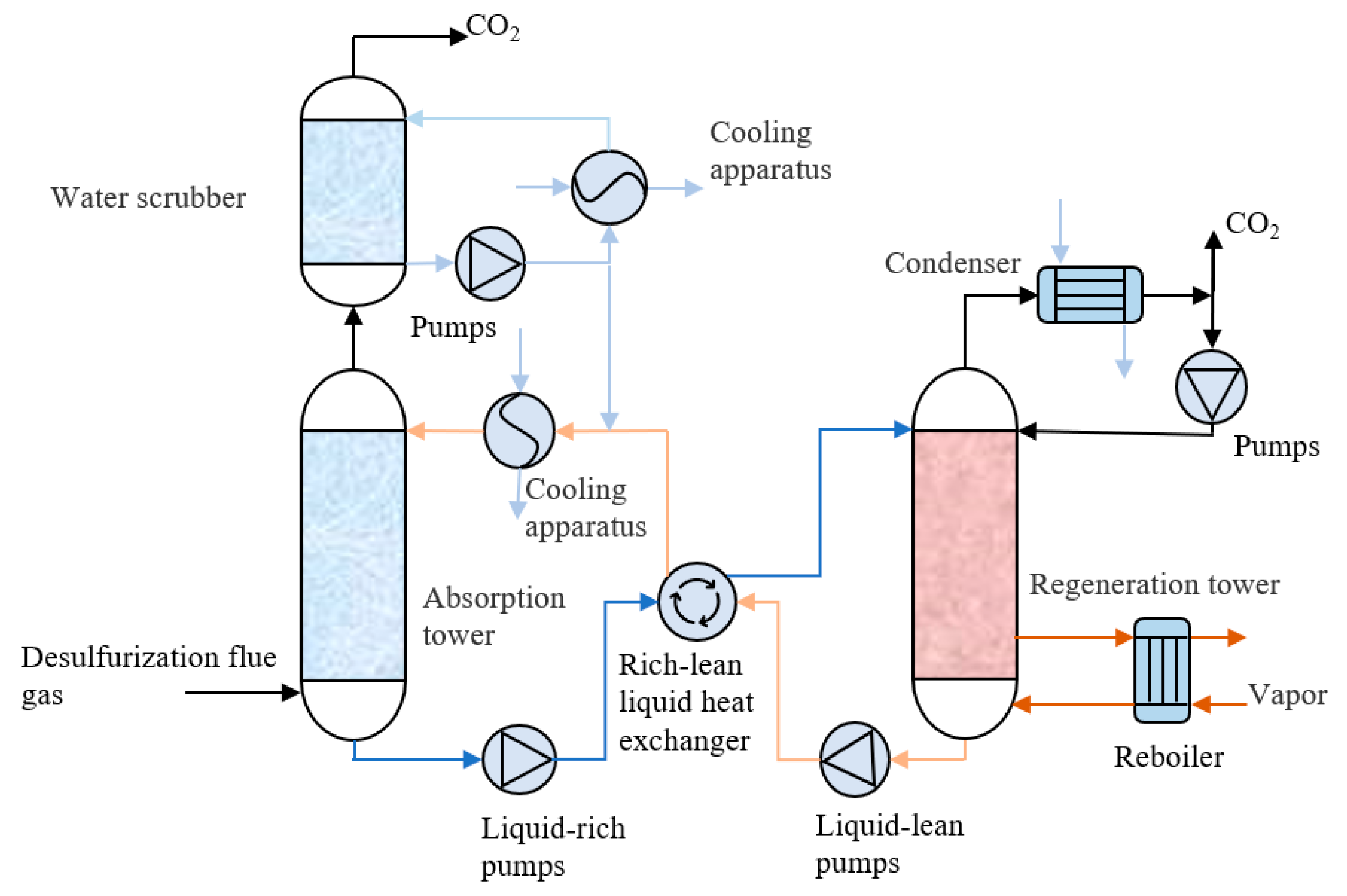
2. Conventional Amine Capture Technologies
2.1. Mixed Amine Solution Absorption Technique
2.2. Sterically Hindered Amine
3. New Liquid CO2 Absorbents and Techniques
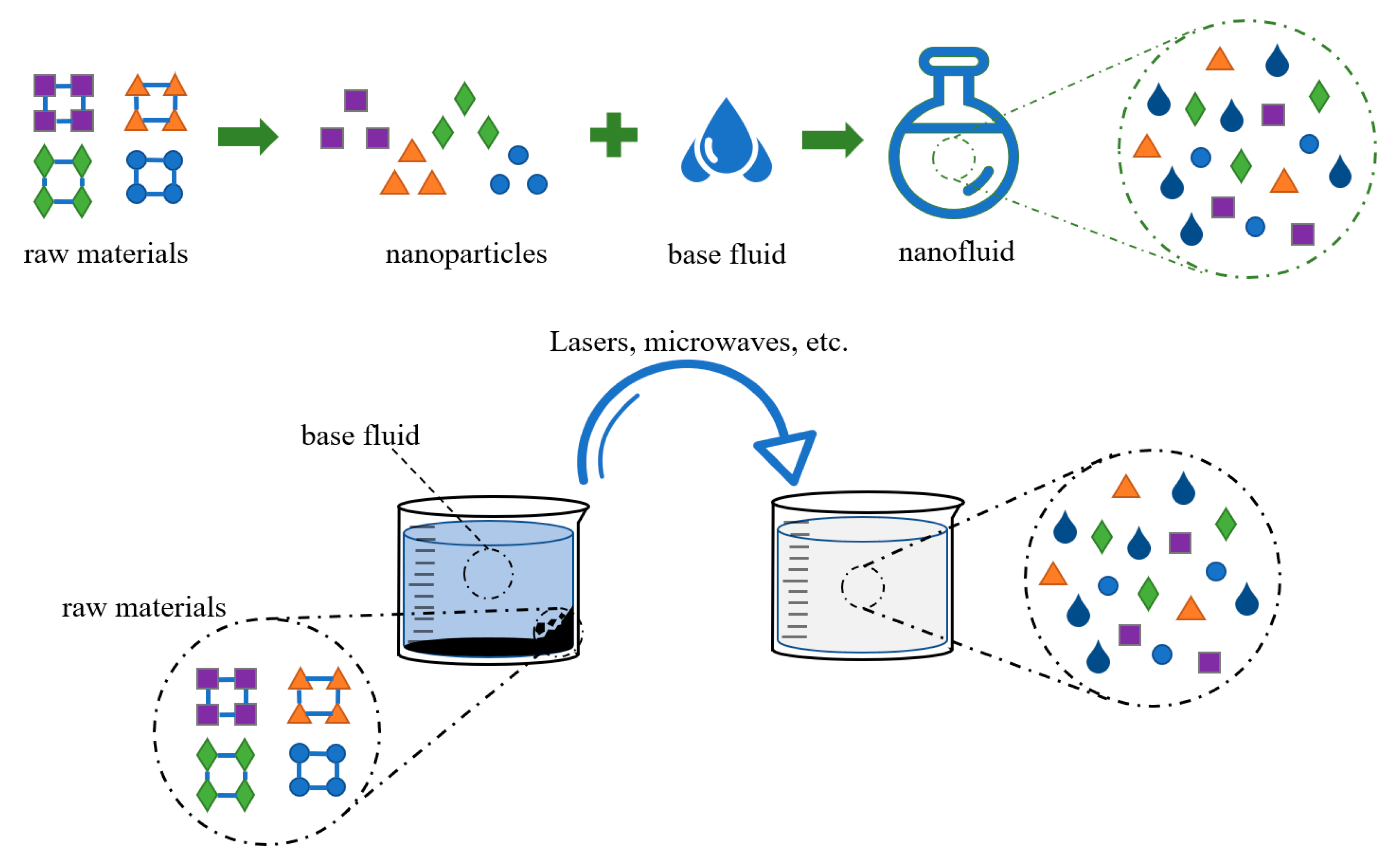
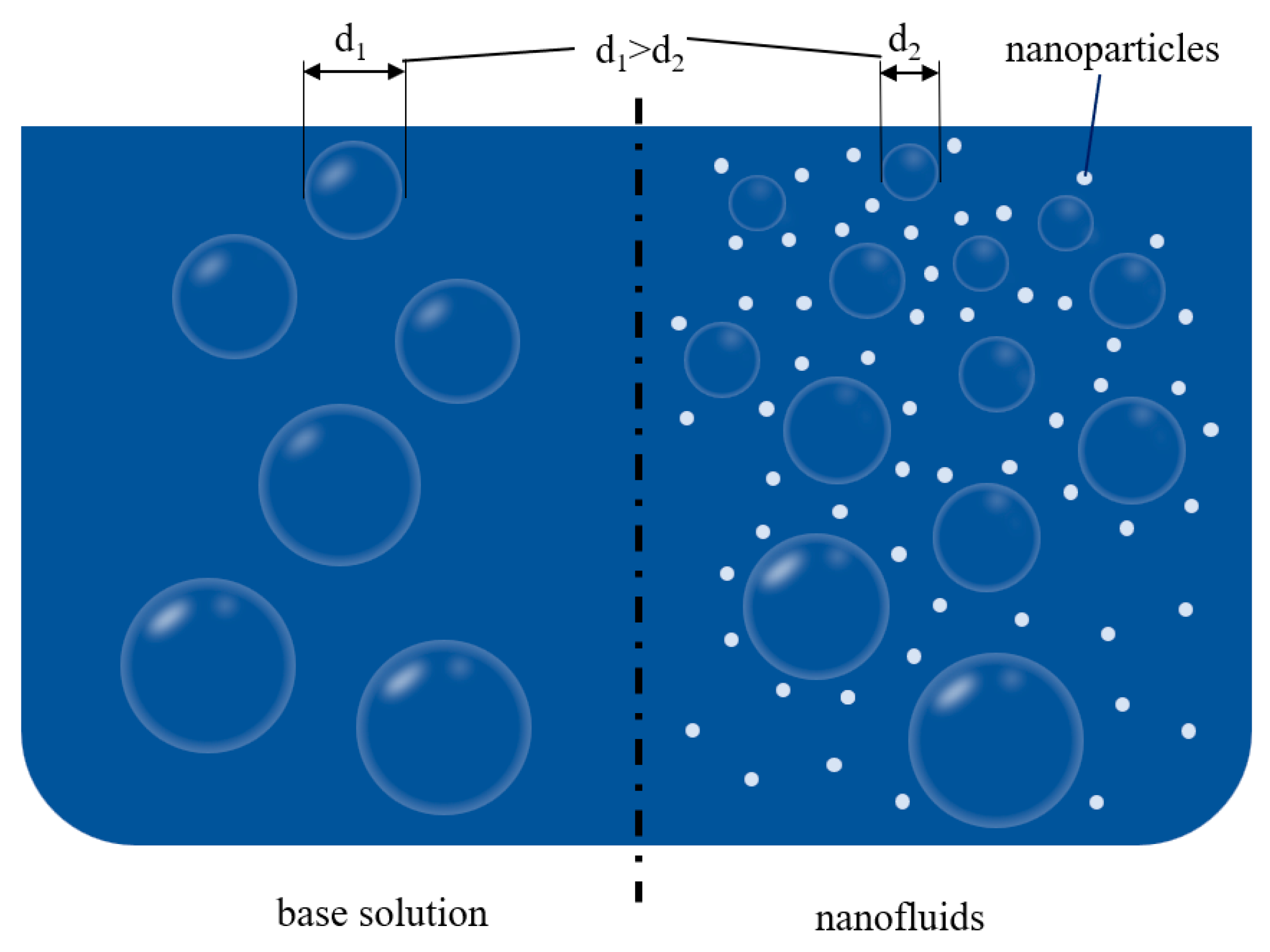
3.1. Nanofluid
3.1.1. Synthesis of Nanofluids
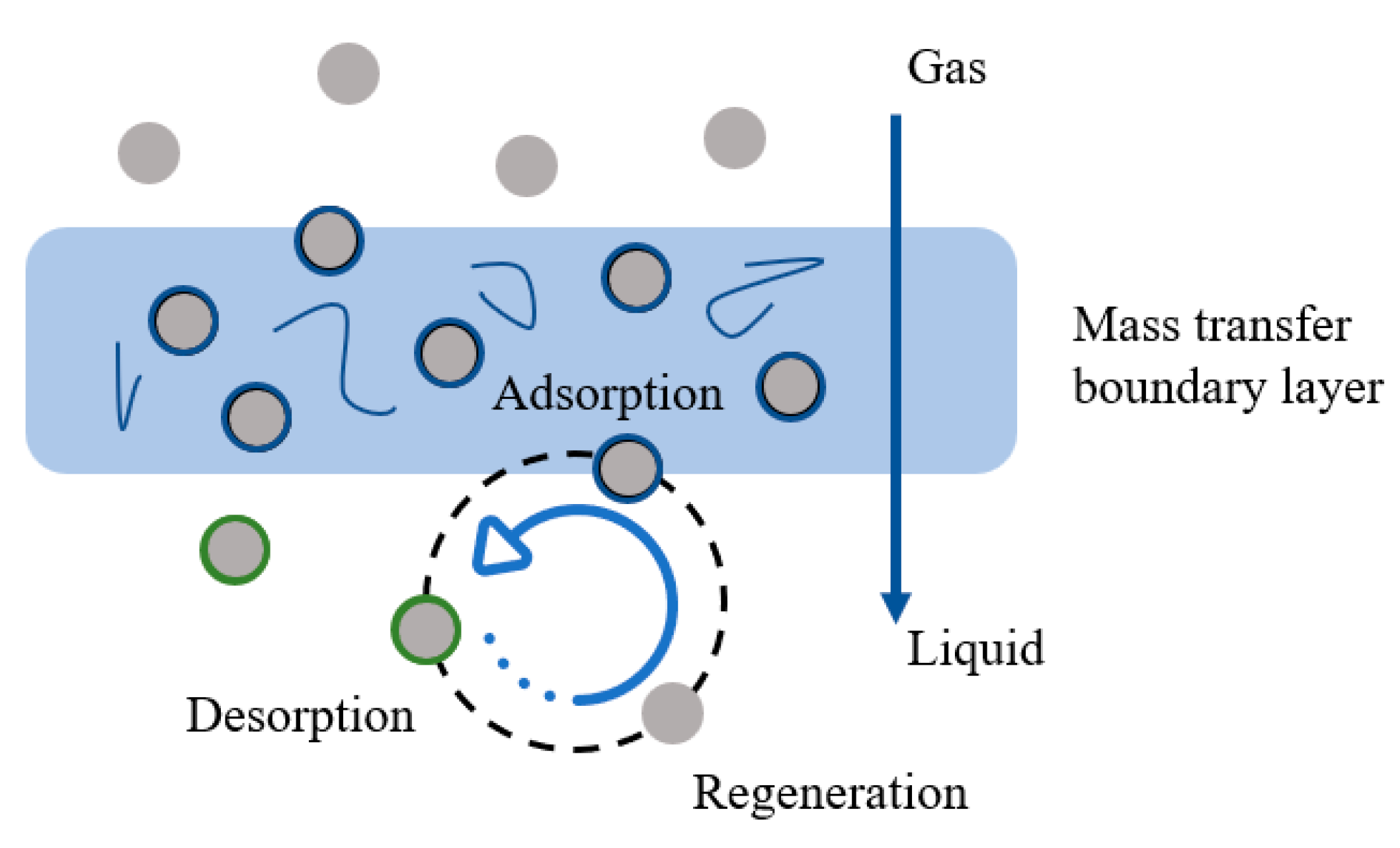
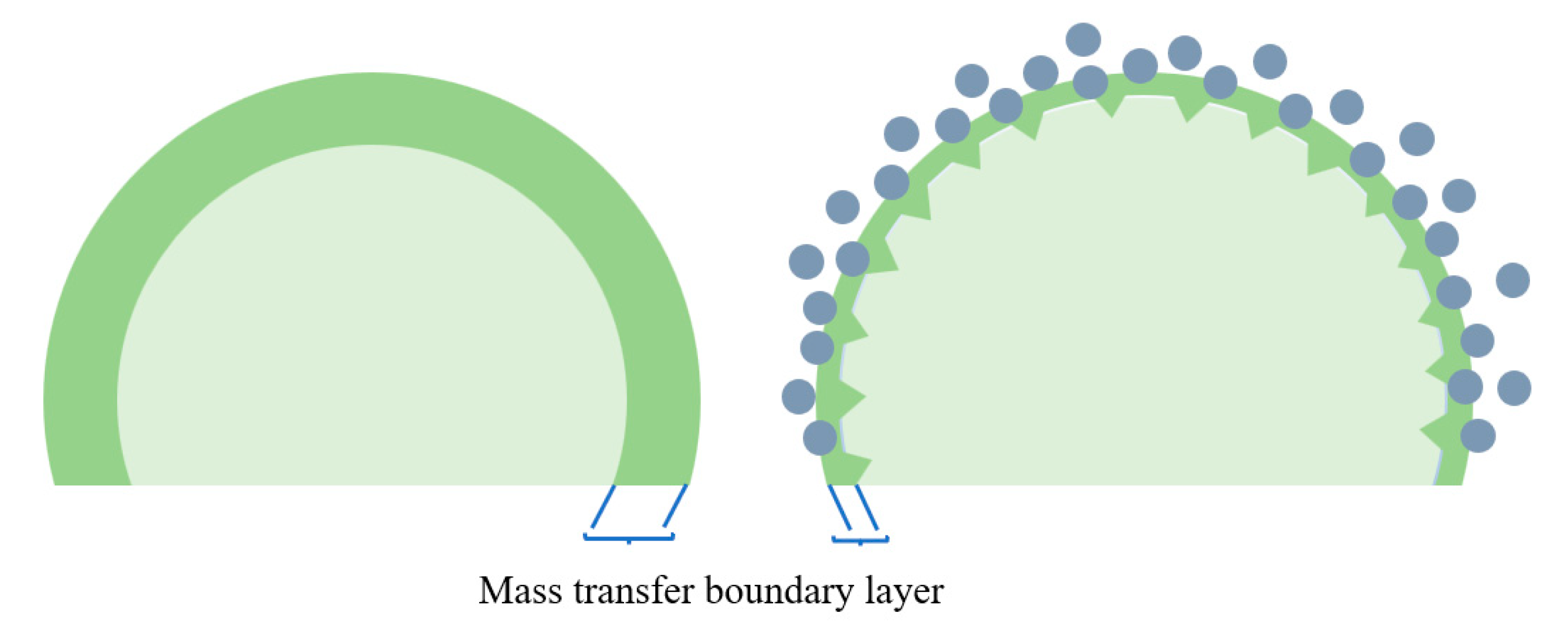
3.1.2. Mechanisms
3.1.3. Dispersion Stability of Nanofluids
3.1.4. Modification
3.1.5. Recycling
3.1.6. Negative Environmental and Health Impacts
3.2. Other New Liquid Absorbents and Techniques
3.2.1. Ionic Liquids
3.2.2. Amino Acids
3.2.3. Phase-Change Solvents
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, K.A.; Cong, P.T.; Thang, P.D.; Uyen, P.T.M.; Anwar, A.; Abbas, A. From brown to green: Are Asian economies on the right path? Assessing the role of green innovations and geopolitical risk on environmental quality. Environ. Sci. Pollut. Res. 2024, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, M.A.; Rauf, A.; Shakir, S.; Abbas, A.M.A.; Sun, H.P.; Abid, S. Exploring the Intertwined Nexus between Globalization, Energy Usage, Economic Complexity, and Environmental Quality in Emerging Asian Economies: A Pathway Towards a Greener Future. Environ. Sci. Pollut. Res. 2023, 30, 100431–100449. [Google Scholar] [CrossRef] [PubMed]
- Bottoms, R.R. Organic Bases for Gas Purification. Ind. Eng. Chem. 1931, 23, 501–504. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, Z.; Mahurin, S.M.; Dai, S.; Jiang, D.-E. Ionic liquids for carbon capture. MRS Bull. 2022, 47, 395–404. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, Y.; Wang, L.; Chen, J.; Lu, Y. Phase change solvents for post-combustion CO2 capture: Principle, advances, and challenges. Appl. Energy 2019, 239, 876–897. [Google Scholar] [CrossRef]
- Tavakoli, A.; Rahimi, K.; Saghandali, F.; Scott, J.; Lovell, E. Nanofluid preparation, stability and performance for CO2 absorption and desorption enhancement: A review. J. Environ. Manag. 2022, 313, 114955. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, W.; Li, X.; Zhao, Z.; Jiang, C.; Luo, C.; Wu, F.; Zhang, L. Novel assessment of highly efficient polyamines for post-combustion CO2 capture: Absorption heat, reaction rate, CO2 cyclic capacity, and phase change behavior. Sep. Purif. Technol. 2023, 306, 122615. [Google Scholar] [CrossRef]
- Dubey, A.; Arora, A. Advancements in carbon capture technologies: A review. J. Clean. Prod. 2022, 373, 133932. [Google Scholar] [CrossRef]
- Tarafdar, A.; Sowmya, G.; Yogeshwari, K.; Rattu, G.; Negi, T.; Awasthi, M.K.; Hoang, A.; Sindhu, R.; Sirohi, R. Environmental pollution mitigation through utilization of carbon dioxide by microalgae. Environ. Pollut. 2023, 328, 121623. [Google Scholar] [CrossRef]
- Lu, G.C.; Wang, Z.; Yue, Z.Y.; Wei, W.J.; Huang, Y.; Zhang, X.L.; Fan, X.F. Development of novel AMP-based absorbents for efficient CO2 capture with low energy consumption through modifying the electrostatic potential. Chem. Eng. J. 2023, 474, 145929. [Google Scholar] [CrossRef]
- Smerigan, A.; Uludag-Demirer, S.; Cutshaw, A.; Marks, A.; Liao, W. High-efficiency carbon dioxide capture using an algal amino acid salt solution. J. CO2 Util. 2023, 69, 102394. [Google Scholar] [CrossRef]
- Ma, D.; Zhu, C.; Fu, T.; Yuan, X.; Ma, Y. An effective hybrid solvent of MEA/DEEA for CO2 absorption and its mass transfer performance in microreactor. Sep. Purif. Technol. 2020, 242, 116795. [Google Scholar] [CrossRef]
- Lv, B.; Guo, B.; Zhou, Z.; Jing, G. Mechanisms of CO2 capture into monoethanolamine solution with different CO2 loading during the absorption/desorption processes. Environ. Sci. Technol. 2015, 49, 10728–10735. [Google Scholar] [CrossRef] [PubMed]
- Caplow, M. Kinetics of carbamate formation and breakdown. J. Am. Chem. Soc. 1968, 90, 6795–6803. [Google Scholar] [CrossRef]
- Gautam, A.; Mondal, M.K. Review of recent trends and various techniques for CO2 capture: Special emphasis on biphasic amine solvents. Fuel 2023, 334, 126616. [Google Scholar] [CrossRef]
- Xiang, J.; Wei, D.; Mao, W.; Liu, T.; Luo, Q.; Huang, Y.; Liang, Z.; Luo, X. Comprehensive kinetic study of carbon dioxide absorption in blended tertiary/secondary amine solutions: Experiments and simulations. Sep. Purif. Technol. 2024, 330, 125310. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, B.; Luo, Y.; Guo, K.; Wang, Z.; Liu, K.; Mei, X.; Liu, C. Mass transfer dynamics of single CO2 bubbles rising in monoethanolamine solutions: Experimental study and mathematical model. Chem. Eng. J. 2023, 465, 142761. [Google Scholar] [CrossRef]
- Gouedard, C.; Picq, D.; Launay, F.; Carrette, P.-L. Amine degradation in CO2 capture. I. A review. Int. J. Greenh. Gas Control 2012, 10, 244–270. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Wang, B.; Klemeš, J.J. A graphical approach for mixed ratio optimisation in the binary mixed amine solution. J. Environ. Manag. 2022, 311, 114779. [Google Scholar] [CrossRef]
- He, X.; He, H.; Barzagli, F.; Amer, M.W.; Li, C.E.; Zhang, R. Analysis of the energy consumption in solvent regeneration processes using binary amine blends for CO2 capture. Energy 2023, 270, 126903. [Google Scholar] [CrossRef]
- Chen, M.; Gao, H.; Sema, T.; Xiao, M.; Sun, Q.; Liang, Z. Study on the mechanism and kinetics of amine with steric hindrance absorbing CO2 in non-aqueous/aqueous solution. Sep. Purif. Technol. 2022, 303, 122202. [Google Scholar] [CrossRef]
- Ji, L.; Zheng, X.; Zhang, L.; Feng, L.; Li, K.; Yu, H.; Yan, S. Feasibility and mechanism of an amine-looping process for efficient CO2 mineralization using alkaline ashes. Chem. Eng. J. 2022, 430, 133118. [Google Scholar] [CrossRef]
- Zhang, R.; Luo, X.; Yang, Q.; Yu, H.; Puxty, G.; Liang, Z. Analysis for the speciation in CO2 loaded aqueous MEDA and MAPA solution using 13C NMR technology. Int. J. Greenh. Gas Control 2018, 71, 1–8. [Google Scholar] [CrossRef]
- Yoon, B.; Calabro, D.C.; Baugh, L.S.; Raman, S.; Hwang, G.S. Probing strong steric hindrance effects in aqueous alkanolamines for CO2 capture from first principles. J. Environ. Chem. Eng. 2022, 10, 108987. [Google Scholar] [CrossRef]
- Tagiuri, A.; Mohamedali, M.; Henni, A. Dissociation constant (pKa) and thermodynamic properties of some tertiary and cyclic amines from (298 to 333) K. J. Chem. Eng. Data 2016, 61, 247–254. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.; Lim, H.; Kang, J.H.; Park, H.S.; Park, J.; Song, H. Structural investigation of aqueous amine solutions for CO2 capture: CO2 loading, cyclic capacity, absorption–desorption rate, and pKa. J. Environ. Chem. Eng. 2024, 12, 112664. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y.; Sun, J.; Gu, Y.; Zhang, X.; Tang, Z. The process intensification of CO2 absorption in Hilbert fractal reactor fabricated by a 3D printer. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 481–492. [Google Scholar] [CrossRef]
- Saha, S.; Chakma, A. Separation of CO2 from gas mixtures with liquid membranes. Energy Convers. Manag. 1992, 33, 413–420. [Google Scholar] [CrossRef]
- Ye, B.; Jiang, J.; Zhou, Y.; Liu, J.; Wang, K. Technical and economic analysis of amine-based carbon capture and sequestration at coal-fired power plants. J. Clean. Prod. 2019, 222, 476–487. [Google Scholar] [CrossRef]
- Choi, S.U.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles; Argonne National Lab. (ANL): Argonne, IL, USA, 1995. [Google Scholar]
- Irani, V.; Maleki, A.; Tavasoli, A. CO2 absorption enhancement in graphene-oxide/MDEA nanofluid. J. Environ. Chem. Eng. 2019, 7, 102782. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Lu, T.; Lai, F. Experimental verification of the effects of three metal oxide nanoparticles on mass transfer at gas-liquid interface. J. Pet. Sci. Eng. 2022, 211, 110122. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Adenutsi, C.D.; Wang, C. An experimental study of the effect of three metallic oxide nanoparticles on oil-water relative permeability curves derived from the JBN and extended JBN methods. J. Pet. Sci. Eng. 2020, 192, 107257. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, N.; Kumar, A.; Thakur, V.K.; Kalia, S.; Arya, V.; Kumar, A.; Kumar, S.; Kyzas, G.Z. A critical review on the recent trends of photocatalytic, antibacterial, antioxidant and nanohybrid applications of anatase and rutile TiO2 nanoparticles. Sci. Total Environ. 2024, 914, 169815. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Chuc, M.C.; Ramos-Castillo, C.M.; Rodríguez-Pérez, M.; Ruiz-Gómez, M.Á.; Rodríguez-Gattorno, G.; Villanueva-Cab, J. Synergistic correlation in the colloidal properties of TiO2 nanoparticles and its impact on the photocatalytic activity. Inorganics 2022, 10, 125. [Google Scholar] [CrossRef]
- Darvanjooghi, M.H.K.; Esfahany, M.N.; Esmaeili-Faraj, S.H. Investigation of the effects of nanoparticle size on CO2 absorption by silica-water nanofluid. Sep. Purif. Technol. 2018, 195, 208–215. [Google Scholar] [CrossRef]
- Du, W.; Ma, J.; Wang, W.; Zhang, L. Surface-tension change of graphene-based water nanofluid and its effects on heat-transfer process. J. Mol. Liq. 2023, 392, 123457. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Luo, Y.; Guo, K.; Zheng, L.; Liu, C. A mathematical model for single CO2 bubble motion with mass transfer and surfactant adsorption/desorption in stagnant surfactant solutions. Sep. Purif. Technol. 2023, 308, 122888. [Google Scholar] [CrossRef]
- Lemaire, P.; Alenzi, A.; Lee, J.; Beckman, E.; Enick, R. Thickening CO2 with direct thickeners, CO2-in-Oil emulsions, or nanoparticle dispersions: Literature review and experimental validation. Energy Fuels 2021, 35, 8510–8540. [Google Scholar] [CrossRef]
- Salafi, T.; Zeming, K.K.; Zhang, Y. Advancements in microfluidics for nanoparticle separation. Lab Chip 2017, 17, 11–33. [Google Scholar] [CrossRef]
- Das, P.K.; Santra, A.K.; Ganguly, R.; Dash, S.K.; Muthusamy, S.; Sha, M.; Sadasivuni, K.K. An extensive review of preparation, stabilization, and application of single and hybrid nanofluids. J. Therm. Anal. Calorim. 2024, 149, 9523–9557. [Google Scholar] [CrossRef]
- Yu, W.; Wang, T.; Park, A.-H.A.; Fang, M. Review of liquid nano-absorbents for enhanced CO2 capture. Nanoscale 2019, 11, 17137–17156. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Kazi, S.N.; Chowdhury, Z.Z.; Johan, M.R.B.; Mehmood, S.; Soudagar, M.E.M.; Mujtaba, M.; Gul, M.; Ahmad, M.S. Heat transfer growth of sonochemically synthesized novel mixed metal oxide ZnO + Al2O3 + TiO2/DW based ternary hybrid nanofluids in a square flow conduit. Renew. Sustain. Energy Rev. 2021, 145, 111025. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996. [Google Scholar] [CrossRef] [PubMed]
- Mudidana, R.K.; Miditana, V.; Rambabu, V. Synthesis of nanofluids preparation—A review. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Brilman, D.W.F.; van Swaaij, W.P.M.; Versteeg, G. A one-dimensional instationary heterogeneous mass transfer model for gas absorption in multiphase systems. Chem. Eng. Process. Process Intensif. 1998, 37, 471–488. [Google Scholar] [CrossRef]
- Lee, W.; Xu, R.; Kim, S.; Park, J.H.; Kang, Y.T. Nanofluid and nanoemulsion absorbents for the enhancement of CO2 absorption performance. J. Clean. Prod. 2021, 291, 125848. [Google Scholar] [CrossRef]
- Feng, X.; Johnson, D.W. Mass transfer in SiO2 nanofluids: A case against purported nanoparticle convection effects. Int. J. Heat Mass Transf. 2012, 55, 3447–3453. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Xiong, M.; Gao, C.; Ren, H.; Ma, L. Process intensification in gas-liquid mass transfer by nanofluids: Mechanism and current status. J. Mol. Liq. 2022, 346, 118268. [Google Scholar] [CrossRef]
- Morán, J.; Yon, J.; Henry, C.; Kholghy, M.R. Approximating the van der Waals interaction potentials between agglomerates of nanoparticles. Adv. Powder Technol. 2023, 34, 104269. [Google Scholar] [CrossRef]
- Sharma, V.; Park, K.; Srinivasarao, M. Colloidal dispersion of gold nanorods: Historical background, optical properties, seed-mediated synthesis, shape separation and self-assembly. Mater. Sci. Eng. R Rep. 2009, 65, 1–38. [Google Scholar] [CrossRef]
- Wan, M.; Xu, B.; Shi, L.; Zheng, N.; Sun, Z. The dynamic stability of silicone oil-based MWCNT nanofluids under high-temperature, high-flux irradiation, and shear-flow conditions. Powder Technol. 2023, 424, 118508. [Google Scholar] [CrossRef]
- Kamalgharibi, M.; Hormozi, F.; Zamzamian, S.A.H.; Sarafraz, M. Experimental studies on the stability of CuO nanoparticles dispersed in different base fluids: Influence of stirring, sonication and surface active agents. Heat Mass Transf. 2016, 52, 55–62. [Google Scholar] [CrossRef]
- Li, F.; Li, L.; Zhong, G.; Zhai, Y.; Li, Z. Effects of ultrasonic time, size of aggregates and temperature on the stability and viscosity of Cu-ethylene glycol (EG) nanofluids. Int. J. Heat Mass Transf. 2019, 129, 278–286. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Yan, X.; Wang, X.; Feng, B. Investigation on viscosity of Fe3O4 nanofluid under magnetic field. Int. Commun. Heat Mass Transf. 2016, 72, 23–28. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Liang, X.; Xu, J.; Lee, C.; Liang, Q.; Tao, P.; Deng, T. Dispersion stability of thermal nanofluids. Prog. Nat. Sci. Mater. Int. 2017, 27, 531–542. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, P.C.; Chaudhuri, P. Stability of heat transfer nanofluids—A review. ChemBioEng Rev. 2018, 5, 312–333. [Google Scholar] [CrossRef]
- Irani, V.; Tavasoli, A.; Vahidi, M. Preparation of amine functionalized reduced graphene oxide/methyl diethanolamine nanofluid and its application for improving the CO2 and H2S absorption. J. Colloid Interface Sci. 2018, 527, 57–67. [Google Scholar] [CrossRef]
- Elhambakhsh, A.; Keshavarz, P. Investigation of carbon dioxide absorption using different functionalized Fe3O4 magnetic nanoparticles. Energy Fuels 2020, 34, 7198–7208. [Google Scholar] [CrossRef]
- Chen, Y.; Abed, A.M.; Raheem, A.B.F.; Altamimi, A.S.; Yasin, Y.; Sheekhoo, W.A.; Smaisim, G.F.; Ghabra, A.A.; Naseer, N.A. Current advancements towards the use of nanofluids in the reduction of CO2 emission to the atmosphere. J. Mol. Liq. 2023, 371, 121077. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.; Pineda, I.T.; Kang, Y.T. Review of nanoabsorbents for capture enhancement of CO2 and its industrial applications with design criteria. Renew. Sustain. Energy Rev. 2021, 138, 110524. [Google Scholar] [CrossRef]
- Arshadi, M.; Taghvaei, H.; Abdolmaleki, M.; Lee, M.; Eskandarloo, H.; Abbaspourrad, A. Carbon dioxide absorption in water/nanofluid by a symmetric amine-based nanodendritic adsorbent. Appl. Energy 2019, 242, 1562–1572. [Google Scholar] [CrossRef]
- Zhang, H.; Qing, S.; Gui, Q.; Zhang, X.; Zhang, A. Effects of surface modification and surfactants on stability and thermophysical properties of TiO2/water nanofluids. J. Mol. Liq. 2022, 349, 118098. [Google Scholar] [CrossRef]
- Han, X.; Yao, Y.; Zhao, X.; Huang, J.; Khosa, A.A. Investigations of stable surface-modified gold nanofluids optical filters based on optical optimization for photovoltaic/thermal systems. Sustain. Energy Technol. Assess. 2023, 57, 103203. [Google Scholar] [CrossRef]
- D’Addio, S.M.; Kafka, C.; Akbulut, M.; Beattie, P.; Saad, W.; Herrera, M.; Kennedy, M.T.; Prud’homme, R.K. Novel method for concentrating and drying polymeric nanoparticles: Hydrogen bonding coacervate precipitation. Mol. Pharm. 2010, 7, 557–564. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Nakamura, N.; Ohta, S. Centrifugal Field-Flow Fractionation Enables Detection of Slight Aggregation of Nanoparticles That Impacts Their Biomedical Applications. Anal. Chem. 2024, 96, 5976–5984. [Google Scholar] [CrossRef]
- Roca, M.; Pandya, N.H.; Nath, S.; Haes, A.J. Linear assembly of gold nanoparticle clusters via centrifugation. Langmuir 2010, 26, 2035–2041. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, Y.; Jia, F.; Song, S.; Li, Y. Recyclable Fe3O4@ Polydopamine (PDA) nanofluids for highly efficient solar evaporation. Green Energy Environ. 2022, 7, 35–42. [Google Scholar] [CrossRef]
- Simonsen, G.; Strand, M.; Øye, G. Potential applications of magnetic nanoparticles within separation in the petroleum industry. J. Pet. Sci. Eng. 2018, 165, 488–495. [Google Scholar] [CrossRef]
- Elsaid, K.; Olabi, A.; Wilberforce, T.; Abdelkareem, M.A.; Sayed, E.T. Environmental impacts of nanofluids: A review. Sci. Total Environ. 2021, 763, 144202. [Google Scholar] [CrossRef]
- Lourenço, M.J.; Alexandre, J.; Huisman, C.; Paredes, X.; Nieto de Castro, C. The balance between energy, environmental security, and technical performance: The regulatory challenge of nanofluids. Nanomaterials 2021, 11, 1871. [Google Scholar] [CrossRef]
- Elmobarak, W.F.; Almomani, F.; Tawalbeh, M.; Al-Othman, A.; Martis, R.; Rasool, K. Current status of CO2 capture with ionic liquids: Development and progress. Fuel 2023, 344, 128102. [Google Scholar] [CrossRef]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J. State-of-the-art of CO2 capture with ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 capture by a task-specific ionic liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ke, Q.; Zhang, Z.; Zhou, B.; Cui, G.; Lu, H. Tuning functionalized ionic liquids for CO2 capture. Int. J. Mol. Sci. 2022, 23, 11401. [Google Scholar] [CrossRef]
- Liu, F.; Shen, Y.; Shen, L.; Sun, C.; Chen, L.; Wang, Q.; Li, S.; Li, W. Novel amino-functionalized ionic liquid/organic solvent with low viscosity for CO2 capture. Environ. Sci. Technol. 2020, 54, 3520–3529. [Google Scholar] [CrossRef]
- Qu, Y.; Zhao, Y.; Li, D.; Sun, J. Task-specific ionic liquids for carbon dioxide absorption and conversion into value-added products. Curr. Opin. Green Sustain. Chem. 2022, 34, 100599. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Zhang, W.; Wang, J.; Soltanian, M.R.; Olabi, A.G. Effectiveness of amino acid salt solutions in capturing CO2: A review. Renew. Sustain. Energy Rev. 2018, 98, 179–188. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, S.; Bian, Y.; Yang, Y.-n.; Ghosh, U. CO2 solubility in aqueous potassium lysinate solutions at absorber conditions. J. Chem. Thermodyn. 2017, 111, 100–105. [Google Scholar] [CrossRef]
- Ramezani, R.; Mazinani, S.; Di Felice, R. State-of-the-art of CO2 capture with amino acid salt solutions. Rev. Chem. Eng. 2022, 38, 273–299. [Google Scholar] [CrossRef]
- Hu, G.; Smith, K.H.; Wu, Y.; Mumford, K.A.; Kentish, S.E.; Stevens, G.W. Carbon dioxide capture by solvent absorption using amino acids: A review. Chin. J. Chem. Eng. 2018, 26, 2229–2237. [Google Scholar] [CrossRef]
- Erga, O.; Juliussen, O.; Lidal, H. Carbon dioxide recovery by means of aqueous amines. Energy Convers. Manag. 1995, 36, 387–392. [Google Scholar] [CrossRef]
- Hu, L. CO2 Capture from Flue Gas by Phase Transitional Absorption; Hampton University: Hampton, VA, USA, 2009. [Google Scholar]
- Shen, S.; Shi, X.; Li, C.; Guo, H.; Long, Q.; Wang, S.; Yin, X. Nonaqueous (amine+ glycol ether) solvents for energy-efficient CO2 capture: New insights into phase change behaviors and assessment of capture performance. Sep. Purif. Technol. 2022, 300, 121908. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.S. Energy analysis of an absorption-based CO2 capture process. Int. J. Greenh. Gas Control 2017, 56, 250–260. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zhang, H.; Li, T.; Deng, T.; Zou, H.; Li, Y.; Yang, D. Liquid CO2-Capture Technologies: A Review. Nanomaterials 2024, 14, 1910. https://doi.org/10.3390/nano14231910
Zhu J, Zhang H, Li T, Deng T, Zou H, Li Y, Yang D. Liquid CO2-Capture Technologies: A Review. Nanomaterials. 2024; 14(23):1910. https://doi.org/10.3390/nano14231910
Chicago/Turabian StyleZhu, Jie, Haokun Zhang, Tingting Li, Tingting Deng, Hao Zou, Yongqi Li, and Dingyu Yang. 2024. "Liquid CO2-Capture Technologies: A Review" Nanomaterials 14, no. 23: 1910. https://doi.org/10.3390/nano14231910
APA StyleZhu, J., Zhang, H., Li, T., Deng, T., Zou, H., Li, Y., & Yang, D. (2024). Liquid CO2-Capture Technologies: A Review. Nanomaterials, 14(23), 1910. https://doi.org/10.3390/nano14231910




