Abstract
Escalating global carbon dioxide (CO2) emissions have significantly exacerbated the climate impact, necessitating imperative advancements in CO2-capture technology. Liquid absorbents have received considerable attention in carbon capture for engineering applications, due to their high flexibility, reliability, and recyclability. Nonetheless, the existing technologies of liquid CO2 capture suffer from various issues that cannot be ignored, such as corrosion, elevated costs, and pronounced secondary pollution. More efforts are required to realize process optimization and novel absorbent innovation. This review presents nanofluids and other novel liquid absorbents such as ionic liquids, amino acids, and phase-change absorbents. The preparation, mechanisms of action, and influencing factors of nanofluid absorbents are discussed in detail to provide researchers with a comprehensive understanding of their potential applications. Further, the challenges (including energy loss, environmental and human health, barriers to application and capture performance, etc.) encountered by these innovative absorbents and techniques are also commented on. This facilitates side-by-side comparisons by researchers.
1. Introduction
With the advancement of industrial innovation and the intensification of global energy consumption, the rise in carbon dioxide emissions across various industries has heightened concerns about climate change and resource reserves. According to the “CO2 Emissions in 2022” report published by the International Energy Agency (IEA), global energy-related CO2 emissions surpassed 3.68 billion tons in 2022, marking a 0.9% increase compared to 2021. Furthermore, emissions from emerging markets and developing economies in Asia (excluding China) witnessed a notable increase of 4.2% [1,2]. In the face of this substantial growth, various carbon capture and storage (CCS) technologies have become available for decreasing CO2 emissions to mitigate global warming and secondary climate issues.
Since the 1930s, the utilization of monoethanolamine (MEA) has been documented for separating acidic gases [3] Contemporary research studies have expanded the number of liquid CO2-capture options, such as ionic liquids [4], phase-change solvents [5], and nanofluids [6]. However, the application of liquid CO2-capture technologies has given rise to associated challenges, including the suboptimal absorption efficiency of absorbents [7], heightened energy consumption [8] during the regeneration process, and a substantial environmental impact [9] linked to absorbent contamination. Extensive research efforts have been devoted to these challenges. Lu et al. [10] introduced polar solvents (DMF/DMSO/NMF) into the AMP (2-amino-2-methyl-1-propanol)-ethylene glycol system. This formulation enhanced the absorption capacity of the absorbent by 28.4% compared to the AMP aqueous solution. Smerigan et al. [11] developed a new highly efficient bio-based CO2 absorbent, which is composed of microalgal amino acid salts, thereby having an extremely low environmental impact.
With the rapid development of nanocatalytic materials and supramolecular chemistry, nanofluids have been invented and gradually become one of the potentially absorbents with liquid-phase mass-transfer enhancement. The main focus of this paper is to summarize the preparation of nanofluids, the enhancement mechanisms in gas–liquid mass transfer, and recovery methods, while also discussing the development trends of nanofluids in the field of carbon dioxide capture. Additionally, other absorbents such as ionic liquids, phase-change solvents, and amino acid salt solutions are briefly introduced.
2. Conventional Amine Capture Technologies
The MEA solution is one of the most traditional and mature CO2 absorbents. In the post-combustion capture of the power plant, the MEA-based CO2 technological process is shown in Figure 1. MEA embraces many advantages as an absorbent, mainly including the high theoretical reaction ratio and its multifunctionality for trapping a variety of pollutants [12].
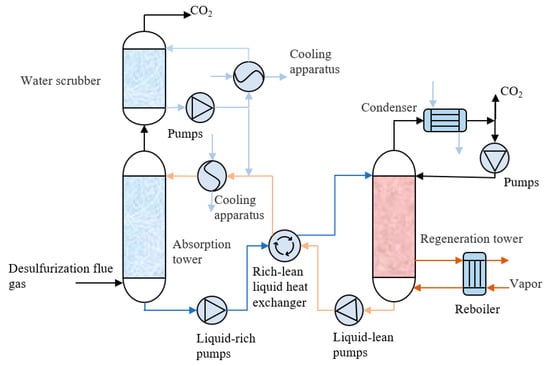
Figure 1.
Process flow diagram of an amine absorption process.
As far as we know, the overall reaction of CO2 with aqueous MEA can be expressed as follows [13]:
Caplow’s seminal work [14] verifies a theory that the most probable early event in the capture from aqueous MEA is the formation of a zwitterion. This theory is in accordance with the analyses of the kinetics of the reactions [15,16,17]. Moreover, the carbamate is the prevailing product of the CO2 interaction with the MEA solution, the decomposition of which requires considerable energy. In summary, the two stages of reactions implied in the zwitterion mechanism can be written as follows [18]:
where B represents the alkali component.
2.1. Mixed Amine Solution Absorption Technique
The absorption method using mixed amine solutions has been investigated as a superior alternative to single-component amine solutions in the CO2-capture process, aimed at overcoming their limitations and enhancing capture efficiency. Commonly used improvement methods usually refer to mixing a primary or secondary amine with a tertiary or sterically hindered amine [19] to give the absorbent the properties of the above components. Thus, the mixed solution possesses both a high CO2-absorption capacity and an excellent regeneration effect.
For example, He et al. [20] compared four amine-based blends and found that the blend of AMP (2-amino-2-methyl-1-propanol) with 2DMA2M1P (N,N-dimethylacetamide-2-methyl-1-propanamine) exhibited a higher desorption rate and lower energy consumption. BZA (benzylamine) + 2DMA2M1P showed an excellent absorption rate, attributed to AMP and BZA having the highest equilibrium solubility and second-order reaction rate among them. These studies demonstrated that mixed amines represented by AMP have an unstable steric hindrance, which inhibits the formation of carbamate and thus promotes the reaction [21]. Ge et al. [7] investigated seven different polyamines containing varying numbers of secondary amine and primary amine groups. They directly measured the heat of CO2 absorption using a high-precision microcalorimeter and compared their cyclic capacities to evaluate the CO2 cyclic capture times, absorption rate, and desorption rate. The experiment result showed that (1) the higher proportion of primary amino functional groups in the molecule, the higher the CO2 absorption heat; and (2) an increase in the proportion of secondary amino functional groups in the molecule causes a quicker CO2 desorption rate and encourages more complete desorption progress. This conclusion on exploring the factors influencing the heat of absorption can help to reduce the cost of mixed amine applications.
2.2. Sterically Hindered Amine
The amine absorption capacity is influenced by the spatial structure of the amine molecule. The initial reaction between the sterically hindered amine and CO2 still follows the zwitterionic theory; however, the steric hindrance renders the resulting carbamate unstable, leading to its rapid hydrolysis into bicarbonate and free amine. Furthermore, the free amine produced from this hydrolysis can continuously react with carbon dioxide, facilitating ongoing CO2 consumption [22,23]. This dynamic process highlights that the interplay between steric effects and reaction stability is important in the context of carbon capture or related applications. Sterically hindered amines [24] and cyclic amines [25] are widely studied in experimental environments. In Kim et al. [26], the effects of the molecular structure of amine on CO2 loading, cyclic capacity, the absorption–desorption rate, and pKa were investigated, and promising candidates for CO2 capture, along with an amine blending strategy, were proposed. The general formula for the reaction is as follows:
3. New Liquid CO2 Absorbents and Techniques
Amine absorbents have a high absorption capacity, while some disadvantages limit their development, such as high energy consumption during regeneration, corrosiveness, and degradation. Researchers have carried out various optimizations for amine-based solvents in the CO2-capture process, including fractal reactors [27] and the use of liquid membranes [28] for absorption. However, a study reported that the CO2-capture cost for a newly constructed power plant is CNY 150 per tonne, whereas, for an existing CCS retrofitted power plant, this cost ranges from CNY 162 to 185 per tonne [29]. Large-scale industrial equipment optimization is clearly not cost-effective. In order to promote industrial applications, it is necessary to develop new absorbers with better performance than amine-based absorbents, which include amine-based solutions, ionic liquid, amino acid salt, and phase-change absorbents. In comparison with the previous items, nanofluid is a better choice. Nanofluids offer a promising solution by enhancing the liquid-phase mass-transfer process without requiring modifications to existing facilities. Adding nanoparticles to solvents could improve CO2 absorption performance and reduce energy requirements.
3.1. Nanofluid
Choi first introduced the concept of nanofluids at the Argonne National Laboratory in the United States [30]. The nanofluid was initially applied in the field of heat transfer and applied to gas-separation applications after the early 21st century. Adding solid nanoparticles of the third dispersed phase into the absorbents is an important method used to enhance gas-separation efficiency. This way is also helpful in enhancing capture performance and reducing the vapor pressure of the absorbent. According to the research by Irani et al. [31], it was shown that a 9.1% increase in CO2 solubility was achieved via adding 0.1% graphene oxide (GO) into a 40% solution of N-methyl diethanolamine (MDEA), with no significantly higher absorption capacity when 0.2 wt. % GO was added to solutions than at the 0.1% addition level. The solubility of CO2 increases because the oxygen-containing groups on GO provide a wide range of reaction sites and interlayer space for gas adsorption. Irani stated that this enhancement has a reverse and direct relationship with increasing temperature and pressure. Some research has demonstrated different improvements in mass-transfer effects with the use of different types of the third dispersed phase [32]. The enhancing effect of TiO2 is more prominent in SiO2, Al2O3, and TiO2 [33]. Under certain conditions, the surface properties [34] and photocatalytic characteristics [35] of TiO2 may enhance the mass-transfer pathways at the gas–liquid interface. Another important factor is the size of the nanoparticles. Darvanjooghi et al. [36] investigated the effects of nanoparticle size on carbon dioxide (CO2) absorption in silica/water nanofluid by use of a bubble column absorption system, and the results showed that the surface renewal rate increased with the decreasing of nanoparticle size. However, nanoparticles of too small a size may have other negative effects, such as dispersion defects.
Some difficulties also hinder the development of nanofluid applications. The poor dispersion stability of nanofluids has been considered a long-existing issue that limits their further development and practical application. A prevalent strategy to mitigate this issue involves the introduction of dispersants and surfactants. The use of surfactants can improve the charge or functional groups on the surface of nanoparticles. However, it is imperative to recognize that the presence of dispersants and surfactants may influence the mass-transfer coefficient and surface tension of the liquid phase in gas–liquid mass-transfer processes [37,38]. Furthermore, these dispersants and surfactants may pose a threat to the environment [39] and increase the cost of manufacturing nanofluids.
On the other hand, the recovery of nanoparticles from nanofluids is an inevitable practice to reduce production costs. Nanoparticles have intrinsic properties, such as particle size, density, magnetic properties, electric properties, and aggregation tendency. These properties could be used to fractionate nanoparticles using external fields, such as centrifugal force or an electric field. These two external forces have been commonly applied for nanoparticle sorting, namely ultracentrifugation and gel electrophoresis [40]. The following text will provide a detailed introduction to the preparation methods, enhanced gas–liquid mass-transfer mechanisms, stability, recovery methods, and modification of nanofluids.
3.1.1. Synthesis of Nanofluids
The preparation process of nanofluids exerts a significant influence on the agglomeration of particles and other pertinent properties [41]. To our knowledge, nanofluids are produced by the uniform dispersion of solid nanoparticles into different base fluids. The synthesis of nanofluids can be divided into two methods, namely the single-step method and the two-step method, as presented in Figure 2.
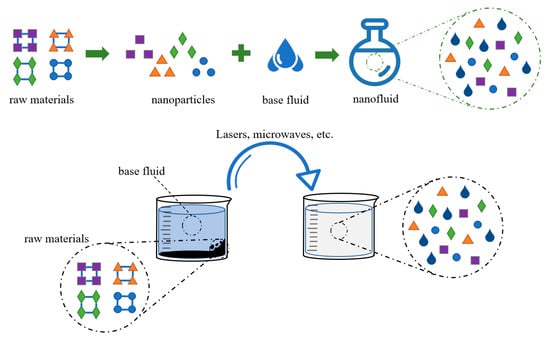
Figure 2.
Single-step technique and two-step technique.
In the two-step technique, the steps of preparing granules and dispersing granules are split up. Many researchers have prepared nanofluids with this method due to its simplicity and suitability [42]. However, aggregation and clustering will inevitably occur due to the high surface energy of the nanoparticles. The issue of particle dispersion and reduction in agglomeration in nanoparticles can be effectively addressed through the application of physical or chemical methods. For instance, a three-component ZnO+Al2O3+TiO2/water-based composite nanofluid was prepared by Ahmed et al. [43] using a two-step process and ultrasonic dispersion. The use of ultrasound to uniformly disperse nanoparticles into the liquid phase is a simple and reliable physical method commonly used in laboratories. Some researchers have synthesized silver nanoparticles of different sizes by changing temperature conditions in the preparation process [44] and summarized the law that particle size increases with increasing temperature. This law helps the research process of synthesizing nanoparticles of specified sizes.
In the one-step method, nanoparticles are produced and suspended in a base fluid directly to avoid drying and aid in the storage, transportation, and dispersion of nanoparticles. Thus, the purity of the nanoparticles and the stability of fluids are guaranteed. Aberoumand et al. [45] prepared nanofluid by a one-step method of the electrical explosion of wire (EEW), and this nanofluid could remain stable for several months. The method generates nanoscale particles or droplets by spraying or atomizing a liquid under a high electric field strength. It has good generalizability and environmental friendliness. Under normal circumstances, the purity and stability of nanofluids prepared by the one-step method are better than those prepared by the two-step method.
3.1.2. Mechanisms
An exact comprehensive mechanism for the enhancement of mass transfer by nanoparticles has not yet been established. Studies have proposed three major models, which are widely accepted by the scientific community.
(1) Shuttle mechanism: The schematic diagram is presented in Figure 3. Nanoparticles adsorb a certain amount of mass-transfer components. Subsequently, the gas adsorbed by the nanoparticles enters the liquid phase. Under the influence of the concentration gradient around the components, significant desorption phenomena occur in the vicinity of the nanoparticles. This results in nanoparticle repositioning and re-exposure to the vapor-phase components. The effect of the shuttle phenomenon can be characterized by measuring the diffusion coefficient (Dco2) within the liquid phase [46].
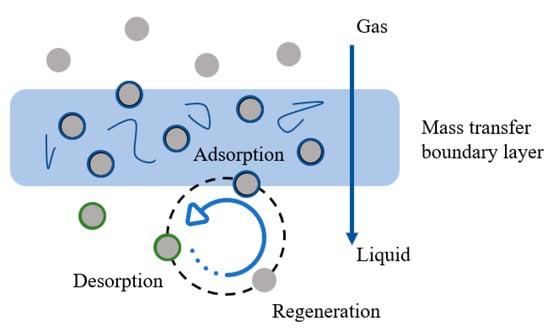
Figure 3.
Schematic diagram of the shuttle mechanism.
(2) Fluid dynamics mechanism: Brownian motion and micro-convection are the foundation of the hydrodynamic mechanism. According to the two-film theory, the primary resistances in gas–liquid mass transfer encompass gas film resistance, interfacial resistance, and liquid film resistance. These three kinds of resistance are all impacted by Brownian motion in the gas–liquid interface, micro convections, and velocity disturbances caused by nanoparticles. The schematic diagram is shown in Figure 4, the liquid-phase mass-transfer resistance can be weakened due to the reduction in the thickness of the boundary layer. The hydrodynamic effect in CO2 absorption was demonstrated by Lee et al. [47] using the shadowgraph method. In the visualization results at 8 s, the plume reconstituted more actively in the nanofluids as compared with pure methanol. The diffusion coefficient was calculated from the visualized test results, and the result indicates that fluid dynamic effects can enhance the mass transfer of nanofluids. However, some researchers disagree with the mechanism. The impact of SiO2 nanoparticles on the mass transfer of O2 and NaCl was investigated by Feng et al. [48]. It was observed that in the presence of nanoparticles, mass-transfer enhancement did not occur. The authors concluded that the promotion of mass transfer by nanoparticles through Brownian motion and microscale convective effects was not evident.
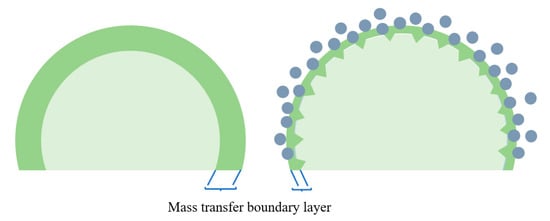
Figure 4.
Schematic diagram of the hydrodynamic effect.
(3) Mechanisms of bubble aggregation inhibition: This mechanism is commonly used to explain the phenomenon of compositional exchange of bubbles in liquids. The schematic diagram is shown in Figure 5. The mass transfer between gas and liquid is highly pronounced around bubbles in the liquid phase, while the bubbles in nanofluids have smaller volumes and shorter lifetimes than those in ordinary liquids. The main reason for this type of phenomenon is that nanoparticles attach to the surface of large bubbles and collide with each other, causing the bubbles to burst, and this behavior of nanoparticles is called the ultra-small size effect. There are five main physical properties affecting the behavior of nanoparticles, including the surface tension of the solution, the density difference between particle and solution, the solution viscosity, the solid particle size, and the hydrophobicity of particles. More importantly, the Laplace–Young equation explains the cause of bubble rupture in terms of size; bubble pressure is directly proportional to bubble size. The Kelvin equation explains the relationship between the internal pressure and solubility of the bubble and relates it to the force of mass transfer [49]. This equation explains the phenomenon that the solubility of the gas increases with an increase in the bubble’s internal pressure, thus leading to an increase in the driving force for mass transfer.
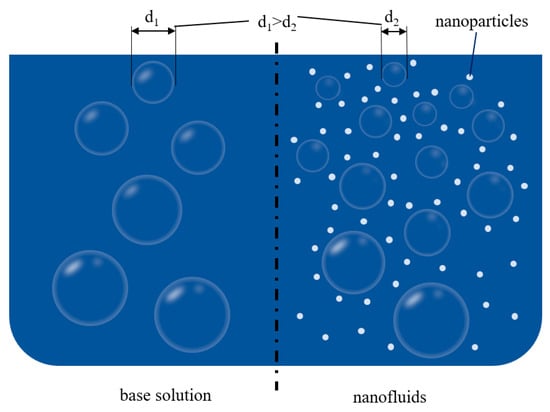
Figure 5.
Schematic diagram of the mechanisms of bubble aggregation inhibition.
In conclusion, the aforementioned three theories regarding the enhancement of mass transfer in nanofluids can reasonably explain the phenomenon of enhanced mass transfer in nanofluids within a certain range. It is speculated by some researchers that the actual mechanism may be a combination of two or more mainstream theories, which requires in-depth study.
3.1.3. Dispersion Stability of Nanofluids
The dispersed state of nanofluids depends on the microscopic forces that are exerted on the nanoparticles, such as van der Waals forces and electrostatic forces [50]. The magnitude of forces between particles depends on the distance; the tendency of nanoparticles to aggregate and precipitate often occurs in short distances and is also affected by gravity.
In addition to the interparticle distance, the shape of the nanoparticles may also lead to aggregation and precipitation phenomena. By comparison, the neighboring rod-shaped nanoparticles have a larger contact area than the spherical nanoparticles. Thus, rod-shaped nanoparticles have a stronger attraction between neighbors and a stronger tendency to form aggregates [51].
Some factors also contribute to the reduced likelihood of aggregation, such as shear flow and irradiation [52]. Finally, the settling force of the nanofluid is calculated by Equation (5), and the viscous resistance during motion by Equation (6) [53]:
where Fd represents the settling force, and Fr is the viscous resistance. μ0 is the settling velocity, μ is the dynamic viscosity, ρp is the density of the nanoparticles, and ρl is the density of the base fluid.
The stability of nanofluids is related to the velocity of Brownian motion; some big nanoparticle clusters break into smaller ones and reduce the flow resistance of particles in nanofluids [54]. In the high-temperature regeneration of absorbents, this low resistance leads to a significant increase in heat-transfer efficiency. The temperature gradient in the vertical direction of the regeneration tower is particularly pronounced, thereby enhancing the micro-convective movement of the fluid, which is beneficial for the regeneration of the absorbents [55,56].
3.1.4. Modification
Surface modification refers to functionalizing the nanoparticle surface, which is known as a promising technique for expanding the different functions of nanofluids (as shown in Figure 6). Nanofluids with functionalized nanoparticles offer excellent physical and chemical properties with low pollution [57]. For instance, the NH2-rGO (amine-functionalized reduced graphene oxide)/MDEA nanofluid synthesized by Vahid et al. [58] has a richer reaction potential and results in a 16.2% enhancement of mass transfer compared to the rGO/MDEA nanofluid. To further minimize the environmental impact, researchers frequently choose environmentally friendly functional groups, such as amino acids, which are then grafted onto nanoparticles. The synthesis and preparation of Fe3O4-proline, Fe3O4-lysine, and Fe3O4@SiO2-NH2 nanofluids were conducted by Elhambakhsh et al. [59] to enhance CO2 absorption. Fe3O4-proline, Fe3O4-lysine, and Fe3O4@SiO2-NH2 nanoparticles showed a 9.6%, 14.83%, and 17.61% higher uptake capacity, respectively, compared to Fe3O4 nanofluids. However, it is noteworthy that the number of active sites in the nanofluid tends to decrease with an increasing number of cycles, leading to a reduction in the CO2-absorption performance of the modified nanofluid after multiple cycles.
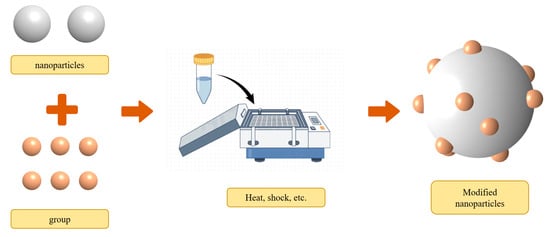
Figure 6.
Typical modification method of nanoparticles.
There are another set of factors influencing the enhancement effects of nanofluids, such as the space structure of the group, the type of nanoparticles, the concentration of nanofluids [60], and the type of base fluid [61]. Symmetric branched amino functional groups were synthesized by Arshadi et al. [62] and incorporated onto Fe3O4@SiO2, yielding Fe3O4@SiO2-NH2 nanoparticles. The CO2 absorption capacity of Fe3O4@SiO2-NH2 was observed to increase by 37.3% compared to Fe3O4, with a slightly higher CO2-capture rate. This enhancement can be attributed to the higher nucleophilic reactivity and density of active sites for Fe3O4@SiO2-NH2, facilitating better chemical bonding with CO2. It can be speculated that optimizing the structure of the functional groups and increasing active sites could potentially be the direction or focal point of future research on modified nanoparticles.
In addition, the stability of nanofluids can be enhanced through modification. Zhang et al. [63] used a silane coupling agent (APTS) to modify nanoscale TiO2. The principle of this behavior lies in the binding of APTS with hydroxyl groups on the surface of TiO2 nanoparticles, resulting in a spatial hindrance effect that prevents the agglomeration of the nanoparticles. Han et al. [64] prepared surface-modified gold nanoparticles and dispersed them into water with polyethylene glycol (PEG) or polyvinylpyrrolidone (PVP) polymers, and succeeded in improving the stability of gold nanoparticles. PEG and PVP as hydrophilic polymers may enhance the hydrophilicity of gold nanoparticles to a certain degree.
3.1.5. Recycling
The recycling and reuse of nanofluids can mitigate environmental pollution and reduce costs. Dialysis and ultrafiltration are widely utilized in laboratory settings because of their crucial role in testing small-scale recycled nanoparticles. However, the shortcomings of the above methods are a long processing duration and high costs, which would be exposed on a larger industrial scale [65]. Centrifugation is another reliable separation method. However, nanoparticles were confirmed to have the tendency to aggregate randomly under gravity conditions (centrifugal condition) by the study of Tsuchiya [66]. For example, gold nanoparticles tend to be assembled into linear clusters under high gravitational acceleration in centrifugal forces [67]. The presence of linear clusters shortens the stable existence of nanofluids, which is not conducive to preservation and transportation.
Magnetic recovery is considered to be a potential recovery route compared to centrifugation. By exploiting the magnetic properties of Fe3O4, Fe3O4@PDA nanoparticles have been successfully recovered via a magnetic field [68]; this provides an effective and efficient method for converting nanoparticle dispersions into a stable dry powder form. The separation of magnetic particles is facilitated by magnetic separation techniques that manipulate magnetic flux and field gradients. There are primarily three technical approaches for the isolation of nanoparticles from liquid phases: High-Gradient Magnetic Separation (HGMS), Magnetic Field Flow Fractionation (MFFF), and Electric Field Flow Fractionation (EFFF). HGMS is an analytical technique used to isolate magnetic species from a nonmagnetic environment utilizing column flow. It was originally proposed to serve mineral beneficiation, water and waste treatment, and chemical processing, as well as the separation of micron-size magnetic particles. HGMS provides a way to capture magnetic material in column flow by dehomogenizing the magnetic field through the column to create a large field gradient. MFFF has been reported as one of the most promising approaches to separation based on the application of external magnetic fields to initiate nanoparticle magnetization. EFFF is another innovative technology that can be utilized primarily for the separation of water/nanoparticle dispersions in narrow channels through the application of an electric field. In this method, the magnetic nanoparticles are separated based on their electrophoretic mobilities and sizes [69]. The magnetic recovery approach is only applicable to magnetic nanofluids, and for the recovery of nonmagnetic nanofluids, the most promising technology remains centrifugal.
3.1.6. Negative Environmental and Health Impacts
The hazards of nanomaterials including nanofluids and nanoparticles to the human body and the environment cannot be ignored. The main health effects of nanomaterials are inflammation, allergy, genotoxicity, and carcinogenicity. In addition, nanomaterials have toxic effects on development and reproduction, including fetal development, the central nervous system, the reproductive system, and the immune system [70].
Nanoparticles released into the environment combine with natural colloidal substances to form mixtures, which are deposited into soil and water and eventually absorbed or ingested by humans. The use of non-toxic nanoparticles has been proposed by researchers as a way to mitigate the risks of nanoparticles to humans and the environment. An approach to adapting the existing regulatory framework has been adopted by the EU to address the nano-form issue [71]. Along with further research on nanofluids, it is believed that more comprehensive regulations will be proposed to limit the misuse of nanofluids.
3.2. Other New Liquid Absorbents and Techniques
3.2.1. Ionic Liquids
Ionic liquids, composed entirely of ions and existing in liquid form at room temperature, exhibit strong electrostatic interactions and hydrogen bonding, which enhance the solubility of CO2 [72]. Ionic liquids are characterized by a low vapor pressure, low reaction enthalpy [73], wide liquid range, and high thermal stability. These advantages of ionic liquids have great potential for application in the field of carbon capture. However, the absorption capacity of conventional ionic liquids is lower than that of alcohol amines. The current solution given by researchers is to add an amine moiety to the ionic liquid. Furthermore, the adjustability of ionic liquids suggests that the chances for preparing a broad array of ionic liquids with ions incorporating functional groups are rather good and functionalized ionic liquids have been studied since 2002 [74]. According to the different types of ions at the CO2-philic sites, functional ionic liquids can be divided into cation-functionalized ionic liquids, anion-functionalized ionic liquids, and cation–anion dual-functionalized ionic liquids [75]. Compared with conventional ionic liquids absorbing CO2, functionalized ionic liquids or task-specific ionic liquids could chemically absorb CO2 through single-site mechanisms or multiple-site mechanisms. Unfortunately, the application of these functionalized ionic liquids in CO2 capture is compromised by their high viscosity [76] and high cost [77].
3.2.2. Amino Acids
The absorption mechanism of amino acid absorbents is analogous to that of primary and secondary amines [78]. In comparison with the intensively investigated MEA, amino acids exhibit distinctive advantages as compared with the intensively investigated MEA. Amino acid salts are noteworthy for their low energy consumption, as well as their safety and environmental friendliness [79]. The CO2 absorption heat of K-Lys (lysine potassium salt) was estimated by Zhao [80] and others using the Gibbs–Helmholtz equation. In comparison with a 30 wt% MEA solution (84.5 kJ/mol), lower energy is represented by 20–30 wt% K-Lys (55–70 kJ/mol). Additionally, Rouzbeh et al. [81] summarized the toxicity of amine compounds revealing an order of PZ > MEA > MDEA, with all amino acids exhibiting lower toxicity. The non-toxic and non-hazardous nature of amino acids allows them to be useful in certain specific locations (e.g., hospitals and kindergartens) and areas. However, Erga et al. [82] pointed out that glycine produces unpleasant odors during the regeneration process, so it is necessary to install purification facilities when using some amino acid absorbents. This will increase the cost of CO2 capture and hinder the large-scale application of amino acid absorbents.
3.2.3. Phase-Change Solvents
The concept of phase-change absorbents was first proposed by Liang Hu [83] at Hampton University. Phase-change solvents are homogeneous (single-phase) solvents under normal conditions, but undergo a phase transition into a heterogenic (two-phase) system, triggered by changes in polarity, hydrophilicity, ionic strength, or hydrogen bond strength to form a CO2-lean liquid phase and a CO2-enriched liquid or solid phase. In contrast with traditional MEA absorbents, phase-change absorbents possess characteristics such as a low regeneration temperature and high reaction rates [84]. Kim and Lee [85] revealed that the CO2 loading in the rich phase was a critical factor influencing the energy demand for the generic biphasic solvent-based process. The energy penalty decreased with increasing CO2 loading in the rich phase. This feature is ideal for large-scale CO2-capture applications. A specific disadvantage of phase-change solvents is unavoidable, the liquid–liquid phase-separation requires. Additionally, some of the absorbent is still corrosive and has a low fault tolerance in each step due to the risk of incomplete phase separation which will affect the subsequent regeneration process [5].
4. Conclusions and Future Perspectives
The liquid CO2-capture technologies are considered to be one of the most useful CO2-capture routes because of their significant absorption capacity, high reliability, and rich engineering foundation. The main discussion throughout the text is summarized in points as follows:
(1) Nanofluids are one of the most valuable applications of liquid carbon dioxide-capture technology and show the ability to significantly improve mass transfer. The enhancement mechanism of nanofluids may be related to the shuttle effect, the hydrodynamic effect, and bubble aggregation inhibition. However, there are differences in the views of different researchers. There are also different conclusions about the enhancement effect of modified nanofluids. In addition, the effect of the nature of nanofluids on gas–liquid mass transfer has been widely discussed. At the application level, existing preparation and recovery methods do not fully meet industrial requirements, and innovative approaches need to be sought. In conclusion, nanofluids technology is a controversial and innovative liquid CO2-capture technology.
(2) Ionic liquids have excellent physical and chemical properties. Not only do they increase the solubility of CO2 significantly, but they also have a low energy requirement for regeneration. The anions and cations in ionic liquids offer a wealth of functionalization possibilities. However, the high viscosity and high cost of functionalized ionic liquids need to be considered.
(3) Amino acids and amine solutions have several points of identity. For example, both absorb CO2 by binding RNH2 groups, and the absorption is affected by the spatial structure and species. However, amino acids have superior application value due to their low toxicity and low pollution properties. With further development, they may be able to replace amine solutions.
(4) The phase-transition behavior of phase-transition absorption has two sides. On the one hand, the phase-change behavior effectively enriches CO2, resulting in an increase in the regeneration efficiency of the phase-change solvents. On the other hand, the phase-change behavior increases the complexity of the process. When designing the process, it is necessary to balance the energy, process complexity, equipment life, and efficiency according to the CO2 load.
A few outlooks for liquid CO2-capture technologies are as follows:
(1) The traditional amine solution CO2-capture technology has a certain engineering hardware basis, but its widespread adoption is hindered by absorbent capacity and rate and regeneration energy consumption. Researchers have developed mixed amine absorbents based on the characteristics of primary, secondary, and tertiary amines, which to some extent compensate for the drawbacks in absorption rate and the capacity of single amine absorbents. However, the use of alcohol amine solutions still faces drawbacks such as high energy consumption, strong corrosiveness, and significant pollution. Future work will focus on finding better combinations of amine systems with regard to the greater CO2 absorption activity, faster CO2 desorption rate, and lower regeneration heat duty. Also, amino acid salts, ammonia water, and other absorbents are new raw materials for absorbent combinations.
(2) Nanofluids offer the potential to improve mass-transfer efficiency and reduce energy consumption based on traditional amine-based CO2-capture technology. The practical usage of nanofluids in engineering applications would raise even harsher requirements on the function, stability, and environmental protection properties. In the future, the positive effects of different nanoparticle and functional groups on liquid carbon-capture technologies need to be considered. Secondly, based on their bonding principles, the bond strength could be increased to extend the circulation cycles of nanofluids.
(3) Other novel liquid absorbents (including ionic liquids and phase-change absorbents) are of undeniable value. The application of ionic liquids is hampered by high viscosity and high cost. Reducing the cost and viscosity of ionic liquids while maintaining their excellent physicochemical properties is an area to focus on in the future. Secondly, the environmental and health impacts of ionic liquids need to be fully assessed in subsequent studies. Phase-change solvents have high requirements for processes and equipment. In practical applications, efficient and cost-effective solutions can be designed based on the specific conditions of the plant.
(4) CO2 absorption in solution is a complicated process involving gas–liquid or gas–liquid-solid mass transfer and chemical reactions. Exploring new absorption materials and revealing the kinetic limiting step is key to facilitating the carbon-capture process. Until now, novel liquid absorbents and techniques exhibit distinct advantages. For instance, amino acid salts demonstrate low pollution potential, ionic liquids exhibit higher physical solubility, and phase-change absorbents result in lower energy consumption. However, the kinetic law of the mass-transfer reaction has not been studied deeply, and this has greatly limited the improvement of carbon-capture technology because gas–liquid mass transfer is a phenomenon of multiple factors, and a single increase in the equilibrium constant of a chemical reaction can only enhance carbon-capture technology to a certain extent. Therefore, multi-factor theoretical research and better model design are essential.
Author Contributions
H.Z. (Haokun Zhang): Conceptualization, writing—original draft. T.L.: Visualization. T.D., Y.L. and H.Z. (Hao Zou): Investigation. J.Z. and D.Y.: Supervision and editing. All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully thank the Natural Science Foundation of Sichuan Province (Grant No. 2022NSFSC1255) and the National Natural Science Foundation of China (Grant No. 22408028).
Data Availability Statement
This manuscript does not report data generation or analysis.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Khan, K.A.; Cong, P.T.; Thang, P.D.; Uyen, P.T.M.; Anwar, A.; Abbas, A. From brown to green: Are Asian economies on the right path? Assessing the role of green innovations and geopolitical risk on environmental quality. Environ. Sci. Pollut. Res. 2024, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, M.A.; Rauf, A.; Shakir, S.; Abbas, A.M.A.; Sun, H.P.; Abid, S. Exploring the Intertwined Nexus between Globalization, Energy Usage, Economic Complexity, and Environmental Quality in Emerging Asian Economies: A Pathway Towards a Greener Future. Environ. Sci. Pollut. Res. 2023, 30, 100431–100449. [Google Scholar] [CrossRef] [PubMed]
- Bottoms, R.R. Organic Bases for Gas Purification. Ind. Eng. Chem. 1931, 23, 501–504. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, Z.; Mahurin, S.M.; Dai, S.; Jiang, D.-E. Ionic liquids for carbon capture. MRS Bull. 2022, 47, 395–404. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, Y.; Wang, L.; Chen, J.; Lu, Y. Phase change solvents for post-combustion CO2 capture: Principle, advances, and challenges. Appl. Energy 2019, 239, 876–897. [Google Scholar] [CrossRef]
- Tavakoli, A.; Rahimi, K.; Saghandali, F.; Scott, J.; Lovell, E. Nanofluid preparation, stability and performance for CO2 absorption and desorption enhancement: A review. J. Environ. Manag. 2022, 313, 114955. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, W.; Li, X.; Zhao, Z.; Jiang, C.; Luo, C.; Wu, F.; Zhang, L. Novel assessment of highly efficient polyamines for post-combustion CO2 capture: Absorption heat, reaction rate, CO2 cyclic capacity, and phase change behavior. Sep. Purif. Technol. 2023, 306, 122615. [Google Scholar] [CrossRef]
- Dubey, A.; Arora, A. Advancements in carbon capture technologies: A review. J. Clean. Prod. 2022, 373, 133932. [Google Scholar] [CrossRef]
- Tarafdar, A.; Sowmya, G.; Yogeshwari, K.; Rattu, G.; Negi, T.; Awasthi, M.K.; Hoang, A.; Sindhu, R.; Sirohi, R. Environmental pollution mitigation through utilization of carbon dioxide by microalgae. Environ. Pollut. 2023, 328, 121623. [Google Scholar] [CrossRef]
- Lu, G.C.; Wang, Z.; Yue, Z.Y.; Wei, W.J.; Huang, Y.; Zhang, X.L.; Fan, X.F. Development of novel AMP-based absorbents for efficient CO2 capture with low energy consumption through modifying the electrostatic potential. Chem. Eng. J. 2023, 474, 145929. [Google Scholar] [CrossRef]
- Smerigan, A.; Uludag-Demirer, S.; Cutshaw, A.; Marks, A.; Liao, W. High-efficiency carbon dioxide capture using an algal amino acid salt solution. J. CO2 Util. 2023, 69, 102394. [Google Scholar] [CrossRef]
- Ma, D.; Zhu, C.; Fu, T.; Yuan, X.; Ma, Y. An effective hybrid solvent of MEA/DEEA for CO2 absorption and its mass transfer performance in microreactor. Sep. Purif. Technol. 2020, 242, 116795. [Google Scholar] [CrossRef]
- Lv, B.; Guo, B.; Zhou, Z.; Jing, G. Mechanisms of CO2 capture into monoethanolamine solution with different CO2 loading during the absorption/desorption processes. Environ. Sci. Technol. 2015, 49, 10728–10735. [Google Scholar] [CrossRef] [PubMed]
- Caplow, M. Kinetics of carbamate formation and breakdown. J. Am. Chem. Soc. 1968, 90, 6795–6803. [Google Scholar] [CrossRef]
- Gautam, A.; Mondal, M.K. Review of recent trends and various techniques for CO2 capture: Special emphasis on biphasic amine solvents. Fuel 2023, 334, 126616. [Google Scholar] [CrossRef]
- Xiang, J.; Wei, D.; Mao, W.; Liu, T.; Luo, Q.; Huang, Y.; Liang, Z.; Luo, X. Comprehensive kinetic study of carbon dioxide absorption in blended tertiary/secondary amine solutions: Experiments and simulations. Sep. Purif. Technol. 2024, 330, 125310. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, B.; Luo, Y.; Guo, K.; Wang, Z.; Liu, K.; Mei, X.; Liu, C. Mass transfer dynamics of single CO2 bubbles rising in monoethanolamine solutions: Experimental study and mathematical model. Chem. Eng. J. 2023, 465, 142761. [Google Scholar] [CrossRef]
- Gouedard, C.; Picq, D.; Launay, F.; Carrette, P.-L. Amine degradation in CO2 capture. I. A review. Int. J. Greenh. Gas Control 2012, 10, 244–270. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Wang, B.; Klemeš, J.J. A graphical approach for mixed ratio optimisation in the binary mixed amine solution. J. Environ. Manag. 2022, 311, 114779. [Google Scholar] [CrossRef]
- He, X.; He, H.; Barzagli, F.; Amer, M.W.; Li, C.E.; Zhang, R. Analysis of the energy consumption in solvent regeneration processes using binary amine blends for CO2 capture. Energy 2023, 270, 126903. [Google Scholar] [CrossRef]
- Chen, M.; Gao, H.; Sema, T.; Xiao, M.; Sun, Q.; Liang, Z. Study on the mechanism and kinetics of amine with steric hindrance absorbing CO2 in non-aqueous/aqueous solution. Sep. Purif. Technol. 2022, 303, 122202. [Google Scholar] [CrossRef]
- Ji, L.; Zheng, X.; Zhang, L.; Feng, L.; Li, K.; Yu, H.; Yan, S. Feasibility and mechanism of an amine-looping process for efficient CO2 mineralization using alkaline ashes. Chem. Eng. J. 2022, 430, 133118. [Google Scholar] [CrossRef]
- Zhang, R.; Luo, X.; Yang, Q.; Yu, H.; Puxty, G.; Liang, Z. Analysis for the speciation in CO2 loaded aqueous MEDA and MAPA solution using 13C NMR technology. Int. J. Greenh. Gas Control 2018, 71, 1–8. [Google Scholar] [CrossRef]
- Yoon, B.; Calabro, D.C.; Baugh, L.S.; Raman, S.; Hwang, G.S. Probing strong steric hindrance effects in aqueous alkanolamines for CO2 capture from first principles. J. Environ. Chem. Eng. 2022, 10, 108987. [Google Scholar] [CrossRef]
- Tagiuri, A.; Mohamedali, M.; Henni, A. Dissociation constant (pKa) and thermodynamic properties of some tertiary and cyclic amines from (298 to 333) K. J. Chem. Eng. Data 2016, 61, 247–254. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.; Lim, H.; Kang, J.H.; Park, H.S.; Park, J.; Song, H. Structural investigation of aqueous amine solutions for CO2 capture: CO2 loading, cyclic capacity, absorption–desorption rate, and pKa. J. Environ. Chem. Eng. 2024, 12, 112664. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y.; Sun, J.; Gu, Y.; Zhang, X.; Tang, Z. The process intensification of CO2 absorption in Hilbert fractal reactor fabricated by a 3D printer. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 481–492. [Google Scholar] [CrossRef]
- Saha, S.; Chakma, A. Separation of CO2 from gas mixtures with liquid membranes. Energy Convers. Manag. 1992, 33, 413–420. [Google Scholar] [CrossRef]
- Ye, B.; Jiang, J.; Zhou, Y.; Liu, J.; Wang, K. Technical and economic analysis of amine-based carbon capture and sequestration at coal-fired power plants. J. Clean. Prod. 2019, 222, 476–487. [Google Scholar] [CrossRef]
- Choi, S.U.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles; Argonne National Lab. (ANL): Argonne, IL, USA, 1995. [Google Scholar]
- Irani, V.; Maleki, A.; Tavasoli, A. CO2 absorption enhancement in graphene-oxide/MDEA nanofluid. J. Environ. Chem. Eng. 2019, 7, 102782. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Lu, T.; Lai, F. Experimental verification of the effects of three metal oxide nanoparticles on mass transfer at gas-liquid interface. J. Pet. Sci. Eng. 2022, 211, 110122. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Adenutsi, C.D.; Wang, C. An experimental study of the effect of three metallic oxide nanoparticles on oil-water relative permeability curves derived from the JBN and extended JBN methods. J. Pet. Sci. Eng. 2020, 192, 107257. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, N.; Kumar, A.; Thakur, V.K.; Kalia, S.; Arya, V.; Kumar, A.; Kumar, S.; Kyzas, G.Z. A critical review on the recent trends of photocatalytic, antibacterial, antioxidant and nanohybrid applications of anatase and rutile TiO2 nanoparticles. Sci. Total Environ. 2024, 914, 169815. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Chuc, M.C.; Ramos-Castillo, C.M.; Rodríguez-Pérez, M.; Ruiz-Gómez, M.Á.; Rodríguez-Gattorno, G.; Villanueva-Cab, J. Synergistic correlation in the colloidal properties of TiO2 nanoparticles and its impact on the photocatalytic activity. Inorganics 2022, 10, 125. [Google Scholar] [CrossRef]
- Darvanjooghi, M.H.K.; Esfahany, M.N.; Esmaeili-Faraj, S.H. Investigation of the effects of nanoparticle size on CO2 absorption by silica-water nanofluid. Sep. Purif. Technol. 2018, 195, 208–215. [Google Scholar] [CrossRef]
- Du, W.; Ma, J.; Wang, W.; Zhang, L. Surface-tension change of graphene-based water nanofluid and its effects on heat-transfer process. J. Mol. Liq. 2023, 392, 123457. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Luo, Y.; Guo, K.; Zheng, L.; Liu, C. A mathematical model for single CO2 bubble motion with mass transfer and surfactant adsorption/desorption in stagnant surfactant solutions. Sep. Purif. Technol. 2023, 308, 122888. [Google Scholar] [CrossRef]
- Lemaire, P.; Alenzi, A.; Lee, J.; Beckman, E.; Enick, R. Thickening CO2 with direct thickeners, CO2-in-Oil emulsions, or nanoparticle dispersions: Literature review and experimental validation. Energy Fuels 2021, 35, 8510–8540. [Google Scholar] [CrossRef]
- Salafi, T.; Zeming, K.K.; Zhang, Y. Advancements in microfluidics for nanoparticle separation. Lab Chip 2017, 17, 11–33. [Google Scholar] [CrossRef]
- Das, P.K.; Santra, A.K.; Ganguly, R.; Dash, S.K.; Muthusamy, S.; Sha, M.; Sadasivuni, K.K. An extensive review of preparation, stabilization, and application of single and hybrid nanofluids. J. Therm. Anal. Calorim. 2024, 149, 9523–9557. [Google Scholar] [CrossRef]
- Yu, W.; Wang, T.; Park, A.-H.A.; Fang, M. Review of liquid nano-absorbents for enhanced CO2 capture. Nanoscale 2019, 11, 17137–17156. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Kazi, S.N.; Chowdhury, Z.Z.; Johan, M.R.B.; Mehmood, S.; Soudagar, M.E.M.; Mujtaba, M.; Gul, M.; Ahmad, M.S. Heat transfer growth of sonochemically synthesized novel mixed metal oxide ZnO + Al2O3 + TiO2/DW based ternary hybrid nanofluids in a square flow conduit. Renew. Sustain. Energy Rev. 2021, 145, 111025. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996. [Google Scholar] [CrossRef] [PubMed]
- Mudidana, R.K.; Miditana, V.; Rambabu, V. Synthesis of nanofluids preparation—A review. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Brilman, D.W.F.; van Swaaij, W.P.M.; Versteeg, G. A one-dimensional instationary heterogeneous mass transfer model for gas absorption in multiphase systems. Chem. Eng. Process. Process Intensif. 1998, 37, 471–488. [Google Scholar] [CrossRef]
- Lee, W.; Xu, R.; Kim, S.; Park, J.H.; Kang, Y.T. Nanofluid and nanoemulsion absorbents for the enhancement of CO2 absorption performance. J. Clean. Prod. 2021, 291, 125848. [Google Scholar] [CrossRef]
- Feng, X.; Johnson, D.W. Mass transfer in SiO2 nanofluids: A case against purported nanoparticle convection effects. Int. J. Heat Mass Transf. 2012, 55, 3447–3453. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Xiong, M.; Gao, C.; Ren, H.; Ma, L. Process intensification in gas-liquid mass transfer by nanofluids: Mechanism and current status. J. Mol. Liq. 2022, 346, 118268. [Google Scholar] [CrossRef]
- Morán, J.; Yon, J.; Henry, C.; Kholghy, M.R. Approximating the van der Waals interaction potentials between agglomerates of nanoparticles. Adv. Powder Technol. 2023, 34, 104269. [Google Scholar] [CrossRef]
- Sharma, V.; Park, K.; Srinivasarao, M. Colloidal dispersion of gold nanorods: Historical background, optical properties, seed-mediated synthesis, shape separation and self-assembly. Mater. Sci. Eng. R Rep. 2009, 65, 1–38. [Google Scholar] [CrossRef]
- Wan, M.; Xu, B.; Shi, L.; Zheng, N.; Sun, Z. The dynamic stability of silicone oil-based MWCNT nanofluids under high-temperature, high-flux irradiation, and shear-flow conditions. Powder Technol. 2023, 424, 118508. [Google Scholar] [CrossRef]
- Kamalgharibi, M.; Hormozi, F.; Zamzamian, S.A.H.; Sarafraz, M. Experimental studies on the stability of CuO nanoparticles dispersed in different base fluids: Influence of stirring, sonication and surface active agents. Heat Mass Transf. 2016, 52, 55–62. [Google Scholar] [CrossRef]
- Li, F.; Li, L.; Zhong, G.; Zhai, Y.; Li, Z. Effects of ultrasonic time, size of aggregates and temperature on the stability and viscosity of Cu-ethylene glycol (EG) nanofluids. Int. J. Heat Mass Transf. 2019, 129, 278–286. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Yan, X.; Wang, X.; Feng, B. Investigation on viscosity of Fe3O4 nanofluid under magnetic field. Int. Commun. Heat Mass Transf. 2016, 72, 23–28. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Liang, X.; Xu, J.; Lee, C.; Liang, Q.; Tao, P.; Deng, T. Dispersion stability of thermal nanofluids. Prog. Nat. Sci. Mater. Int. 2017, 27, 531–542. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, P.C.; Chaudhuri, P. Stability of heat transfer nanofluids—A review. ChemBioEng Rev. 2018, 5, 312–333. [Google Scholar] [CrossRef]
- Irani, V.; Tavasoli, A.; Vahidi, M. Preparation of amine functionalized reduced graphene oxide/methyl diethanolamine nanofluid and its application for improving the CO2 and H2S absorption. J. Colloid Interface Sci. 2018, 527, 57–67. [Google Scholar] [CrossRef]
- Elhambakhsh, A.; Keshavarz, P. Investigation of carbon dioxide absorption using different functionalized Fe3O4 magnetic nanoparticles. Energy Fuels 2020, 34, 7198–7208. [Google Scholar] [CrossRef]
- Chen, Y.; Abed, A.M.; Raheem, A.B.F.; Altamimi, A.S.; Yasin, Y.; Sheekhoo, W.A.; Smaisim, G.F.; Ghabra, A.A.; Naseer, N.A. Current advancements towards the use of nanofluids in the reduction of CO2 emission to the atmosphere. J. Mol. Liq. 2023, 371, 121077. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.; Pineda, I.T.; Kang, Y.T. Review of nanoabsorbents for capture enhancement of CO2 and its industrial applications with design criteria. Renew. Sustain. Energy Rev. 2021, 138, 110524. [Google Scholar] [CrossRef]
- Arshadi, M.; Taghvaei, H.; Abdolmaleki, M.; Lee, M.; Eskandarloo, H.; Abbaspourrad, A. Carbon dioxide absorption in water/nanofluid by a symmetric amine-based nanodendritic adsorbent. Appl. Energy 2019, 242, 1562–1572. [Google Scholar] [CrossRef]
- Zhang, H.; Qing, S.; Gui, Q.; Zhang, X.; Zhang, A. Effects of surface modification and surfactants on stability and thermophysical properties of TiO2/water nanofluids. J. Mol. Liq. 2022, 349, 118098. [Google Scholar] [CrossRef]
- Han, X.; Yao, Y.; Zhao, X.; Huang, J.; Khosa, A.A. Investigations of stable surface-modified gold nanofluids optical filters based on optical optimization for photovoltaic/thermal systems. Sustain. Energy Technol. Assess. 2023, 57, 103203. [Google Scholar] [CrossRef]
- D’Addio, S.M.; Kafka, C.; Akbulut, M.; Beattie, P.; Saad, W.; Herrera, M.; Kennedy, M.T.; Prud’homme, R.K. Novel method for concentrating and drying polymeric nanoparticles: Hydrogen bonding coacervate precipitation. Mol. Pharm. 2010, 7, 557–564. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Nakamura, N.; Ohta, S. Centrifugal Field-Flow Fractionation Enables Detection of Slight Aggregation of Nanoparticles That Impacts Their Biomedical Applications. Anal. Chem. 2024, 96, 5976–5984. [Google Scholar] [CrossRef]
- Roca, M.; Pandya, N.H.; Nath, S.; Haes, A.J. Linear assembly of gold nanoparticle clusters via centrifugation. Langmuir 2010, 26, 2035–2041. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, Y.; Jia, F.; Song, S.; Li, Y. Recyclable Fe3O4@ Polydopamine (PDA) nanofluids for highly efficient solar evaporation. Green Energy Environ. 2022, 7, 35–42. [Google Scholar] [CrossRef]
- Simonsen, G.; Strand, M.; Øye, G. Potential applications of magnetic nanoparticles within separation in the petroleum industry. J. Pet. Sci. Eng. 2018, 165, 488–495. [Google Scholar] [CrossRef]
- Elsaid, K.; Olabi, A.; Wilberforce, T.; Abdelkareem, M.A.; Sayed, E.T. Environmental impacts of nanofluids: A review. Sci. Total Environ. 2021, 763, 144202. [Google Scholar] [CrossRef]
- Lourenço, M.J.; Alexandre, J.; Huisman, C.; Paredes, X.; Nieto de Castro, C. The balance between energy, environmental security, and technical performance: The regulatory challenge of nanofluids. Nanomaterials 2021, 11, 1871. [Google Scholar] [CrossRef]
- Elmobarak, W.F.; Almomani, F.; Tawalbeh, M.; Al-Othman, A.; Martis, R.; Rasool, K. Current status of CO2 capture with ionic liquids: Development and progress. Fuel 2023, 344, 128102. [Google Scholar] [CrossRef]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J. State-of-the-art of CO2 capture with ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 capture by a task-specific ionic liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ke, Q.; Zhang, Z.; Zhou, B.; Cui, G.; Lu, H. Tuning functionalized ionic liquids for CO2 capture. Int. J. Mol. Sci. 2022, 23, 11401. [Google Scholar] [CrossRef]
- Liu, F.; Shen, Y.; Shen, L.; Sun, C.; Chen, L.; Wang, Q.; Li, S.; Li, W. Novel amino-functionalized ionic liquid/organic solvent with low viscosity for CO2 capture. Environ. Sci. Technol. 2020, 54, 3520–3529. [Google Scholar] [CrossRef]
- Qu, Y.; Zhao, Y.; Li, D.; Sun, J. Task-specific ionic liquids for carbon dioxide absorption and conversion into value-added products. Curr. Opin. Green Sustain. Chem. 2022, 34, 100599. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Zhang, W.; Wang, J.; Soltanian, M.R.; Olabi, A.G. Effectiveness of amino acid salt solutions in capturing CO2: A review. Renew. Sustain. Energy Rev. 2018, 98, 179–188. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, S.; Bian, Y.; Yang, Y.-n.; Ghosh, U. CO2 solubility in aqueous potassium lysinate solutions at absorber conditions. J. Chem. Thermodyn. 2017, 111, 100–105. [Google Scholar] [CrossRef]
- Ramezani, R.; Mazinani, S.; Di Felice, R. State-of-the-art of CO2 capture with amino acid salt solutions. Rev. Chem. Eng. 2022, 38, 273–299. [Google Scholar] [CrossRef]
- Hu, G.; Smith, K.H.; Wu, Y.; Mumford, K.A.; Kentish, S.E.; Stevens, G.W. Carbon dioxide capture by solvent absorption using amino acids: A review. Chin. J. Chem. Eng. 2018, 26, 2229–2237. [Google Scholar] [CrossRef]
- Erga, O.; Juliussen, O.; Lidal, H. Carbon dioxide recovery by means of aqueous amines. Energy Convers. Manag. 1995, 36, 387–392. [Google Scholar] [CrossRef]
- Hu, L. CO2 Capture from Flue Gas by Phase Transitional Absorption; Hampton University: Hampton, VA, USA, 2009. [Google Scholar]
- Shen, S.; Shi, X.; Li, C.; Guo, H.; Long, Q.; Wang, S.; Yin, X. Nonaqueous (amine+ glycol ether) solvents for energy-efficient CO2 capture: New insights into phase change behaviors and assessment of capture performance. Sep. Purif. Technol. 2022, 300, 121908. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.S. Energy analysis of an absorption-based CO2 capture process. Int. J. Greenh. Gas Control 2017, 56, 250–260. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).