Innovative Carbonaceous Materials and Metal/Metal Oxide Nanoparticles for Electrochemical Biosensor Applications
Abstract
1. Introduction
2. Advantages and Applications of Carbonaceous Materials, Metal, and Metal Oxide NPs
3. Carbonaceous Materials, Metal, and Metal Oxide NPs for Sensor Applications
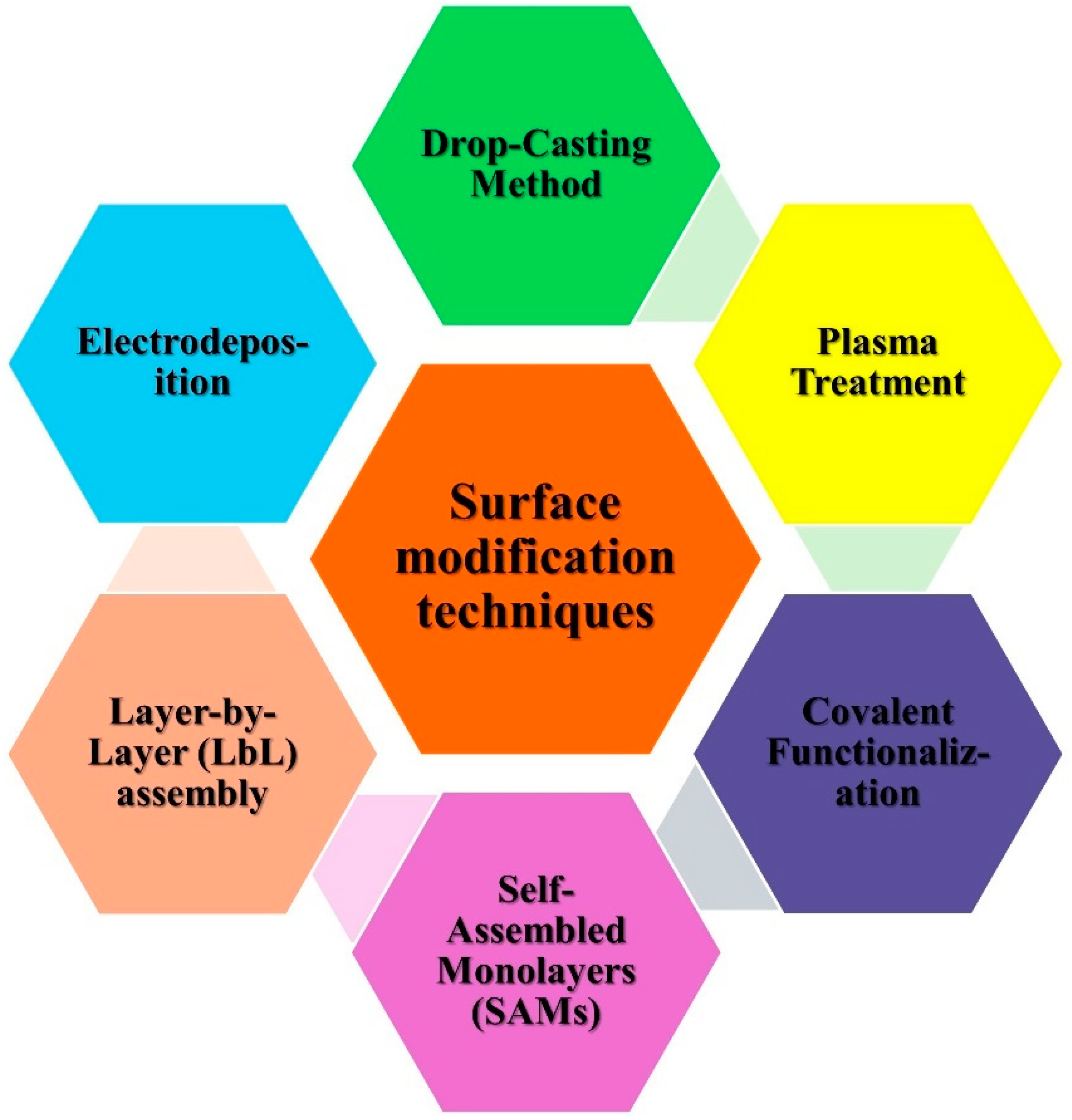
3.1. Surface Modification Techniques
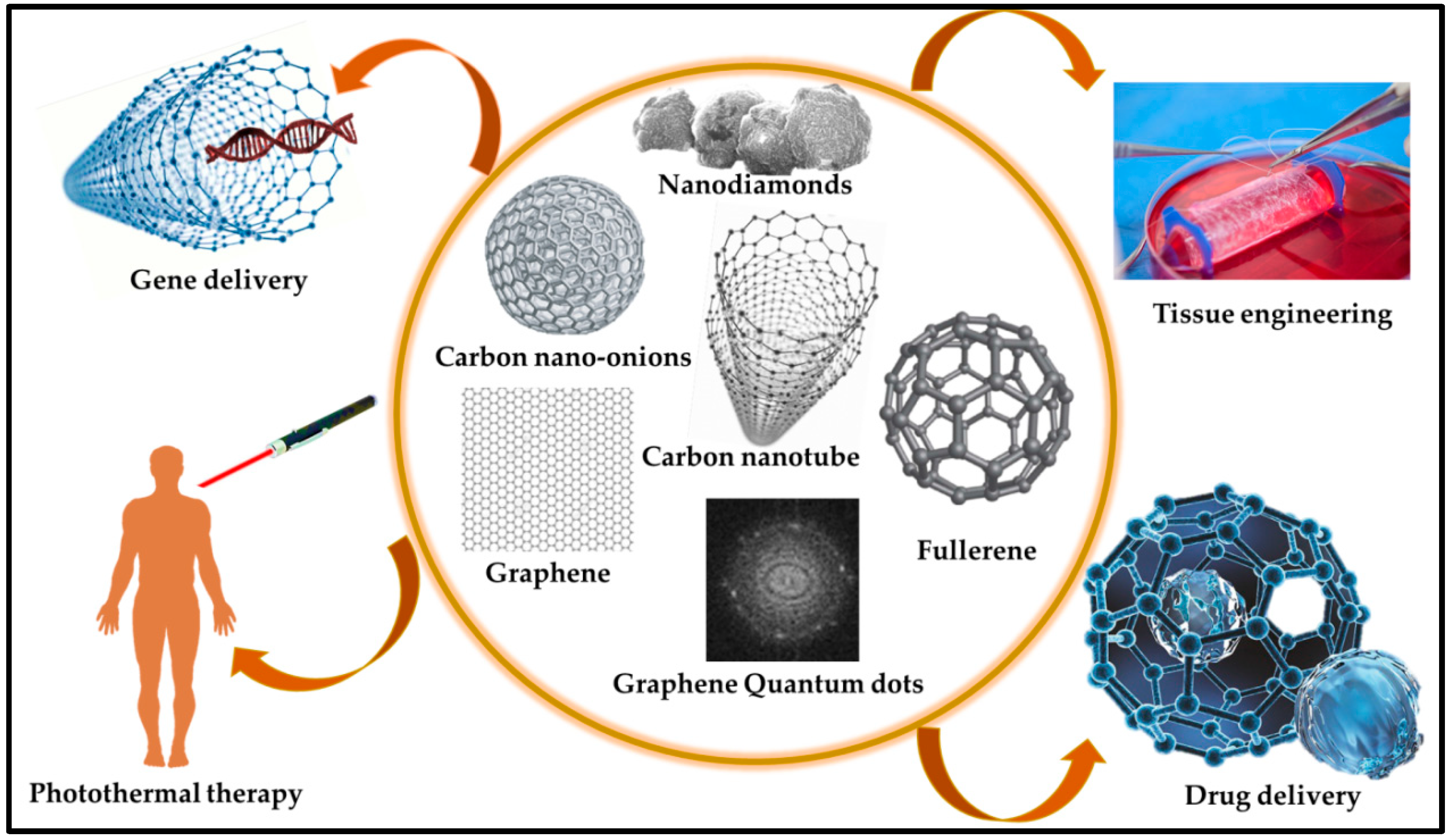
3.2. Carbonaceous Materials (CBN Materials)
3.3. Metal and Metal Oxide NPs
4. Conclusions, Challenges, and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Menon, S.; Mathew, M.R.; Sam, S.; Keerthi, K.; Kumar, K.G. Recent advances and challenges in electrochemical biosensors for emerging and re-emerging infectious diseases. J. Electroanal. Chem. 2020, 878, 114596. [Google Scholar] [CrossRef] [PubMed]
- Bakirhan, N.K.; Topal, B.D.; Ozcelikay, G.; Karadurmus, L.; Ozkan, S.A. Current advances in electrochemical biosensors and nanobiosensors. Crit. Rev. Anal. Chem. 2022, 52, 519–534. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical methods for the analysis of clinically relevant biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, X.; Chen, J. Development of biosensor technologies for analysis of environmental contaminants. Trends Environ. Anal. Chem. 2014, 2, 25–32. [Google Scholar] [CrossRef]
- Puttaningaiah, K.P.C.H.; Hur, J. Recent Advances in Phthalocyanine-Based Hybrid Composites for Electrochemical Biosensors. Micromachines 2024, 15, 1061. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mohamed, M.A.; Vinu Mohan, A.M.; Zhu, Z.; Sharma, V.; Mishra, G.K.; Mishra, R.K. Application of electrochemical aptasensors toward clinical diagnostics, food, and environmental monitoring. Sensors 2019, 19, 5435. [Google Scholar] [CrossRef]
- Songa, E.A.; Okonkwo, J.O. Recent approaches to improving selectivity and sensitivity of enzyme-based biosensors for organophosphorus pesticides: A review. Talanta 2016, 155, 289–304. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef]
- Kempahanumakkagari, S.; Vellingiri, K.; Deep, A.; Kwon, E.E.; Bolan, N.; Kim, K.H. Metal–organic framework composites as electrocatalysts for electrochemical sensing applications. Coord. Chem. Rev. 2018, 357, 105–129. [Google Scholar] [CrossRef]
- Gaddimath, S.; Payamalle, S.; Channabasavana Hundi Puttaningaiah, K.P.; Hur, J. Recent advances in pH and redox responsive polymer nanocomposites for cancer therapy. J. Compos. Sci. 2024, 8, 28. [Google Scholar] [CrossRef]
- Sadik, O.A.; Aluoch, A.O.; Zhou, A. Status of biomolecular recognition using electrochemical techniques. Biosens. Bioelectron. 2009, 24, 2749–2765. [Google Scholar] [CrossRef]
- CP, K.P.; Naveen, K.R.; Hur, J. Acceptor–donor–acceptor based thermally activated delayed fluorescent materials: Structure–property insights and electroluminescence performances. Mater. Chem. Front. 2024, 8, 769–784. [Google Scholar]
- Menon, S.; Sam, S.; Keerthi, K.; Kumar, K.G. Carbon nanomaterial-based sensors: Emerging trends, markets, and concerns. In Carbon Nanomaterials-Based Sensors; Elsevier: Amsterdam, The Netherlands, 2022; pp. 347–379. [Google Scholar]
- Fu, Y.; Liu, T.; Wang, H.; Wang, Z.; Hou, L.; Jiang, J.; Xu, T. Applications of nanomaterial technology in biosensing. J. Sci. Adv. Mater. Devices 2024, 9, 100694. [Google Scholar] [CrossRef]
- Ashraf, G.; Chen, W.; Asif, M.; Aziz, A.; Zhong, Z.T.; Iftikhar, T.; Zhao, Y.D. Topical advancements in electrochemical and optical signal amplification for biomolecules detection: A comparison. Mater. Today Chem. 2022, 26, 101119. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Prabhu, C.K.; Naveen, K.R.; Hur, J. Cobalt–benzimidazole swapped metal–organic macrocycle with reduced graphene oxide as a hybrid electrocatalyst for highly efficient oxygen evolution reaction. RSC Appl. Interfaces 2024, 1, 301–312. [Google Scholar] [CrossRef]
- Yahyazadeh, A.; Nanda, S.; Dalai, A.K. Carbon Nanotubes: A Review of Synthesis Methods and Applications. Reactions 2024, 5, 429–451. [Google Scholar] [CrossRef]
- Huang, H.; Shi, H.; Das, P.; Qin, J.; Li, Y.; Wang, X.; Su, F.; Wen, P.; Li, S.; Lu, P.; et al. The chemistry and promising applications of graphene and porous graphene materials. Adv. Funct. Mater. 2020, 30, 1909035. [Google Scholar] [CrossRef]
- Shepherd, C.; Hadzifejzovic, E.; Shkal, F.; Jurkschat, K.; Moghal, J.; Parker, E.M.; Sawangphruk, M.; Slocombe, D.R.; Foor, J.S.; Moloney, M.G. New routes to functionalize carbon black for polypropylene nanocomposites. Langmuir 2016, 32, 7917–7928. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Redín, G.; Silva, T.A.; Vicentini, F.C.; Fatibello-Filho, O. Effect of carbon black functionalization on the analytical performance of a tyrosinase biosensor based on glassy carbon electrode modified with dihexadecylphosphate film. Enzym. Microb. Technol. 2018, 116, 41–47. [Google Scholar] [CrossRef]
- Maciulis, V.; Ramanaviciene, A.; Plikusiene, I. Recent advances in synthesis and application of metal oxide nanostructures in chemical sensors and biosensors. Nanomaterials 2022, 12, 4413. [Google Scholar] [CrossRef]
- Pal, D.; Roy, E.; Karandikar, P.; Chaudhary, A. TiO2, ZnO and Fe2O3 Thin Film Nanomaterials: Preparation to Applications. In Thin Film Nanomaterials: Synthesis, Properties and Innovative Energy Applications; Bentham Science Publishers: Sharjah, United Arab Emirates, 2024; pp. 199–230. [Google Scholar]
- Salari, H.; Sadeghinia, M. MOF-templated synthesis of nano Ag2O/ZnO/CuO heterostructure for photocatalysis. J. Photochem. Photobiol. A Chem. 2019, 376, 279–287. [Google Scholar] [CrossRef]
- Zheng, Q.; Wu, H.; Wang, N.; Yan, R.; Ma, Y.; Guang, W.; Junzhong, W.; Ding, K. Graphene-based biosensors for biomolecules detection. Curr. Nanosci. 2014, 10, 627–637. [Google Scholar] [CrossRef]
- Parnianchi, F.; Nazari, M.; Maleki, J.; Mohebi, M. Combination of graphene and graphene oxide with metal and metal oxide nanoparticles in fabrication of electrochemical enzymatic biosensors. Int. Nano Lett. 2018, 8, 229–239. [Google Scholar] [CrossRef]
- Adil, S.F.; Ashraf, M.; Khan, M.; Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Tremel, W.; Tahir, M.N. Advances in graphene/inorganic nanoparticle composites for catalytic applications. Chem. Rec. 2022, 22, e202100274. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, Y.; Zan, X.; Liao, K.; Xu, R.; Duan, H. Growth of metal–metal oxide nanostructures on freestanding graphene paper for flexible biosensors. Adv. Funct. Mater. 2012, 22, 2487–2494. [Google Scholar] [CrossRef]
- Gao, J.; Yuan, Q.; Ye, C.; Guo, P.; Du, S.; Lai, G.; Yu, A.; Jiang, N.; Fu, L.; Lin, C.-T.; et al. Label-free electrochemical detection of vanillin through low-defect graphene electrodes modified with Au nanoparticles. Materials 2018, 11, 489. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Choi, M.; Kim, S.; Heo, J.; Yeom, E.; Kim, S.; Lee, H.; Kim, S. Simply controlling the surface structure of graphene oxide films using multiple drop-casting. Diam. Relat. Mater. 2023, 139, 110327. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Yang, Z.; Li, Y.; Yu, S.; Xu, Q.; Hu, X. Graphene–Au nanoparticles nanocomposite film for selective electrochemical determination of dopamine. Anal. Methods 2012, 4, 1725–1728. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Y.; Wang, H.; Hou, S. Gold nanoparticles decorated on single layer graphene applied for electrochemical ultrasensitive glucose biosensor. J. Electroanal. Chem. 2019, 855, 113495. [Google Scholar]
- Mohammadzadeh Kakhki, R. Recent developments on application of nanometal-oxide based gas sensor arrays. Russ. J. Appl. Chem. 2017, 90, 1030–1039. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, L.; Li, S.; Chang, X.; Liu, X.; Zhang, J. Atomic layer deposition of SnO2 on In2O3 enables ultrasensitive NO2 detection at room temperature and DFT mechanism research. Appl. Surf. Sci. 2023, 640, 158410. [Google Scholar] [CrossRef]
- Spampinato, V.; Parracino, M.A.; La Spina, R.; Rossi, F.; Ceccone, G. Surface analysis of gold nanoparticles functionalized with thiol-modified glucose SAMs for biosensor applications. Front. Chem. 2016, 4, 8. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Abu Serea, E.S.; Salah-Eldin, R.E.; Al-Hafiry, S.A.; Ali, M.K.; Shalan, A.E.; Lanceros-Méndez, S. Recent progress in graphene-and related carbon-nanomaterial-based electrochemical biosensors for early disease detection. ACS Biomater. Sci. Eng. 2022, 8, 964–1000. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Wazeer, A. Graphene and carbon nanotubes (CNTs)-based biosensor for life sciences applications. In Next Generation Smart Nano-Bio-Devices; Springer: Singapore, 2022; pp. 61–79. [Google Scholar]
- Oliveira, S.F.; Bisker, G.; Bakh, N.A.; Gibbs, S.L.; Landry, M.P.; Strano, M.S. Protein functionalized carbon nanomaterials for biomedical applications. Carbon 2015, 95, 767–779. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Y.; Yang, Y.; Liang, H.; Wang, L.; Song, Y. Electroactive covalent organic frameworks/carbon nanotubes composites for electrochemical sensing. ACS Appl. Nano Mater. 2020, 3, 1412–1419. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, Y.; Zhang, X.; Shen, G.; Yu, R. Microwave plasma treated carbon nanotubes and their electrochemical biosensing application. Talanta 2007, 72, 1336–1341. [Google Scholar] [CrossRef]
- Mikuła, E. Recent Advancements in Electrochemical Biosensors for Alzheimer’s Disease Biomarkers Detection. Curr. Med. Chem. 2021, 28, 4049–4073. [Google Scholar] [CrossRef]
- Channabasavana Hundi Puttaningaiah, K.P.; D Ramegowda, S.; Hur, J. Polymer Tetrabenzimidazole Aluminum Phthalocyanine Complex with Carbon Nanotubes: A Promising Approach for Boosting Lithium-Ion Battery Anode Performance. ACS Appl. Energy Mater. 2024, 7, 6793–6806. [Google Scholar] [CrossRef]
- Kim, J.; Park, G.; Lee, S.; Hwang, S.W.; Min, N.; Lee, K.M. Single wall carbon nanotube electrode system capable of quantitative detection of CD4+ T cells. Biosens. Bioelectron. 2017, 90, 238–244. [Google Scholar] [CrossRef]
- Wang, L.; Jia, W.; Wu, Y.; Wang, F.; Kui, L. Direct Fabrication of 3D Graphene–Multi Walled Carbon Nanotubes Network and Its Application for Sensitive Electrochemical Determination of Hyperin. Int. J. Electrochem. Sci. 2019, 14, 481–493. [Google Scholar] [CrossRef]
- Chaithra, K.P.; Akshaya, K.B.; Maiyalagan, T.; Gurumurthy, H.; Anitha, V.; Louis, G. Unique Host Matrix to Disperse Pd Nanoparticles for Electrochemical Sensing of Morin: Sustainable Engineering Approach. ACS Biomater. Sci. Eng. 2020, 6, 5264–52731. [Google Scholar] [CrossRef]
- Diao, P.; Liu, Z.; Wu, B.; Nan, X.; Zhang, J.; Wei, Z. Chemically assembled single-wall carbon nanotubes and their electrochemistry. Chemphyschem. 2002, 3, 898–901. [Google Scholar] [CrossRef]
- Singh, C.; Srivastava, S.; Ali, M.A.; Gupta, T.K.; Sumana, G.; Srivastava, A.; Mathur, R.B.; Malhotra, B.D. Carboxylated multiwalled carbon nanotubes-based biosensor for aflatoxin detection. Sens. Actuators Chem. 2013, 185, 258–264. [Google Scholar] [CrossRef]
- Mahor, A.; Singh, P.P.; Bharadwaj, P.; Sharma, N.; Yadav, S.; Rosenholm, J.M.; Bansal, K.K. Carbon-based nanomaterials for delivery of biologicals and therapeutics: A cutting-edge technology. C 2021, 7, 19. [Google Scholar] [CrossRef]
- Ge, S.; Sun, M.; Liu, W.; Li, S.; Wang, X.; Chu, C.; Yan, M.; Yu, J. Disposable electrochemical immunosensor based on peroxidase-like magnetic silica–graphene oxide composites for detection of cancer antigen 153. Sens. Actuators B Chem. 2014, 192, 317–326. [Google Scholar] [CrossRef]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S.; et al. Nanomaterials-based biosensors and their applications: A review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Joshi, P.; Mishra, R.; Narayan, R.J. Biosensing applications of carbon-based materials. Curr. Opin. Biomed. Eng. 2021, 18, 100274. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon nanomaterials and their application to electrochemical sensors: A review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- CP, K.P.; Naveen, K.R.; Aralekallu, S.; Sannegowda, L.K. Novel polymeric cobalt tetrabenzimidazole phthalocyanine for nanomolar detection of hydrogen peroxide. RSC Sustain. 2023, 1, 128–138. [Google Scholar]
- Mohammed, I.; Nemakal, M.; Sajjan, V.A.; Puttappashetty, D.B.; Sannegowda, L.K. Electropolymerized film of cobalt tetrabenzimidazolephthalocyanine for the amperometric detection of H2O2. J. Electroanal. Chem. 2018, 826, 96–103. [Google Scholar] [CrossRef]
- Zribi, B.; Roy, E.; Pallandre, A.; Chebil, S.; Koubaa, M.; Mejri, N.; Gomez, H.M.; Sola, C.; Korri-Youssoufi, H.; Haghiri-Gosnet, A.M. A microfluidic electrochemical biosensor based on multiwall carbon nanotube/ferrocene for genomic DNA detection of Mycobacterium tuberculosis in clinical isolates. Biomicrofluidics 2016, 10, 14115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Liang, Q.L.; Wang, Y.M.; Luo, G.A. Carbon nanotube-intercalated graphite electrodes for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. J. Electroanal. Chem. 2003, 540, 129–134. [Google Scholar] [CrossRef]
- Khan, R.; Pal, M.; Kuzikov, A.V.; Bulko, T.; Suprun, E.V.; Shumyantseva, V.V. Impedimetric immunosensor for detection of cardiovascular disorder risk biomarker. Mater. Sci. Eng. C 2016, 68, 52–58. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Yan, S.; Wang, M.; Liu, P.; Dong, Y.; Zhang, C. Single-walled carbon nanotubes–carboxyl-functionalized graphene oxide-based electrochemical DNA biosensor for thermolabile hemolysin gene detection. Anal. Methods 2015, 7, 5303–5310. [Google Scholar] [CrossRef]
- Xing, R.; Yang, H.; Li, S.; Yang, J.; Zhao, X.; Wang, Q.; Liu, S.; Liu, X. A sensitive and reliable rutin electrochemical sensor based on palladium phthalocyanine-MWCNTs-Nafion nanocomposite. J. Solid State Electrochem. 2017, 21, 1219–1228. [Google Scholar] [CrossRef]
- Yang, H.; Li, B.; Cui, R.; Xing, R.; Liu, S. Electrochemical sensor for rutin detection based on Au nanoparticle-loaded helical carbon nanotubes. J. Nanopart. Res. 2017, 19, 354. [Google Scholar] [CrossRef]
- Jing, S.S.; Zheng, H.J.; Zhao, L.; Qu, L.B.; Yu, L.L. A novel electrochemical sensor based on WO3 nanorods-decorated poly(sodium 4-styrenesulfonate) functionalized graphene nanocomposite modified electrode for detecting of puerarin. Talanta 2017, 174, 477–485. [Google Scholar] [CrossRef]
- Li, H.F.; Wang, S.; Cui, F.; Zhuo, B.B.; Zhao, C.R.; Liu, W.L. Sensitive and selective detection of puerarin based on the hybrid of reduced graphene oxide and molecularly imprinted polymer. J. Pharmaceut. Biomed. 2020, 185, 113221. [Google Scholar] [CrossRef]
- Meng, R.Q.; Li, Q.L.; Zhang, S.J.; Tang, J.K.; Ma, C.L.; Jin, R.Y. GQDs/PEDOT Bilayer Films Modified Electrode as a Novel Electrochemical Sensing Platform for Rutin Detection. Int. J. Electrochem. Sci. 2019, 14, 11000–11011. [Google Scholar] [CrossRef]
- Zhao, P.; Ni, M.; Xu, Y.; Wang, C.; Chen, C.; Zhang, X.; Li, C.; Xie, Y.; Fei, J. A novel ultrasensitive electrochemical quercetin sensor based on MoS2-carbon nanotube@ graphene oxide nanoribbons/HS-cyclodextrin/graphene quantum dots composite film. Sens. Actuators B Chem. 2019, 299, 126997. [Google Scholar] [CrossRef]
- Senocak, A.; Khataee, A.; Demirbas, E.; Doustkhah, E. Ultrasensitive detection of rutin antioxidant through a magnetic micro-mesoporous graphitized carbon wrapped Co nanoarchitecture. Sensor Actuat. B-Chem. 2020, 312, 127939. [Google Scholar] [CrossRef]
- Malekzad, H.; Zangabad, P.S.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble metal nanoparticles in biosensors: Recent studies and applications. Nanotechnol. Rev. 2017, 6, 301–329. [Google Scholar] [CrossRef] [PubMed]
- CP, K.P.; Aralekallu, S.; Sajjan, V.A.; Palanna, M.; Kumar, S.; Sannegowda, L.K. Non-precious cobalt phthalocyanine-embedded iron ore electrocatalysts for hydrogen evolution reactions. Sustain. Energy Fuels 2021, 5, 1448–1457. [Google Scholar] [CrossRef]
- Gaddimath, S.; Chandrakala, K.B.; Lagashetty, A.; Dani, S.; Prabhu, C.K.; Giddaerappa; Sannegowda, L.K. Ilmenite-type NiTiO3 nanoparticles for oxygen evolution reaction. J. Appl. Electrochem. 2024, 54, 2519–2536. [Google Scholar] [CrossRef]
- Tripathy, N.; Kim, D.H. Metal oxide modified ZnO nanomaterials for biosensor applications. Nano Converg. 2018, 5, 27. [Google Scholar] [CrossRef]
- Sannegowda, L.K.; Reddy, K.V.; Shivaprasad, K.H. Stable nano-sized copper and its oxide particles using cobalt tetraamino phthalocyanine as a stabilizer; application to electrochemical activity. RSC Adv. 2014, 4, 11367–11374. [Google Scholar] [CrossRef]
- Guo, X.; Liang, B.; Jian, J.; Zhang, Y.; Ye, X. Glucose biosensor based on a platinum electrode modified with rhodium nanoparticles and with glucose oxidase immobilized on gold nanoparticles. Microchim. Acta 2014, 181, 519–525. [Google Scholar] [CrossRef]
- Han, W.; Yang, J.; Jiang, B.; Wang, X.; Wang, C.; Guo, L.; Sun, Y.; Liu, F.; Sun, P.; Lu, G. Conductometric ppb-Level CO Sensors Based on In2O3 Nanofibers Co-Modified with Au and Pd Species. Nanomaterials 2022, 12, 3267. [Google Scholar] [CrossRef]
- Fallatah, A.; Kuperus, N.; Almomtan, M.; Padalkar, S. Sensitive Biosensor Based on Shape-Controlled ZnONanostructures Grown on Flexible Porous Substrate for Pesticide Detection. Sensors 2022, 22, 3522. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.F.; Li, J.J.; Zhao, J.W. Tuning the fluorescence quenching properties of plasmonic Ag-coated-Au triangular nanoplates: Application in ultrasensitive detection of CEA. Plasmonics 2016, 11, 565–572. [Google Scholar] [CrossRef]
- Lan, D.; Li, B.; Zhang, Z. Chemiluminescence flow biosensor for glucose based on gold nanoparticle-enhanced activities of glucose oxidase and horseradish peroxidase. Biosens. Bioelectron. 2008, 24, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Su, M.; Gao, C.; Tao, X.; Ge, S. Application of Au cage/Ru (bpy) 32+ nanostructures for the electrochemiluminescence detection of K562 cancer cells based on aptamer. Sens. Actuator B 2015, 214, 144–151. [Google Scholar] [CrossRef]
- Liang, W.; Zhuo, Y.; Xiong, C.; Zheng, Y.; Chai, Y.; Yuan, R. Ultrasensitive cytosensor based on self-enhanced electrochemiluminescent ruthenium-silica composite nanoparticles for efficient drug screening with cell apoptosis monitoring. Anal. Chem. 2015, 87, 12363–12371. [Google Scholar] [CrossRef] [PubMed]
- Mohadeseh, S.; Foroughi, M.M.; Nasser, E.; Shohreh, J.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Prasad, G.J.; Namrata, W.G.; Abdul, H.B.; Mohan, G.K. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Q.; Zhang, X.; Hou, H.; You, T. PdCo alloy nanoparticle-embedded carbon nanofiber for ultrasensitive nonenzymatic detection of hydrogen peroxide and nitrite. J. Colloid Interface Sci. 2015, 450, 168–173. [Google Scholar] [CrossRef]
- Bernardo-Boongaling, V.R.R.; Serrano, N.; García-Guzmán, J.J.; Palacios-Santander, J.M.; Díaz-Cruz, J.M. Screen-PrintedElectrodes Modified with Green-Synthesized Gold Nanoparticles for the Electrochemical Determination of Aminothiols. J. Electroanal. Chem. 2019, 847, 113184. [Google Scholar] [CrossRef]
- Ravalli, A.; Dos Santos, G.P.; Ferroni, M.; Faglia, G.; Yamanaka, H.; Marrazza, G. New label free CA125 detection based on gold nanostructured screen-printed electrode. Sens. Actuators B Chem. 2013, 179, 194–200. [Google Scholar] [CrossRef]
- Baradoke, A.; Jose, B.; Pauliukaite, R.; Forster, R.J. Properties of Anti-CA125 antibody layers on screen-printed carbon electrodes modified by gold and platinum nanostructures. Electrochim. Acta 2019, 306, 299–306. [Google Scholar] [CrossRef]
- Phetsang, S.; Jakmunee, J.; Mungkornasawakul, P.; Laocharoensuk, R.; Ounnunkad, K. Sensitive amperometric biosensors for detection of glucose and cholesterol using a platinum/reduced graphene oxide/poly (3-aminobenzoic acid) film-modified screen-printed carbon electrode. Bioelectrochemistry 2019, 127, 125–135. [Google Scholar] [CrossRef]
- Reitz, E.; Jia, W.; Gentile, M.; Wang, Y.; Lei, Y. CuO nanospheres based nonenzymatic glucose sensor. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 2482–2486. [Google Scholar] [CrossRef]
- Naik, K.K.; Rout, C.S. Electrodeposition of ZnCo2O4 nanoparticles for biosensing applications. RSC Adv. 2015, 5, 79397–79404. [Google Scholar] [CrossRef]
- Liao, J.; Lin, S.; Yang, Y.; Liu, K.; Du, W. Highly selective and sensitive glucose sensors based on organic electrochemical transistors using TiO2 nanotube arrays-based gate electrodes. Sens. Actuators B Chem. 2015, 208, 457–463. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, A.; Zhao, M.; Dong, W.; Zhao, T.; Wang, J.; Tang, W. Bimetallic PdCu nanoparticle decorated three-dimensional graphene hydrogel for non-enzymatic amperometric glucose sensor. Sens. Actuators B Chem. 2014, 190, 707–714. [Google Scholar] [CrossRef]
- Chang, G.; Shu, H.; Ji, K.; Oyama, M.; Liu, X.; He, Y. Gold nanoparticles directly modified glassy carbon electrode for non-enzymatic detection of glucose. Appl. Surf. Sci. 2014, 288, 524–529. [Google Scholar] [CrossRef]
- Yin, G.; Xing, L.; Ma, X.J.; Wan, J. Non-enzymatic hydrogen peroxide sensor based on a nanoporous gold electrode modified with platinum nanoparticles. Chem. Pap. 2014, 68, 435–441. [Google Scholar] [CrossRef]
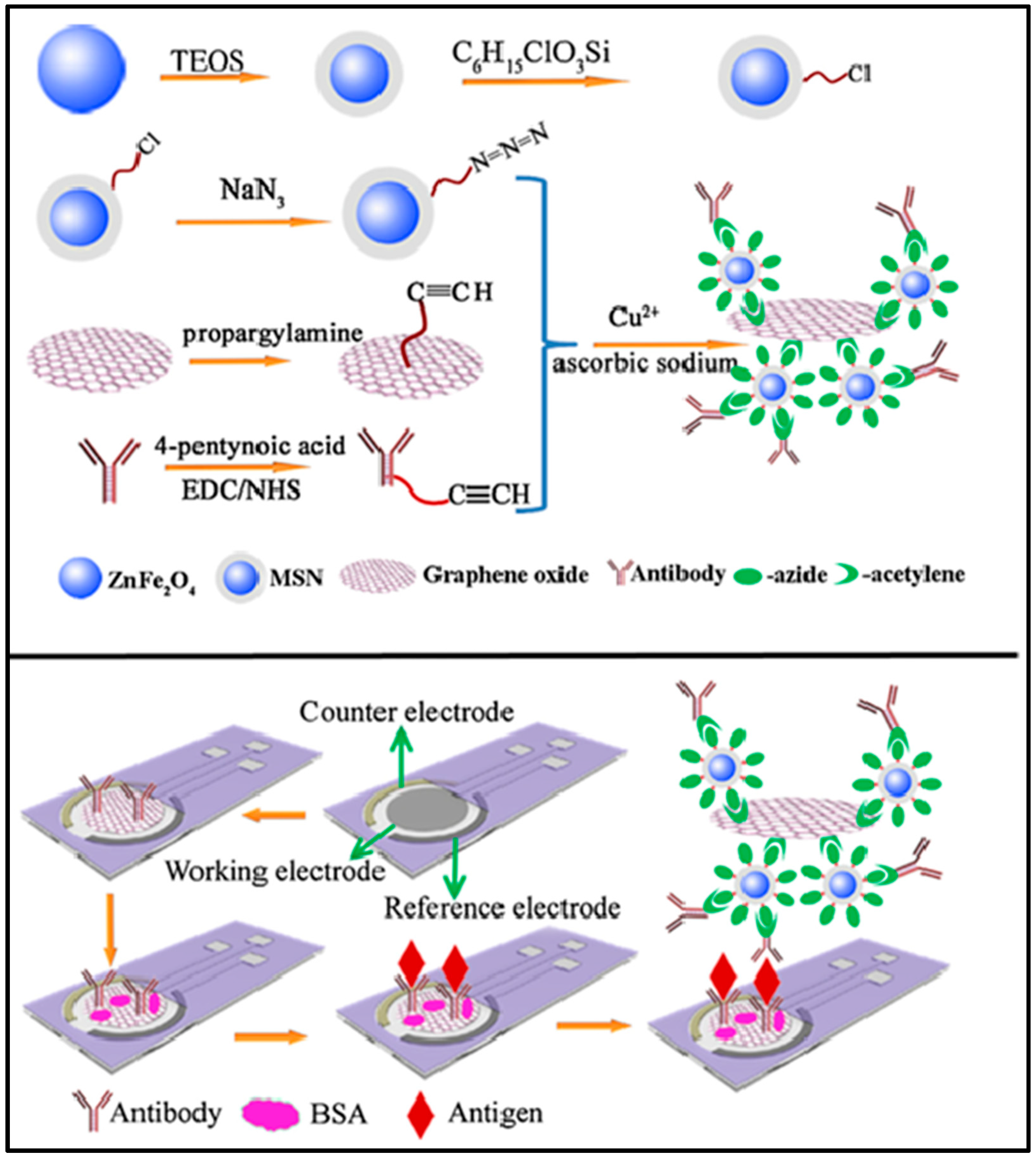
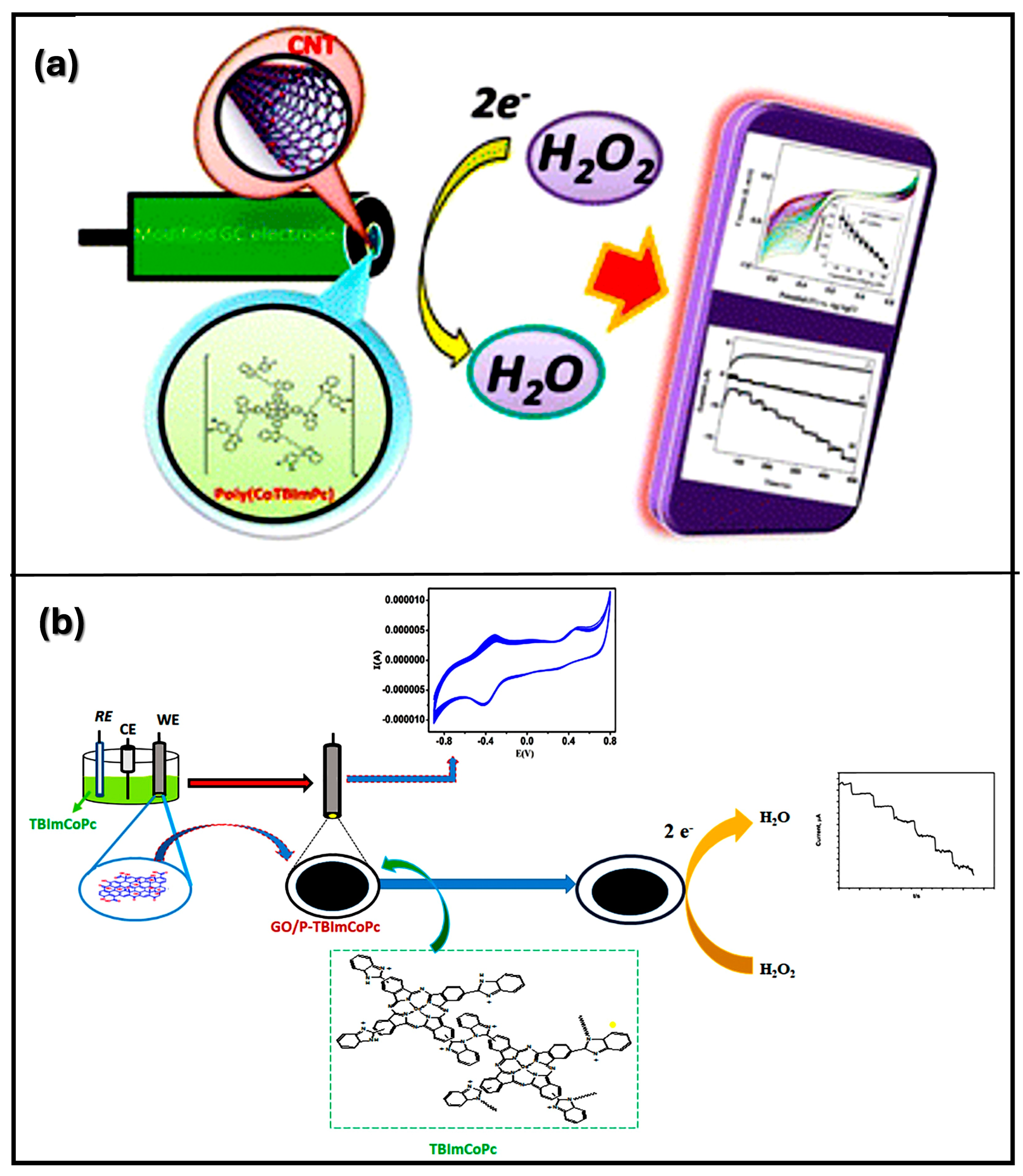
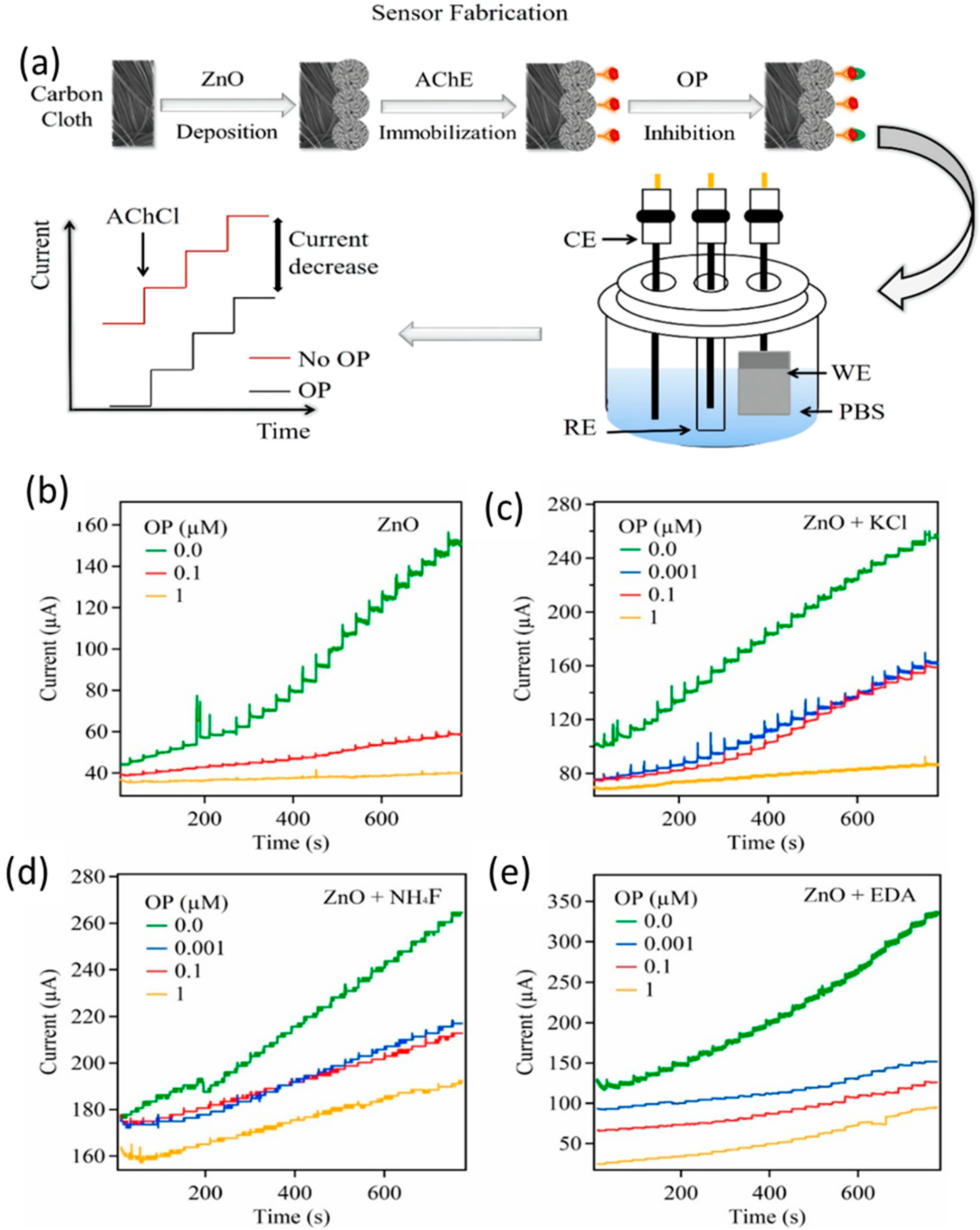
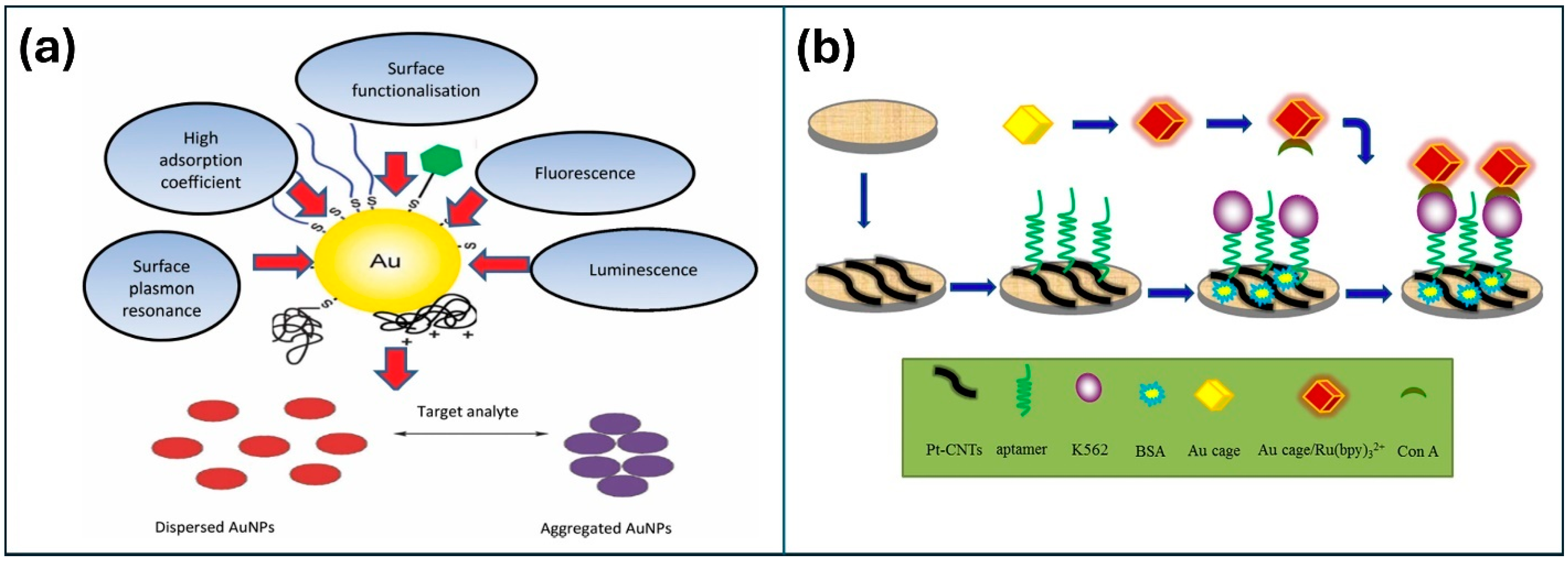
| Material | Method | Analyte | LOD | Linear Range | Ref |
|---|---|---|---|---|---|
| MSN/GO | DPV | CA 153 | 2.8 × 10−4 Um/L | 10−3–200 U/mL | [49] |
| MWCNT/ | CV | Hepatitis C and tuberculosis genomic DNA | 7 fM | 0.1 fM–1 pM | [55] |
| ferrocene | |||||
| CNT | DPV | Dopamine (DA) | 0.1 μM | 0.5–10 μM | [56] |
| MWCNTs | EIS | Mb | 0.08 ng m/L | 0.1–90 ng m/L | [57] |
| SWCNT | DPV | Vibroparahaemolytics thermolabile hemolysin (tlh) gene | 7.27 μM | 1.0 × 10−6–1 × 10−13 mol/L | [59] |
| CNTs | CV | Rutin | 0.075 μM | 0.10–51 μM | [59] |
| CNTs | CV | Rutin | 0.081 μM | 0.10–31 μM | [60] |
| Graphene | CV, LSV | Puerarin | 0.04 μM | 0.06–6.0 μM | [61] |
| Graphene | CV | Puerarin | 0.006 μM | 0.02–40 μM | [62] |
| CNTs–graphene | CV | Hyperin | 0.001 μM | 0.005–1.5 μM | [44] |
| Graphene quantum dots (GQDs) | CV | Rutin | 0.011 μM | 0.05–10 μM | [63] |
| GQD | DPV | Quercentin (Que) | 8.2 × 10−4 μM | 0.002–1.6 μM | [64] |
| Mesoporous carbon | CV | Rutin | 0.002 μM | 0.1–30 μM | [65] |
| Material | Method | Analyte | LOD | Linear Range | Ref |
|---|---|---|---|---|---|
| Au | Amperometry | Cysteine | 3.1 μM | 10–80 μM | [81] |
| Glutathione | 0.1 μM | 0.3–10 μM | |||
| Methionine | 1 μM | 3.3–39 μM | |||
| Homocysteine | 0.6 μM | 2.2–30 μM | |||
| Au | EIS | Carcinoma antigen 125 | 6.7 U/mL | 0–100 U/mL | [82] |
| Carcinoma antigen 125 | 419 ng/mL | 450 ng/mL–2.916 μg/mL | |||
| Pt | EIS | Carcinoma antigen 125 | 386 ng/mL | 450 ng/mL–2.916 μg/mL | [83] |
| Pt | Amperometry | Glucose | 44.3 μM | 0.25–6.0 mM | [84] |
| CuO nanospheres | Amperometry | Glucose | 1 μM | 2.5–20 mM | [85] |
| ZnCo2O4 | Amperometry | DA | 15.5 μM | 5–100 μM | [86] |
| TiO2 | Amperometry | Glucose | 100 nM | 100 nM–5 mM | [87] |
| Pd–Cu | - | Glucose | 20 μM | 2–18 mM | [88] |
| Au | - | Glucose | 0.05 μM | 0.1–25 mM | [89] |
| Pt | - | Glucose | 7.2 × 10−8 M | 1.0 × 10−7–2.0 × 10−5 M | [90] |
| Material | Selectivity | Detection Limit | Sensitivity | Technical Benefits | Application Examples |
|---|---|---|---|---|---|
| Graphene & Graphene Oxide (GO) | Selective with modifications (e.g., enzymes, antibodies) for biomolecules like glucose, DNA | Low (fM–pM levels for DNA) | High due to excellent electron transfer properties | High surface area, stability, tunable functional groups | Glucose sensors, DNA biosensors |
| Carbon Nanotubes (CNTs) | Functionalized for specific biomolecule binding, selective to neurotransmitters, DNA | Low (nM–µM range) | High, due to conductivity and large surface area | Excellent conductivity, stability under diverse conditions | Dopamine, glucose, and DNA sensing |
| Carbon Dots (CDs) | Modified for ion/molecule targeting, high specificity for small molecules | Low (nM range) | High, with luminescent properties enhancing signal | Stable luminescence, customizable surface functionality | Ion sensing, environmental pollutant detection |
| Activated Carbon | Porous structure allows selective adsorption, suited for various chemical sensors | Moderate (µM range) | Moderate, depending on surface modifications | High porosity, low cost, good stability | VOCs, pollutant detection |
| Gold Nanoparticles (AuNPs) | Functionalized with thiol groups, antibodies for specific proteins, DNA | Ultra-low (fM–pM levels) | Very high due to surface plasmon resonance (SPR) | Biocompatible, excellent stability, SPR-enhanced signals | Cancer biomarkers, virus detection |
| Silver Nanoparticles (AgNPs) | Targeted ion detection, antimicrobial properties | Low (pM–nM levels) | High, SPR enhances sensitivity | Antimicrobial, strong SPR, surface modifiable | Glucose, H2O2, pathogen detection |
| Platinum (Pt) & Palladium (Pd) Nanoparticles | Catalytic for reactions (e.g., glucose oxidation), selective in nonenzymatic sensors | Very low (nM–pM for glucose) | Very high due to catalytic efficiency | Catalytic properties, stable under harsh conditions | Nonenzymatic glucose biosensors |
| Metal Oxides (ZnO, TiO2, Fe2O3) | Selective to gases, ions (e.g., ZnO for pH, TiO2 for pollutants) | Moderate to low (µM–nM range) | High with strong response to analyte concentration | Tunable semiconducting properties, stable | pH sensors, pollutant, and gas detection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Channabasavana Hundi Puttaningaiah, K.P. Innovative Carbonaceous Materials and Metal/Metal Oxide Nanoparticles for Electrochemical Biosensor Applications. Nanomaterials 2024, 14, 1890. https://doi.org/10.3390/nano14231890
Channabasavana Hundi Puttaningaiah KP. Innovative Carbonaceous Materials and Metal/Metal Oxide Nanoparticles for Electrochemical Biosensor Applications. Nanomaterials. 2024; 14(23):1890. https://doi.org/10.3390/nano14231890
Chicago/Turabian StyleChannabasavana Hundi Puttaningaiah, Keshavananda Prabhu. 2024. "Innovative Carbonaceous Materials and Metal/Metal Oxide Nanoparticles for Electrochemical Biosensor Applications" Nanomaterials 14, no. 23: 1890. https://doi.org/10.3390/nano14231890
APA StyleChannabasavana Hundi Puttaningaiah, K. P. (2024). Innovative Carbonaceous Materials and Metal/Metal Oxide Nanoparticles for Electrochemical Biosensor Applications. Nanomaterials, 14(23), 1890. https://doi.org/10.3390/nano14231890





