A Facile Microwave-Promoted Formation of Highly Photoresponsive Au-Decorated TiO2 Nanorods for the Enhanced Photo-Degradation of Methylene Blue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Microwave Synthesis of TiO2 and Au/TiO2
2.3. Characterization
2.4. Photocatalytic Activity Tests
3. Results and Discussion
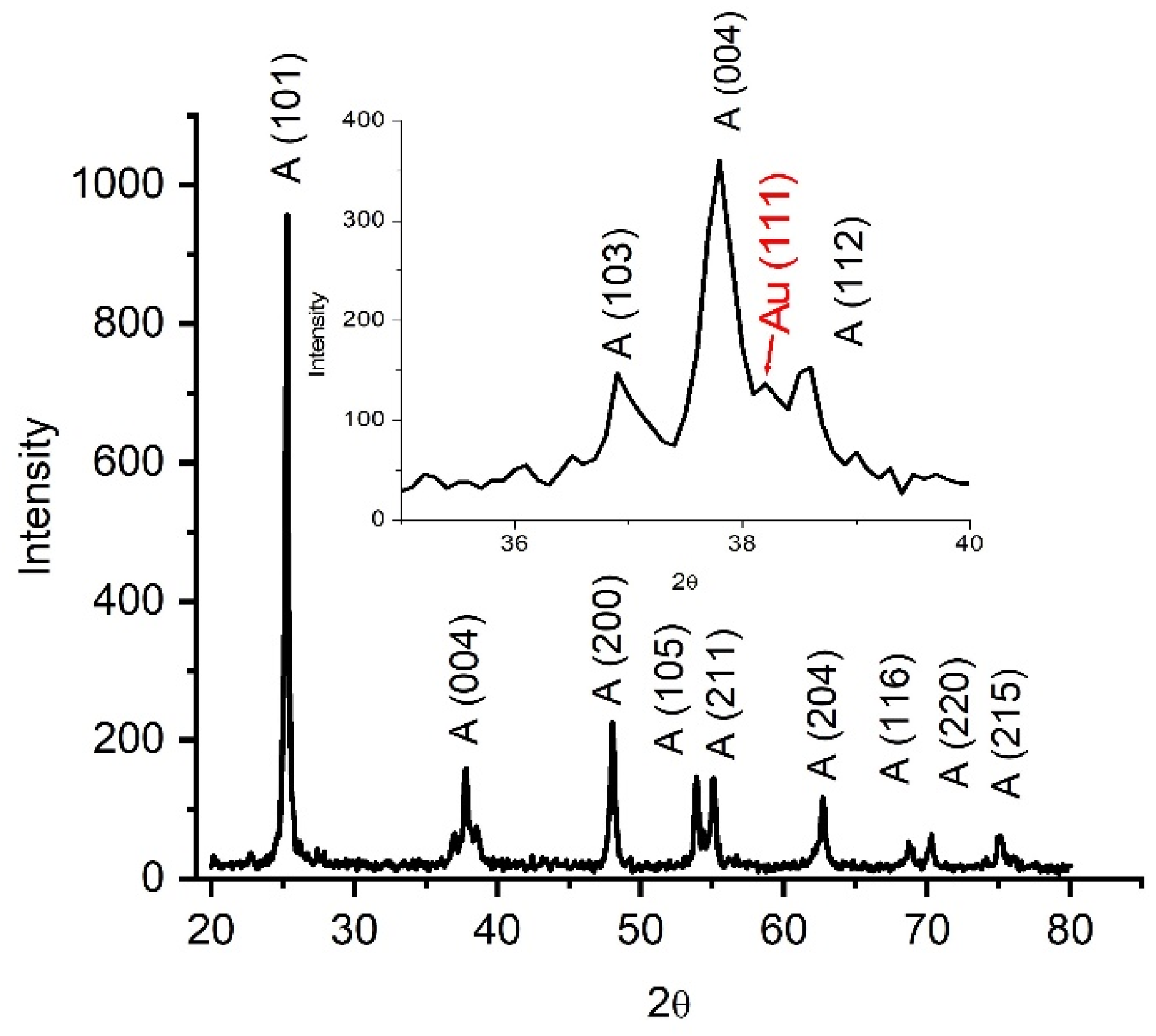
3.1. XRD Analysis
3.2. XPS Analysis
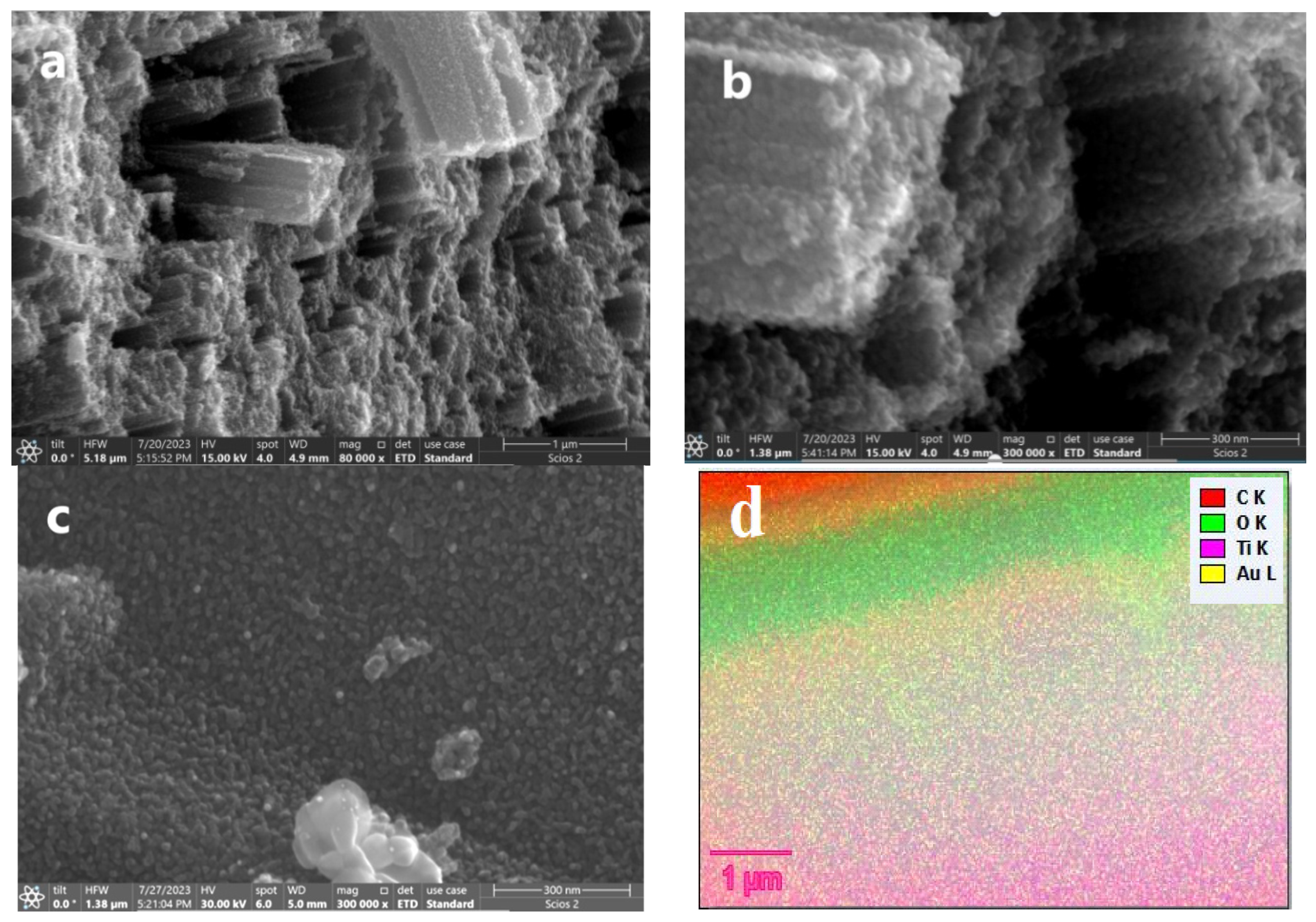
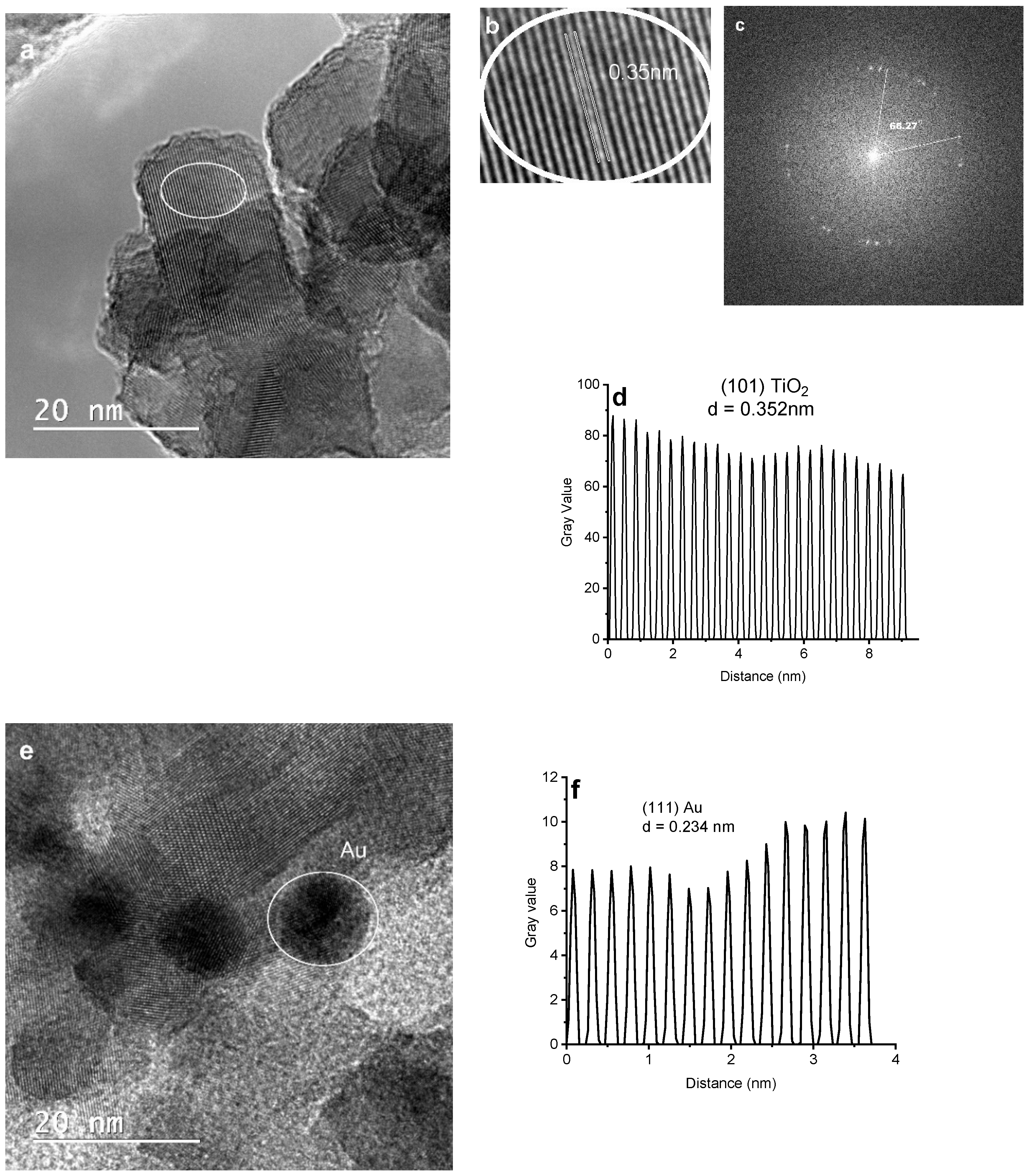
3.3. Morphological Studies
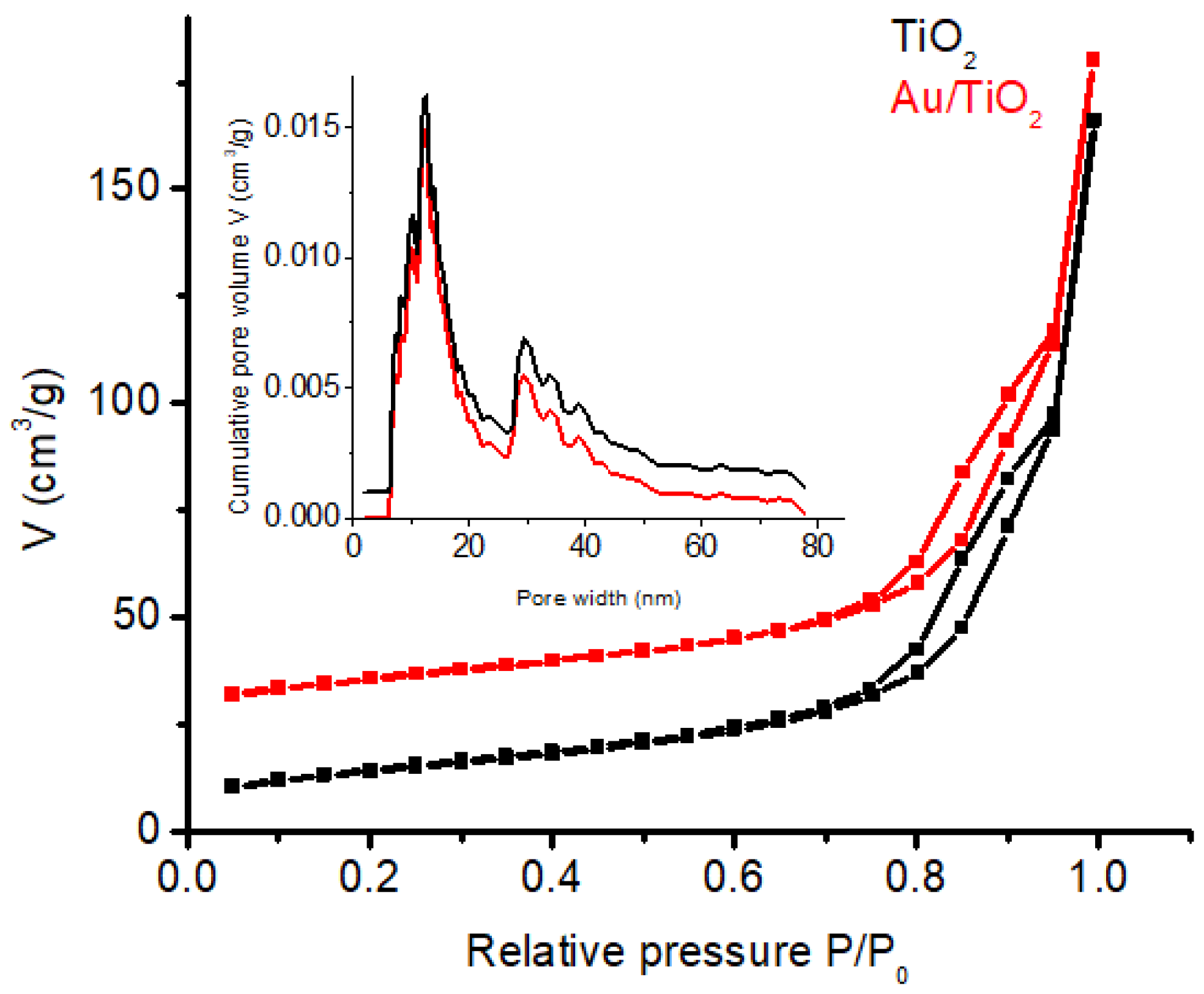
3.4. N2 Physisorption
3.5. Optical Studies
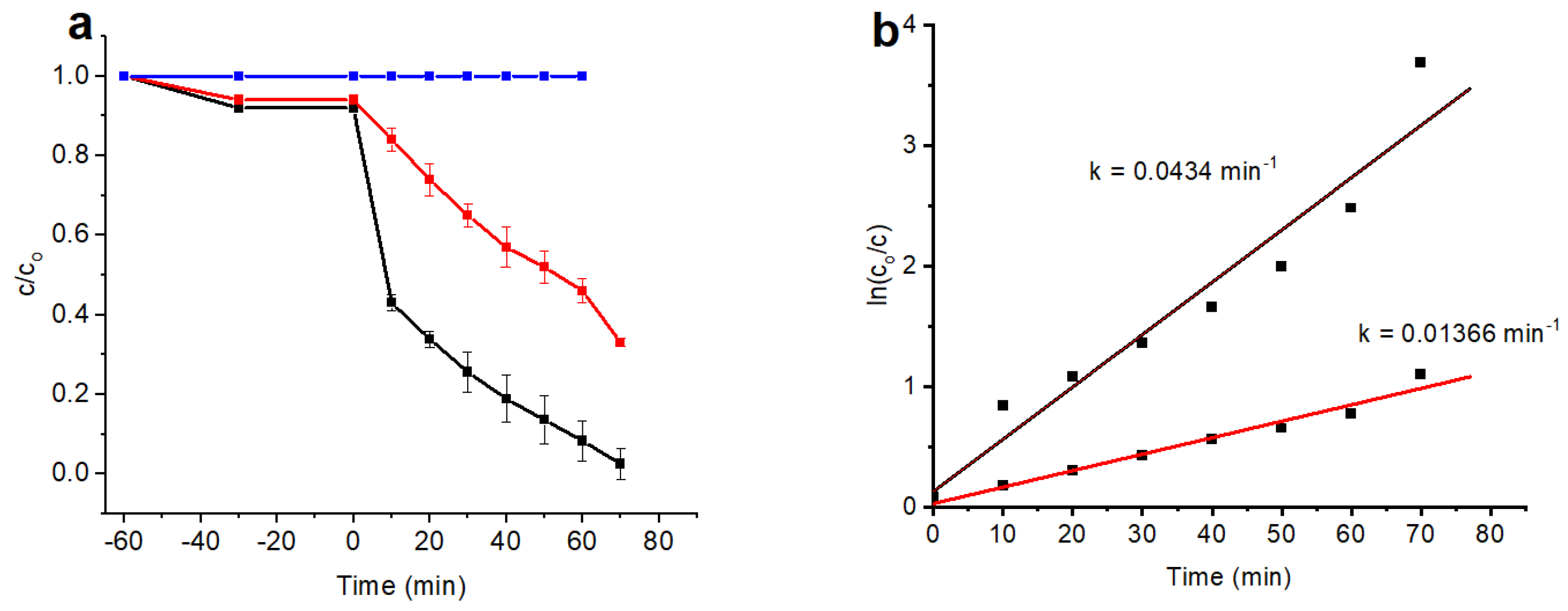
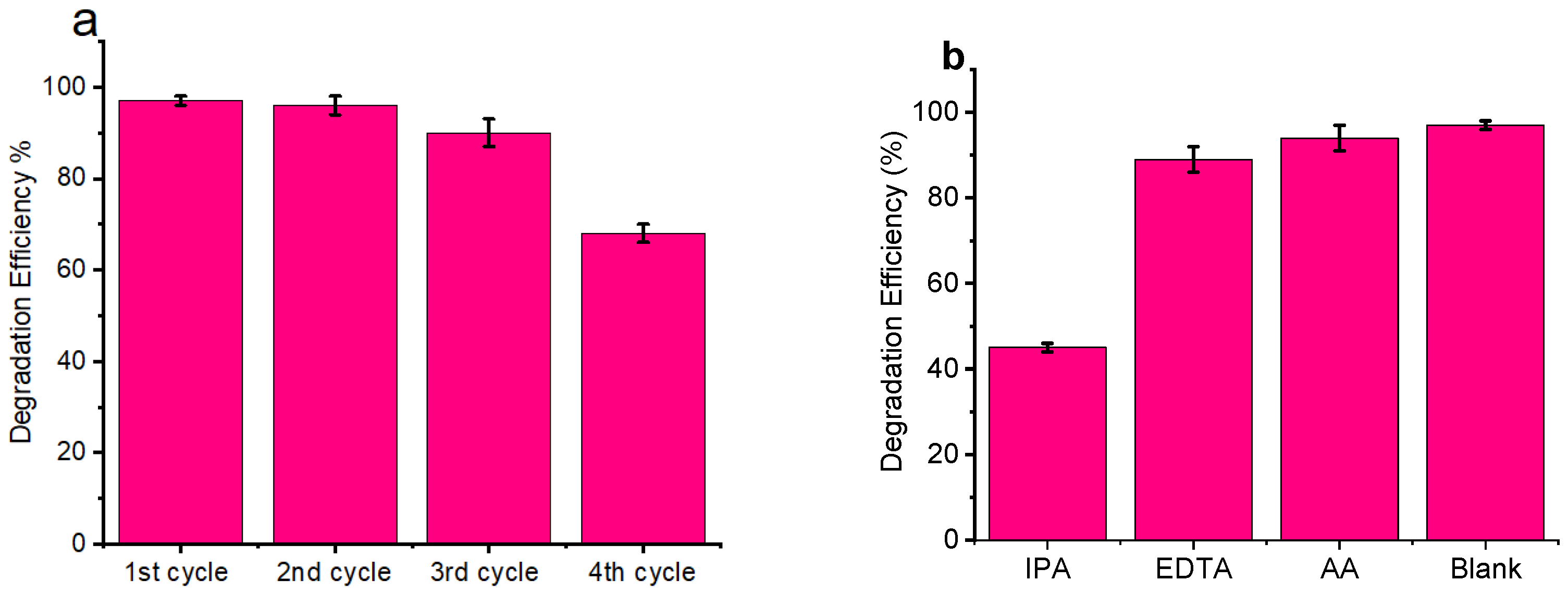
3.6. Photocatalytic Activity
3.7. Scavenger Test
3.8. Proposed Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lakhani, L. Impact of pollution on human health: A mini review article. Environ. Conserv. J. 2018, 19, 11–15. [Google Scholar] [CrossRef]
- Cui, H.; Wang, K.; Ma, E.; Wang, H. Multifunctional Biotemplated Micromotors for In Situ Decontamination of Antibiotics and Heavy Metals in Soil and Groundwater. Nanomaterials 2023, 13, 2710. [Google Scholar] [CrossRef] [PubMed]
- McKeown, E.; Bugyi, G. Impact of Water Pollution on Human Health and Environmental Sustainability; IGI Global: Hershey, PA, USA, 2016. [Google Scholar] [CrossRef]
- Hanif, M.A.; Miah, R.; Islam, M.A.; Marzia, S. Impact of Kapotaksha river water pollution on human health and environment. Progress. Agric. 2020, 31, 1–9. [Google Scholar] [CrossRef]
- Farhan, M.; Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Ismail, M.; Akhtar, K.; Khan, M.; Kamal, T.; Khan, M.A.; Asiri, A.M.; Seo, J.; Khan, S.B. Pollution, Toxicity and Carcinogenicity of Organic Dyes and their Catalytic Bio-Remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, H.; Lee, K.; Noh, S.; Jin, S.; Jae, J.; Jeong, Y.; Noh, J. ZnO/Graphene Oxide on Halloysite Nanotubes as a Superabsorbent Nanocomposite Photocatalyst for the Degradation of Organic Dyes. Nanomaterials 2023, 13, 1895. [Google Scholar] [CrossRef] [PubMed]
- Patle, D.S.; Khajone, V.B.; Bhagat, P.R.; Jaiswal, A.K.; Kumar, S. Functionalized Ionic Liquids for the Photodegradation of Dyes. In Water Pollution and Remediation: Photocatalysis Environmental Chemistry for a Sustainable World; Springer: Cham, Switzerland, 2020; pp. 391–409. [Google Scholar] [CrossRef]
- Maheshwari, K.; Agrawal, M.; Gupta, A.B. Dye Pollution in Water and Wastewater. In Novel Materials for Dye-Containing Wastewater Treatment; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Mittal, Y.; Dash, S.; Srivastava, P.; Mishra, P.M.; Aminabhavi, T.M.; Yadav, A.K. Azo Dye Containing Wastewater Treatment in Earthen Membrane Based Unplanted Two Chambered Constructed Wetlands-microbial Fuel Cells: A New Design for Enhanced Performance. Chem. Eng. J. 2022, 427, 131856. [Google Scholar] [CrossRef]
- Mecha, A.C.; Chollom, M.N. Photocatalytic ozonation of wastewater: A review. Environ. Chem. Lett. 2020, 18, 1491–1507. [Google Scholar] [CrossRef]
- Caracciolo, D.; Vaiano, V.; Gambicorti, T.; Sacco, O. Photocatalytic Treatment of Industrial Wastewaters Using Structured Photocatalysts. Chem. Eng. Trans. 2021, 84, 151–156. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Li, J.; Zhang, Y. Effects of calcination temperature on morphology and structure of CeO2 nanofibers and their photocatalytic activity. Mater. Lett. 2019, 241, 76–79. [Google Scholar] [CrossRef]
- Parrino, F.; D’Arienzo, M.; Mostoni, S.; Dirè, S.; Ceccato, R.; Bellardita, M.; Palmisano, L. Electron and Energy Transfer Mechanisms: The Double Nature of TiO2 Heterogeneous Photocatalysis. Top. Curr. Chem. 2022, 380, 2. [Google Scholar] [CrossRef] [PubMed]
- Shoneye, A.; Chang, S.J.; Chong, M.N.; Tang, J. Recent progress in photocatalytic degradation of chlorinated phenols and reduction of heavy metal ions in water by TiO2-based catalysts. Int. Mater. Rev. 2021, 67, 47–64. [Google Scholar] [CrossRef]
- Miyabe, H.; Kohtani, S. Photocatalytic single electron transfer reactions on TiO2 semiconductor. Sci. China-Chem. 2019, 62, 1439–1449. [Google Scholar] [CrossRef]
- Su, R.; Besenbacher, F.; Hutchings, G.J. Alternative Materials to TiO2. In Heterogeneous Photocatalysis: From Fundamentals to Green Applications; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Zeng, G.; Qiu, J.; Li, Z.; Pavaskar, P.; Cronin, S. CO2 Reduction to Methanol on TiO2-Passivated GaP Photocatalysts. ACS Catal. 2014, 4, 3512–3516. [Google Scholar] [CrossRef]
- Wick-Joliat, R.; Musso, T.; Prabhakar, R.R.; Löckinger, J.; Siol, S.; Cui, W.; Sévery, L.; Moehl, T.; Suh, J.; Hutter, J.; et al. Stable and tunable phosphonic acid dipole layer for band edge engineering of photoelectrochemical and photovoltaic heterojunction devices. Energy Environ. Sci. 2019, 12, 1901–1909. [Google Scholar] [CrossRef]
- Luna, M.; Barawi, M.; Gomez-Monivas, S.; Colchero, J.; Rodríguez-Peña, M.; Yang, S.; Zhao, X.; Lu, Y.X.; Chintala, R.C.; Reñones, P.; et al. Photoinduced Charge Transfer and Trapping on Single Gold Metal Nanoparticles on TiO2. ACS Appl. Mater. Interfaces 2021, 13, 50531–50538. [Google Scholar] [CrossRef]
- Li, X.; Han, T.; Zhou, Y.T.; Xie, Y.; Luo, Y.D.; Huang, J.T.; Chen, Z.; Deng, F. Photoelectrocatalytic hydrogen evolution and synchronous degradation of organic pollutants by pg-C3N4/β-FeOOH S-scheme heterojunction. Sci. China Technol. Sci. 2024, 67, 1238–1250. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Gutiérrez, M.; Cohen, B.; Valverde-González, A.; Iglesias, M.; Abderrazzak, D. How does the Metal Doping in Mixed Metal MOFs Influence their Photodynamics? A Direct Evidence for Improved Photocatalysts. Mater. Today Energy 2022, 29, 101125. [Google Scholar] [CrossRef]
- Mao, T.; Liu, M.; Lin, L.; Chen, Y.; Fang, C. A Study on Doping and Compound of Zinc Oxide Photocatalysts. Polymers 2022, 14, 4484. [Google Scholar] [CrossRef]
- Al-Naggar, A.H.; Shinde, N.M.; Kim, J.S.; Mane, R.S. Water splitting performance of metal and non-metal-doped transition metal oxide electrocatalysts. Coord. Chem. Rev. 2023, 474, 214864. [Google Scholar] [CrossRef]
- Wang, K.; Liu, B.; Liu, Z.; Jiang, X.; Zhang, Z.; Han, W. Improved photocarrier separation in Ga/Fe gradient (Ga, Fe)2O3 thin films. Appl. Phys. Lett. 2023, 122, 203901. [Google Scholar] [CrossRef]
- Hernández, J.V.; Coste, S.; Murillo, A.G.; Romo, F.J.C.; Kassiba, A. Effects of metal doping (Cu, Ag, Eu) on the electronic and optical behavior of nanostructured TiO2. J. Alloys Compd. 2017, 710, 355–363. [Google Scholar] [CrossRef]
- Huang, H.; Wang, J.; Kong, Q.; Wei, Z.S.; Chang, Y.C. Noble Metal Modified TiO2 Hierarchically Structured Microspheres with Enhanced Photocatalytic Activity. Catalysts 2023, 13, 995. [Google Scholar] [CrossRef]
- Seman, N.; Tarmitzi, Z.I.; Ali, R.R.; Taib, S.H.M.; Salleh, M.S.N.; Zhe, J.C.; Mohamad Sukri, S.N.A. Preparation method of titanium dioxide nanoparticles and its application: An Update. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1091. [Google Scholar]
- Kong, X.; Zeng, C.; Wang, X.; Huang, J.; Li, C.; Fei, J.; Li, J.; Feng, Q. Ti-O-O coordination bond caused visible light photocatalytic property of layered titanium oxide. Sci. Rep. 2016, 6, 29049. [Google Scholar] [CrossRef]
- Ng, J.; Xu, S.; Zhang, X.; Yang, H.; Sun, D. Hybridized nanowires and cubes: A novel architecture of a heterojunctionedTiO2/SrTiO3 thin film for efficient water splitting. Adv. Funct. Mater. 2010, 20, 4287–4294. [Google Scholar] [CrossRef]
- Singha, J.; Sahua, K.; Satpatib, B.; Shahc, J.; Kotnalac, R.K.; Mohapatra, S. Facile synthesis, structural and optical properties of Au-TiO2 plasmonic nanohybrids for photocatalytic applications. J. Phys. Chem. Solids 2019, 135, 109100. [Google Scholar] [CrossRef]
- Ye, S.; Connell, S.D.; McLaughlan, J.R.; Roach, L.; Aslam, Z.; Chankhunthod, N.; Brown, A.P.; Brydson, R.; Bushby, R.J.; Critchley, K.; et al. One-Step Preparation of Biocompatible Gold Nanoplates with Controlled Thickness and Adjustable Optical Properties for Plasmon-Based Applications. Adv. Funct. Mater. 2020, 30, 2003512. [Google Scholar] [CrossRef]
- Liu, M.; Piao, L.; Zhao, L.; Ju, S.; Yan, Z.; He, T.; Zhou, C.; Wang, W. Anatase TiO2 single crystals with exposed {001} and {110} facets: Facile synthesis and enhanced photocatalysis. Chem. Commun. 2010, 46, 1664–1666. [Google Scholar] [CrossRef]
- Blahovec, J.; Yanniotis, S. Modified classification of sorption isotherms. J. Food Eng. 2009, 91, 72–77. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, S.S.; Han, L.; Tian, B.C.; Li, N.; Chen, W.; Li, L.B. Ferroelectric perovskite PbTiO3 for advanced photocatalysis. Rare Met. 2024, 43, 4038–4055. [Google Scholar] [CrossRef]
- Rubesh Ashok Kumar, S.; Vasvini Mary, D.; Suganya Josephine, G.; Sivasamy, A. Hydrothermally Synthesized WO3:CeO2 Supported g-C3N4 Nanolayers for Rapid Photocatalytic Degradation of Azo Dye under Natural Sunlight. Inorg. Chem. Commun. 2024, 164, 112366. [Google Scholar] [CrossRef]
- Wu, X.; Chen, G.; Wang, J.; Li, J.; Wang, G. Review on S-Scheme Heterojunctions for Photocatalytic Hydrogen Evolution. Acta Phys.-Chim. Sin. 2023, 39, 2212016. [Google Scholar] [CrossRef]
- Balsamo, S.A.; Sciré, S.; Condorelli, M.; Fiorenza, R. Photocatalytic H2 Production on Au/TiO2: Effect of Au Photodeposition on Different TiO2 Crystalline Phases. J 2022, 5, 92–104. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Hsiao, Y.-S.; Wei, P.-C.; Liu, Y.-T.; Chu, C.-C.; Hsiao, V.K.S. Visible Light-Induced Photocatalyst with Au/TiO2 Nanocomposites Fabricated through Pulsed Laser-Induced Photolysis. Catalysts 2022, 11, 1459. [Google Scholar] [CrossRef]
- Usman, A.K.; Cursaru, D.-L.; Brănoiu, G.; Şomoghi, R.; Manta, A.-M.; Matei, D.; Mihai, S. A Modified Sol–Gel Synthesis of Anatase {001}-TiO2/Au Hybrid Nanocomposites for Enhanced Photodegradation of Organic Contaminants. Gels 2022, 8, 728. [Google Scholar] [CrossRef]


| Variable | Total Count | Mean | SE Mean | StDev | Minimum | Maximum |
|---|---|---|---|---|---|---|
| L1 | 101 | 14.712 | 0.325 | 3.249 | 5.595 | 24.060 |
| L2 | 105 | 14.368 | 0.257 | 2.620 | 8.986 | 22.318 |
| L3 | 80 | 14.997 | 0.320 | 2.843 | 10.025 | 23.288 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondarev, A.; Mihai, S.; Usman, A.K.; Cursaru, D.L.; Matei, D.; Sătulu, V.; Gheorghe, C.; Brănoiu, G.; Şomoghi, R. A Facile Microwave-Promoted Formation of Highly Photoresponsive Au-Decorated TiO2 Nanorods for the Enhanced Photo-Degradation of Methylene Blue. Nanomaterials 2024, 14, 1780. https://doi.org/10.3390/nano14221780
Bondarev A, Mihai S, Usman AK, Cursaru DL, Matei D, Sătulu V, Gheorghe C, Brănoiu G, Şomoghi R. A Facile Microwave-Promoted Formation of Highly Photoresponsive Au-Decorated TiO2 Nanorods for the Enhanced Photo-Degradation of Methylene Blue. Nanomaterials. 2024; 14(22):1780. https://doi.org/10.3390/nano14221780
Chicago/Turabian StyleBondarev, Andreea, Sonia Mihai, Abubakar Katsina Usman, Diana Luciana Cursaru, Dănuţa Matei, Veronica Sătulu, Cătălina Gheorghe, Gheorghe Brănoiu, and Raluca Şomoghi. 2024. "A Facile Microwave-Promoted Formation of Highly Photoresponsive Au-Decorated TiO2 Nanorods for the Enhanced Photo-Degradation of Methylene Blue" Nanomaterials 14, no. 22: 1780. https://doi.org/10.3390/nano14221780
APA StyleBondarev, A., Mihai, S., Usman, A. K., Cursaru, D. L., Matei, D., Sătulu, V., Gheorghe, C., Brănoiu, G., & Şomoghi, R. (2024). A Facile Microwave-Promoted Formation of Highly Photoresponsive Au-Decorated TiO2 Nanorods for the Enhanced Photo-Degradation of Methylene Blue. Nanomaterials, 14(22), 1780. https://doi.org/10.3390/nano14221780









