Organic–Inorganic Hybridization of Silkworm Cocoon Filaments Using Nano Pastes of Silica–Phosphate–M (M = Cu, Fe, or Al)
Abstract
1. Introduction
2. Materials and Methods
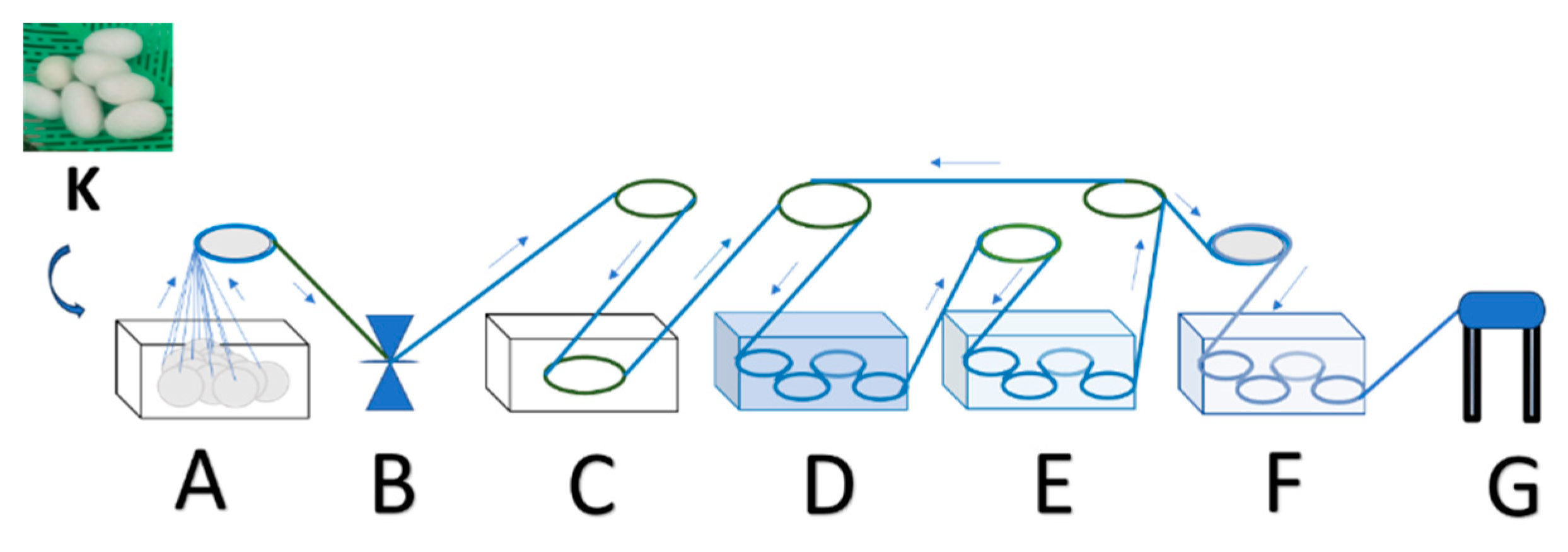
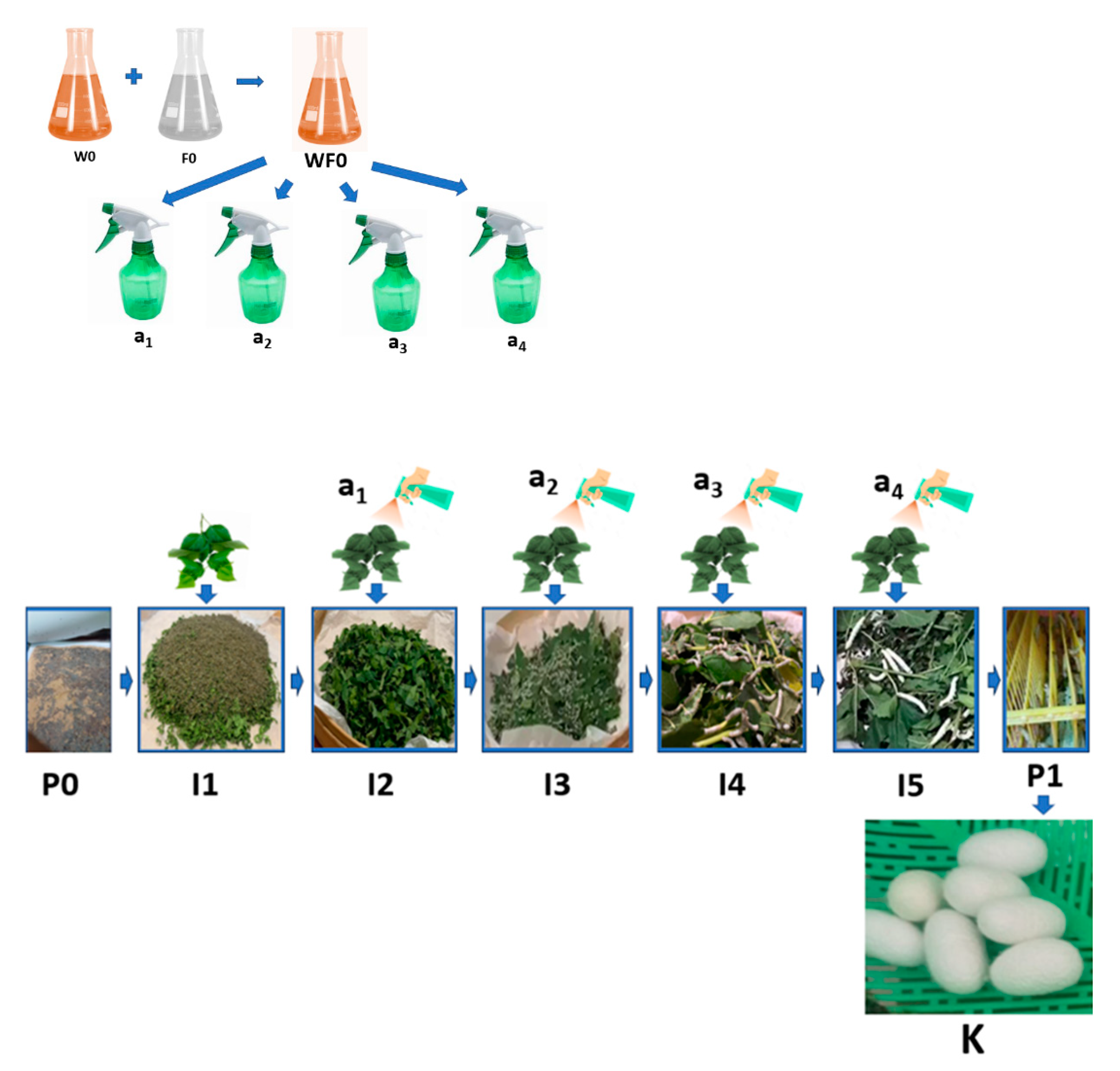
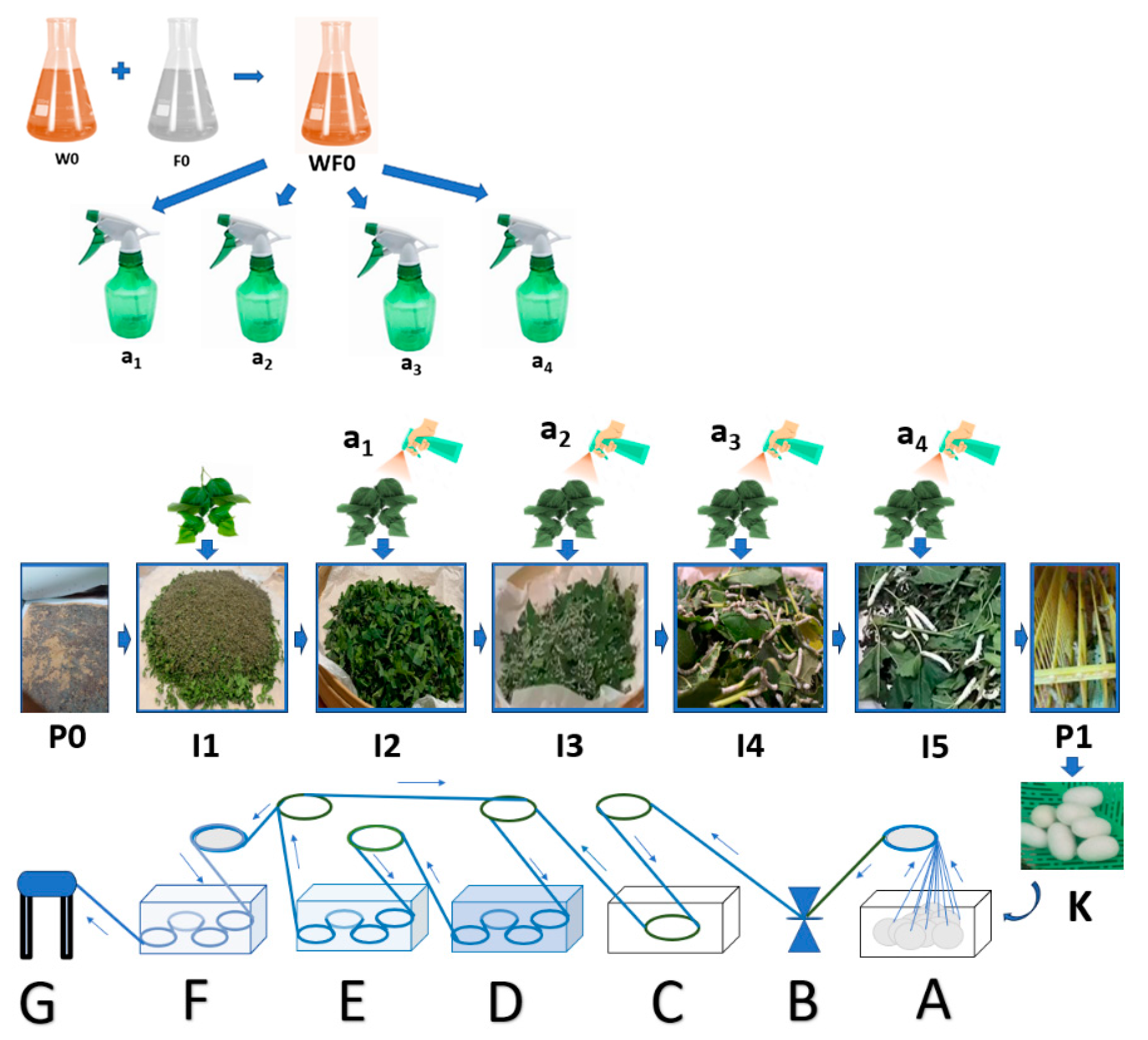
- The stage of hatching the eggs of the silkworm Bombyx mori (P0) was conducted by placing the silkworm in a place not exposed to sunlight for 10–12 days, then placing the silkworm seeds in a dark atmosphere with a humidity of 80–90% and an air temperature of 25–28 °C for 2 days, and preparing a sterile hatchery through the addition of chlorine powder (CaOCl2) and calcined lime (CaO) with a mass ratio of 5–7:95–93 that was evenly sprinkled over the surface of the newly hatched silkworm cluster.
- The stage of preparing natural color nano paste (W0) was conducted by extracting natural colors from natural materials, such as yellow natural color extract from turmeric rhizomes (Curcuma longa Linn.), red color extract from areca nut fruit (Areca cathecu), and blue color extract from indigo leaves (Strobilanthes cusia), then thickening the extract by heating slowly at low to medium temperature and/or adding coagulants such as CaO solution for indigo leaf extract until a natural color paste is obtained. Then, we prepared nano silica-based natural color fixator nano paste (F0) by mixing nano silica (made from rice husk ash) with nano calcium phosphate (made from hydroxyapatite of cow bone waste) and copper sulphate mineral with a ratio of 500–600 g of nano silica powder: 200–300 g of nano calcium phosphate powder: 100–200 g of copper sulphate (CuSO4), then made a mixture of natural color nano paste and natural color fixator nano paste (WF0) with a mass ratio of 1:1, using this mixture as an additional ingredient stock (WF0) in silkworm feed engineering.
- The stage of maintaining silkworms in the 1st life phase of instar 1 (I1) began the 1st day after hatching and lasted until the 4th or 5th day, when the small caterpillars fell asleep. It included preparing young mulberry (Morus alba) leaves, namely, the third to fifth leaves of the mulberry shoots, by washing the mulberry leaves with water, air-drying the washed mulberry leaves by placing the leaves in the shade until dry, and cutting or slicing the dried leaves to a width of 1–2 cm. Then, we fed the silkworms 3 times a day (morning at 07.00–08.00, afternoon at 12.00–13.00, night at 19.00–20.00), and repeating daily until the I1 ends, namely, the caterpillars begin to sleep, thus ending the I1 phase and progressing to the I2 (24–36 h) phase by evenly sprinkling the CaO mixed with CaOCl2 powders (95:5) on the surface of the caterpillars, while maintaining the room temperature and humidity during the 1st instar life phase of the caterpillars at 25–28 °C and 80–90%, respectively, by using air conditioning (air conditioning and air cooler) and spraying the walls of the room with water.
- The stage of raising silkworms in the 2nd life phase of instar 2 (I2) began with feed engineering through preparing fresh mulberry leaves from the 3rd to 10th leaf from the shoots, washing the leaves, then cutting or slicing them to a width of 3–5 cm. Then, we evenly sprayed a mixture of natural color nano paste and nano silica-based natural color fixator nano paste with a concentration of 1–1.25% m/v (or 10–12.5 mL of a mixture of natural color paste and nano silica-based natural color fixator nano paste in water until the volume becomes 1000 mL) (A1) on the surface of the sliced mulberry leaves, then we let the leaves sit in an open and shady space for 8–10 h until they are dry, and fed the silkworms 3 times a day (morning 07.00–08.00, noon 12.00–13.00, night 19.00–20.00). This is repeated daily until the I2 phase end, namely, the caterpillars begin to sleep. The 2nd instar silkworm progress to the I3 (24–36 h) phase by evenly sprinkling the CaO powder mixed with the CaOCl2 powder (95:5), while maintaining the room temperature and humidity during the I2 phase of the caterpillar life at 25–28 °C and 80–90%, respectively, by using air conditioning (air conditioner and air cooler) and spraying the walls of the room with water.
- The stage of raising silkworms in the 3rd life phase of instar 3 (I3) began with feed engineering through preparing fresh mulberry leaves from the 3rd to 10th leaf from the shoots, washing the leaves, evenly spraying a mixture of natural color nano paste and nano silica-based natural color fixator nano paste with a concentration of 2.5–3.0% volume/volume (or 25–30 mL of a mixture of natural color paste and nano silica-based natural color fixator nano paste in water until the volume becomes 1000 mL) (A2) on the surface of the mulberry leaf. Then, we let the leaves sit in an open and shady space for 8–10 h until they are dry, and feed the silkworms 3 times a day (morning 07.00–08.00, afternoon 12.00–13.00, night 19.00–20.00), repeating daily until the I3 phase ends, namely, the caterpillars begin to sleep, and the I3 phase progresses to the I4 (24–36 h) phase by evenly sprinkling the CaO powders mixed with CaOCl2 powders (95:5) while maintaining the room temperature and humidity during the I3 phase of the caterpillar life at 25–28 °C and 80–90%, respectively, by using air conditioning (air conditioner and air cooler) and spraying the walls of the room with water.
- The stage of raising silkworms in the 4th life phase of instar 4 (I4) began with feed engineering through preparing fresh mulberry leaves and stems/stalks with a size of 20–30 cm from the shoots, washing the leaves, evenly spraying a mixture of natural color nano paste and nano silica-based natural color fixator nano paste, with a concentration of 5–6% m/v (or 50–60 mL of a mixture of natural color paste and nano silica-based natural color fixator nano paste in water until the volume becomes 1000 mL) (A3), on the surface of the mulberry leaves and stems. Then, we let the leaves sit in an open and shady space for 8–10 h until they become dry, and feed the silkworms 3 times a day (morning 07.00–08.00, afternoon 12.00–13.00, night 19.00–20.00), repeating daily until the I4 phase ends, namely, the caterpillars begin to sleep. The 4th instar phase silkworms progress to the 5th instar (24–36 h) phase by evenly sprinkling the calcined lime powder (CaO) mixed with the chlorine powder (95:5) while maintaining the room temperature and humidity during the 4th phase of life at 25–28 °C and 80–90%, respectively, by using air conditioning (air conditioner and air cooler) and spraying the walls of the room with water.
- The stage of raising silkworms in the 5th life phase of instar 5 (I5) began with feed engineering through preparing fresh mulberry leaves and stems/stalks with a size of 50–60 cm from the shoots, washing the leaves, evenly spraying a mixture of natural color nano paste and nano silica-based natural color fixator nano paste with a concentration of 7.5–10% volume/volume (or 75–100 mL of a mixture of natural color paste and nano silica-based natural color fixator nano paste in water until the volume becomes 1000 mL) (A4) on the surface of the mulberry leaves and stems. Then, we let the leaves sit in an open and shady space for 8–10 until they become dry, and feed the silkworms 4 times a day (morning 07.00–08.00, afternoon 12.00–13.00, afternoon 16.00–17.00 and night 19.00–20.00), repeating daily until the I5 phase ends (about 6–7 days) with signs that the caterpillars would be nostalgic, namely, the caterpillars begin to stop eating, their movement slows down, the color of the caterpillars become bright yellow, and they secrete a saliva of fine fibers from their mouths, spin, and wrap themselves with the fibers that come out of their mouths. During the I5 phase, the temperature and humidity of the room is maintained at 25–28 °C and 80–90%, respectively, by using air conditioning and spraying the walls of the room with water.
- The cocooning stage (P1) was conducted by moving the caterpillars that were ready to nest to seeding sites (well known as Seri frames) that have been disinfected with lime powder mixed with chlorine in a ratio of 90:10 beforehand. Furthermore, we can harvest the cocoon after the 4th or 5th day from the time the caterpillar first grows and produces a cocoon (K) as expected.
3. Results
3.1. Implementation Test of PTPD-A and Its Product Characterization
3.2. Implementation of PTPD-B Test and Product Characterization
3.3. Implementation of PTPD-C Test and Product Characterization
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shamsheer, B.; Mughal, T.A.; Yousaf, Z.; Riaz, N.; Aftab, A.; Zahoor, M. Green Synthesis of Dyes and Appliance on Silk by Using Metamordanting Technique. Biol. Sci. PJSIR 2021, 64, 116–125. [Google Scholar] [CrossRef]
- Some, S.; Bulut, O.; Biswas, K.; Kumar, A.; Roy, A.; Sen, I.K.; Mandal, A.; Franco, O.L.; Ince, I.A.; Neog, K.; et al. Effect of Feed Supplementation with Biosynthesized Silver Nanoparticles using Leaf Extract of Morus indica L. V1 on Bombyx mori L. (Lepidoptera: Bombycidae). Sci. Rep. 2019, 9, 14839. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zou, F.; Zhang, J.; Tang, B.; Wang, J. Enhanced Properties of Silk Fabric through Immobilization of Gold and Titanium Dioxide Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 128018. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, H.; Chen, S.; Wang, W.; Dai, F.; Zhao, H. Characterization of Silkworm Larvae Growth and Properties of Silk Fibres after Direct Feeding of Copper or Silver Nanoparticles. Mater. Des. 2017, 129, 125–134. [Google Scholar] [CrossRef]
- Canabady-Rochelle, L.L.; Belton, D.J.; Deschaume, O.; Currie, H.A.; Kaplan, D.L.; Perry, C.C. Bioinspired Silicification of Silica-binding Peptide-silk Protein Chimeras: Comparison of Chemically and Genetically Produced Proteins. Biomacromolecules 2012, 13, 683–690. [Google Scholar] [CrossRef]
- Martín-Moldes, Z.; López Barreiro, D.; Buehler, M.J.; Kaplan, D.L. Effect of the Silica Nanoparticle Size on the Osteoinduction of Biomineralized Silk-silica Nanocomposites. Acta Biomater. 2021, 120, 203–212. [Google Scholar] [CrossRef]
- Baburaj, A.; Das, S. Dye Fed Silkworms to Produce Naturally Coloured Silk Cocoons. J. Nat. Fibers 2021, 19, 5651–5662. [Google Scholar] [CrossRef]
- Khyade, V.B.; Kandel, E.R. Influence of Aqueous Solution of Eurhodin Treated Mulberry Leaves on the Quality of Cocoons and Silk Filament in Silkworm, Bombyx mori (L) Races: Bivoltine Crossbreed [(CSR6 × CSR26) × (CSR2 × CSR27)] and multivoltine crossbreed [(PM × CSR2)]. Int. J. Res. Sci. Eng. 2018, 6, 22–37. [Google Scholar]
- Guo, Z.; Xie, W.; Gao, Q.; Wang, D.; Gao, F.; Li, S.; Zhao, L. In situ Biomineralization by Silkworm Feeding with Ion Precursors for the Improved Mechanical Properties of Silk Fiber. Int. J. Biol. Macromol. 2018, 109, 21–26. [Google Scholar] [CrossRef]
- Widihastuti. Teknologi Pencelupan Bahan Tekstil. In Mata Kuliah Teknologi Tekstil; UNY: Yogyakarta, Indonesia, 2018. [Google Scholar]
- Siva, R. Status of natural dyes and dye-yielding plants in India. Curr. Sci. 2007, 92, 916–925. [Google Scholar]
- Minko, S.; Sharma, S.; Hardin, I.; Luzinov, I.; Daubenmire, S.W.; Zakharchenko, A.; Saremi, R.; Kim, Y.S. Textile Dyeing Using Nanocellulosc Fibers. U.S. Patent US00,950,618,7B2, 14 January 2016. [Google Scholar]
- Rezaee, S.A.; Oey, M.; Nevejan, C.; Brazier, F. Participatory Demand-Supply Systems. Procedia Comput. Sci. 2015, 44, 105–144. [Google Scholar] [CrossRef]
- Sachdev, R. Method for Dyeing Textile Product Using Neemand Holy Basil Extract. U.S. Patent US00,869,742,9B2, 15 April 2014. [Google Scholar]
- Blank, S. The Four Steps to the Epiphany: Successful Strategies for Products that Win; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Jacobs, G.; Konrad, C.; Berroth, J.; Zerwas, T.; Höpfner, G.; Spütz, K. Function-Oriented Model-Based Product Development. In Design Methodology for Future Products; Springer: Cham, Switzerland, 2022; pp. 243–263. [Google Scholar] [CrossRef]
- Elango, M.; Deepa, M.; Subramanian, R.; Musthafa, A.M. Synthesis, characterization, and antibacterial activity of polyindole/Ag-CuO nanocomposites by reflux condensation method. Polym. Technol. Eng. 2017, 57, 1440–1451. [Google Scholar] [CrossRef]
- Ulfah, U.; Asmi, D. Gugus Fungsional, Mikrostruktur dan Struktur Keramik Kalsium Silikat Berbasis Silika Sekam Padi pada Suhu 1100 °C dengan Teknik Reaksi Padatan. J. Teor. Dan Apl. Fis. 2013, 1, 1. [Google Scholar]
- Sunardi. Kajian Spektroskopi FTIR, XRD dan SEM Kaolin Alam Asal Tatakan, Kalimantan Selatan Hasil Purifikasi dengan Metode Sedimentasi. Sains Dan Terap. Kim. 2010, 4, 137–149. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; Analytical Chemistry: Mumbai, India, 1994. [Google Scholar]
- Khan, S.A.; Ashames, A.; Ullah, H.; Ullah, K.; Murtaza, G.; Hassan, N. Synthesis, Characterization and Safety Evaluation of Sericin-Based Hydrogels for Controlled Delivery of Acyclovir. Pharmaceuticals 2021, 14, 234. [Google Scholar] [CrossRef]
- Bruno, C.; Blasi, M.F.; Mattei, D.; Martellone, L.; Brancaleone, E.; Savoca, S.; Favero, G. Polymer Composition Analysis of Plastic Debris Ingested by Loggerhead Turtles (Caretta Caretta) in Souther Tyrrhemiam Sea Through ATR-FTIR Spectroscopy. Mar. Environ. Res. 2022, 179, 105676. [Google Scholar] [CrossRef]
- Prasdiantika, R.; Suseno. Preparasi dan Penentuan Jenis Oksida Besi pada Material Magnetik Pasir Besi Lansilowo. J. Teknosains 2016, 6, 1–58. [Google Scholar] [CrossRef][Green Version]
- Borah, M.P.; Kalita, B.B.; Jose, S.; Baruah, S. Fabrication of Hydrophobic Surface on Eri Silk/Wool Fabric Using Nano Silica Extracted from Rice Husk. Silicon 2023, 15, 7039–7046. [Google Scholar] [CrossRef]
- Abd El-Aziz, E.; El-Desoky, S.; El-Bahrawy, G.; Ezat, H.; Abd El-Rahman, R.; Abdelraouff, A.; Hassabo, A. Nanotechnology to Improve the Performance of Silk Fabric. J. Text. Color. Polym. Sci. 2024, 21, 33–38. [Google Scholar] [CrossRef]
- Liang, Z.; Zhou, Z.; Li, J.; Zhang, S.; Dong, B.; Zhao, L.; Wu, C.; Yang, H.; Chen, F.; Wang, S. Multi-functional silk fibers/fabrics with a negligible impact on comfortable and wearability properties for fiber bulk. Chem. Eng. J. 2021, 415, 128980. [Google Scholar] [CrossRef]
- Wahyuningsih, S.; Handayani, S.R. Chemistry of Natural Dyes of Batik Crafts Colouring Process. IOP Conf. Ser. Mater. Sci. Eng. 2020, 858, 012052. [Google Scholar] [CrossRef]
- Tong, T.; Xu, A.; Tan, S.; Jiang, H.; Liu, L.; Deng, S.; Wang, H. Biological effects and biomedical applications of areca nut and its extract. Pharmaceuticals 2024, 17, 228. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The natural product curcumin as an antibacterial agent: Current achievements and problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, S.M.; Salvador, R.; Guedes, M.D.; de Francisco, A.C. Opportunities for improving the environmental profile of silk cocoon production under Brazilian conditions. Sustainability 2020, 12, 3214. [Google Scholar] [CrossRef]
- Munasinghe, P.; Druckman, A.; Dissanayake, D.G. A systematic review of the life cycle inventory of clothing. J. Clean. Prod. 2021, 320, 128852. [Google Scholar] [CrossRef]
- Astudillo, M.F.; Thalwitz, G.; Vollrath, F. Life cycle assessment of Indian silk. J. Clean. Prod. 2014, 81, 158–167. [Google Scholar] [CrossRef]
- Silva, A.S.; Costa, E.C.; Reis, S.; Spencer, C.; Calhelha, R.C.; Miguel, S.P.; Ribeiro, M.P.; Barros, L.; Vaz, J.A.; Coutinho, P. Silk sericin: A promising sustainable biomaterial for biomedical and pharmaceutical applications. Polymers 2022, 14, 4931. [Google Scholar] [CrossRef]
- Bernardes, B.G.; Veiga, A.; Barros, J.; García-González, C.A.; Oliveira, A.L. Sustainable Silk-Based Particulate Systems for the Controlled Release of Pharmaceuticals and Bioactive Agents in Wound Healing and Skin Regeneration. Int. J. Mol. Sci. 2024, 25, 3133. [Google Scholar] [CrossRef]
- Opálková Šišková, A.; Kozma, E.; Opálek, A.; Kroneková, Z.; Kleinová, A.; Nagy, Š.; Kronek, J.; Rydz, J.; Eckstein Andicsová, A. Diclofenac embedded in silk fibroin fibers as a drug delivery system. Materials 2020, 13, 3580. [Google Scholar] [CrossRef]
- Florczak, A.; Deptuch, T.; Kucharczyk, K.; Dams-Kozlowska, H. Systemic and local silk-based drug delivery systems for cancer therapy. Cancers 2021, 13, 5389. [Google Scholar] [CrossRef]
- Ahmed, A.; Bain, S.; Prottoy, Z.H.; Morsada, Z.; Islam, M.T.; Hossain, M.M.; Shkir, M. Silk-templated nanomaterial interfaces for wearables and bioelectronics: Advances and prospects. ACS Mater. Lett. 2021, 4, 68–86. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Mohammadi, A.; Aghamirza Moghim Aliabadi, H.; Kashtiaray, A.; Bani, M.S.; Karimi, A.H.; Maleki, A.; Mahdavi, M. A novel ternary magnetic nanobiocomposite based on tragacanth-silk fibroin hydrogel for hyperthermia and biological properties. Sci. Rep. 2024, 14, 8166. [Google Scholar] [CrossRef]
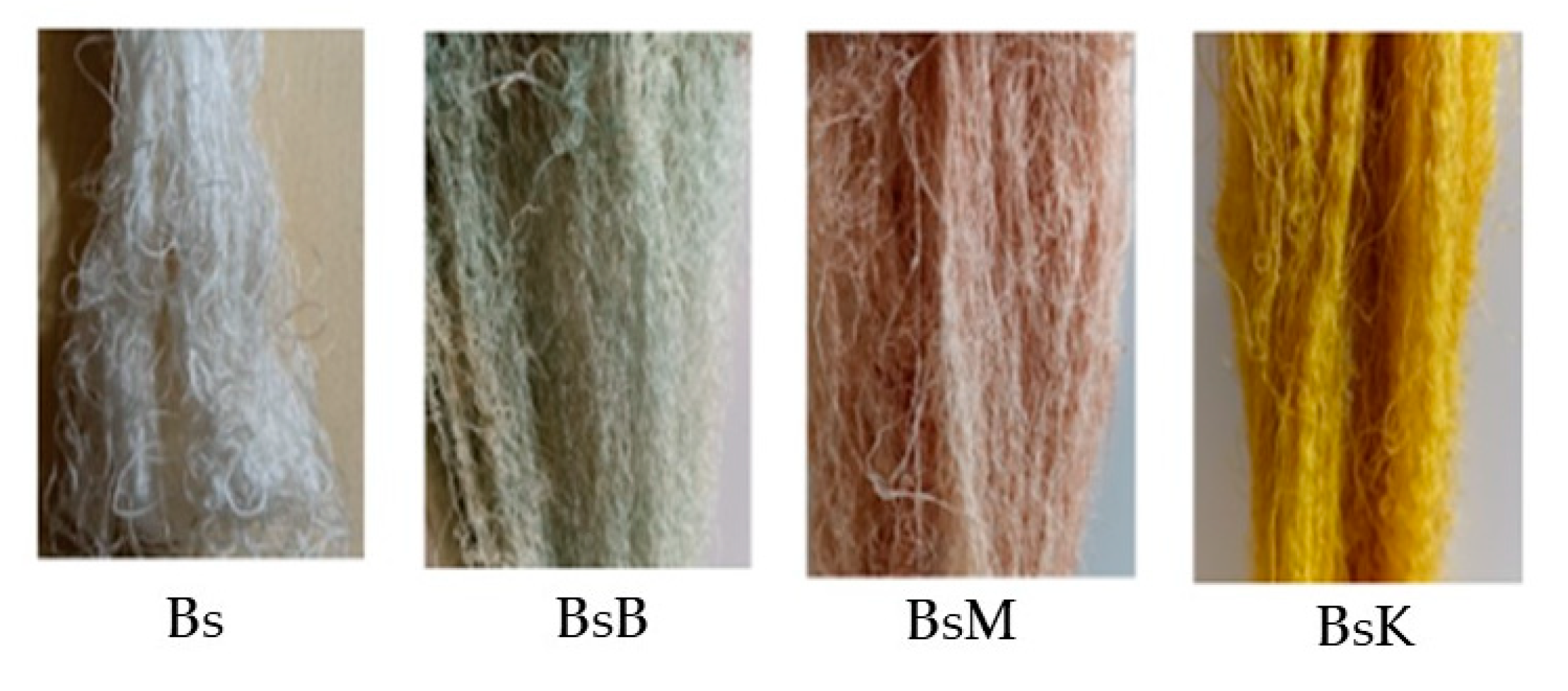
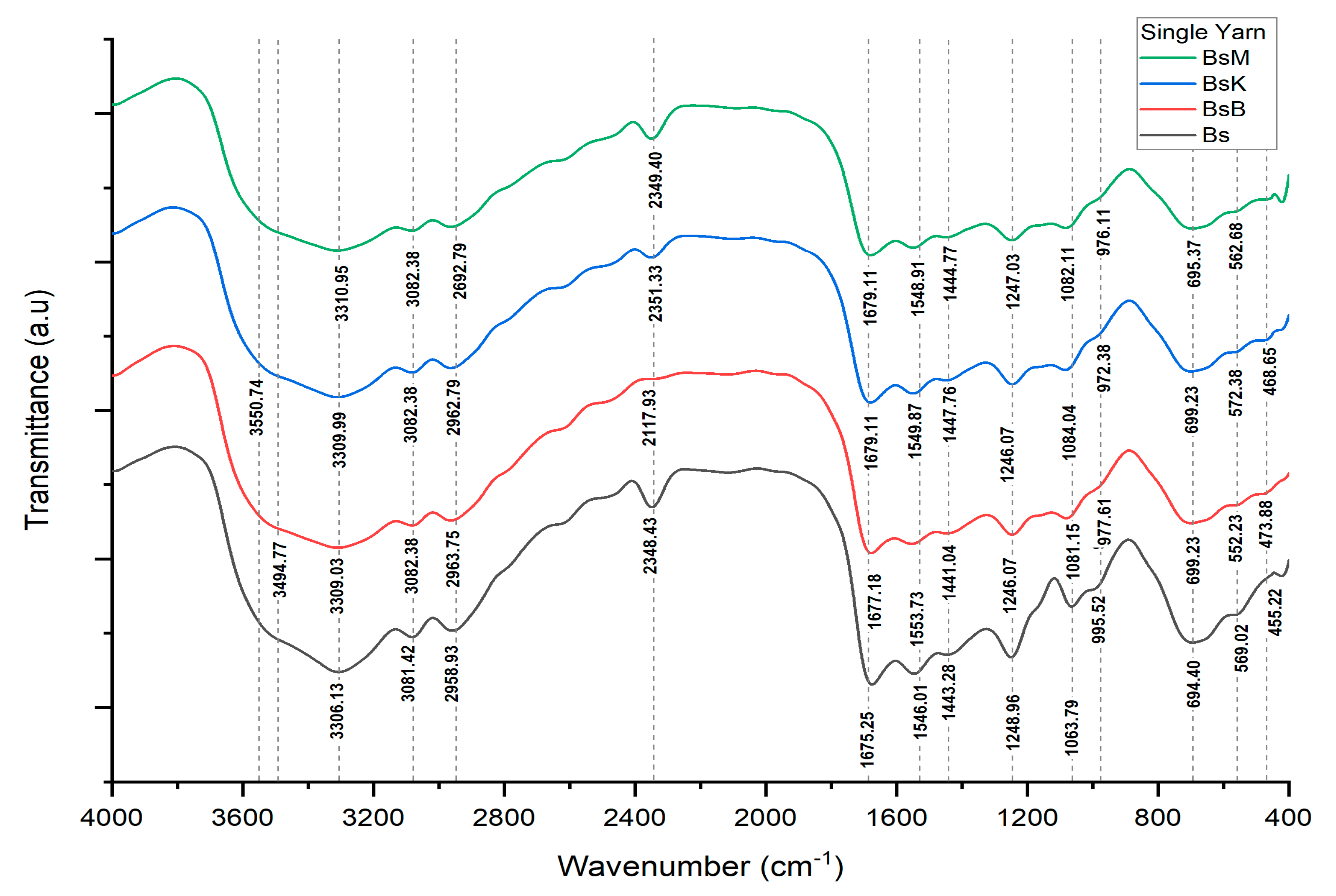


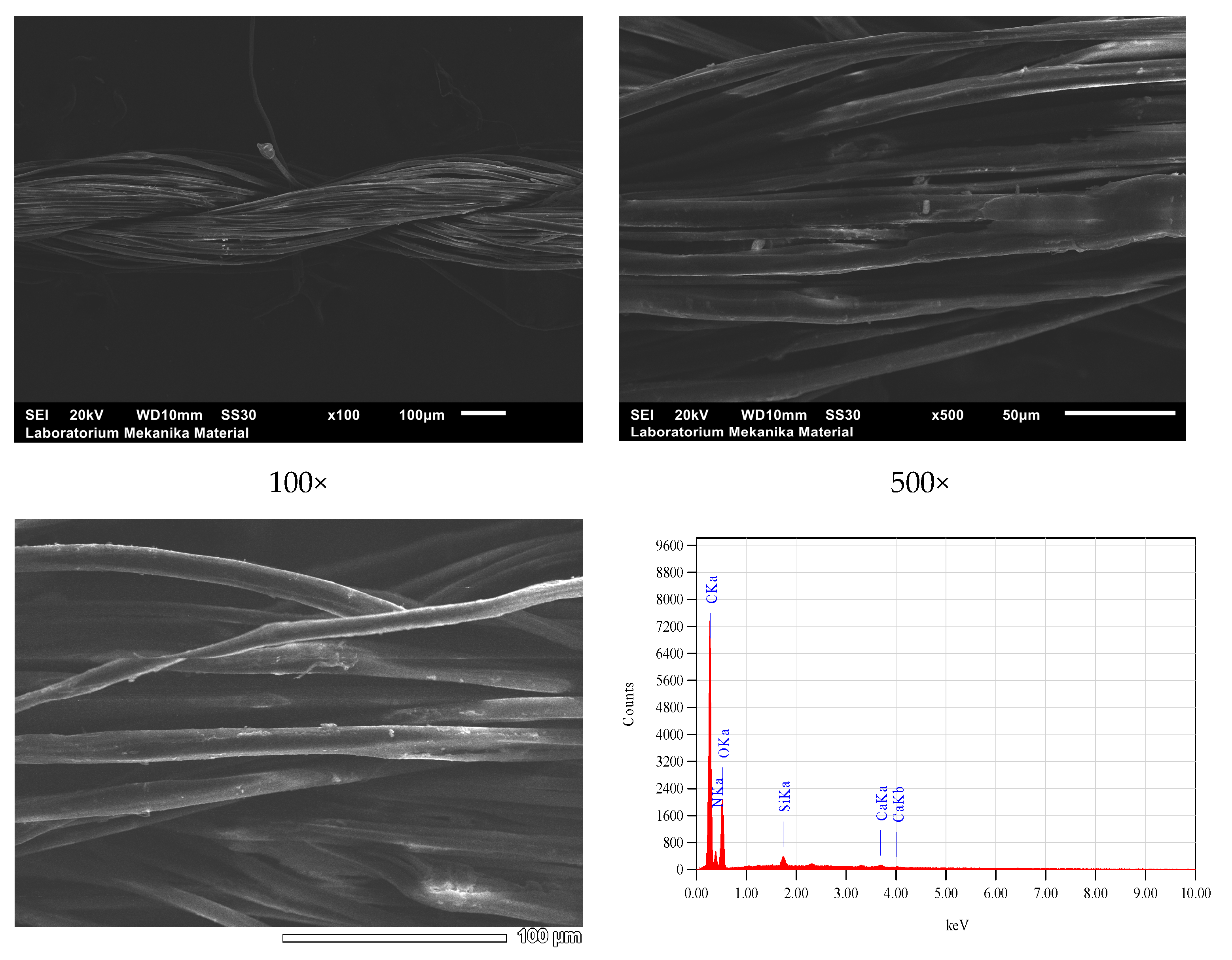
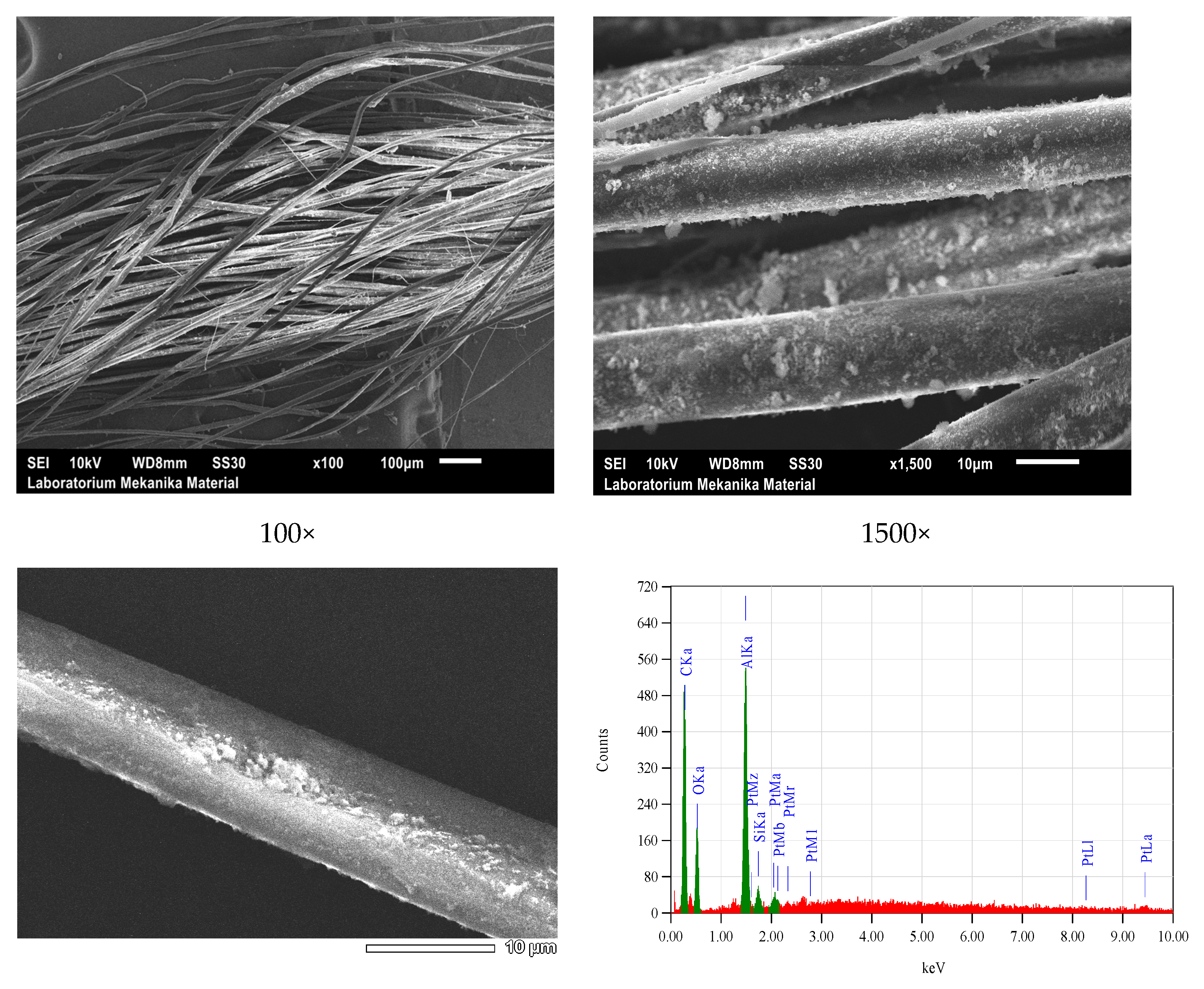

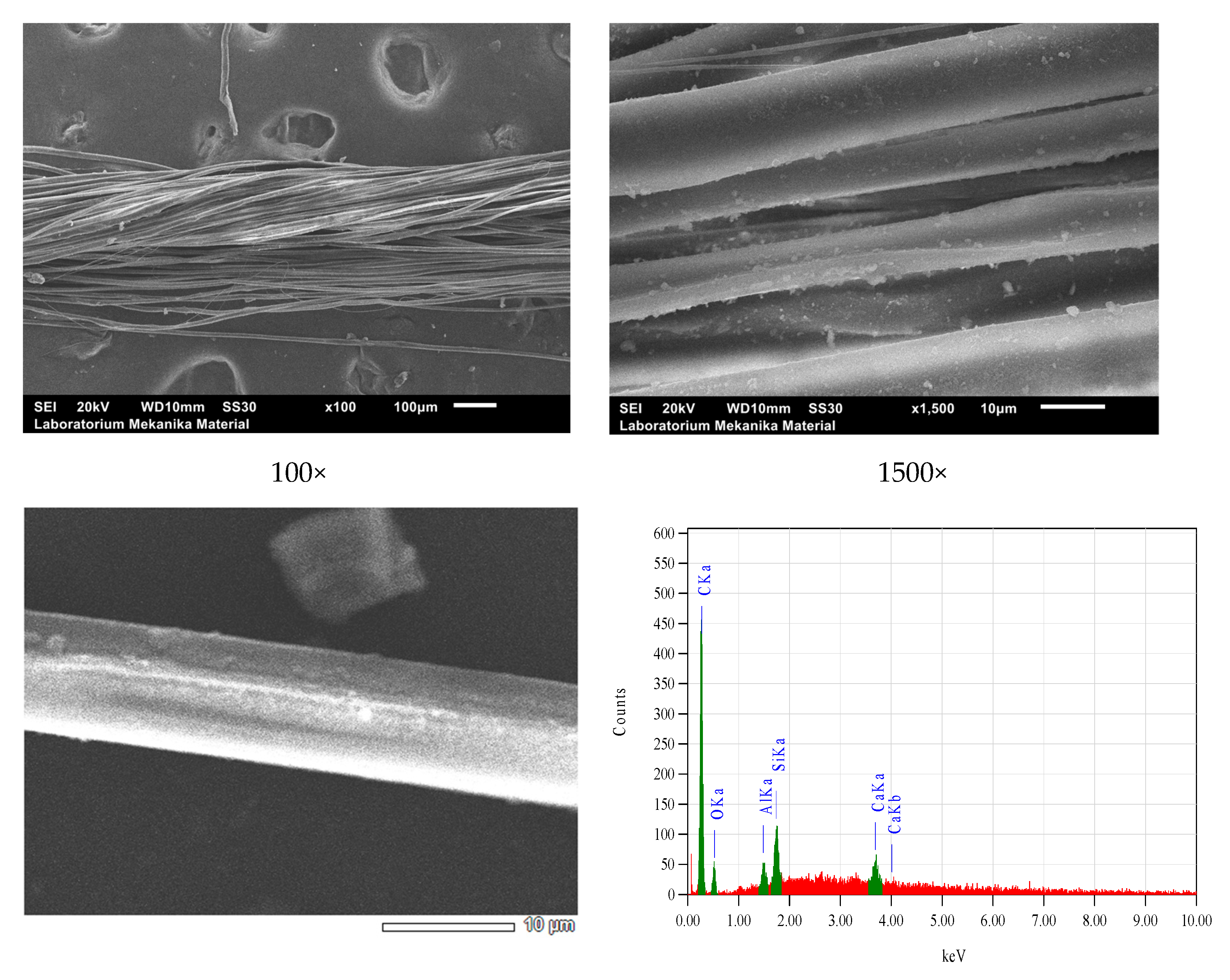

| Bonding | Wave Numbers (cm−1) | Reference Wave Numbers (cm−1) | |||
|---|---|---|---|---|---|
| Bs | BsK | BsM | BsB | ||
| Cu-O | 569.02 | 572.38 | 562.68 | 552.23 | 437–580 [17] |
| O-Si-O | 455.22 | 468.65 | −1082.11 | 473.88 | 467–532 bending [18] 1111–1114 asymmetric [19] |
| N-H | 694.40 | 699.23 | 695.37 | 699.23 | 665–910 stretching [20] |
| P-O-H | 995.52 | 972.38 | 976.11 | 977.61 | 910–1040 [20] |
| P-O-P | 995.52 | 972.38 | 976.11 | 977.61 | 807–1000 [20] |
| C-N | 1248.96 | 1246.07 | 1247.03 | 1246.07 | 1240–1340 [20] |
| N-O | 1443.28 | 1447.76 | 1444.77 | 1441.04 | 1475–1550 asymmetric [19] 1290–1400 stretching [20] |
| N=N | 1546.01 | 1549.87 | 1548.91 | 1553.73 | 1429–1576 [20] |
| O-H | 1675.25 | 1679.11 | 1679.11 | 1677.18 | 1632–1742 [21] |
| Si-O-H | 3306 | 3309.99 | 3310.95 | 3309.03 | 3200–3390 [21,22] |
| Si-O | 1063.79 | 1084.04 | 1082.11 | 1081.15 | 1002–1034 [19] |
| Al-O-H | - | 3550.74 | - | - | 3618–3696 [19] |
| Fe-O-H | - | - | - | 3494.77 | 3425 [23] |
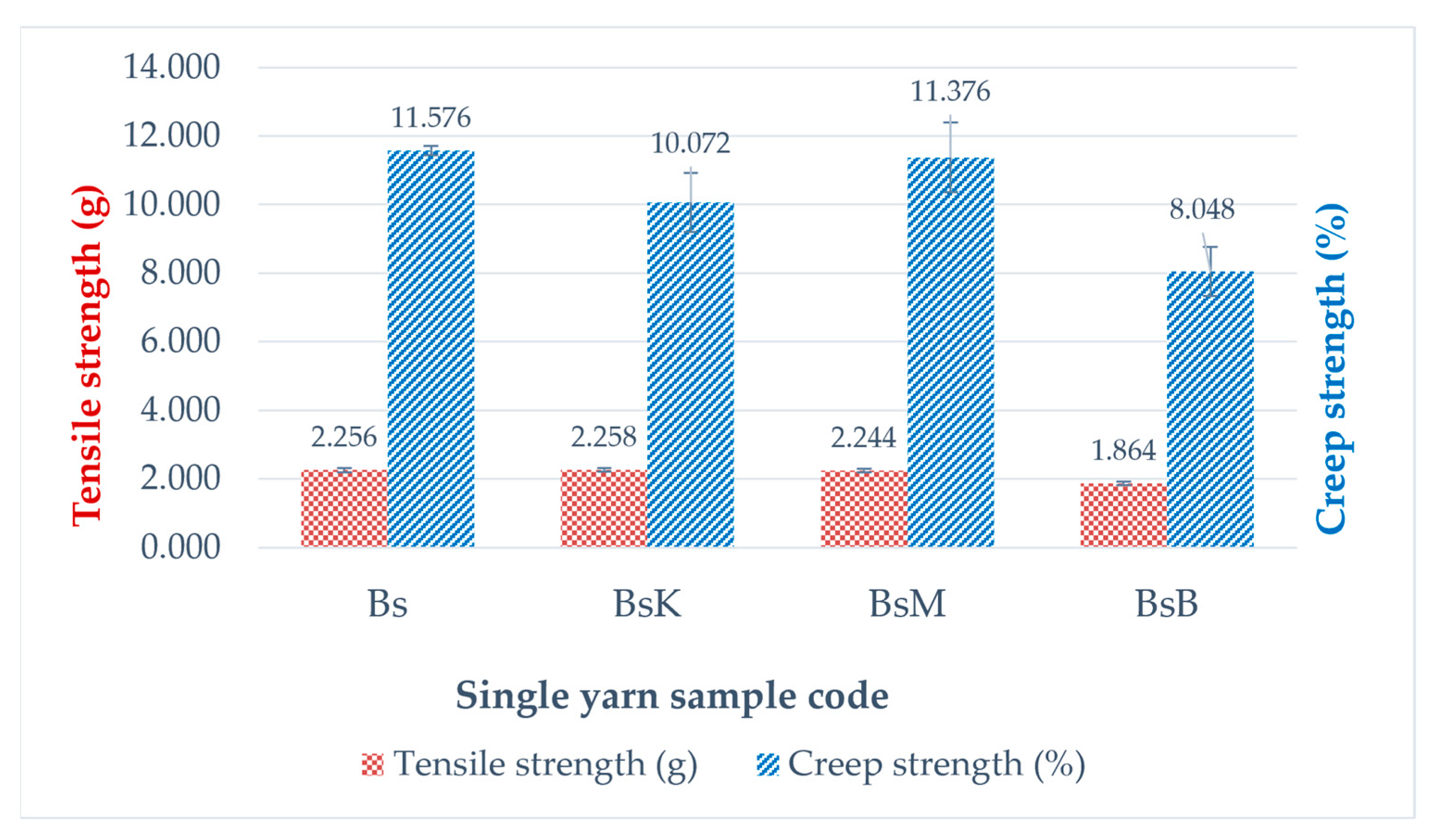
| Sample Code | Tensile Strength (g) | Creep Strength (%) | ||||
|---|---|---|---|---|---|---|
| Measured Data | Average | STDeV | Measured Data | Average | STDeV | |
| Bs | 2.308 | 2.256 | 0.1079 | 11.571 | 11.5760 | 0.1326 |
| 2.328 | 11.711 | |||||
| 2.132 | 11.446 | |||||
| BsK | 2.254 | 2.258 | 0.0656 | 11.155 | 10.0717 | 1.0548 |
| 2.325 | 10.012 | |||||
| 2.194 | 9.048 | |||||
| BsM | 2.211 | 2.244 | 0.0523 | 11.344 | 11.3763 | 1.0129 |
| 2.304 | 10.380 | |||||
| 2.216 | 12.405 | |||||
| BsB | 1.844 | 1.864 | 0.0510 | 8.143 | 8.0477 | 0.7098 |
| 1.922 | 8.705 | |||||
| 1.826 | 7.295 | |||||
| Sample Code | The Value of Color Fastness of Yarns Against Soap Washing (Gray Scale) | The Value of Color Fastness of Yarns Against Sunlight (Gray Scale) |
|---|---|---|
| Bs | - | - |
| BsK-1 | 4 (Good) | 3–4 (Sufficient) |
| BsK-2 | 4 (Good) | 3–4 (Sufficient) |
| BsK-3 | 4 (Good) | 3–4 (Sufficient) |
| BsM-1 | 4 (Good) | 4 (Good) |
| BsM-2 | 4 (Good) | 4 (Good) |
| BsM-3 | 4 (Good) | 4 (Good) |
| BsB-1 | 4–5 (Good) | 3–4 (Sufficient) |
| BsB-2 | 4–5 (Good) | 3–4 (Sufficient) |
| BsB-3 | 4–5 (Good) | 3–4 (Sufficient) |
| Sample Code | Color Difference Test Value | Color Intensity Test Values (R%) | |||
|---|---|---|---|---|---|
| L* | a* | b* | dE*ab | ||
| Bs | 91 | 1.08 | −0.58 | 0.00 | 101.43 |
| BsK-1 | 53.28 | 16.12 | 39.09 | 56.76 | 17.06 |
| BsK-2 | 61.35 | 14.25 | 42.63 | 54.05 | 13.13 |
| BsK-3 | 61.16 | 14.21 | 40.48 | 52.42 | 12.04 |
| BsM-1 | 29.37 | 22.33 | 25.77 | 70.31 | 7.53 |
| BsM-2 | 29.33 | 21.64 | 26.61 | 70.46 | 7.06 |
| BsM-3 | 33.88 | 21.06 | 20.26 | 64 | 7.59 |
| BsB-1 | 52.24 | −2.06 | −3.64 | 38.92 | 29.37 |
| BsB-2 | 53.07 | −1.1 | 1.1 | 38.26 | 27.07 |
| BsB-3 | 53.28 | −3.18 | 3.96 | 38.33 | 30.87 |
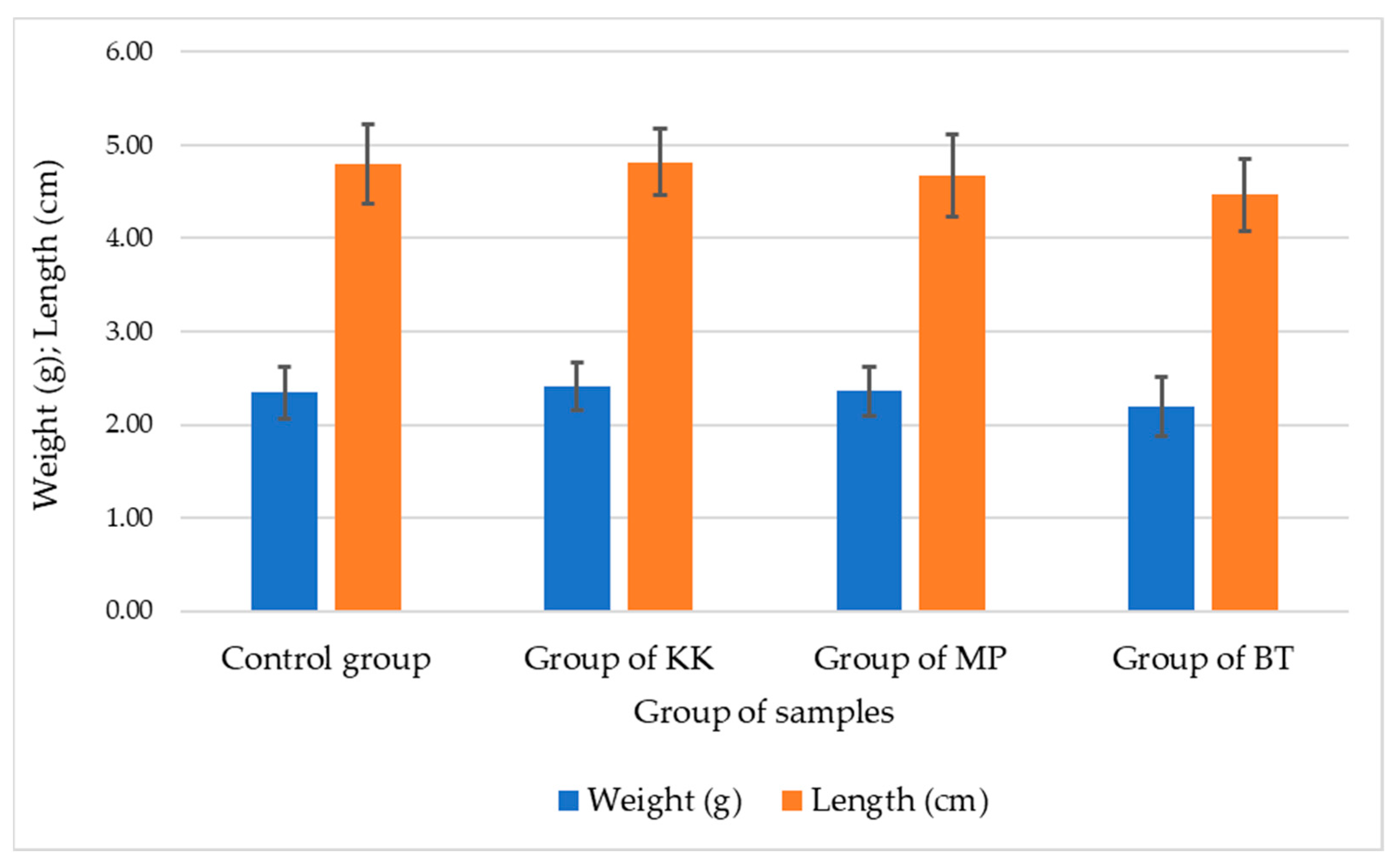
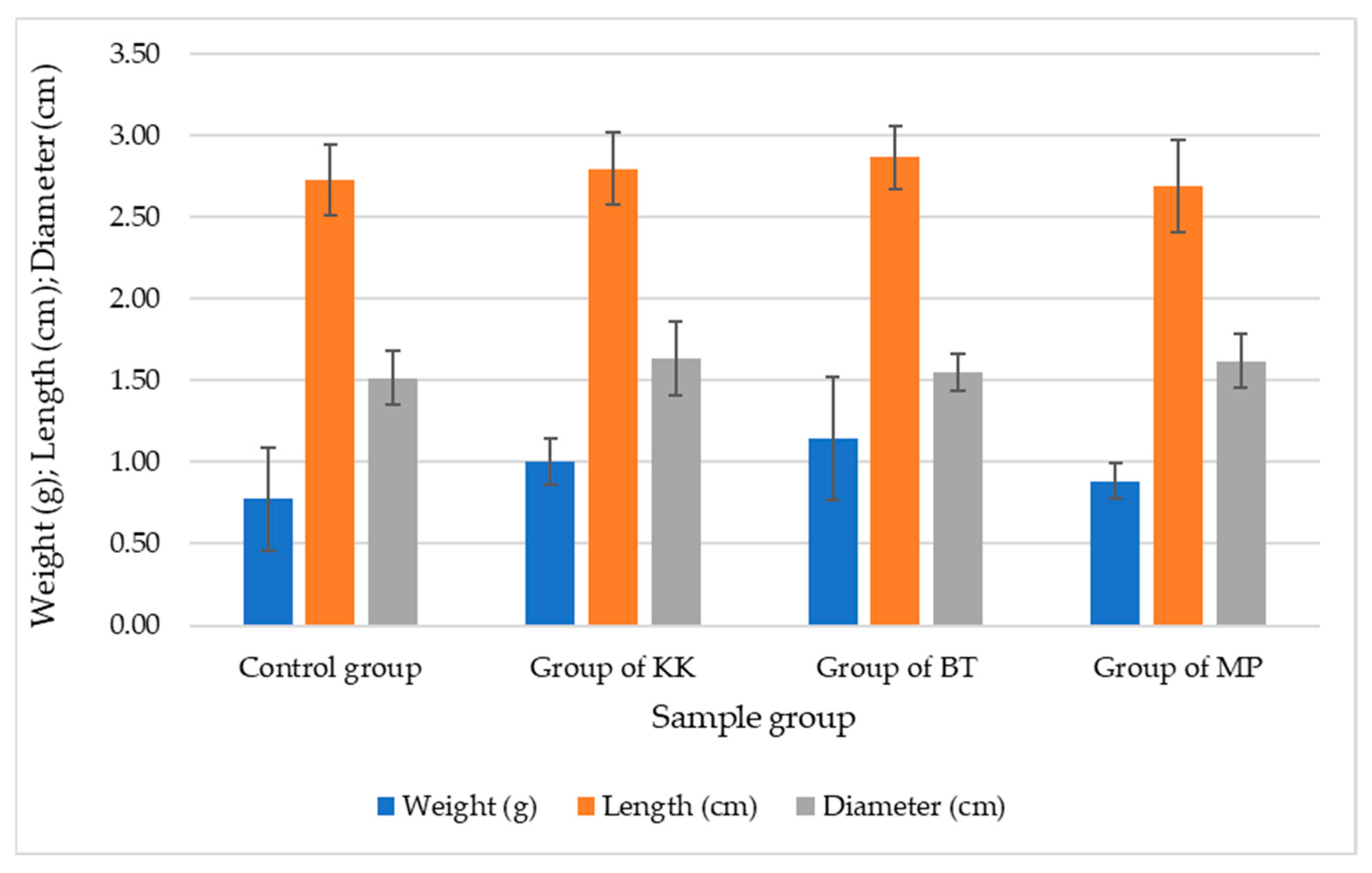
| Sample Number | Control Group | Experimental Group of KK | Experimental Group of BT | Experimental Group of MP | ||||
|---|---|---|---|---|---|---|---|---|
| Weight (g) | Length (m) | Weight (g) | Length (m) | Weight (g) | Length (m) | Weight (g) | Length (m) | |
| 1 | 0.23 | 578.25 | 0.29 | 694.74 | 0.28 | 732.25 | 0.34 | 922.25 |
| 2 | 0.25 | 590.62 | 0.31 | 725.25 | 0.33 | 768.94 | 0.36 | 936.75 |
| 3 | 0.21 | 547.92 | 0.28 | 694.95 | 0.27 | 678.45 | 0.31 | 870.75 |
| 4 | 0.21 | 550.21 | 0.27 | 687.85 | 0.28 | 680.25 | 0.29 | 825.78 |
| 5 | 0.23 | 571.95 | 0.26 | 675.76 | 0.27 | 670.95 | 0.28 | 815.88 |
| Average | 0.23 | 567.79 | 0.28 | 695.71 | 0.29 | 706.17 | 0.32 | 874.28 |
| St. Dev. | 0.02 | 18.38 | 0.02 | 18.26 | 0.03 | 42.72 | 0.03 | 54.72 |
| Sample Code | Test Nr. | Value of Color Fastness Against Soap Washing (Gray Scale) | Value of Color Fastness Against Sunlight (Gray Scale) | Value of Color Differences | Value of Color Intensity Test (R%) | |||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | dE*ab | |||||
| Bd | - | - | 97.7 | 0.41 | −2.49 | 0.00 | 193.3 | |
| BdK | 1 | 4 (Good) | 4 (Good) | 77.88 | 35.75 | 40.31 | 60.79 | 34.2 |
| 2 | 4 (Good) | 4 (Good) | 72.35 | 29.78 | 42.18 | 57.2 | 35.62 | |
| 3 | 4 (Good) | 4 (Good) | 72.22 | 35.15 | 36.42 | 58.04 | 39.08 | |
| BdM | 1 | 4–5 (Good) | 4–5 (Good) | 54.69 | 30.89 | −0.88 | 52.74 | 55.08 |
| 2 | 4–5 (Good) | 4–5 (Good) | 55.51 | 27.93 | 5.64 | 51.03 | 59.79 | |
| 3 | 4–5 (Good) | 4–5 (Good) | 54.54 | 30.21 | 1.07 | 52.58 | 54 | |
| BdB | 1 | 4 (Good) | 4 (Good) | 22.21 | 5.32 | −28.24 | 80.01 | 38.18 |
| 2 | 4 (Good) | 4 (Good) | 24.79 | 9.64 | −34.56 | 80.19 | 30.72 | |
| 3 | 4 (Good) | 4 (Good) | 24.72 | 7.49 | −34.46 | 80 | 39.9 | |
| Sample Code | Tensile Strength (g) | Creep Strength (%) | ||||
|---|---|---|---|---|---|---|
| Measured Data | Average | STDeV | Measured Data | Average | STDeV | |
| Bd | 2.387 | 2.276 | 0.1026 | 4.902 | 4.6517 | 0.2939 |
| 2.185 | 4.725 | |||||
| 2.255 | 4.328 | |||||
| BdK | 3.640 | 3.531 | 0.1729 | 11.062 | 11.4123 | 0.3344 |
| 3.332 | 11.728 | |||||
| 3.622 | 11.447 | |||||
| BdM | 3.215 | 3.315 | 0.1652 | 11.984 | 11.5840 | 0.3722 |
| 3.506 | 11.248 | |||||
| 3.225 | 11.52 | |||||
| BdB | 2.687 | 2.785 | 0.1738 | 7.068 | 7.3680 | 0.3056 |
| 2.986 | 7.679 | |||||
| 2.683 | 7.357 | |||||
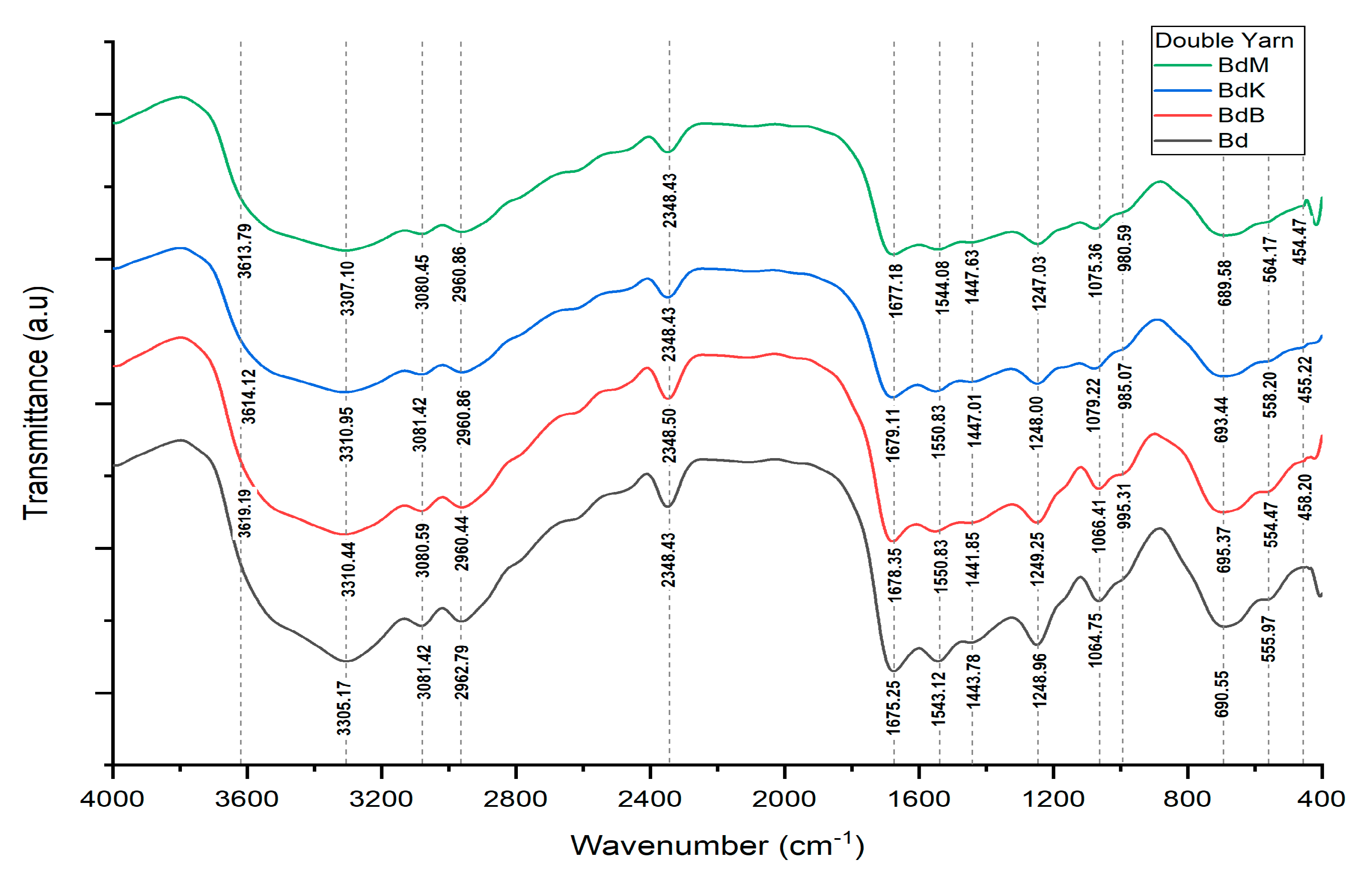
| Bonding | Wave Numbers (cm−1) | Reference Wave Numbers (cm−1) | |||
|---|---|---|---|---|---|
| Bd | BdK | BdM | BdB | ||
| Cu-O | 555.97 | 558.20 | 564.17 | 554.47 | 437–580 [17] |
| O-Si-O | - | 455.22 | 454.47 | 458.20 | 467–532 bending [18] |
| N-H | 690.55 | 693.44 | 689.58 | 695.37 | 665–910 stretching [20] |
| P-O-H | - | 985.07 | 980.59 | 995.31 | 910–1040 [20] |
| P-O-P | - | 985.07 | 980.59 | 995.31 | 807–1000 [20] |
| C-N | 1248.96 | 1248.00 | 1247.03 | 1249.25 | 1240–1340 [20] |
| N-O | 1443.78 | 1447.01 | 1447.63 | 1441.85 | 1475–1550 asymmetric [19] |
| N=N | 1543.12 | 1550.83 | 1544.08 | 1550.83 | 1429–1576 [20] |
| O-H | 1675.25 | 1679.11 | 1677.18 | 1678.35 | 1632–1742 [21] |
| Si-O-H | 3305.17 | 3310.95 | 3307.10 | 3310.44 | 3200–3390 [21,22] |
| Si-O | 1064.75 | 1079.22 | 1075.36 | 1066.41 | 1002–1034 [19] |
| Al-O-H | - | 3614.12 | 3613.79 | 3619.19 | 3618–3696 [19] |
| Fe-O-H | - | - | - | 3310.44 | 3425 [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karyasa, I.W.; Kusumawati, E.D.; Agustarini, R.; Andadari, L.; Sari, H. Organic–Inorganic Hybridization of Silkworm Cocoon Filaments Using Nano Pastes of Silica–Phosphate–M (M = Cu, Fe, or Al). Nanomaterials 2024, 14, 1697. https://doi.org/10.3390/nano14211697
Karyasa IW, Kusumawati ED, Agustarini R, Andadari L, Sari H. Organic–Inorganic Hybridization of Silkworm Cocoon Filaments Using Nano Pastes of Silica–Phosphate–M (M = Cu, Fe, or Al). Nanomaterials. 2024; 14(21):1697. https://doi.org/10.3390/nano14211697
Chicago/Turabian StyleKaryasa, I Wayan, Enike Dwi Kusumawati, Retno Agustarini, Lincah Andadari, and Herman Sari. 2024. "Organic–Inorganic Hybridization of Silkworm Cocoon Filaments Using Nano Pastes of Silica–Phosphate–M (M = Cu, Fe, or Al)" Nanomaterials 14, no. 21: 1697. https://doi.org/10.3390/nano14211697
APA StyleKaryasa, I. W., Kusumawati, E. D., Agustarini, R., Andadari, L., & Sari, H. (2024). Organic–Inorganic Hybridization of Silkworm Cocoon Filaments Using Nano Pastes of Silica–Phosphate–M (M = Cu, Fe, or Al). Nanomaterials, 14(21), 1697. https://doi.org/10.3390/nano14211697








