Flexible All-Carbon Nanoarchitecture Built from In Situ Formation of Nanoporous Graphene Within “Skeletal-Capillary” Carbon Nanotube Networks for Supercapacitors
Abstract
1. Introduction
2. Experimental Methodology
2.1. Synthesis of the CNT/Graphene Nanocomposites
2.2. Material Characterization
2.3. Electrochemical Performance
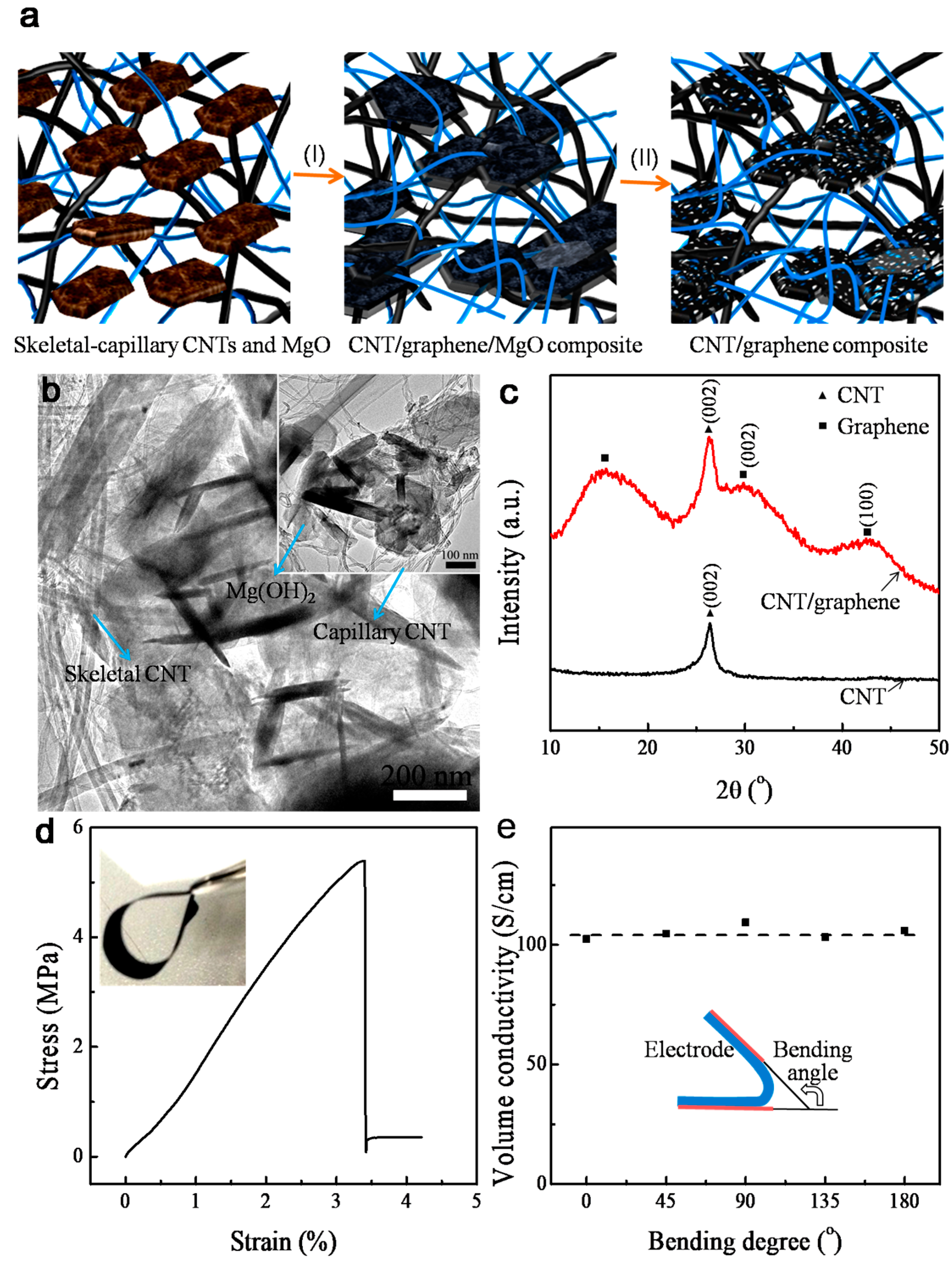
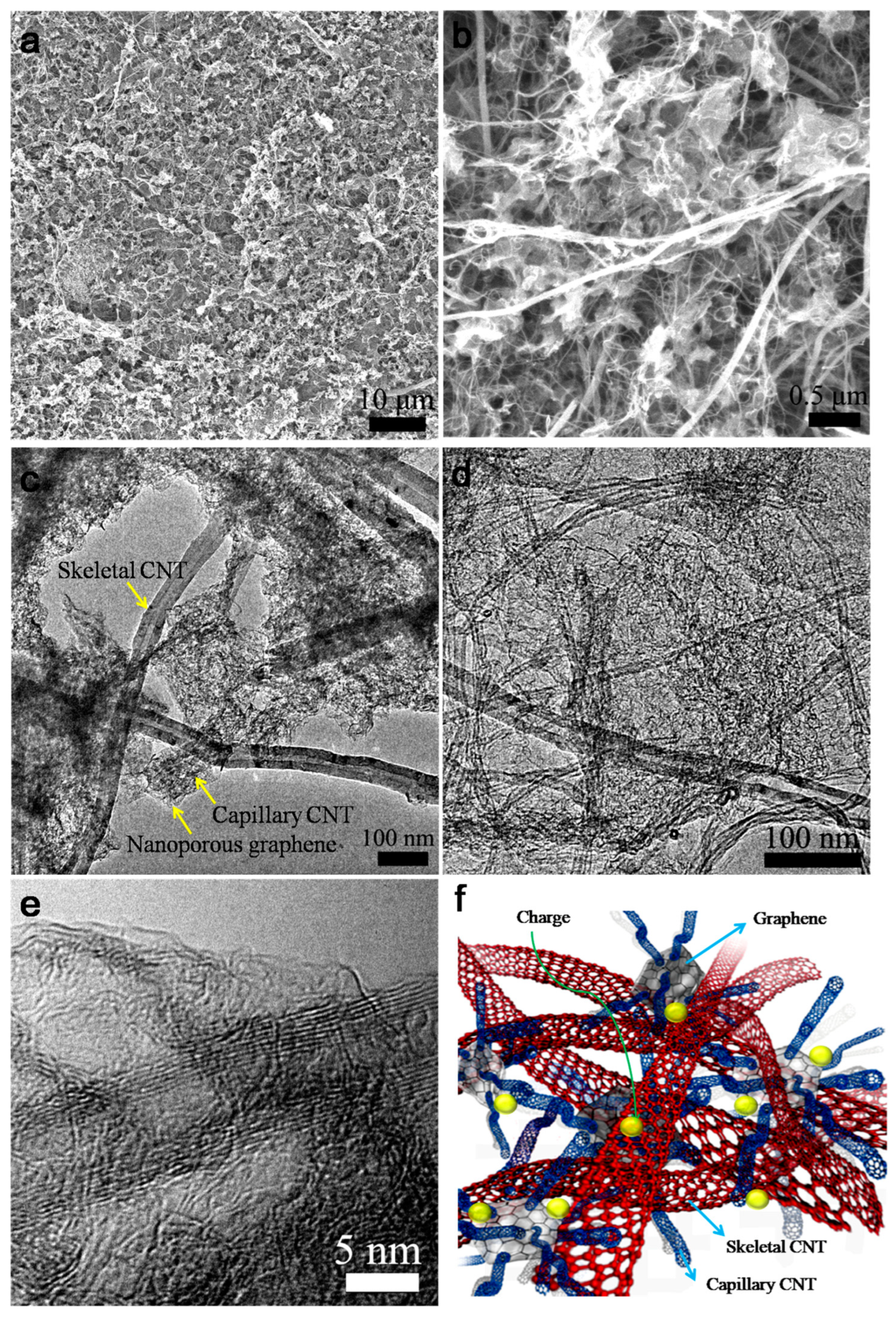
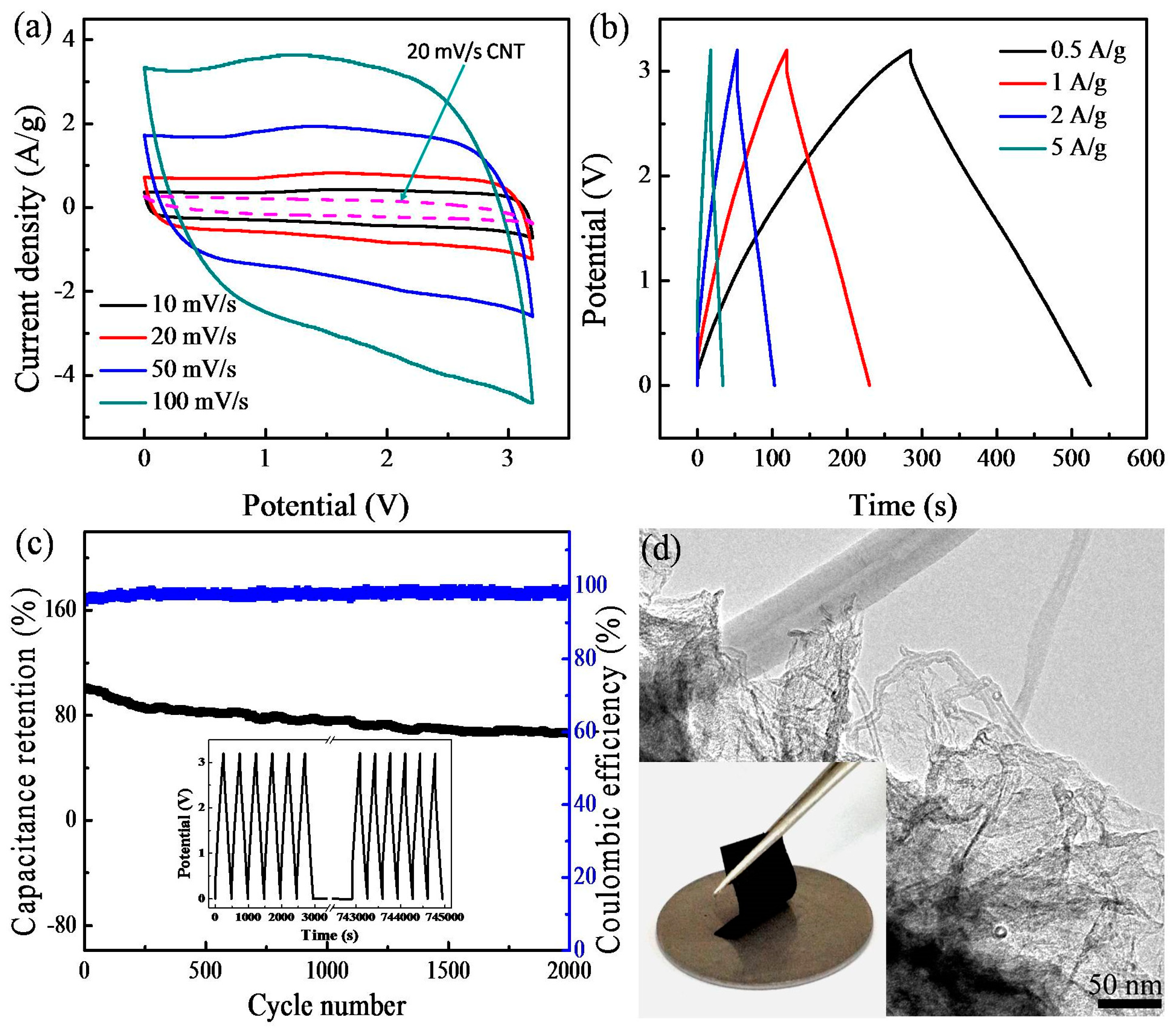
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Sources 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Ru, M.; Huang, A.; Hua, J.; Huang, R.; Yan, S.; Zhang, Q. A novel carbonized natural silk nanofiber/graphene oxide aerogel for supercapacitor. Mater. Lett. 2024, 359, 135901. [Google Scholar] [CrossRef]
- Zheng, C.; Qian, W.; Yu, Y.; Wei, F. Ionic liquid coated single-walled carbon nanotube buckypaper as supercapacitor electrode. Particuology 2013, 11, 409–414. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Omar, M.F.; Abidin, M.S.Z.; Akil, H.M.; Abdullah, M.M.A.B. Recent progress in the three-dimensional structure of graphene-carbon nanotubes hybrid and their supercapacitor and high-performance battery applications. Comp. Part A Appl. Sci. Manufactur. 2022, 154, 106756. [Google Scholar] [CrossRef]
- Kinloch, I.A.; Suhr, J.; Lou, J.; Young, R.J.; Ajayan, P.M. Composites with carbon nanotubes and graphene: An outlook. Science 2018, 362, 547–553. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube-and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Xu, G.; Zheng, C.; Zhang, Q.; Huang, J.; Zhao, M.; Nie, J.; Wang, X.; Wei, F. Binder-free activated carbon/carbon nanotube paper electrodes for use in supercapacitors. Nano Res. 2011, 4, 870–881. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, B.-S.; Chen, S.; Shao-Horn, Y.; Hammond, P.T. Layer-by-layer assembly of all carbon nanotube ultrathin films for electrochemical applications. J. Am. Chem. Soc. 2008, 131, 671–679. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Zhi, L.; Zhang, Q.; Wei, T.; Feng, J.; Zhang, M.; Qian, W.; Wei, F. A Three-Dimensional Carbon Nanotube/Graphene Sandwich and Its Application as Electrode in Supercapacitors. Adv. Mater. 2010, 22, 3723–3728. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Yu, D.; Dai, L.; Ganguli, S.; Varshney, V.; Roy, A. Preparation of tunable 3D pillared carbon nanotube–graphene networks for high-performance capacitance. Chem. Mater. 2011, 23, 4810–4816. [Google Scholar] [CrossRef]
- Ma, X.; Ning, G.; Qi, C.; Xu, C.; Gao, J. Phosphorus and nitrogen dual-doped few-layered porous graphene: A high-performance anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 14415–14422. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xu, Y.; Yao, Z.; Liu, A.; Shi, G. Supercapacitors based on flexible graphene/polyaniline nanofiber composite films. ACS Nano 2010, 4, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ning, G.; Fan, Z.; Gao, J.; Xu, C.; Qian, W.; Wei, F. One-step synthesis of a graphene-carbon nanotube hybrid decorated by magnetic nanoparticles. Carbon 2012, 50, 2764–2771. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, X.; Mao, S.; Bo, Z.; Kim, H.; Cui, S.; Lu, G.; Feng, X.; Chen, J. Crumpled nitrogen-doped graphene nanosheets with ultrahigh pore volume for high-performance supercapacitor. Adv. Mater. 2012, 24, 5610–5616. [Google Scholar] [CrossRef]
- Zan, G.; Li, S.; Chen, P.; Dong, K.; Wu, Q.; Wu, T. Mesoporous Cubic Nanocages Assembled by Coupled Monolayers With 100% Theoretical Capacity and Robust Cycling. ACS Cent. Sci. 2024, 10, 1283–1294. [Google Scholar] [CrossRef]
- Kong, D.; Lv, W.; Liu, R.; He, Y.B.; Wu, D.; Li, F.; Fu, R.; Yang, Q.H.; Kang, F. Superstructured carbon materials: Design and energy applications. Energy Mater. Devices 2023, 1, 9370017. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Bulla, M.; Devi, R.; Dahiya, R.; Sisodiya, A.K.; Singh, R.B.; Mishra, A.K. Hydrothermally reduced graphene oxide based electrodes for high-performance symmetric supercapacitor. Mater. Lett. 2024, 364, 136364. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Zhang, H.; Sun, X.; Zhang, D.; Ma, Y. High-performance supercapacitors based on a graphene–activated carbon composite prepared by chemical activation. RSC Adv. 2012, 2, 7747–7753. [Google Scholar] [CrossRef]
- Jia, X.; Chen, Z.; Suwarnasarn, A.; Rice, L.; Wang, X.; Sohn, H.; Zhang, Q.; Wu, B.M.; Wei, F.; Lu, Y. High-performance flexible lithium-ion electrodes based on robust network architecture. Energy Environ. Sci. 2012, 5, 6845–6849. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, Q.; Zhao, M.-Q.; Xu, G.-H.; Huang, J.-Q.; Qian, W.; Lu, Y.; Wei, F. Dramatic enhancements in toughness of polyimide nanocomposite via long-CNT-induced long-range creep. J. Mater. Chem. 2012, 22, 7050–7056. [Google Scholar] [CrossRef]
- He, X.; Ling, P.; Qiu, J.; Yu, M.; Zhang, X.; Yu, C.; Zheng, M. Efficient preparation of biomass-based mesoporous carbons for supercapacitors with both high energy density and high power density. J. Power Sources 2013, 240, 109–113. [Google Scholar] [CrossRef]
- Chen, Z.; Augustyn, V.; Wen, J.; Zhang, Y.; Shen, M.; Dunn, B.; Lu, Y. High-Performance Supercapacitors Based on Intertwined CNT/V2O5 Nanowire Nanocomposites. Adv. Mater. 2011, 23, 791–795. [Google Scholar] [CrossRef]
- Kim, T.; Jung, G.; Yoo, S.; Suh, K.S.; Ruoff, R.S. Activated graphene-based carbons as supercapacitor electrodes with macro- and mesopores. ACS Nano 2013, 7, 6899–6905. [Google Scholar] [CrossRef]
- Naoi, K. ‘Nanohybrid Capacitor’: The Next Generation Electrochemical Capacitors. Fuel Cells 2010, 10, 825–833. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Li, H.; Wang, J.; Jia, X. Flexible All-Carbon Nanoarchitecture Built from In Situ Formation of Nanoporous Graphene Within “Skeletal-Capillary” Carbon Nanotube Networks for Supercapacitors. Nanomaterials 2024, 14, 1683. https://doi.org/10.3390/nano14201683
Chen T, Li H, Wang J, Jia X. Flexible All-Carbon Nanoarchitecture Built from In Situ Formation of Nanoporous Graphene Within “Skeletal-Capillary” Carbon Nanotube Networks for Supercapacitors. Nanomaterials. 2024; 14(20):1683. https://doi.org/10.3390/nano14201683
Chicago/Turabian StyleChen, Tao, Hongyan Li, Jiaziyi Wang, and Xilai Jia. 2024. "Flexible All-Carbon Nanoarchitecture Built from In Situ Formation of Nanoporous Graphene Within “Skeletal-Capillary” Carbon Nanotube Networks for Supercapacitors" Nanomaterials 14, no. 20: 1683. https://doi.org/10.3390/nano14201683
APA StyleChen, T., Li, H., Wang, J., & Jia, X. (2024). Flexible All-Carbon Nanoarchitecture Built from In Situ Formation of Nanoporous Graphene Within “Skeletal-Capillary” Carbon Nanotube Networks for Supercapacitors. Nanomaterials, 14(20), 1683. https://doi.org/10.3390/nano14201683




