Core-Shell Fe3O4@C Nanoparticles as Highly Effective T2 Magnetic Resonance Imaging Contrast Agents: In Vitro and In Vivo Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis
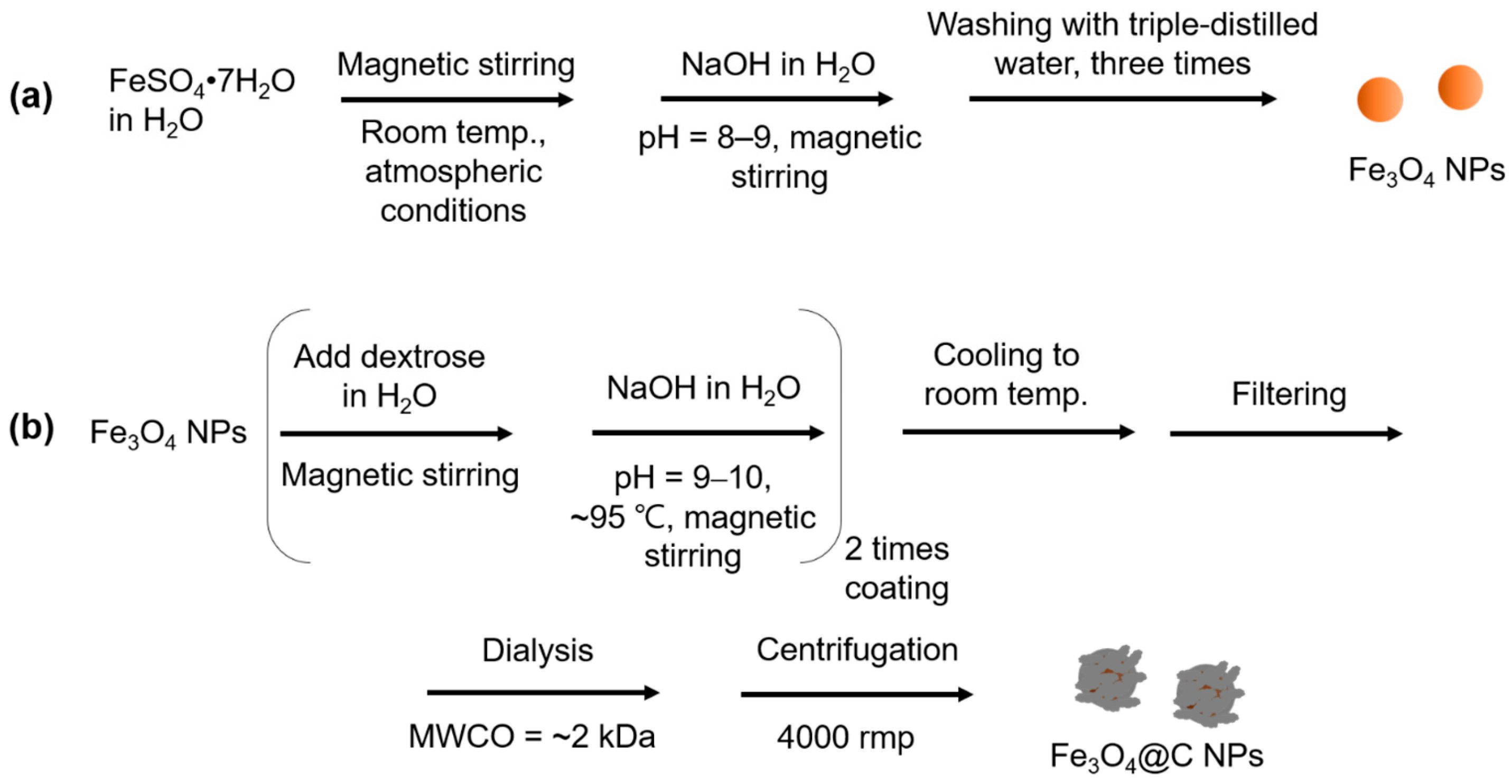
2.2.1. Synthesis of the Fe3O4 NPs
2.2.2. Synthesis of the Core-Shell Fe3O4@C NPs
2.3. Characterization
2.4. Water Proton Spin Relaxivities and Map Images
2.5. In Vitro Cellular Cytotoxicity
2.6. In Vivo MRI Experiments
3. Results
3.1. Particle Diameter and Crystallinity of the Fe3O4@C NPs
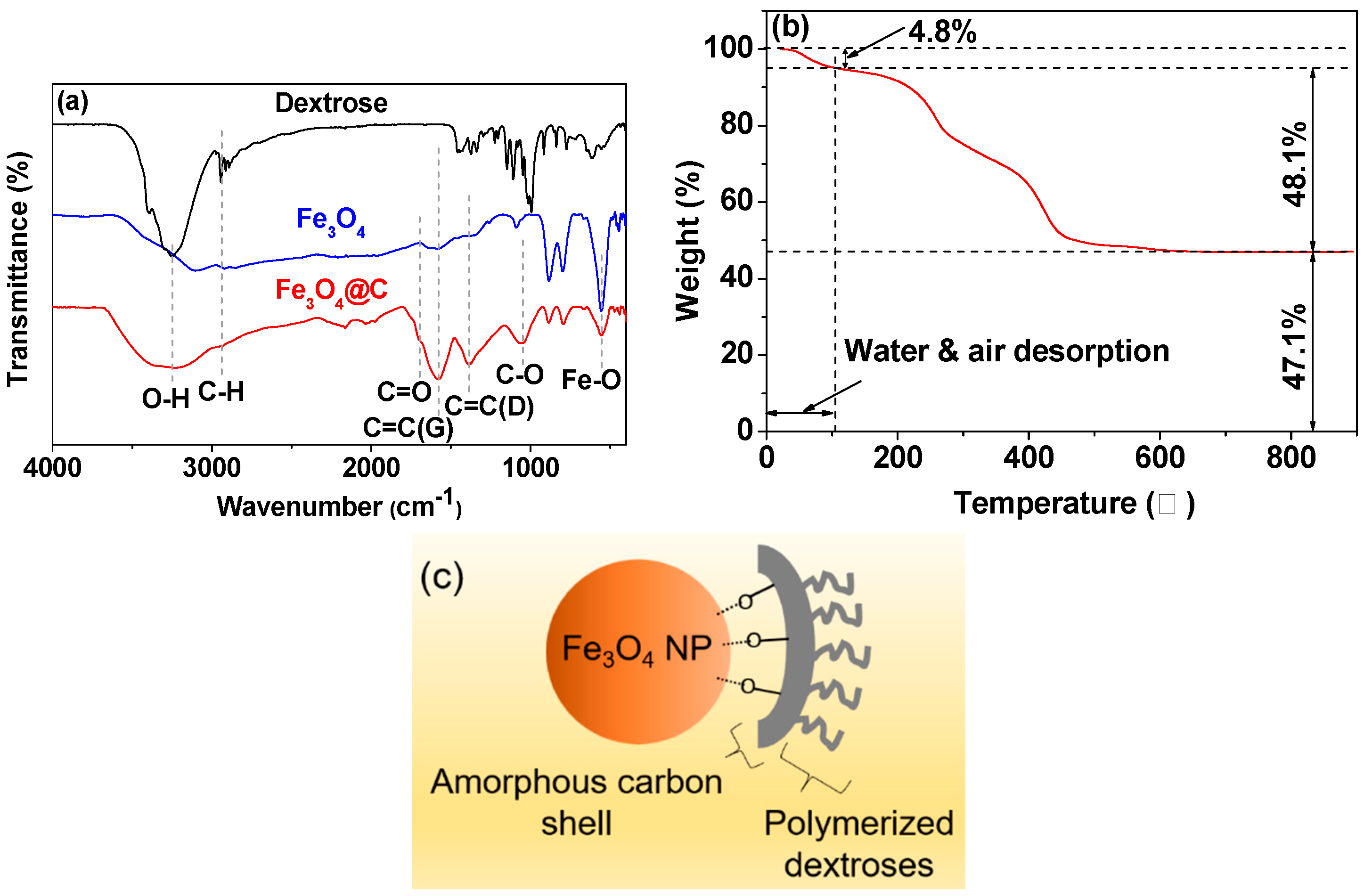
3.2. Surface-Coating Analysis
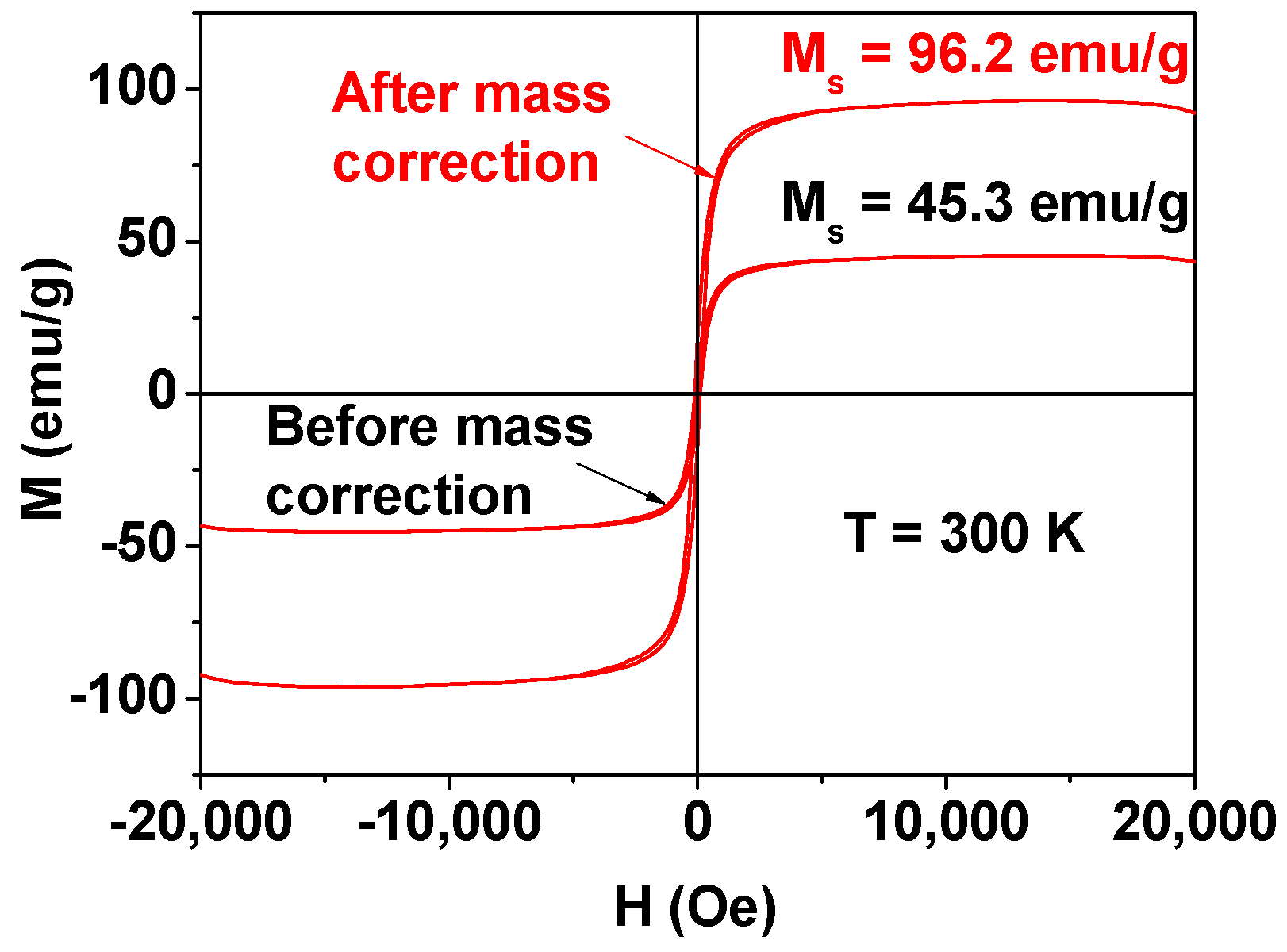
3.3. Magnetic Property
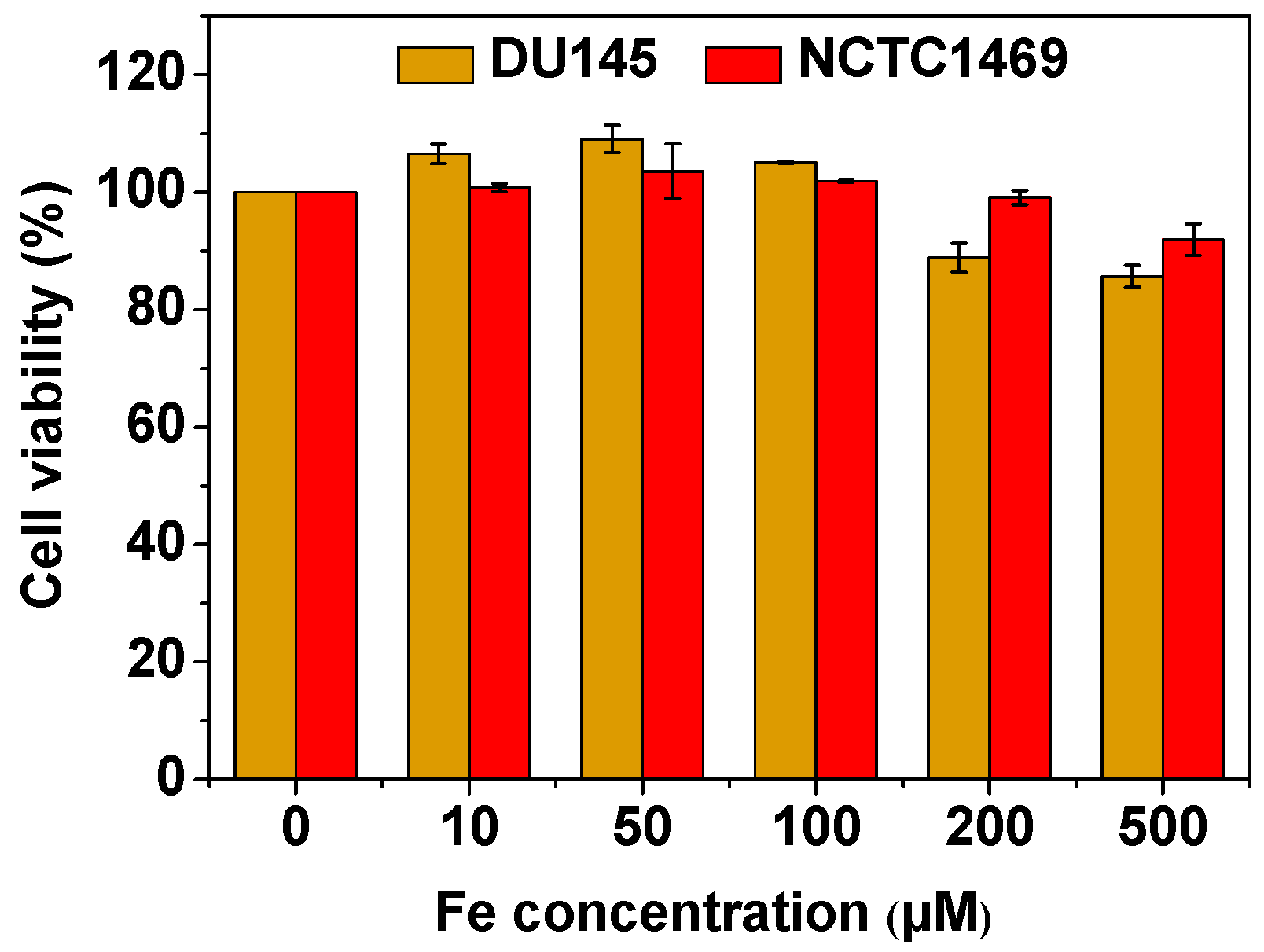
3.4. In Vitro Cellular Cytotoxicity
3.5. r1 and r2 Values and R1 and R2 Map Images
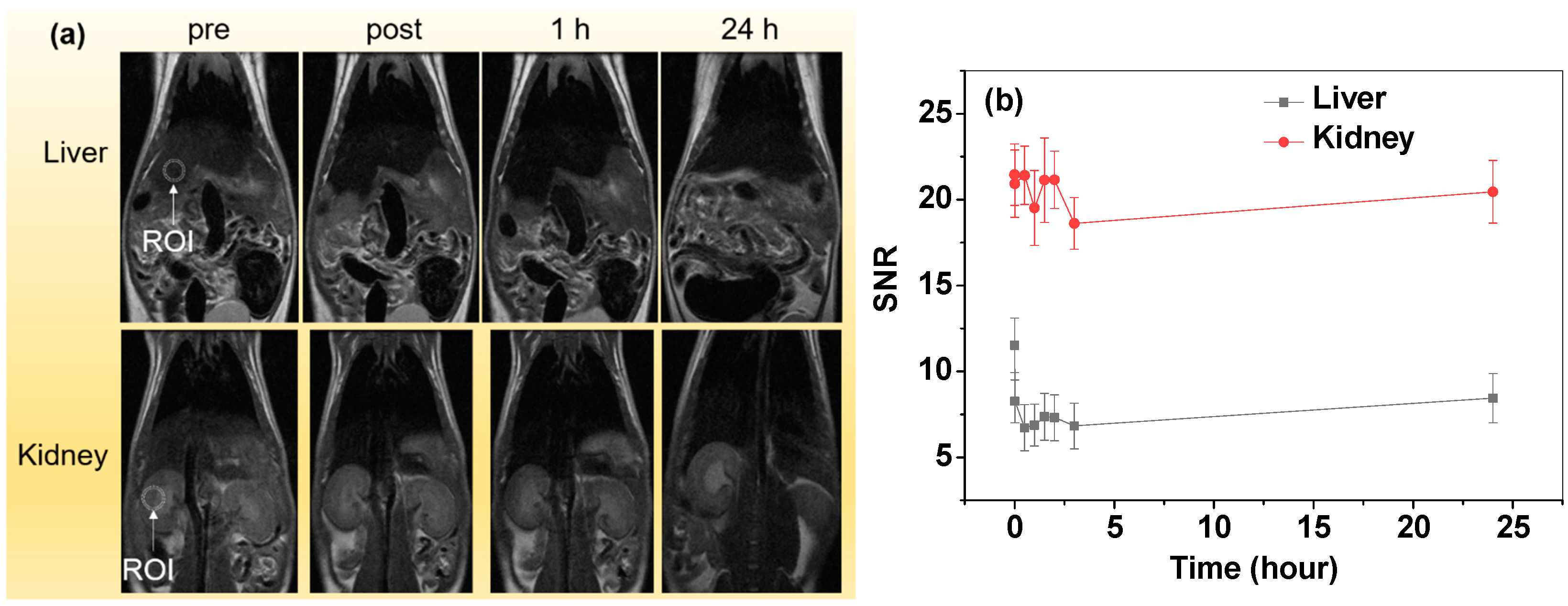
3.6. In Vivo T2 MR Images
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nezhadheydari, H.; Tavabe, K.R.; Mirvaghefi, A.; Heydari, A.; Frinsko, M. Effects of different concentrations of Fe3O4@ZnO and Fe3O4@CNT magnetic nanoparticles separately and in combination on aquaculture waste water treatment. Environ. Technol. Innov. 2019, 15, 100414. [Google Scholar] [CrossRef]
- Luo, T.; Xu, Q.; Wei, W.; Sun, J.; Dai, X.; Ni, B.J. Performance and mechanism of Fe3O4 improving biotransformation of waste activated sludge into liquid high-value products. Environ. Sci. Technol. 2022, 56, 3658–3668. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Gao, W.; Zhang, Y.; He, D. Removal of microplastics from water by magnetic nano-Fe3O4. Sci. Total Environ. 2022, 802, 149838. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, L.; Uwaya, G.E.; Meier, F.; Bisetty, K. A novel MB-tagged aptasensor for Aflatoxin B1 detection in food using Fe3O4 nanoparticles substantiated with in silico modelling. Biosens. Bioelectron. X 2023, 15, 100416. [Google Scholar] [CrossRef]
- Naidoo, L.; Uwaya, G.E.; Meier, F.; Bisetty, K. A novel electrochemical sensor for the detection of zearalenone in food matrices using PEGylated Fe3O4 nanoparticles supported by in-silico and multidetector AF4. J. Electroanal. Chem. 2023, 935, 117363. [Google Scholar] [CrossRef]
- Khetagoudar, M.C.; Jinendra, U.; Kumar, A.P.; Bilehal, D.; Kollur, S.P. Multiresidue pesticide analysis in green chilli using GC–MS/MS using modified QuEChERS method with highly efficient Fe3O4@CFR@GO nanocomposite. Inorg. Chem. Commun. 2022, 137, 109195. [Google Scholar] [CrossRef]
- Antarnusa, G.; Jayanti, P.D.; Denny, Y.R.; Suherman, A. Utilization of co-precipitation method on synthesis of Fe3O4/PEG with different concentrations of PEG for biosensor applications. Materialia 2022, 25, 101525. [Google Scholar] [CrossRef]
- Liu, Q.; Xin, S.; Tan, X.; Yang, Q.; Hou, X. Ionic liquids functionalized Fe3O4-based colorimetric biosensor for rapid determination of ochratoxin A. Mikrochim. Acta 2023, 190, 364. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.; Zhou, R.; Zeng, B.; Liu, X.; Li, X.; Zhang, G. Peptide-inspired one-step synthesis of surface-functionalized Fe3O4 magnetic nanoparticles for oriented enzyme immobilization and biocatalytic applications. ACS Appl. Nano Mater. 2022, 5, 8260–8270. [Google Scholar] [CrossRef]
- Fattahi Nafchi, R.; Ahmadi, R.; Heydari, M.; Rahimipour, M.R.; Molaei, M.J.; Unsworth, L. In vitro study: Synthesis and evaluation of Fe3O4/CQD magnetic/fluorescent nanocomposites for targeted drug delivery, MRI, and cancer cell labeling applications. Langmuir 2022, 38, 3804–3816. [Google Scholar] [CrossRef]
- Nordin, A.H.; Ahmad, Z.; Husna, S.M.N.; Ilyas, R.A.; Azemi, A.K.; Ismail, N.; Nordin, M.L.; Ngadi, N.; Siti, N.H.; Nabgan, W. The state of the art of natural polymer functionalized Fe3O4 magnetic nanoparticle composites for drug delivery applications: A review. Gels 2023, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Bekaroğlu, M.G.; Kiriş, A.; Başer, H.N.; İşçi, S. Stabilizer effect of tumor-targeting ligands on the drug delivering Fe3O4 nanoparticles. Appl. Phys. A 2023, 129, 182. [Google Scholar] [CrossRef]
- Vahabi, L.; Ranjbar, P.R.; Davar, F. Cladosporium protease/doxorubicin decorated Fe3O4@SiO2 nanocomposite: An efficient nanoparticle for drug delivery and combating breast cancer. J. Drug Deliv. Sci. Technol. 2023, 80, 104144. [Google Scholar] [CrossRef]
- Moradi, S.; Najjar, R.; Hamishehkar, H.; Lotfi, A. Triple-responsive drug nanocarrier: Magnetic core-shell nanoparticles of Fe3O4@poly(N-isopropylacrylamide)-grafted-chitosan, synthesis and in vitro cytotoxicity evaluation against human lung and breast cancer cells. J. Drug Deliv. Sci. Technol. 2022, 72, 103426. [Google Scholar] [CrossRef]
- Yue, H.; Shin, J.M.; Tegafaw, T.; Han, H.S.; Chae, K.S.; Chang, Y.; Lee, G.H. Magnetic separation of nucleic acids from various biological samples using silica-coated iron oxide nanobeads. J. Nanopart. Res. 2020, 22, 366. [Google Scholar] [CrossRef]
- He, H.; Yuan, D.H.; Gao, Z.Q.; Xiao, D.L.; He, H.; Dai, H.; Peng, J.; Li, N. Mixed hemimicelles solid-phase extraction based on ionic liquid-coated Fe3O4/SiO2 nanoparticles for the determination of flavonoids in bio-matrix samples coupled with high performance liquid chromatography. J. Chromatogr. A 2014, 1324, 78–85. [Google Scholar] [CrossRef]
- Ma, W.F.; Zhang, Y.; Li, L.L.; You, L.J.; Zhang, P.; Zhang, Y.T.; Li, J.M.; Yu, M.; Guo, J.; Lu, H.J.; et al. Tailor-made magnetic Fe3O4@mTiO2 microspheres with a tunable mesoporous anatase shell for highly selective and effective enrichment of phosphopeptides. ACS Nano 2012, 6, 3179–3188. [Google Scholar] [CrossRef]
- Do, X.H.; Nguyen, T.D.; Le, T.T.H.; To, T.T.; Bui, T.V.K.; Pham, N.H.; Ha, P.T. High biocompatibility, MRI enhancement, and dual chemo-and thermal-therapy of curcumin-encapsulated alginate/Fe3O4 nanoparticles. Pharmaceutics 2023, 15, 1523. [Google Scholar] [CrossRef]
- Lipengolts, A.A.; Finogenova, Y.A.; Skribitsky, V.A.; Shpakova, K.E.; Anaki, A.; Motiei, M.; Semkina, A.S.; Abakumov, M.A.; Smirnova, A.V.; Grigorieva, E.Y. CT and MRI Imaging of theranostic bimodal Fe3O4@Au nanoparticles in tumor bearing mice. Int. J. Mol. Sci. 2023, 24, 70. [Google Scholar] [CrossRef]
- Malekzadeh, R.; Babaye Abdollahi, B.; Ghorbani, M.; Pirayesh Islamian, J.; Mortezazadeh, T. Trastuzumab conjugated PEG–Fe3O4@Au nanoparticle as an MRI biocompatible nano-contrast agent. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 759–770. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, P.; Wang, Z.; Zhao, Y.; Xiao, W.; Bian, Y.; Liang, D.; Lin, Q.; Song, W.; Jiang, W.; et al. Herceptin-conjugated DOX-Fe3O4/P(NIPAM-AA-MAPEG) nanogel system for HER2-targeted breast cancer treatment and magnetic resonance imaging. ACS Appl. Mater. Interfaces 2022, 14, 15956–15969. [Google Scholar] [CrossRef] [PubMed]
- Rezayan, A.H.; Kheirjou, S.; Edrisi, M.; Shafiee Ardestani, M.; Alvandi, H. A modified PEG-Fe3O4 magnetic nanoparticles conjugated with D(+)glucosamine (DG): MRI contrast agent. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1988–1998. [Google Scholar] [CrossRef]
- Mushtaq, A.; Tang, Z.; Hou, Y.; Zhu, Z.; Tian, C.; Wu, Y.; Lu, Y.; Iqbal, M.Z.; Kong, X. Biocompatible magnetic hydroxyapatite Fe3O4-HAp nanocomposites for T1-magnetic resonance imaging guided photothermal therapy of breast cancer. Mater. Today Commun. 2022, 31, 103734. [Google Scholar] [CrossRef]
- Divband, B.; Gharehaghaji, N.; Hassani, S. Fe3O4/graphene-based nanotheranostics for bimodal magnetic resonance/fluorescence imaging and cancer therapy. J. Inorg. Organomet. Polym. Mater. 2022, 32, 4443–4460. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Xu, X.; Rong, M.; Lu, L.; Zhao, X.; Wang, Y.; Jiang, Y. Fe3O4-rhodamine 6G nanoparticles: An iron enhanced pH sensitive multimodal probe for fluorescence and magnetic resonance imaging of tumor cell. J. Mater. Sci. Technol. 2023, 160, 128–138. [Google Scholar] [CrossRef]
- Kushwaha, P.; Chauhan, P. Facile synthesis of water-soluble Fe3O4 and Fe3O4@PVA nanoparticles for dual-contrast T1- and T2-weighted magnetic resonance imaging. Magn. Reson. Imaging 2023, 95, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, J.; Gao, X.; Hao, C.; Hu, S.; Qu, A.; Xu, L. Chiral Iron Oxide supraparticles enable enantiomer-dependent tumor-targeted magnetic resonance imaging. Adv. Mater. 2023, 35, 2308198. [Google Scholar] [CrossRef]
- González-Gómez, M.A.; Seco-Gudiña, R.; García-Acevedo, P.; Arnosa-Prieto, Á.; de Castro-Alves, L.; Piñeiro, Y.; Rivas, J. Fluorescent magnetic mesoporous nanoprobes for biotechnological enhancement procedures in gene therapy. Magnetochemistry 2023, 9, 67. [Google Scholar] [CrossRef]
- Morishita, N.; Nakagami, H.; Morishita, R.; Takeda, S.; Mishima, F.; Terazono, B.; Nishijima, S.; Kaneda, Y.; Tanaka, N. Magnetic nanoparticles with surface modification enhanced gene delivery of HVJ-E vector. Biochem. Biophys. Res. Commun. 2005, 334, 1121–1126. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Rizwan, M.; Razak, S.I.A.; Hassan, A.; Rasheed, T.; Bilal, M. Electroactive polymeric nanocomposite BC-g-(Fe3O4/GO) materials for bone tissue engineering: In vitro evaluations. J. Biomater. Sci. Polym. Ed. 2022, 33, 1349–1368. [Google Scholar] [CrossRef]
- Sun, R.; Chen, H.; Zheng, J.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Composite scaffolds of gelatin and Fe3O4 nanoparticles for magnetic hyperthermia-based breast cancer treatment and adipose tissue regeneration. Adv. Healthc. Mater. 2023, 12, 2202604. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, Y.; Wang, W.; Tang, X.; Yang, Y. Polyetherimide- and folic acid-modified Fe3O4 nanospheres for enhanced magnetic hyperthermia performance. J. Biomed. Mater. Res. 2023, 111, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Seehra, M. Magnetic Spinels: Synthesis, Properties and Applications; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Shen, L.; Li, B.; Qiao, Y. Fe3O4 nanoparticles in targeted drug/gene delivery systems. Materials 2018, 11, 324. [Google Scholar] [CrossRef]
- Yue, H.; Park, J.; Ho, S.; Ahmad, M.; Cha, H.; Liu, S.; Tegafaw, T.; Marasini, S.; Ghazanfari, A.; Kim, S.; et al. New class of efficient T2 magnetic resonance imaging contrast agent: Carbon-coated paramagnetic dysprosium oxide nanoparticles. Pharmaceuticals 2020, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Marasini, S.; Ahmad, M.Y.; Ho, S.L.; Cha, H.; Liu, S.; Jang, Y.J.; Tegafaw, T.; Ghazanfari, A.; Miao, X.; et al. Carbon-coated ultrasmall gadolinium oxide (Gd2O3@C) nanoparticles: Application to magnetic resonance imaging and fluorescence properties. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 586, 124261. [Google Scholar] [CrossRef]
- Bianchetti, E.; Di Valentin, C. Effect of surface functionalization on the magnetization of Fe3O4 nanoparticles by hybrid density functional theory calculations. J. Phys. Chem. Lett. 2022, 13, 9348–9354. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.V.; Xu, S.; Lee, T.R. Fe3O4 nanoparticles: Structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Lazarov, V.K.; Lari, L.; Hosono, H.; Susaki, T. Fe3O4(1 1 1) thin films with bulk-like properties: Growth and atomic characterization. J. Phys. D Appl. Phys. 2013, 46, 022001. [Google Scholar] [CrossRef]
- Bae, H.; Ahmad, T.; Rhee, I.; Chang, Y.; Jin, S.U.; Hong, S. Carbon-coated iron oxide nanoparticles as contrast agents in magnetic resonance imaging. Nanoscale Res. Lett. 2012, 7, 44. [Google Scholar] [CrossRef]
- Wang, Y.X.; Wang, D.W.; Zhu, X.M.; Zhao, F.; Leung, K.C. Carbon coated superparamagnetic iron oxide nanoparticles for sentinel lymph nodes mapping. Quant. Imaging Med. Surg. 2012, 2, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Basta, E.Z. Accurate determination of the cell dimensions of magnetite. Mineral. Mag. J. Mineral. Soc. 1957, 31, 431–442. [Google Scholar] [CrossRef]
- Tegafaw, T.; Oh, I.T.; Cha, H.; Yue, H.; Miao, X.; Ho, S.L.; Ahmad, M.Y.; Marasini, S.; Ghazanfari, A.; Kim, H.-K.; et al. Facile synthesis of stable colloidal suspension of amorphous carbon nanoparticles in aqueous medium and their characterization. J. Phys. Chem. Solids 2018, 120, 96–103. [Google Scholar] [CrossRef]
- Kaufman, J.H.; Metin, S.; Saperstein, D.D. Symmetry breaking in nitrogen-doped amorphous carbon: Infrared observation of the Raman-active G and D bands. Phys. Rev. B: Condens. Matter Mater. Phys. 1989, 39, 13053. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Li, Y.D. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 597–601. [Google Scholar] [CrossRef]
- Hara, M.; Yoshida, T.; Takagaki, A.; Takata, T.; Kondo, J.N.; Hayashi, S.; Domen, K. A carbon material as a strong protonic acid. Angew. Chem. Int. Ed. 2004, 43, 2955–2958. [Google Scholar] [CrossRef]
- Kolhatkar, A.; Jamison, A.; Litvinov, D.; Willson, R.; Lee, T. Tuning the magnetic properties of nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef]
- Wang, Y.-X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar] [CrossRef]

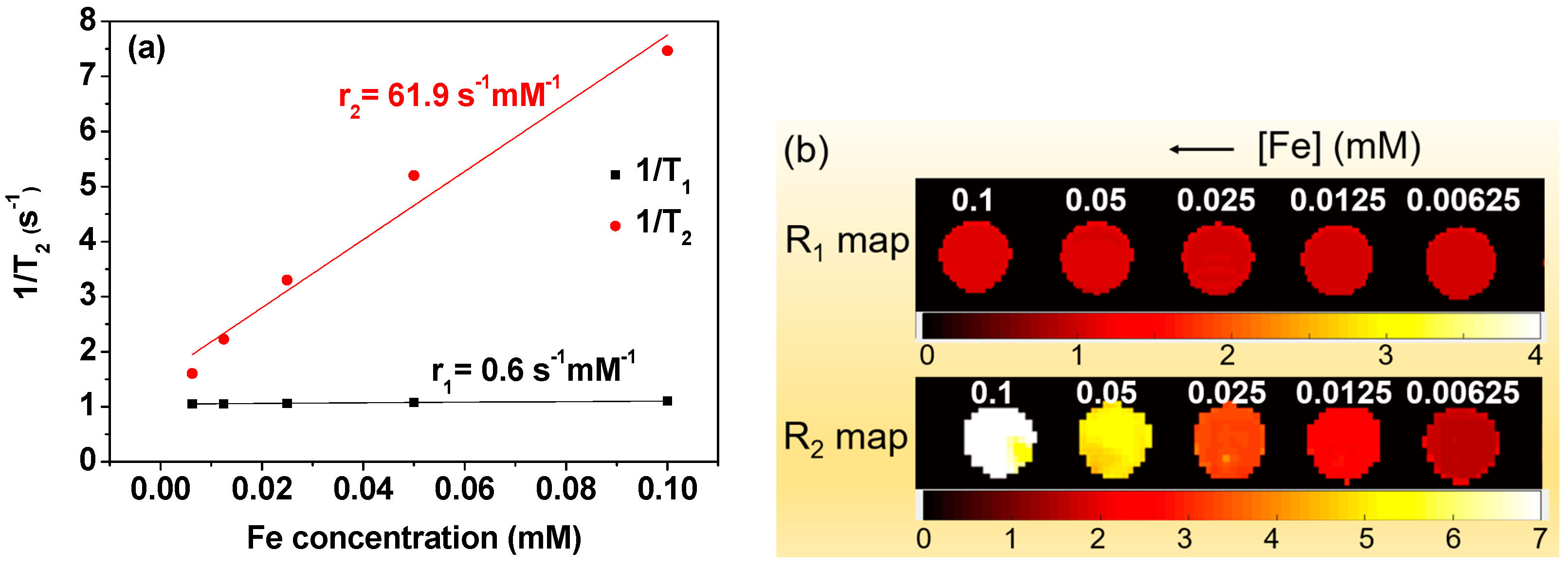
| davg (nm) | ζ (mV) | Carbon Coating (wt.%) | Saturation Magnetization (emu/g) | Relaxivity (s−1 mM−1) | |||
|---|---|---|---|---|---|---|---|
| TGA 1 | EA 2 | r1 | r2 | r2/r1 | |||
| 35.1 ± 1.4 | −32.2 | 48.1 | 58.04 | 96.2 | 0.6 | 61.9 | 103.2 |
| Sample | O–H | C–H | C=O | C=O (D) | C=O (G) | C–O | Fe–O |
|---|---|---|---|---|---|---|---|
| Dextrose | 3248 | 2943 | - | - | - | 1010 | - |
| Fe3O4@C NP | 3248 | 2943 | 1699 | 1384 | 1578 | 1048 | 556 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, H.; Zhao, D.; Tegafaw, T.; Ahmad, M.Y.; Saidi, A.K.A.A.; Liu, Y.; Cha, H.; Yang, B.W.; Chae, K.S.; Nam, S.-W.; et al. Core-Shell Fe3O4@C Nanoparticles as Highly Effective T2 Magnetic Resonance Imaging Contrast Agents: In Vitro and In Vivo Studies. Nanomaterials 2024, 14, 177. https://doi.org/10.3390/nano14020177
Yue H, Zhao D, Tegafaw T, Ahmad MY, Saidi AKAA, Liu Y, Cha H, Yang BW, Chae KS, Nam S-W, et al. Core-Shell Fe3O4@C Nanoparticles as Highly Effective T2 Magnetic Resonance Imaging Contrast Agents: In Vitro and In Vivo Studies. Nanomaterials. 2024; 14(2):177. https://doi.org/10.3390/nano14020177
Chicago/Turabian StyleYue, Huan, Dejun Zhao, Tirusew Tegafaw, Mohammad Yaseen Ahmad, Abdullah Khamis Ali Al Saidi, Ying Liu, Hyunsil Cha, Byeong Woo Yang, Kwon Seok Chae, Sung-Wook Nam, and et al. 2024. "Core-Shell Fe3O4@C Nanoparticles as Highly Effective T2 Magnetic Resonance Imaging Contrast Agents: In Vitro and In Vivo Studies" Nanomaterials 14, no. 2: 177. https://doi.org/10.3390/nano14020177
APA StyleYue, H., Zhao, D., Tegafaw, T., Ahmad, M. Y., Saidi, A. K. A. A., Liu, Y., Cha, H., Yang, B. W., Chae, K. S., Nam, S.-W., Chang, Y., & Lee, G. H. (2024). Core-Shell Fe3O4@C Nanoparticles as Highly Effective T2 Magnetic Resonance Imaging Contrast Agents: In Vitro and In Vivo Studies. Nanomaterials, 14(2), 177. https://doi.org/10.3390/nano14020177









