Advancements in Engineering Planar Model Cell Membranes: Current Techniques, Applications, and Future Perspectives
Abstract
1. Introduction
2. Classification of Planar Model Cell Membranes
| Planar Model Cell Membranes | Advantages | Limitations | Cartoon | Ref |
|---|---|---|---|---|
| LM | Stable and facile to assemble, possessing a composition similar to real membranes. | LM just replicates one side of the bilayer, lacking the capability to functionalize transmembrane proteins. | 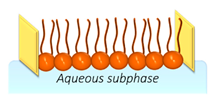 | [43,44,45] |
| SLB | Easily to characterize and stable, capable of forming lipid domains, and amenable to functionalization with other substances. | Interference in interactions stems from the substrate effect, coupled with the inability to functionalize transmembrane proteins. | 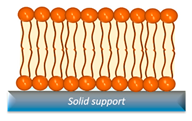 | [46,47] |
| BLM | Free from substrate disturbances, allowing for functionalization by transmembrane proteins on both sides of the bilayer. | Prone to instability in the surrounding medium, leading to membrane fluctuations caused by variations in tension at the edges. | 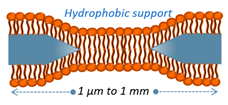 | [48] |
| SAM | They readily incorporate cholesterol and experience minimal perturbation from the substrate due to the strong anchoring of molecules within the lipid layer to the underlying substrate. | High rate of lipid oxidation. | 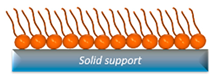 | [46,49,50] |
2.1. Lipid Monolayers (LMs)
2.2. Supported Lipid Bilayers (SLBs)
2.3. Black Lipid Membranes (BLMs)
2.4. Self-Assembled Monolayers (SAMs)
3. Techniques for the Preparation of Planar Model Cell Membranes
3.1. Vesicle Fusion Method
- (1)
- Vesicles may rupture if the mechanical stress induced by the support is sufficiently strong, leading to pore formation and subsequent nucleation until complete vesicle rupture [118].
- (2)
- If the vesicles do not rupture and continue to adsorb, they may interact with each other and fuse, resulting in larger vesicles with a higher mean diameter until they reach a critical vesicular radius. At this point, the forces of bending and support attraction are strong enough to promote vesicle rupture (B) and the formation of discs or bilayer patches [116,119,120]. The fusion of vesicles with one another and their subsequent rupture is a complex process, and its occurrence depends on various factors, including the nature of the lipid components within the vesicles (lipid charge, polarity, headgroup size, acyl chain length, and degree of unsaturation); the size and concentration of the vesicles; the flow conditions; the nature of the substrate (hydrophilicity and roughness); osmotic stress; pH; and temperature [42]. Later in this section, we will discuss in more detail the different parameters that can be optimized in the laboratory to promote the formation of planar supported membranes by the vesicle fusion method.
- (3)
- The bilayer patches formed are thermodynamically unstable due to their exposed edges, which can disrupt neighboring intact vesicles (C). This disruption promotes rupture and subsequent growth into a uniform lipid bilayer, a process known as coalescence (D) [119].
3.2. Langmuir Technique
| Systems | Aim of the Work | Reference |
|---|---|---|
| Anesthetics | Evaluation of the interaction of lidocaine with a lipid monolayer composed of POPC and cholesterol in a solvent mixture to demonstrate its effect on packaging and permeability. | [78] |
| Phospholipidic drugs | Study of the action of HePC, a phospholipid used as a treatment against visceral leishmaniosis, on POPC monolayers and sterols to evaluate HePC affinity for the parasite membrane. | [152] |
| Antifungals | Recreation of a fungal membrane using POPC and sterols to evaluate the interaction of AmB and its effect on the formation of lipid rafts and pores through which ions pass, triggering cell death. | [79] |
| Antifungal Antibiotics | Study of AmB and Am3 interactions with lipids and cholesterol/ergosterol into the model cell membrane for understanding its biological activity and mechanism of action. | [153,154,155] |
| Lipid mixtures | Reconstruction of the microbial membrane of E. coli using a monolayer formed with varying percentages of PE, PG, and CL to study their interactions and thermodynamic properties. | [74] |
| Antineoplastic drug | Study of the interaction of paclitaxel in monolayers formed by ternary mixtures of DPPC, cholesterol, and sphingomyelin and its effect on compressibility and lipid raft formation as a function of cholesterol concentration. | [156] |
| Study of the interactions of docetaxel in DPPC monolayers at several surface pressures to evaluate its absorption and penetration ability into the phospholipid matrix. | [20] | |
| Antiprotozoals | Use of PTF as a treatment agent for Chagas disease by reconstructing a protozoal monolayer from DPPG to study its cytotoxicity and its effects on lipid fluidity and rearrangement. | [157] |
| Antiparasitic | Cyclosporine A, an immunosuppressive agent that has been studied to analyze its potential to be incorporated into model cell membranes that inhibit the development of the parasite. | [19,158,159] |
| Monoterpenoids | The incorporation of thymol (a biocidal drug) in monolayers formed by DPPC with analysis of the effect on the physicochemical properties of the membrane. | [160] |
| Antimicrobial peptides | Analysis of the interaction of defense peptides that target the cell membrane and organelles of malignant cells, altering their metabolism. | [45,77] |
| Anti-inflamatory drugs | Study of the interaction of ibuprofen with a phospholipidic monolayer (DPPC and DPPG) probing that ibuprofen penetrates into the hydrophobic region of the monolayer, accompanied by a fluidizing effect. | [161,162] |
| Anesthetics | Studies at the air–water interface of lidocaine with model cell membranes incorporating DPPC, DPPE, and SM indicate that the most probable mechanism of anesthetic action is the adsorption of lidocaine to the protein ion channel of the membrane. | [148] |
| Anti-histaminic drugs | Olopatadine and ketotifen interactions with the components of a model cell membrane offer information for the mechanism of action of these compounds. | [163] |
| Xenobiotics | Analysis of the interactions of curcumin with a model cell membrane (DPPC+Chol) probing that this compound tends to fluidize the monolayer. | [21] |
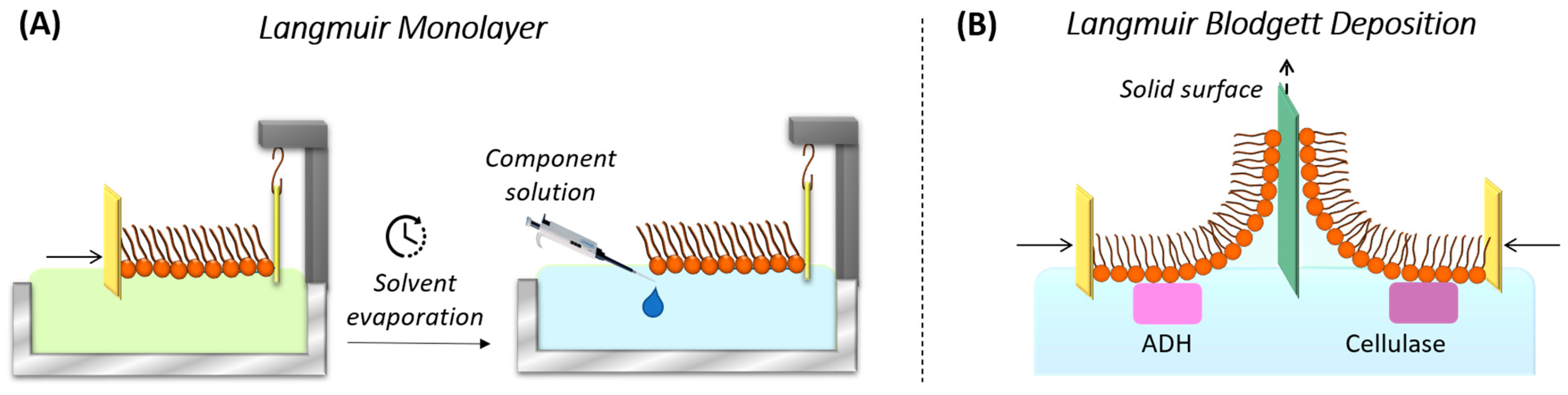
3.3. Langmuir–Blodgett (LB) and Langmuir–Schaefer (LS) Technique
4. Incorporation of Membrane Components into Planar Model Cell Membranes
4.1. Membrane Components and Their Role
| Glycerophospholipids | Sterols | Sphingolipids | Enzymes | Proteins/Glycoproteins | |
|---|---|---|---|---|---|
| 1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) [137,177] | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) [27,178,179,180,181,182] | Cholesterol (Chol) [27,126,149,150,153,155,178,183] | Glycosphingolipids: Ganglioside GM1 [179] | Alcohol Dehydrogenase (ADH) E. coli [164] | Enterotoxin: Cholera toxin b-subunit (CTB) [184] |
| 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) [183,185,186] | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) [42] | 7-ketocholesterol (7-KC) [178,187] | Ceramide galactosylceramide (GalCer) [179] | Cellulase [164] | Ephrin-A5 Fc Chimera (CF) [181] |
| 1,2-dioleyl-snglycro-3-phosphocholine (DOPC) [173,182,188] | 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS) [42] | Ergosterol [154,155] | Brain sphingomyelin (BSM) [42] | Catalase [170] | Annexin A5 (AnxA5) [173] |
| 1,2-distearoyl-sn-glycero-3-phosphatidylcholine (DSPC) [189,190] | 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine (DOEPC) [173] | 25-hydroxycholesterol (25-OH) [187] | Sphingomyelin (SM) [27,158,182,187] | Tyrosinase [191,192] | gp41-antibodies 2F5/4E10 MPER peptide [182,193] |
| 1,2-diooleoyl-sn-glycero-3-phosphoserine (DOPS) [173,194] | 1,2 ditetradecanoyl-sn-glycero-3-phosphate (DMPA) [177] | 7β-hydroxycholesterol (7β-OH) [187] | Urease [171] | Type I collagen (rat tail) [195] | |
| 1,2-diooleoyl-sn-glycero-3-phosphatidylglycerol (DOPG) [173] | 1,5-Odihexadecyl-N-succinyl-L-glutamate (DHSG) [196] | Horseradich peroxidase [197,198,199] | Glycosylphosphatidylinositol (GPI) anchored [200] | ||
| 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC) [179] | Dipalmitoyl phosphatidylserine (DPPS) [5] | Asparaginase [201] | Heparan sulfate proteoglycan HSPG [202] | ||
| L-α-Phosphatidylethanolamine (PE) [203,204] | 1,2-dipalmitoyl-sn-glycero-3-ethylphosphocholine (DPEPC) [194] | β-lactoglobulin [205] | |||
| Phosphatidylglycerol (PG) [204] | Cardiolipin (CL) [149,204,206] | α-lactalbumin (α-LA) [207] | |||
| Dipalmitoyl phosphatidylglycerol (DPPG) [170,171,197,198,208] | 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(glutaryl) (DP-NGPE) [195] | Syndecan-4 [202] | |||
| L -α–phosphatidylinositol (PI) [206] | G-protein-coupled receptors [168] | ||||
| Membrane Components | Study objective | Ref. |
|---|---|---|
| Langmuir Films | ||
| DPPE:GM1-CTB | Membrane study:
| [184] |
| PE:PG (3:1) and CL (5–20%) | Membrane study:
| [204] |
| 7-KC, Chol, SM, and POPC | Membrane study:
| [178] |
| DPPC and Syndecan-4 HSPG | Membrane study:
| [202] |
| POPC, SM, Chol, and trans-resveratrol | Drug delivery:
| [27] |
| DPPC, DPPG, and Chol Mehylene blue MB and Acridine orange AO | Treatment of diseases and encapsulation:
| [208] |
| PE and penicillin | Drug delivery:
| [203] |
| Membrane Components | Molecular Incorporation Method | Method—Support Type | Study Objective | Ref. |
|---|---|---|---|---|
| Supported Monolayer | ||||
| Palmitic acid-PA, normal human lung cells MRC-5, 2-methyltriclisine (drug) | Langmuir monolayer PA | Langmuir–Blodgett MRC-5-Mica | Drug delivery:
| [209] |
| DPPG, HRP, and chitosan | Langmuir monolayer: The enzyme solution was injected in the subphase under a pre-formed lipid monolayer | Langmuir–Blodgett Optical glass Gold AT-cut quartz crystal coated with Au | Biosensor:
| [197,198] |
| DMPA and DMPC | Preparation of vesicles: Lipid hydration | LB |
| [177,210] |
| DPPC, DPPA, DPEPC, DOPS, 1,2-dihexadecanoyl-3-trimethylammonium-propane (DPTAP)/ pluronic F-127 cubosomes | Langmuir monolayer | LB | Drug delivery: nanoparticles:
| [194] |
| DPPC, DPPS, PI, CL, SM, TAT–ritonavir-loaded poly (L-lactide) NPs | Langmuir monolayer | Injection NPs: Langmuir–Schaeffer—Silicon substrate | Drug delivery:
| [206] |
| Cis-9-octadecenoic acid (OA), α-LA, CaCl2 (Ca2+) | Langmuir monolayer CaCl2 was dissolved into suphase before spreading of amphiphilic molecules | LB-solid support | Drug test:
| [207] |
| DPPC and β-sheet peptide nanofibers NFs | Langmuir monolayer | Langmuir: Suspension NFs was injected slowly into the buffer subphase | Drug delivery: nanoparticles:
| [211] |
| DPPC and hydrophobic fumed silica NPs | Langmuir monolayer | Langmuir: Spreading of SiO2 NPs | Nanoparticles:
| [186] |
| DPPC and chitosan, PVA, functionalized Fe3O4 NPs | Langmuir monolayer | Langmuir: Spreading of NPs | Nanoparticles:
| [185] |
| (GPCRs: CXCR4) -Ishiwaka cells/BSA—CXCL12 α | Double incubation, first the cells and then the ligand CXCL12α | LS-Cr/Au-coated glass slides | Drug delivery:
| [168] |
| DPPG–Ureasa | Langmuir monolayer based on the injection of molecular solutions below the air–water interface after having spread the lipid components and evaporated the solvent. | LB–quartz crystal, quartz plate, indium tin oxide (ITO) substrates | Biosensor:
| [171] |
| DPPG–Catalase | LB-optical glass and gold | Biosensor:
| [170] | |
| ADH/cellulase− DPPC | LB-solid glass supports | Membrane study:
| [164] | |
| Membrane Components | Molecular Incorporation Method | Method—Support Type | Study Objective | Ref. |
|---|---|---|---|---|
| Lipid Bilayer | ||||
| Sulfated butyl oleate (SBO), phospholipids, and β-lactoglobulin | Electrostatic SA: First layer: SBO Second layer: SBO, or phospholipids | - | Vehicle for bioactive substances (nutritional, pharmaceutical, and/or cosmetic applications):
| [205] |
| DOPC, DPPC, ciprofloxacin, and moxifloxacin | SUV preparation: lipidic hydration | Vesicle fusion and rupture method-mica | Drug test:
| [212,213] |
| POPC, Ephrin-A5 Fc Chimera (CF) | SUV’s fusion and rupture method (POPC): Clean and O2-plasma activated glass cover slips | Detergent-mediated reconstitution method: NOG-EA5/Fc proteoliposomes | Membrane study:
| [181] |
| POPC or DOPC: SM: Chol Interaction with gp41-2F5/4E10 | SUV’s fusion and rupture method-mica | Addition and incubation on SLB |
| [182] |
| DPPC/POPC and GM1-CTB | Vesicle fusion and rupture method-SiO2/Si substrate |
| [179,214] | |
| DOPC, DOPS, DOPG, DOEPC, and annexin A5 (AnxA5) | Vesicle fusion and rupture method (addition of divalent cations and osmotic gradients)-SiO2 | Injection and adsorption on SLB |
| [173] |
| DP-NGPE: POPC and type I collagen (rat tail) | SUV’s fusion and rupture method-SiO2 | Membrane study:
| [195] | |
| DLPC-GalCer | Vesicle fusion and rupture method: SiO2/Si substrate |
| [179] | |
| POPC, POPE, POPS, BSM, Chol | Use of AH peptides for vesicle fusion: Silica substrate |
| [42] | |
4.1.1. Lipids
4.1.2. Proteins
4.1.3. Enzymes
4.2. Methodologies for the Incorporation of External and Cell Membrane Components onto the Lipid Planar Model Cell Membranes
4.2.1. Monolayers
4.2.2. Bilayers
5. Applications of Planar Model Cell Membranes
5.1. Model Cell Membranes as Platforms for Fundamental Knowledge Acquisition
5.2. Alteration of Membrane Components to Promote Changes in the Performance of the Cell Membrane
5.3. Transport and Encapsulation
5.4. Functionalization of Nanoparticles
5.5. Biosensors
6. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, H. Biological Membranes. Essays Biochem. 2015, 59, 43–70. [Google Scholar] [CrossRef] [PubMed]
- Vogel, V.; Sheetz, M. Local Force and Geometry Sensing Regulate Cell Functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Pomorski, T.G.; Nylander, T.; Cárdenas, M. Model Cell Membranes: Discerning Lipid and Protein Contributions in Shaping the Cell. Adv. Colloid. Interface Sci. 2014, 205, 207–220. [Google Scholar] [CrossRef]
- Tanaka, M.; Sackmann, E. Polymer-Supported Membranes as Models of the Cell Surface. Nature 2005, 437, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Peetla, C.; Stine, A.; Labhasetwar, V. Biophysical Interactions with Model Lipid Membranes: Applications in Drug Discovery and Drug Delivery. Mol. Pharm. 2009, 6, 1264–1276. [Google Scholar] [CrossRef]
- Castellana, E.T.; Cremer, P.S. Solid Supported Lipid Bilayers: From Biophysical Studies to Sensor Design. Surf. Sci. Rep. 2006, 61, 429–444. [Google Scholar] [CrossRef]
- Martin, D.K. Nanobiotechnology of Biomimetic Membranes, 1st ed.; Ferrari, M., Ed.; Springer: New York, NY, USA, 2007. [Google Scholar]
- Kucik, D.F.; Elson, E.L.; Sheetz, M.P. Weak Dependence of Mobility of Membrane Protein Aggregates on Aggregate Size Supports a Viscous Model of Retardation of Diffusion. Biophys. J. 1999, 76, 314–322. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, S.; Ding, Q.; Fan, Q.; Dai, Y.; Guo, S.; Ye, Y.; Li, C.; Zhou, M. Recent Advances in Cell Membrane-Camouflaged Nanoparticles for Inflammation Therapy. Drug Deliv. 2021, 28, 1109–1119. [Google Scholar] [CrossRef]
- Escribá, P.V.; González-Ros, J.M.; Goñi, F.M.; Kinnunen, P.K.J.; Vigh, L.; Sánchez-Magraner, L.; Fernández, A.M.; Busquets, X.; Horváth, I.; Barceló-Coblijn, G. Membranes: A Meeting Point for Lipids, Proteins and Therapies: Translational Medicine. J. Cell Mol. Med. 2008, 12, 829–875. [Google Scholar] [CrossRef]
- Robertson, J. The Ultrastructure of Cell Membranes and Their Derivatives. Biochem. Soc. Symp. 1959, 16, 3–43. [Google Scholar]
- Kusumi, A.; Fujiwara, T.K.; Chadda, R.; Xie, M.; Tsunoyama, T.A.; Kalay, Z.; Kasai, R.S.; Suzuki, K.G.N. Dynamic Organizing Principles of the Plasma Membrane That Regulate Signal Transduction: Commemorating the Fortieth Anniversary of Singer and Nicolson’s Fluid-Mosaic Model. Annu. Rev. Cell Dev. Biol. 2012, 28, 215–250. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, A.; Suzuki, K.G.N.; Kasai, R.S.; Ritchie, K.; Fujiwara, T.K. Hierarchical Mesoscale Domain Organization of the Plasma Membrane. Trends Biochem. Sci. 2011, 36, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; de Mattos, G.F. A Brief Introduction to Some Aspects of the Fluid–Mosaic Model of Cell Membrane Structure and Its Importance in Membrane Lipid Replacement. Membranes 2021, 11, 947. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, Y.; Iwamoto, M.; Oiki, S. Asymmetric Lipid Bilayers and Potassium Channels Embedded Therein in the Contact Bubble Bilayer. Methods Mol. Biol. 2024, 2796, 1–21. [Google Scholar] [CrossRef]
- Nicolson, G.L. The Fluid—Mosaic Model of Membrane Structure: Still Relevant to Understanding the Structure, Function and Dynamics of Biological Membranes after More than 40 Years. Biochim. Biophys. Acta (BBA)—Biomembr. 2014, 1838, 1451–1466. [Google Scholar] [CrossRef]
- Viljetić, B.; Blažetić, S.; Labak, I.; Ivić, V.; Zjalić, M.; Heffer, M.; Balog, M. Lipid Rafts: The Maestros of Normal Brain Development. Biomolecules 2024, 14, 362. [Google Scholar] [CrossRef]
- Ai, X.; Wang, S.; Duan, Y.; Zhang, Q.; Chen, M.S.; Gao, W.; Zhang, L. Emerging Approaches to Functionalizing Cell Membrane-Coated Nanoparticles. Biochemistry 2021, 60, 941–955. [Google Scholar] [CrossRef]
- Jurak, M.; Szafran, K.; Cea, P.; Martín, S. Characteristics of Phospholipid–Immunosuppressant–Antioxidant Mixed Langmuir–Blodgett Films. J. Phys. Chem. B 2022, 126, 6936–6947. [Google Scholar] [CrossRef]
- Fernández-Botello, A.; Comelles, F.; Asunción Alsina, M.; Cea, P.; Reig, F. A Monolayer Study on Interactions of Docetaxel with Model Lipid Membranes. J. Phys. Chem. B 2008, 112, 13834–13841. [Google Scholar] [CrossRef]
- Dotor, L.; García-Pinilla, J.M.; Martín, S.; Cea, P. Langmuir and Langmuir–Blodgett Technologies as Nanoarchitectonic Tools for the Incorporation of Curcumin in Membrane Systems. Nanoscale 2023, 15, 2891–2903. [Google Scholar] [CrossRef]
- Cunill Semanat, E. Activity of Apoptotic Peptides in Model Membranes. Biomedicine and Biotechnology. Ph.D. Thesis, Universitat de València, Valencia, Spain, 2017. [Google Scholar]
- Kleinzeller, A. Chapter 1 Charles Ernest Overton’s Concept of a Cell Membrane. Curr. Top. Membr. Transp. 1999, 48, 1–22. [Google Scholar] [CrossRef]
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of Cell Membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef]
- Edidin, M. Lipids on the Frontier: A Century of Cell-Membrane Bilayers. Nat. Rev. Mol. Cell Biol. 2003, 4, 414–418. [Google Scholar] [CrossRef]
- Becker, W.; Kleinsmith, L.; Hardin, J.; Raasch, J. Membranas: Estructura, Química y Función. In El Mundo de la Célula; Pearson: Madrid, Spain, 2003; pp. 172–177. [Google Scholar]
- Płachta, Ł.; Mach, M.; Kowalska, M.; Wydro, P. The Effect of Trans-Resveratrol on the Physicochemical Properties of Lipid Membranes with Different Cholesterol Content. Biochim. Biophys. Acta (BBA)—Biomembr. 2024, 1866, 184212. [Google Scholar] [CrossRef]
- Gould, S.B. Membranes and Evolution. Curr. Biol. 2018, 28, R381–R385. [Google Scholar] [CrossRef]
- Zhukov, A.; Popov, V. Eukaryotic Cell Membranes: Structure, Composition, Research Methods and Computational Modelling. Int. J. Mol. Sci. 2023, 24, 11226. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Zimmerberg, J.; Gawrisch, K. The Physical Chemistry of Biological. Nat. Chem. Biol. 2006, 2, 564–567. [Google Scholar] [CrossRef]
- Doktorova, M.; Symons, J.L.; Levental, I. Structural and Functional Consequences of Reversible Lipid Asymmetry in Living Membranes. Nat. Chem. Biol. 2020, 16, 1321–1330. [Google Scholar] [CrossRef]
- Megías, M.; Molist, P.; Pombal, M. La Célula. 3. Membrana Celular. Proteínas. Atlas de Histología Vegetal y Animal. Available online: https://mmegias.webs.uvigo.es/5-celulas/3-proteinas.php (accessed on 9 November 2021).
- Ripa, M.I. Módulo: Membrana Celular. Cátedra de Biología. Facultad de Ciencias Agrarias. Universidad de Lomas de Zamora, Buenos Aires, Argentina. Available online: https://xdoc.mx/preview/membrana-celular-facultad-de-ciencias-agrarias-5eaf30914f13e (accessed on 11 September 2024).
- Zhao, W.; Tian, Y.; Cai, M.; Wang, F.; Wu, J.; Gao, J.; Liu, S.; Jiang, J.; Jiang, S.; Wang, H. Studying the Nucleated Mammalian Cell Membrane by Single Molecule Approaches. PLoS ONE 2014, 9, e91595. [Google Scholar] [CrossRef]
- Lee, A.G. Lipid-Protein Interactions in Biological Membranes: A Structural Perspective. Biochim. Biophys. Acta Biomembr. 2003, 1612, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Penkauskas, T.; Preta, G. Biological Applications of Tethered Bilayer Lipid Membranes. Biochimie 2019, 157, 131–141. [Google Scholar] [CrossRef]
- Fuster, M.M.; Esko, J.D. The Sweet and Sour of Cancer: Glycans as Novel Therapeutic Targets. Nat. Rev. Cancer 2005, 5, 526–542. [Google Scholar] [CrossRef]
- Efremov, R.G. Dynamic “Molecular Portraits” of Biomembranes Drawn by Their Lateral Nanoscale Inhomogeneities. Int. J. Mol. Sci. 2021, 22, 6250. [Google Scholar] [CrossRef] [PubMed]
- Rascol, E.; Devoisselle, J.M.; Chopineau, J. The Relevance of Membrane Models to Understand Nanoparticles–Cell Membrane Interactions. Nanoscale 2016, 8, 4780–4798. [Google Scholar] [CrossRef]
- Siontorou, C.G.; Nikoleli, G.P.; Nikolelis, D.P.; Karapetis, S.K. Artificial Lipid Membranes: Past, Present, and Future. Membranes 2017, 7, 38. [Google Scholar] [CrossRef]
- Hardy, G.J.; Nayak, R.; Zauscher, S. Model Cell Membranes: Techniques to Form Complex Biomimetic Supported Lipid Bilayers via Vesicle Fusion. Curr. Opin. Colloid. Interface Sci. 2013, 18, 448–458. [Google Scholar] [CrossRef]
- Méndez, M.A.; Nazemi, Z.; Uyanik, I.; Lu, Y.; Girault, H.H. Melittin Adsorption and Lipid Monolayer Disruption at Liquid-Liquid Interfaces. Langmuir 2011, 27, 13918–13924. [Google Scholar] [CrossRef][Green Version]
- Santos, H.A.; Ferreira, E.S.; Pereira, E.J.; Pereira, C.M.; Kontturi, K.; Silva, F. Adsorption-Penetration Studies of Glucose Oxidase into Phospholipid Monolayers at the 1,2-Dichloroethane/Water Interface. ChemPhysChem 2007, 8, 1540–1547. [Google Scholar] [CrossRef]
- Travkova, O.G.; Moehwald, H.; Brezesinski, G. The Interaction of Antimicrobial Peptides with Membranes. Adv. Colloid. Interface Sci. 2017, 247, 521–532. [Google Scholar] [CrossRef]
- Jackman, J.A.; Cho, N.-J. Supported Lipid Bilayer Formation: Beyond Vesicle Fusion. Langmuir 2020, 36, 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Ferhan, A.R.; Yoon, B.K.; Park, S.; Sut, T.N.; Chin, H.; Park, J.H.; Jackman, J.A.; Cho, N.J. Solvent-Assisted Preparation of Supported Lipid Bilayers. Nat. Protoc. 2019, 14, 2091–2118. [Google Scholar] [CrossRef] [PubMed]
- Winterhalter, M. Black Lipid Membranes. Curr. Opin. Colloid. Interface Sci. 2000, 5, 250–255. [Google Scholar] [CrossRef]
- Schlenoff, J.B.; Li, M.; Ly, H. Stability and Self-Exchange in Alkanethiol Monolayers. J. Am. Chem. Soc. 1995, 117, 12528–12536. [Google Scholar] [CrossRef]
- Li, Z.; Munro, K.; Ebralize, I.I.; Narouz, M.R.; Padmos, J.D.; Hao, H.; Crudden, C.M.; Horton, J.H. N-Heterocyclic Carbene Self-Assembled Monolayers on Gold as Surface Plasmon Resonance Biosensors. Langmuir 2017, 33, 13936–13944. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. II. Liquids 1. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef]
- Gorter, E.; Grendel, F. On Bimolecular Layers of Lipoids on the Chromocytes of the Blood. J. Exp. Med. 1925, 41, 439–443. [Google Scholar] [CrossRef]
- Blodgett, K.B. Films Built by Depositing Successive Monomolecular Layers on a Solid Surface. J. Am. Chem. Soc. 1935, 57, 1007–1022. [Google Scholar] [CrossRef]
- Gorter, E. Protein-Films. Trans. Faraday Soc. 1937, 33, 1125. [Google Scholar] [CrossRef]
- Langmuir, I.; Waugh, D.F. The Adsorption of Proteins at Oil-Water Interfaces and Artificial Protein-Lipoid Membranes. J. Gen. Physiol. 1938, 21, 745–755. [Google Scholar] [CrossRef]
- Langmuir, I.; Schaefer, V.J. Activities of Urease and Pepsin Monolayers. J. Am. Chem. Soc. 1938, 60, 1351–1360. [Google Scholar] [CrossRef]
- LEVINE, Y.K.; BAILEY, A.I.; WILKINS, M.H.F. Multilayers of Phospholipid Bimolecular Leaflets. Nature 1968, 220, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Mueller, P.; Rudin, D.O.; Ti Tien, H.; Wescott, W.C. Reconstitution of Cell Membrane Structure in Vitro and Its Transformation into an Excitable System. Nature 1962, 194, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Montal, M.; Mueller, P. Formation of Bimolecular Membranes from Lipid Monolayers and a Study of Their Electrical Properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef]
- Hafeman, D.G.; von Tscharner, V.; McConnell, H.M. Specific Antibody-Dependent Interactions between Macrophages and Lipid Haptens in Planar Lipid Monolayers. Proc. Natl. Acad. Sci. USA 1981, 78, 4552–4556. [Google Scholar] [CrossRef]
- Tamm, L.K.; McConnell, H.M. Supported Phospholipid Bilayers. Biophys. J. 1985, 47, 105–113. [Google Scholar] [CrossRef]
- Brian, A.A.; McConnell, H.M. Allogeneic Stimulation of Cytotoxic T Cells by Supported Planar Membranes. Proc. Natl. Acad. Sci. USA 1984, 81, 6159–6163. [Google Scholar] [CrossRef]
- Bigelow, W.C.; Pickett, D.L.; Zisman, W.A. Oleophobic Monolayers. J. Colloid. Sci. 1946, 1, 513–538. [Google Scholar] [CrossRef]
- Blackman, L.C.F.; Dewar, M.J.S. 27. Promoters for the Dropwise Condensation of Steam. Part I. Preparation of Compounds Containing Monofunctional Sulphur Groups. J. Chem. Soc. (Resumed) 1957, 162–165. [Google Scholar] [CrossRef]
- Murphy, J.G. Silver Polish. U.S. Patent Application US 2841501, 17 April 1957. [Google Scholar]
- Nuzzo, R.G.; Allara, D.L. Adsorption of Bifunctional Organic Disulfides on Gold Surfaces. J. Am. Chem. Soc. 1983, 105, 4481–4483. [Google Scholar] [CrossRef]
- Plant, A.L. Self-Assembled Phospholipid/Alkanethiol Biomimetic Bilayers on Gold. Langmuir 1993, 9, 2764–2767. [Google Scholar] [CrossRef]
- Lang, H.; Duschl, C.; Vogel, H. A New Class of Thiolipids for the Attachment of Lipid Bilayers on Gold Surfaces. Langmuir 1994, 10, 197–210. [Google Scholar] [CrossRef]
- Vockenroth, I.K.; Ohm, C.; Robertson, J.W.F.; McGillivray, D.J.; Lösche, M.; Köper, I. Stable Insulating Tethered Bilayer Lipid Membranes. Biointerphases 2008, 3, FA68–FA73. [Google Scholar] [CrossRef]
- Rebaud, S.; Maniti, O.; Girard-Egrot, A.P. Tethered Bilayer Lipid Membranes (TBLMs): Interest and Applications for Biological Membrane Investigations. Biochimie 2014, 107, 135–142. [Google Scholar] [CrossRef]
- Eeman, M.; Deleu, M. From Biological Membranes to Biomimetic Model Membranes. Biotechnol. Agron. Société Environ. 2010, 14, 719–736. [Google Scholar]
- O’Shea, P. Intermolecular Interactions with/within Cell Membranes and the Trinity of Membrane Potentials: Kinetics and Imaging. Biochem. Soc. Trans. 2003, 31, 990–996. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional Rafts in Cell Membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Hoyo, J.; Torrent-Burgués, J.; Tzanov, T. Physical States and Thermodynamic Properties of Model Gram-Negative Bacterial Inner Membranes. Chem. Phys. Lipids 2019, 218, 57–64. [Google Scholar] [CrossRef]
- Santos, H.A.; García-Morales, V.; Roozeman, R.J.; Manzanares, J.A.; Kontturi, K. Interfacial Interaction between Dextran Sulfate and Lipid Monolayers: An Electrochemical Study. Langmuir 2005, 21, 5475–5484. [Google Scholar] [CrossRef]
- Verger, R.; Pattus, F. Lipid-Protein Interactions in Monolayers. Chem. Phys. Lipids 1982, 30, 189–227. [Google Scholar] [CrossRef]
- Ciumac, D.; Gong, H.; Hu, X.; Lu, J.R. Membrane Targeting Cationic Antimicrobial Peptides. J. Colloid. Interface Sci. 2019, 537, 163–185. [Google Scholar] [CrossRef] [PubMed]
- Cavassin, P.; Pappa, A.M.; Pitsalidis, C.; Barbosa, H.F.P.; Colucci, R.; Saez, J.; Tuchman, Y.; Salleo, A.; Faria, G.C.; Owens, R.M. Organic Transistors Incorporating Lipid Monolayers for Drug Interaction Studies. Adv. Mater. Technol. 2020, 5, 1900680. [Google Scholar] [CrossRef]
- Foglia, F.; Fragneto, G.; Clifton, L.A.; Lawrence, M.J.; Barlow, D.J. Interaction of Amphotericin B with Lipid Monolayers. Langmuir 2014, 30, 9147–9156. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Auffret, N. Interaction of Xenobiotics with Mercury-Adsorbed Phospholipid Monolayers. Mar. Environ. Res. 1988, 24, 51–56. [Google Scholar] [CrossRef]
- Matyszewska, D.; Nazaruk, E.; Campbell, R.A. Interactions of Anticancer Drugs Doxorubicin and Idarubicin with Lipid Monolayers: New Insight into the Composition, Structure and Morphology. J. Colloid. Interface Sci. 2021, 581, 403–416. [Google Scholar] [CrossRef]
- Contini, C.; Schneemilch, M.; Gaisford, S.; Quirke, N. Nanoparticle–Membrane Interactions. J. Exp. Nanosci. 2018, 13, 62–81. [Google Scholar] [CrossRef]
- Boucher, P.A.; Joós, B.; Zuckermann, M.J.; Fournier, L. Pore Formation in a Lipid Bilayer under a Tension Ramp: Modeling the Distribution of Rupture Tensions. Biophys. J. 2007, 92, 4344–4355. [Google Scholar] [CrossRef]
- Roy, M.T.; Gallardo, M.; Estelrich, J. Bilayer Distribution of Phosphatidylserine and Phosphatidylethanolamine in Lipid Vesicles. Bioconjug Chem. 1997, 8, 941–945. [Google Scholar] [CrossRef]
- Bavi, N.; Nakayama, Y.; Bavi, O.; Cox, C.D.; Qin, Q.-H.; Martinac, B. Biophysical Implications of Lipid Bilayer Rheometry for Mechanosensitive Channels. Proc. Natl. Acad. Sci. USA 2014, 111, 13864–13869. [Google Scholar] [CrossRef]
- Siontorou, C.G.; Batzias, F.A. Innovation in Biotechnology: Moving from Academic Research to Product Development—The Case of Biosensors. Crit. Rev. Biotechnol. 2010, 30, 79–98. [Google Scholar] [CrossRef]
- Ruiz-Rincón, S.; González-Orive, A.; de la Fuente, J.M.; Cea, P. Reversible Monolayer–Bilayer Transition in Supported Phospholipid LB Films under the Presence of Water: Morphological and Nanomechanical Behavior. Langmuir 2017, 33, 7538–7547. [Google Scholar] [CrossRef]
- Gözen, I.; Jesorka, A. Instrumental Methods to Characterize Molecular Phospholipid Films on Solid Supports. Anal. Chem. 2012, 84, 822–838. [Google Scholar] [CrossRef]
- Griebenow, K.; Klibanov, A.M. On Protein Denaturation in Aqueous-Organic Mixtures but Not in Pure Organic Solvents. J. Am. Chem. Soc. 1996, 118, 11695–11700. [Google Scholar] [CrossRef]
- Siontorou, C.G. Bilayer Lipid Membrane Constructs: A Strategic Technology Evaluation Approach. In Advanced Bioelectronic Materials; Wiley: Hoboken, NJ, USA, 2015; pp. 309–353. [Google Scholar]
- Bamberg, E.; Alpes, H.; Apell, H.J.; Bradley, R.; Härter, B.; Quelle, M.J.; Urry, D.W. Formation of Ionic Channels in Black Lipid Membranes by Succinic Derivatives of Gramicidin A. J. Membr. Biol. 1979, 50, 257–270. [Google Scholar] [CrossRef]
- Kresák, S.; Hianik, T.; Naumann, R.L.C. Giga-Seal Solvent-Free Bilayer Lipid Membranes: From Single Nanopores to Nanopore Arrays. Soft Matter 2009, 5, 4021–4032. [Google Scholar] [CrossRef]
- Schmidt, C.; Mayer, M.; Vogel, H. ZUSCHRIFTEN-A Chip-Based Biosensor for the Functional Analysis of Single Ion Channels. Angew. Chem.-Ger. Ed. 2000, 112, 3267–3269. [Google Scholar] [CrossRef]
- Angelova, M.I.; Dimitrov, D.S. Liposome Electroformation. Faraday Discuss. Chem. Soc. 1986, 81, 303–311. [Google Scholar] [CrossRef]
- Kloda, A.; Lua, L.; Hall, R.; Adams, D.J.; Martinac, B. Liposome Reconstitution and Modulation of Recombinant N -Methyl- d -Aspartate Receptor Channels by Membrane Stretch. Proc. Natl. Acad. Sci. USA 2007, 104, 1540–1545. [Google Scholar] [CrossRef]
- Tadaki, D.; Yamaura, D.; Araki, S.; Yoshida, M.; Arata, K.; Ohori, T.; Ishibashi, K.; Kato, M.; Ma, T.; Miyata, R.; et al. Mechanically Stable Solvent-Free Lipid Bilayers in Nano- and Micro-Tapered Apertures for Reconstitution of Cell-Free Synthesized HERG Channels. Sci. Rep. 2017, 7, 17736. [Google Scholar] [CrossRef]
- Han, X.; Studer, A.; Sehr, H.; Geissbühler, I.; Di Berardino, M.; Winkler, F.K.; Tiefenauer, L.X. Nanopore Arrays for Stable and Functional Free-Standing Lipid Bilayers. Adv. Mater. 2007, 19, 4466–4470. [Google Scholar] [CrossRef]
- Komiya, M.; Kato, M.; Tadaki, D.; Ma, T.; Yamamoto, H.; Tero, R.; Tozawa, Y.; Niwano, M.; Hirano-Iwata, A. Advances in Artificial Cell Membrane Systems as a Platform for Reconstituting Ion Channels. Chem. Rec. 2020, 20, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Park, S.; Yuan, F.; Hayden, C.C.; Wang, L.; Lafer, E.M.; Choi, S.Q.; Stachowiak, J.C. Transmembrane Coupling of Liquid-like Protein Condensates. Nat. Commun. 2023, 14, 8015. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, S.Q. Sphingomyelinase-Mediated Multitimescale Clustering of Ganglioside GM1 in Heterogeneous Lipid Membranes. Adv. Sci. 2021, 8, 202101766. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.; Oh, S.S.; Choi, S.Q. Ultra-Stable Freestanding Lipid Membrane Array: Direct Visualization of Dynamic Membrane Remodeling with Cholesterol Transport and Enzymatic Reactions. Small 2020, 16, e202002541. [Google Scholar] [CrossRef]
- Jenkins, A.T.A.; Boden, N.; Bushby, R.J.; Evans, S.D.; Knowles, P.F.; Miles, R.E.; Ogier, S.D.; Schönherr, H.; Vancso, G.J. Microcontact Printing of Lipophilic Self-Assembled Monolayers for the Attachment of Biomimetic Lipid Bilayers to Surfaces. J. Am. Chem. Soc. 1999, 121, 5274–5280. [Google Scholar] [CrossRef]
- Crudden, C.M.; Horton, J.H.; Ebralidze, I.I.; Zenkina, O.V.; McLean, A.B.; Drevniok, B.; She, Z.; Kraatz, H.B.; Mosey, N.J.; Seki, T.; et al. Ultra Stable Self-Assembled Monolayers of N-Heterocyclic Carbenes on Gold. Nat. Chem. 2014, 6, 409–414. [Google Scholar] [CrossRef]
- Alharbi, A.R.M.; Andersson, J.M.; Köper, I.; Andersson, G.G. Investigating the Structure of Self-Assembled Monolayers Related to Biological Cell Membranes. Langmuir 2019, 35, 14213–14221. [Google Scholar] [CrossRef]
- Hohner, A.O.; David, M.P.C.; Rädler, J.O. Controlled Solvent-Exchange Deposition of Phospholipid Membranes onto Solid Surfaces. Biointerphases 2010, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tabaei, S.R.; Choi, J.H.; Haw Zan, G.; Zhdanov, V.P.; Cho, N.J. Solvent-Assisted Lipid Bilayer Formation on Silicon Dioxide and Gold. Langmuir 2014, 30, 10363–10373. [Google Scholar] [CrossRef]
- Tabaei, S.R.; Jackman, J.A.; Kim, S.O.; Liedberg, B.; Knoll, W.; Parikh, A.N.; Cho, N.J. Formation of Cholesterol-Rich Supported Membranes Using Solvent-Assisted Lipid Self-Assembly. Langmuir 2014, 30, 13345–13352. [Google Scholar] [CrossRef]
- Tian, Z.; Gong, J.; Crowe, M.; Lei, M.; Li, D.; Ji, B.; Diao, J. Biochemical Studies of Membrane Fusion at the Single-Particle Level. Prog. Lipid Res. 2019, 73, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, P.; Lingden, D.; Stine, K.J. Structure, Formation, and Biological Interactions of Supported Lipid Bilayers (SLB) Incorporating Lipopolysaccharide. Coatings 2020, 10, 981. [Google Scholar] [CrossRef]
- Simonsen, A.C.; Bagatolli, L.A. Structure of Spin-Coated Lipid Films and Domain Formation in Supported Membranes Formed by Hydration. Langmuir 2004, 20, 9720–9728. [Google Scholar] [CrossRef]
- Furukawa, K.; Hibino, H. Self-Spreading of Supported Lipid Bilayer on Sio2 Surface Bearing Graphene Oxide. Chem. Lett. 2012, 41, 1259–1261. [Google Scholar] [CrossRef]
- Richter, R.; Mukhopadhyay, A.; Brisson, A. Pathways of Lipid Vesicle Deposition on Solid Surfaces: A Combined QCM-D and AFM Study. Biophys. J. 2003, 85, 3035–3047. [Google Scholar] [CrossRef]
- Richter, R.P.; Brisson, A. QCM-D on Mica for Parallel QCM-D—AFM Studies. Langmuir 2004, 20, 4609–4613. [Google Scholar] [CrossRef]
- Richter, R.P.; Brisson, A. Characterization of Lipid Bilayers and Protein Assemblies Supported on Rough Surfaces by Atomic Force Microscopy. Langmuir 2003, 19, 1632–1640. [Google Scholar] [CrossRef]
- Kalb, E.; Frey, S.; Tamm, L.K. Formation of Supported Planar Bilayers by Fusion of Vesicles to Supported Phospholipid Monolayers. Biochim. Biophys. Acta (BBA)—Biomembr. 1992, 1103, 307–316. [Google Scholar] [CrossRef]
- Richter, R.P.; Bérat, R.; Brisson, A.R. Formation of Solid-Supported Lipid Bilayers: An Integrated View. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef]
- Bottacchiari, M.; Gallo, M.; Bussoletti, M.; Casciola, C.M. Topological Transitions in Fluid Lipid Vesicles: Activation Energy and Force Fields. Commun. Phys. 2022, 5, 283. [Google Scholar] [CrossRef] [PubMed]
- Brochard-Wyart, F.; de Gennes, P.G.; Sandre, O. Transient Pores in Stretched Vesicles: Role of Leak-Out. Phys. A Stat. Mech. Its Appl. 2000, 278, 32–51. [Google Scholar] [CrossRef]
- Reviakine, I.; Brisson, A. Formation of Supported Phospholipid Bilayers from Unilamellar Vesicles Investigated by Atomic Force Microscopy. Langmuir 2000, 16, 1806–1815. [Google Scholar] [CrossRef]
- Lipowsky, R.; Seifert, U. Adhesion of Vesicles and Membranes. Mol. Cryst. Liq. Cryst. 1991, 202, 17–25. [Google Scholar] [CrossRef]
- Lind, T.K.; Cárdenas, M.; Wacklin, H.P. Formation of Supported Lipid Bilayers by Vesicle Fusion: Effect of Deposition Temperature. Langmuir 2014, 30, 7259–7263. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.J.; Jackman, J.A.; Liu, M.; Frank, C.W. PH-Driven Assembly of Various Supported Lipid Platforms: A Comparative Study on Silicon Oxide and Titanium Oxide. Langmuir 2011, 27, 3739–3748. [Google Scholar] [CrossRef]
- Dacic, M.; Jackman, J.A.; Yorulmaz, S.; Zhdanov, V.P.; Kasemo, B.; Cho, N.J. Influence of Divalent Cations on Deformation and Rupture of Adsorbed Lipid Vesicles. Langmuir 2016, 32, 6486–6495. [Google Scholar] [CrossRef]
- Anderson, T.H.; Min, Y.; Weirich, K.L.; Zeng, H.; Fygenson, D.; Israelachvili, J.N. Formation of Supported Bilayers on Silica Substrates. Langmuir 2009, 25, 6997–7005. [Google Scholar] [CrossRef] [PubMed]
- Tero, R. Substrate Effects on the Formation Process, Structure and Physicochemical Properties of Supported Lipid Bilayers. Materials 2012, 5, 2658–2680. [Google Scholar] [CrossRef]
- Drücker, P.; Grill, D.; Gerke, V.; Galla, H.J. Formation and Characterization of Supported Lipid Bilayers Containing Phosphatidylinositol-4,5-Bisphosphate and Cholesterol as Functional Surfaces. Langmuir 2014, 30, 14877–14886. [Google Scholar] [CrossRef]
- Jackman, J.A.; Kim, M.C.; Zhdanov, V.P.; Cho, N.J. Relationship between Vesicle Size and Steric Hindrance Influences Vesicle Rupture on Solid Supports. Phys. Chem. Chem. Phys. 2016, 18, 3065–3072. [Google Scholar] [CrossRef]
- Lu, Y.; Allegri, G.; Huskens, J. Vesicle-Based Artificial Cells: Materials, Construction Methods and Applications. Mater. Horiz. 2022, 9, 892–907. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, C.F.; Calori, I.R.; Tessaro, A.L.; Caetano, W.; Hioka, N. Rapid Formation of Small Unilamellar Vesicles (SUV) through Low-Frequency Sonication: An Innovative Approach. Colloids Surf. B Biointerfaces 2019, 181, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.A.; Glasmästar, K.; Zhdanov, V.P.; Kasemo, B. Formation of Supported Membranes from Vesicles. Phys. Rev. Lett. 2000, 84, 5443–5446. [Google Scholar] [CrossRef]
- Cevc, G. Membrane Electrostatics. BBA—Rev. Biomembr. 1990, 1031, 311–382. [Google Scholar] [CrossRef]
- Wilschut, J.; Hoekstra, D. Membrane Fusion: Lipid Vesicles as a Model System. Chem. Phys. Lipids 1986, 40, 145–166. [Google Scholar] [CrossRef]
- Seantier, B.; Kasemo, B. Influence of Mono- And Divalent Ions on the Formation of Supported Phospholipid Bilayers via Vesicle Adsorption. Langmuir 2009, 25, 5767–5772. [Google Scholar] [CrossRef]
- Mornet, S.; Lambert, O.; Duguet, E.; Brisson, A. The Formation of Supported Lipid Bilayers on Silica Nanoparticles Revealed by Cryoelectron Microscopy. Nano Lett. 2005, 5, 281–285. [Google Scholar] [CrossRef]
- Richter, R.P.; Brisson, A.R. Following the Formation of Supported Lipid Bilayers on Mica: A Study Combining AFM, QCM-D, and Ellipsometry. Biophys. J. 2005, 88, 3422–3433. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.A.; Kasemo, B. Surface Specific Kinetics of Lipid Vesicle Adsorption Measured with a Quartz Crystal Microbalance. Biophys. J. 1998, 75, 1397–1402. [Google Scholar] [CrossRef]
- Zawisza, I.; Bin, X.; Lipkowski, J. Potential-Driven Structural Changes in Langmuir-Blodgett DMPC Bilayers Determined by in Situ Spectroelectrochemical PM IRRAS. Langmuir 2007, 23, 5180–5194. [Google Scholar] [CrossRef]
- Rossetti, F.F.; Bally, M.; Michel, R.; Textor, M.; Reviakine, I. Interactions between Titanium Dioxide and Phosphatidyl Serine-Containing Liposomes: Formation and Patterning of Supported Phospholipid Bilayers on the Surface of a Medically Relevant Material. Langmuir 2005, 21, 6443–6450. [Google Scholar] [CrossRef] [PubMed]
- Goksu, E.I.; Hoopes, M.I.; Nellis, B.A.; Xing, C.; Faller, R.; Frank, C.W.; Risbud, S.H.; Satcher, J.H.; Longo, M.L. Silica Xerogel/Aerogel-Supported Lipid Bilayers: Consequences of Surface Corrugation. Biochim. Biophys. Acta Biomembr. 2010, 1798, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, T.; Husen, P.; Brewer, J.; Bagatolli, L.A.; Hansen, P.L.; Ipsen, J.H.; Mouritsen, O.G. Preparing Giant Unilamellar Vesicles (GUVs) of Complex Lipid Mixtures on Demand: Mixing Small Unilamellar Vesicles of Compositionally Heterogeneous Mixtures. Biochim. Biophys. Acta Biomembr. 2015, 1848, 3175–3180. [Google Scholar] [CrossRef]
- Meher, G.; Chakraborty, H. Membrane Composition Modulates Fusion by Altering Membrane Properties and Fusion Peptide Structure. J. Membr. Biol. 2019, 252, 261–272. [Google Scholar] [CrossRef]
- Joardar, A.; Pattnaik, G.P.; Chakraborty, H. Mechanism of Membrane Fusion: Interplay of Lipid and Peptide. J. Membr. Biol. 2022, 255, 211–224. [Google Scholar] [CrossRef]
- Nobre, T.M.; Pavinatto, F.J.; Caseli, L.; Barros-Timmons, A.; Dynarowicz-Łatka, P.; Oliveira, O.N. Interactions of Bioactive Molecules & Nanomaterials with Langmuir Monolayers as Cell Membrane Models. Thin Solid Film. 2015, 593, 158–188. [Google Scholar] [CrossRef]
- Kurniawan, J.; Ventrici De Souza, J.F.; Dang, A.T.; Liu, G.Y.; Kuhl, T.L. Preparation and Characterization of Solid-Supported Lipid Bilayers Formed by Langmuir-Blodgett Deposition: A Tutorial. Langmuir 2018, 34, 15622–15639. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; John Wiley & Sons: New York, NY, USA, 1997; ISBN 978-0-471-14873-9. [Google Scholar]
- Dynarowicz-Latka, P.; Wnętrzak, A.; Chachaj-Brekiesz, A. Advantages of the Classical Thermodynamic Analysis of Single—And Multi-Component Langmuir Monolayers from Molecules of Biomedical Importance—Theory and Applications. J. R. Soc. Interface 2024, 21, 20230559. [Google Scholar] [CrossRef]
- Torp, K.D.; Metheny, E.; Simon, L.V. Lidocaine Toxicity; StatPearls: Treasure Island, FL, USA, 2022; pp. 1–4. [Google Scholar]
- Choi, S.Y.; Oh, S.G.; Lee, J.S. Effects of Lidocaine on the Expansion of Lipid Monolayer at Air/Water Interface in Relation to the Local Anesthesia. Colloids Surf. B Biointerfaces 2000, 17, 255–264. [Google Scholar] [CrossRef]
- Mildner, J.; Wnętrzak, A.; Dynarowicz-Latka, P. Cholesterol and Cardiolipin Importance in Local Anesthetics–Membrane Interactions: The Langmuir Monolayer Study. J. Membr. Biol. 2019, 252, 31–39. [Google Scholar] [CrossRef]
- Silvius, J.R. Role of Cholesterol in Lipid Raft Formation: Lessons from Lipid Model Systems. Biochim. Biophys. Acta (BBA)—Biomembr. 2003, 1610, 174–183. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid Rafts as a Membrane-Organizing Principle. Science (1979) 2010, 327, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Rakotomanga, M.; Loiseau, P.M.; Saint-Pierre-Chazalet, M. Hexadecylphosphocholine Interaction with Lipid Monolayers. Biochim. Biophys. Acta Biomembr. 2004, 1661, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, D.M. Recent Progress in the Study of the Interactions of Amphotericin B with Cholesterol and Ergosterol in Lipid Environments. Eur. Biophys. J. 2014, 43, 453–467. [Google Scholar] [CrossRef]
- Kasai, Y.; Matsumori, N.; Ueno, H.; Nonomura, K.; Yano, S.; Michio, M.; Oishi, T. Synthesis of 6-F-Ergosterol and Its Influence on Membrane-Permeabilization of Amphotericin B and Amphidinol 3. Org. Biomol. Chem. 2011, 9, 1437–1442. [Google Scholar] [CrossRef]
- Kamiński, D.M.; Czernel, G.; Murphy, B.; Runge, B.; Magnussen, O.M.; Gagoś, M. Effect of Cholesterol and Ergosterol on the Antibiotic Amphotericin B Interactions with Dipalmitoylphosphatidylcholine Monolayers: X-Ray Reflectivity Study. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2014, 1838, 2947–2953. [Google Scholar] [CrossRef]
- Pereira, A.R.; Shimizu, F.M.; Oliveira, O.N. Cholesterol Modulates the Interaction between Paclitaxel and Langmuir Monolayers Simulating Cell Membranes. Colloids Surf. B Biointerfaces 2021, 205, 111889. [Google Scholar] [CrossRef]
- Parolin, G.A.; Gonçalves, G.E.G.; Costa-Silva, T.A.; Tempone, A.G.; Caseli, L.; Lago, J.H.G.; Péres, L.O. Evaluation of the Effects in Cellular Membrane Models of Antitrypanosomal Poly-Thymolformaldehyde (PTF) Using Langmuir Monolayers. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183500. [Google Scholar] [CrossRef]
- Azouzi, S.; El Kirat, K.; Morandat, S. The Potent Antimalarial Drug Cyclosporin A Preferentially Destabilizes Sphingomyelin-Rich Membranes. Langmuir 2010, 26, 1960–1965. [Google Scholar] [CrossRef]
- Jurak, M.; Szafran, K.; Cea, P.; Martín, S. Analysis of Molecular Interactions between Components in Phospholipid-Immunosuppressant-Antioxidant Mixed Langmuir Films. Langmuir 2021, 37, 5601–5616. [Google Scholar] [CrossRef]
- Ferreira, J.V.N.; Capello, T.M.; Siqueira, L.J.A.; Lago, J.H.G.; Caseli, L. Mechanism of Action of Thymol on Cell Membranes Investigated through Lipid Langmuir Monolayers at the Air-Water Interface and Molecular Simulation. Langmuir 2016, 32, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, V.P.N.; Pavinatto, F.J.; Nobre, T.M.; Caseli, L.; Oliveira, O.N. Langmuir Films Containing Ibuprofen and Phospholipids. Chem. Phys. Lett. 2013, 559, 99–106. [Google Scholar] [CrossRef]
- Jabłonowska, E.; Bilewicz, R. Interactions of Ibuprofen with Langmuir Monolayers of Membrane Lipids. Thin Solid Film. 2007, 515, 3962–3966. [Google Scholar] [CrossRef]
- Brockman, H.; Graff, G.; Spellman, J.; Yanni, J. A Comparison of the Effects of Olopatadine and Ketotifen on Model Membranes. Acta Ophthalmol. Scand. 2000, 78, 10–15. [Google Scholar] [CrossRef]
- Rodrigues, D.; Camilo, F.F.; Caseli, L. Cellulase and Alcohol Dehydrogenase Immobilized in Langmuir and Langmuir-Blodgett Films and Their Molecular-Level Effects upon Contact with Cellulose and Ethanol. Langmuir 2014, 30, 1855–1863. [Google Scholar] [CrossRef]
- Girard-Egrot, A.P.; Blum, L.J. Langmuir-Blodgett Technique for Synthesis of Biomimetic Lipid Membranes. In Nanobiotechnology of Biomimetic Membranes; Springer: Boston, MA, USA, 2007; pp. 23–74. [Google Scholar] [CrossRef]
- Steinem, C.; Janshoff, A.; Ulrich, W.-P.; Sieber, M.; Galla, H.-J. Impedance Analysis of Supported Lipid Bilayer Membranes: A Scrutiny of Different Preparation Techniques. Biochim. Biophys. Acta (BBA)—Biomembr. 1996, 1279, 169–180. [Google Scholar] [CrossRef]
- Yang, J.; Kleijn, J.M. Order in Phospholipid Langmuir-Blodgett Layers and the Effect of the Electrical Potential of the Substrate. Biophys. J. 1999, 76, 323–332. [Google Scholar] [CrossRef][Green Version]
- Kriechbaumer, V.; Nabok, A.; Widdowson, R.; Smith, D.P.; Abell, B.M. Quantification of Ligand Binding to G-Protein Coupled Receptors on Cell Membranes by Ellipsometry. PLoS ONE 2012, 7, e46221. [Google Scholar] [CrossRef][Green Version]
- Clifton, L.A.; Skoda, M.W.A.; Daulton, E.L.; Hughes, A.V.; Le Brun, A.P.; Lakey, J.H.; Holt, S.A. Asymmetric Phospholipid: Lipopolysaccharide Bilayers; a Gram-Negative Bacterial Outer Membrane Mimic. J. R. Soc. Interface 2013, 10, 20130810. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.E.; Lopez, R.F.; Oliveira, O.N.; Caseli, L. Enzyme Activity of Catalase Immobilized in Langmuir−Blodgett Films of Phospholipids. Langmuir 2010, 26, 11135–11139. [Google Scholar] [CrossRef]
- Caseli, L.; Crespilho, F.N.; Nobre, T.M.; Zaniquelli, M.E.D.; Zucolotto, V.; Oliveira, O.N. Using Phospholipid Langmuir and Langmuir-Blodgett Films as Matrix for Urease Immobilization. J. Colloid. Interface Sci. 2008, 319, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Bikerman, J.J. On the Formation and Structure of Multilayers. Proc. R. Soc. Lond. A Math. Phys. Sci. 1939, 170, 130–144. [Google Scholar] [CrossRef]
- Nielsen, S.B.; Otzen, D.E. Quartz Crystal Microbalances as Tools for Probing Protein-Membrane Interactions. Methods Mol. Biol. 2019, 2003, 31–52. [Google Scholar] [CrossRef]
- Cho, N.-J.; Cho, S.-J.; Cheong, K.H.; Glenn, J.S.; Frank, C.W. Employing an Amphipathic Viral Peptide to Create a Lipid Bilayer on Au and TiO 2. J. Am. Chem. Soc. 2007, 129, 10050–10051. [Google Scholar] [CrossRef] [PubMed]
- Fezoua-Boubegtiten, Z.; Desbat, B.; Brisson, A.; Gounou, C.; Laguerre, M.; Lecomte, S. Effect of Mg2+ versus Ca2+ on the Behavior of Annexin A5 in a Membrane-Bound State. Eur. Biophys. J. 2011, 40, 641–649. [Google Scholar] [CrossRef]
- Dutta, D.; Kam, L.C. Micropatterned, Multicomponent Supported Lipid Bilayers for Cellular Systems. Methods Cell Biol. 2014, 120, 53–67. [Google Scholar] [CrossRef]
- Leonard-Latour, M.; Morelis, R.M.; Coulet, P.R. Interaction of DMPC Liposomes with a DMPA Monolayer: Related Study of Langmuir-Blodgett Films. Supramol. Sci. 1997, 4, 357–363. [Google Scholar] [CrossRef]
- Wnętrzak, A.; Makyła-Juzak, K.; Filiczkowska, A.; Kulig, W.; Dynarowicz-Łątka, P. Oxysterols Versus Cholesterol in Model Neuronal Membrane. I. The Case of 7-Ketocholesterol. The Langmuir Monolayer Study. J. Membr. Biol. 2017, 250, 553–564. [Google Scholar] [CrossRef]
- Howland, M.C.; Szmodis, A.W.; Sanii, B.; Parikh, A.N. Characterization of Physical Properties of Supported Phospholipid Membranes Using Imaging Ellipsometry at Optical Wavelengths. Biophys. J. 2007, 92, 1306–1317. [Google Scholar] [CrossRef]
- Mizogami, M.; Tsuchiya, H. Lipid Composition-, Medium PH-, and Drug-Concentration-Dependent Membrane Interactions of Ibuprofen, Diclofenac, and Celecoxib: Hypothetical Association with Their Analgesic and Gastrointestinal Toxic Effects. Future Pharmacol. 2024, 4, 437–448. [Google Scholar] [CrossRef]
- Moulick, R.G.; Afanasenkau, D.; Choi, S.-E.; Albers, J.; Lange, W.; Maybeck, V.; Utesch, T.; Offenhäusser, A. Reconstitution of Fusion Proteins in Supported Lipid Bilayers for the Study of Cell Surface Receptor–Ligand Interactions in Cell–Cell Contact. Langmuir 2016, 32, 3462–3469. [Google Scholar] [CrossRef] [PubMed]
- Franquelim, H.G.; Chiantia, S.; Veiga, A.S.; Santos, N.C.; Schwille, P.; Castanho, M.A. Anti-HIV-1 Antibodies 2F5 and 4E10 Interact Differently with Lipids to Bind Their Epitopes. AIDS 2011, 25, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gkeka, P.; Fuchs, J.E.; Liedl, K.R.; Cournia, Z. DPPC-Cholesterol Phase Diagram Using Coarse-Grained Molecular Dynamics Simulations. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2846–2857. [Google Scholar] [CrossRef]
- Miller, C.E.; Majewski, J.; Faller, R.; Satija, S.; Kuhl, T.L. Cholera Toxin Assault on Lipid Monolayers Containing Ganglioside GM1. Biophys. J. 2004, 86, 3700–3708. [Google Scholar] [CrossRef]
- Piosik, E.; Modlińska, A.; Gołaszewski, M.; Chełminiak-Dudkiewicz, D.; Ziegler-Borowska, M. Influence of the Type of Biocompatible Polymer in the Shell of Magnetite Nanoparticles on Their Interaction with DPPC in Two-Component Langmuir Monolayers. J. Phys. Chem. B 2024, 128, 781–794. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Interfacial Properties of Mixed DPPC–Hydrophobic Fumed Silica Nanoparticle Layers. J. Phys. Chem. C 2015, 119, 21024–21034. [Google Scholar] [CrossRef]
- Wnętrzak, A.; Chachaj–Brekiesz, A.; Stępniak, A.; Kobierski, J.; Dynarowicz–Latka, P. Different Effects of Oxysterols on a Model Lipid Raft—Langmuir Monolayer Study Complemented with Theoretical Calculations. Chem. Phys. Lipids 2022, 244, 105182. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Karmakar, P.; Karmakar, S. Supported Planar Single and Multiple Bilayer Formation by DOPC Vesicle Rupture on Mica Substrate: A Mechanism as Revealed by Atomic Force Microscopy Study. J. Membr. Biol. 2020, 253, 205–219. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Zhou, Q.A. PEGylated Lipid Nanoparticle Formulations: Immunological Safety and Efficiency Perspective. Bioconjug. Chem. 2023, 34, 941–960. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to MRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Fernandes, E.G.R.; Alessio, P.; Constantino, C.J.L.; De Saja, J.A.; Zucolotto, V.; Apetrei, C.; Oliveira, O.N.; Rodriguez-Mendez, M.L. Optimized Architecture for Tyrosinase-Containing Langmuir–Blodgett Films to Detect Pyrogallol. J. Mater. Chem. 2011, 21, 4995–5003. [Google Scholar] [CrossRef]
- Pereira, M.S.; Maximino, M.D.; Martin, C.S.; Aoki, P.H.B.; Oliveira, O.N.; Alessio, P. Lipid-Matrix Effects on Tyrosinase Immobilization in Langmuir and Langmuir-Blodgett Films. An. Acad. Bras. Cienc. 2021, 93, e20200019. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Huang, W.-C.; Lin, C.; Hicar, M.D.; LaBranche, C.C.; Montefiori, D.C.; Lovell, J.F. An Engineered Biomimetic MPER Peptide Vaccine Induces Weakly HIV Neutralizing Antibodies in Mice. Ann. Biomed. Eng. 2020, 48, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Jabłonowska, E.; Nazaruk, E.; Matyszewska, D.; Speziale, C.; Mezzenga, R.; Landau, E.M.; Bilewicz, R. Interactions of Lipidic Cubic Phase Nanoparticles with Lipid Membranes. Langmuir 2016, 32, 9640–9648. [Google Scholar] [CrossRef]
- Huang, C.J.; Cho, N.J.; Hsu, C.J.; Tseng, P.Y.; Frank, C.W.; Chang, Y.C. Type i Collagen-Functionalized Supported Lipid Bilayer as a Cell Culture Platform. Biomacromolecules 2010, 11, 1231–1240. [Google Scholar] [CrossRef]
- Kure, T.; Sakai, H. Preparation of Artificial Red Blood Cells (Hemoglobin Vesicles) Using the Rotation–Revolution Mixer for High Encapsulation Efficiency. ACS Biomater. Sci. Eng. 2021, 7, 2835–2844. [Google Scholar] [CrossRef]
- Schmidt, T.F.; Caseli, L.; Viitala, T.; Oliveira, O.N. Enhanced Activity of Horseradish Peroxidase in Langmuir–Blodgett Films of Phospholipids. Biochim. Biophys. Acta (BBA)—Biomembr. 2008, 1778, 2291–2297. [Google Scholar] [CrossRef]
- Schmidt, T.F.; Caseli, L.; dos Santos, D.S.; Oliveira, O.N. Enzyme Activity of Horseradish Peroxidase Immobilized in Chitosan Matrices in Alternated Layers. Mater. Sci. Eng. C 2009, 29, 1889–1892. [Google Scholar] [CrossRef]
- Schmidt, T.F.; Caseli, L.; Nobre, T.M.; Zaniquelli, M.E.D.; Oliveira, O.N. Interaction of Horseradish Peroxidase with Langmuir Monolayers of Phospholipids. Colloids Surf. A Physicochem. Eng. Asp. 2008, 321, 206–210. [Google Scholar] [CrossRef]
- Caseli, L.; Masui, D.C.; Furriel, R.P.M.; Leone, F.A.; Zaniquelli, M.E.D. Incorporation Conditions Guiding the Aggregation of a Glycosylphosphatidyl Inositol (GPI)-Anchored Protein in Langmuir Monolayers. Colloids Surf. B Biointerfaces 2005, 46, 248–254. [Google Scholar] [CrossRef]
- da Rocha Junior, C.; Caseli, L. Adsorption and Enzyme Activity of Asparaginase at Lipid Langmuir and Langmuir-Blodgett Films. Mater. Sci. Eng. C 2017, 73, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Caseli, L.; Cavalheiro, R.P.; Nader, H.B.; Lopes, C.C. Probing the Interaction between Heparan Sulfate Proteoglycan with Biologically Relevant Molecules in Mimetic Models for Cell Membranes: A Langmuir Film Study. Biochim. Biophys. Acta (BBA)—Biomembr. 2012, 1818, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.M.; Ivanova, K.; Francesko, A.; Rivera, D.; Torrent-Burgués, J.; Gedanken, A.; Mendonza, E.; Tzanov, T. Escherichia Coli and Pseudomonas Aeruginosa Eradication by Nano-Penicillin G. Nanomedicine 2016, 12, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Wydro, P. The Influence of Cardiolipin on Phosphatidylglycerol/Phosphatidylethanolamine Monolayers--Studies on Ternary Films Imitating Bacterial Membranes. Colloids Surf. B Biointerfaces 2013, 106, 217–223. [Google Scholar] [CrossRef]
- Pouzot, M. Food Protein and Charged Emulsifier Interaction 2008. U.S. Patent Application US 12/439,624, 25 February 2010. [Google Scholar]
- Peetla, C.; Rao, K.S.; Labhasetwar, V. Relevance of Biophysical Interactions of Nanoparticles with a Model Membrane in Predicting Cellular Uptake: Study with TAT Peptide-Conjugated Nanoparticles. Mol. Pharm. 2009, 6, 1311. [Google Scholar] [CrossRef]
- Krajewska, M.; Dopierała, K.; Prochaska, K. Lipid–Protein Interactions in Langmuir Monolayers under Dynamically Varied Conditions. J. Phys. Chem. B 2020, 124, 302–311. [Google Scholar] [CrossRef]
- Pivetta, T.P.; Jochelavicius, K.; Wrobel, E.C.; Balogh, D.T.; Oliveira, O.N.; Ribeiro, P.A.; Raposo, M. Incorporation of Acridine Orange and Methylene Blue in Langmuir Monolayers Mimicking Releasing Nanostructures. Biochim. Biophys. Acta (BBA)—Biomembr. 2023, 1865, 184156. [Google Scholar] [CrossRef]
- Fernández, L.; Reviglio, A.L.; Heredia, D.A.; Morales, G.M.; Santo, M.; Otero, L.; Alustiza, F.; Liaudat, A.C.; Bosch, P.; Larghi, E.L.; et al. Langmuir-Blodgett Monolayers Holding a Wound Healing Active Compound and Its Effect in Cell Culture. A Model for the Study of Surface Mediated Drug Delivery Systems. Heliyon 2021, 7, e06436. [Google Scholar] [CrossRef]
- Barenholzt, Y.; Amselem, S.; Lichtenberg, D. A New Method for Preparation of Phospholipid Vesicles (Liposomes)—French Press. FEBS Lett. 1979, 99, 210–214. [Google Scholar] [CrossRef]
- Waku, T.; Kasai, A.; Kobori, A.; Tanaka, N. Investigation on the Interactions between Self-Assembled β-Sheet Peptide Nanofibers and Model Cell Membranes. Int. J. Mol. Sci. 2020, 21, 9518. [Google Scholar] [CrossRef]
- Bensikaddour, H.; Fa, N.; Burton, I.; Deleu, M.; Lins, L.; Schanck, A.; Brasseur, R.; Dufrêne, Y.F.; Goormaghtigh, E.; Mingeot-Leclercq, M.P. Characterization of the Interactions between Fluoroquinolone Antibiotics and Lipids: A Multitechnique Approach. Biophys. J. 2008, 94, 3035. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Has, C.; Pan, S. Vesicle Formation Mechanisms: An Overview. J. Liposome Res. 2021, 31, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Van Heyningen, S. The Interaction of Cholera Toxin with Gangliosides and the Cell Membrane. Curr. Top. Membr. Transp. 1983, 18, 445–471. [Google Scholar] [CrossRef]
- Prinz, W.A. Lipid Trafficking sans Vesicles: Where, Why, How? Cell 2010, 143, 870. [Google Scholar] [CrossRef]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; et al. LMSD: LIPID MAPS Structure Database. Nucleic Acids Res. 2007, 35, D527. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Patra, S.K. Lipid Raft Facilitated Receptor Organization and Signaling: A Functional Rheostat in Embryonic Development, Stem Cell Biology and Cancer. Stem Cell Rev. Rep. 2023, 19, 2–25. [Google Scholar] [CrossRef]
- Codini, M.; Garcia-Gil, M.; Albi, E. Cholesterol and Sphingolipid Enriched Lipid Rafts as Therapeutic Targets in Cancer. Int. J. Mol. Sci. 2021, 22, 726. [Google Scholar] [CrossRef]
- Westphal, P.; Bornmann, A. Biomolecular Detection by Surface Plasmon Enhanced Ellipsometry. Sens. Actuators B Chem. 2002, 84, 278–282. [Google Scholar] [CrossRef]
- Schöneberg, T.; Schulz, A.; Biebermann, H.; Hermsdorf, T.; Römpler, H.; Sangkuhl, K. Mutant G-Protein-Coupled Receptors as a Cause of Human Diseases. Pharmacol. Ther. 2004, 104, 173–206. [Google Scholar] [CrossRef]
- Girard-Egrot, A.P.; Godoy, S.; Blum, L.J. Enzyme Association with Lipidic Langmuir-Blodgett Films: Interests and Applications in Nanobioscience. Adv. Colloid. Interface Sci. 2005, 116, 205–225. [Google Scholar] [CrossRef]
- Campioni, S.; Carret, G.; Jordens, S.; Nicoud, L.; Mezzenga, R.; Riek, R. The Presence of an Air–Water Interface Affects Formation and Elongation of α-Synuclein Fibrils. J. Am. Chem. Soc. 2014, 136, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Zanon, N.C.M.; Oliveira, O.N.; Caseli, L. Immbolization of Uricase Enzyme in Langmuir and Langmuir-Blodgett Films of Fatty Acids: Possible Use as a Uric Acid Sensor. J. Colloid. Interface Sci. 2012, 373, 69–74. [Google Scholar] [CrossRef]
- Bussières, S.; Cantin, L.; Desbat, B.; Salesse, C. Binding of a Truncated Form of Lecithin:Retinol Acyltransferase and Its N- and C-Terminal Peptides to Lipid Monolayers. Langmuir 2012, 28, 3516–3523. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, J.-L.; Pitard, B.; Levy, D. Reconstitution of Membrane Proteins into Liposomes: Application to Energy-Transducing Membrane Proteins. Biochim. Biophys. Acta—Bioenerg. 1995, 1231, 223–246. [Google Scholar] [CrossRef]
- Seddon, A.M.; Curnow, P.; Booth, P.J. Membrane Proteins, Lipids and Detergents: Not Just a Soap Opera. Biochim. Biophys. Acta 2004, 1666, 105–117. [Google Scholar] [CrossRef]
- Grassi, S.; Chiricozzi, E.; Mauri, L.; Sonnino, S.; Prinetti, A. Sphingolipids and Neuronal Degeneration in Lysosomal Storage Disorders. J. Neurochem. 2019, 148, 600–611. [Google Scholar] [CrossRef]
- Sántha, P.; Dobos, I.; Kis, G.; Jancsó, G. Role of Gangliosides in Peripheral Pain Mechanisms. Int. J. Mol. Sci. 2020, 21, 1005. [Google Scholar] [CrossRef]
- Dopico, A.M.; Tigyi, G.J. A Glance at the Structural and Functional Diversity of Membrane Lipids. Methods Mol. Biol. 2007, 400, 1–13. [Google Scholar] [CrossRef]
- Sackmann, E. Biological Membranes Architecture and Function. Handb. Biol. Phys. 1995, 1, 1–63. [Google Scholar] [CrossRef]
- Javadi, A.; Dowlati, S.; Shourni, S.; Miller, R.; Kraume, M.; Kopka, K.; Eckert, K. Experimental Techniques to Study Protein–Surfactant Interactions: New Insights into Competitive Adsorptions via Drop Subphase and Interface Exchange. Adv. Colloid. Interface Sci. 2022, 301, 102601. [Google Scholar] [CrossRef]
- Amrein, M.; von Nahmen, A.; Sieber, M. A Scanning Force- and Fluorescence Light Microscopy Study of the Structure and Function of a Model Pulmonary Surfactant. Eur. Biophys. J. 1997, 26, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Tempra, C.; Ollila, O.H.S.; Javanainen, M. Accurate Simulations of Lipid Monolayers Require a Water Model with Correct Surface Tension. J. Chem. Theory Comput. 2022, 18, 1862–1869. [Google Scholar] [CrossRef] [PubMed]
- van Oss, C.J.; Giese, R.F.; Docoslis, A. Hyperhydrophobicity of the Water-Air Interface. J. Dispers. Sci. Technol. 2005, 26, 585–590. [Google Scholar] [CrossRef]
- Kiessling, V.; Crane, J.M.; Tamm, L.K. Transbilayer Effects of Raft-Like Lipid Domains in Asymmetric Planar Bilayers Measured by Single Molecule Tracking. Biophys. J. 2006, 91, 3313–3326. [Google Scholar] [CrossRef]
- Adamala, K.P.; Dogterom, M.; Elani, Y.; Schwille, P.; Takinoue, M.; Tang, T.-Y.D. Present and Future of Synthetic Cell Development. Nat. Rev. Mol. Cell Biol. 2024, 25, 162–167. [Google Scholar] [CrossRef]
- Lee, Y.; Fracassi, A.; Devaraj, N.K. Light-Driven Membrane Assembly, Shape-Shifting, and Tissue Formation in Chemically Responsive Synthetic Cells. J. Am. Chem. Soc. 2023, 145, 25815–25823. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.L.; Nishi, K.; Klawa, S.J.; Hinton, K.Y.; Gao, Y.; Freeman, R. Designer Peptide–DNA Cytoskeletons Regulate the Function of Synthetic Cells. Nat. Chem. 2024, 16, 1229–1239. [Google Scholar] [CrossRef]
- Davis, F.F. The Origin of Pegnology. Adv. Drug Deliv. Rev. 2002, 54, 457–458. [Google Scholar] [CrossRef]
- Blume, G.; Cevc, G. Liposomes for the Sustained Drug Release in Vivo. Biochim. Biophys. Acta 1990, 1029, 91–97. [Google Scholar] [CrossRef]
- Gaines, G.L. Insoluble Monolayers at Liquid-Gas Interface; John Wiley & Sons Interscience: New York, NY, USA, 1966. [Google Scholar]
- Vogel, V.; Möbius, D. Local Surface Potentials and Electric Dipole Moments of Lipid Monolayers: Contributions of the Water/Lipid and the Lipid/Air Interfaces. J. Colloid. Interface Sci. 1988, 126, 408–420. [Google Scholar] [CrossRef]
- Taylor, D.M.; Oliveira, O.N.; Morgan, H. The Effect of Water Quality on the Electrical Characteristics of Langmuir Monolayers. Thin Solid Film. 1989, 173, L141–L147. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Luchowski, R.; Gagoś, M.; Arczewska, M.; Sarkar, P.; Hereć, M.; Myśliwa-Kurdziel, B.; Strzałka, K.; Gryczynski, I.; Gryczynski, Z. Molecular Organization of Antifungal Antibiotic Amphotericin B in Lipid Monolayers Studied by Means of Fluorescence Lifetime Imaging Microscopy. Biophys. Chem. 2009, 143, 95–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moore, B.; Knobler, C.M.; Broseta, D.; Rondelez, F. Studies of Phase Transitions in Langmuir Monolayers by Fluorescence Microscopy. J. Chem. Soc. Faraday Trans. 1986, 82, 1753. [Google Scholar] [CrossRef]
- Möbius, D. Morphology and Structural Characterization of Organized Monolayers by Brewster Angle Microscopy. Curr. Opin. Colloid. Interface Sci. 1998, 3, 137–142. [Google Scholar] [CrossRef]
- Kaganer, V.M.; Möhwald, H.; Dutta, P. Structure and Phase Transitions in Langmuir Monolayers. Rev. Mod. Phys. 1999, 71, 779–819. [Google Scholar] [CrossRef]
- Kjaer, K.; Als-Neilsen, J.; Heln, C.A.; Tippmann-Krayer, P.; Möhwald, H. An X-Ray Scattering Study of Lipid Monolayers at the Air-Water Interface and on Solid Supports. Thin Solid Film. 1988, 159, 17–28. [Google Scholar] [CrossRef]
- Mobius, D.; Miller, R.; Jensen, T.R.; Kjaer, K. Novel Methods to Study Interfacial Layers; Elsevier Science: Amsterdam, The Netherlands, 2001; Volume 11. [Google Scholar]
- Kjaer, K.; Als-Nielsen, J.; Helm, C.A.; Laxhuber, L.A.; Möhwald, H. Ordering in Lipid Monolayers Studied by Synchrotron X-Ray Diffraction and Fluorescence Microscopy. Phys. Rev. Lett. 1987, 58, 2224–2227. [Google Scholar] [CrossRef]
- Mann, J.A. Dynamics, Structure, and Function of Interfacial Regions. Langmuir 1985, 1, 10–23. [Google Scholar] [CrossRef]
- Als-Nielsen, J.; Jacquemain, D.; Kjaer, K.; Leveiller, F.; Lahav, M.; Leiserowitz, L. Principles and Applications of Grazing Incidence X-Ray and Neutron Scattering from Ordered Molecular Monolayers at the Air-Water Interface. Phys. Rep. 1994, 246, 251–313. [Google Scholar] [CrossRef]
- Krueger, S. Neutron Reflection from Interfaces with Biological and Biomimetic Materials. Curr. Opin. Colloid. Interface Sci. 2001, 6, 111–117. [Google Scholar] [CrossRef]
- Zaborowska, M.; Dziubak, D.; Fontaine, P.; Matyszewska, D. Influence of Lipophilicity of Anthracyclines on the Interactions with Cholesterol in the Model Cell Membranes—Langmuir Monolayer and SEIRAS Studies. Colloids Surf. B Biointerfaces 2022, 211, 112297. [Google Scholar] [CrossRef]
- Ducharme, D.; Max, J.J.; Salesse, C.; Leblanc, R.M. Ellipsometric Study of the Physical States of Phosphatidylcholines at the Air-Water Interface. J. Phys. Chem. 1990, 94, 1925–1932. [Google Scholar] [CrossRef]
- Pérez-Morales, M.; Pedrosa, J.M.; Muñoz, E.; Martín-Romero, M.T.; Möbius, D.; Camacho, L. Ellipsometric Study of a Phospholipid Monolayer at the Air–Water Interface in Presence of Large Organic Counter Ions. Thin Solid Film. 2005, 488, 247–253. [Google Scholar] [CrossRef]
- Dluhy, R.A.; Stephens, S.M.; Widayati, S.; Williams, A.D. Vibrational Spectroscopy of Biophysical Monolayers. Applications of IR and Raman Spectroscopy to Biomembrane Model Systems at Interfaces. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1995, 51, 1413–1447. [Google Scholar] [CrossRef]
- Dluhy, R.A.; Reilly, K.E.; Hunt, R.D.; Mitchell, M.L.; Mautone, A.J.; Mendelsohn, R. Infrared Spectroscopic Investigations of Pulmonary Surfactant. Surface Film Transitions at the Air-Water Interface and Bulk Phase Thermotropism. Biophys. J. 1989, 56, 1173–1181. [Google Scholar] [CrossRef]
- Blaudez, D.; Turlet, J.-M.; Dufourcq, J.; Bard, D.; Buffeteau, T.; Desbat, B. Investigations at the Air/Water Interface Using Polarization Modulation IR Spectroscopy. J. Chem. Soc. Faraday Trans. 1996, 92, 525. [Google Scholar] [CrossRef]
- Cea, P.; Martín, S.; Villares, A.; Möbius, D.; López, M.C. Use of UV−vis Reflection Spectroscopy for Determining the Organization of Viologen and Viologen Tetracyanoquinodimethanide Monolayers. J. Phys. Chem. B 2006, 110, 963–970. [Google Scholar] [CrossRef]
- Thakur, G.; Wang, C.; Leblanc, R.M. Surface Chemistry and in Situ Spectroscopy of a Lysozyme Langmuir Monolayer. Langmuir 2008, 24, 4888–4893. [Google Scholar] [CrossRef]
- Thakur, G.; Micic, M.; Leblanc, R.M. Surface Chemistry of Alzheimer’s Disease: A Langmuir Monolayer Approach. Colloids Surf. B Biointerfaces 2009, 74, 436–456. [Google Scholar] [CrossRef]
- Kanazawa, K.; Cho, N.J. Quartz Crystal Microbalance as a Sensor to Characterize Macromolecular Assembly Dynamics. J. Sens. 2009, 2009, 824947. [Google Scholar] [CrossRef]
- Tatarko, M.; Spagnolo, S.; Csiba, M.; Šubjaková, V.; Hianik, T. Analysis of the Interaction between DNA Aptamers and Cytochrome C on the Surface of Lipid Films and on the MUA Monolayer: A QCM-D Study†. Biosensors 2023, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Parkkila, P.; Elderdfi, M.; Bunker, A.; Viitala, T. Biophysical Characterization of Supported Lipid Bilayers Using Parallel Dual-Wavelength Surface Plasmon Resonance and Quartz Crystal Microbalance Measurements. Langmuir 2018, 34, 8081–8091. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, F.; Ng, T.; Vanderah, D.J.; Shekhar, P.; Mihailescu, M.; Nanda, H.; Lösche, M. A New Lipid Anchor for Sparsely Tethered Bilayer Lipid Membranes. Langmuir 2009, 25, 4219–4229. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.W.; Jeon, W.-Y.; Yoon, B.K.; Jackman, J.A. Mechanistic Evaluation of Antimicrobial Lipid Interactions with Tethered Lipid Bilayers by Electrochemical Impedance Spectroscopy. Sensors 2022, 22, 3712. [Google Scholar] [CrossRef]
- Kučerka, N.; Nieh, M.P.; Pencer, J.; Harroun, T.; Katsaras, J. The Study of Liposomes, Lamellae and Membranes Using Neutrons and X-Rays. Curr. Opin. Colloid. Interface Sci. 2007, 12, 17–22. [Google Scholar] [CrossRef]
- Heinrich, F.; Lösche, M. Zooming in on Disordered Systems: Neutron Reflection Studies of Proteins Associated with Fluid Membranes. Biochim. Biophys. Acta (BBA)—Biomembr. 2014, 1838, 2341–2349. [Google Scholar] [CrossRef]
- Caselli, L.; Nylander, T.; Malmsten, M. Neutron Reflectometry as a Powerful Tool to Elucidate Membrane Interactions of Drug Delivery Systems. Adv. Colloid. Interface Sci. 2024, 325, 103120. [Google Scholar] [CrossRef]
- Stefaniu, C.; Brezesinski, G. X-Ray Investigation of Monolayers Formed at the Soft Air/Water Interface. Curr. Opin. Colloid. Interface Sci. 2014, 19, 216–227. [Google Scholar] [CrossRef]
- Tikhonov, A.M.; Asadchikov, V.E.; Volkov, Y.O.; Roshchin, B.S.; Nuzhdin, A.D.; Makrinsky, K.I.; Ermakov, Y.A. X-Ray Reflectivity Study of Polylysine Adsorption on the Surface of DMPS Monolayers. Membranes 2022, 12, 1223. [Google Scholar] [CrossRef]
- Bykov, A.G.; Panaeva, M.A.; Milyaeva, O.Y.; Michailov, A.V.; Rafikova, A.R.; Guzman, E.; Rubio, R.; Miller, R.; Noskov, B.A. Structural Changes in Layers of Lipid Mixtures at Low Surface Tensions. Chem. Phys. Lipids 2024, 258, 105365. [Google Scholar] [CrossRef]
- Brezesinski, G.; Schneck, E. Investigating Ions at Amphiphilic Monolayers with X-Ray Fluorescence. Langmuir 2019, 35, 8531–8542. [Google Scholar] [CrossRef] [PubMed]
- Tawa, K.; Morigaki, K. Substrate-Supported Phospholipid Membranes Studied by Surface Plasmon Resonance and Surface Plasmon Fluorescence Spectroscopy. Biophys. J. 2005, 89, 2750–2758. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.; Hadinoto, K.; Park, J.W. Change in Gastric-Lipase Adsorption on Lipid Layer by Stigmasterols. Tenside Surfactants Deterg. 2023, 60, 409–413. [Google Scholar] [CrossRef]
- Garcia-Ortiz, C.E.; Cortes, R.; Garcia-Gonzalez, A.; Tellez-Limon, R.; Rodriguez-Cobos, A.; Coello, V. Real-Time Topography Inspection of DPPC Monolayers Using a Surface-Plasmon Resonance Sensor. AIP Adv. 2023, 13, 105023. [Google Scholar] [CrossRef]
- Jackman, J.A.; Špačková, B.; Linardy, E.; Kim, M.C.; Yoon, B.K.; Homola, J.; Cho, N.-J. Nanoplasmonic Ruler to Measure Lipid Vesicle Deformation. Chem. Commun. 2016, 52, 76–79. [Google Scholar] [CrossRef]
- Jackman, J.A.; Yorulmaz Avsar, S.; Ferhan, A.R.; Li, D.; Park, J.H.; Zhdanov, V.P.; Cho, N.-J. Quantitative Profiling of Nanoscale Liposome Deformation by a Localized Surface Plasmon Resonance Sensor. Anal. Chem. 2017, 89, 1102–1109. [Google Scholar] [CrossRef]
- Ferhan, A.R.; Špačková, B.; Jackman, J.A.; Ma, G.J.; Sut, T.N.; Homola, J.; Cho, N.-J. Nanoplasmonic Ruler for Measuring Separation Distance between Supported Lipid Bilayers and Oxide Surfaces. Anal. Chem. 2018, 90, 12503–12511. [Google Scholar] [CrossRef]
- Zan, G.H.; Jackman, J.A.; Kim, S.; Cho, N. Controlling Lipid Membrane Architecture for Tunable Nanoplasmonic Biosensing. Small 2014, 10, 4828–4832. [Google Scholar] [CrossRef]
- Arima, Y.; Teramura, Y.; Takiguchi, H.; Kawano, K.; Kotera, H.; Iwata, H. Surface Plasmon Resonance and Surface Plasmon Field-Enhanced Fluorescence Spectroscopy for Sensitive Detection of Tumor Markers. Biosens. Biodetect. 2009, 503, 3–20. [Google Scholar]
- Shen, Y.R. Surface Properties Probed by Second-Harmonic and Sum-Frequency Generation. Nature 1989, 337, 519–525. [Google Scholar] [CrossRef]
- Ye, S.; Nguyen, K.T.; Le Clair, S.V.; Chen, Z. In Situ Molecular Level Studies on Membrane Related Peptides and Proteins in Real Time Using Sum Frequency Generation Vibrational Spectroscopy. J. Struct. Biol. 2009, 168, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Jasensky, J.; Li, Y.; Chen, Z. Engineering and Characterization of Peptides and Proteins at Surfaces and Interfaces: A Case Study in Surface-Sensitive Vibrational Spectroscopy. Acc. Chem. Res. 2016, 49, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.F.M.; vandenAkker, C.C.; Schleeger, M.; Velikov, K.P.; Koenderink, G.H.; Bonn, M. The Polyphenol EGCG Inhibits Amyloid Formation Less Efficiently at Phospholipid Interfaces than in Bulk Solution. J. Am. Chem. Soc. 2012, 134, 14781–14788. [Google Scholar] [CrossRef]
- de Souza, M.L.; Machado, A.C.; Barbosa, H.; Lago, J.H.G.; Caseli, L. Interaction of Sakuranetin with Unsaturated Lipids Forming Langmuir Monolayers at the Air-Water Interface: A Biomembrane Model. Colloids Surf. B Biointerfaces 2024, 234, 113747. [Google Scholar] [CrossRef]
- Crane, J.M.; Tamm, L.K. Fluorescence Microscopy to Study Domains in Supported Lipid Bilayers. Methods Membr. Lipids 2007, 400, 481–488. [Google Scholar]
- Chattopadhyay, M.; Krok, E.; Orlikowska, H.; Schwille, P.; Franquelim, H.G.; Piatkowski, L. Hydration Layer of Only a Few Molecules Controls Lipid Mobility in Biomimetic Membranes. J. Am. Chem. Soc. 2021, 143, 14551–14562. [Google Scholar] [CrossRef]
- Cho, N.-J.; Frank, C.W. Fabrication of a Planar Zwitterionic Lipid Bilayer on Titanium Oxide. Langmuir 2010, 26, 15706–15710. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Lai, A.C.-K.; Liao, K.; Corridon, P.R.; Graves, D.J.; Chan, V. Recent Advances in Fluorescence Recovery after Photobleaching for Decoupling Transport and Kinetics of Biomacromolecules in Cellular Physiology. Polymers 2022, 14, 1913. [Google Scholar] [CrossRef]
- Lind, T.K.; Cárdenas, M. Understanding the Formation of Supported Lipid Bilayers via Vesicle Fusion—A Case That Exemplifies the Need for the Complementary Method Approach (Review). Biointerphases 2016, 11, 020801. [Google Scholar] [CrossRef]
- Shin, G.; Hadinoto, K.; Lee, S.; Park, J.W. Binding Behavior between Transforming-Growth-Factor-Beta1 and Its Receptor Reconstituted in Biomimetic Membranes. Membranes 2023, 13, 446. [Google Scholar] [CrossRef]
- Ruiz-Rincón, S.; González-Orive, A.; Grazú, V.; Fratila, R.M.; de la Fuente, J.M.; Cea, P. Altering Model Cell Membranes by Means of Localized Magnetic Heating. Colloids Surf. B Biointerfaces 2020, 196, 111315. [Google Scholar] [CrossRef]
- Chiodini, S.; Ruiz-Rincón, S.; Garcia, P.D.; Martin, S.; Kettelhoit, K.; Armenia, I.; Werz, D.B.; Cea, P. Bottom Effect in Atomic Force Microscopy Nanomechanics. Small 2020, 16, e202000269. [Google Scholar] [CrossRef]
- Weroński, K.J.; Cea, P.; Diez-Peréz, I.; Busquets, M.A.; Prat, J.; Girona, V. Time-Lapse Atomic Force Microscopy Observations of the Morphology, Growth Rate, and Spontaneous Alignment of Nanofibers Containing a Peptide-Amphiphile from the Hepatitis G Virus (NS3 Protein). J. Phys. Chem. B 2010, 114, 620–625. [Google Scholar] [CrossRef]
- Tabaei, S.R.; Jackman, J.A.; Liedberg, B.; Parikh, A.N.; Cho, N.-J. Observation of Stripe Superstructure in the β-Two-Phase Coexistence Region of Cholesterol–Phospholipid Mixtures in Supported Membranes. J. Am. Chem. Soc. 2014, 136, 16962–16965. [Google Scholar] [CrossRef]
- Alsaker, N.E.; Halskau, Ø.; Haug, B.E.; Reuter, N.; Nerdal, W. Phospholipid Membrane Interactions of Model Ac-WL-X-LL-OH Peptides Investigated by Solid-State Nuclear Magnetic Resonance. Membranes 2024, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.W.; Andersen, O.S.; Roux, B. Structure of Gramicidin A in a Lipid Bilayer Environment Determined Using Molecular Dynamics Simulations and Solid-State NMR Data. J. Am. Chem. Soc. 2003, 125, 9868–9877. [Google Scholar] [CrossRef]
- Tanaka, M.; Sackmann, E. Supported Membranes as Biofunctional Interfaces and Smart Biosensor Platforms. Phys. Status Solidi (A) Appl. Mater. Sci. 2006, 203, 3452–3462. [Google Scholar] [CrossRef]
- Escribá, P.V.; Busquets, X.; Inokuchi, J.I.; Balogh, G.; Török, Z.; Horváth, I.; Harwood, J.L.; Vígh, L. Membrane Lipid Therapy: Modulation of the Cell Membrane Composition and Structure as a Molecular Base for Drug Discovery and New Disease Treatment. Prog. Lipid Res. 2015, 59, 38–53. [Google Scholar] [CrossRef]
- Gao, P.; Yan, Z.; Zhu, Z. Mitochondria-Associated Endoplasmic Reticulum Membranes in Cardiovascular Diseases. Front. Cell Dev. Biol. 2020, 8, 604240. [Google Scholar] [CrossRef]
- Eckmann, J.; Eckert, S.H.; Leuner, K.; Muller, W.E.; Eckert, G.P. Mitochondria: Mitochondrial Membranes in Brain Ageing and Neurodegeneration. Int. J. Biochem. Cell Biol. 2013, 45, 76–80. [Google Scholar] [CrossRef]
- Chen, Z.; Wen, D.; Gu, Z. Cargo-Encapsulated Cells for Drug Delivery. Sci. China Life Sci. 2020, 63, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.T.; Fang, R.H.; Hu, C.M.J.; Copp, J.A.; Thamphiwatana, S.; Dehaini, D.; Gao, W.; Zhang, K.; Li, S.; Zhang, L. Safe and Immunocompatible Nanocarriers Cloaked in RBC Membranes for Drug Delivery to Treat Solid Tumors. Theranostics 2016, 6, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wu, T.; Zhang, D.; Zhang, Z. Cell or Cell Membrane-Based Drug Delivery Systems. Theranostics 2015, 5, 863–881. [Google Scholar] [CrossRef]
- Zucker, D.; Marcus, D.; Barenholz, Y.; Goldblum, A. Liposome Drugs’ Loading Efficiency: A Working Model Based on Loading Conditions and Drug’s Physicochemical Properties. J. Control. Release 2009, 139, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.H.; Zhang, Y.H.; Han, L.J.; Zhang, C.Z.; Wu, J.H.; Wang, X.R.; Gao, J.Q.; Mao, Z.W. Cell Membrane Capsules for Encapsulation of Chemotherapeutic and Cancer Cell Targeting in Vivo. ACS Appl. Mater. Interfaces 2015, 7, 18628–18637. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A Doxorubicin Delivery Platform Using Engineered Natural Membrane Vesicle Exosomes for Targeted Tumor Therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Yeo, R.W.Y.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal Stem Cell: An Efficient Mass Producer of Exosomes for Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef]
- Li, M.; Zhang, F.; Chen, K.; Wang, C.; Su, Y.; Liu, Y.; Zhou, J.; Wang, W. Nanoparticles and Mesenchymal Stem Cells: A Win-Win Alliance for Anticancer Drug Delivery. RSC Adv. 2016, 6, 36910–36922. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.; Pan, J.; Liu, S.; Lu, G.Q. (Max) Advances in Multicompartment Mesoporous Silica Micro/Nanoparticles for Theranostic Applications. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 389–411. [Google Scholar] [CrossRef]
- Maurya, C.K.; Misra, R.; Sharma, P.; Singh, N.; Awasthi, H.; Agrawal, R.; Misra, S.; Dwivedi, S. Novel Stem Cells and Nucleic Acid-Based Vaccine Trials Against Viral Outbreak: A Systematic Evaluation During COVID-2019 Pandemic. Indian J. Clin. Biochem. 2020, 35, 397. [Google Scholar] [CrossRef]
- Popowski, K.D.; Dinh, P.-U.C.; George, A.; Lutz, H.; Cheng, K. Exosome Therapeutics for COVID-19 and Respiratory Viruses. View 2021, 2, 20200186. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Allergy and Infectious Diseases (NIAID). Safety and Immunogenicity Study of 2019-NCoV Vaccine (MRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19). Available online: https://clinicaltrials.gov/study/NCT04283461 (accessed on 23 July 2024).
- Nsairat, H.; Alshaer, W.; Odeh, F.; Esawi, E.; Khater, D.; Al Bawab, A.; El-Tanani, M.; Awidi, A.; Mubarak, M.S. Recent Advances in Using Liposomes for Delivery of Nucleic Acid-Based Therapeutics. OpenNano 2023, 11, 100132. [Google Scholar] [CrossRef]
- Mockey, M.; Bourseau, E.; Chandrashekhar, V.; Chaudhuri, A.; Lafosse, S.; Le Cam, E.; Quesniaux, V.F.J.; Ryffel, B.; Pichon, C.; Midoux, P. MRNA-Based Cancer Vaccine: Prevention of B16 Melanoma Progression and Metastasis by Systemic Injection of MART1 MRNA Histidylated Lipopolyplexes. Cancer Gene Ther. 2007, 14, 802–814. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Gao, J.; Saeed, M.; Li, T.; Wang, W.; Yu, H. Nanobiomaterial-Based Vaccination Immunotherapy of Cancer. Biomaterials 2021, 270, 120709. [Google Scholar] [CrossRef]
- Guo, J.; Liu, C.; Qi, Z.; Qiu, T.; Zhang, J.; Yang, H. Engineering Customized Nanovaccines for Enhanced Cancer Immunotherapy. Bioact. Mater. 2024, 36, 330–357. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Sun, X.; Jiang, Q.; Sun, B.; He, Z.; Zhang, S.; Luo, C.; Sun, J. Lymph Node-Targeting Nanovaccines for Cancer Immunotherapy. J. Control. Release 2022, 351, 102–122. [Google Scholar] [CrossRef]
- Shishparenok, A.N.; Furman, V.V.; Zhdanov, D.D. DNA-Based Nanomaterials as Drug Delivery Platforms for Increasing the Effect of Drugs in Tumors. Cancers 2023, 15, 2151. [Google Scholar] [CrossRef]
- Liang, S.; Xu, H.; Ye, B.C. Membrane-Decorated Exosomes for Combination Drug Delivery and Improved Glioma Therapy. Langmuir 2022, 38, 299–308. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on Metal Nanoparticles as Nanocarriers: Current Challenges and Perspectives in Drug Delivery Systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Martano, S.; De Matteis, V.; Cascione, M.; Rinaldi, R. Inorganic Nanomaterials versus Polymer-Based Nanoparticles for Overcoming Neurodegeneration. Nanomaterials 2022, 12, 2337. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, W.; Wang, M.; Liao, Z. Magnetic Nanoparticles for Cancer Theranostics: Advances and Prospects. J. Control. Release 2021, 335, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Aqil, M. Lyotropic Liquid Crystalline Nanoparticles: Scaffolds for Delivery of Myriad Therapeutics and Diagnostics. J. Mol. Liq. 2021, 338, 116919. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996. [Google Scholar] [CrossRef]
- Roger, M.; Clavreul, A.; Venier-Julienne, M.C.; Passirani, C.; Montero-Menei, C.; Menei, P. The Potential of Combinations of Drug-Loaded Nanoparticle Systems and Adult Stem Cells for Glioma Therapy. Biomaterials 2011, 32, 2106–2116. [Google Scholar] [CrossRef]
- Li, L.; Guan, Y.; Liu, H.; Hao, N.; Liu, T.; Meng, X.; Fu, C.; Li, Y.; Qu, Q.; Zhang, Y.; et al. Silica Nanorattle–Doxorubicin-Anchored Mesenchymal Stem Cells for Tumor-Tropic Therapy. ACS Nano 2011, 5, 7462–7470. [Google Scholar] [CrossRef]
- Stephan, M.T.; Moon, J.J.; Um, S.H.; Bersthteyn, A.; Irvine, D.J. Therapeutic Cell Engineering with Surface-Conjugated Synthetic Nanoparticles. Nat. Med. 2010, 16, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Suryaprakash, S.; Lao, Y.-H.; Cho, H.-Y.; Li, M.; Ji, H.Y.; Shao, D.; Hu, H.; Quek, C.H.; Huang, D.; Mintz, R.L.; et al. Engineered Mesenchymal Stem Cell/Nanomedicine Spheroid as an Active Drug Delivery Platform for Combinational Glioblastoma Therapy. Nano Lett. 2019, 19, 1701–1705. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Wang, D.W.; Zhu, X.M.; Lee, S.F.; Chan, H.M.; Li, H.W.; Kong, S.K.; Yu, J.C.; Cheng, C.H.K.; Wang, Y.X.J.; Leung, K.C.F. Folate-Conjugated Fe3O4@SiO2@gold Nanorods@mesoporous SiO2 Hybrid Nanomaterial: A Theranostic Agent for Magnetic Resonance Imaging and Photothermal Therapy. J. Mater. Chem. B 2013, 1, 2934–2942. [Google Scholar] [CrossRef]
- Liu, H.; Chen, D.; Li, L.; Liu, T.; Tan, L.; Wu, X.; Tang, F. Multifunctional Gold Nanoshells on Silica Nanorattles: A Platform for the Combination of Photothermal Therapy and Chemotherapy with Low Systemic Toxicity. Angew. Chem. Int. Ed. 2011, 50, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Escoto, J.L.; Slowing, I.I.; Wu, C.W.; Lin, V.S.Y. Photoinduced Intracellular Controlled Release Drug Delivery in Human Cells by Gold-Capped Mesoporous Silica Nanosphere. J. Am. Chem. Soc. 2009, 131, 3462–3463. [Google Scholar] [CrossRef]
- Luo, G.F.; Chen, W.H.; Lei, Q.; Qiu, W.X.; Liu, Y.X.; Cheng, Y.J.; Zhang, X.Z. A Triple-Collaborative Strategy for High-Performance Tumor Therapy by Multifunctional Mesoporous Silica-Coated Gold Nanorods. Adv. Funct. Mater. 2016, 26, 4339–4350. [Google Scholar] [CrossRef]
- Siqueira, J.R.; Caseli, L.; Crespilho, F.N.; Zucolotto, V.; Oliveira, O.N. Immobilization of Biomolecules on Nanostructured Films for Biosensing. Biosens. Bioelectron. 2010, 25, 1254–1263. [Google Scholar] [CrossRef]
- Liu, Q.; Boyd, B.J. Liposomes in Biosensors. Analyst 2013, 138, 391–409. [Google Scholar] [CrossRef]
- Santos, W.d.J.R.; Sousa, A.L.; Sotomayor, M.d.P.T.; Damos, F.S.; Tanaka, S.M.C.N.; Kubota, L.T.; Tanaka, A.A. Manganese Phthalocyanine as a Biomimetic Electrocatalyst for Phenols in the Development of an Amperometric Sensor. J. Braz. Chem. Soc. 2009, 20, 1180–1187. [Google Scholar] [CrossRef]
- Kidner, N.J.; Meier, A.; Homrighaus, Z.J.; Wessels, B.W.; Mason, T.O.; Garboczi, E.J. Complex Electrical (Impedance/Dielectric) Properties of Electroceramic Thin Films by Impedance Spectroscopy with Interdigital Electrodes. Thin Solid Film. 2007, 515, 4588–4595. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, R.; Zhang, R.; Liang, J.; Ren, S.; Gao, Z. Application of DNA-Fueled Molecular Machines in Food Safety Testing. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13299. [Google Scholar] [CrossRef]
- Lu, C.; Luo, S.; Wang, X.; Li, J.; Li, Y.; Shen, Y.; Wang, J. Illuminating the Nanomaterials Triggered Signal Amplification in Electrochemiluminescence Biosensors for Food Safety: Mechanism and Future Perspectives. Coord. Chem. Rev. 2024, 501, 215571. [Google Scholar] [CrossRef]
- Kim, S.N.; Rusling, J.F.; Papadimitrakopoulos, F. Carbon Nanotubes for Electronic and Electrochemical Detection of Biomolecules. Adv. Mater. 2007, 19, 3214–3228. [Google Scholar] [CrossRef]
- Sharotri, N.; Ahmed, K.; Sharma, D.; Agrawal, N. Nanobiosensing Disease Diagnostics for in Vivo Applications. In Handbook of Nanomaterials, Volume 2: Biomedicine, Environment, Food, and Agriculture; Elsevier: Amsterdam, The Netherlands, 2024; pp. 179–206. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, T.; Jiang, J.; Wu, C.; Zhu, G.; You, M.; Chen, X.; Zhang, L.; Cui, C.; Yu, R.; et al. Cell Membrane-Anchored Biosensors for Real-Time Monitoring of the Cellular Microenvironment. J. Am. Chem. Soc. 2014, 136, 13090–13093. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Tian, Q.; Bagheri, Y.; You, M. Lipid–Oligonucleotide Conjugates for Simple and Efficient Cell Membrane Engineering and Bioanalysis. Curr. Opin. Biomed. Eng. 2020, 13, 76–83. [Google Scholar] [CrossRef] [PubMed]
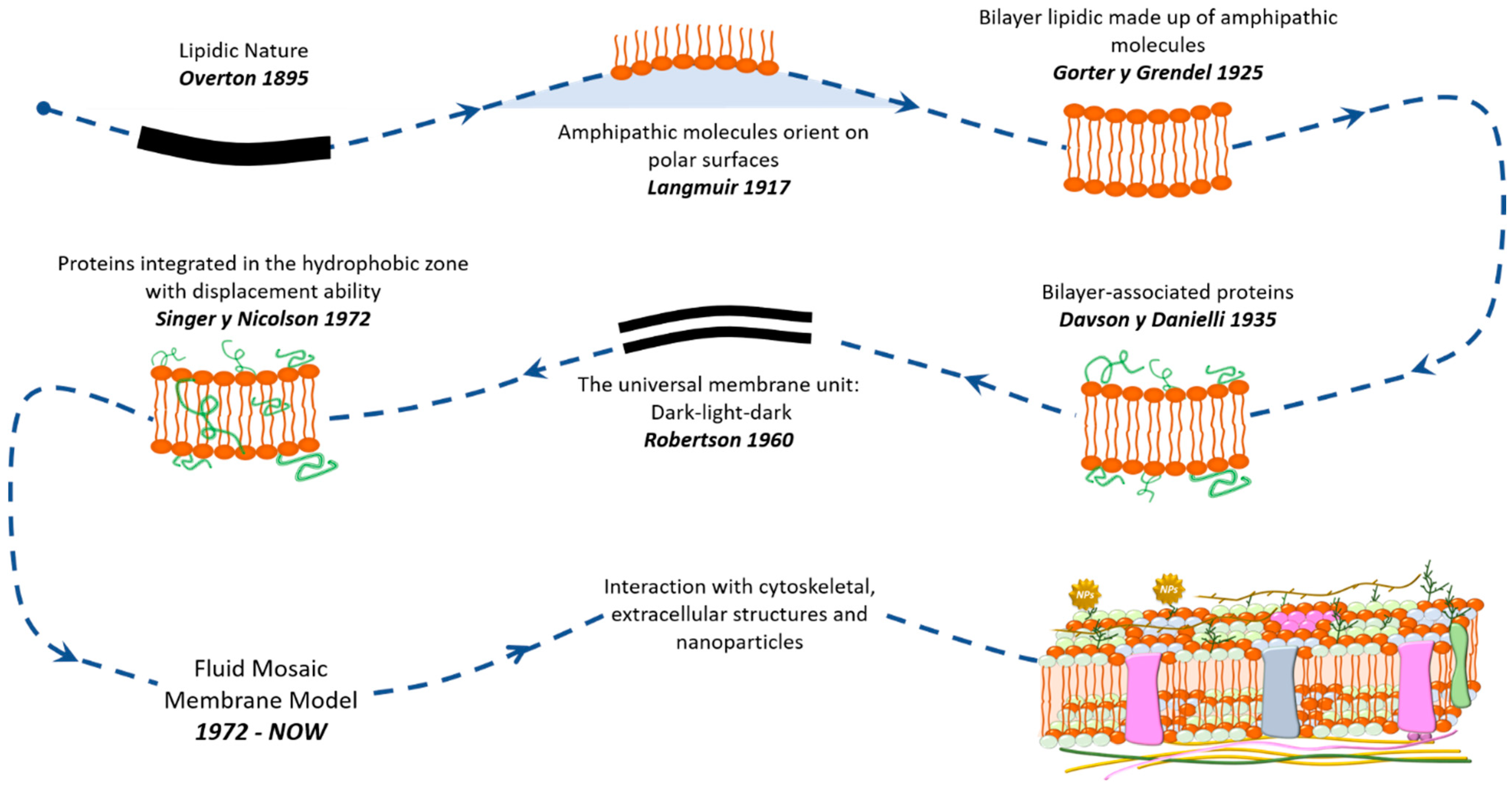
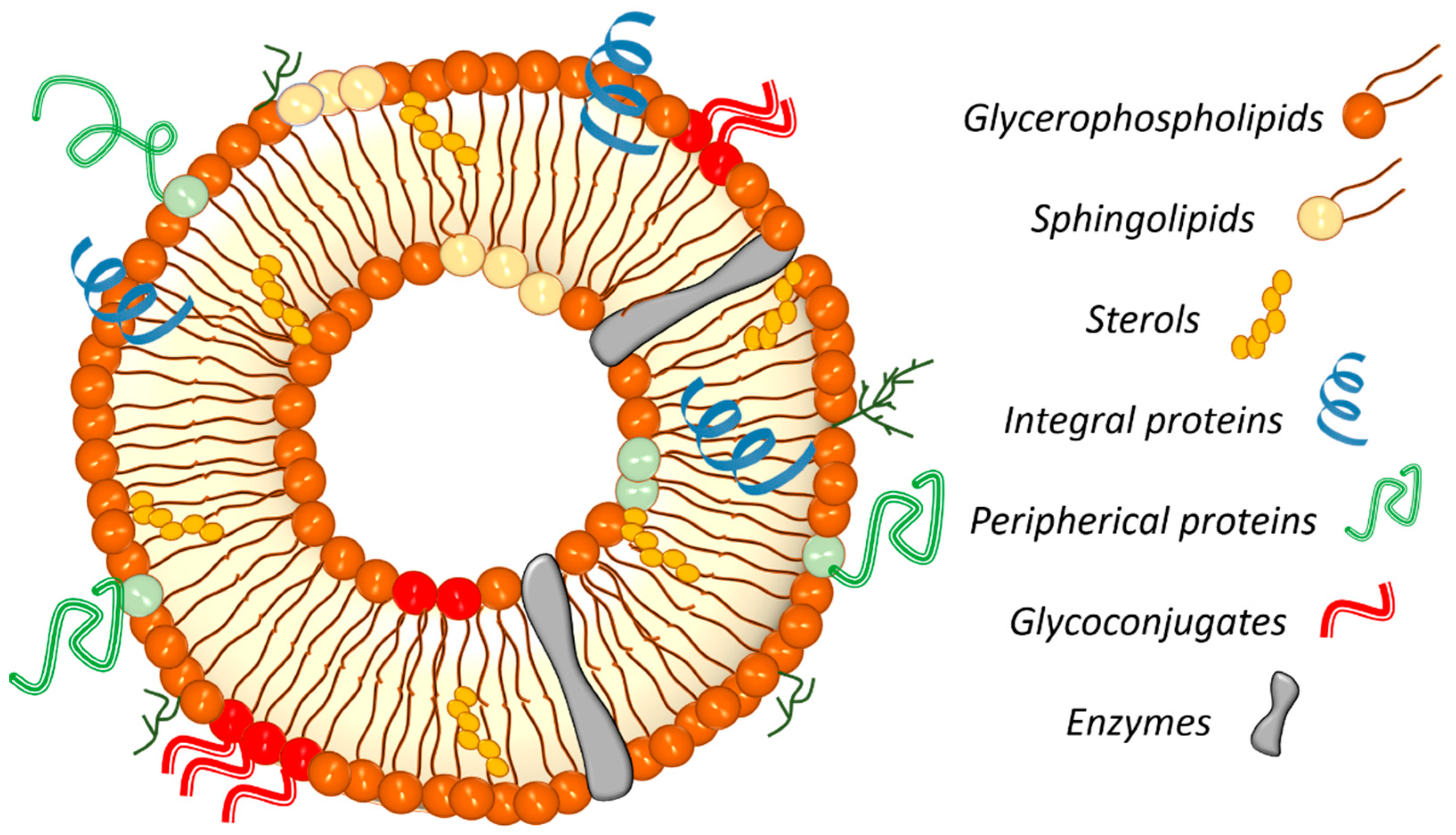
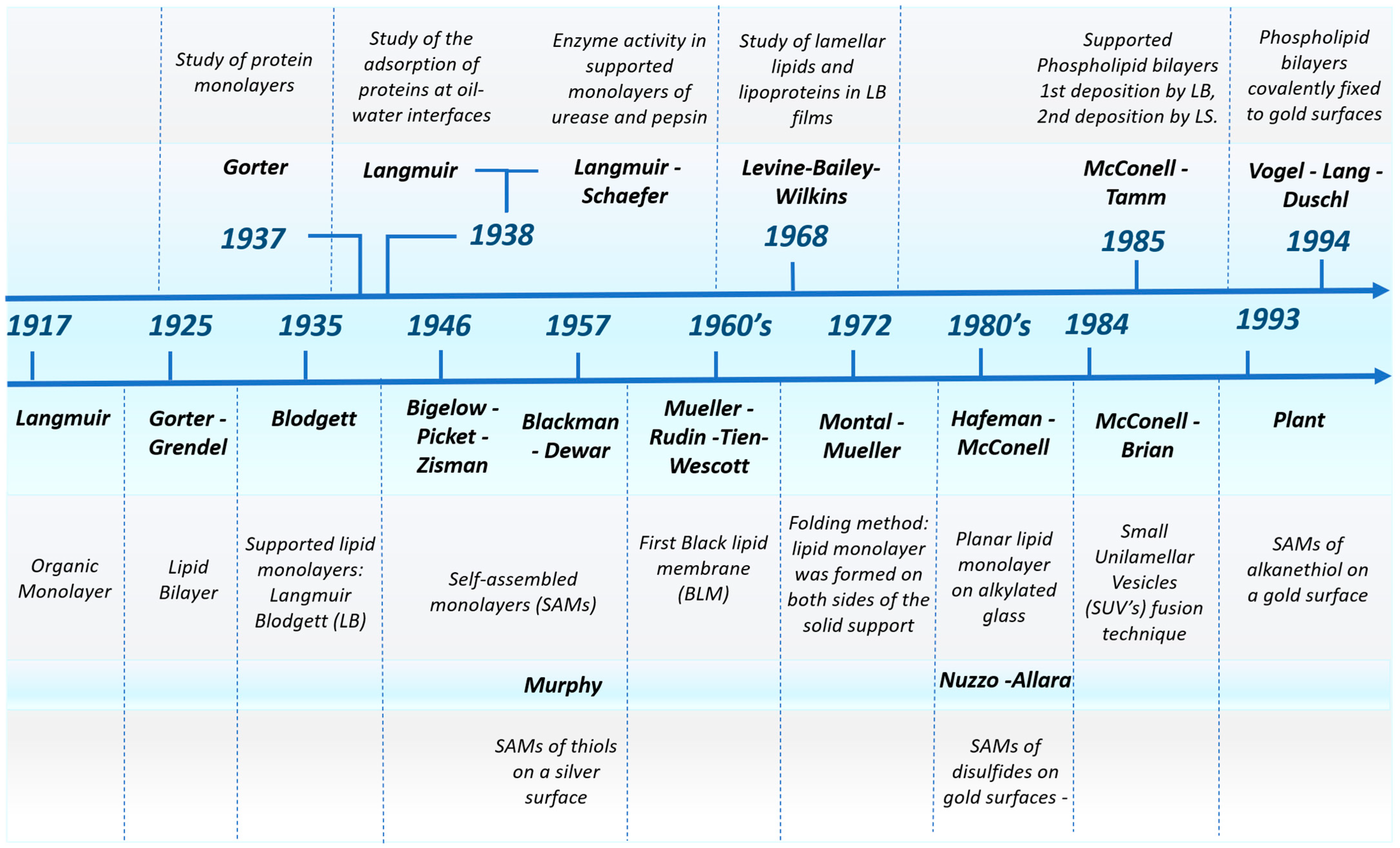
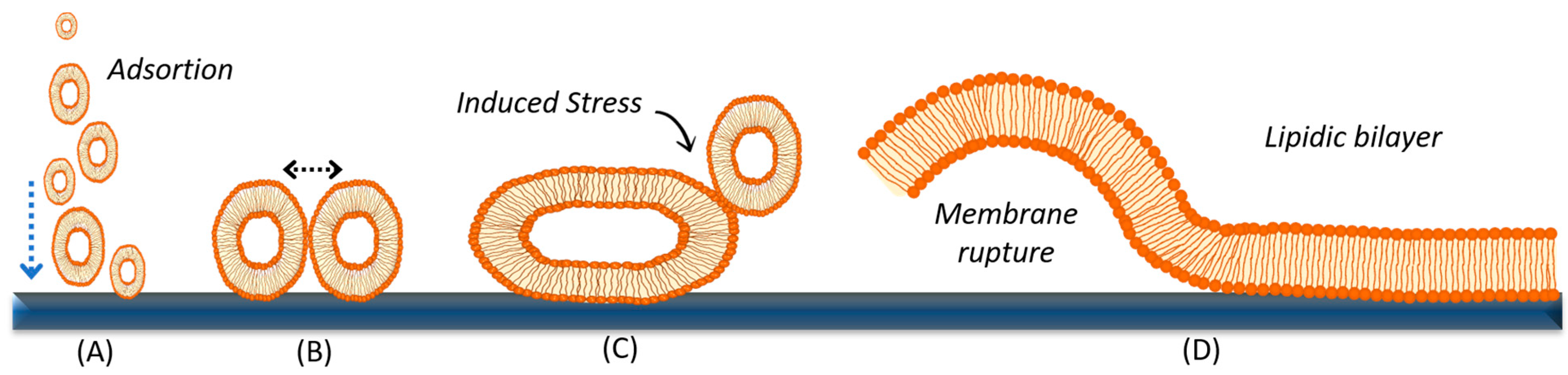
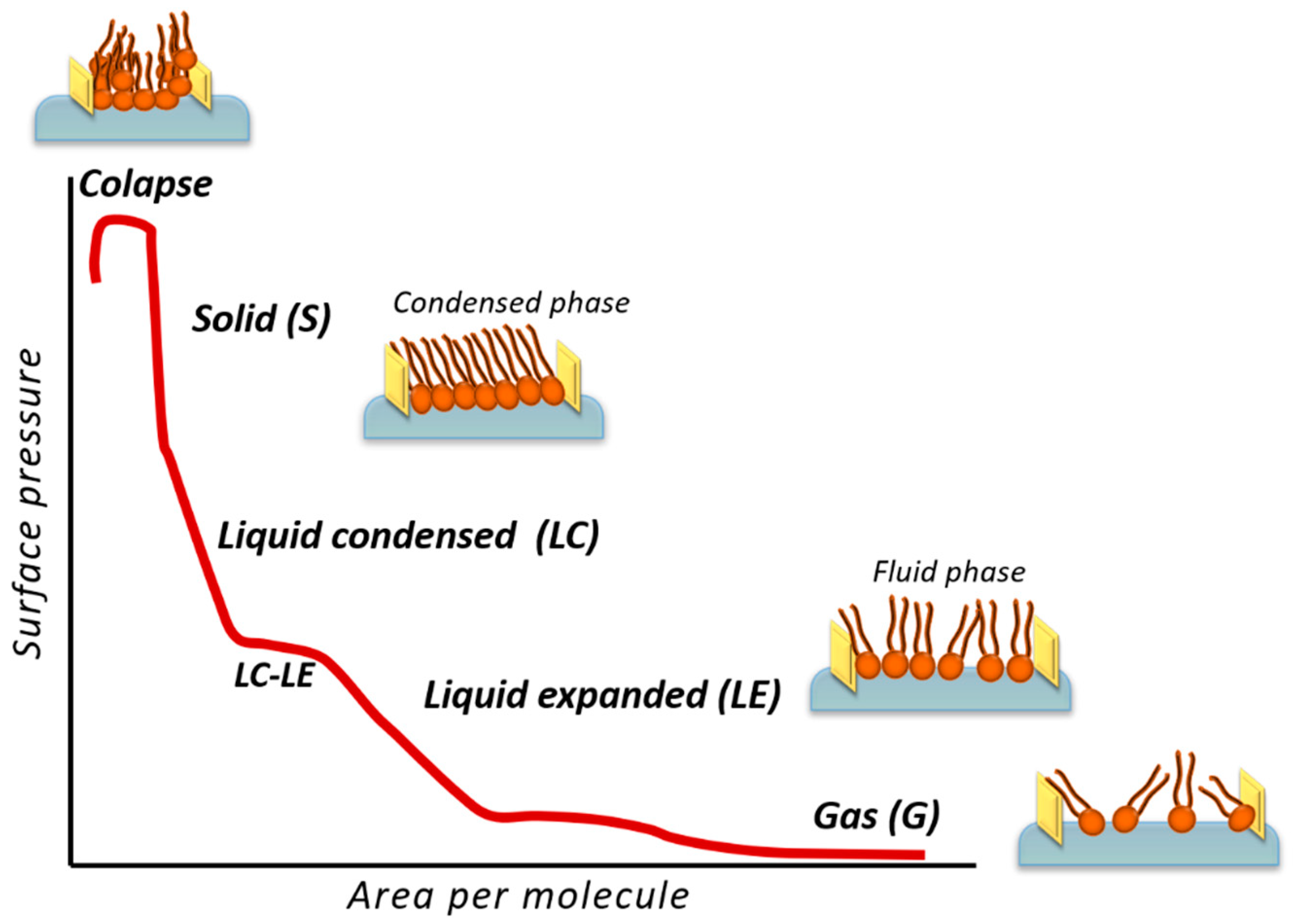


| Characterization Technique | Principle | Information Provided | References |
|---|---|---|---|
| Surface pressure vs. area per molecule isotherms | Measurement of surface tension changes as molecules are compressed on the air–water interface | Measurement of surface tension changes as molecules are compressed on the air–water interface. Compression modulus. Excess thermodynamic properties in multicomponent films. | [145,241] |
| Surface potential vs. area per molecule isotherms | Measurement of the electrical potential difference across the air–water interface | Surface potential changes related to molecular orientation and interactions | [159,242,243] |
| Fluorescence Microscopy | Fluorescence detection at the air–water interface | Visualization of morphology, domain formation, and phase separation | [244,245] |
| Brewster Angle Microscopy (BAM) | Reflection of a p-polarized laser beam at the Brewster angle (~53° for water) on the air–water interface containing a monolayer | Real-time visualization of monolayer organization and molecular orientation | [246,247,248] |
| X-ray diffraction (XRD), grazing incidence X-ray diffraction (GIXD), X-ray specular reflectivity (XR), and neutron specular reflectivity | Diffraction of X-rays by ordered molecular structures at the air–water interface | Structural information, molecular packing, and phase behavior | [249,250,251,252,253,254] |
| Ellipsometry | Change of ellipsometry angles associated with reflection | Layer thickness and density | [255,256] |
| Reflection/Absorption of electronic (UV-vis and fluorescence) and vibrational spectroscopies (IR and Raman) | Measurement of electron transitions or vibrational modes of molecules at the air–water interface | Molecular composition, chemical interactions, formation of aggregates, and structural changes | [257,258,259,260,261,262] |
| Characterization Technique | Principle | Information Provided | References |
|---|---|---|---|
| Quartz crystal electrochemical microbalance, QCM with dissipation (QCM-D), and impedance-based QCM (QCM-Z) | Oscillation frequencies of a quartz crystal that depends on the amount of material deposited on the surface | Real-time adsorbed mass and energy dissipation | [263,264,265] |
| Electrochemisty: cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIC) | Resistance of an electrochemical system to the flow of electrical current | Real time: Interfacial properties related to bio-recognition | [266,267] |
| Neutron reflectometry (NR) | Measures the intensity of neutrons reflected from a surface to determine the structure and composition of thin films and interfaces | Structure, composition, and interactions | [268,269,270] |
| X-ray reflectometry (XRR) | Measures the intensity of X-rays reflected from a surface to assess the thickness, density, and roughness of thin films and interfaces | Structural properties and phase transitions | [271,272] |
| Ellipsometry | Change of ellipsometry angles associated with reflection | Interfacial mass and layer thickness and density | [135,273] |
| X-ray fluorescence | Monochromatic synchrotron X-ray beam irradiating the sample causing the atoms in the sample to emit secondary (or fluorescent) X-rays | Analyze the composition for detecting the presence and distribution of metal ions or other elements | [274] |
| Surface plasmon resonance (SPR) | Excitation of molecules with lasers and monitoring of the emitted spectrum | Real-time monitoring of morphology and physicochemical properties | [275,276,277] |
| Total internal reflection ellipsometry (TIRE) | Combination of ellipsometry and SPR to detect changes in the polarization of reflected light | Adsorption/desorption Quantify membrane receptor interactions at the surface | [168] |
| Nanoplasmonic Sensor (NPS) | Localized surface plasmon resonance induced by refractive index changes around the model cell membrane induced by molecular binding events | Mass, thickness, and conformation of adsorbates, interaction kinetics, and binding avidity | [278,279,280,281] |
| Surface plasmon fluorescence spectroscopy | Intensity of electromagnetic field and excitation of surface plasmons | Topography and physicochemical properties | [275,282] |
| Sum Frequency Generation Vibrational Spectroscopy (SFG) | Nonlinear optical technique based on the generation of sum frequency from two incident light beams | Information on interfacial peptide and protein structure (e.g., conformation and orientation) and interactions between peptides and proteins with lipid layers | [283,284,285,286] |
| Attenuated-total reflectance Infrared spectroscopy (ATR-FTIR); surface enhanced infrared absorption spectroscopy (SEIRAS); and polarization modulated infrared reflection absorption spectroscopy (PM-IRRAS) | Infrared beam intensity or absorption of polarized light at specific angles/incident light, which is characteristic of the functional groups and order state of the material | Chemical composition, molecular orientation, lipid phase, and protein interactions | [57,137,208,287] |
| Fluorescence microscopy | Fluorescence detection (UV or blue light) | Interfacial morphology and phase structure | [288,289] |
| FRAP: fluorescence recovery after photobleaching | Fluorescence recovery in a photobleached area | Protein diffusion rates based on the fluorescence recovery rate within a photobleached region in the model membrane | [290,291] |
| Atomic force microscopy (AFM) | Interaction force between surface and detection tip | Surface topography, layer thickness, mechanical properties, interactions on a surface, phase separation, nanomechanical properties | [292,293,294,295,296,297] |
| Solid-state Nuclear magnetic resonance (NMR) | Radiation absorption in atomic nuclei under a magnetic field | Composition, lipid–protein interactions, and structure 3D of proteins embedded in a lipid bilayer | [298,299] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coronado, S.; Herrera, J.; Pino, M.G.; Martín, S.; Ballesteros-Rueda, L.; Cea, P. Advancements in Engineering Planar Model Cell Membranes: Current Techniques, Applications, and Future Perspectives. Nanomaterials 2024, 14, 1489. https://doi.org/10.3390/nano14181489
Coronado S, Herrera J, Pino MG, Martín S, Ballesteros-Rueda L, Cea P. Advancements in Engineering Planar Model Cell Membranes: Current Techniques, Applications, and Future Perspectives. Nanomaterials. 2024; 14(18):1489. https://doi.org/10.3390/nano14181489
Chicago/Turabian StyleCoronado, Sara, Johan Herrera, María Graciela Pino, Santiago Martín, Luz Ballesteros-Rueda, and Pilar Cea. 2024. "Advancements in Engineering Planar Model Cell Membranes: Current Techniques, Applications, and Future Perspectives" Nanomaterials 14, no. 18: 1489. https://doi.org/10.3390/nano14181489
APA StyleCoronado, S., Herrera, J., Pino, M. G., Martín, S., Ballesteros-Rueda, L., & Cea, P. (2024). Advancements in Engineering Planar Model Cell Membranes: Current Techniques, Applications, and Future Perspectives. Nanomaterials, 14(18), 1489. https://doi.org/10.3390/nano14181489








