Research and Analysis on Enhancement of Surface Flashover Performance of Epoxy Resin Based on Dielectric Barrier Discharge Plasma Fluorination Modification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. DBD Plasma Experimental Devices
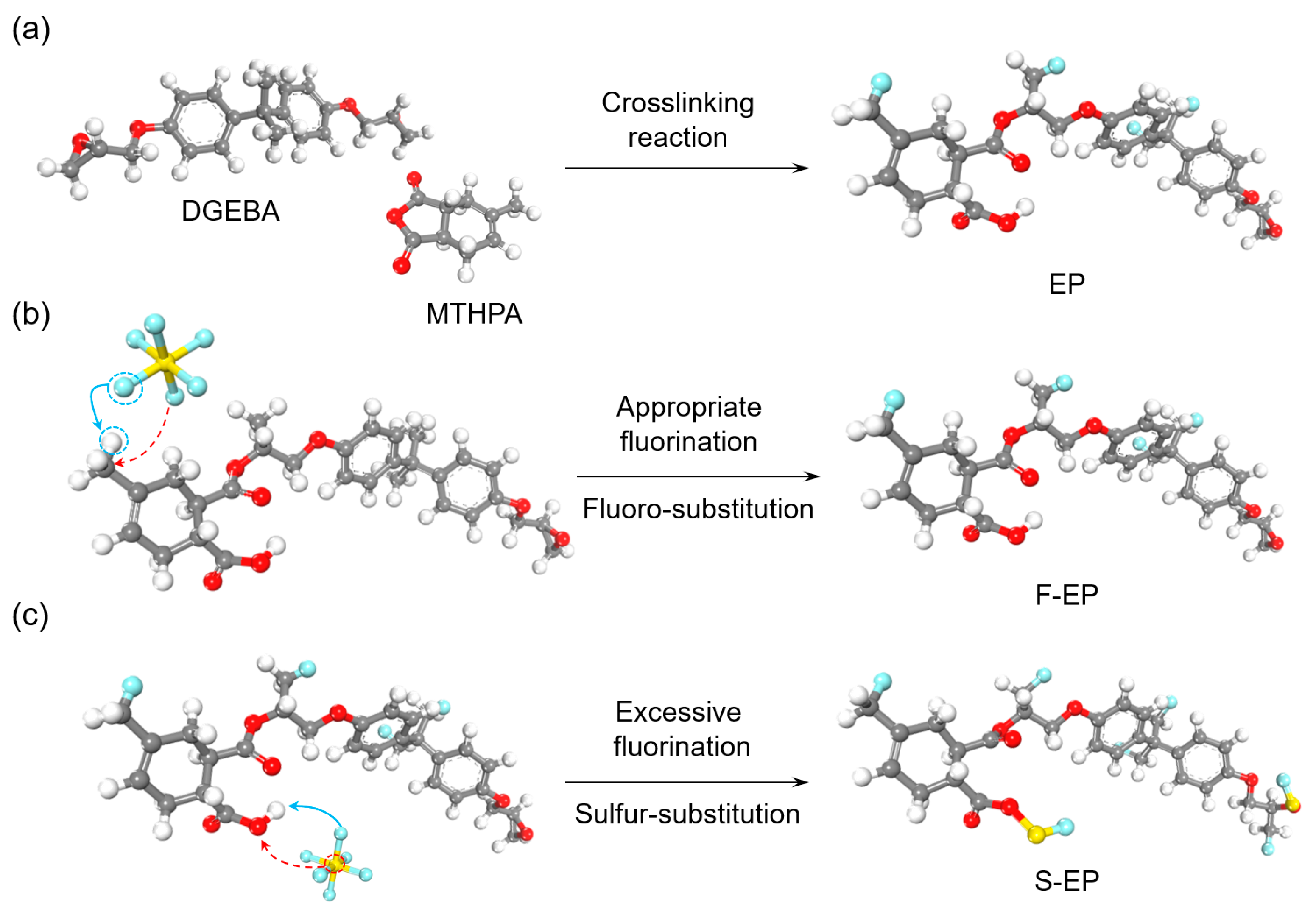
2.3. Fluorination Treatment Process with Plasma
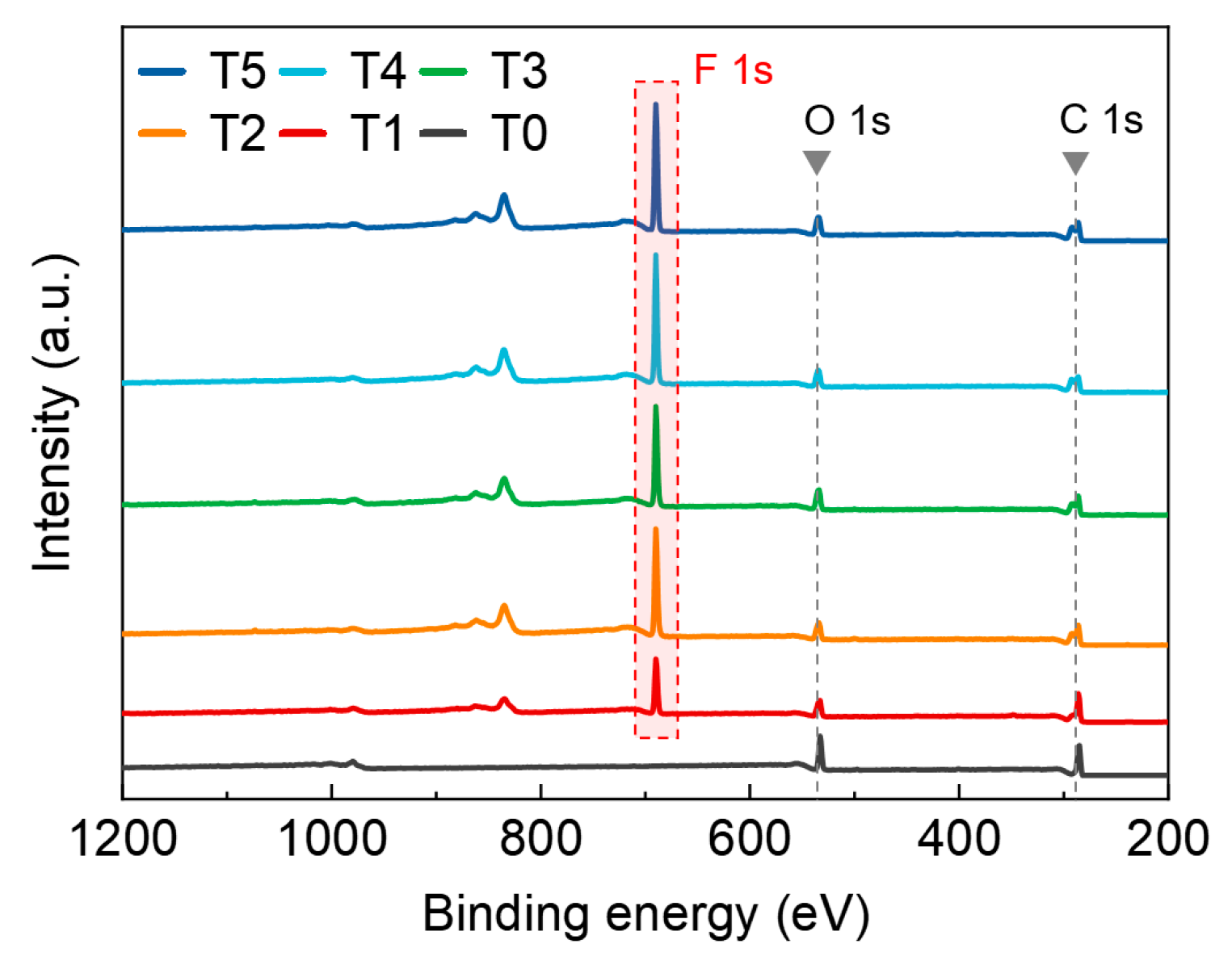
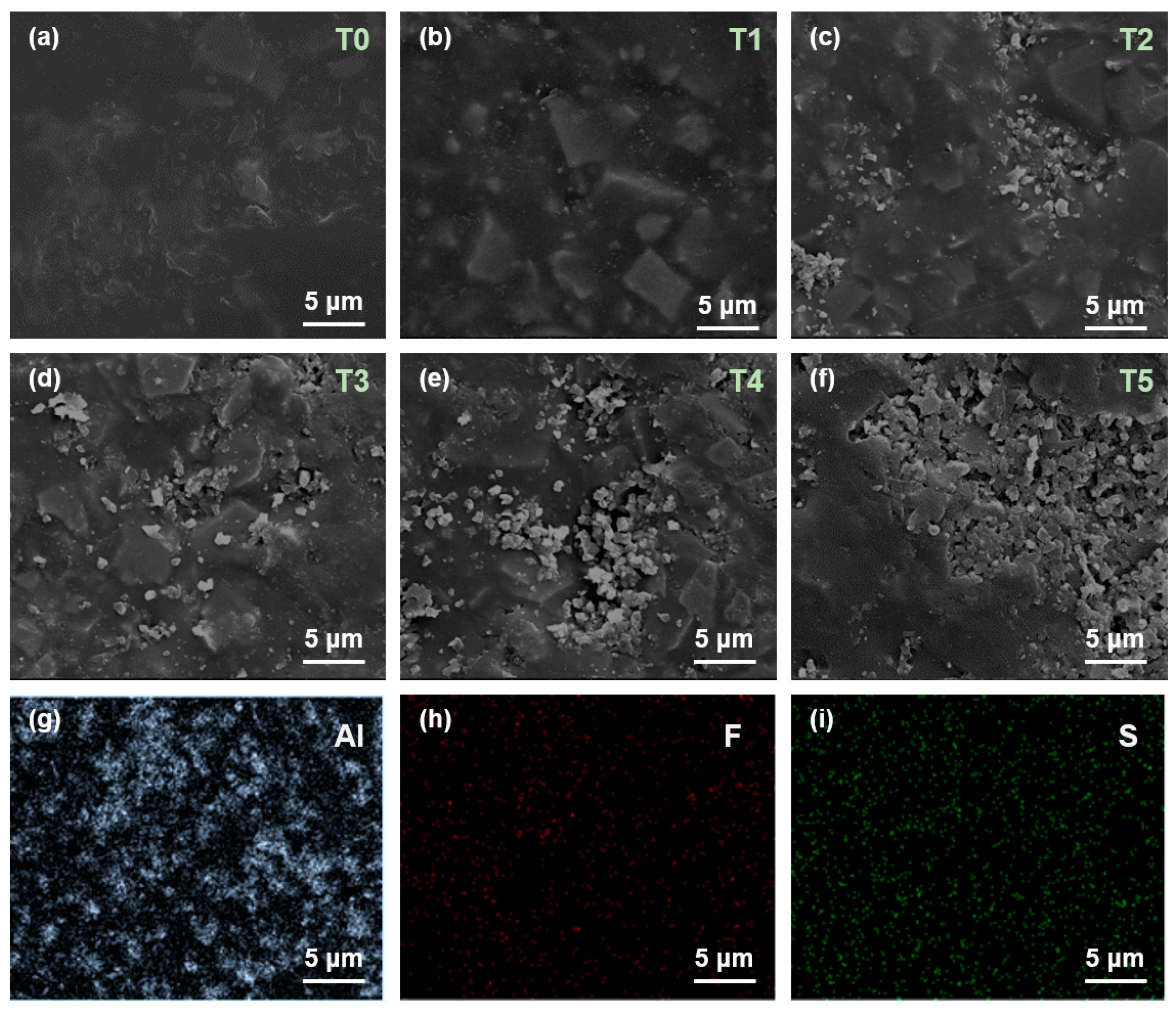
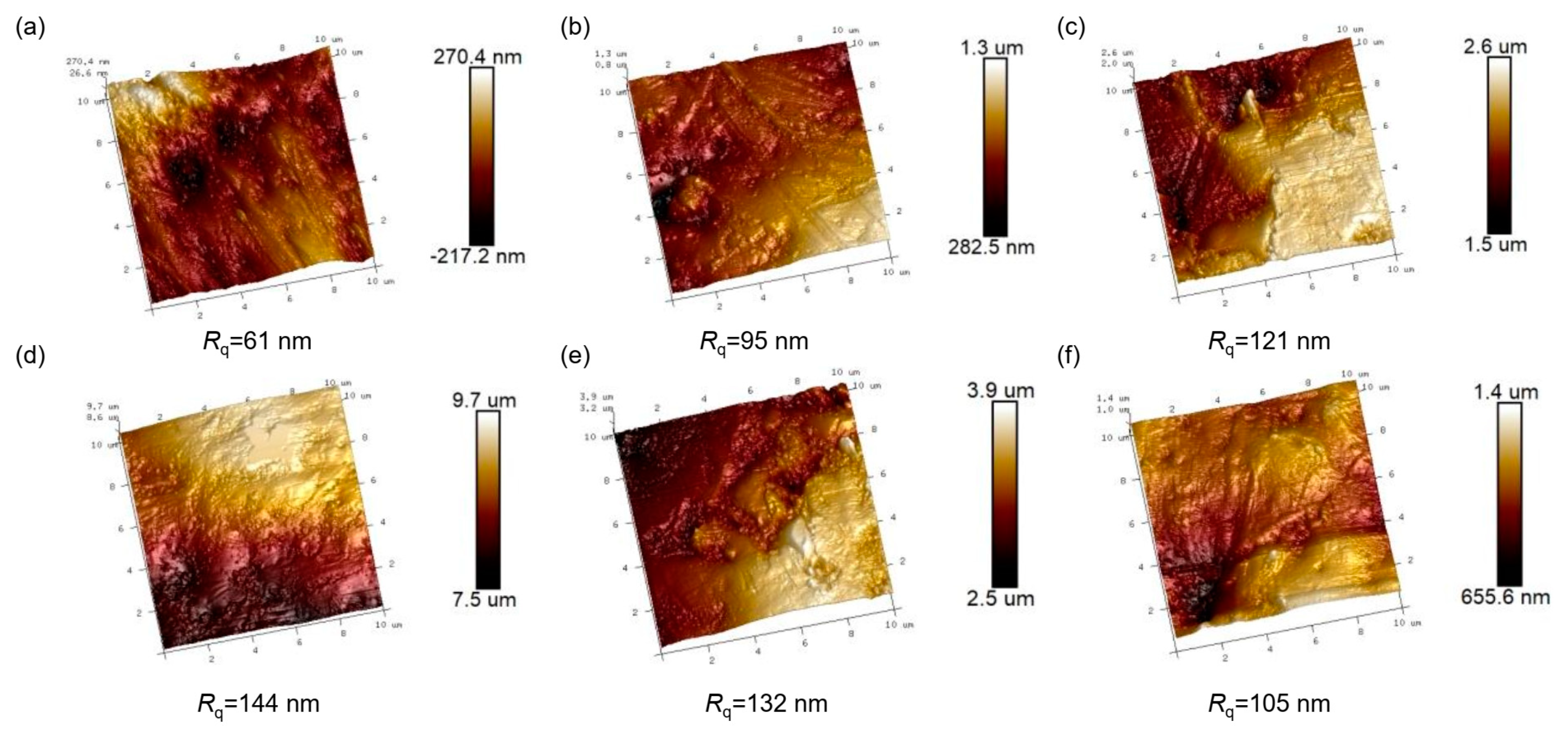
2.4. Characterizations
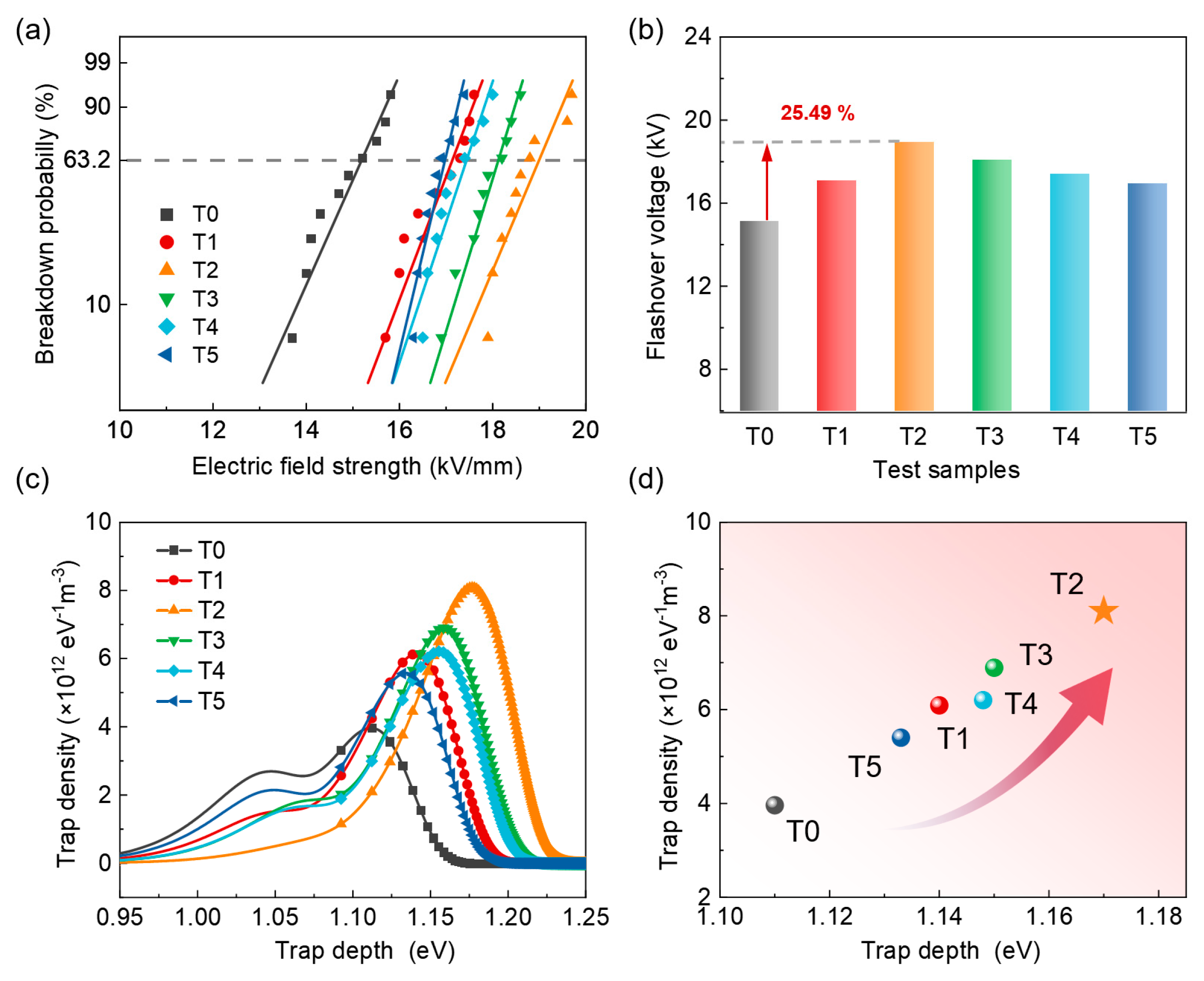
2.5. Performance Testing
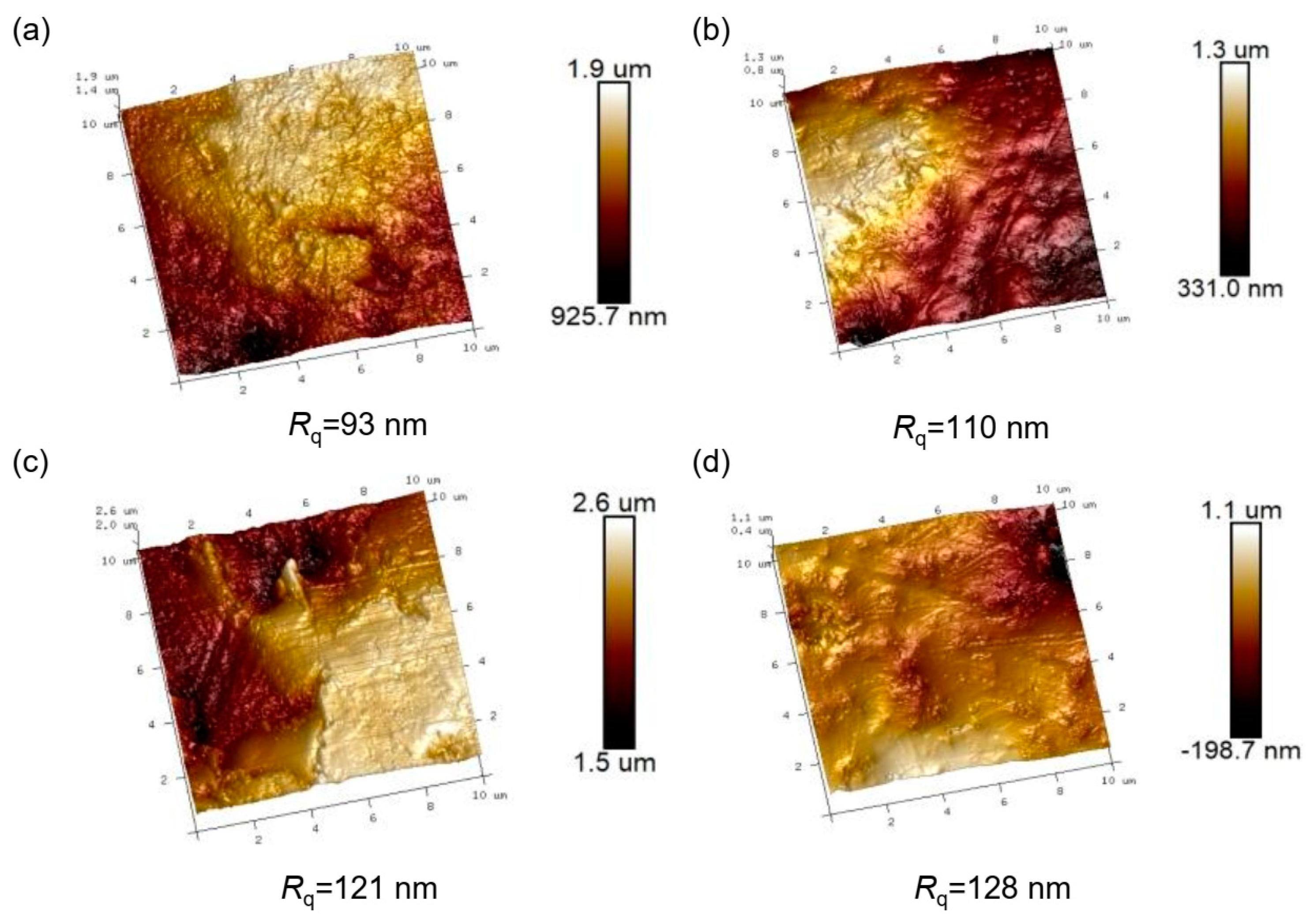
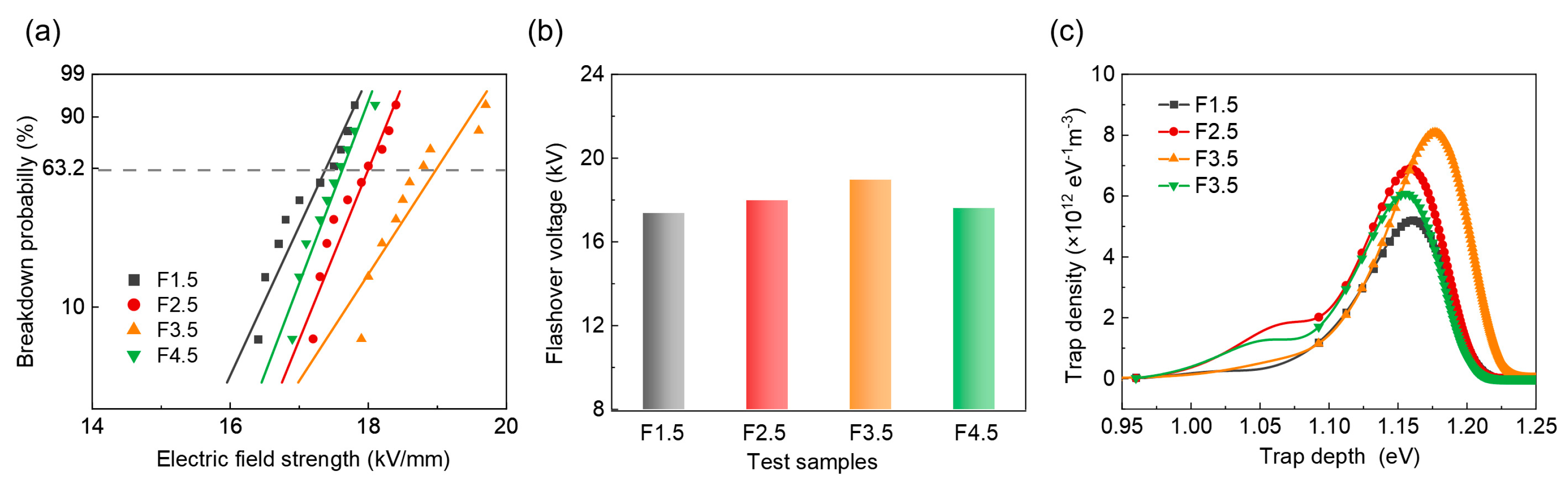
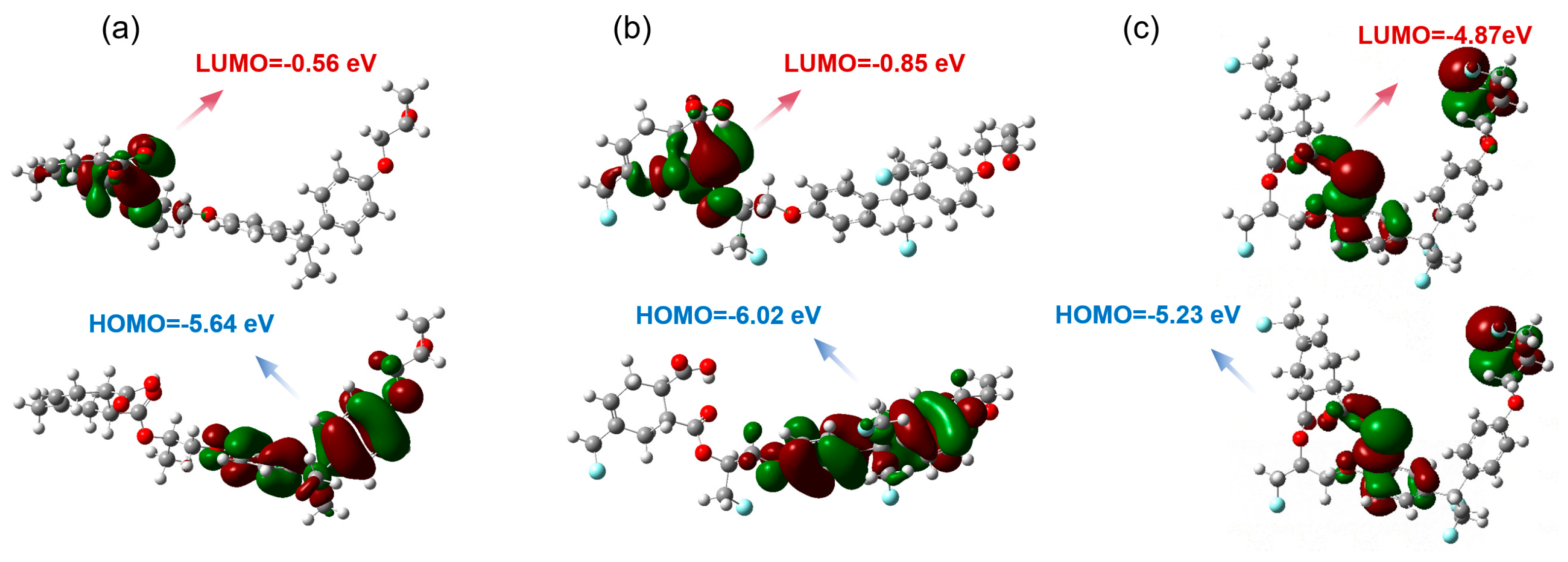
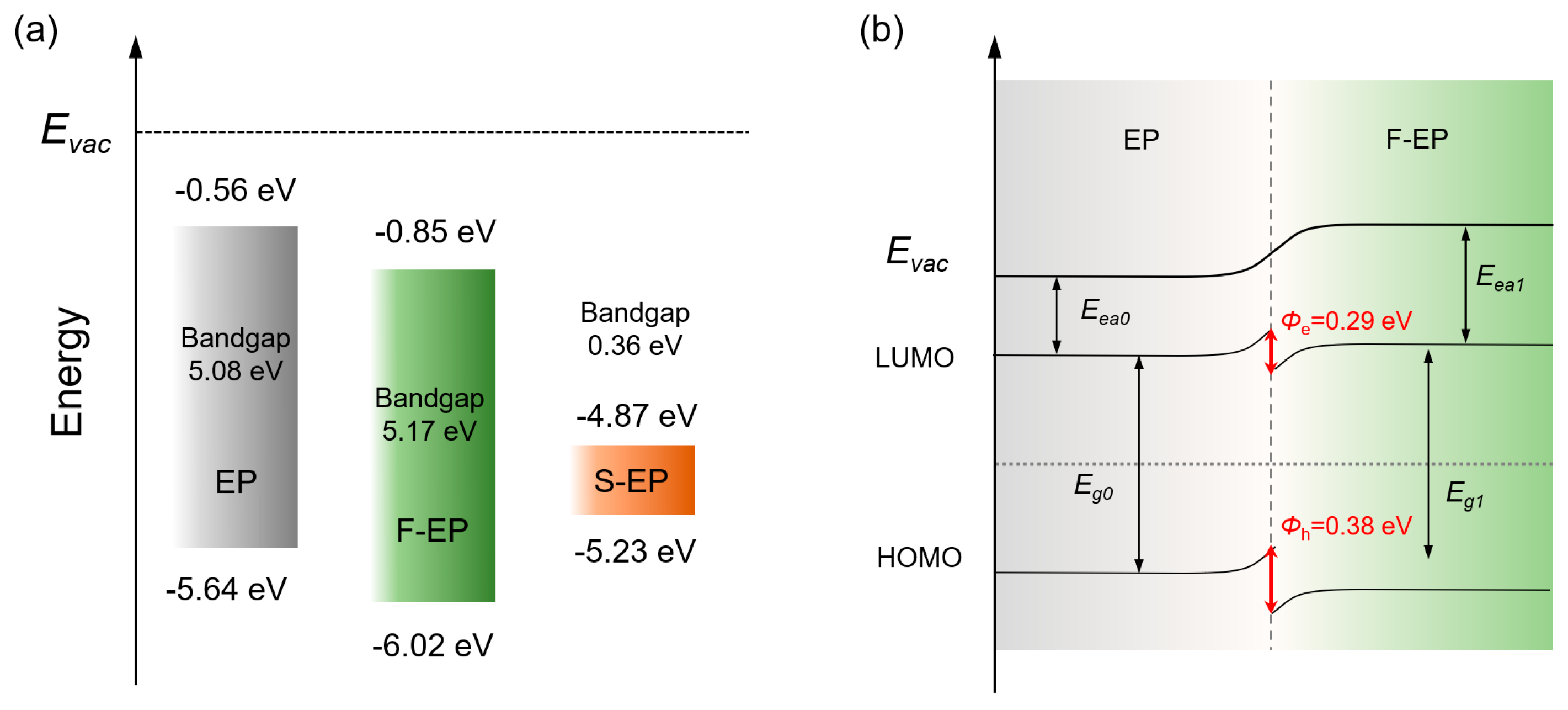
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Şomoghi, R.; Semenescu, A.; Pasăre, V.; Chivu, O.R.; Nițoi, D.F.; Marcu, D.F.; Florea, B. The Impact of ZnO Nanofillers on the Mechanical and Anti-Corrosion Performances of Epoxy Composites. Polymers 2024, 16, 2054. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Zhang, T.; Wang, X.; Yin, C.; Liu, X.; Chi, Q. High-temperature resistance and excellent electrical insulation in epoxy resin blends. J. Appl. Polym. Sci. 2023, 140, e54359. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Z.; Sun, J.; Li, Z. Enhanced switching electric field and breakdown strength of epoxy composites with core-shell silicon carbide nanoparticles. High Volt. 2023, 8, 583–589. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, X.; Wang, Z.; Zhang, Z.; Liu, Z. A mini-review of ultra-low dielectric constant intrinsic epoxy resins: Mechanism, preparation and application. Polym. Adv. Technol. 2024, 35, e6241. [Google Scholar] [CrossRef]
- Yang, K.; Chen, W.; Zhao, Y.; Ding, L.; Du, B.; Zhang, S.; Yang, W. Enhancing dielectric strength of thermally conductive epoxy composites by preventing interfacial charge accumulation using micron-sized diamond. Compos. Sci. Technol. 2022, 221, 109178. [Google Scholar] [CrossRef]
- Zhao, Y.; He, Y.; Yang, K.; Wang, X.; Bai, J.; Du, B. Improving the surface insulating performance of epoxy resin/Al2O3 composite materials by extending chain of liquid epoxy resin with Me-THPA. High Volt. 2020, 5, 472–481. [Google Scholar] [CrossRef]
- Huang, Y.; Min, D.; Li, S.; Li, Z.; Xie, D.; Wang, X.; Lin, S. Surface flashover performance of epoxy resin microcomposites improved by electron beam irradiation. Appl. Surf. Sci. 2017, 406, 39–45. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, Y.; Liu, X.; Huang, H.; Zhang, C.; Shao, T. Characteristics of microsecond-pulse surface flashover on epoxy resin surfaces in SF6. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2328–2336. [Google Scholar] [CrossRef]
- Shao, T.; Kong, F.; Lin, H.; Ma, Y.; Xie, Q.; Zhang, C. Correlation between surface charge and DC surface flashover of plasma treated epoxy resin. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1267–1274. [Google Scholar] [CrossRef]
- Fan, L.; Yin, Y.; Wang, Y. Surface Potential Dynamics and Flashover Resistance Evaluation of Epoxy Resin Under DC Flashover. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 575–582. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Zhang, B.-Y.; Li, D.-Y.; Hou, Y.-C.; Zhang, G.-X. Metal nanoparticle-doped epoxy resin to suppress surface charge accumulation on insulators under DC voltage. Nanotechnology 2020, 31, 324001. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Sun, J.; Yang, Y.; Liu, F.; Liu, X. DC Flashover Performance of GIS Spacers Fluorinated at Different Temperatures. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1200–1207. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, G.; Li, D.; Hou, Y.; Zhang, B. MXene-doped epoxy resin to suppress surface charge accumulation on insulators in a DC gas-insulated system. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 939–946. [Google Scholar] [CrossRef]
- Sun, J.; Song, S.; Zheng, J.; Li, Z.; Huo, J.; Wang, Y.; Xiao, P.; Akram, S.; Qin, D. A Review on Surface Flashover Phenomena at DC Voltage in Vacuum and Compressed Gas. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1–14. [Google Scholar] [CrossRef]
- Wang, T.; Liu, C.; Li, D.; Hou, Y.; Zhang, G.; Zhang, B. Nano ZnO/epoxy coating to promote surface charge dissipation on insulators in DC gas-insulated systems. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1322–1329. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Wang, S.; He, J.; Zhang, T.; Wang, J.; Wu, G. Simultaneously Enhanced Thermal Conductivity and Dielectric Breakdown Strength in Sandwich AlN/Epoxy Composites. Nanomaterials 2021, 11, 1898. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Min, D.; Niu, H.; Li, S.; Zhang, Y.; Huang, Y.; Li, S. Enhanced DC breakdown strength of epoxy nanocomposites at elevated temperature and its mechanisms. J. Appl. Phys. 2021, 130, 065101. [Google Scholar] [CrossRef]
- Xue, J.; Li, Y.; Dong, J.; Chen, J.; Li, W.; Deng, J.; Zhang, G.-J. Surface charge transport behavior and flashover mechanism on alumina/epoxy spacers coated by SiC/epoxy composites with varied SiC particle size. J. Phys. D Appl. Phys. 2020, 53, 155503. [Google Scholar] [CrossRef]
- Lü, F.-C.; Ruan, H.-O.; Song, J.-X.; Yin, K.; Zhan, Z.-Y.; Jiao, Y.-F.; Xie, Q. Enhanced surface insulation and depressed dielectric constant for Al2O3/epoxy composites through plasma fluorination of filler. J. Phys. D Appl. Phys. 2019, 52, 155201. [Google Scholar] [CrossRef]
- Phan, T.T.M.; Chu, N.C.; Luu, V.B.; Nguyen Xuan, H.; Martin, I.; Carriere, P. The role of epoxy matrix occlusions within BaTiO3 nanoparticles on the dielectric properties of functionalized BaTiO3/epoxy nanocomposites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 528–535. [Google Scholar] [CrossRef]
- Zare, Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos. Part A Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Vu, M.-T.; Monsalve-Bravo, G.M.; Lin, R.; Li, M.; Bhatia, S.K.; Smart, S. Mitigating the Agglomeration of Nanofiller in a Mixed Matrix Membrane by Incorporating an Interface Agent. Membranes 2021, 11, 328. [Google Scholar] [CrossRef]
- Asadian, M.; Chan, K.V.; Norouzi, M.; Grande, S.; Cools, P.; Morent, R.; De Geyter, N. Fabrication and Plasma Modification of Nanofibrous Tissue Engineering Scaffolds. Nanomaterials 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Vandenabeele, C.R.; Lucas, S. Technological challenges and progress in nanomaterials plasma surface modification—A review. Mater. Sci. Eng. R Rep. 2020, 139, 100521. [Google Scholar] [CrossRef]
- Yan, J.; Liang, G.; Lian, H.; Song, Y.; Ruan, H.; Duan, Q.; Xie, Q. Improving the surface flashover performance of epoxy resin by plasma treatment: A comparison of fluorination and silicon deposition under different modes. Plasma Sci. Technol. 2021, 23, 115501. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, H.; Zhang, S.; Zhang, Z.; Feng, T.; Xu, Z.; Wu, M. Surface De-Fluorination and Bond Modification of CFx by High-Density Hydrogen Plasma Processing. ACS Appl. Energy Mater. 2021, 4, 8615–8620. [Google Scholar] [CrossRef]
- He, J.; Wen, X.; Wu, L.; Chen, H.; Hu, J.; Hou, X. Dielectric barrier discharge plasma for nanomaterials: Fabrication, modification and analytical applications. TrAC Trends Anal. Chem. 2022, 156, 116715. [Google Scholar] [CrossRef]
- Li, S.; Dang, X.; Yu, X.; Abbas, G.; Zhang, Q.; Cao, L. The application of dielectric barrier discharge non-thermal plasma in VOCs abatement: A review. Chem. Eng. J. 2020, 388, 124275. [Google Scholar] [CrossRef]
- Li, M.; Shang, K.; Zhao, J.; Jiang, L.; Sun, J.; Wang, X.; Niu, H.; Feng, Y.; An, Z.; Li, S. Constructing Fluorine Hybrid Epoxy Polymers with Excellent Breakdown Strength by Surface Fluorination. ACS Appl. Polym. Mater. 2023, 5, 10226–10233. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ruan, H.O.; Wang, S.S.; Zhou, H.H.; Xie, Q.; Lü, F.C. Enhanced DC Insulation Strength of PMIA Paper by CF4/Ar Plasma Treatment. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 874–882. [Google Scholar] [CrossRef]
- Kim, S.-H.; Yun, U.-H.; Kim, J.-G. Low-Density Polyethylene Degradation and Energy Yield Using Dielectric Barrier Discharge under Various Electrical Conditions. Energies 2023, 16, 2403. [Google Scholar] [CrossRef]
- Shao, T.; Yang, W.; Zhang, C.; Niu, Z.; Yan, P.; Schamiloglu, E. Enhanced surface flashover strength in vacuum of polymethylmethacrylate by surface modification using atmospheric-pressure dielectric barrier discharge. Appl. Phys. Lett. 2014, 105, 071607. [Google Scholar] [CrossRef]
- Shen, F.; Liu, W.; Zhang, L.; Li, X.; Xiang, K.; Liu, H. Nonthermal Plasma Coupled with Al2O3/Carbon for High-Performance CF4 Decomposition at Low Temperatures. Ind. Eng. Chem. Res. 2023, 62, 13046–13052. [Google Scholar] [CrossRef]
- Guo, W.; Chen, X.; Lin, S.; Ji, J.; Wang, X. Numerical Investigation on Mechanisms of Particle Generation in He/CF4 Pulsed Dielectric Barrier Discharge Plasma at Atmospheric Pressure. IEEE Trans. Plasma Sci. 2023, 51, 1932–1940. [Google Scholar] [CrossRef]
- Jun, D.; Wei, J.; Eng Png, C.; Guangyuan, S.; Son, J.; Yang, H.; Danner, A.J. Deep anisotropic LiNbO3 etching with SF6/Ar inductively coupled plasmas. J. Vac. Sci. Technol. B 2012, 30, 011208. [Google Scholar] [CrossRef]
- Kechkar, S.; Babu, S.K.; Swift, P.; Gaman, C.; Daniels, S.; Turner, M. Investigation of absolute atomic fluorine density in a capacitively coupled SF6/O2/Ar and SF6/Ar discharge. Plasma Sources Sci. Technol. 2014, 23, 065029. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, S.-X.; Wen, D.-Q.; Liu, W.; Liu, Y.-X.; Li, X.-C.; Wang, Y.-N. F-atom kinetics in SF6/Ar inductively coupled plasmas. J. Vac. Sci. Technol. A 2016, 34, 031305. [Google Scholar] [CrossRef]
- Hussain, S.Q.; Ahn, S.; Park, H.; Kwon, G.; Raja, J.; Lee, Y.; Balaji, N.; Kim, H.; Le, A.H.T.; Yi, J. Light trapping scheme of ICP-RIE glass texturing by SF6/Ar plasma for high haze ratio. Vacuum 2013, 94, 87–91. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, X.; Sun, K.; Yang, R.; Fan, S.; Lv, F.; Yu, X. Synergistic enhancement of dielectric strength and thermal conductivity in meta-aramid-based composite films via honeycomb cavities filling strategy. Appl. Mater. Today 2024, 38, 102183. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Xu, B.; Ye, B.; Du, B. Effect of chemical corrosion on charge transport behaviour in epoxy/Al2O3 nanocomposite irradiated by gamma ray. High Volt. 2022, 7, 52–63. [Google Scholar] [CrossRef]
- Brundle, C.R.; Crist, B.V. X-ray photoelectron spectroscopy: A perspective on quantitation accuracy for composition analysis of homogeneous materials. J. Vac. Sci. Technol. A 2020, 38, 041001. [Google Scholar] [CrossRef]
- Románszki, L.; Klébert, S.; Héberger, K. Estimating Nanoscale Surface Roughness of Polyethylene Terephthalate Fibers. ACS Omega 2020, 5, 3670–3677. [Google Scholar] [CrossRef] [PubMed]
- Yahya, M.; Fadavieslam, M.R. The effects of argon plasma treatment on ITO properties and the performance of OLED devices. Opt. Mater. 2021, 120, 111400. [Google Scholar] [CrossRef]
- Chen, X.; Guan, H.; Jiang, T.; Du, H.; Paramane, A.; Zhou, H. Surface charge dissipation and DC flashover characteristic of DBD plasma treated epoxy resin/AlN nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 504–511. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, T.; Chen, Z.; Zhang, G. Mechanism on improved surface flashover performances in vacuum of epoxy resin using fluorocarbon plasma treatment. High Volt. 2022, 7, 420–428. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Gong, Y.; Hou, Q.; Liu, H.; Wang, J.; Ren, H. Long-term corona behaviour and performance enhancing mechanism of SiC/epoxy nanocomposite in SF6 gas environment. Plasma Sci. Technol. 2023, 25, 035501. [Google Scholar] [CrossRef]
- Mumit, M.A.; Pal, T.K.; Alam, M.A.; Islam, M.A.-A.-A.-A.; Paul, S.; Sheikh, M.C. DFT studies on vibrational and electronic spectra, HOMO–LUMO, MEP, HOMA, NBO and molecular docking analysis of benzyl-3-N-(2,4,5-trimethoxyphenylmethylene)hydrazinecarbodithioate. J. Mol. Struct. 2020, 1220, 128715. [Google Scholar] [CrossRef]
- Sengupta, A.; Li, B.; Svatunek, D.; Liu, F.; Houk, K.N. Cycloaddition Reactivities Analyzed by Energy Decomposition Analyses and the Frontier Molecular Orbital Model. Acc. Chem. Res. 2022, 55, 2467–2479. [Google Scholar] [CrossRef]
- Yuan, H.; Zhou, Y.; Zhu, Y.; Hu, S.; Yuan, C.; Song, W.; Shao, Q.; Zhang, Q.; Hu, J.; Li, Q.; et al. Origins and effects of deep traps in functional group grafted polymeric dielectric materials. J. Phys. D Appl. Phys. 2020, 53, 475301. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, X.; Sui, Y.; Li, C.; Yan, Z. Research and Analysis on Enhancement of Surface Flashover Performance of Epoxy Resin Based on Dielectric Barrier Discharge Plasma Fluorination Modification. Nanomaterials 2024, 14, 1382. https://doi.org/10.3390/nano14171382
Chang X, Sui Y, Li C, Yan Z. Research and Analysis on Enhancement of Surface Flashover Performance of Epoxy Resin Based on Dielectric Barrier Discharge Plasma Fluorination Modification. Nanomaterials. 2024; 14(17):1382. https://doi.org/10.3390/nano14171382
Chicago/Turabian StyleChang, Xizhe, Yueyi Sui, Changyu Li, and Zhanyuan Yan. 2024. "Research and Analysis on Enhancement of Surface Flashover Performance of Epoxy Resin Based on Dielectric Barrier Discharge Plasma Fluorination Modification" Nanomaterials 14, no. 17: 1382. https://doi.org/10.3390/nano14171382
APA StyleChang, X., Sui, Y., Li, C., & Yan, Z. (2024). Research and Analysis on Enhancement of Surface Flashover Performance of Epoxy Resin Based on Dielectric Barrier Discharge Plasma Fluorination Modification. Nanomaterials, 14(17), 1382. https://doi.org/10.3390/nano14171382




