Performance Enhancement of Ti/IrO2-Ta2O5 Anode through Introduction of Tantalum–Titanium Interlayer via Double-Glow Plasma Surface Alloying Technology
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Interlayer
2.2. Preparation of Electrode
2.3. Surface Analysis and Electrochemical Measurement
3. Results and Discussion
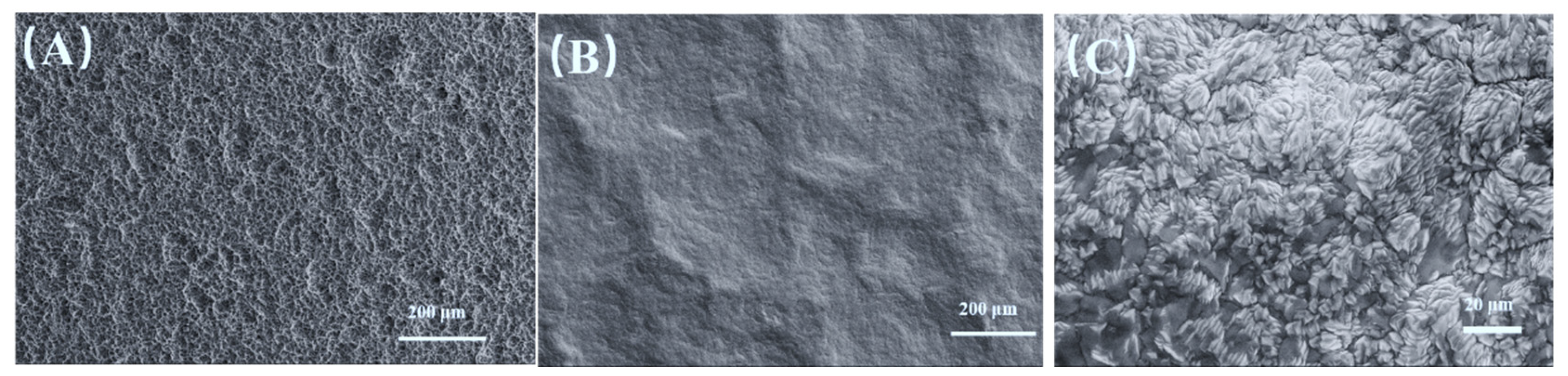
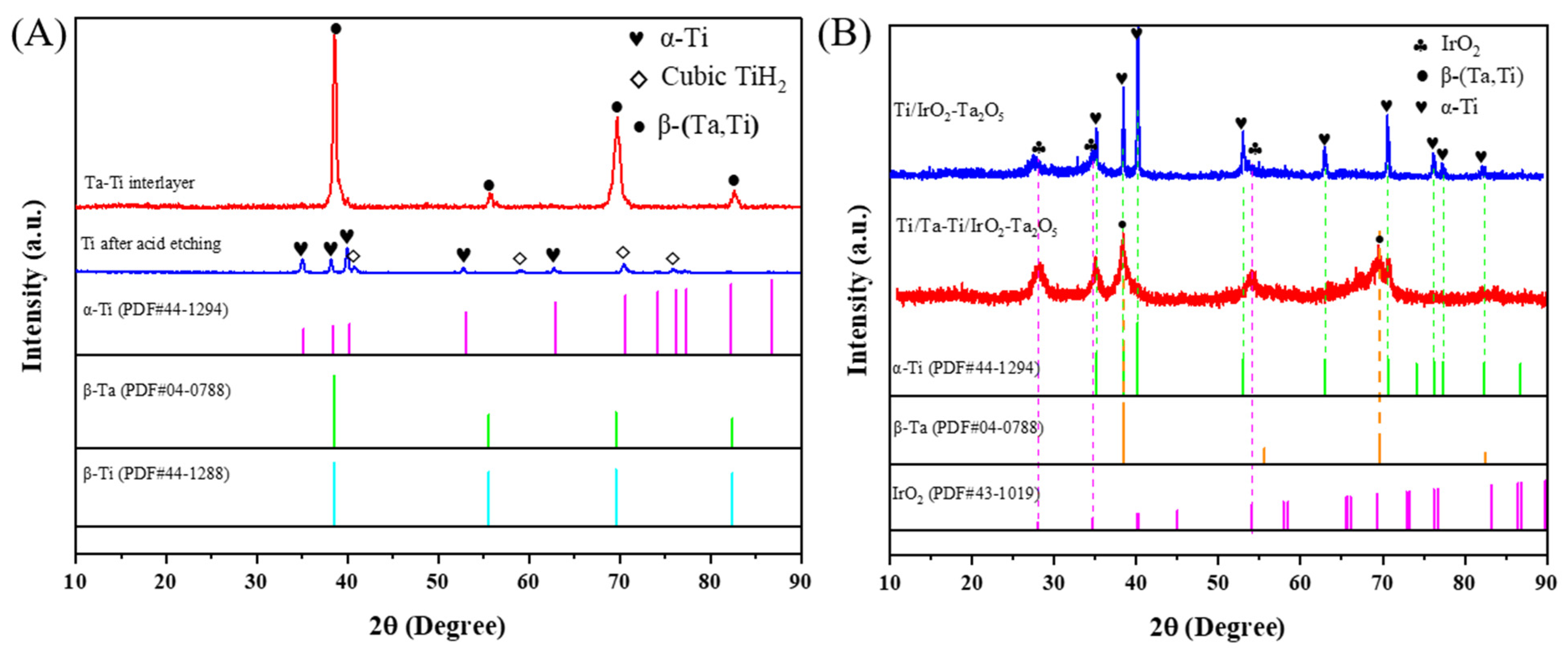
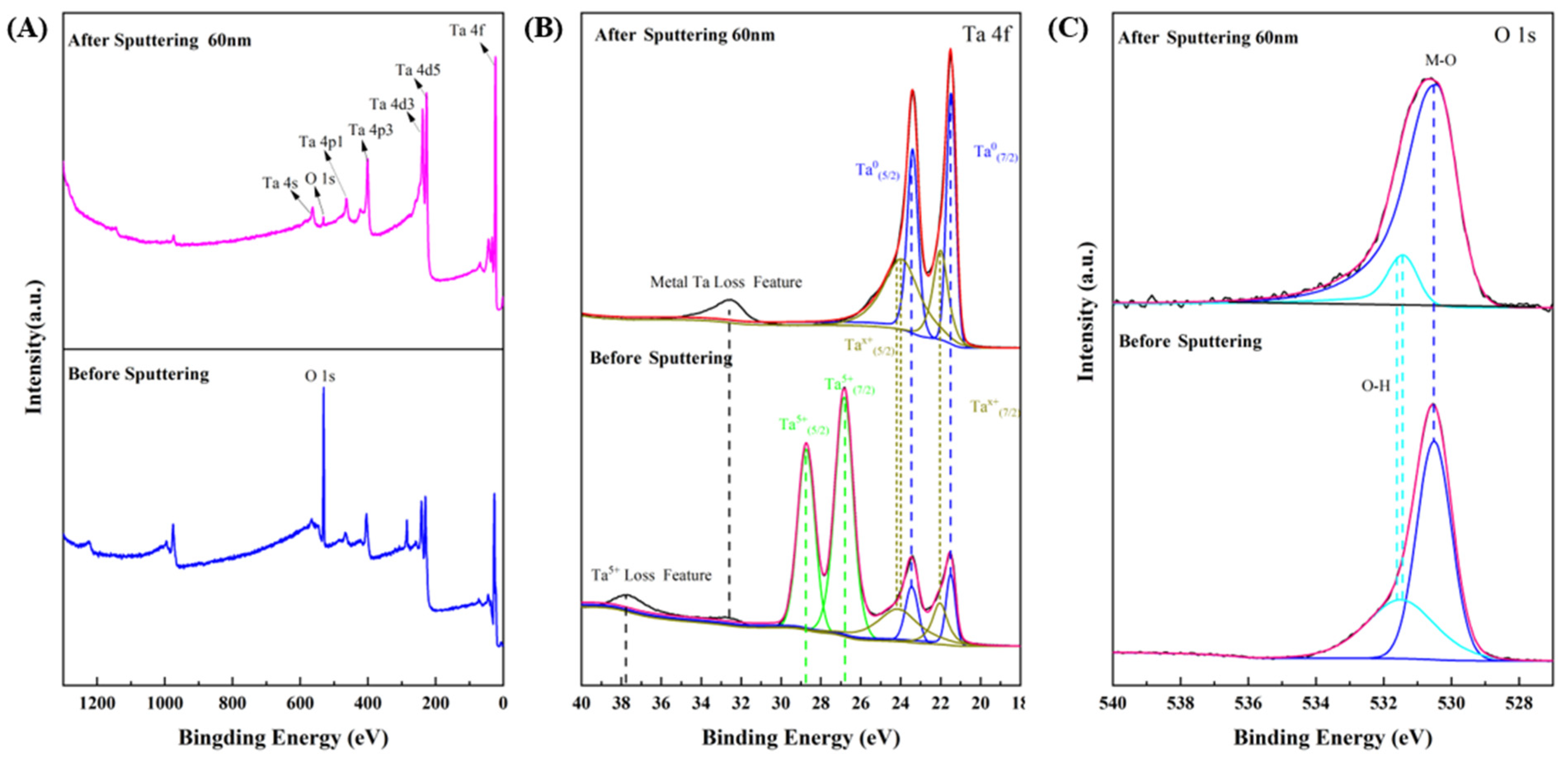
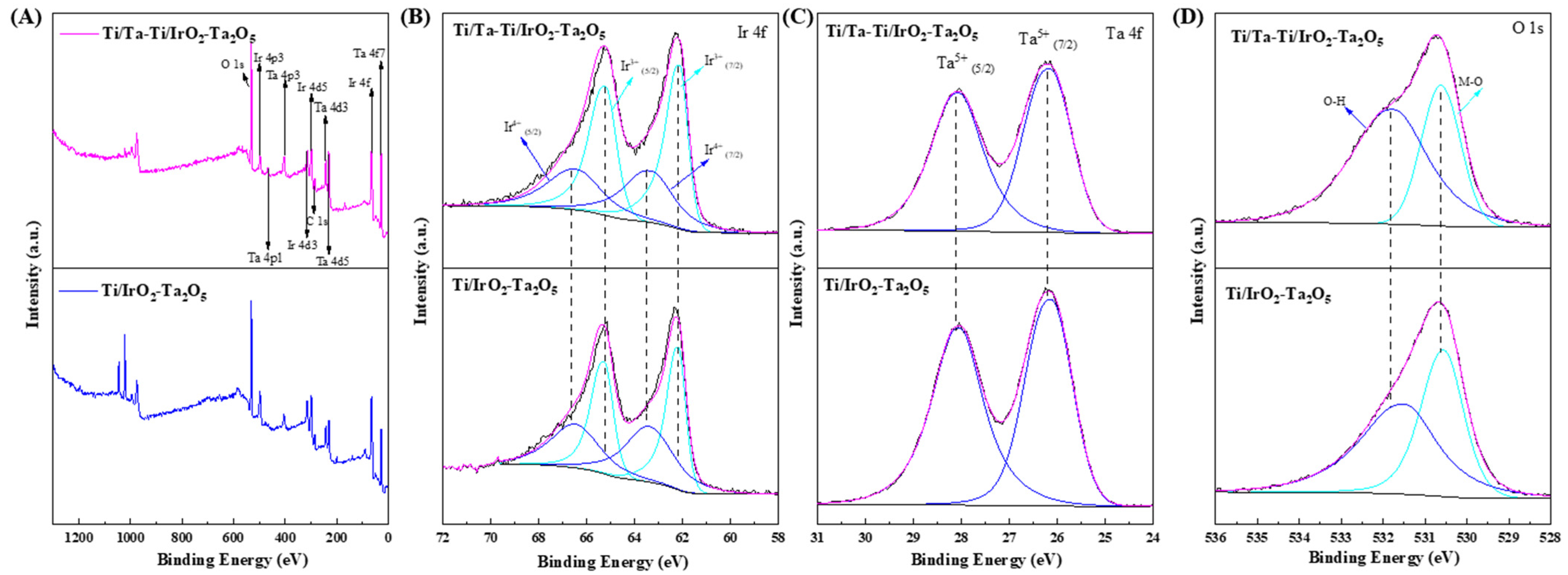
3.1. Microstructure
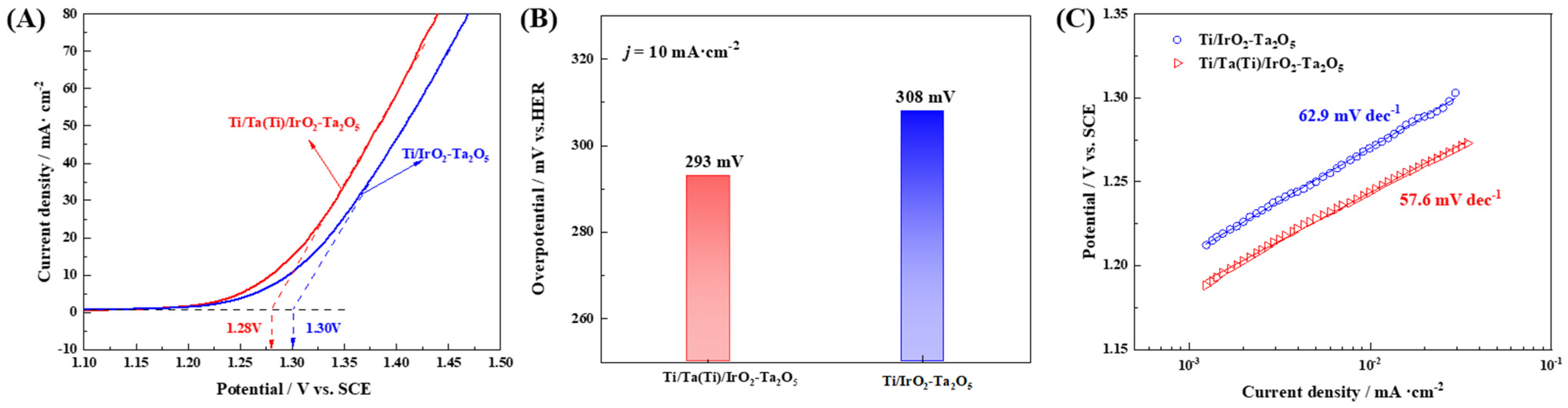
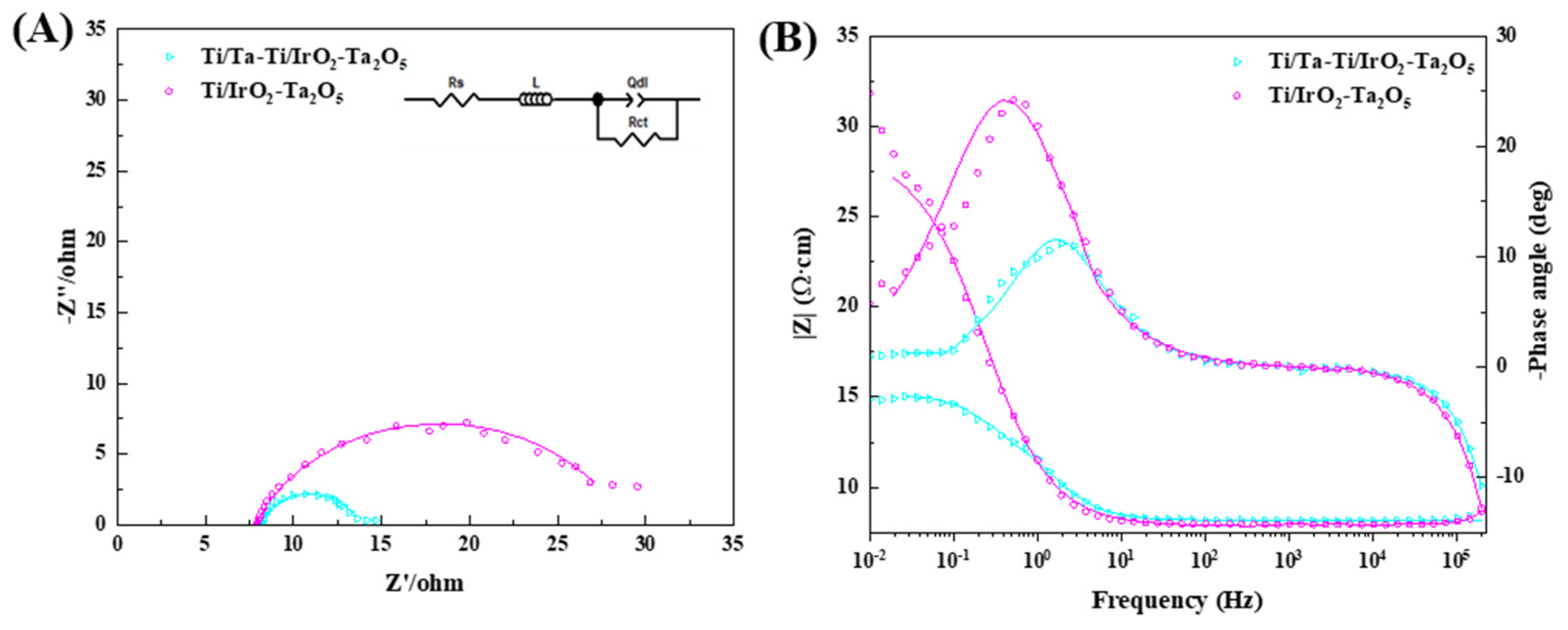
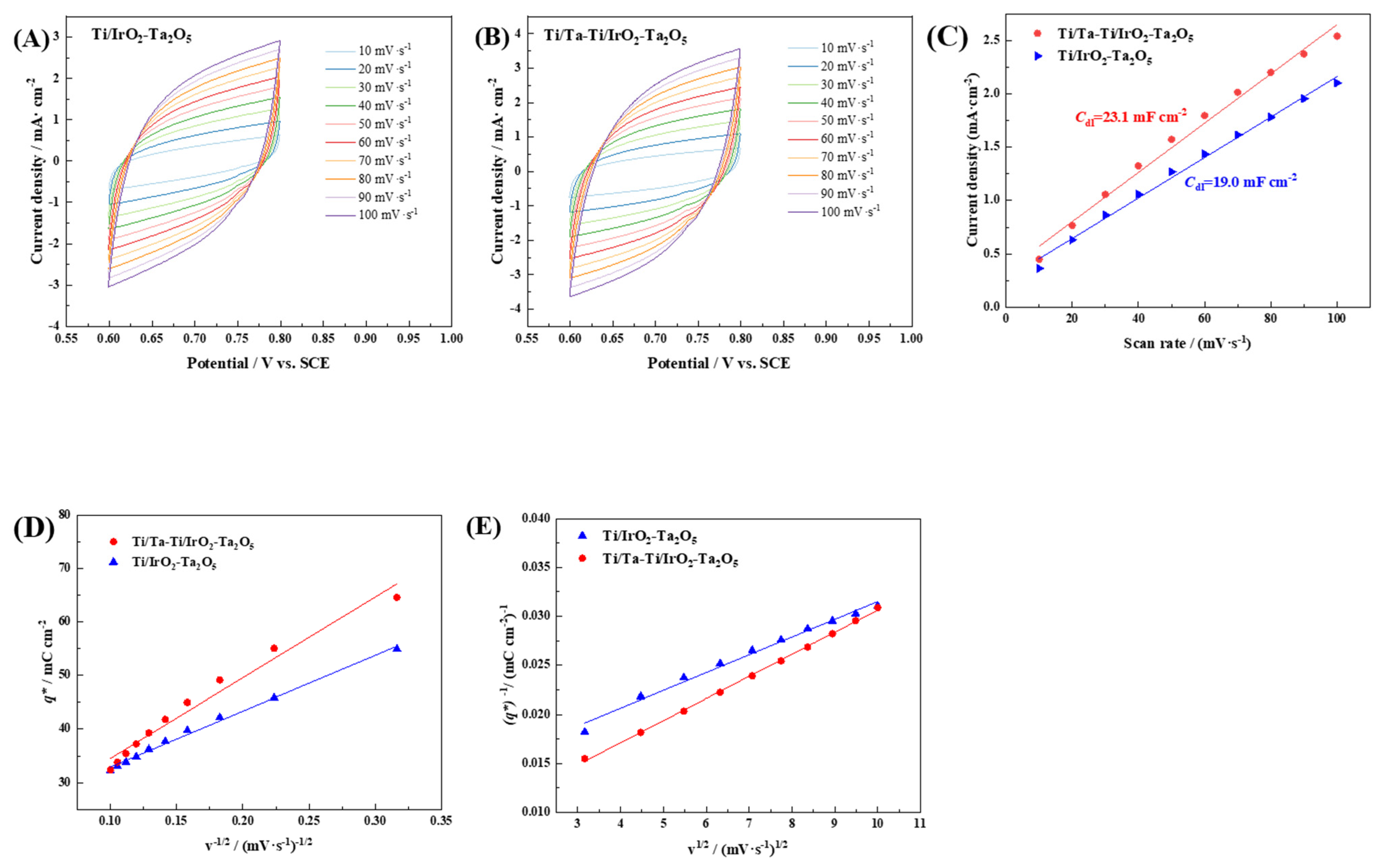
3.2. Electrocatalytic Activity
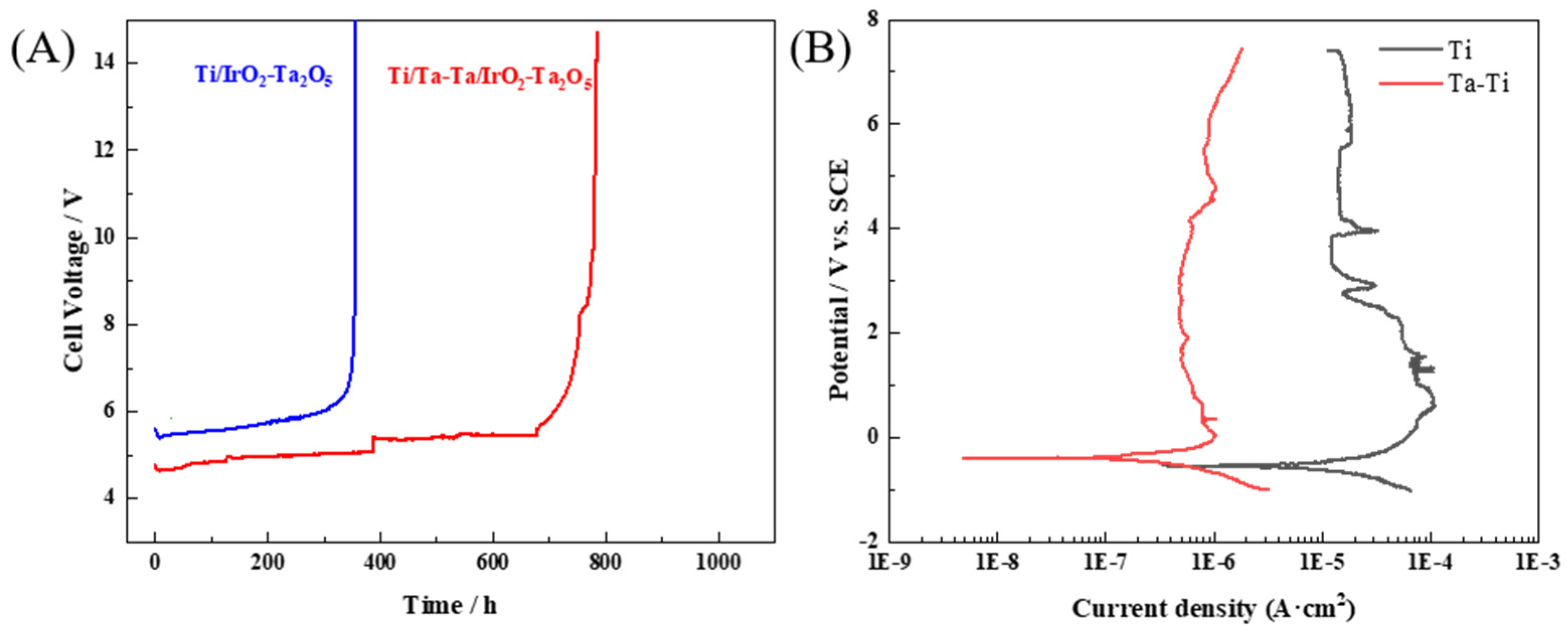
3.3. Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Q.; Liu, D.; Yuan, G.; Tang, Y.; Cui, K.; Jiang, S.; Xia, Y.; Xiong, W. Efficient degradation of phenolic wastewaters by a novel Ti/PbO2-Cr-PEDOT electrode with enhanced electrocatalytic activity and chemical stability. Sep. Purif. Technol. 2022, 281, 119735. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.M.; Li, J.; Meng, W.; Yang, C.; Sun, C.; Lan, X. Metal modified (Ni, Ce, Ta) Ti/SnO2-Sb2O5-RuO2 electrodes for enhanced electrochemical degradation of Orange G. J. Appl. Electrochem. 2022, 52, 573–581. [Google Scholar] [CrossRef]
- Yu, S.; Xie, Z.; Li, K.; Ding, L.; Wang, W.; Yang, G.; Zhang, F.-Y. Morphology engineering of iridium electrodes via modifying titanium substrates with controllable pillar structures for highly efficient oxygen evolution reaction. Electrochim. Acta 2022, 405, 139797. [Google Scholar] [CrossRef]
- Wang, W.; Wang, K.; Hao, W.; Zhang, T.; Liu, Y.; Yu, L.; Li, W. Preparation of Ti-based Yb-doped SnO2-RuO2 electrode and electrochemical oxidation treatment of coking wastewater. J. Rare Earth 2022, 40, 763–771. [Google Scholar] [CrossRef]
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biot. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Xu, L.K.; Scantlebury, J.D. Microstructure and electrochemical properties of IrO2-Ta2O5-coated titanium anodes. J. Electrochem. Soc. 2003, 150, B254. [Google Scholar] [CrossRef]
- Li, B.S.; Lin, A.; Gan, F.X. Preparation and electrocatalytic properties of Ti/IrO2-Ta2O5 anodes for oxygen evolution. T. Nonferr. Metal Soc. 2006, 16, 1193–1199. [Google Scholar] [CrossRef]
- Herrada, R.A.; Rodil, S.E.; Sepúlveda-Guzmán, S.; Manríquez, J.; Exner, K.S.; Bustos, E. Characterization of Ti electrodes electrophoretically coated with IrO2-Ta2O5 films with different Ir:Ta molar ratios. J. Alloy Compd. 2021, 862, 158015. [Google Scholar] [CrossRef]
- Yan, Z.; Li, G.; Wang, J.; Zhang, Z.; Feng, Z.; Tang, M.; Zhang, R. Electro-catalytic study of IrO2-Ta2O5 coated anodes with pretreated titanium substrates. J. Alloy Compd. 2016, 680, 60–66. [Google Scholar] [CrossRef]
- Xu, W.; Haarberg, G.M.; Seland, F.; Sunde, S.; Ratvik, A.P.; Holmin, S.; Gustavsson, J.; Afvander, Å.; Zimmerman, E.; Åkre, T. The durability of the thermally decomposed IrO2-Ta2O5 coated titanium anode in a sulfate solution. Corros. Sci. 2019, 150, 76–90. [Google Scholar] [CrossRef]
- Han, Z.; Zhu, P.; Xu, L.; Kannan, C.S.; Guo, S.; Liu, J.; Koppala, S.; Ju, S. Electrochemical properties of the IrO2-Ta2O5 coated anodes with Al/Ti and Cu/Ti layered composites substrates. J. Alloy Compd. 2018, 769, 210–217. [Google Scholar] [CrossRef]
- Huang, C.A.; Yang, S.W.; Chen, C.Z.; Hsu, F.-Y. Electrochemical behavior of IrO2-Ta2O5/Ti anodes prepared with different surface pretreatments of Ti substrate. Surf. Coat. Technol. 2017, 320, 270–278. [Google Scholar] [CrossRef]
- Comninellis, C.; Vercesi, G.P. Characterization of DSA®-type oxygen evolving electrodes: Choice of a Coating. J. Appl. Electrochem. 1991, 21, 335–345. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Xuan, J.; Xin, Y.; Li, Y.; Duan, T.; Liu, F. A comparative study on Ti/IrO2–Ta2O5 anodes prepared by microwave plasma-assisted sintering and conventional thermal decomposition methods. J. Mater. Res. Technol. 2023, 23, 1447–1457. [Google Scholar] [CrossRef]
- Xu, L.K.; Scantlebury, J.D. A study on the deactivation of an IrO2–Ta2O5 coated titanium anode. Corros. Sci. 2003, 45, 2729–2740. [Google Scholar] [CrossRef]
- Hu, J.M.; Meng, H.M.; Zhang, J.Q.; Cao, C.N. Degradation mechanism of long service life Ti/IrO2-Ta2O5 oxide anodes in sulphuric acid. Corros. Sci. 2002, 44, 1655–1668. [Google Scholar] [CrossRef]
- Chen, A.; Nigro, S. Influence of a nanoscale gold thin layer on Ti/SnO2-Sb2O5 electrodes. J. Phys. Chem. B 2003, 107, 13341–13348. [Google Scholar] [CrossRef]
- Tang, C.; Liu, Z.; Cui, D.; Yu, L.; Xue, J.; Yin, X. Enhancing the stability and electrocatalytic activity of Ti-based PbO2 anodes by introduction of an arc-sprayed TiN interlayer. Electrochim. Acta 2021, 399, 139398. [Google Scholar] [CrossRef]
- Li, X.; Yan, J.; Zhu, K.G. Effects of IrO2 interlayer on the electrochemical performance of Ti/Sb-SnO2 electrodes. J. Electroanal. Chem. 2020, 878, 114471. [Google Scholar] [CrossRef]
- Li, X.L.; Xu, H.; Yan, W. Electrochemical oxidation of aniline by a novel Ti/TiOxHy/Sb-SnO2 electrode. Chin. J. Catal. 2016, 37, 1860–1870. [Google Scholar] [CrossRef]
- Xu, H.B.; Lu, Y.H.; Li, C.H.; Hu, J.Z. A novel IrO2 electrode with iridium-titanium oxide interlayers from a mixture of TiN nanoparticle and H2IrCl6 solution. J. Appl. Electrochem. 2010, 40, 719–727. [Google Scholar] [CrossRef]
- Salgado, L.; Pozos, C.B.; Zayas, T.; Galicia, L. Preparation and characterization of Ti/SnO2-Sb electrodes without or with a platinum interlayer using the polymeric precursor method and thermal decomposition. Int. J. Electrochem. Sci. 2019, 14, 7281–7292. [Google Scholar] [CrossRef]
- Tang, C.B.; Zheng, C.; Yu, L.H.; Xue, J.Q. Effect of electroplating nickel interlayer on performance of Ti-based lead dioxide electrodes. Rare Metal. Mat. Eng. 2019, 48, 143–151. [Google Scholar]
- Piercy, B.; Allen, C.; Gullá, A.F. Ta and Ti anti-passivation interlayers for oxygen-evolving anodes produced by cold gas spray. J. Therm. Spray. Technol. 2015, 24, 702–710. [Google Scholar] [CrossRef]
- Jin, H.C.; Zhang, X.J.; Yu, Y.; Chen, X.M. High-performance Ti/IrO2-RhOx-TiO2/alpha-PbO2/beta-PbO2 electrodes for scale inhibitors degradation. Chem. Eng. J. 2022, 435, 135167. [Google Scholar] [CrossRef]
- Zanta, C.L.P.S.; Michaud, P.A.; Comninellis, C.; De Andrade, A.R.; Boodts, J.F.C. Electrochemical oxidation of p-chlorophenol on SnO2-Sb2O5 based anodes for wastewater treatment. J. Appl. Electrochem. 2003, 33, 1211–1215. [Google Scholar] [CrossRef]
- Mameda, N.; Park, H.; Shah, S.S.A.; Lee, K.; Li, C.W.; Naddeo, V.; Choo, K.H. Highly robust and efficient Ti-based Sb-SnO2 anode with a mixed carbon and nitrogen interlayer for electrochemical 1,4-dioxane removal from water. Chem. Eng. J. 2020, 393, 124794. [Google Scholar] [CrossRef]
- Cardarelli, F.; Taxil, P.; Savall, A.; Comninellis, C.; Manoli, G.; Leclerc, O. Preparation of oxygen evolving electrodes with long service life under extreme conditions. J. Appl. Electrochem. 1998, 28, 245–250. [Google Scholar] [CrossRef]
- Vercesi, G.P.; Rolewicz, J.; Comninellis, C.; Hinder, J. Characterization of dsa-type oxygen evolving electrodes. Choice Base Metal. Thermochim. Acta 1991, 176, 31–47. [Google Scholar] [CrossRef]
- Meng, F.; Li, Z.; Liu, X. Synthesis of tantalum thin films on titanium by plasma immersion ion implantation and deposition. Surf. Coat. Technol. 2013, 229, 205–209. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Xin, Y.; Liu, F.; Xuan, J.; Guo, M.; Duan, T. IrO2-Ta2O5 anode for oxygen evolution with TaOx interlayer prepared by thermal decomposition in inert atmosphere. J. Electrochem. Soc. 2022, 169, 6516. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, X.; Zhang, P.; Zhang, Y.; Zhang, G.; He, Z. Double glow plasma surface alloying and plasma nitriding. Surf. Coat. Technol. 2007, 201, 4822–4825. [Google Scholar] [CrossRef]
- Song, J.; Zhang, P.-Z.; Wei, D.-B.; Wei, X.-F.; Wang, Y. Isothermal oxidation behavior and microstructure of plasma surface Ta coating on γ-TiAl. Mater. Charact. 2014, 98, 54–59. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, P.; Wang, L.; Zhao, H.; Xu, Z. The role of process parameters in plasma surface chromising of Ti2AlNb-based alloys. Appl. Surf. Sci. 2009, 256, 1333–1340. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, H.; Wang, L.; Yang, X.; Pei, H.; Wei, Y.; Ren, J.; Huang, K.; Hu, L. Role of the double-glow plasma pre-sputtering in the growth mechanisms and metal–insulator transition of VO2 film. Appl. Surf. Sci. 2022, 603, 154043. [Google Scholar] [CrossRef]
- Wei, D.B.; Chen, X.H.; Zhang, P.Z.; Ding, F.; Li, F.K.; Yao, Z.J. Plasma surface tantalum alloying on titanium and its corrosion behavior in sulfuric acid and hydrochloric acid. Appl. Surf. Sci. 2018, 441, 448–457. [Google Scholar] [CrossRef]
- Xuan, W.; Zhang, P.; Huang, J. Corrosion resistance of tantalum modified layer on pure titanium surface in H2SO4 solution. Mater. Mech. Eng. 2010, 34, 50–53. [Google Scholar]
- Xu, L.; Xin, Y.; Wang, J. A comparative study on IrO2–Ta2O5 coated titanium electrodes prepared with different methods. Electrochim. Acta 2009, 54, 1820–1825. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, Y.; Zhang, Z.; Li, G.; Li, H.; Wang, J.; Feng, Z.; Tang, M.; Yuan, X.; Zhang, R.; et al. A study on the performance of IrO2–Ta2O5 coated anodes with surface treated Ti substrates. Electrochim. Acta 2015, 157, 345–350. [Google Scholar] [CrossRef]
- Kim, K.-W.; Lee, E.-H.; Kim, J.-S.; Shin, K.-H.; Kim, K.-H. Effect of an etching Ti substrate on a catalytic oxide electrode. J. Electrochem. Soc. 2001, 148, B111–B115. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Jiang, W.Q.; Dong, H.; Hu, X.Y.; Fang, B.H.; Gao, G.F.; Zhao, R. Study on the electrochemical removal mechanism of oxytetracycline by a Ti/IrO2-Ta2O5 Plate. Int. J. Environ. Res. Public Health 2021, 18, 1708. [Google Scholar] [CrossRef] [PubMed]
- Kodintsev, I.M.; Trasatti, S.; Rubel, M.; Wieckowski, A.; Kaufher, N. X-ray photoelectron spectroscopy and electrochemical surface characterization of iridium(IV) oxide + ruthenium(IV) oxide electrodes. Langmuir 1992, 8, 283–290. [Google Scholar] [CrossRef]
- Barksdale, J. Titanium, Its Occurrence, Chemistry, and Technology. Soil. Sci. 1950, 70, 414. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, Y.; Peng, M.; Qi, H. The interstitial diffusion behaviors and mechanisms of boron in α-Ti and β-Ti: A first-principles calculation. Comp. Mater. Sci. 2020, 184, 109866. [Google Scholar] [CrossRef]
- Murray, J.L. The Ta−Ti (Tantalum-Titanium) system. Bull. Alloy Phase Diagr. 1981, 2, 62–66. [Google Scholar] [CrossRef]
- Maykuth, D.J.; Ogden, H.R.; Jaffee, R.I. Titanium-tungsten and titanium-tantalum systems. Trans. Am. Inst. Min. Metall. Eng. 1953, 197, 231–237. [Google Scholar]
- Liu, Y.; Li, K.; Wu, H.; Song, M.; Wang, W.; Li, N.; Tang, H. Synthesis of Ti–Ta alloys with dual structure by incomplete diffusion between elemental powders. J. Mech. Behav. Biomed. 2015, 51, 302–312. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, L.-C.; Zhang, J.; Zhang, X.; Ni, Q.; Chen, Y.; Wu, M.; Yang, C. Distinction in corrosion resistance of selective laser melted Ti-6Al-4V alloy on different planes. Corros. Sci. 2016, 111, 703–710. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.Y.; Chen, Y.Q.; Ma, B.Z. Electrochemical behavior and corrosion mechanism of Ti/IrO2-RuO2 anodes in sulphuric acid solution. J. Electroanal. Chem. 2019, 837, 175–183. [Google Scholar] [CrossRef]
- Simpson, R.; White, R.G.; Watts, J.F.; Baker, M.A. XPS investigation of monatomic and cluster argon ion sputtering of tantalum pentoxide. Appl. Surf. Sci. 2017, 405, 79–87. [Google Scholar] [CrossRef]
- Wilks, J.A.; Kelber, J.A. Nitridation of organo-silicate glass: A self-limiting process for PVD Ta1+xN/Ta barrier formation. Appl. Surf. Sci. 2009, 255, 9543–9547. [Google Scholar] [CrossRef]
- Wilks, J.A.; Magtoto, N.P.; Kelber, J.A.; Arunachalam, V. Interfacial reactions during sputter deposition of Ta and TaN films on organosilicate glass: XPS and TEM results. Appl. Surf. Sci. 2007, 253, 6176–6184. [Google Scholar] [CrossRef]
- Manso, A.P.; Marzo, F.F.; Garicano, X.; Alegre, C.; Lozano, A.; Barreras, F. Corrosion behavior of tantalum coatings on AISI 316L stainless steel substrate for bipolar plates of PEM fuel cells. Int. J. Hydrogen Energy 2020, 45, 20679–20691. [Google Scholar] [CrossRef]
- McNamara, K.; Beloshapkin, S.; Hossain, K.M.; Dhoubhadel, M.S.; Tofail, S.A.M. Tantalum coating inhibits Ni-migration from titanium out-diffusion in NiTi shape memory biomedical alloy. Appl. Surf. Sci. 2021, 535, 147621. [Google Scholar] [CrossRef]
- Zhou, J.J.; Wang, T.; Cheng, C.; Pan, F.; Zhu, Y.Q.; Ma, H.R.; Niu, J.F. Ultralong-lifetime Ti/RuO2-IrO2@Pt anodes with a strong metal-support interaction for efficient electrochemical mineralization of perfluorooctanoic acid. Nanoscale 2022, 14, 3579–3588. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, M.; Tabaian, S.H.; Firoozi, S. Effect of IrO2 crystallinity on electrocatalytic behavior of IrO2-Ta2O5/MWCNT composite as anodes in chlor-alkali membrane cell. Ceram. Int. 2019, 45, 19971–19980. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L.; Cappelletti, G.; Ionita, M.; Minguzzi, A.; Rondinini, S.; Vertova, A. Composite ternary SnO2–IrO2–Ta2O5 oxide electrocatalysts. J. Electroanal. Chem. 2006, 589, 160–166. [Google Scholar] [CrossRef]
- Fan, Y.; Cheng, X. Porous IrO2-Ta2O5 coating modified with carbon nanotubes for oxygen evolution reaction. J. Electrochem. Soc. 2016, 163, E209. [Google Scholar] [CrossRef]
- Rolison, D.R.; Kuo, K.; Umana, M.; Brundage, D.; Murray, R.W. Properties of RuOx working electrodes in nonaqueous solvents. J. Electrochem. Soc. 1979, 126, 407. [Google Scholar] [CrossRef]
- De Battisti, A.; Lodi, G.; Cappadonia, M.; Battaglin, G.; Kötz, R. Influence of the valve metal oxide on the properties of ruthenium based mixed oxide electrodes: II. coatings. J. Electrochem. Soc. 1989, 136, 2596. [Google Scholar] [CrossRef]
- Duan, T.G.; Wen, Q.; Chen, Y.; Zhou, Y.D.; Duan, Y. Enhancing electrocatalytic performance of Sb-doped SnO2 electrode by compositing nitrogen-doped graphene nanosheets. J. Hazard. Mater. 2014, 280, 304–314. [Google Scholar] [CrossRef]
- Ltaief, A.H.; Sabatino, S.; Proietto, F.; Ammar, S.; Gadri, A.; Galia, A.; Scialdone, O. Electrochemical treatment of aqueous solutions of organic pollutants by electro-fenton with natural heterogeneous catalysts under pressure using Ti/IrO2-Ta2O5 or BDD anodes. Chemosphere 2018, 202, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Tahar, N.B.; Savall, A. A comparison of different lead dioxide coated electrodes for the electrochemical destruction of phenol. J. New Mater. Electrochem. Syst. 1999, 2, 19–26. [Google Scholar]


| Items | Parameters |
|---|---|
| Working temperature (°C) | 900 |
| Deposition time (h) | 2.5 |
| Working pressure (Pa) | 35 |
| Cathode (workpiece) voltage (V) | 400–450 |
| Cathode power (W) | 700–750 |
| Source voltage (V) | 900–950 |
| Source power (W) | 1100–1200 |
| Chamber gas | Ar2 |
| Gas flow rates (SCCM) | 35 |
| Sample | Ti | Ta | O | Ir | Cl |
|---|---|---|---|---|---|
| Ta-Ti interlayer | 1.59 | 92.83 | 5.58 | – | – |
| Ti/IrO2-Ta2O5 | 8.12 | 24.34 | 12.09 | 54.24 | 1.21 |
| Ti/Ta-Ti/IrO2-Ta2O5 | 2.12 | 31.16 | 12.39 | 53.19 | 1.14 |
| Sample | Ta | O | Ir |
|---|---|---|---|
| Ti/Ta-Ti interlayer before sputtering | 75.1 | 24.9 | – |
| Ti/Ta-Ti interlayer after sputtering | 94.1 | 5.9 | – |
| Ti/IrO2-Ta2O5 | 13.54 | 73 | 13.46 |
| Ti/Ta-Ti/IrO2-Ta2O5 | 15.75 | 71.91 | 12.34 |
| Electrode | Rs/Ω·cm2 | Rct/Ω·cm2 | Qdl/Ω−1·cm−2·Sn | n |
|---|---|---|---|---|
| Ti/IrO2-Ta2O5 | 8.1 ± 0.1 | 22.42 ± 1.54 | 0.013 ± 0.004 | 0.92 ± 0.01 |
| Ti/Ta-Ti/IrO2-Ta2O5 | 7.8 ± 0.1 | 5.26 ± 0.81 | 0.031 ± 0.003 | 0.91 ± 0.01 |
| Electrode | Cdl/mF·cm−2 | /mC·cm−2 | /mC·cm−2 | |
|---|---|---|---|---|
| Ti/Ta-Ti/IrO2-Ta2O5 | 23.1 ± 0.9 | 175.4 ± 5.7 | 22.5 ± 1.2 | 152.9 ± 4.8 |
| Ti/IrO2-Ta2O5 | 19.0 ± 0.5 | 123.2 ± 4.3 | 19.5 ± 0.8 | 103.7 ± 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Liu, Y.; Xin, Y.; Xu, L.; Xue, L.; Duan, T.; Zhao, R.; Xuan, J.; Li, L. Performance Enhancement of Ti/IrO2-Ta2O5 Anode through Introduction of Tantalum–Titanium Interlayer via Double-Glow Plasma Surface Alloying Technology. Nanomaterials 2024, 14, 1219. https://doi.org/10.3390/nano14141219
Guo M, Liu Y, Xin Y, Xu L, Xue L, Duan T, Zhao R, Xuan J, Li L. Performance Enhancement of Ti/IrO2-Ta2O5 Anode through Introduction of Tantalum–Titanium Interlayer via Double-Glow Plasma Surface Alloying Technology. Nanomaterials. 2024; 14(14):1219. https://doi.org/10.3390/nano14141219
Chicago/Turabian StyleGuo, Mingshuai, Yueren Liu, Yonglei Xin, Likun Xu, Lili Xue, Tigang Duan, Rongrong Zhao, Junji Xuan, and Li Li. 2024. "Performance Enhancement of Ti/IrO2-Ta2O5 Anode through Introduction of Tantalum–Titanium Interlayer via Double-Glow Plasma Surface Alloying Technology" Nanomaterials 14, no. 14: 1219. https://doi.org/10.3390/nano14141219
APA StyleGuo, M., Liu, Y., Xin, Y., Xu, L., Xue, L., Duan, T., Zhao, R., Xuan, J., & Li, L. (2024). Performance Enhancement of Ti/IrO2-Ta2O5 Anode through Introduction of Tantalum–Titanium Interlayer via Double-Glow Plasma Surface Alloying Technology. Nanomaterials, 14(14), 1219. https://doi.org/10.3390/nano14141219






