Thermal Energy Storage in Concrete by Encapsulation of a Nano-Additivated Phase Change Material in Lightweight Aggregates
Abstract
1. Introduction
2. Materials and Methods
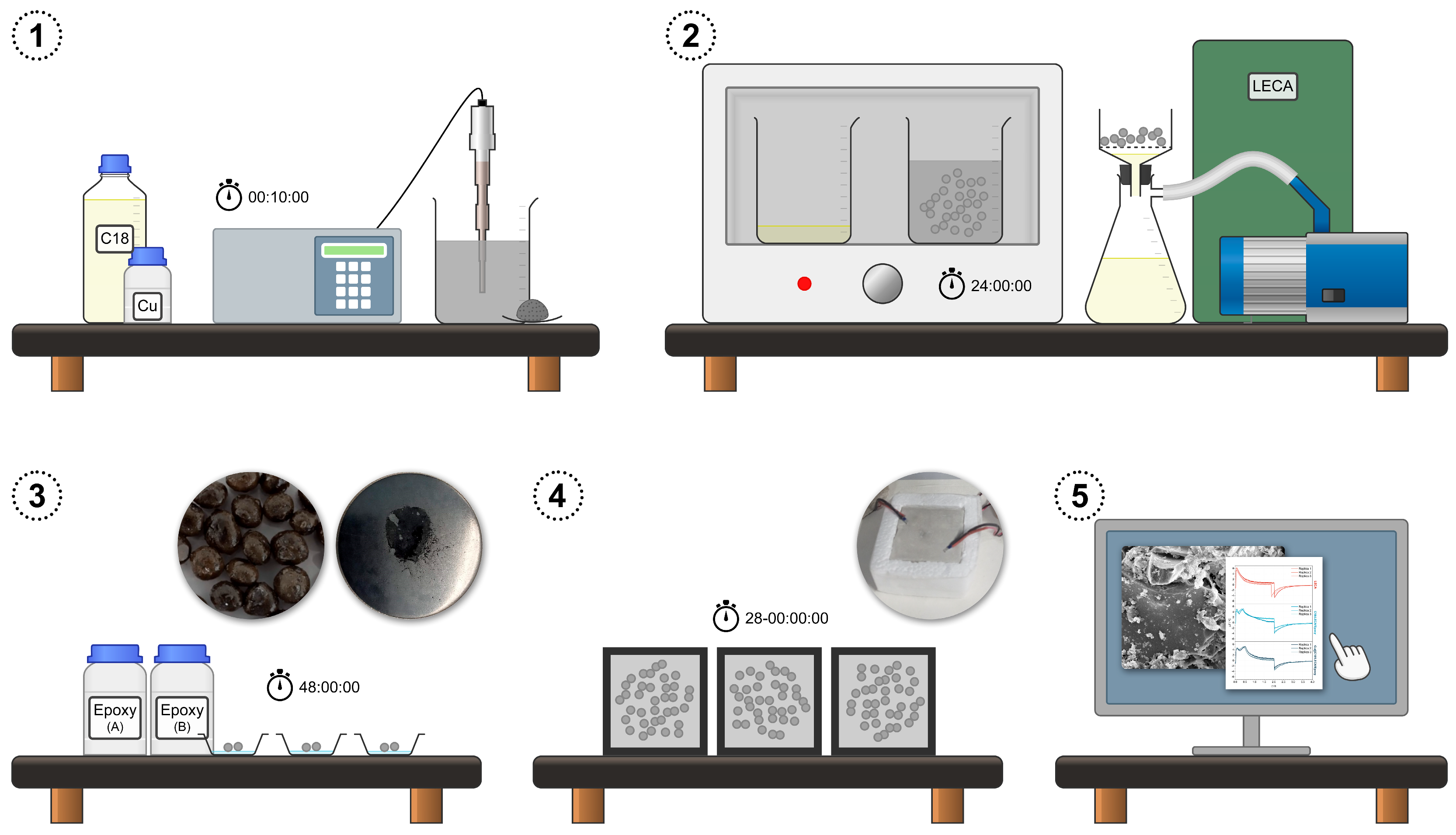
2.1. Preparation of Aggregate-Encapsulated PCM Samples
2.2. Characterization of the Microstructure, Composition, and Thermal Characteristics
2.3. Testing of the Thermal Response under Artificial Sunlight
3. Results and Discussion
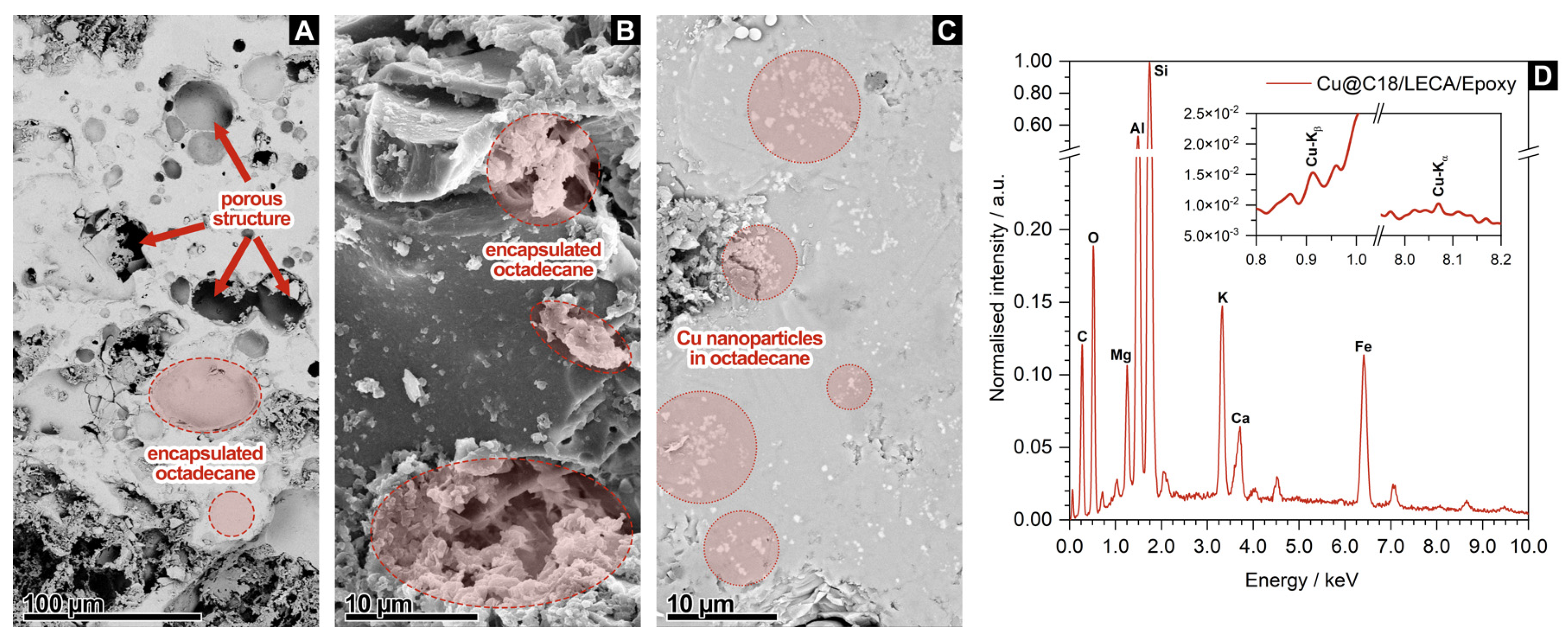
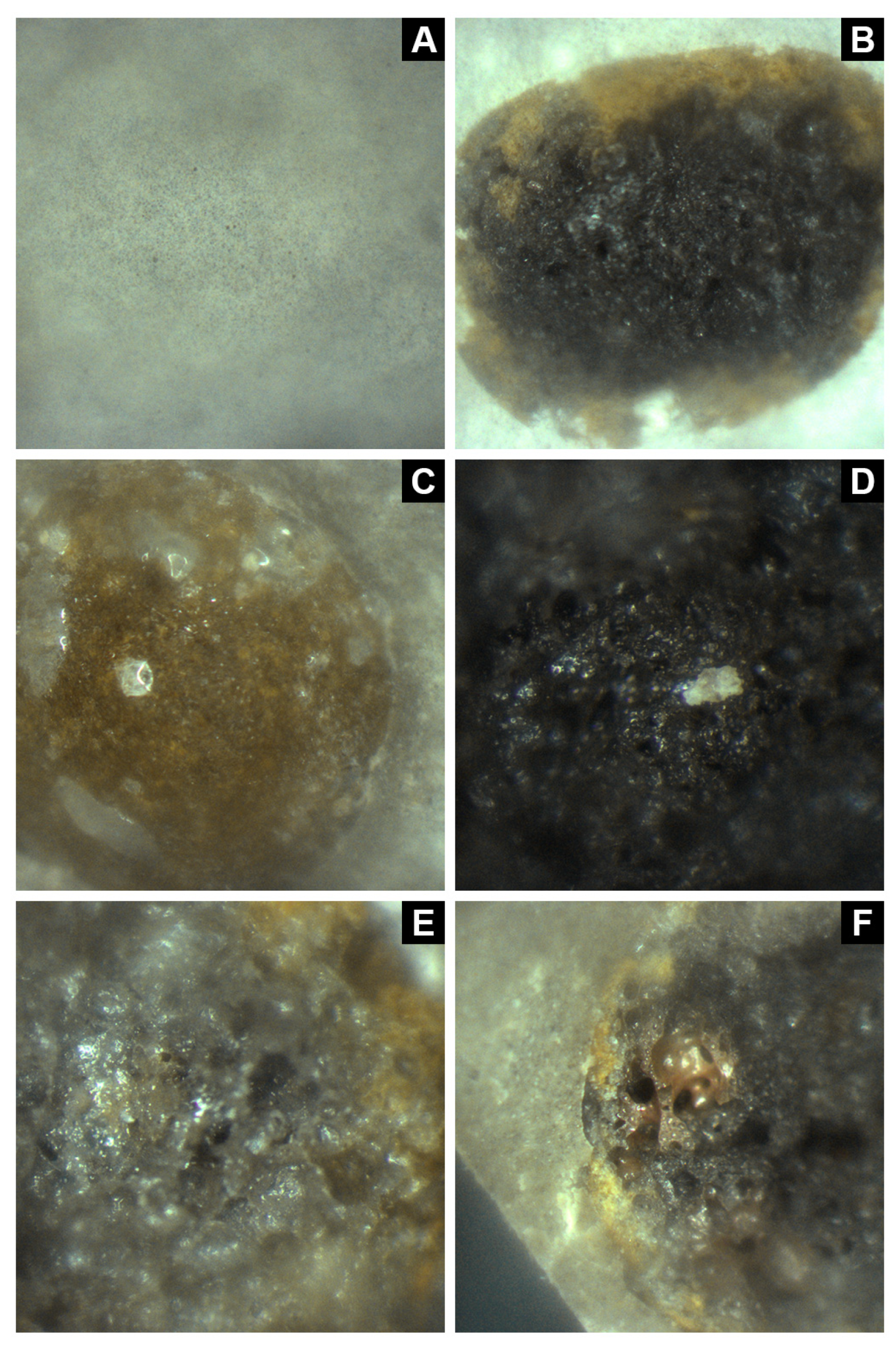
3.1. Core Microstructure and Composition of Aggregate-Encapsulated PCM Samples
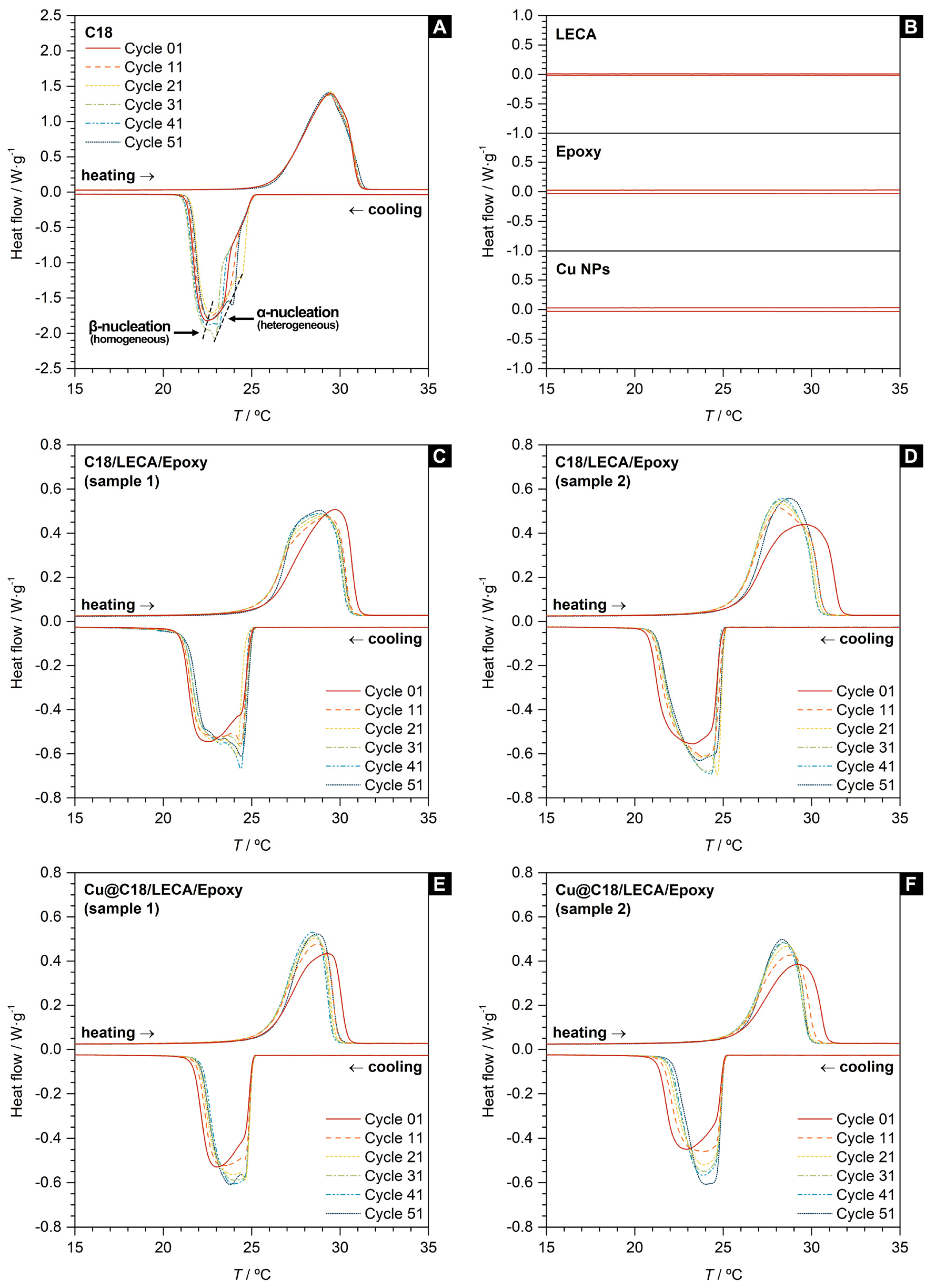
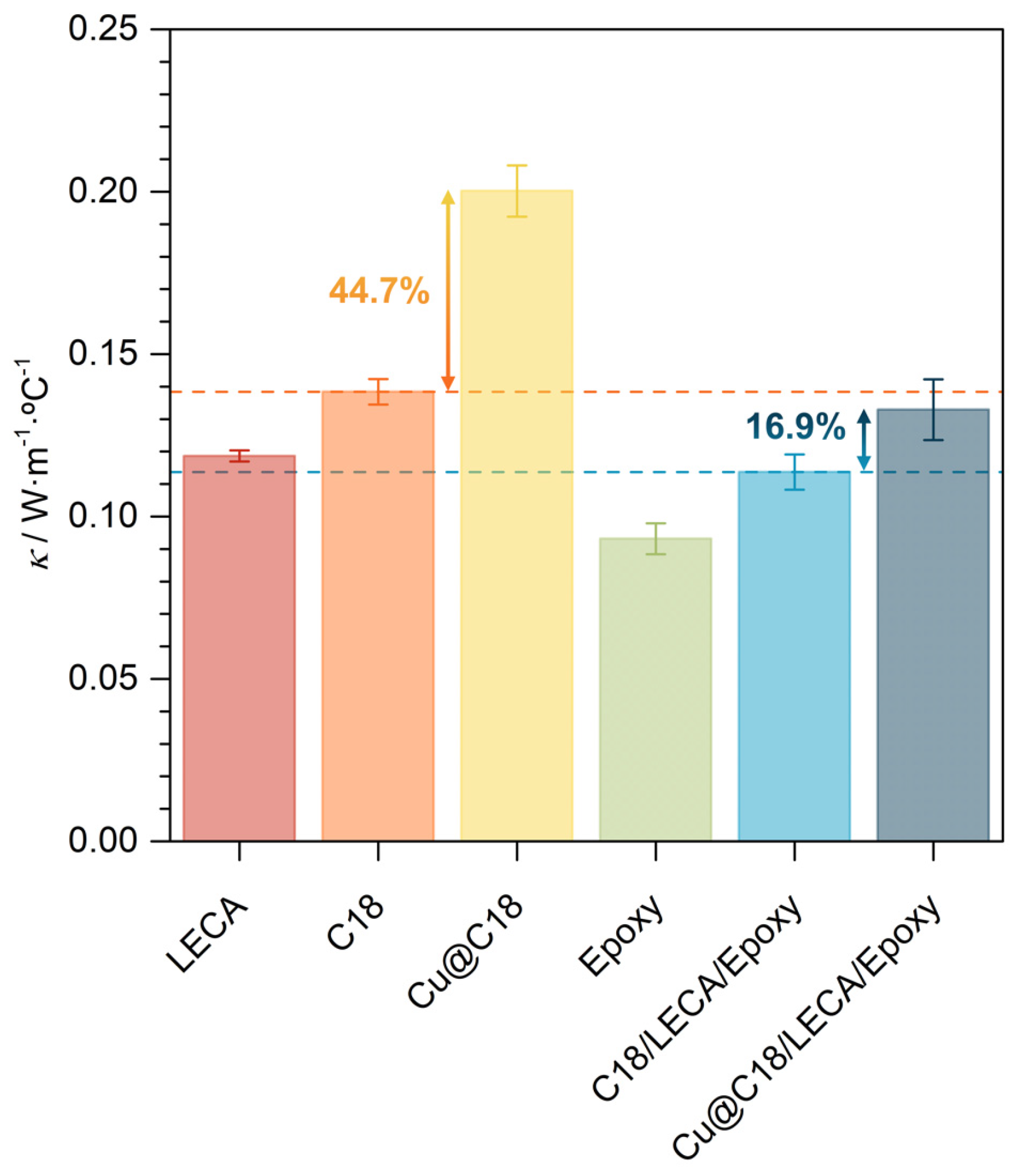
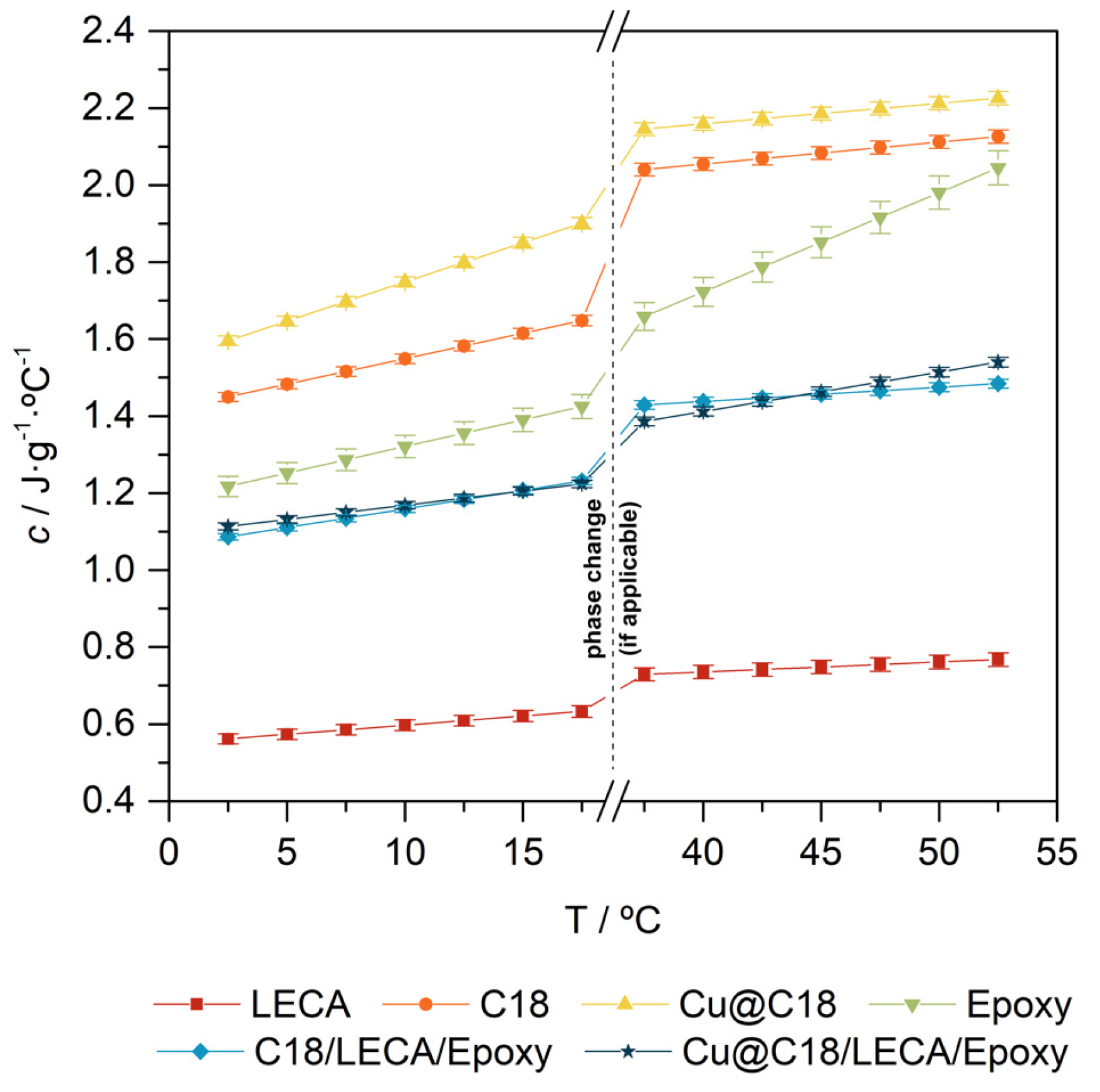
3.2. Phase Change and Thermal Properties of Aggregate-Encapsulated PCM Samples
3.3. Thermal Response of Concrete with Aggregate-Encapsulated PCM
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sust. Energ. Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Castellón, C.; Castell, A.; Medrano, M.; Martorell, I.; Cabeza, L.F. Experimental Study of PCM Inclusion in Different Building Envelopes. J. Sol. Energy Eng. 2009, 131, 041006. [Google Scholar] [CrossRef]
- Kant, K.; Shukla, A.; Sharma, A. Heat transfer studies of building brick containing phase change materials. Sol. Energy 2017, 155, 1233–1242. [Google Scholar] [CrossRef]
- Gupta, M.K.; Rathore, P.K.S.; Singh, A.K. Experimental investigation of clay brick with sensible, latent, and hybrid thermal energy storage in buildings. Mater. Today Proc. 2022, 69, 112–118. [Google Scholar] [CrossRef]
- Memon, S.A.; Cui, H.Z.; Zhang, H.; Xing, F. Utilization of macro encapsulated phase change materials for the development of thermal energy storage and structural lightweight aggregate concrete. Appl. Energy 2015, 139, 43–55. [Google Scholar] [CrossRef]
- Memon, S.A.; Cui, H.Z.; Lo, T.Y.; Li, Q. Development of structural–functional integrated concrete with macro-encapsulated PCM for thermal energy storage. Appl. Energy 2015, 150, 245–257. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Li, L.; Zhao, M. Experimental assessment on the use of phase change materials (PCMs)-bricks in the exterior wall of a full-scale room. Energy Convers. Manag. 2016, 120, 81–89. [Google Scholar] [CrossRef]
- Cunha, S.; Aguiar, I.; Aguiar, J.B. Phase change materials composite boards and mortars: Mixture design, physical, mechanical and thermal behavior. J. Energy Stor. 2022, 53, 105135. [Google Scholar] [CrossRef]
- Xin, H.; Li, D.; Yang, J.; Yang, Z.; Gong, Y.; Luo, W.; Zhou, W. Preparation and performance research of functional geopolymer filled with microencapsulated phase change materials enhanced by modified graphite. J. Build. Eng. 2022, 60, 105169. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Lin, K.P.; Yang, R.; Di, H.F.; Jiang, Y. Preparation, thermal performance and application of shape-stabilized PCM in energy efficient buildings. Energy Build. 2006, 38, 1262–1269. [Google Scholar] [CrossRef]
- Oliver, A. Thermal characterization of gypsum boards with PCM included: Thermal energy storage in buildings through latent heat. Energy Build. 2012, 48, 1–7. [Google Scholar] [CrossRef]
- Lee, K.O.; Medina, M.A.; Raith, E.; Sun, X. Assessing the integration of a thin phase change material (PCM) layer in a residential building wall for heat transfer reduction and management. Appl. Energy 2015, 137, 699–706. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sari, A.; Biçer, A. Thermal regulating performance of gypsum/(C18–C24) composite phase change material (CPCM) for building energy storage applications. Appl. Them. Eng. 2016, 107, 55–62. [Google Scholar] [CrossRef]
- Sari, A.; Biçer, A.; Karaipekli, A.; Al-Sulaiman, F.A. Preparation, characterization and thermal regulation performance of cement based-composite phase change material. Sol. Energ. Mat. Sol. Cells 2018, 174, 523–529. [Google Scholar] [CrossRef]
- Hattan, H.A.; Madhkhan, M.; Marani, A. Thermal and mechanical properties of building external walls plastered with cement mortar incorporating shape-stabilized phase change materials (SSPCMs). Constr. Build. Mater. 2021, 270, 121385. [Google Scholar] [CrossRef]
- Sari, A. Form-stable paraffin/high density polyethylene composites as solid–liquid phase change material for thermal energy storage: Preparation and thermal properties. Energy Convers. Manag. 2004, 45, 2033–2042. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Wu, D. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: Synthesis, microstructure, and performance evaluation. Appl. Energy 2014, 114, 632–643. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Zhang, W.; Zhao, J.; Fang, X.; Sun, J.; Xia, R.; Guo, H.; Liu, Y. Processing wood into a phase change material with high solar-thermal conversion efficiency by introducing stable polyethylene glycol-based energy storage polymer. Energy 2022, 254, 124206. [Google Scholar] [CrossRef]
- Simó-Solsona, M.; Palumbo, M.; Bosch, M.; Fernández, A.I. Why it’s so hard? Exploring social barriers for the deployment of thermal energy storage in Spanish buildings. Energy Res. Soc. Sci. 2021, 76, 102057. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; de Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sust. Energ. Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Parhizi, M.; Jain, A. The impact of thermal properties on performance of phase change based energy storage systems. Appl. Them. Eng. 2019, 162, 114154. [Google Scholar] [CrossRef]
- Stefan, J. Ueber die Theorie der Eisbildung, insbesondere über die Eisbildung im Polarmeere. Ann. Phys. 1891, 278, 269–286. [Google Scholar] [CrossRef]
- Hahn, D.W.; Özisik, M.N. Heat Conduction, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bergman, T.L.; Lavine, A.S.; Incropera, F.P.; Dewitt, D.P. Fundamentals of Heat and Mass Transfer, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Liu, Z.; Yu, Z.J.; Yang, T.; Qin, D.; Li, S.; Zhang, G.; Haghighat, F.; Joybari, M.M. A review on macro-encapsulated phase change material for building envelope applications. Build. Environ. 2018, 144, 281–294. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castellón, C.; Nogués, M.; Medrano, M.; Leppers, R.; Zubillaga, O. Use of microencapsulated PCM in concrete walls for energy savings. Energy Build. 2007, 39, 113–119. [Google Scholar] [CrossRef]
- Marani, A.; Nehdi, M.L. Integrating phase change materials in construction materials: Critical review. Constr. Build. Mater. 2019, 217, 36–49. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Luo, Z.; Wang, K.; Shah, S.P. A critical review on phase change materials (PCM) for sustainable and energy efficient building: Design, characteristic, performance and application. Energy Build. 2022, 260, 111923. [Google Scholar] [CrossRef]
- Sheng, N.; Zhu, C.; Saito, G.; Hiraki, T.; Haka, M.; Hasegawa, Y.; Sakai, H.; Akiyama, T.; Nomura, T. Development of a microencapsulated Al–Si phase change material with high-temperature thermal stability and durability over 3000 cycles. J. Mater. Chem. A 2018, 6, 18143–18153. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol–gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interf. Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Chan, Z.; Li, H. Synthesis and properties of microencapsulated paraffin composites with SiO2 shell as thermal energy storage materials. Chem. Eng. J. 2010, 163, 154–159. [Google Scholar] [CrossRef]
- Liu, L.; Miao, X.; Cheng, X.; Wang, H.; Guo, M.; Cheng, F.; Zhang, M. Preparation and characterization of ZnO/SiO2@n-octadecane nanocapsule for ultraviolet absorbing and photothermal conversion energy storage. J. Energy Stor. 2022, 54, 105363. [Google Scholar] [CrossRef]
- Bui, T.H.; Nguyen, C.T. Enhanced Thermal Energy Storage of n-Octadecane-Impregnated Mesoporous Silica as a Novel Shape-Stabilized Phase Change Material. ACS Omega 2022, 7, 12222–12230. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Liu, C.; Li, L.; Tian, W.; Yang, Y.; Liu, Y. Carbon-negative heat-stored limestone calcined clay cement mortar containing form-stable phase change materials. J. Clean. Prod. 2024, 437, 140703. [Google Scholar] [CrossRef]
- Topçu, İ.B.; Bayram, M.; Ustaoğlu, A.; Hekimoğlu, G.; Erdoğmuş, E.; Sarı, A.; Gencel, O.; Ozbakkaloglu, T. Innovative cementitious mortar incorporated with sepiolite based shape-stable phase change material for thermal controlling of buildings. Constr. Build. Mater. 2024, 426, 136124. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, X.; Gao, H.; Zhang, X.; Tang, Z.; Li, A.; Wang, G. Different dimensional nanoadditives for thermal conductivity enhancement of phase change materials: Fundamentals and applications. Nano Energy 2021, 85, 105948. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, R.; Peng, F.; Fang, Y.; Akiyama, T.; Wang, S. Synthesis, characterization and thermal properties of paraffin microcapsules modified with nano-Al2O3. Appl. Energy 2015, 137, 731–737. [Google Scholar] [CrossRef]
- Zhan, W.; Zhao, Y.; Yuan, Y.; Yi, H.; Song, S. Development of 2D-Mt/SA/AgNPs microencapsulation phase change materials for solar energy storage with enhancement of thermal conductivity and latent heat capacity. Sol. Energy Mater. Sol. Cells 2019, 201, 110090. [Google Scholar] [CrossRef]
- Zhang, L.; An, L.; Wang, Y.; Lee, A.; Schuman, Y.; Ural, A.; Fleischer, A.S.; Feng, G. Thermal enhancement and shape stabilization of a phase-change energy-storage material via copper nanowire aerogel. Chem. Eng. J. 2019, 373, 857–869. [Google Scholar] [CrossRef]
- Ji, H.; Sellan, D.P.; Pettes, M.T.; Kong, X.; Ji, J.; Shi, L.; Ruoff, R.S. Enhanced thermal conductivity of phase change materials with ultrathin-graphite foams for thermal energy storage. Energy Environ. Sci. 2014, 7, 1185–1192. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Ali, H.M.; Khushnood, S. Recent advances on thermal conductivity enhancement of phase change materials for energy storage system: A review. Int. J. Heat Mass Transfer 2018, 127, 838–856. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.-M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Al-Absi, Z.A.; Hafizal, M.I.M.; Ismail, M. Innovative PCM-incorporated foamed concrete panels for walls’ exterior cladding: An experimental assessment in real-weather conditions. Energy Build. 2023, 288, 113003. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, G.; Wu, G.; Liu, S.; Gao, L. Thermally adaptive walls for buildings applications: A state of the art review. Energy 2022, 271, 112314. [Google Scholar] [CrossRef]
- Torres, A.; Brandt, J.; Lear, K.; Liu, J. A looming tragedy of the sand commons. Science 2017, 357, 970–971. [Google Scholar] [CrossRef] [PubMed]
- Tittarelli, F.; Carsana, M.; Ruello, M.L. Effect of hydrophobic admixture and recycled aggregate on physical–mechanical properties and durability aspects of no-fines concrete. Constr. Build. Mater. 2014, 66, 30–37. [Google Scholar] [CrossRef]
- Günther, E.; Hiebler, S.; Mehling, H.; Redlich, R. Enthalpy of Phase Change Materials as a Function of Temperature: Required Accuracy and Suitable Measurement Methods. Int. J. Thermophys. 2009, 30, 1257–1269. [Google Scholar] [CrossRef]
- Fortuniak, W.; Slomkowski, S.; Chojnowski, J.; Kurjata, J.; Tracz, A.; Mizerska, U. Synthesis of a paraffin phase change material microencapsulated in a siloxane polymer. Colloid Polym. Sci. 2013, 291, 725–733. [Google Scholar] [CrossRef] [PubMed]
- UNE EN 197-1:2011; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. AENOR: Madrid, Spain, 2011.
- Deslattes, R.D.; Kessler, E.G.; Indelicato, P.; de Billy, L.; Lindroth, E.; Anton, J.; Coursey, J.S.; Schwab, D.J.; Chang, J.; Sukumar, R.; et al. X-ray Transition Energies Database; NIST, Physical Measurment Laboratory: Gaithersburg, MD, USA, 2005. [CrossRef]
- Rashad, A.M. Lightweight expanded clay aggregate as a building material—An overview. Constr. Build. Mater. 2018, 170, 757–775. [Google Scholar] [CrossRef]
- Faden, M.; Höhlein, S.; Wanner, J.; König-Haagen, A.; Brüggemann, D. Review of Thermophysical Property Data of Octadecane for Phase-Change Studies. Materials 2019, 12, 2974. [Google Scholar] [CrossRef]
- Liu, X.; Rao, Z. Thermal diffusion and phase transition of n-octadecane as thermal energy storage material on nanoscale copper surface: A molecular dynamics study. J. Energy Inst. 2019, 92, 161–176. [Google Scholar] [CrossRef]
- Warrier, P.; Teja, A. Effect of particle size on the thermal conductivity of nanofluids containing metallic nanoparticles. Nanoscale Res. Lett. 2011, 6, 247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; Cheng, Y.; Zhang, Y. A molecular dynamics study on thermal and mechanical properties of graphene–paraffin nanocomposites. RSC Adv. 2015, 5, 82638–82644. [Google Scholar] [CrossRef]
- Xiong, S.; Qi, W.; Cheng, Y.; Huang, B.; Wang, M.; Li, Y. Universal relation for size dependent thermodynamic properties of metallic nanoparticles. Phys. Chem. Chem. Phys. 2011, 13, 10652–10660. [Google Scholar] [CrossRef] [PubMed]

| Aggregate Type | Cement | Aggregate | LECA | PCM | Epoxy |
|---|---|---|---|---|---|
| LECA only | 90.0 wt.% | 10.0 wt.% | 10.0 wt.% | 0.0 wt.% | 0.0 wt.% |
| C18/LECA/Epoxy | 84.0 wt.% | 16.0 wt.% | 7.7 wt.% | 7.5 wt.% | 0.7 wt.% |
| Cu@C18/LECA/Epoxy | 84.8 wt.% | 15.2 wt.% | 7.0 wt.% | 7.5 wt.% * | 0.7 wt.% |
| Sample | PCM wt. | ΔHm/J·g−1 | −ΔHc/J·g−1 | EE | Tm/°C | Tc/°C | ΔT*/°C | Tc,α/°C | Tc,β/°C |
|---|---|---|---|---|---|---|---|---|---|
| Bulk C18 | -- | 239.3 ± 0.4 | 240.9 ± 0.5 | -- | 26.2 ± 0.1 | 25.2 ± 0.1 | 1.0 ± 0.1 | 23.7 ± 0.7 | 22.5 ± 0.1 |
| C18/LECA/Epoxy | 38.9% | 98.8 ± 0.2 | 98.1 ± 0.2 | 41.0% | 25.8 ± 0.2 | 25.1 ± 0.1 | 0.7 ± 0.2 | 24.5 ± 0.1 | 22.9 ± 0.2 |
| 43.8% | 105.4 ± 0.7 | 105.4 ± 0.6 | 43.9% | 25.8 ± 0.2 | 25.2 ± 0.1 | 0.6 ± 0.2 | 24.5 ± 0.1 | 23.6 ± 0.2 | |
| Cu@C18/LECA/Epoxy | 33.2% | 80.1 ± 0.3 | 77.9 ± 0.4 | 32.7% | 25.6 ± 0.1 | 25.2 ± 0.1 | 0.4 ± 0.1 | 24.2 ± 0.1 | |
| 30.3% | 74.2 ± 0.2 | 73.7 ± 0.2 | 30.8% | 25.9 ± 0.1 | 25.2 ± 0.1 | 0.7 ± 0.1 | 24.2 ± 0.1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-Berdugo, I.; Gallardo, J.J.; Ruiz-Marín, N.; Guillén-Domínguez, V.; Alcántara, R.; Navas, J.; Poce-Fatou, J.A. Thermal Energy Storage in Concrete by Encapsulation of a Nano-Additivated Phase Change Material in Lightweight Aggregates. Nanomaterials 2024, 14, 1180. https://doi.org/10.3390/nano14141180
Carrillo-Berdugo I, Gallardo JJ, Ruiz-Marín N, Guillén-Domínguez V, Alcántara R, Navas J, Poce-Fatou JA. Thermal Energy Storage in Concrete by Encapsulation of a Nano-Additivated Phase Change Material in Lightweight Aggregates. Nanomaterials. 2024; 14(14):1180. https://doi.org/10.3390/nano14141180
Chicago/Turabian StyleCarrillo-Berdugo, Iván, Juan Jesús Gallardo, Nazaret Ruiz-Marín, Violeta Guillén-Domínguez, Rodrigo Alcántara, Javier Navas, and Juan Antonio Poce-Fatou. 2024. "Thermal Energy Storage in Concrete by Encapsulation of a Nano-Additivated Phase Change Material in Lightweight Aggregates" Nanomaterials 14, no. 14: 1180. https://doi.org/10.3390/nano14141180
APA StyleCarrillo-Berdugo, I., Gallardo, J. J., Ruiz-Marín, N., Guillén-Domínguez, V., Alcántara, R., Navas, J., & Poce-Fatou, J. A. (2024). Thermal Energy Storage in Concrete by Encapsulation of a Nano-Additivated Phase Change Material in Lightweight Aggregates. Nanomaterials, 14(14), 1180. https://doi.org/10.3390/nano14141180










