Effects of Cation Exchange in Rhodamine B Photocatalytic Degradation Using Peroxo-Titanate Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Peroxo-Titanium Complex (PTC) Ion Precursors
2.2. Synthesis of Layered Peroxo-Titanate Nanotube (PTNT)
2.3. Characterization of PTNT Samples
2.4. Photocatalytic Degradation Study of PTNT Samples
2.5. Kinetic Study of Rh B Photodegradation Test
3. Results and Discussion
3.1. Crystallographic Properties of PTNT Samples
3.2. Morphological Properties of the PTNT Samples
3.3. Photocatalytic Degradation of Rhodamine B
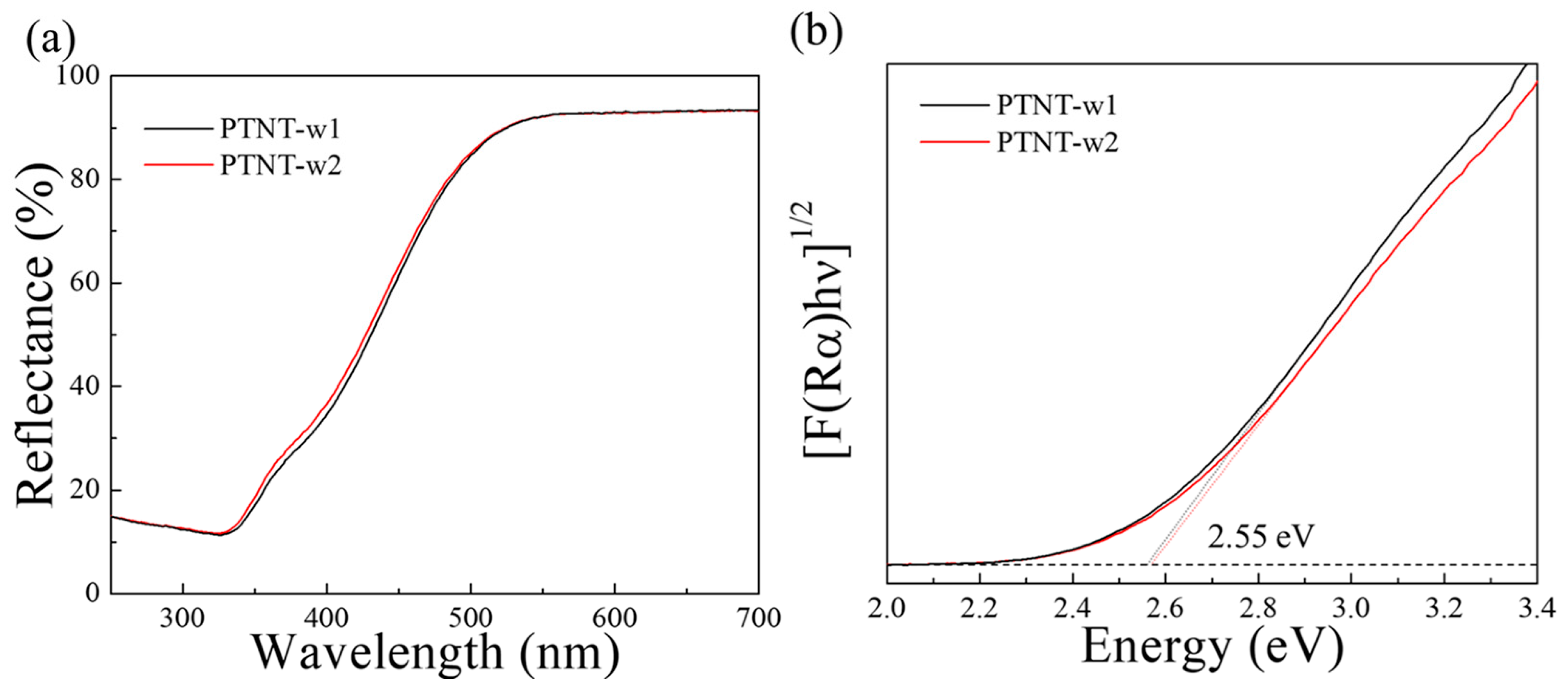
3.3.1. Effects of Sodium Ion Contents on Photocatalytic Properties
3.3.2. Effect of Cation Exchange on Rh B Solution
3.4. Methods for Minimizing the Influence of Cation Exchange
3.4.1. Minimizing Sodium Ion Contents in PTNT Samples
3.4.2. Controlling the pH of the Rhodamine B Solution
3.5. Kinetic Study of Rh B Photodegradation
3.6. Effects of Cation Exchange in TC Photodegradation Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Xiong, Z.; Ma, J.; Ng, W.J.; Waite, T.D.; Zhao, X.S. Silver-Modified Mesoporous TiO2 Photocatalyst for Water Purification. Water Res. 2011, 45, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Sweet, C.W.; Vermette, S.J. Toxic Volatile Organic Compounds in Urban Air in Illinois. Environ. Sci. Technol. 1992, 26, 165–173. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.S. Role of Titanium Dioxide (TiO2) Structural Design/Morphology in Photocatalytic Air Purification. Appl. Catal. B 2020, 269, 118735. [Google Scholar] [CrossRef]
- Lei, X.; Li, X.; Ruan, Z.; Zhang, T.; Pan, F.; Li, Q.; Xia, D.; Fu, J. Adsorption-Photocatalytic Degradation of Dye Pollutant in Water by Graphite Oxide Grafted Titanate Nanotubes. J. Mol. Liq. 2018, 266, 122–131. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Ljubas, D.; Mužina, K.; Bačić, I.; Radošević, T.; Podlogar, M.; Bdikin, I.; Otero-Irurueta, G.; Hortigüela, M.J.; et al. Graphene-Based TiO2 Nanocomposite for Photocatalytic Degradation of Dyes in Aqueous Solution under Solar-like Radiation. Appl. Sci. 2021, 11, 3966. [Google Scholar] [CrossRef]
- Mohamed Mukthar Ali, S.M.Y.; Sandhya, K.Y. A Novel Approach for P25-Carbon Dot Composite and the Reactive Oxygen Species Involved in the Visible Light Photocatalytic Mineralization of Rhodamine B. ChemistrySelect 2017, 2, 11840–11845. [Google Scholar] [CrossRef]
- Saothayanun, T.K.; Sirinakorn, T.T.; Ogawa, M. Layered Alkali Titanates (A2TinO2n+1): Possible Uses for Energy/Environment Issues. Front. Energy 2021, 15, 631–655. [Google Scholar] [CrossRef]
- Wang, Q.; Sohn, J.H.; Chung, J.S. Thermally Stable Pt/K2Ti2O5 as High-Temperature NOx Storage and Reduction Catalyst. Appl. Catal. B 2009, 89, 97–103. [Google Scholar] [CrossRef]
- Zhao, B.; Lin, L.; He, D. Phase and Morphological Transitions of Titania/Titanate Nanostructures from an Acid to an Alkali Hydrothermal Environment. J. Mater. Chem. A Mater. 2013, 1, 1659–1668. [Google Scholar] [CrossRef]
- Shirpour, M.; Cabana, J.; Doeff, M. Lepidocrocite-Type Layered Titanate Structures: New Lithium and Sodium Ion Intercalation Anode Materials. Chem. Mater. 2014, 26, 2502–2512. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, S.H.; Lim, S.K.; Kim, S. Effects of Ion Exchange and Calcinations on the Structure and Photocatalytic Activity of Hydrothermally Prepared Titanate Nanotubes. Cryst. Res. Technol. 2012, 47, 1190–1194. [Google Scholar] [CrossRef]
- Kang, S.; Durand-Vidal, S.; Badot, J.C.; Legein, C.; Body, M.; Borkiewicz, O.J.; Dubrunfaut, O.; Dambournet, D. Intercalation–Exfoliation Processes during Ionic Exchange Reactions from Sodium Lepidocrocite-Type Titanate toward a Proton-Based Trititanate Structure. Phys. Chem. Chem. Phys. 2021, 23, 10498–10508. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, L.; Li, G.; Liu, Y.; Withers, R.L. Atomic-Scale Control of TiO6 Octahedra through Solution Chemistry towards Giant Dielectric Response. Sci. Rep. 2014, 4, 6582. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Kanungo, M.; Hemraj-Benny, T.; Wong, S.S. Synthesis and Growth Mechanism of Titanate and Titania One-Dimensional Nanostructures Self-Assembled into Hollow Micrometer-Scale Spherical Aggregates. J. Phys. Chem. B 2005, 110, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Barim, G.; Dhall, R.; Arca, E.; Kuykendall, T.R.; Yin, W.; Takeuchi, K.J.; Takeuchi, E.S.; Marschilok, A.C.; Doeff, M.M. Heterostructured Lepidocrocite Titanate-Carbon Nanosheets for Electrochemical Applications. ACS Appl. Nano Mater. 2022, 5, 678–690. [Google Scholar] [CrossRef]
- Qamar, M.; Yoon, C.R.; Oh, H.J.; Lee, N.H.; Park, K.; Kim, D.H.; Lee, K.S.; Lee, W.J.; Kim, S.J. Preparation and Photocatalytic Activity of Nanotubes Obtained from Titanium Dioxide. Catal. Today 2008, 131, 3–14. [Google Scholar] [CrossRef]
- Yu, H.; Yu, J.; Cheng, B.; Lin, J. Synthesis, Characterization and Photocatalytic Activity of Mesoporous Titania Nanorod/Titanate Nanotube Composites. J. Hazard. Mater. 2007, 147, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kwak, S.Y. The Hydrothermal Synthesis of Mesoporous TiO2 with High Crystallinity, Thermal Stability, Large Surface Area, and Enhanced Photocatalytic Activity. Appl. Catal. A Gen. 2007, 323, 110–118. [Google Scholar] [CrossRef]
- Guohui, T.; Honggang, F.; Liqiang, J.; Baifu, X.; Kai, P. Preparation and Characterization of Stable Biphase TiO2 Photocatalyst with High Crystallinity, Large Surface Area, and Enhanced Photoactivity. J. Phys. Chem. C 2008, 112, 3083–3308. [Google Scholar]
- Goto, T.; Cho, S.H.; Lee, S.W.; Sekino, T. Sorption Capacity of Cs+ on Titania Nanotubes Synthesized by Solution Processing. J. Ceram. Soc. Jpn. 2018, 126, 801–807. [Google Scholar] [CrossRef]
- Goto, T.; Kondo, Y.; Cho, S.H.; Seino, S.; Sekino, T. Comparative Study of Divalent Cation Sorption on Titania Nanotubes Using Co2+, Ni2+, Zn2+, and Sr2+. Chem. Eng. J. Adv. 2022, 12, 100388. [Google Scholar] [CrossRef]
- Qianqian, Z.; Tang, B.; Guoxin, H. High Photoactive and Visible-Light Responsive Graphene/Titanate Nanotubes Photocatalysts: Preparation and Characterization. J. Hazard. Mater. 2011, 198, 78–86. [Google Scholar] [CrossRef]
- Doong, R.A.; Liao, C.Y. Enhanced Photocatalytic Activity of Cu-Deposited N-TiO2/Titanate Nanotubes under UV and Visible Light Irradiations. Sep. Purif. Technol. 2017, 179, 403–411. [Google Scholar] [CrossRef]
- Park, H.; Goto, T.; Han, D.H.; Cho, S.; Nishida, H.; Sekino, T. Low Alkali Bottom-Up Synthesis of Titanate Nanotubes Using a Peroxo Titanium Complex Ion Precursor for Photocatalysis. ACS Appl. Nano Mater. 2020, 3, 7795–7803. [Google Scholar] [CrossRef]
- Park, H.; Goto, T.; Cho, S.; Nishida, H.; Sekino, T. Enhancing Visible Light Absorption of Yellow-Colored Peroxo-Titanate Nanotubes Prepared Using Peroxo Titanium Complex Ions. ACS Omega 2020, 5, 21753–21761. [Google Scholar] [CrossRef] [PubMed]
- Lorençon, E.; Brandão, F.D.; Krambrock, K.; Alves, D.C.B.; Silva, J.C.C.; Ferlauto, A.S.; Lago, R.M. Generation of Reactive Oxygen Species in Titanates Nanotubes Induced by Hydrogen Peroxide and Their Application in Catalytic Degradation of Methylene Blue Dye. J. Mol. Catal. A Chem. 2014, 394, 316–323. [Google Scholar] [CrossRef]
- Erjavec, B.; Kaplan, R.; Pintar, A. Effects of Heat and Peroxide Treatment on Photocatalytic Activity of Titanate Nanotubes. Catal. Today 2015, 241, 15–24. [Google Scholar] [CrossRef]
- Kinsinger, N.M.; Dudchenko, A.; Wong, A.; Kisailus, D. Synergistic Effect of pH and Phase in a Nanocrystalline Titania Photocatalyst. ACS Appl. Mater. Interfaces 2013, 5, 6247–6254. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, J.; Hidaka, H. Highly Selective Deethylation of Rhodamine B: Adsorption and Photooxidation Pathways of the Dye on the TiO2/SiO2 Composite Photocatalyst. Int. J. Photoenergy 2003, 5, 209–217. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Wang, C.; Wang, P. Kinetics of Heterogeneous Photocatalytic Degradation of Rhodamine B by TiO2-Coated Activated Carbon: Roles of TiO2 Content and Light Intensity. Desalination 2011, 266, 40–45. [Google Scholar] [CrossRef]
- Sharma, M.; Mandal, M.K.; Pandey, S.; Kumar, R.; Dubey, K.K. Visible-Light-Driven Photocatalytic Degradation of Tetracycline Using Heterostructured Cu2O-TiO2 Nanotubes, Kinetics, and Toxicity Evaluation of Degraded Products on Cell Lines. ACS Omega 2022, 7, 33572–33586. [Google Scholar] [CrossRef] [PubMed]
- Yaemsunthorn, K.; Tatarchuk, T.; Danyliuk, N.; Shyichuk, A.; Macyk, W. Yellow TiO2 from Titanium Peroxo Complexes: Verification of the Visible Light Activity and a Rational Enhancement of Its Photocatalytic Efficiency. J. Environ. Chem. Eng. 2023, 11, 111520. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Adsorption of Rhodamine B Dye from Aqueous Solution on Irvingia Gabonensis Biomass: Kinetics and Thermodynamics Studies. S. Afr. J. Chem. 2015, 68, 115–125. [Google Scholar] [CrossRef]
- Zamouche, M.; Hamdaoui, O. Sorption of Rhodamine B by Cedar Cone: Effect of pH and Ionic Strength. Energy Procedia 2012, 18, 1228–1239. [Google Scholar] [CrossRef]










| Sample | 2thata (Degree) | d200 (Å) | Chemical Composition |
|---|---|---|---|
| PTNT-w1 | 9.3 | 9.5 | Na0.5H1.5Ti2O5 |
| PTNT-w2 | 9.6 | 9.2 | Na0.2H1.8Ti2O5 |
| Sample | Specific Surface Area (m2/g) |
|---|---|
| PTNT-w1 | 140.90 |
| PTNT-w2 | 130.01 |
| Sample | Specific Surface Area (m2/g) | Chemical Composition |
|---|---|---|
| PTNT-w3 | 145.49 | Na0.1H1.9Ti2O5 |
| Sample | Irradiation Light | k (s−1) | R-Squared Value |
|---|---|---|---|
| PTNT-w1 | UV | 6.47 × 10−5 | 0.99 |
| Vis | 1.13 × 10−5 | 0.96 | |
| PTNT-w2 | UV | 9.80 × 10−5 | 0.98 |
| Vis | 3.54 × 10−5 | 0.98 | |
| PTNT-w3 | UV | 1.06 × 10−5 | 0.95 |
| Vis | 2.04 × 10−5 | 0.99 | |
| PTNT-w2 + HCl | UV | 2.19 × 10−4 | 0.99 |
| Vis | 3.20 × 10−4 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.H.; Park, H.; Goto, T.; Cho, S.; Seo, Y.; Kondo, Y.; Nishida, H.; Sekino, T. Effects of Cation Exchange in Rhodamine B Photocatalytic Degradation Using Peroxo-Titanate Nanotubes. Nanomaterials 2024, 14, 1170. https://doi.org/10.3390/nano14141170
Han DH, Park H, Goto T, Cho S, Seo Y, Kondo Y, Nishida H, Sekino T. Effects of Cation Exchange in Rhodamine B Photocatalytic Degradation Using Peroxo-Titanate Nanotubes. Nanomaterials. 2024; 14(14):1170. https://doi.org/10.3390/nano14141170
Chicago/Turabian StyleHan, Do Hyung, Hyunsu Park, Tomoyo Goto, Sunghun Cho, Yeongjun Seo, Yoshifumi Kondo, Hisataka Nishida, and Tohru Sekino. 2024. "Effects of Cation Exchange in Rhodamine B Photocatalytic Degradation Using Peroxo-Titanate Nanotubes" Nanomaterials 14, no. 14: 1170. https://doi.org/10.3390/nano14141170
APA StyleHan, D. H., Park, H., Goto, T., Cho, S., Seo, Y., Kondo, Y., Nishida, H., & Sekino, T. (2024). Effects of Cation Exchange in Rhodamine B Photocatalytic Degradation Using Peroxo-Titanate Nanotubes. Nanomaterials, 14(14), 1170. https://doi.org/10.3390/nano14141170






