Analysis of Fluorescent Carbon Nanodot Formation during Pretzel Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
3. Results
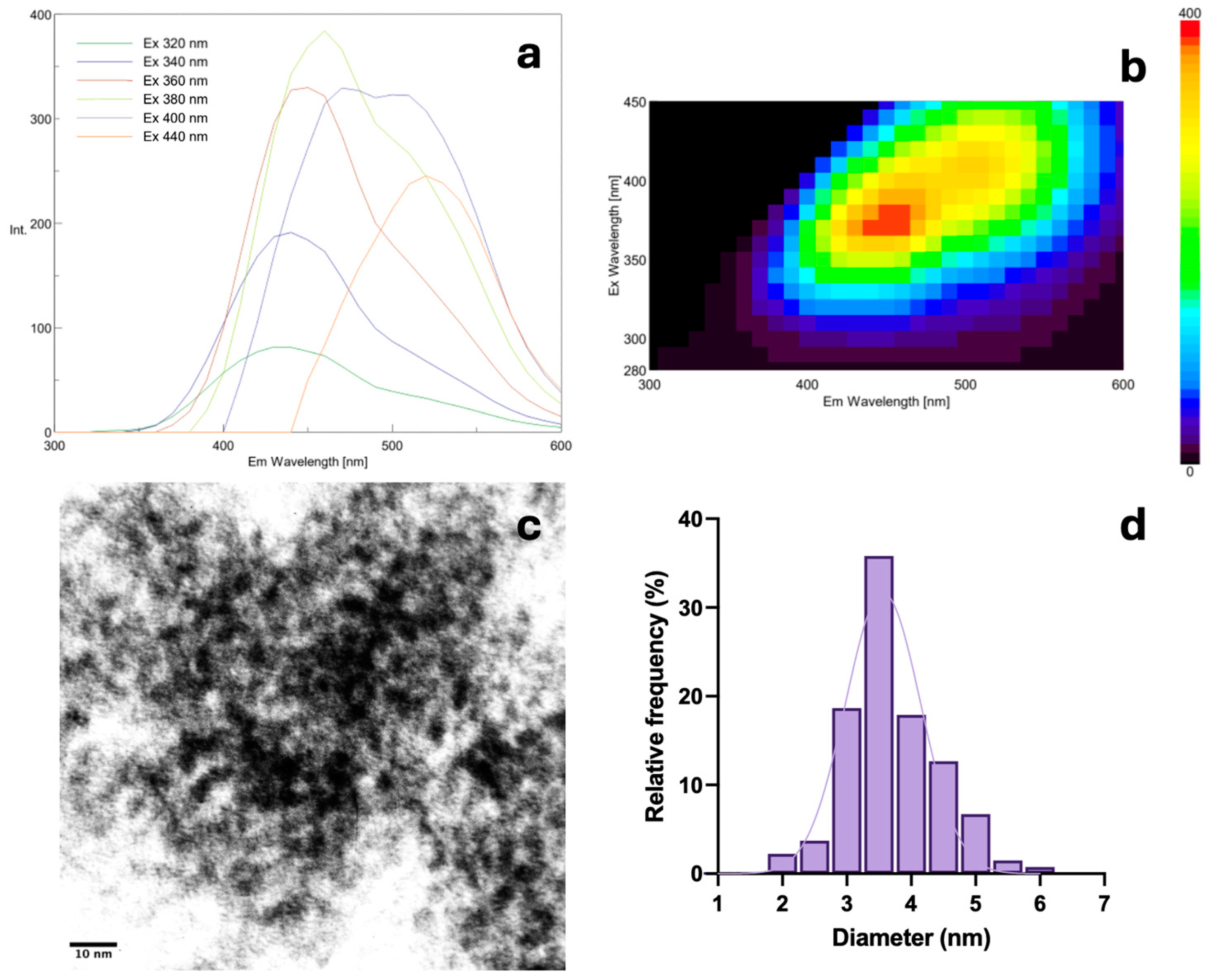
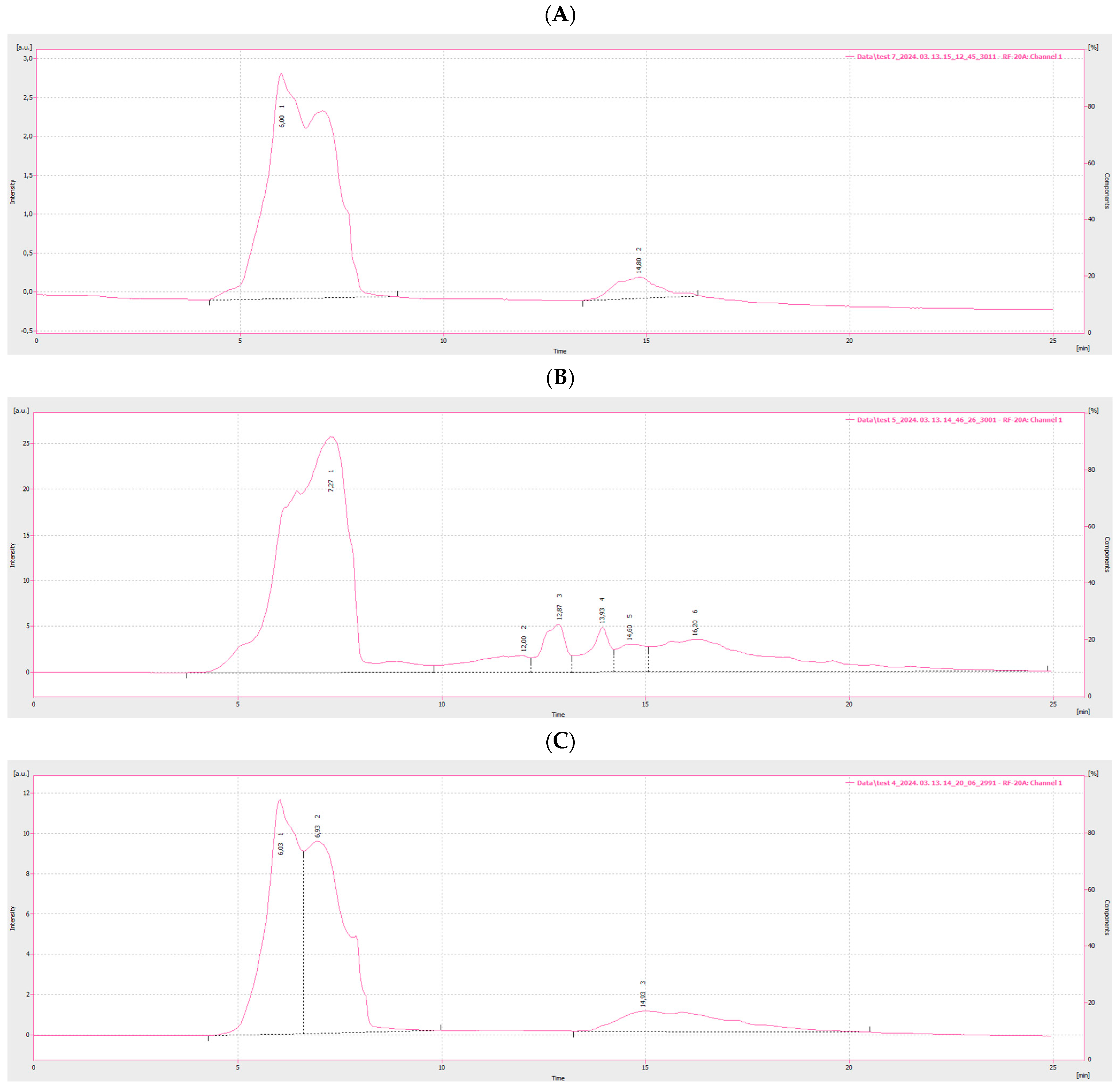
3.1. Characterization of Carbon Nanodots from Bakery Products
3.2. Detection of CNDs in Pretzel Production
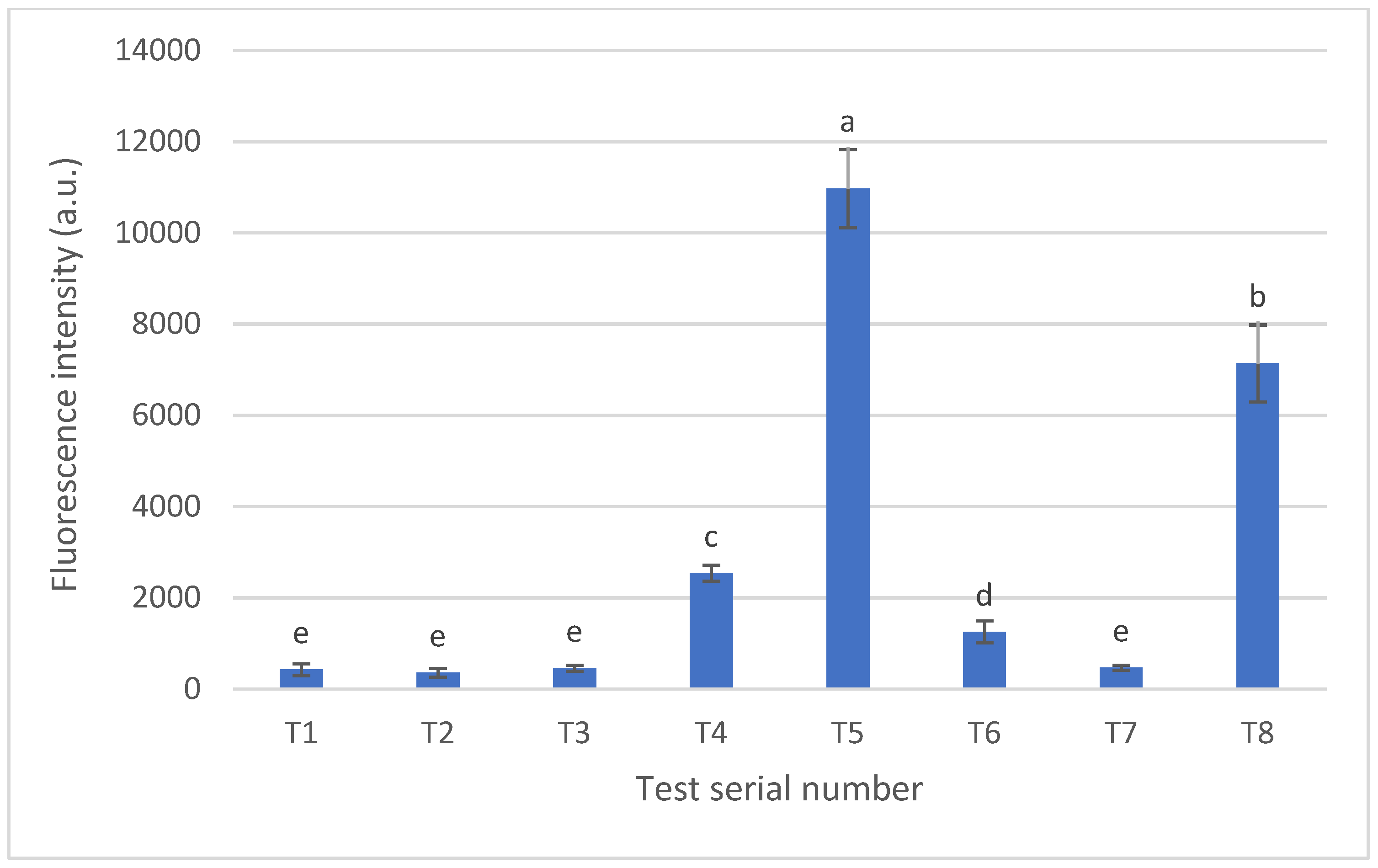
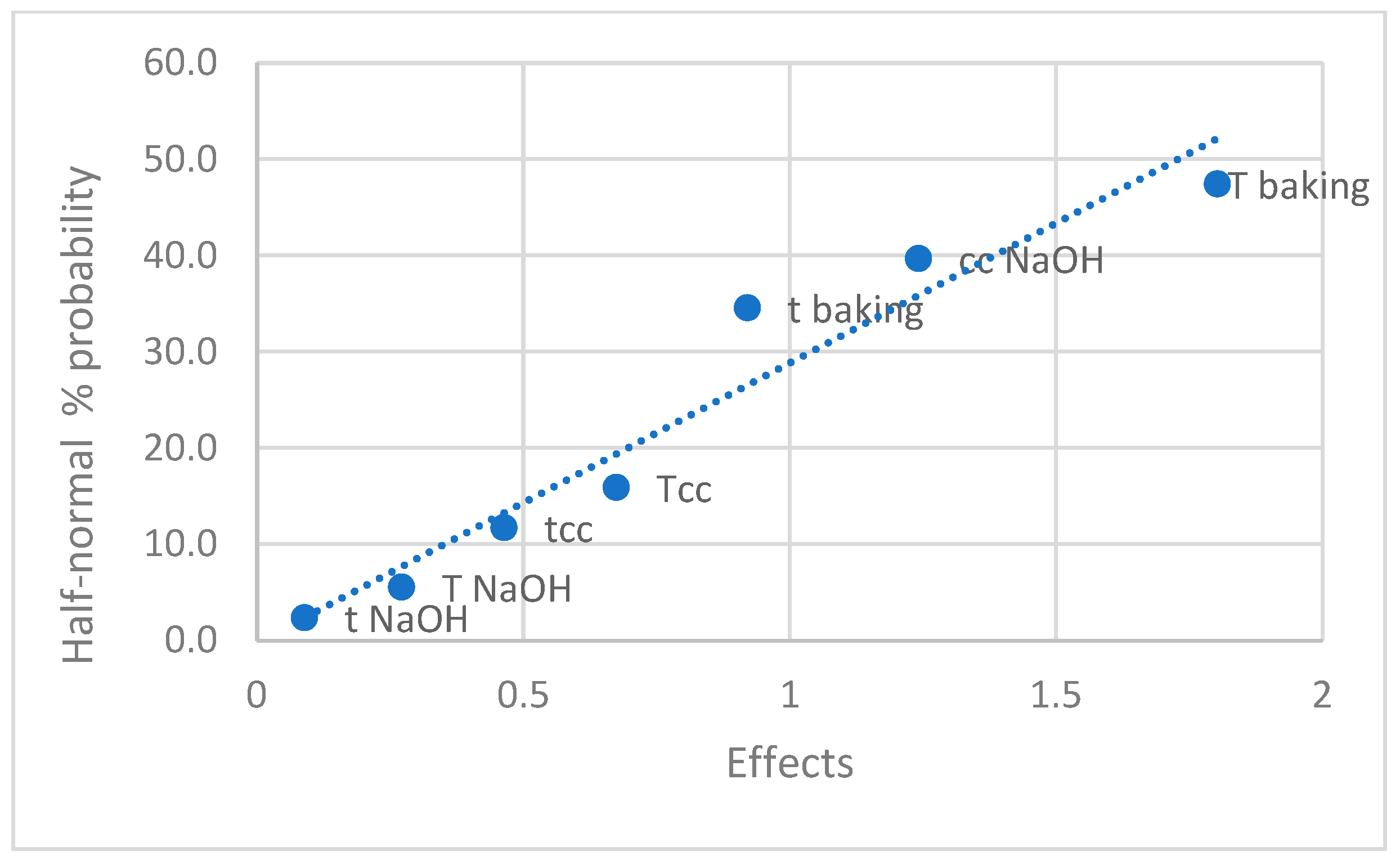
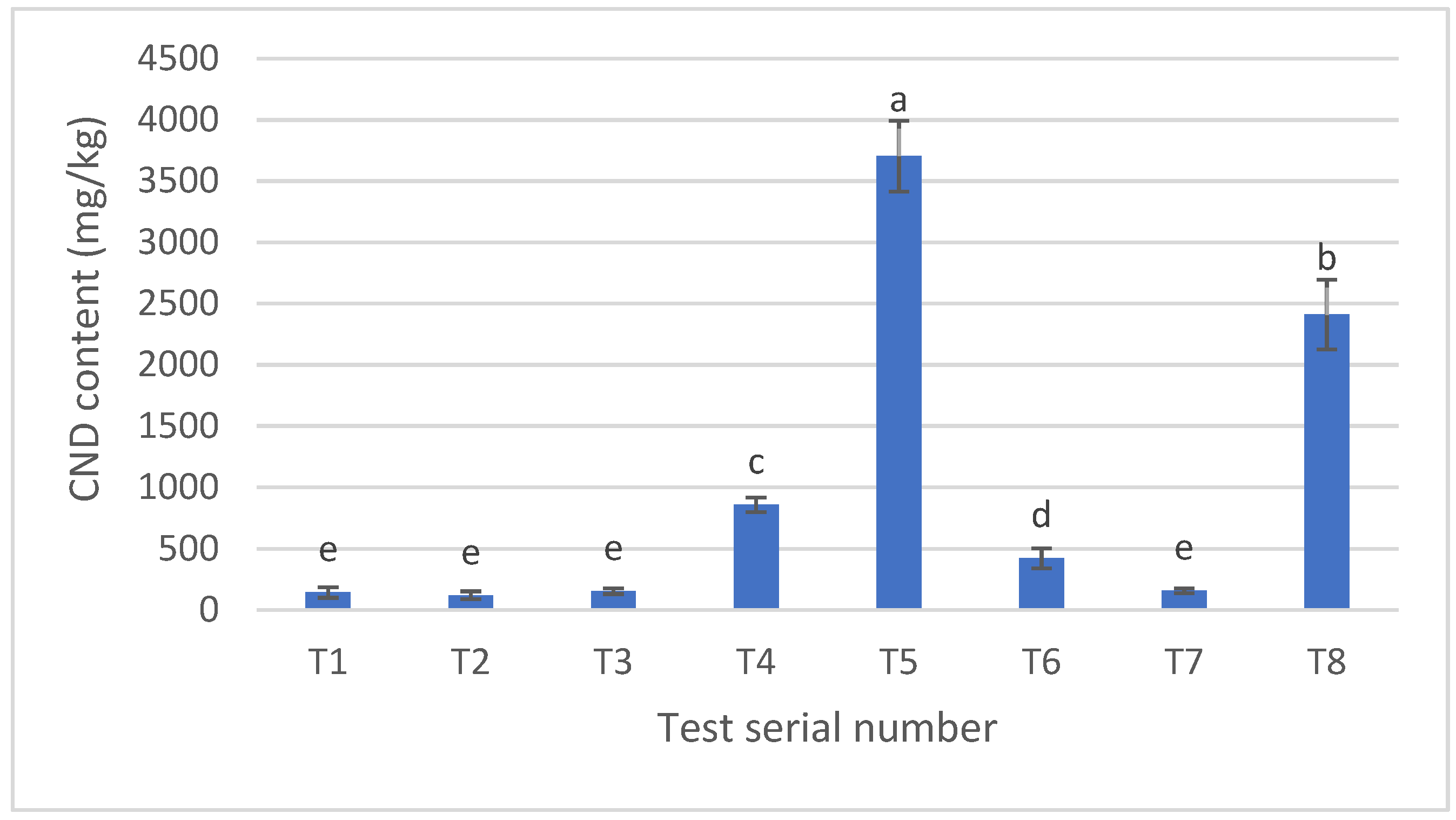
3.3. Carbon Nanodot Concentration (mg/kg) in Pretzel Production
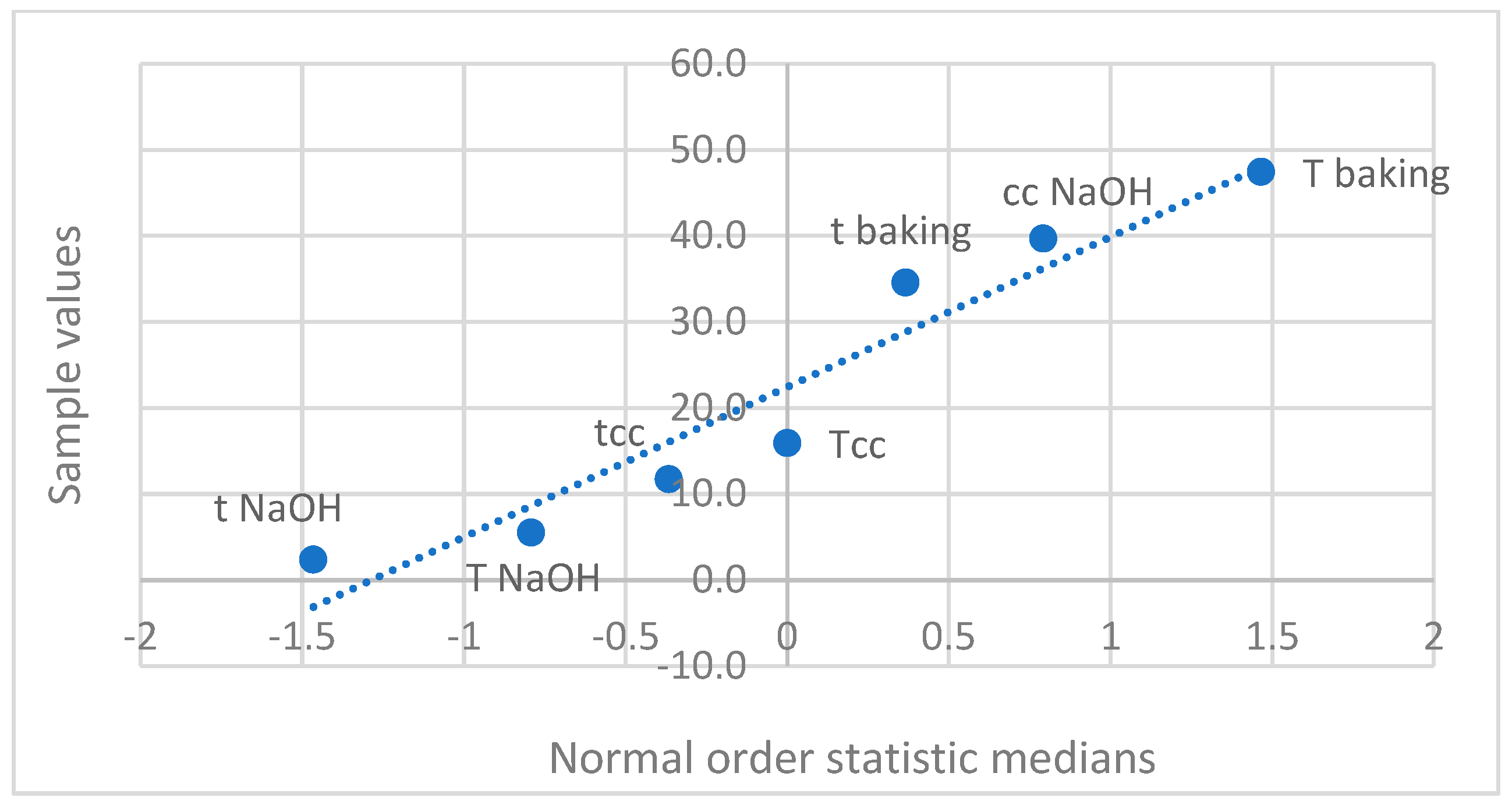
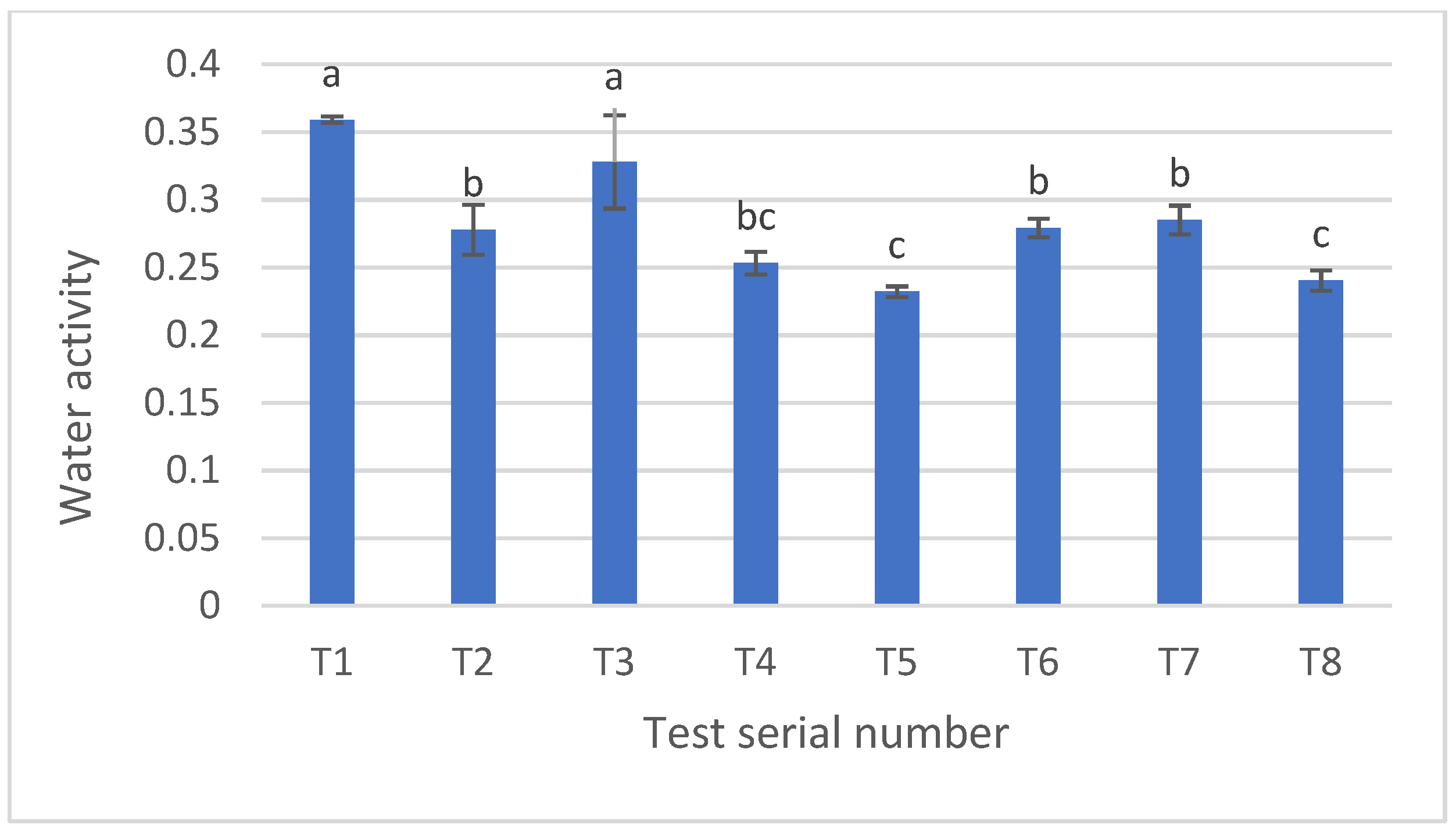
3.4. Water Activity in Baked Pretzels
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bredariol, P.; Vanin, F.M. Bread Baking Review: Insight into Technological Aspects in Order to Preserve Nutrition. Food Rev. Int. 2022, 38, 651–668. [Google Scholar] [CrossRef]
- Parry, R.T. Principles and Applications of Modified Atmosphere Packaging of Foods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Nachi, I.; Fhoula, I.; Smida, I.; Ben Taher, I.; Chouaibi, M.; Jaunbergs, J.; Bartkevics, V.; Hassouna, M. Assessment of Lactic Acid Bacteria Application for the Reduction of Acrylamide Formation in Bread. LWT 2018, 92, 435–441. [Google Scholar] [CrossRef]
- Galal, W.; Abdel-Haleem, A.; Seleem, H. Some Technological Studies on Pretzel. J. Food Dairy Sci. 2007, 32, 7411–7416. [Google Scholar] [CrossRef]
- Hui, Y.H.; Corke, H.; Leyn, I.D.; Nip, W.-K.; Cross, N.A. Bakery Products: Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-27632-7. [Google Scholar]
- Edwards, W.P. The Science of Bakery Products; Royal Society of Chemistry: London, UK, 2007; ISBN 978-1-84755-779-7. [Google Scholar]
- Davies, C.G.A.; Labuza, T.P. The Maillard Reaction: Application to Confectionery Products. Confect. Sci. 1997, 33, 35–66. [Google Scholar]
- Purlis, E. Browning Development in Bakery Products—A Review. J. Food Eng. 2010, 99, 239–249. [Google Scholar] [CrossRef]
- Michalska, A.; Amigo-Benavent, M.; Zielinski, H.; del Castillo, M.D. Effect of Bread Making on Formation of Maillard Reaction Products Contributing to the Overall Antioxidant Activity of Rye Bread. J. Cereal Sci. 2008, 48, 123–132. [Google Scholar] [CrossRef]
- Helou, C.; Jacolot, P.; Niquet-Léridon, C.; Gadonna-Widehem, P.; Tessier, F.J. Maillard Reaction Products in Bread: A Novel Semi-Quantitative Method for Evaluating Melanoidins in Bread. Food Chem. 2016, 190, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.I.F.S.; Jongen, W.M.F.; van Boekel, M.A.J.S. A Review of Maillard Reaction in Food and Implications to Kinetic Modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Li, D.; Na, X.; Wang, H.; Xie, Y.; Cong, S.; Song, Y.; Xu, X.; Zhu, B.-W.; Tan, M. Fluorescent Carbon Dots Derived from Maillard Reaction Products: Their Properties, Biodistribution, Cytotoxicity, and Antioxidant Activity. J. Agric. Food Chem. 2018, 66, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.H.; Muthu, A.; El-Ramady, H.; Daróczi, L.; Nagy, L.; Kéki, S.; Béni, Á.; Csarnovics, I.; Prokisch, J. Optimization of Extraction Conditions to Synthesize Green Carbon Nanodots Using the Maillard Reaction. Mater. Adv. 2024, 5, 3499–3505. [Google Scholar] [CrossRef]
- Li, D.; Xie, Y.; Na, X.; Li, Y.; Dai, C.; Li, Y.; Tan, M. Insights into Melanoidin Conversion into Fluorescent Nanoparticles in the Maillard Reaction. Food Funct. 2019, 10, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Sk, M.P.; Jaiswal, A.; Paul, A.; Ghosh, S.S.; Chattopadhyay, A. Presence of Amorphous Carbon Nanoparticles in Food Caramels. Sci. Rep. 2012, 2, 383. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Sun, H. Novel Properties and Applications of Carbon Nanodots. Nanoscale Horiz. 2018, 3, 565–597. [Google Scholar] [CrossRef]
- Wang, H.; Su, W.; Tan, M. Endogenous Fluorescence Carbon Dots Derived from Food Items. Innovation 2020, 1, 100009. [Google Scholar] [CrossRef] [PubMed]
- Anpalagan, K.; Karakkat, J.V.; Jelinek, R.; Kadamannil, N.N.; Zhang, T.; Cole, I.; Nurgali, K.; Yin, H.; Lai, D.T.H. A Green Synthesis Route to Derive Carbon Quantum Dots for Bioimaging Cancer Cells. Nanomaterials 2023, 13, 2103. [Google Scholar] [CrossRef]
- Goheen, S.C. The Influence of pH and Acetonitrile on the High Performance Size Exclusion Profile of Proteins. J. Liq. Chromatogr. 1988, 11, 1221–1228. [Google Scholar] [CrossRef]

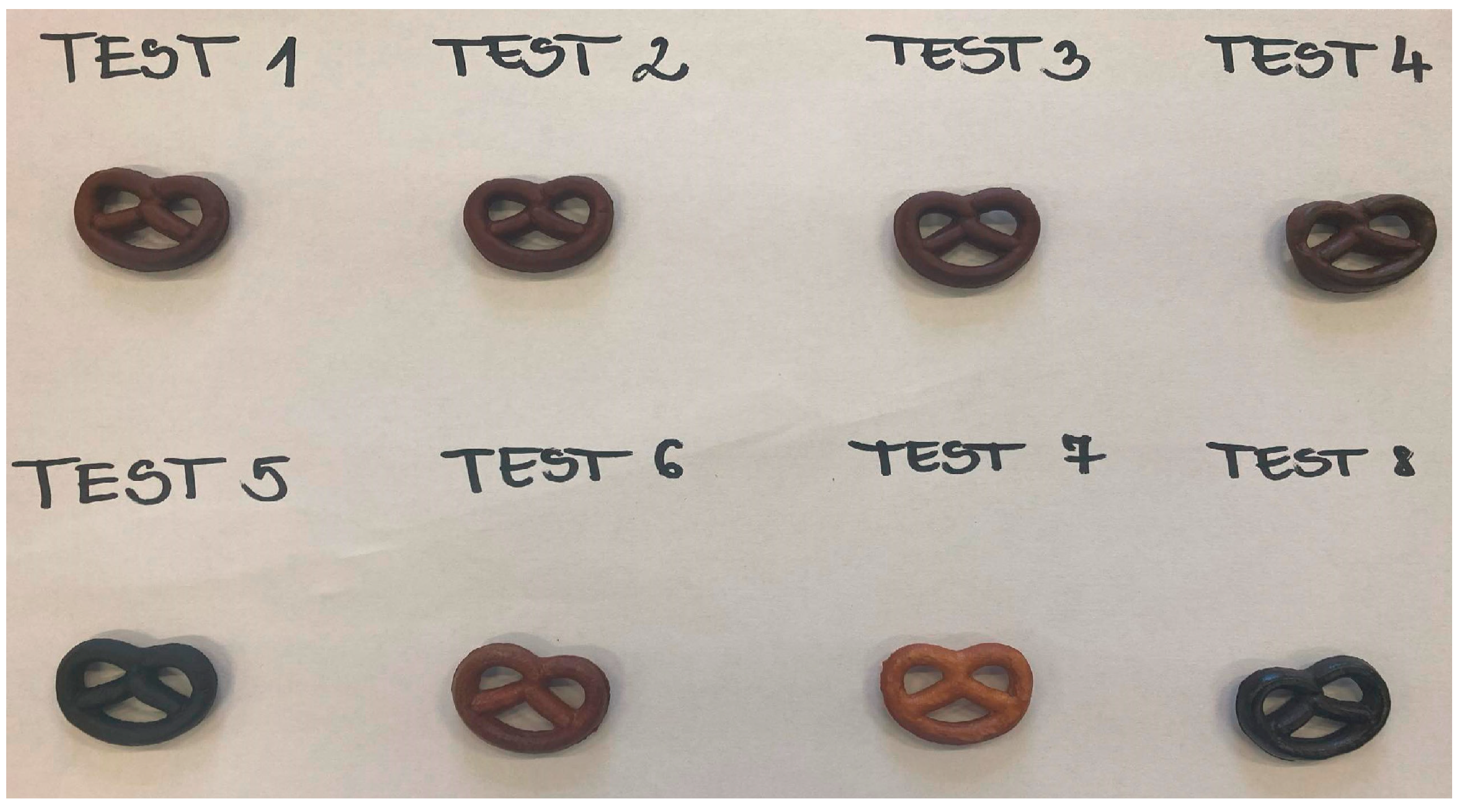
| Ingredients and Factors | Test 1 | Test 2 | Test 3 | Test 4 | Test 5 | Test 6 | Test 7 | Test 8 |
|---|---|---|---|---|---|---|---|---|
| Controlled variables | ||||||||
| BL-55 wheat flour quantity (%) (w/w) | 67.92 | |||||||
| Liquid barley malt extract quantity (%) (w/w) | 1.358 | |||||||
| Native corn starch quantity (%) (w/w) | 2.037 | |||||||
| Dried baker’s yeast quantity (%) (w/w) | 0.6792 | |||||||
| Granulated sugar quantity (%) (w/w) | 0.6792 | |||||||
| High oleic sunflower oil quantity (%) (w/w) | 6.112 | |||||||
| Sodium metabisulfite quantity (%) (w/w) | 0.04292 | |||||||
| L-cysteine quantity (%) (w/w) | 0.008557 | |||||||
| Independent variables | ||||||||
| Immersion time in NaOH solution (s) | 5 | 20 | 5 | 20 | 5 | 20 | 5 | 20 |
| Temperature of NaOH solution (°C) | 40 | 40 | 80 | 80 | 40 | 40 | 80 | 80 |
| Concentration of NaOH solution (g/L) | 10 | 10 | 10 | 10 | 30 | 30 | 30 | 30 |
| Baking time (min) | 10 | 20 | 20 | 10 | 20 | 10 | 10 | 20 |
| Baking temperature (°C) | 240 | 200 | 200 | 240 | 240 | 200 | 200 | 240 |
| Variable | Level | |
|---|---|---|
| Low (−1) | High (+1) | |
| Immersion time in NaOH solution (s) | 5 | 20 |
| Temperature of NaOH solution (°C) | 40 | 80 |
| Concentration of NaOH solution (g/L) | 10 | 30 |
| Baking time (min) | 10 | 20 |
| Baking temperature (°C) | 200 | 240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semsey, D.; Nguyen, D.H.H.; Törős, G.; Muthu, A.; Labidi, S.; El-Ramady, H.; Béni, Á.; Rai, M.; József, P. Analysis of Fluorescent Carbon Nanodot Formation during Pretzel Production. Nanomaterials 2024, 14, 1142. https://doi.org/10.3390/nano14131142
Semsey D, Nguyen DHH, Törős G, Muthu A, Labidi S, El-Ramady H, Béni Á, Rai M, József P. Analysis of Fluorescent Carbon Nanodot Formation during Pretzel Production. Nanomaterials. 2024; 14(13):1142. https://doi.org/10.3390/nano14131142
Chicago/Turabian StyleSemsey, Dávid, Duyen H. H. Nguyen, Gréta Törős, Arjun Muthu, Safa Labidi, Hassan El-Ramady, Áron Béni, Mahendra Rai, and Prokisch József. 2024. "Analysis of Fluorescent Carbon Nanodot Formation during Pretzel Production" Nanomaterials 14, no. 13: 1142. https://doi.org/10.3390/nano14131142
APA StyleSemsey, D., Nguyen, D. H. H., Törős, G., Muthu, A., Labidi, S., El-Ramady, H., Béni, Á., Rai, M., & József, P. (2024). Analysis of Fluorescent Carbon Nanodot Formation during Pretzel Production. Nanomaterials, 14(13), 1142. https://doi.org/10.3390/nano14131142












