Abstract
Two novel samples of nanoparticles based on chitosan were greenly synthesized using pomegranate peel extract. The extract served as a nanoparticle precursor, facilitating the precipitation of nanosized chitosan through the ionic gelation method. Additionally, by mixing the green chitosan nanoparticles with copper ions, a nanoscale composite of chitosan and copper oxide was also produced. Structural and morphological investigations (FTIR, XRD, SEM, EDX, and TGA analyses) were performed for greenly synthesized chitosan nanoparticles and their copper oxide composite to determine all the significant characteristics of those nanoparticles. In addition, both samples were tested using some biological investigations, such as antimicrobial activity and hematological effects. The antimicrobial tests yielded promising results for both the green chitosan nanoparticles and the CuO composite when tested using two bacterial strains and two fungal strains. Moreover, the results showed that using a similar concentration of both green-based chitosan samples resulted in a slightly larger inhibition zone and a lower minimum inhibition concentration (MIC) for the copper oxide chitosan composite compared to the chitosan nanoparticles for all microorganisms included in the test. The mean count of blood components (RBCs and platelets), clotting time, and cholesterol levels in three different blood samples were used to indicate the hematological activity of both greenly synthesized nanoparticles. The results verified a slight reduction in blood component count after the addition of green chitosan nanoparticles, but the chitosan copper oxide composite did not have a noticeable effect on the three blood samples. The chitosan nanoparticles were able to cause a considerable reduction in clotting time and cholesterol levels for all blood samples, thus acting as procoagulants. However, the mixing of CuO with chitosan nanoparticles prolonged the rate of clotting in blood samples from hypercholesteremic individuals, and thus, the mixture acted as an anticoagulant agent.
1. Introduction
Green approaches were utilized for the production of chitosan, a well-known cationic biopolymer, and its nanoparticles. These green approaches provide economic and environmental benefits when compared to conventional chemical and physical protocols, making them a more advantageous choice [1]. Chitosan nanoparticles are typically synthesized through chemical methods, specifically ionotrophic gelation using sodium tripolyphosphate (TPP), alongside physical methods. Although TPP is generally considered safe, it has the potential to cause variations in the size, stability, and biological properties of the resulting nanoparticles [2,3]. In sustainable processes, nontoxic, eco-friendly, and biosafe materials are employed [4]. The main goal of synthesizing green chitosan nanoparticles is to attain particle sizes smaller than 100 nm. This characteristic is vital for numerous applications that rely on the significant role of specific surface area [5]. Nanoparticles were synthesized using microorganisms such as bacteria and fungi, whose biosynthetic capabilities were harnessed during synthesis [6,7]. Moreover, the biosynthesis process involved the utilization of secondary metabolites derived from plant leaf extracts, which served as effective reducing agents [8]. Biological substances act as reducers, stabilizers, or both during the nanoparticle formation process. Punica granatum L., also known as “pomegranate” fruit, along with its derivatives, particularly peels, is known to contain beneficial secondary metabolites (phytochemicals). These metabolites possess various unique properties, including antimicrobial, antioxidant, anti-allergic, anti-atherosclerotic, and anti-inflammatory characteristics [9]. As a result, pomegranates and their byproducts have gained significant global recognition for their significant economic, nutritional, and medicinal potential [10]. Utilizing pomegranate peel as a capping agent contributes to reductions in cholesterol levels. This effect is attributed to the presence of its phytochemical contents, particularly polyphenols. Therefore, using pomegranate peel and its bioactive compounds could be a natural way to help lower the risk factors for heart disease [11].
Overall, nanochitosan provides significant advantages over traditional chitosan in various applications [12,13]. Its small size and increased surface area enable targeted delivery, making it effective in drug delivery systems [14]. Additionally, its biocompatibility and low toxicity make it promising for biomedical applications, including tissue engineering and regenerative medicine [15,16]. By incorporating nanomaterials such as graphene oxide, carbon nanotubes, and metal oxides like TiO2, ZnO [17], Fe3O4 [18], AgO [17], Au2O3 and CuO [19], the functionality of the material can be enhanced, enabling its utilization in various industries such as packaging, textiles, and environmental remediation [20,21]. The process of synthesizing chitosan–metal oxide nanocomposites involves the solution casting method for chitosan–silver oxide [17], and the chemical reduction of gold ions using X-rays as an alternative energy source in the presence of chitosan results in chitosan–Au NPs [22].
Among these notable metal oxides, considerable attention has been focused on copper oxide nanoparticles as cost-effective substitutes for noble metal nanoparticles such as silver and gold [23]. The remarkable antimicrobial [24], antiviral, and antitumor properties of copper oxide nanoparticles, coupled with their ability to enhance cell proliferation and migration and their applications in wound dressings and biocidal properties [25], have rendered them highly attractive for diverse applications [26]. The eco-friendly methods for the preparation of chitosan–copper oxide composites are important because they are less expensive than other methods [27].
It is well known that abnormal blood coagulation, injuries, surgical complications, or vascular malformations can cause severe hemorrhage, leading to shock and local tissue damage [28]. Immediate and effective hemorrhage control is crucial for saving lives. In recent times, advanced hemostatic dressings have been utilized to manage profuse bleeding [29]. Notably, chitosan-based dressings have proven effective in suppressing hemorrhage. The impact of chitosan on various blood components, including red blood cells (RBCs), coagulation factors, and platelets, must be considered. Surprisingly, the hemocompatibility compatibility of chitosan remains under investigation. When in contact with whole blood, chitosan forms a coagulum, leading to distinct platelet adhesion on its surface within a short timeframe. Notably, clotting time is reduced by 40% compared to whole blood alone. Consequently, chitosan has a practical application as a hemostatic dressing in clinical settings to promote wound healing rather than as a blood-contact medical device [30].
This research article focuses on two main objectives. Firstly, it describes the synthesis of green nanochitosan, a versatile biopolymer, using a green extract from pomegranate peel. The goal is to develop an environmentally sustainable approach to nanochitosan synthesis by eliminating the use of chemicals or traditional agents like tripolyphosphate (TPP). Secondly, this study aims to outline the creation of a novel bifunctional composite material using a combination of the unique properties of biogenic copper oxide nanoparticles and the versatility and biocompatibility of nanochitosan. This article also details an investigation of the antimicrobial properties of these nanoparticles and an evaluation of their potential for promoting healing processes.
2. Experimental Work
2.1. Plant Extract Preparation (Pomegranate Peel Extract)
The natural extract was prepared by combining an equivalent of 40 g of raw pomegranate peel powder with one liter of distilled water. The mixture was subjected to boiling for a duration of 15 min, followed by cooling and subsequent filtration to obtain the desired natural extract.
2.2. Synthesis of Green Chitosan Nanoparticles (PP–Cs–NPs)
To prepare a sample of chitosan nanoparticles (PP–Cs–NPs), the process involved dissolving half a gram of commercial chitosan in one percent glacial acetic acid until complete dissolution. Fifty milliliters of pomegranate peel extract were carefully added to the chitosan solution while it was being heated at 80 °C, and the mixture was continuously stirred for an hour. Subsequently, the mixture was washed multiple times with distilled water and filtered using decantation. Finally, the sample was left to dry overnight in an oven at 80 °C.
2.3. Synthesis of Green Chitosan–Copper Oxide Composite (PP–CS–CuO–NPs)
The synthesis of the second sample (PP–Cs–CuO–NPs) involved a three-step process. Firstly, half a gram of commercial chitosan was fully dissolved in a one percent glacial acetic acid solution. Secondly, 50 millimoles of anhydrous copper sulfate was added to the previous mixture, followed by the careful addition of 50 mL of pomegranate peel extract. This step was conducted at a temperature of 80 °C, with continuous stirring for an hour. Finally, the mixture was washed multiple times with distilled water, filtered using decantation, and dried overnight in an oven at 80 °C.
2.4. Characterization
2.4.1. Structural Assessment
To examine the characteristic functional groups of the synthesized nanoparticles, FTIR spectra using Shimadzu Fourier Transform Infrared spectrophotometer were used [31]. The thermal stability of all greenly synthesized nanoparticles was evaluated through thermo-gravimetric analysis (TGA-DTA7300, Exstar, Hitachi, Tokyo, Japan) at ambient temperature. An X-ray powder diffractometer using (Shimadzu, Kyoto, Japan) XRD with Cu Kα radiation (λ = 1.5418 Å) at a scanning speed of 0.2 s was used to identify the characteristic peaks of both synthesized nanoparticles (details are provided in the Supplementary Materials).
2.4.2. Morphological Characterizations
SEM (FEI, Inspect S 50, Czech Republic) was used to examine the morphology and arrangement of the synthesized nanoparticles. Furthermore, using energy dispersive X-ray spectroscopy, the EDAX analyses were conducted with an EDX-8000 instrument (Shimadzu, Kyoto, Japan) for elemental analysis and identification of the synthesized nanoparticles [32] (details are provided in the Supplementary Materials).
2.5. Antibacterial Test
The susceptibility tests were performed according to the guidelines provided by the National Committee for Clinical Laboratory Standards (NCCLS, 1993). Screening tests to determine inhibition zones were performed using the well diffusion method [33] (details are provided in the Supplementary Materials).
2.6. Hematological Test
2.6.1. Chitosan Solution Preparation
The chitosan solution was prepared by dissolving 10 mg/mL of each of the 2 samples, the PP–Cs–NPs and PP–Cs–CuO–NPs, in 1% acetic acid, with continuous stirring at ambient temperature for 2 h.
2.6.2. Blood Collection
The blood samples were obtained from individuals with overall good health, diabetes, or hypercholesterolemia.
The Institutional Review Board of Imam Abdulrahman Bin Faisal, Kingdom of Saudi Arabia, granted ethical approval for this research (reference number: IRB-2020-03-339). This study adhered to the principles outlined in the Declaration of Protecting Human Research Participants Online Training (PHRP No. 2852904) for conducting research involving human subjects. Additionally, all participants provided informed consent by signing a consent form prior to their involvement in the study (details are provided in the Supplementary Materials).
2.6.3. Complete Blood Count (CBC) and Coagulation Time (BCT)
Hematological analysis, including erythrocyte count (RBC, count/mm3) and platelet count (PLT, count/mm3), was conducted using the CELLTAC hematology analyzer. The complete blood count and coagulation time were assessed by introducing half a mL of the PP–Cs–NPs and PP–Cs–CuO–NPs solutions to one and a half ml of each blood sample, each of which had been previously divided into tubes containing 3.8% sodium citrate. Following a 5-min incubation period in a water bath at 37 °C, the blood samples were analyzed using the CELLTAC instrument. To observe blood coagulation, the tube was gently inclined at 30-s intervals until the blood clot formed. The coagulation time was recorded when no blood flow was observed upon perpendicularly tilting the tube. CBC was analyzed using the same procedure. The mixture was then incubated in a water bath at 37 °C for 5 min.
3. Results and Discussion
3.1. Characterization of Chitosan Nanoparticles
3.1.1. FTIR Analysis
Figure 1 presents the spectra of both PP–Cs–NPs and PP–Cs–CuO–NPs, which were obtained using FTIR. In the spectrum of PP–Cs–NPs, a prominent peak at 3400 cm−1 is observed, indicating the overlap between the N–H of chitosan, the O–H stretching of phenolic compounds, and the polysaccharides present in the pomegranate peel extract. Another peak appeared at 1640 cm−1, indicating the existence of a –CONH group characteristic of chitosan. Additionally, a sharp peak observed at 1214 cm−1 corresponds to a C–O group characteristic of phenolic compounds found in the pomegranate peel extract [34,35]. On the other hand, the spectrum of the PP–Cs–CuO–NPs exhibited several bands in different regions. A broad band ranging from 2890 to 3575 cm−1 could be attributed to the merging of bands of the N–H group of chitosan, the O–H stretching of the organic compounds from the pomegranate peel, which is found in the range of 3400 cm−1, and a band of C–H asymmetric stretching at 2920 cm−1 [36]. The vibrational bending of an N–H group was observed at 1530 cm−1. Furthermore, a band at 2070 cm−1 indicates the existence of silicon compounds. Two additional bands in the range between 1635 and 1098 cm−1 represent the existence of the –CONH group of chitosan and the C–O group of phenolic compounds and polysaccharides in pomegranate peel, respectively. A small peak corresponding to the stretching vibration of metal oxide was found at 895 cm−1 [37]. Evidence of CuO–NPs formation was found in the sharp band at 618 cm−1 [19,38,39]. Finally, the difference between the spectra of CuO–NPs and PP–Cs–CuO–NPs was mainly indicated by the existence of bands at 895 and 618 cm−1 that are characteristic of the metal oxide group. Also, the peaks of PP–Cs–CuO–NPs at 2920, 2070, and 1530 cm−1 provide evidence of more organic matter from the pomegranate peel extract, which may be needed to precipitate CuO–NPs. Based on this data, it can be inferred that pomegranate polysaccharides serve as a highly effective alternative to conventional TPP as a nanoparticle precursor [5].
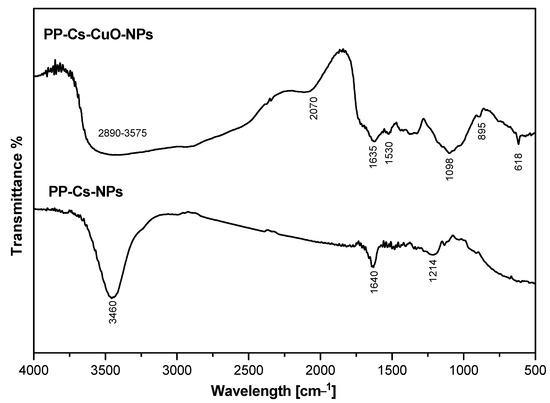
Figure 1.
FTIR for green chitosan PP–Cs–NPs and green chitosan–CuO nanocomposites (PP–Cs–CuO–NPs).
3.1.2. XRD Analysis
Figure 2 exhibited no distinct peaks in the XRD diffractogram of PP–Cs–NPs, indicating the amorphous nature of synthesized nanoparticles. This characteristic enhances the sorption properties of the material [3]. However, PP–Cs–CuO–NPs showed weak CuO pattern peaks of 2θ = 32.9°, 35.0°, 38.5°, 48.0°, and 53.2°, corresponding to (−110), (002), (111), (−202), and (020) reflections, respectively [40]. These peaks confirmed the presence of the CuO phase (monoclinic structure, JCPDS card no. 45-0937). Some peaks representing impurities from pomegranate peel capping were overlaid on the broad, amorphous band of the chitosan matrix [41]. These peaks suggest the existence of K2O and K2CO3 at 2θ = 25.2°, 28.2°, and 40.7° (JCPDS 77-2176 and 87-0730), while the weak peaks at 2θ = 25.0°, 32.9°, 42.1°, and 51.26° were attributed to SiO2, Fe2O3, P2O5, and carbon (JCPDS 41-1413, 33-0664, 5-0488, 75-1621 and 34-0941) [19].
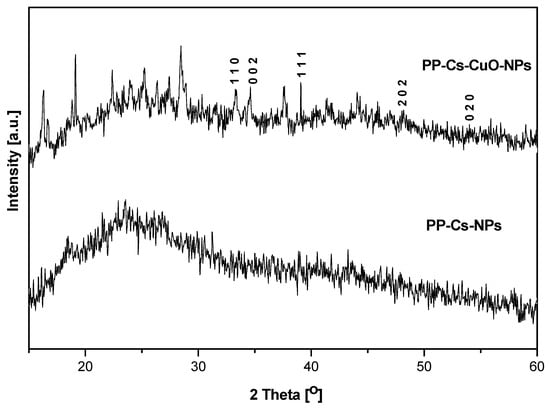
Figure 2.
XRD patterns for green chitosan (PP–Cs–NPs) and green chitosan–CuO nanocomposites (PP–Cs–CuO–NPs).
3.1.3. TGA and DTA
To explore the thermoanalytical characteristics of the green chitosan samples, thermal analysis was performed across a controlled temperature range. The thermal gravimetric analysis (TGA) and differential thermal gravimetric (DTA) curves of PP–Cs–NPs and PP–Cs–CuO–NPs are depicted in (Figure 3). Table 1 provides the temperature values corresponding to each stage of thermal decomposition, along with their respective weight losses. The poor thermal stability of conventional chitosan represents a significant challenge, especially for its biomedical applications [42,43]. However, both samples examined in this study (PP–Cs–NPs and PP–Cs–CuO–NPs) exhibited improved thermal stability compared to regular chitosan.
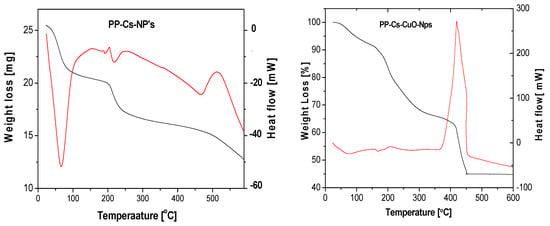
Figure 3.
TGA (black) and DTA (red) for green chitosan (PP–Cs–NPs) and green chitosan–CuO nanocomposites (PP–Cs–CuO–NPs).
During the initial stages of TGA, the decrease in mass observed with chitosan nanoparticles can be attributed to the drying process, where residual water bound to the polar groups in chitosan nanoparticles is lost. This weight loss is not associated with any chemical reactions and is primarily due to the removal of water molecules. It is evident that PP–Cs–NPs undergo a first weight loss of approximately 16.7% between 25 °C and 87 °C, primarily due to dehydration.
The high weight loss during the dehydration stages may be due to the increased hydrophilicity of PP–Cs–NPs compared to regular chitosan. The subsequent decomposition of PP–Cs–NPs takes place in the second and third stages in which a residue of about 42.8% at 600 °C is left behind. On the other hand, PP–Cs–CuO–NPs exhibited a different thermal behavior, with water elimination occurring within a temperature range from 25 °C to 117 °C with 8.9% weight loss. The decomposition of chitosan and the capping green extract residue appeared in the following two stages in a temperature range of 117–450 °C. Figure 3 confirms the improved stability of PP–Cs–CuO–NPs by showing no significant mass loss beyond 450 °C. The material exhibits a residue of 45%, as shown in Table 1. This suggests that PP–Cs–CuO–NPs maintain their structural integrity and do not undergo further decomposition at higher temperatures. This finding suggests that these modified forms of chitosan show potential in overcoming the thermal stability challenges encountered in biomedical applications.

Table 1.
Sample weight loss and thermal degradation stages.
Table 1.
Sample weight loss and thermal degradation stages.
| Sample | Degradation Stage | Temperature Range (°C) | Peak Temperature (°C) | % Weight Loss |
|---|---|---|---|---|
| PP–Cs–NPs | First | 25–87 | 68 | 16.7% |
| Second | 87–240 | 218 | 31.5% | |
| Third | 240–600 | 470 | 51.8% | |
| PP–Cs–CuO–NPs | First | 25–117 | 79 | 8.9% |
| Second | 117–314 | 200 | 23.1% | |
| Third | 314–451 | 415 | 54.8% |
3.1.4. SEM and EDX
Figure 4 reveals the morphological distribution (size, shape) and the elemental structure of synthesized samples. Figure 4a shows a uniform dispersion of exemplary spherical nanoparticles that exhibit a narrow particle size distribution in an average range from 22.5 nm 1.2 nm to 26.2 nm 1.3 nm for the sample of PP–Cs–NPs and from 18.7 nm 1.0 nm to 26.5 nm 1.3 nm for the sample of PP–Cs–CuO–NPs. Meanwhile, the SEM image (Figure 4c) depicts two distinct types of nanoparticle aggregates on the surface within the same particle size range. Furthermore, Figure 4b,d verify that PP–Cs–NPs primarily consisted of carbon and oxygen, whereas PP–Cs–CuO–NPs comprised carbon, oxygen, nitrogen, and copper.
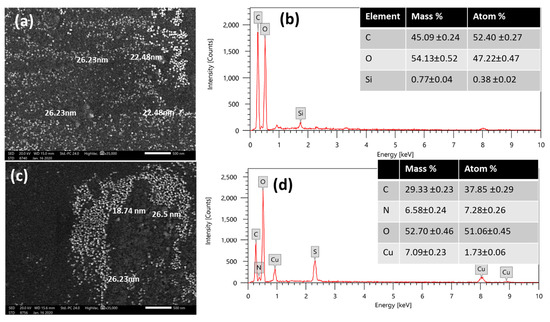
Figure 4.
SEM images of (a) green chitosan (PP–CS–NPs), (c) green chitosan–CuO nanocomposites (PP–CS–CuO–NPs); EDX elemental % (b) green chitosan (PP–CS–NPs), and (d) green chitosan–CuO nanocomposites (PP–CS–CuO–NPs).
3.2. Evaluation of Antimicrobial Activity of Green PP–Cs–NPs and PP–Cs–CuO–NPs
The antimicrobial activity of both green PP–Cs–NPs and PP–Cs–CuO–NPs was evaluated using the gram-positive bacterium Bacillus subtilis (B. subtilis) and the gram-negative bacterium Pseudomonas aeruginosa (P. aeruginosa), as well as two types of fungi, Cryptococcus neoformans (C. neoformans) and Candida albicans (C. albicans). The diameter of the inhibition zone and minimum inhibitory concentration (MIC) were measured for each sample. Interestingly, both samples demonstrated good activity against the microorganisms tested (Figure 5). The results demonstrate that the antimicrobial activity of PP–Cs–CuO–NPs resulted in slightly larger inhibition zones (16, 10, 10, and 9 mm) compared to the antimicrobial activity of PP–Cs–NPs (15, 9, 8, 7 mm) at the same concentration for all microorganisms used, indicating enhanced antimicrobial activity, as previously found in the literature [44].
Also, the MIC values showed that both chitosan samples exhibited antimicrobial activity. PP–Cs–CuO–NPs generally demonstrated MIC values that were comparable to or slightly lower than PP–Cs–NPs, suggesting similar or improved antimicrobial efficacy. It is important to note that the MIC results generally align with results of the diameter of the inhibition zone, indicating a correlation between the two measures of antimicrobial activity. However, it is noteworthy that for the C. albicans fungus, PP–Cs–NPs exhibited a lower MIC value (20 mg/mL) compared to that of PP–Cs–CuO–NPs (25 mg/mL). This discrepancy suggests that while PP–Cs–NPs might have a smaller inhibition zone diameter (9 mm) against C. albicans, a lower concentration is needed to effectively inhibit fungal growth. This discrepancy could potentially be attributed to variations in the susceptibility of C. albicans to different chitosan formulations [27].
The findings revealed a marginally reduced antimicrobial efficacy for the green samples compared to the previously prepared TPP-based samples, with the exception of C. neoformans treated with PP–Cs–NPs, which exhibited superiority (15 mm) over the chemical-based chitosan nanoparticles from previous work (9 mm) [19].
Our study introduces a groundbreaking green synthesis method, utilizing raw pomegranate peel extract instead of TPP, thereby eliminating the requirement for chemicals and complex extraction processes. This substitution marks a significant advancement, as TPP has traditionally been a key precursor in chitosan synthesis. Additionally, despite a minor decline in antimicrobial activity, our chitosan samples, which were synthesized in an environmentally friendly manner, demonstrated comparable antimicrobial effectiveness, affirming the practicality and efficacy of employing nanochitosan and its composites derived from natural extracts for antimicrobial applications.
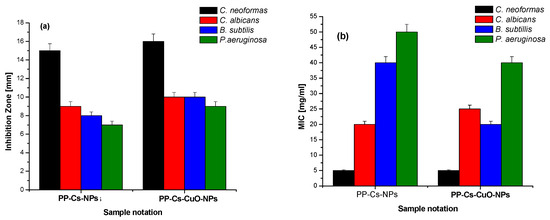
Figure 5.
(a) Inhibition zone [mm] and (b) minimum inhibitory concentrations [mg/mL] of green chitosan (PP–Cs–NPs) and green chitosan–CuO nanocomposites (PP–Cs–CuO–NPs).
3.3. Hematological Analysis
Compared to control blood samples, slight reductions in the mean blood component counts were observed in all blood samples after the addition of PP–Cs–NPs; by contrast, the addition of PP–Cs–CuO–NPs did not result in noticeable changes in the RBC or platelet counts in hematological specimens from healthy, diabetic, and hypercholesteremic individuals., as shown in Figure 6a,b. The addition of PP–Cs–NPs led to a 6.5% reduction in the mean RBC count in blood specimens from healthy and hypercholesterolemic individuals compared to a 4% reduction in blood samples from diabetic individuals (Figure 6a). Decreases in platelet count of 18.7% and 14% were observed in samples from healthy and diabetic individuals, respectively, without any noticeable change in platelet count in blood samples from hypercholesterolemic individuals.
Figure 6c demonstrates the in vitro effects of both biosynthesized samples on the coagulation time of all tested blood samples. PP–Cs–NPs reduced the time of blood clotting for healthy and diabetic blood specimens, whereas the clotting time of blood samples from hypercholesteremic individuals was increased. PP–Cs–CuO–NPs increased the clotting time of blood samples from healthy and hypercholesterolemic individuals. That means that both synthesized nanoparticles may be able to accelerate the wound healing process in diabetic individuals by reducing clotting time. Figure 6c,d show the effect of both synthesized samples, especially PP–Cs–NPs, on cholesterol concentration reduction in blood samples from hypercholesteremic individuals.
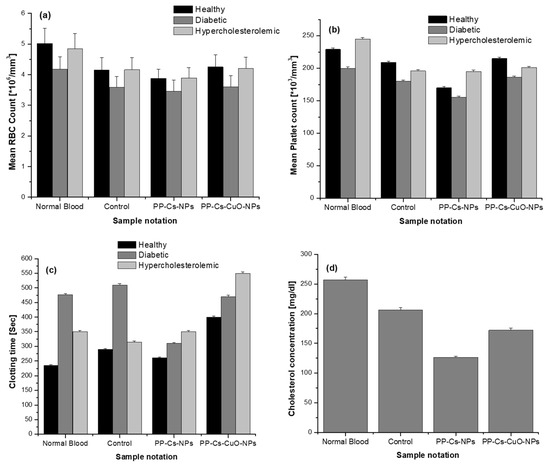
Figure 6.
The effect of green chitosan (PP–Cs–NPs) and green chitosan–CuO nanocomposites (PP–Cs–CuO–NPs) on (a) mean RBC count, (b) mean platelet count, (c) clotting time, and (d) cholesterol concentration in blood samples from healthy, diabetic, and hypercholesteremic individuals.
Chitosan has a crucial impact on the prevention of atherosclerosis by transporting glucosamine groups to the cell via glucose receptors. Previous studies have verified that the surface of the human blood cell contains approximately 107 negative ionic groups due to the presence of a carboxylic acid group of N-acetylneuraminic acid at the peripheral end of a protein called glycophorin that penetrates the lipid bilayer, which is responsible for the majority of the negative charges [45]. The net positive charge of chitosan nanoparticles (Cs–NPs), which relies on the degree of deacetylation (DD) and the number of protonated amine groups, is responsible for their hemostatic properties [46,47]. These amine groups promote the interaction between the negatively charged blood components (red blood cells and platelets) and positively charged chitosan (13 mV), enabling chitosan to form a spatial structure resembling a mesh, which facilitates blood clot formation (Figure 6a,b). Previous studies have demonstrated that chitosan with moderate DD exhibits the most significant procoagulant effect [48].
On the other side, copper nanoparticles play a crucial role in inhibiting and masking the inflammatory effect of chitosan, as well as assisting copper-dependent enzymes in collagen synthesis, which aids in wound healing [49]. The wound healing property of nano-copper (nCu) is enhanced when combined with chitosan. Chitosan is known to be polycationic in acidic media, allowing it to bind easily with metallic ions such as Cu [20].
Additionally, a P. granatum extract led to prolonged clotting time without any noticeable change in the blood component count, which could be attributable to the existence of anthocyanidins in P. granatum that play a vital role in suppressing cyclooxygenase [50] or diminishing the level of fibrinogen [51], thus preventing platelet aggregation.
The nonsignificant reduction in the RBC and platelet counts after the addition of PP–Cs–CuO–NPs (Figure 6a,b) is attributed to the chelating effect of Cu, which suppresses the interaction of chitosan nanoparticles and blood components. This verifies that the incorporation of CuO with peel extract in Cs–NPs serves as an anticoagulant that inhibits thrombin and intrinsic coagulation factor [52]. The significant reduction in cholesterol levels achieved by adding PP–Cs–NPs may be attributed to the potential interaction between the positive nanochitosan particles and the lone pair electrons of the hydroxyl group in cholesterol [53]. The preparation of PP–Cs–NPs and PP–Cs–CuO–NPs through the gelation of pomegranate peel extract in an acidic environment results in the formation of a cationic form of chitosan (–NH3+), as indicated by the zeta results (details are provided in the Supplementary Materials) [54]. The cationic chitosan binds to the hydroxyl group of cholesterol, creating a composite that effectively conceals the cholesterol molecules during the testing process. This composite is believed to inhibit the absorption of cholesterol in vivo.
4. Conclusions
This study investigated the successful synthesis of two types of nanoparticles, PP–Cs–NPs and PP–Cs–CuO–NPs, using a fully green technique. It introduced a novel approach to replacing TPP as a nanoparticle precursor by utilizing polysaccharides from pomegranate peel extract, known for its sustainability, eco-friendly nature, and green attributes. The characterization of both PP–Cs–NPs and PP–Cs–CuO–NPs confirmed their spherical uniformity, positive charge, and particle size ranging from 20 to 27 nm.
Biological evaluations of these newly synthesized nanoparticles (PP–Cs–NPs and PP–Cs–CuO–NPs) demonstrated their efficacy as bifunctional agents (antimicrobial agents and wound healers). Both PP–Cs–NPs and PP–Cs–CuO–NPs exhibited significant inhibition of bacterial and fungal growth, with the highest inhibition observed against the fungus C. neoformans at MIC values of 5 mg/mL. Furthermore, PP–Cs–CuO–NPs displayed a slightly higher antimicrobial activity compared to PP–Cs–NPs. Additionally, both nanoparticles reduced clotting time in blood samples from diabetic patients.
Finally, the study revealed a crucial role of PP–Cs–CuO–NPs and PP–Cs–NPs in reducing cholesterol levels in blood samples from both normal and hypercholesterolemic individuals through the creation of a hybrid structure between nanochitosan and cholesterol molecules.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano14131111/s1, Supplementary Files S1 and S2.
Author Contributions
Conceptualization, N.M.R.M. and S.B.A.; Methodology, N.M.R.M. and S.B.A.; Investigation, N.M.R.M., A.M.A.-S. and S.B.A.; Resources, A.R.; Writing—original draft, H.I.M., N.M.R.M. and S.B.A.; Writing—review & editing, H.I.M., N.M.R.M., A.R. and S.B.A.; Project administration, H.I.M. and A.M.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was primarily funded by Imam Abdulrahman Bin Faisal University through the IAU-2019 Newly Recruited Faculty Members Program, which is supported by the Deanship of Scientific Research (DSR) under the grant number 2019-224-AMSJ.
Data Availability Statement
The original data presented in the study are openly available in the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El-Naggar, N.E.A.; Saber, W.E.I.A.; Zweil, A.M.; Bashir, S.I. An Innovative Green Synthesis Approach of Chitosan Nanoparticles and Their Inhibitory Activity against Phytopathogenic Botrytis Cinerea on Strawberry Leaves. Sci. Rep. 2022, 12, 3515. [Google Scholar] [CrossRef]
- Jogaiah, S.; Satapute, P.; De Britto, S.; Konappa, N.; Udayashankar, A.C. Exogenous Priming of Chitosan Induces Upregulation of Phytohormones and Resistance against Cucumber Powdery Mildew Disease Is Correlated with Localized Biosynthesis of Defense Enzymes. Int. J. Biol. Macromol. 2020, 162, 1825–1838. [Google Scholar] [CrossRef] [PubMed]
- Sathiyabama, M.; Boomija, R.V.; Muthukumar, S.; Gandhi, M.; Salma, S.; Prinsha, T.K.; Rengasamy, B. Green Synthesis of Chitosan Nanoparticles Using Tea Extract and Its Antimicrobial Activity against Economically Important Phytopathogens of Rice. Sci. Rep. 2024, 14, 7381. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, D.; Geetha, N.; Khawar, K.M.; Jogaiah, S.; Mujtaba, M. Current Trends and Challenges in the Synthesis and Applications of Chitosan-Based Nanocomposites for Plants: A Review. Carbohydr. Polym. 2021, 261, 117904. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Shiha, A.M.; Mahrous, H.; Mohammed, A.B.A. Green Synthesis of Chitosan Nanoparticles, Optimization, Characterization and Antibacterial Efficacy against Multi Drug Resistant Biofilm-Forming Acinetobacter Baumannii. Sci. Rep. 2022, 12, 19869. [Google Scholar] [CrossRef]
- Sachin, K.; Karn, S.K. Microbial Fabricated Nanosystems: Applications in Drug Delivery and Targeting. Front. Chem. 2021, 9, 617353. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, R.; Daskum, A.; Unyah, Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Singh, S.; Nayik, G.A. Bioactive Compounds from Pomegranate Peels—Biological Properties, Structure–Function Relationships, Health Benefits and Food Applications—A Comprehensive Review. J. Funct. Foods 2024, 116, 106132. [Google Scholar] [CrossRef]
- Elfalleh, W. Total Phenolic Contents and Antioxidant Activities of Pomegranate Peel, Seed, Leaf and Flower. J. Med. Plants Res. 2012, 6, 4724–4730. [Google Scholar] [CrossRef]
- Mo, Y.; Ma, J.; Gao, W.; Zhang, L.; Li, J.; Li, J.; Zang, J. Pomegranate Peel as a Source of Bioactive Compounds: A Mini Review on Their Physiological Functions. Front. Nutr. 2022, 9, 887113. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.M.; Ahmed Rather, G.; Patrício, A.; Haq, Z.; Sheikh, A.A.; Shah, M.Z.u.H.; Singh, H.; Khan, A.A.; Imtiyaz, S.; Ahmad, S.B.; et al. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan Nanoparticles as a Promising Tool in Nanomedicine with Particular Emphasis on Oncological Treatment. Cancer Cell Int. 2021, 21, 318. [Google Scholar] [CrossRef]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Baranwal, A.; Kumar, A.; Priyadharshini, A.; Oggu, G.S.; Bhatnagar, I.; Srivastava, A.; Chandra, P. Chitosan: An Undisputed Bio-Fabrication Material for Tissue Engineering and Bio-Sensing Applications. Int. J. Biol. Macromol. 2018, 110, 110–123. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan–Silver Oxide Nanocomposite Film: Preparation and Antimicrobial Activity. Bull. Mater. Sci. 2011, 34, 29–35. [Google Scholar] [CrossRef]
- Jiang, R.; Shen, T.-T.; Zhu, H.-Y.; Fu, Y.-Q.; Jiang, S.-T.; Li, J.-B.; Wang, J.-L. Magnetic Fe3O4 Embedded Chitosan–Crosslinked-Polyacrylamide Composites with Enhanced Removal of Food Dye: Characterization, Adsorption and Mechanism. Int. J. Biol. Macromol. 2023, 227, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Mohamed, H.; Al-Subaie, A.; Al-Ohali, A.; Mahmoud, N. Investigation of the Antimicrobial Activity and Hematological Pattern of Nano-Chitosan and Its Nano-Copper Composite. Sci. Rep. 2021, 11, 9540. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, S.; Ikram, S. Adsorption of Heavy Metal Ions: Role of Chitosan and Cellulose for Water Treatment Saiqa Ikram Jamia Millia Islamia adsorption of heavy metal ions: Role of chitosan and cellulose for water treatment. Int. J. Pharmacogn. 2015, 2, 280–289. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Hashem, A.H.; Al-Askar, A.A.; Haponiuk, J.; Saied, E. A Novel Nanocomposite Based on Mycosynthesized Bimetallic Zinc-Copperoxide Nanoparticles, Nanocellulose and Chitosan: Characterization, Antimicrobial and Photocatalytic Activities. Electron. J. Biotechnol. 2023, 65, 45–55. [Google Scholar] [CrossRef]
- Mescola, A.; Canale, C.; Fragouli, D.; Athanassiou, A. Controlled Formation of Gold Nanostructures on Biopolymer Films upon Electromagnetic Radiation. Nanotechnology 2017, 28, 415601. [Google Scholar] [CrossRef]
- Bejan, A.; Anisiei, A.; Andreica, B.-I.; Rosca, I.; Marin, L. Chitosan Nanofibers Encapsulating Copper Oxide Nanoparticles: A New Approach towards Multifunctional Ecological Membranes with High Antimicrobial and Antioxidant Efficiency. Int. J. Biol. Macromol. 2024, 260, 129377. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, C.; Lin, I.H.; Krisnawati, D.I.; Khaerunnisa, S.; Widodo; Hsiao, Y.C.; Kuo, T.R.; Khafid, M. Antibacterial Pathways in Transition Metal-Based Nanocomposites: A Mechanistic Overview. Int. J. Nanomed. 2022, 17, 6821–6842. [Google Scholar] [CrossRef]
- Jadhav, S.; Gaikwad, S.; Nimse, M.; Rajbhoj, A. Copper Oxide Nanoparticles: Synthesis, Characterization and Their Antibacterial Activity. J. Clust. Sci. 2011, 22, 121–129. [Google Scholar] [CrossRef]
- Sajjad, H.; Sajjad, A.; Haya, R.T.; Khan, M.M.; Zia, M. Copper Oxide Nanoparticles: In Vitro and in Vivo Toxicity, Mechanisms of Action and Factors Influencing Their Toxicology. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 271, 109682. [Google Scholar] [CrossRef] [PubMed]
- Syame, S.; Mohamed, W.; Mahmoud, R.; Tawfeeq, S. Synthesis of Copper-Chitosan Nanocomposites and Its Application in Treatment of Local Pathogenic Isolates Bacteria. Orient. J. Chem. 2017, 33, 2959–2969. [Google Scholar] [CrossRef]
- Wang, Y.W.; Liu, C.C.; Cherng, J.H.; Lin, C.S.; Chang, S.J.; Hong, Z.J.; Liu, C.C.; Chiu, Y.K.; Der Hsu, S.; Chang, H. Biological Effects of Chitosan-Based Dressing on Hemostasis Mechanism. Polymers 2019, 11, 1906. [Google Scholar] [CrossRef]
- Silva, M.M.P.; de Aguiar, M.I.F.; Rodrigues, A.B.; Miranda, M.D.C.; Araújo, M.Â.M.; Rolim, I.L.T.P.; e Souza, A.M.A. The Use of Nanoparticles in Wound Treatment: A Systematic Review. Rev. da Esc. Enferm. 2017, 51, 1–9. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, X.; Liu, L.; Guan, J.; Liu, M.; Li, Z.; Yang, J.; Huang, S.; Wu, J.; Tian, F.; et al. Blood Coagulation Evaluation of N-Alkylated Chitosan. Carbohydr. Polym. 2017, 173, 259–268. [Google Scholar] [CrossRef]
- Abdel-fattah, W.I.; Eid, M.M.; El-moez, S.I.A.; Mohamed, E.; Ali, G.W. Synthesis of Biogenic Ag @ Pd Core-Shell Nanoparticles Having Anti-Cancer / Anti-Microbial Functions. Life Sci. 2017, 183, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Gabr, D.G. Significance of Fruit and Seed Coat Morphology in Taxonomy and Identification for Some Species of Brassicaceae. Am. J. Plant Sci. 2018, 9, 380–402. [Google Scholar] [CrossRef]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Sivakami, M.S.; Gomathi, T.; Venkatesan, J.; Jeong, H.-S.; Kim, S.-K.; Sudha, P.N. Preparation and Characterization of Nano Chitosan for Treatment Wastewaters. Int. J. Biol. Macromol. 2013, 57, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.J.; Cruz-Romero, M.; Collins, T.; Cummins, E.; Kerry, J.P.; Morris, M.A. Synthesis of Monodisperse Chitosan Nanoparticles. Food Hydrocoll. 2018, 83, 355–364. [Google Scholar] [CrossRef]
- Mohamed, E.A. Green Synthesis of Copper & Copper Oxide Nanoparticles Using the Extract of Seedless Dates. Heliyon 2020, 6, e03123. [Google Scholar] [CrossRef]
- Vara, J.A.; Dave, P.N.; Chaturvedi, S. The Catalytic Activity of Transition Metal Oxide Nanoparticles on Thermal Decomposition of Ammonium Perchlorate. Def. Technol. 2019, 15, 629–635. [Google Scholar] [CrossRef]
- Muthukrishnan, A.M. Green Synthesis of Copper-Chitosan Nanoparticles and Study of Its Antibacterial Activity. J. Nanomed. Nanotechnol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Mahmoud, N.; Mohamed, H.I.; Ahmed, S.B.; Akhtar, S. Efficient Biosynthesis of CuO Nanoparticles with Potential Cytotoxic Activity. Chem. Pap. 2020, 74, 2825–2835. [Google Scholar] [CrossRef]
- Veisi, H.; Karmakar, B.; Tamoradi, T.; Hemmati, S.; Hekmati, M.; Hamelian, M. Biosynthesis of CuO Nanoparticles Using Aqueous Extract of Herbal Tea (Stachys Lavandulifolia) Flowers and Evaluation of Its Catalytic Activity. Sci. Rep. 2021, 11, 1983. [Google Scholar] [CrossRef]
- Patil, R.; Patil, U.; Jagdale, A.; Shinde, S.; Patil, S. Ash of Pomegranate Peels (APP): A Bio-Waste Heterogeneous Catalyst for Sustainable Synthesis of α,A′-Bis(Substituted Benzylidine)Cycloalkanones and 2-Arylidene-1-Tetralones. Res. Chem. Intermed. 2020, 46, 3527–3543. [Google Scholar] [CrossRef]
- Hafizi, T.; Shahriari, M.H.; Abdouss, M.; Kahdestani, S.A. Synthesis and Characterization of Vancomycin-Loaded Chitosan Nanoparticles for Drug Delivery. Polym. Bull. 2023, 80, 5607–5621. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Thakur, R.; Barnela, M.; Chopra, M.; Manuja, A.; Chaudhury, A. Synthesis, Characterization and in Vitro Evaluation of Cytotoxicity and Antimicrobial Activity of Chitosan–Metal Nanocomposites. J. Chem. Technol. Biotechnol. 2015, 90, 867–873. [Google Scholar] [CrossRef]
- Clark, L.J.; Chan, L.S.; Powars, D.R.; Baker, R.F. Negative Charge Distribution and Density on the Surface of Oxygenated Normal and Sickle Red Cells. Blood 1981, 57, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Howley, P.; Prevost, N.; Condon, B.; Arnold, J.; Diegelmann, R. Positively and Negatively Charged Ionic Modifications to Cellulose Assessed as Cotton-Based Protease-Lowering and Hemostatic Wound Agents. Cellulose 2009, 16, 911–921. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Zhou, J.; Li, L. An Investigation of Chitosan and Its Derivatives on Red Blood Cell Agglutination. RSC Adv. 2017, 7, 12247–12254. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, S.; Cheng, Y.; Kong, S.; Li, S.; Li, C.; Yang, L. Investigation of the Effects of Molecular Parameters on the Hemostatic Properties of Chitosan. Molecules 2018, 23, 3147. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tian, F.; Wang, Z.; Wang, Q.; Zeng, Y.-J.; Chen, S.-Q. Effect of Chitosan Molecular Weight and Deacetylation Degree on Hemostasis. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 131–137. [Google Scholar] [CrossRef]
- Aviram, M.; Dornfeld, L.; Rosenblat, M.; Volkova, N.; Kaplan, M.; Coleman, R.; Hayek, T.; Presser, D.; Fuhrman, B. Pomegranate Juice Consumption Reduces Oxidative Stress, Atherogenic Modifications to LDL, and Platelet Aggregation: Studies in Humans and in Atherosclerotic Apolipoprotein E–Deficient Mice12. Am. J. Clin. Nutr. 2000, 71, 1062–1076. [Google Scholar] [CrossRef]
- Marie-Helene, D.; Martine, J.-P.; Jacques, E.; Olivier, B.; Gene, A.H.; Michael, W.M.; Marie-Claude, G. ADP-Induced Platelet Aggregation Depends on the Conformation or Availability of the Terminal Gamma Chain Sequence of Fibrinogen. Study of the Reactivity of Fibrinogen Paris 1. Blood 1987, 70, 558–563. [Google Scholar] [CrossRef]
- Di Cera, E. Thrombin. Mol. Aspects Med. 2008, 29, 203–254. [Google Scholar] [CrossRef] [PubMed]
- Ylitalo, R.; Lehtinen, S.; Wuolijoki, E.; Ylitalo, P.; Lehtimäki, T. Cholesterol-Lowering Properties and Safety of Chitosan. Arzneimittelforschung 2002, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Fylling, C.P.; Parnell, L.K.S. Use of Platelet Rich Plasma Gel on Wound Healing: A Systematic Review and Meta-Analysis. Eplasty 2011, 11, e38. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).