Chemical Bath Deposition of ZnO/ZnGa2O4 Core–Shell Nanowire Heterostructures Using Partial Chemical Conversion
Abstract
1. Introduction
2. Materials and Methods
2.1. Deposition Techniques
2.2. Annealing Process
2.3. Characterization Techniques
3. Results
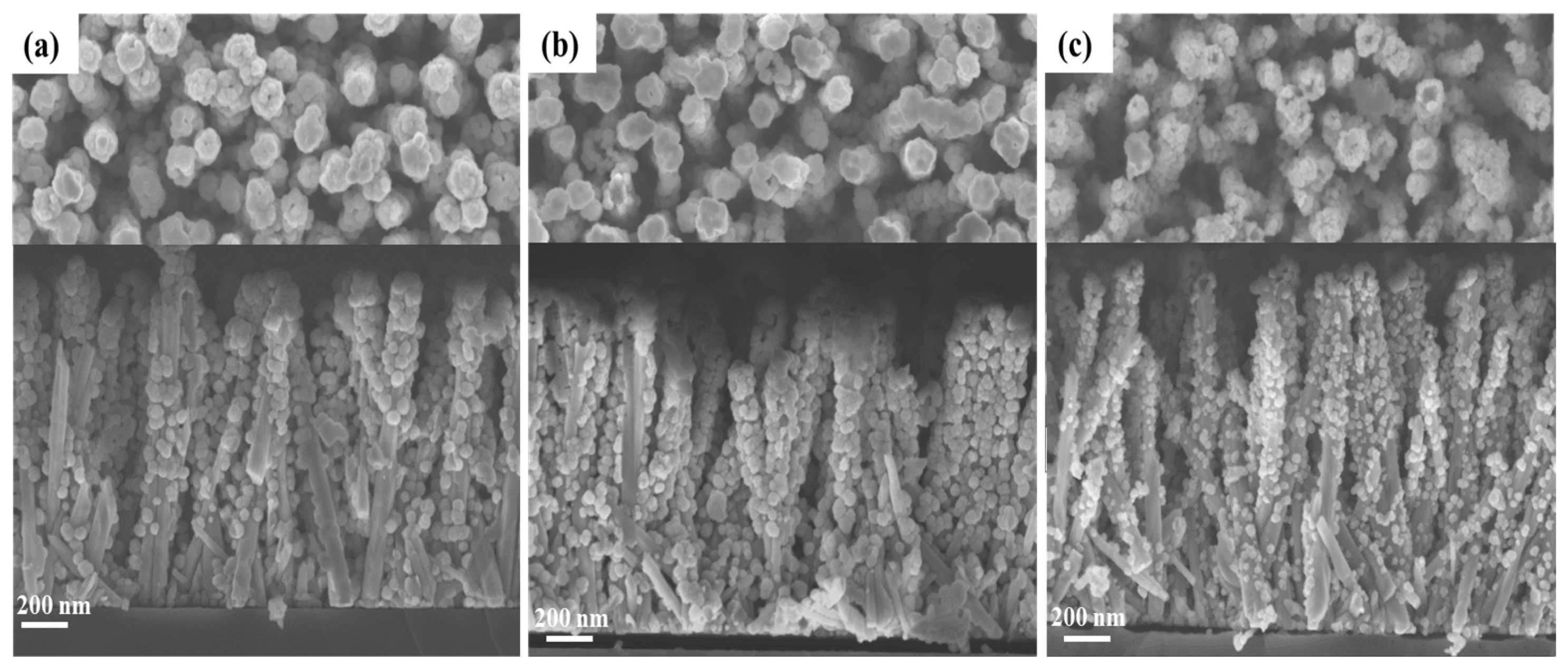
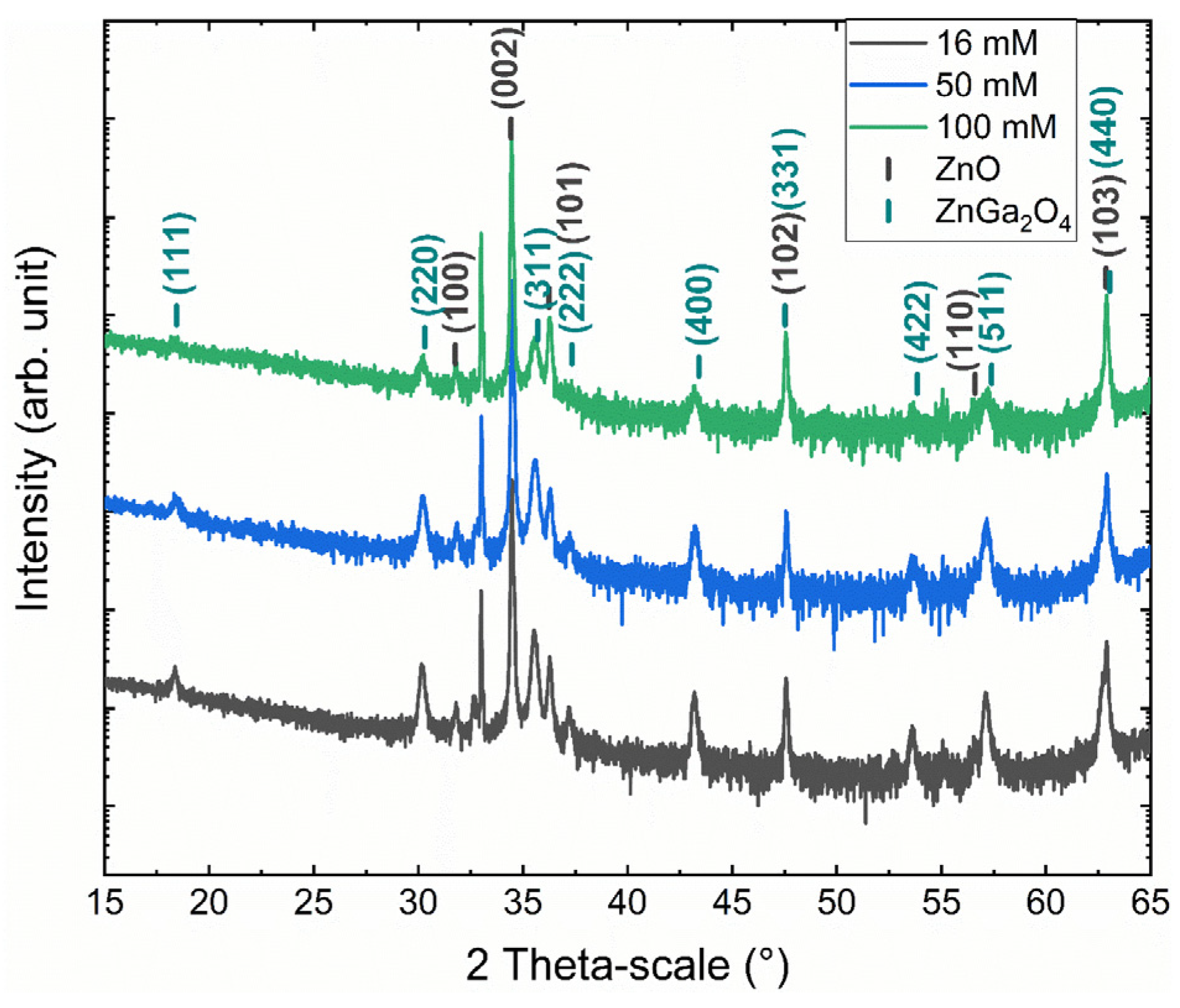
3.1. Effect of the Gallium Nitrate Concentration of the Solution on the ZnGa2O4 Shell Formation
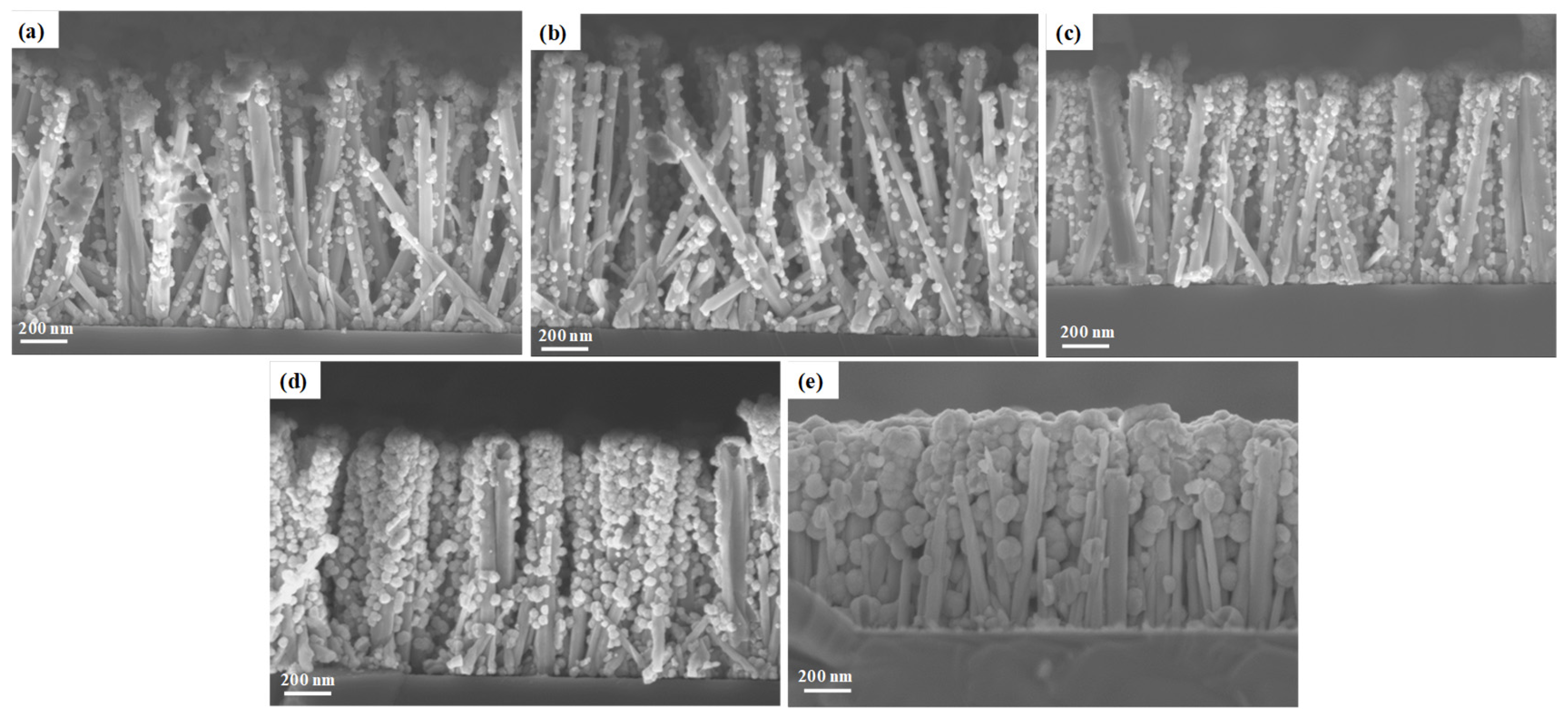
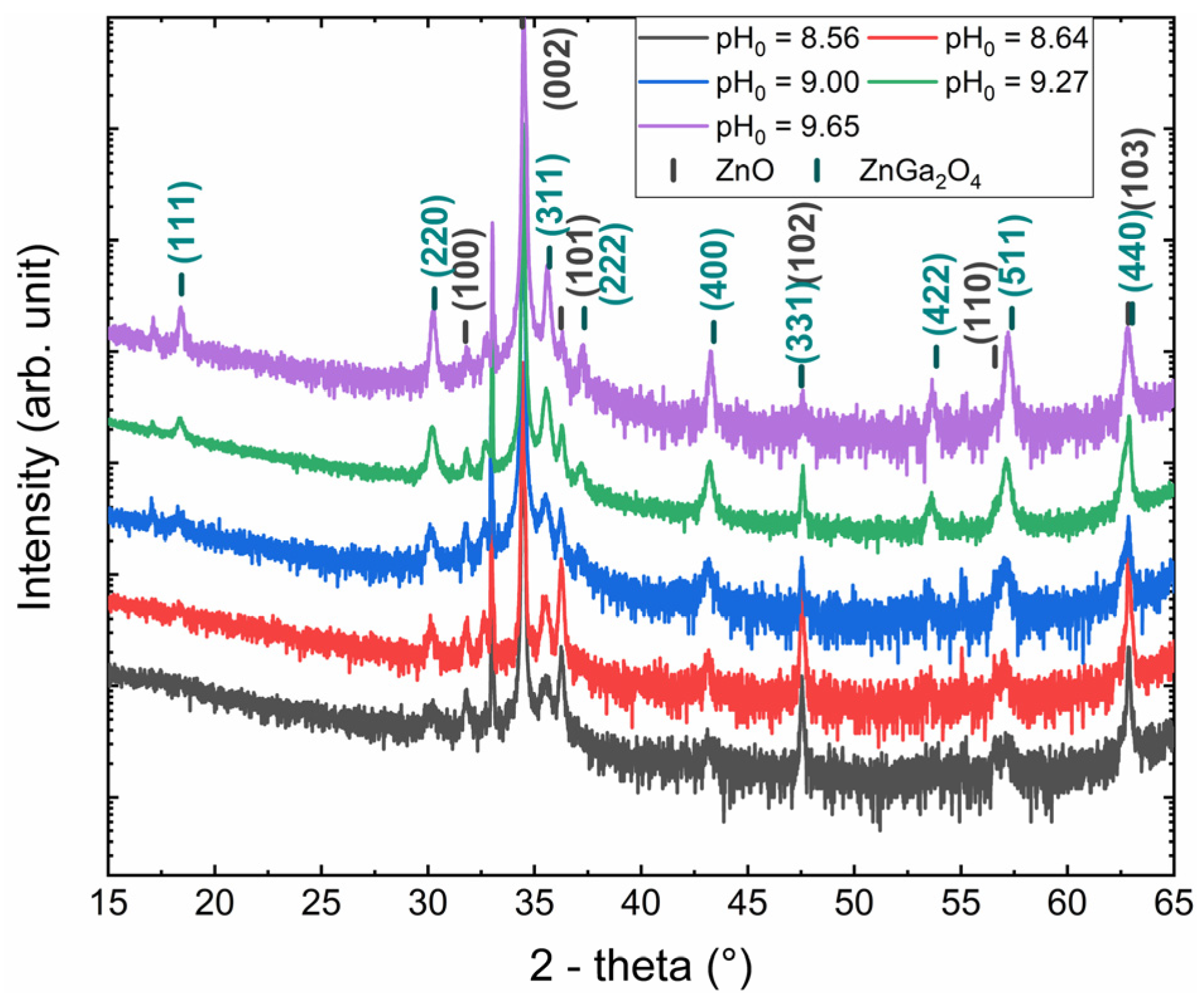
3.2. Effect of the pH0 of the Solution on the ZnGa2O4 Shell Formation
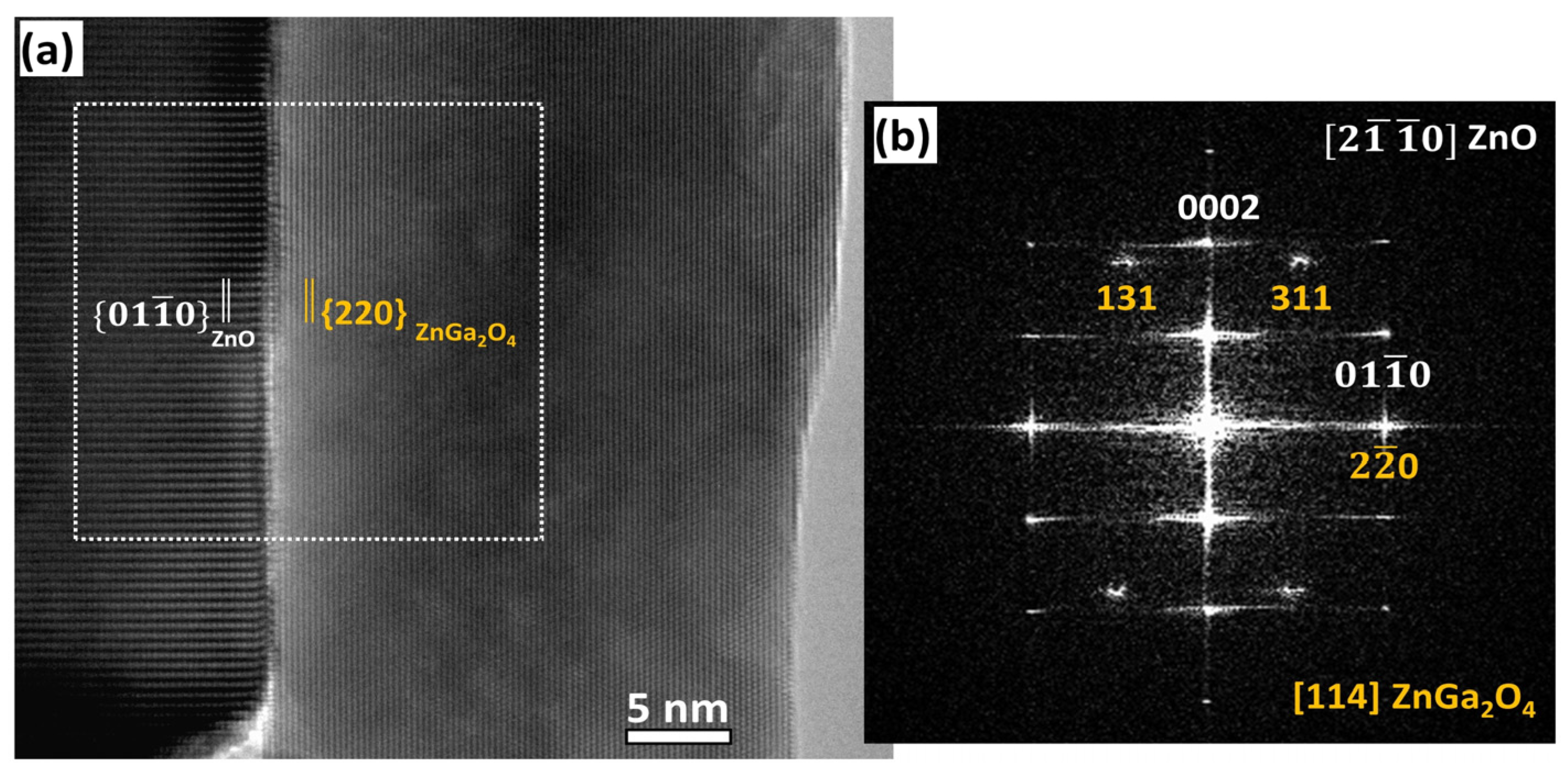
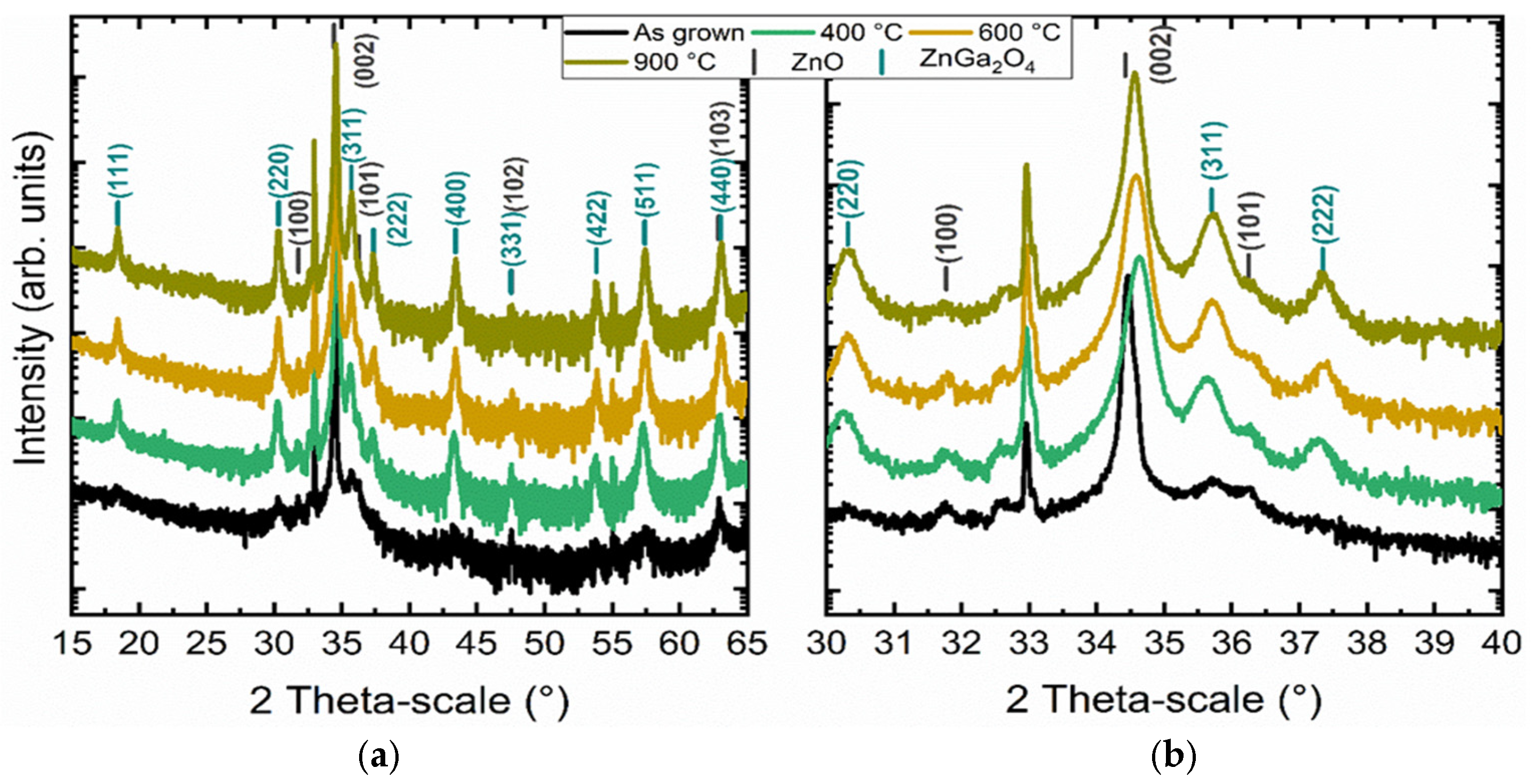
3.3. Study of the Crystallization Process of the ZnGa2O4 Shell
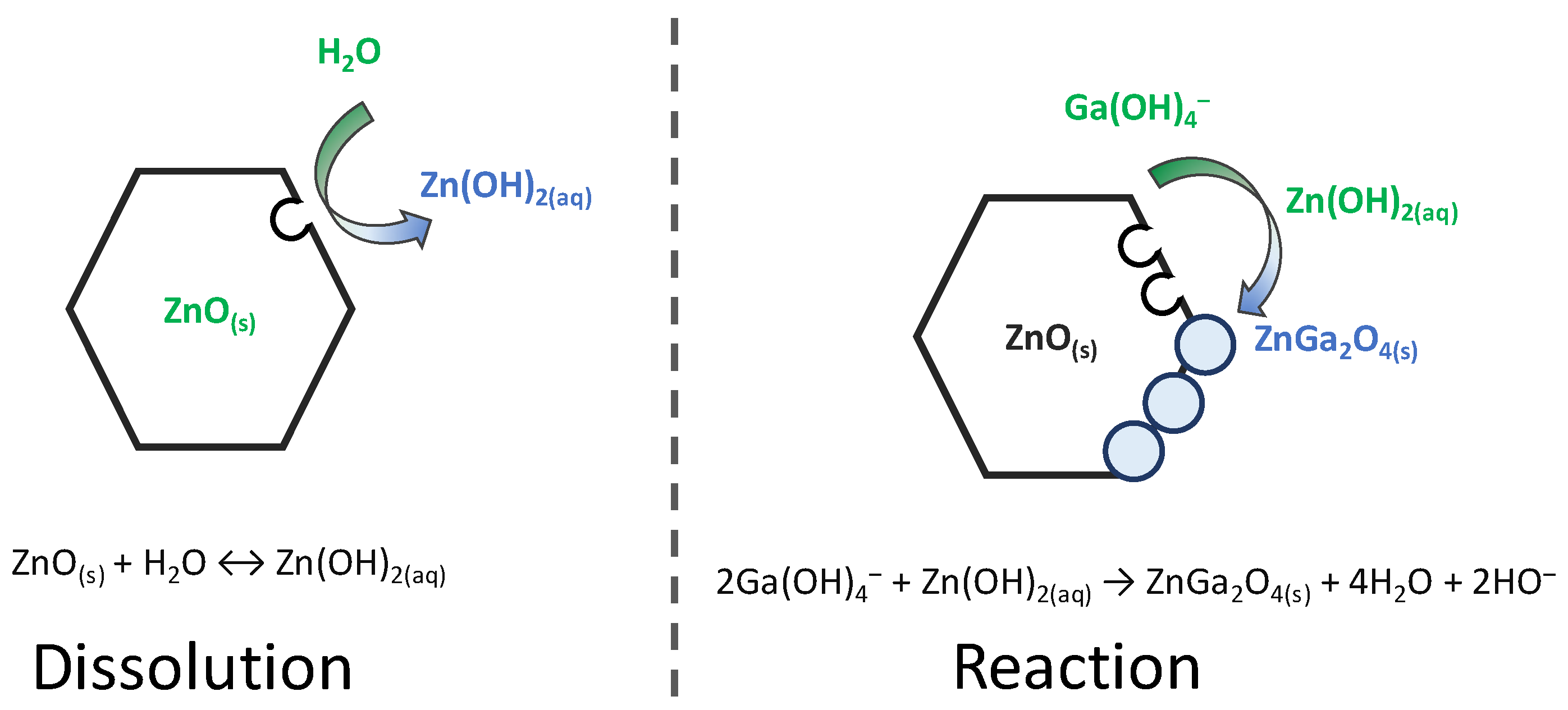
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, M.-I.; Singh, A.K.; Chiang, J.-L.; Horng, R.-H.; Wuu, D.-S. Zinc Gallium Oxide—A Review from Synthesis to Applications. Nanomaterials 2020, 10, 2208. [Google Scholar] [CrossRef]
- Chikoidze, E.; Sartel, C.; Madaci, I.; Mohamed, H.; Vilar, C.; Ballesteros, B.; Belarre, F.; del Corro, E.; Vales-Castro, P.; Sauthier, G.; et al. PType Ultrawide-Band-Gap Spinel ZnGa2O4: New Perspectives for Energy Electronics. Cryst. Growth Des. 2020, 20, 2535–2546. [Google Scholar] [CrossRef]
- Lee, Y.E.; Norton, D.P.; Budai, J.D.; Wei, Y. Enhanced Ultraviolet Photoconductivity in Semiconducting ZnGa2O4 Thin Films. J. Appl. Phys. 2001, 90, 3863–3866. [Google Scholar] [CrossRef]
- Chen, P.; Huang, S.; Yuan, S.; Chen, Y.; Hsiao, P.; Wuu, D. Quasi-Single-Crystalline ZnGa2O4 Films via Solid Phase Epitaxy for Enhancing Deep-Ultraviolet Photoresponse. Adv. Mater. Interfaces 2019, 6, 1901075. [Google Scholar] [CrossRef]
- Zhong, M.; Li, Y.; Tokizono, T.; Zheng, M.; Yamada, I.; Delaunay, J.-J. Vertically Aligned ZnO–ZnGa2O4 Core–Shell Nanowires: From Synthesis to Optical Properties. J. Nanopart. Res. 2012, 14, 804. [Google Scholar] [CrossRef]
- Chang, M.-P.; Chiang, M.-H.; Lin, W.-T.; Lee, C.-T. Growth of ZnGa2O4 Nanowires on a ZnO Buffer Layer by Carbothermal Reduction of Ga2O3 Powder. Mater. Lett. 2011, 65, 1473–1475. [Google Scholar] [CrossRef]
- Sei, T.; Nomura, Y.; Tsuchiya, T. Preparation of ZnGa2O4 Thin Film by Sol-Gel Process and Effect of Reduction on Its Electric Conductivity. J. Non Cryst. Solids 1997, 218, 135–138. [Google Scholar] [CrossRef]
- Hirano, M. Hydrothermal Synthesis and Characterization of ZnGa2O4 Spinel Fine Particles. J. Mater. Chem. 2000, 10, 469–472. [Google Scholar] [CrossRef]
- Li, Y.; Duan, X.; Liao, H.; Qian, Y. Self-Regulation Synthesis of Nanocrystalline ZnGa2O4 by Hydrothermal Reaction. Chem. Mater. 1998, 10, 17–18. [Google Scholar] [CrossRef]
- Katayama, I.; Iseda, A.; Kemori, N.; Kozuka, Z. Measurements of Standard Gibbs Energies of Formation of ZnO and ZnGa2O4 by E.M.F. Method. Trans. Jpn. Inst. Met. 1982, 23, 556–562. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Lu, Z.; Huang, K. Hydrothermal Synthesis and Characterization of ZnGa2O4 Phosphors. Mater. Chem. Phys. 2006, 97, 247–251. [Google Scholar] [CrossRef]
- Yan, S.; Wang, J.; Gao, H.; Wang, N.; Yu, H.; Li, Z.; Zhou, Y.; Zou, Z. An Ion-Exchange Phase Transformation to ZnGa2O4 Nanocube Towards Efficient Solar Fuel Synthesis. Adv. Funct. Mater. 2013, 23, 758–763. [Google Scholar] [CrossRef]
- Liang, H.; Meng, F.; Lamb, B.K.; Ding, Q.; Li, L.; Wang, Z.; Jin, S. Solution Growth of Screw Dislocation Driven α-GaOOH Nanorod Arrays and Their Conversion to Porous ZnGa2O4 Nanotubes. Chem. Mater. 2017, 29, 7278–7287. [Google Scholar] [CrossRef]
- Lu, M.-Y.; Zhou, X.; Chiu, C.-Y.; Crawford, S.; Gradečak, S. From GaN to ZnGa2O4 through a Low-Temperature Process: Nanotube and Heterostructure Arrays. ACS Appl. Mater. Interfaces 2014, 6, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, S.; Shibata, Y.; Hosono, E. Chemical Deposition of Rodlike GaOOH and β-Ga2O3 Films Using Simple Aqueous Solutions. J. Electrochem. Soc. 2005, 152, C764. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, S.; Wan, Y.; Gao, S.; Wang, D.; Wang, J. A Well-Grown β-Ga2O3 Microrod Array Formed from GaOOH on a Si (100) Substrate and Growth Mechanism Study. CrystEngComm 2018, 20, 4329–4335. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-Dimensional ZnO Nanostructures: Solution Growth and Functional Properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef]
- Guillemin, S.; Appert, E.; Roussel, H.; Doisneau, B.; Parize, R.; Boudou, T.; Brémond, G.; Consonni, V. Controlling the Strutcural Properties of Single Step, Dip Coated ZnO Seed Layers for Growing Perfectly Aligned Nanowire Arrays. J. Phys. Chem. C 2015, 119, 21694–21703. [Google Scholar] [CrossRef]
- Parize, R.; Garnier, J.; Chaix-Pluchery, O.; Verrier, C.; Appert, E.; Consonni, V. Effect of Hexamethylenetetramine on the Nucleation and Radial Growth of ZnO Nanowires by Chemical Bath Deposition. J. Phys. Chem. C 2016, 120, 5242–5250. [Google Scholar] [CrossRef]
- Hector, G.; Appert, E.; Sarigiannidou, E.; Matheret, E.; Roussel, H.; Chaix-Pluchery, O.; Consonni, V. Chemical Synthesis of β-Ga2O3 Microrods on Silicon and Its Dependence on the Gallium Nitrate Concentration. Inorg. Chem. 2020, 59, 15696–15706. [Google Scholar] [CrossRef] [PubMed]
- Kunze, C.; Valtiner, M.; Michels, R.; Huber, K.; Grundmeier, G. Self-Localization of Polyacrylic Acid Molecules on Polar ZnO(0001)–Zn Surfaces. Phys. Chem. Chem. Phys. 2011, 13, 12959–12967. [Google Scholar] [CrossRef] [PubMed]
- Valtiner, M.; Borodin, S.; Grundmeier, G. Stabilization and Acidic Dissolution Mechanism of Single-Crystalline ZnO(0001) Surfaces in Electrolytes Studied by in-Situ AFM Imaging and Ex-Situ LEED. Langmuir 2008, 24, 5350–5358. [Google Scholar] [CrossRef] [PubMed]
- Galazka, Z.; Ganschow, S.; Schewski, R.; Irmscher, K.; Klimm, D.; Kwasniewski, A.; Pietsch, M.; Fiedler, A.; Schulze-Jonack, I.; Albrecht, M.; et al. Ultra-Wide Bandgap, Conductive, High Mobility, and High Quality Melt-Grown Bulk ZnGa2O4 Single Crystals. APL Mater. 2019, 7, 022512. [Google Scholar] [CrossRef]
- Yamabi, S.; Imai, H. Growth Conditions for Wurtzite Zinc Oxide Films in Aqueous Solutions. J. Mater. Chem. 2002, 12, 3773–3778. [Google Scholar] [CrossRef]
- Hector, G.; Appert, E.; Roussel, H.; Gélard, I.; Consonni, V. Chemical Bath Deposition of α-GaOOH with Tunable Morphology on Silicon Using the pH Adjustment. Inorg. Chem. 2023, 62, 7764–7771. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga-Pérez, J.; Consonni, V.; Lymperakis, L.; Kong, X.; Trampert, A.; Fernández-Garrido, S.; Brandt, O.; Renevier, H.; Keller, S.; Hestroffer, K.; et al. Polarity in GaN and ZnO: Theory, Measurement, Growth, and Devices. Appl. Phys. Rev. 2016, 3, 041303. [Google Scholar] [CrossRef]
- Cossuet, T.; Appert, E.; Chaix-Pluchery, O.; Roussel, H.; Rapenne, L.; Renou, G.; Sauvage, F.; Consonni, V. Epitaxial TiO2 Shell Grown by Atomic Layer Deposition on ZnO Nanowires Using a Double-Step Process and Its Beneficial Passivation Effect. J. Phys. Chem. C 2020, 124, 13447–13455. [Google Scholar] [CrossRef]
- Cossuet, T.; Rapenne, L.; Renou, G.; Appert, E.; Consonni, V. Template-Assisted Growth of Open-Ended TiO2 Nanotubes with Hexagonal Shape Using Atomic Layer Deposition. Cryst. Growth Des. 2021, 21, 125–132. [Google Scholar] [CrossRef]
- Consonni, V.; Briscoe, J.; Karber, E.; Li, X.; Cossuet, T. ZnO Nanowires for Solar Cells: A Comprehensive Review. Nanotechnology 2019, 30, 362001. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Chen, J.; Shi, Z.; Fang, X. Self-Powered UV Photodetectors Based on ZnO Nanomaterials. Appl. Phys. Rev. 2021, 8, 031315. [Google Scholar] [CrossRef]
- Briscoe, J.; Dunn, S. Piezoelectric Nanogenerators—A Review of Nanostructured Piezoelectric Energy Harvesters. Nano Energy 2015, 14, 15–29. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hector, G.; Appert, E.; Roussel, H.; Bujak, A.; Sarigiannidou, E.; Consonni, V. Chemical Bath Deposition of ZnO/ZnGa2O4 Core–Shell Nanowire Heterostructures Using Partial Chemical Conversion. Nanomaterials 2024, 14, 991. https://doi.org/10.3390/nano14120991
Hector G, Appert E, Roussel H, Bujak A, Sarigiannidou E, Consonni V. Chemical Bath Deposition of ZnO/ZnGa2O4 Core–Shell Nanowire Heterostructures Using Partial Chemical Conversion. Nanomaterials. 2024; 14(12):991. https://doi.org/10.3390/nano14120991
Chicago/Turabian StyleHector, Guislain, Estelle Appert, Hervé Roussel, Anna Bujak, Eirini Sarigiannidou, and Vincent Consonni. 2024. "Chemical Bath Deposition of ZnO/ZnGa2O4 Core–Shell Nanowire Heterostructures Using Partial Chemical Conversion" Nanomaterials 14, no. 12: 991. https://doi.org/10.3390/nano14120991
APA StyleHector, G., Appert, E., Roussel, H., Bujak, A., Sarigiannidou, E., & Consonni, V. (2024). Chemical Bath Deposition of ZnO/ZnGa2O4 Core–Shell Nanowire Heterostructures Using Partial Chemical Conversion. Nanomaterials, 14(12), 991. https://doi.org/10.3390/nano14120991






