Synthesis of Mesoporous Silica Using the Sol–Gel Approach: Adjusting Architecture and Composition for Novel Applications
Abstract
1. Introduction
2. Synthesis of Mesoporous Silica with Different Architectures and Compositions
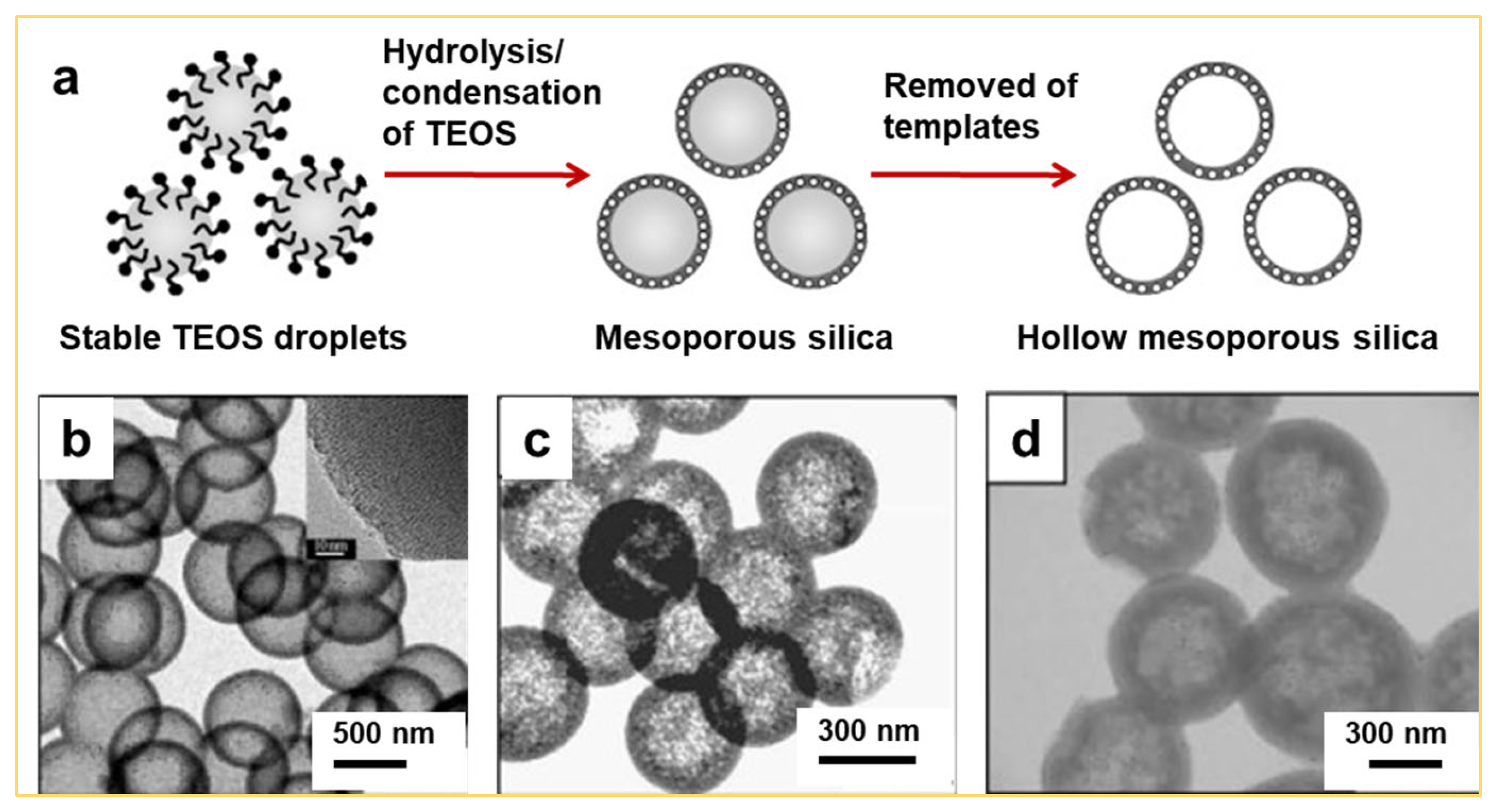
2.1. Synthesis of Hollow Mesoporous Silica Spheres by the Sol–Gel Approach
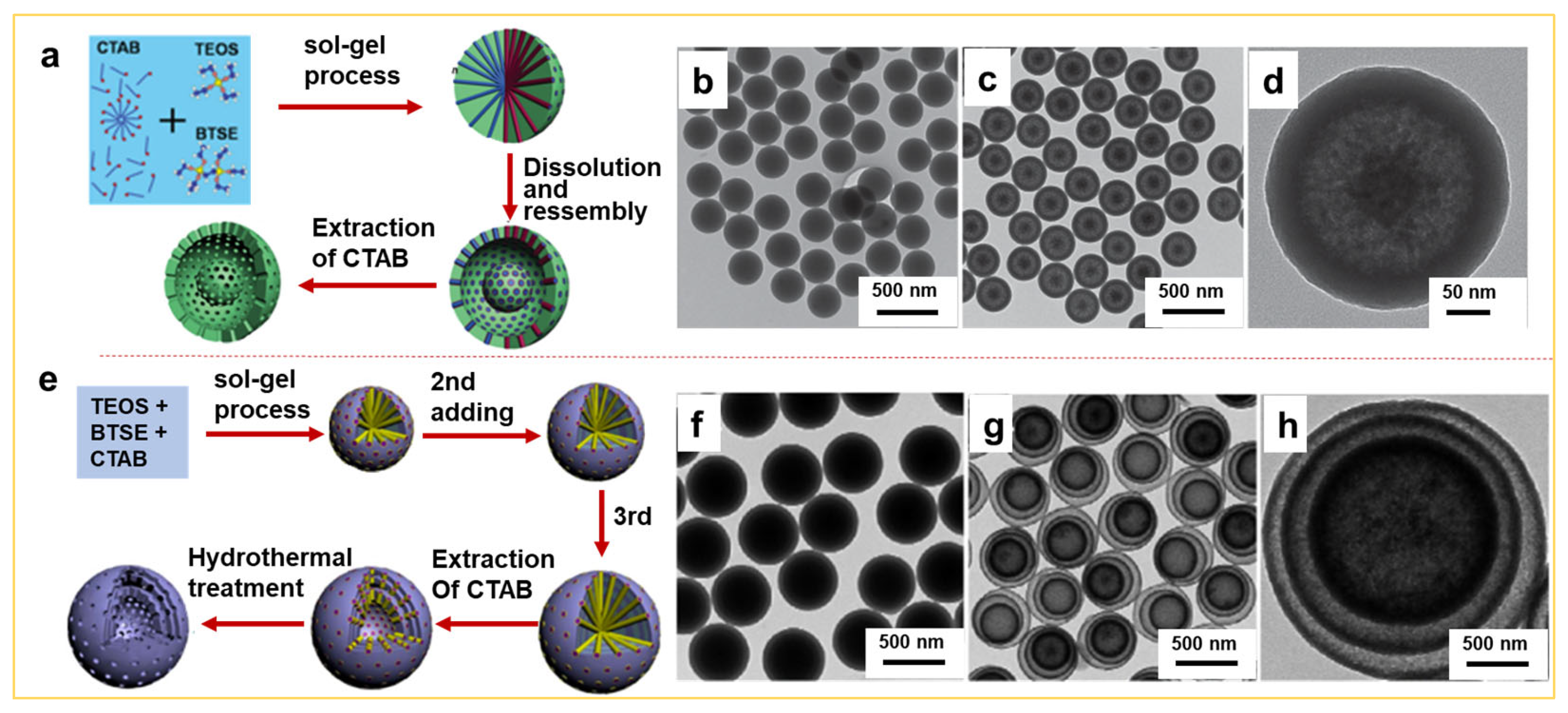
2.2. Synthesis of Yolk-Shell or Multi-Shelled Hollow Silica Spheres by the Sol–Gel Approach
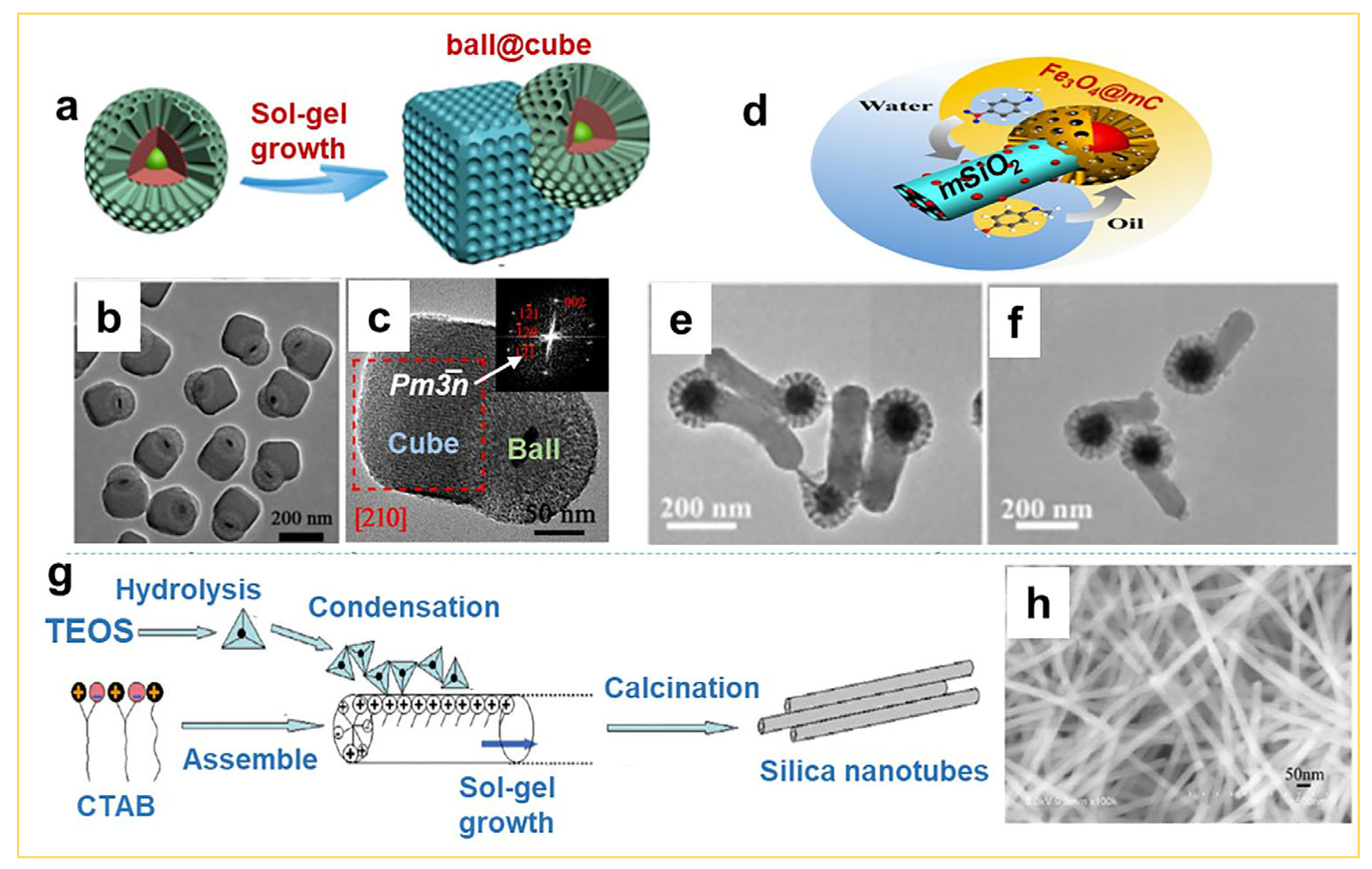
2.3. Synthesis of Non-Spherical Mesoporous Silica by the Sol–Gel Approach
2.4. Synthesis of Supported 2D Mesoporous Silica Membranes by the Sol–Gel Approach
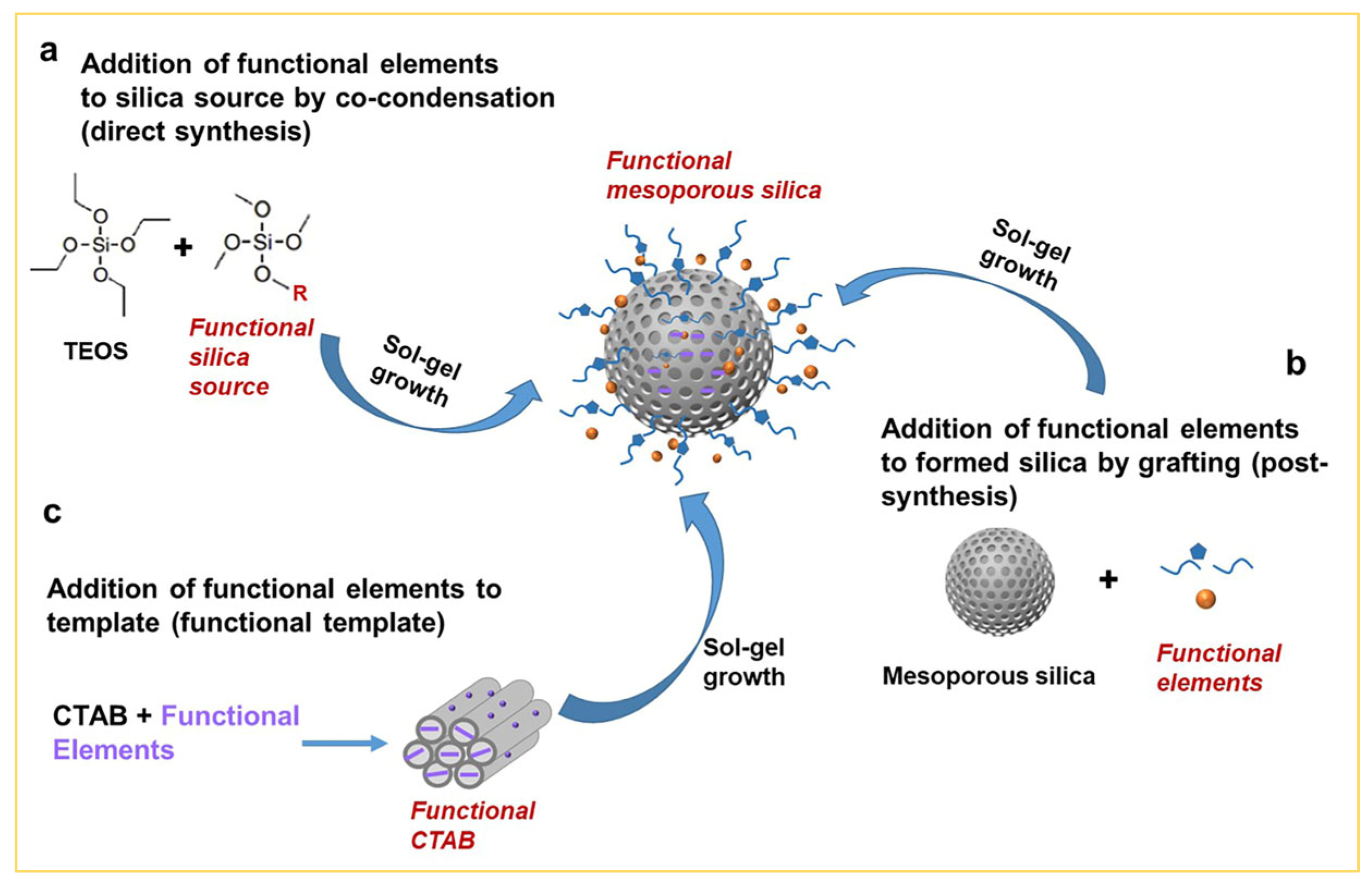
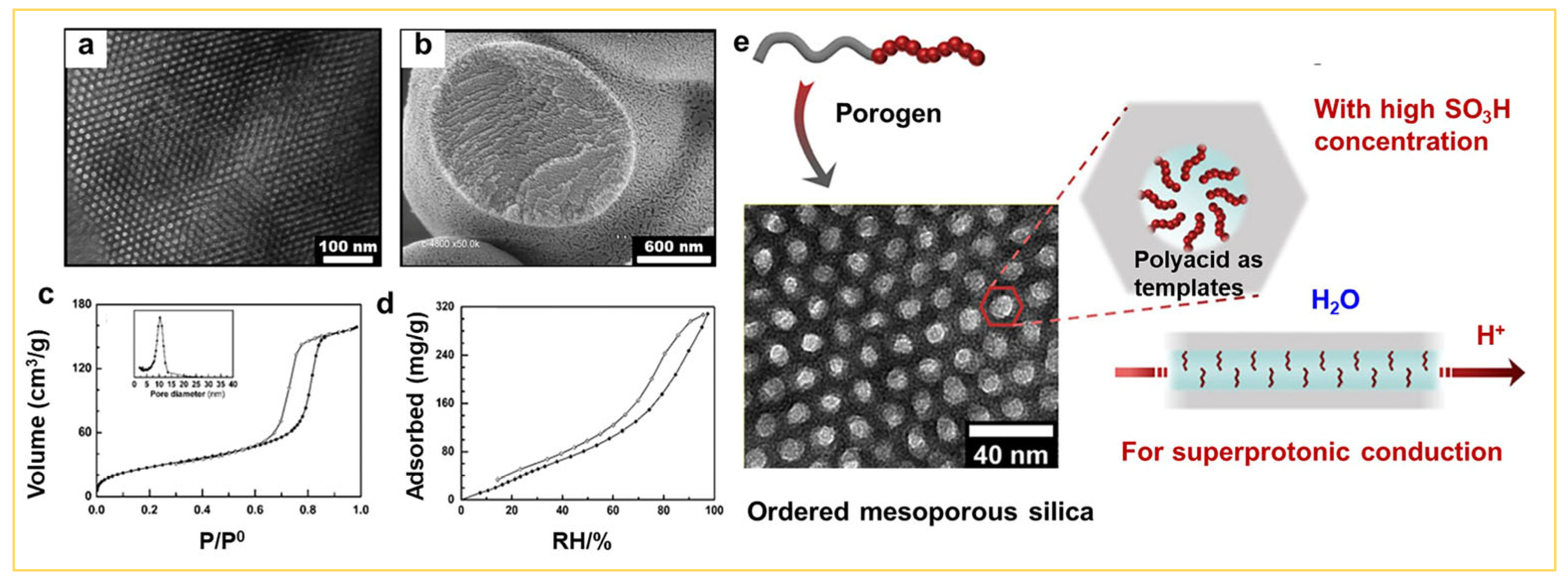
2.5. Functionalization of Mesoporous Silica by the Sol–Gel Approach
3. Applications of Mesoporous Silica
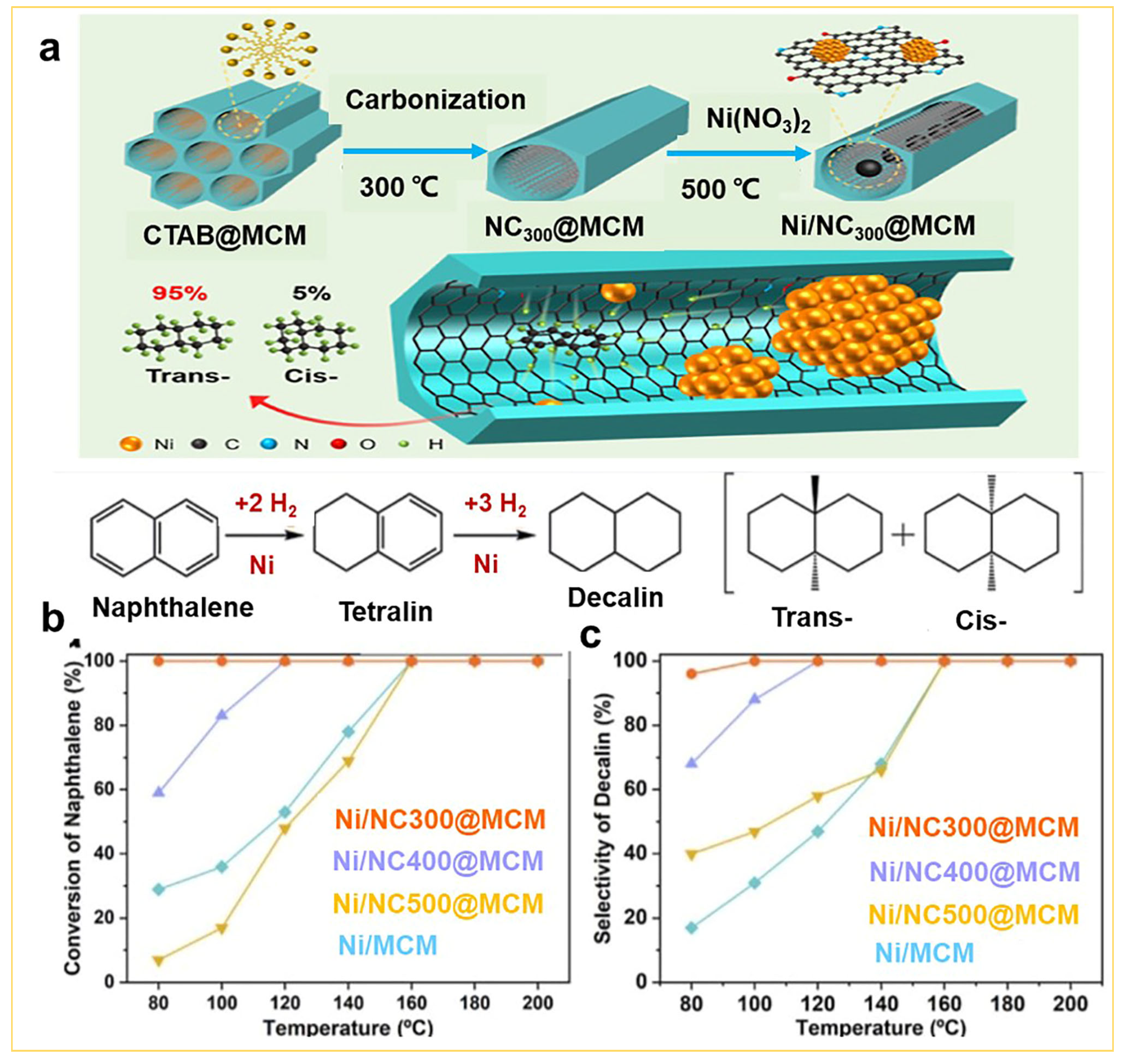
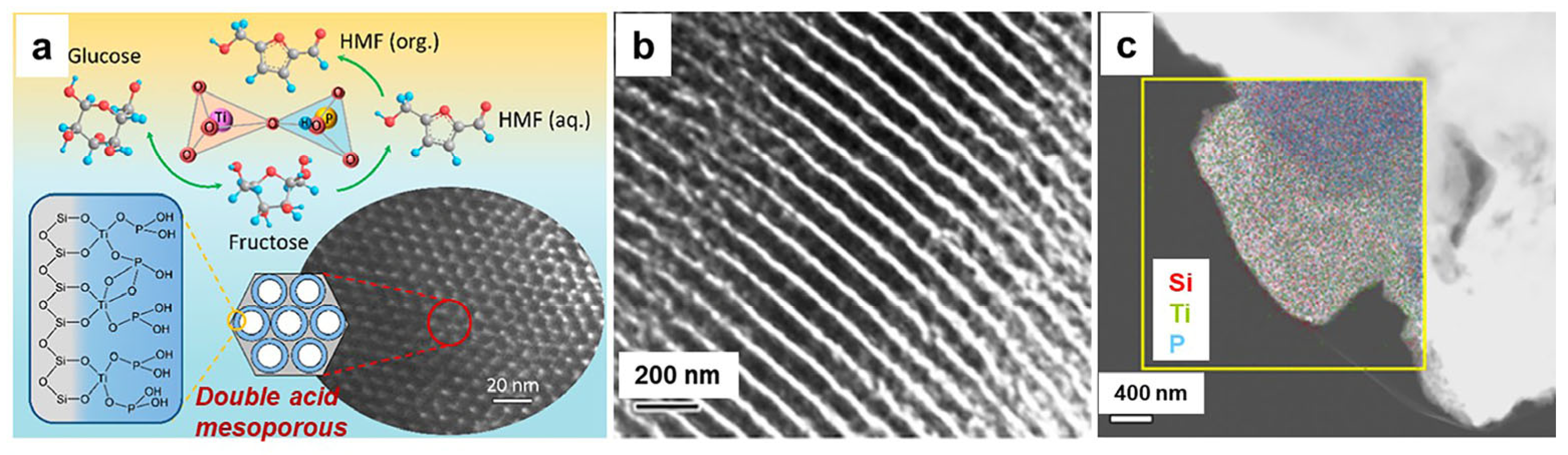
3.1. Catalytic Applications
3.2. Applications in Nanomedicines
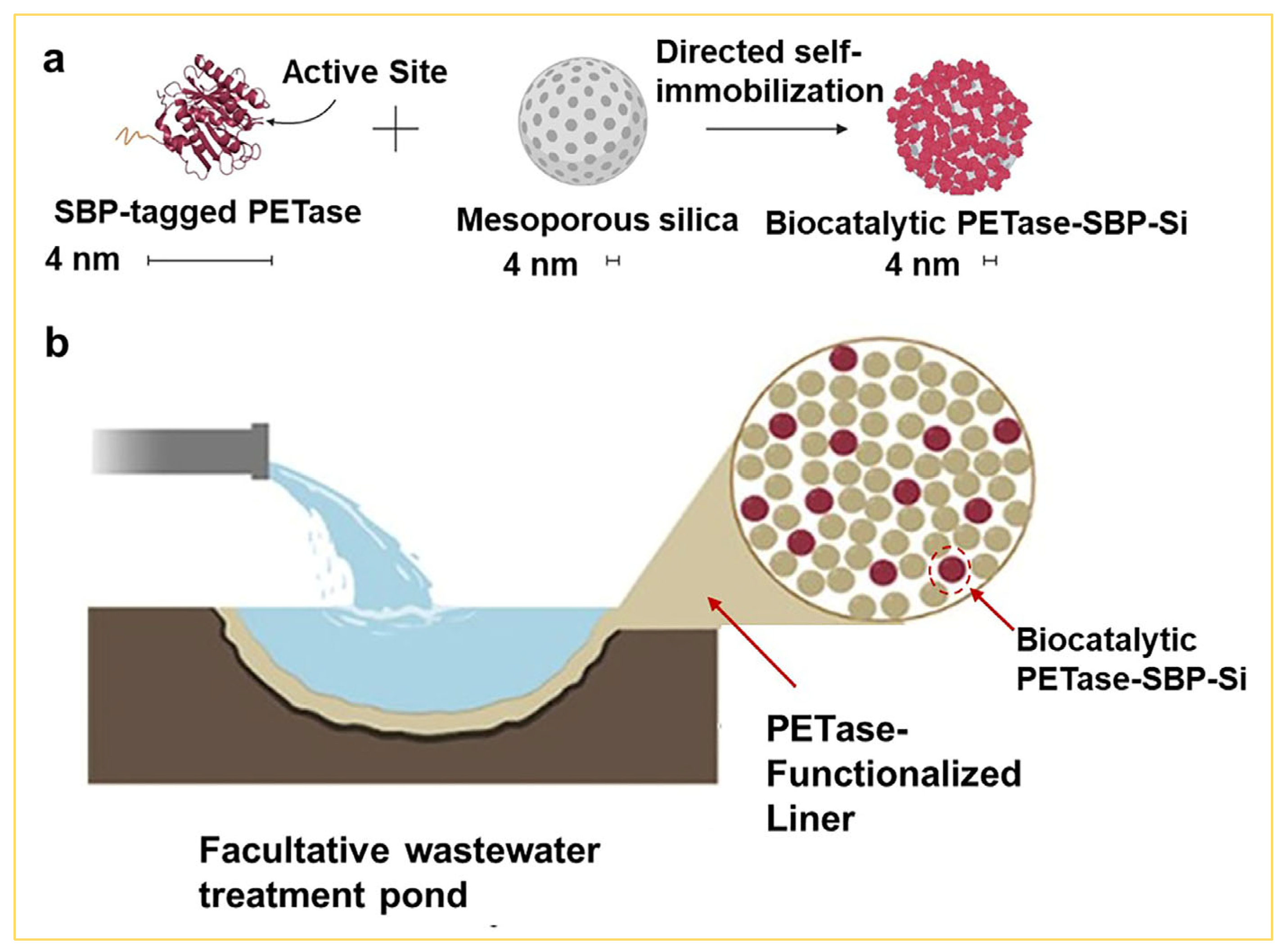
3.3. Environmental Applications
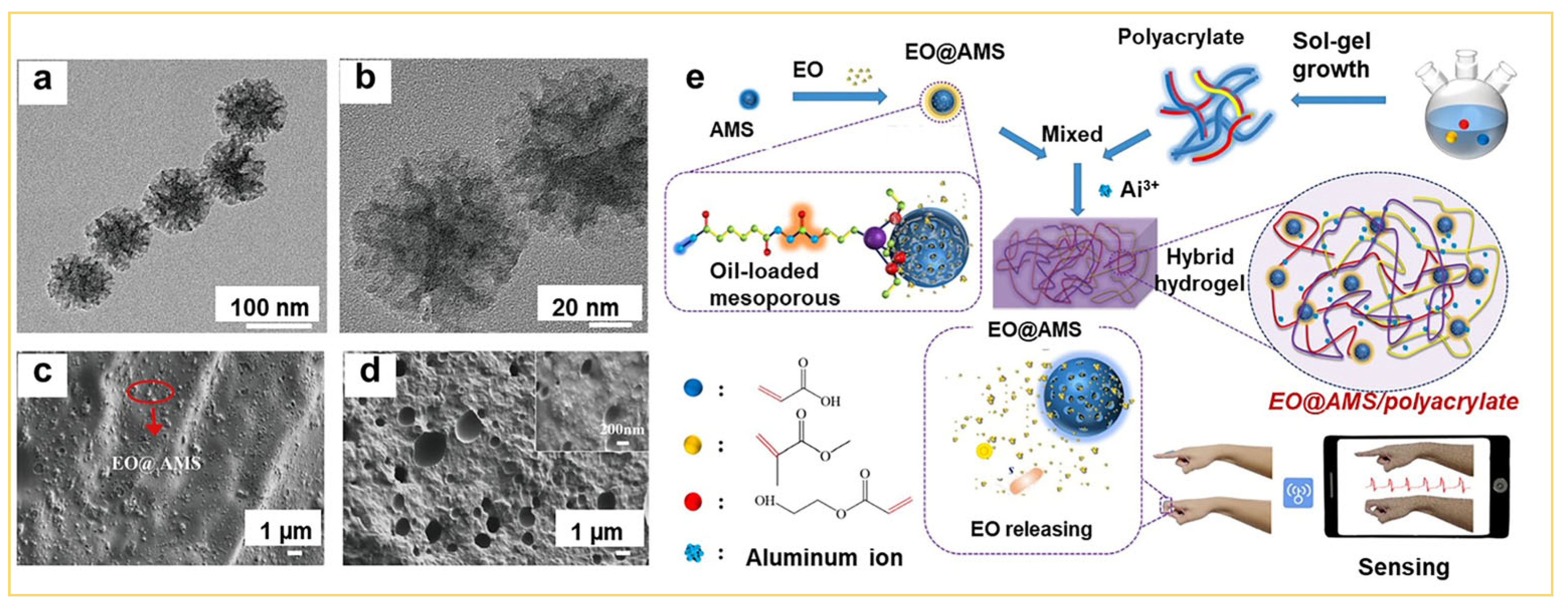
3.4. Other Applications
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Huo, Q.S.; Margoless, D.I.; Ciesla, U.; Stucky, G.D. Organization of organic molecules with inorganic molecular species into nanocomposite biphase arrays. Chem. Mater. 1994, 6, 1176–1191. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Cha, D.; Zhang, X.; Basset, J.M. High-surface-area silica nanospheres (KCC-1) with a fibrous morphology. Angew. Chem. Int. Ed. 2010, 49, 9652–9656. [Google Scholar] [CrossRef]
- Li, X.; Xing, L.; Hu, Y.; Xiong, Z.; Wang, R.; Xu, X.; Du, L.; Shen, M.; Shi, X. An RGD-modified hollow silica@Au core/shell nanoplatform for tumor combination therapy. Acta Biomater. 2017, 62, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Cashin, V.B.; Eldridge, D.S.; Yu, A.; Zhao, D. Surface functionalization and manipulation of mesoporous silica adsorbents for improved removal of pollutants: A review. Environ. Sci. Water Res. Technol. 2018, 4, 110–128. [Google Scholar] [CrossRef]
- Wu, S.H.; Moua, C.Y.; Lin, H.P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Shi, J.L.; Shen, W.H.; Dong, X.P.; Feng, J.W.; Ruan, M.L.; Li, Y.S. Stimuli-responsive controlled drug release from a hollow mesoporous silica sphere/polyelectrolyte multilayer core–shell structure. Angew. Chem. Int. Ed. 2005, 44, 5083–5087. [Google Scholar] [CrossRef]
- Cao, S.S.; Chang, J.; Fang, L.; Wu, L.M. Metal nanoparticles confined in the nanospace of double-shelled hollow silica spheres for highly efficient and selective catalysis. Chem. Mater. 2016, 28, 5596–5600. [Google Scholar] [CrossRef]
- Jang, K.S.; Kim, H.J.; Johnson, J.R.; Kim, W.G.; Koros, W.J.; Jones, C.W.; Nair, S. Modified mesoporous silica gas separation membranes on polymeric hollow fibers. Chem. Mater. 2011, 23, 3025–3028. [Google Scholar] [CrossRef]
- Chen, D.; Li, L.L.; Tang, F.Q.; Qi, S. Facile and scalable synthesis of tailored silica ‘‘nanorattle’’ structures. Adv. Mater. 2009, 21, 3804–3807. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wu, J.; Zhou, L.P.; Zhang, D.Y.; Qi, L.M. Facile synthesis of monodisperse microspheres and gigantic hollow shells of mesoporous silica in mixed water-ethanol solvents. Langmuir 2007, 23, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Shiokawa, K.; Tanaka, Y.; Nakahara, Y. Preparation and formation mechanism of silica microcapsules (hollow sphere) by water/oil/water interfacial reaction. Chem. Mater. 2004, 16, 5420–5426. [Google Scholar] [CrossRef]
- Teng, Z.G.; Han, Y.D.; Li, J.; Yan, F.; Yang, W.S. Preparation of hollow mesoporous silica spheres by a sol–gel/emulsion approach. Micropor. Mesopor. Mat. 2010, 127, 67–72. [Google Scholar] [CrossRef]
- Li, W.J.; Sha, X.X.; Dong, W.J.; Wang, Z.C. Synthesis of stable hollow silica microspheres with mesoporous shell in nonionic W/O emulsion. Chem. Commun. 2002, 20, 2434–2435. [Google Scholar] [CrossRef]
- Li, G.L.; Shi, Q.; Yuan, S.J.; Neoh, K.G.; Kang, E.T.; Yang, X.L. Alternating silica/polymer multilayer hybrid microspheres templates for double-shelled polymer and inorganic hollow microstructures. Chem. Mater. 2010, 22, 1309–1317. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, H.J.; Wu, M.H.; Zhong, Y.F.; Liu, X.W.; Jiao, Z. Dual-templating synthesis of multi-shelled mesoporous silica nanoparticles as catalyst and drug carrier. Micropor. Mesopor. Mat. 2016, 228, 318–328. [Google Scholar] [CrossRef]
- Huang, C.C.; Huang, W.; Yeh, C.S. Shell-by-shell synthesis of multi-shelled mesoporous silica nanospheres for optical imaging and drug delivery. Biomaterials 2011, 32, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Wei, Z.P.; Han, H.J.; Wang, X.B.; Cui, K.Q.; Yang, L. Tunable construction of multi-shell hollow SiO2microspheres with hierarchically porous structure as high-performance anodes for lithiumion batteries. Chem. Eng. J. 2017, 323, 252–259. [Google Scholar] [CrossRef]
- Patoka, P.; Giersig, M. Self-assembly of latex particles for the creation of nanostructures with tunable plasmonic properties. J. Mater. Chem. 2011, 21, 16783–16796. [Google Scholar] [CrossRef]
- Tang, F.Q.; Li, L.L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiao, S.Z.; Chen, J.S.; Lou, X.W.; Xing, X.R.; Lu, G.Q. Yolk/shell nanoparticles: New platforms for nanoreactors, drug delivery and lithium-ion batteries. Chem. Commun. 2011, 47, 12578–12591. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.G.; Su, X.D.; Zheng, Y.Y.; Zhang, J.J.; Liu, Y.; Wang, S.J.; Wu, J.; Chen, G.T.; Wang, J.D.; Zhao, D.Y.; et al. A facile multi-interface transformation approach to monodisperse multiple-shelled periodic mesoporous organosilica hollow spheres. J. Am. Chem. Soc. 2015, 137, 7935–7944. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.G.; Wang, S.J.; Su, X.D.; Chen, G.T.; Liu, Y.; Luo, Z.M.; Luo, W.; Tang, Y.X.; Ju, H.X.; Zhao, D.Y.; et al. Facile synthesis of yolk–shell structured inorganic–organic hybrid spheres with ordered radial mesochannels. Adv. Mater. 2014, 26, 3741–3747. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Wu, S.H.; Tseng, C.T.; Hung, Y.; Chang, C.; Mou, C.Y. Synthesis of hollow silica nanospheres with a microemulsion as the template. Chem. Commun. 2009, 3542–3544. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Zhou, L.; Wei, Y.; El-Toni, A.M.; Zhang, F.; Zhao, D.Y. Anisotropic growth-induced synthesis of dual-compartment Janus mesoporous silica nanoparticles for bimodal triggered drugs delivery. J. Am. Chem. Soc. 2014, 136, 15086–15092. [Google Scholar] [CrossRef]
- Zhao, T.C.; Zhu, X.H.; Hung, C.T.; Wang, P.Y.; Elzatahry, A.; Al-Khalaf, A.A.; Hozzein, W.N.; Zhang, F.; Li, X.M.; Zhao, D.Y. Spatial isolation of carbon and silica in a single janus mesoporous nanoparticle with tunable amphiphilicity. J. Am. Chem. Soc. 2018, 140, 10009–10015. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zhang, J.; Wang, J.R.; Huang, J.B. Self-catalytic sol-gel synergetic replication of uniform silica nanotubes using an amino acid amphiphile dynamically growing fibers as template. Langmuir 2010, 26, 4288–4295. [Google Scholar] [CrossRef]
- Yang, H.; Coombs, N.; Sokolov, I.; Ozin, G.A. Free-standing and oriented mesoporous silica films grown at the air–water interface. Nature 1996, 381, 589–592. [Google Scholar] [CrossRef]
- Schacht, S.; Huo, Q.; Voigt-Martin, I.G.; Stucky, G.D.; Schuth, F. Oil-water interface templating of mesoporous macroscale structures. Science 1996, 273, 768–771. [Google Scholar] [CrossRef]
- Nicole, L.; Boissière, C.; Grosso, D.; Quach, A.; Sanchez, C. Mesostructured hybrid organic–inorganic thin films. J. Mater. Chem. 2005, 15, 3598–3627. [Google Scholar] [CrossRef]
- Hara, M.; Nagano, S.; Seki, T. π-π Interaction-induced vertical alignment of silica mesochannels templated by a discotic lyotropic liquid crystal. J. Am. Chem. Soc. 2010, 132, 13654–13656. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.-W.; Jiang, X.F.; Yamauchi, Y. New trend on mesoporous films: Precise controls of one-dimensional (1D) mesochannels toward innovative applications. J. Mater. Chem. 2011, 21, 8934–8939. [Google Scholar] [CrossRef]
- Teng, Z.G.; Zheng, G.F.; Dou, Y.Q.; Li, W.; Mou, C.-Y.; Zhang, X.H.; Asiri, A.M.; Zhao, D.Y. Highly ordered mesoporous silica films with perpendicular mesochannels by a simple Stöber-solution growth approach. Angew. Chem. Int. Ed. 2012, 51, 2173–2177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Shen, D.K.; Chen, G.; Elzatahry, A.A.; Pal, M.; Zhu, H.W.; Wu, L.L.; Lin, J.J.; Al-Dahyan, D.; Li, W.; et al. Mesoporous silica thin membranes with large vertical mesochannels for nanosize-based separation. Adv. Mater. 2017, 29, 1702274. [Google Scholar] [CrossRef] [PubMed]
- Nooney, R.I.; Kalyanaraman, M.; Kennedy, G.; Maginn, E.J. Heavy metal remediation using functionalized mesoporous silicas with controlled macrostructure. Langmuir 2001, 17, 528–533. [Google Scholar] [CrossRef]
- Yang, C.-M.; Zibrowius, B.; Schüth, F. A novel synthetic route for negatively charged ordered mesoporous silica SBA-15. Chem. Commun. 2003, 14, 1772–1773. [Google Scholar] [CrossRef]
- Ba, J.W.; Han, Y.D.; Zhang, L.; Yang, W.S. Au (III) cross-linked hollow organosilica capsules from 3-aminopropyltriethoxysilane. J. Colloid. Interf. Sci. 2023, 641, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Goubert-Renaudin, S.; Schneider, R.; Walcarius, A. Synthesis of new dithiocarbamate-based organosilanes for grafting on silica. Tetrahedron Lett. 2007, 48, 2113–2116. [Google Scholar] [CrossRef]
- Vinu, A.; Hossain, K.Z.; Ariga, K. Recent advances in functionalization of mesoporous silica. J. Nanosci. Nanotechno. 2005, 5, 347–371. [Google Scholar] [CrossRef]
- Lu, Y.F.; Yang, Y.; Sellinger, A.; Lu, M.C.; Huang, J.M.; Fan, H.Y.; Haddad, R.; Lopez, G.; Burns, A.R.; Sasaki, D.Y.; et al. Self-assembly of mesoscopically ordered chromatic polydiacetylene/silica nanocomposites. Nature 2001, 410, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.L.; Huang, Z.Z.; Wang, Y.F.; Wang, D.Y.; Han, M.-Y.; Yang, W.S. Phase engineering of hydrophobic me-so-environments in silica particles for technical performance enrichment. Langmuir 2018, 34, 7428–7435. [Google Scholar] [CrossRef] [PubMed]
- Fapojuwo, D.P.; Akinnawo, C.A.; Oseghale, C.O.; Meijboom, R. Tailoring the surface wettability of mesoporous silica for selective hydrogenation of cinnamaldehyde to hydrocinnamaldehyde in a Pickering emulsion system. Colloid. Surface. A 2022, 655, 130231. [Google Scholar] [CrossRef]
- Haynes, T.; Bougnouch, O.; Dubois, V.; Hermans, S. Preparation of mesoporous silica nanocapsules with a high specific surface area by hard and soft dual templating approach: Application to biomass valorization catalysis. Micropor. Mesopor. Mat. 2020, 306, 110400. [Google Scholar] [CrossRef]
- Sosa, A.A.; Palermo, V.; Langer, P.; Luque, R.; Romanelli, G.P.; Pizzio, L.R. Tungstophosphoric acid/mesoporous silicas as suitable catalysts in quinoxaline synthesis. Mol. Catal. 2022, 517, 112046. [Google Scholar] [CrossRef]
- Luo, L.; Bock, L.; Liang, Y.; Anwander, R. Gold-loaded mesoporous organosilica-silica core-shell nanoparticles as catalytic nanoreactors. Eur. J. Inorg. Chem. 2020, 41, 3967–3976. [Google Scholar] [CrossRef]
- Ma, Y.Z.; Nagy, G.; Siebenbürger, M.; Kaur, R.; Dooley, K.M.; Bharti, B. Adsorption and catalytic activity of gold nano-particles in mesoporous silica: Effect of pore size and dispersion salinity. J. Phys. Chem. C 2022, 126, 2531–2541. [Google Scholar] [CrossRef]
- Shu, Y.; Song, X.Y.; Lan, F.J.; Zhao, C.Y.; Guan, Q.X.; Li, W. N-Doped carbon interior-modified mesoporous silica-confined nickel nanoclusters for stereoselective hydrogenation. ACS Catal. 2022, 12, 15386–15399. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Guo, W.; Hensen, E.J.M.; Qi, W.; Heeres, H.J.; Jun Yue, J. Titanium phosphate grafted on mesoporous SBA-15 silica as a solid acid catalyst for the synthesis of 5-hydroxymethylfurfural from glucose. ACS Sustain. Chem. Eng. 2022, 10, 10157–10168. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, Z.; Wang, J.; Shen, J.; Hao, Q.; Li, Y.; Yang, J.; Niu, Y.; Xiao, Z.; Chen, L.; et al. Preparation of mesoporous silica nanoparticle with tunable pore diameters for encapsulating and slowly releasing eugenol. Chin. Chem. Lett. 2021, 32, 1755–1758. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Cai, L.; Li, X.; Lin, K.; Wu, X.J.; Wang, Z.Q.; Yu, D.H.; Li, J. Contribution of molecular chiral mesoporous silica nanoparticles in delivering drugs with chiral recognition ability. Mater. Sci. Eng. B 2022, 284, 115864. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Zhang, W.W.; Song, P.; Li, W.Z.; Chen, X.L.; Ge, F.; Gui, L.; Yang, K.; Tao, Y.G.; Du, G.C. Redox-responsive mesoporous silica nanoparticles for chemophotodynamic combination cancer therapy. Mater. Res. Express 2022, 9, 045401. [Google Scholar] [CrossRef]
- Chang, J.; Mo, L.F.; Song, J.F.; Wang, X.C.; Liu, H.H.; Meng, C.C.; Wu, Y.J. A pH-responsive mesoporous silica nano-particlebased drug delivery system for targeted breast cancer therapy. J. Mater. Chem. B 2022, 10, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Kim, C. Mesoporous silica nanoparticles with cyclic peptide gatekeeper for stimulus responsive drug release by conformational transformation. J. Nanopart. Res. 2022, 24, 22. [Google Scholar] [CrossRef]
- Aquib, M.; Zhang, H.; Raza, F.; Banerjee, P.; Bavi, R.; Kesse, S.; Boakye-Yiadom, K.O.; Filli, M.S.; Farooq, M.A.; Wang, B. Delivery of repurposed disulfiram by aminated mesoporous silica nanoparticles for anticancer therapy. J. Mol. Liq. 2022, 346, 117065. [Google Scholar] [CrossRef]
- Zhao, N.; Cai, R.F.; Zhang, Y.T.; Wang, X.L.; Zhou, N.D. A pH-gated functionalized hollow mesoporous silica delivery system for photodynamic sterilization in staphylococcus aureus biofilm. Materials 2022, 15, 2815. [Google Scholar] [CrossRef] [PubMed]
- Candela-Noguera, V.; Vivo-Llorca, G.; de Greñu, B.D.; Alfonso, M.; Aznar, E.; Orzáez, M.; Marcos, M.D.; Sancenón, F.; Martínez-Máñez, R. Gene-directed enzyme prodrug therapy by dendrimer-like mesoporous silica nanoparticles against tumor cells. Nanomaterials 2021, 11, 1298. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Wang, X.L.; Lu, Z.H.; Chang, C.; Zhang, Y.L.; Li, Y.Y.; Yi, M.Q.; Xiong, B.; Lu, B. Lactobionic acid-functionalized hollow mesoporous silica nanoparticles for cancer chemotherapy and phototherapy. Process Biochem. 2022, 121, 698–706. [Google Scholar] [CrossRef]
- Kong, J.; Park, S.S.; Ha, C.-S. pH-sensitive polyacrylic acid-gated mesoporous silica nanocarrier incorporated with calcium ions for controlled drug release. Materials 2022, 15, 5926. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Shi, J.Y.; Pan, H.; Liu, M.X.; Sang, Y.L.; Ai, J.; Liu, Y.; Chen, L.J. Membrane-cloaked polydopamine modified mesoporous silica nanoparticles for cancer therapy. Nanotechnology 2022, 3, 345101. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.; Carvalho, A.; Durães, L.; Faneca, H. Triantennary GalNAc-functionalized multi-responsive mesoporous silica nanoparticles for drug delivery targeted at asialoglycoprotein receptor. Int. J. Mol. Sci. 2022, 23, 6243. [Google Scholar] [CrossRef] [PubMed]
- Gowsalya, K.; Karthikeyan, L.; Vivek, R. Near-infrared light-activated dual targeting with peptide-conjugated mesoporous silica nanoparticles for multimodal anticancer therapy. ACS Appl. Nano Mater. 2022, 5, 17105–17122. [Google Scholar] [CrossRef]
- Carstens, M.R.; Fisher, R.C.; Acharya, A.P.; Butterworth, E.A.; Scott, E.; Huang, E.H.; Keselowsky, B.G. Drug-eluting microarrays to identify effective chemotherapeutic combinations targeting patient-derived cancer stem cells. Proc. Natl. Acad. Sci. USA 2015, 112, 8732–8737. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Z.; Zhu, P.P.; Zhang, Y.S.; Liu, Y.T.; He, Y.L.; Zhang, L.F.; Gao, Y.F. Enhanced sensitivity of cancer stem cells to chemotherapy using functionalized mesoporous silica nanoparticles. Mol. Pharm. 2016, 13, 2749–2759. [Google Scholar] [CrossRef]
- Kobylinska, N.G.; Kessler, V.G.; Seisenbaeva, G.A.; Dudarko, O.A. In situ functionalized mesoporous silicas for sustainable remediation strategies in removal of inorganic pollutants from contaminated environmental water. ACS Omega 2022, 7, 23576–23590. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, H.; Wei, C.; Yang, L.; Cao, C.; Yang, S.; Zhang, A.Z. Feasibility evaluation of the terminated waste energy in situ conversion strategy toward carbon neutralization in metallurgical processes. ACS Sustainable Chem. Eng. 2021, 9, 14079–14089. [Google Scholar] [CrossRef]
- Li, Q. The view of technological innovation in coal industry under the vision of carbon neutralization. Int. J. Coal Sci. Technol. 2021, 8, 1197–1207. [Google Scholar] [CrossRef]
- Rafigh, S.M.; Heydarinasab, A. Mesoporous chitosan-SiO2 nanoparticles: Synthesis, characterization, and CO2 Adsorption Capacity. ACS Sustain. Chem. Eng. 2017, 5, 10379–10386. [Google Scholar] [CrossRef]
- Barbosa, M.N.; Araujo, A.S.; Galvão, L.P.F.C.; Silva, E.F.B.; Santos, A.G.D.; Luz, G.E.; Fernandes, V.J. Carbon dioxide adsorption over DIPA functionalized MCM-41 and SBA-15 molecular sieves. J. Therm. Anal. Calorim. 2011, 106, 779–782. [Google Scholar] [CrossRef]
- Anyanwu, J.T.; Wang, Y.; Yang, R.T. Amine-grafted silica gels for CO2 capture including direct air capture. Ind. Eng. Chem. Res. 2020, 59, 7072–7079. [Google Scholar] [CrossRef]
- Oliveira, D.E.F.; Chagas, J.A.O.; de Lima, A.L.; Mota, C.J.A. CO2 capture over MCM-41 and SBA-15 mesoporous silicas impregnated with chitosan. Ind. Eng. Chem. Res. 2022, 61, 10522–10530. [Google Scholar] [CrossRef]
- Zhang, K.; Hamidian, A.H.; Tubic’, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liu, H.; Gao, S.; Weng, Y.; Zhu, L. Enhanced extracellular production of Is PETase in escherichia coli via engineering of the PelB signal peptide. J. Agric. Food Chem. 2021, 69, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chen, Y.; Liu, X.; Dong, S.; Tian, Y.; Qiao, Y.; Mitra, R.; Han, J.; Li, C.; Han, X.; et al. Computational redesign of a PETase for plastic biodeg-radation under ambient condition by the GRAPE strategy. ACS Catal. 2021, 11, 1340–1350. [Google Scholar] [CrossRef]
- Lu, J.; Nie, M.; Li, Y.; Zhu, H.; Shi, G. Design of composite nanosupports and applications thereof in enzyme immobilization: A review. Colloids Surf. B 2022, 217, 112602. [Google Scholar] [CrossRef]
- Taniguchi, K.; Nomura, K.; Hata, Y.; Nishimura, T.; Asami, Y.; Kuroda, A. The Si-Tag for immobilizing proteins on a silica surface. Biotechnol. Bioeng. 2007, 96, 1023. [Google Scholar] [CrossRef]
- Zurier, H.S.; Goddard, J.M. Directed immobilization of PETase on mesoporous silica enables sustained depolymerase activity in synthetic wastewater conditions. ACS Appl. Bio Mater. 2022, 5, 4981–4992. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, J.; Cai, S.; Long, Z.; Zhang, Y.; Su, L.; He, S.; Tang, C.; Liu, P.; Peng, H.; et al. A real-time wearable UV-radiation monitor based on a high-performance p-CuZnS/n-TiO2 photodetector. Adv. Mater. 2018, 30, 1803165. [Google Scholar] [CrossRef]
- Cho, Y.; Pak, S.; Lee, Y.G.; Hwang, J.S.; Giraud, P.; An, G.H.; Cha, S. Hybrid smart fiber with spontaneous self-charging mechanism for sustainable wearable electronics. Adv. Funct. Mater. 2020, 30, 1908479. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Cao, T.; Wang, T.; Sun, L.; Wang, K.; Fan, X. Antiliquid-interfering, antibacteria, and adhesive wearable strain sensor based on superhydrophobic and conductive composite hydrogel. ACS Appl. Mater. Interfaces 2021, 13, 46022–46032. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Zhang, J.; Duan, L.; Gao, G. A highly conductive hydrogel driven by phytic acid towards a wearable sensor with freezing and dehydration resistance. J. Mater. Chem. A 2021, 9, 22615–22625. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, J.; Wu, C.; Hu, X.; Lu, Y.; Wang, G.; Yu, F.; Zhang, X.; Wang, Y. Flexible and self-healing electrochemical hydrogel sensor with high efficiency toward glucose monitoring. Biosens. Bioelectron. 2020, 155, 112105. [Google Scholar] [CrossRef]
- Liu, X.; Lu, C.; Wu, X.; Zhang, X. Self-healing strain sensors based on nanostructured supramolecular conductive elastomers. J. Mater. Chem. A 2017, 5, 9824–9832. [Google Scholar] [CrossRef]
- Lin, L.; Liu, S.; Fu, S.; Zhang, S.; Deng, H.; Fu, Q. Fabrication of highly stretchable conductors via morphological control of carbon nanotube network. Small 2013, 9, 3620–3629. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ni, Y.Z.; Hu, J.; Jin, Y.M.; Gu, P.; Qiu, H.; Chen, K. Self-healing and antibacterial essential oil-loaded mesoporous silica/polyacrylate hybrid hydrogel for high-performance wearable body-strain sensing. ACS Appl. Mater. Interfaces 2022, 14, 21509–21520. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Mrair, Y.M.; Alharthi, F.A.; Al-Odayni, A.B. Catalytic performance of SBA-15-supported poly (sty-renesulfonic acid) in the esterification of acetic acid with n-heptanol. Appl. Sci. 2020, 10, 5835. [Google Scholar] [CrossRef]
- Tom, J.C.; Appel, C.; Andrieu-Brunsen, A. Fabrication and in situ functionalisation of mesoporous silica films by the physical entrapment of functional and responsive block copolymer structuring agents. Soft Matter 2019, 15, 8077–8083. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xu, L.; Xu, J.; Zhang, R.; Song, G.; Chao, Y.; Feng, L.; Han, F.; Dong, Z.; Li, B.; et al. Smart nanoreactors for pH-responsive tumor homing, mitochondria-targeting, and enhanced photodynamic-immunotherapy of cancer. Nano Lett. 2018, 18, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Tang, Q.; Yang, D.; Zhang, J.Z.; Zhang, F.; Hu, J. Preparation of pH-responsive mesoporous silica nanoparticles and their application in controlled drug delivery. J. Phys. Chem. C 2011, 115, 9926–9932. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Wang, P.; Liu, J.; Yang, Q. An efficient solid acid catalyst: Polypstyrenesulfonic acid supported on SBA-15 via surface-initiated ATRP. Micropor. Mesopor. Mat. 2009, 123, 228–233. [Google Scholar] [CrossRef]
- Richard, J.; Phimphachanh, A.; Schneider, J.; Nandi, S.; Laurent, E.; Lacroix-Desmazes, P.; Trens, P.; Devautour-Vinot, S.; Marcotte, N.; Gérardin, C. Integrated process for structuring and functionalizing ordered mesoporous silica to achieve superprotonic conductivity. Chem. Mater. 2022, 34, 7828–7836. [Google Scholar] [CrossRef]



| Mesoporous Materials | Shape | Diameter (nm) | Pore Size (nm) | Functional Groups | Preparation Method | Medicinal Application | Ref. |
|---|---|---|---|---|---|---|---|
| Chiral MCM-41 | Spheres | 100 | 3.5 | -NH2/ L-tartaric acid | Co-condensation | Chiral delivery | [53] |
| MCM-41 | Spheres | 158 | 4 | -NH2/-S-S-/-COOH/ DOX/Ce6 | Grafting | Chemo- photodynamic therapy | [54] |
| MCM-41 | Spheres | 100 | 3.4 | -COOH/ PEI-AA/ DOX | Grafting | pH-responsive delivery DOX | [55] |
| MCM-41 | Spheres | 50 | 2.5 | -NH2/ peptides/ DOX | Grafting | Peptides -responsive delivery DOX | [56] |
| MCM-41 | Spheres | 184 | 8.9 | -NH2/ disulfiram | Co-condensation | Delivery of DSF for cancer therapy | [57] |
| Hollow mesoporous silica | Hollow spheres | 155 | - | -NH2/GA/PEI/Cur | Grafting | pH-responsive release of curcumin | [58] |
| Dendrimer- like mesoporous silica | Dendrimer- Like spheres | 110 | 4.0 | -SH/ PEG/DOX | Grafting | pH-responsive delivery DOX | [59] |
| Hollow mesoporous silica | Hollow spheres | 170 | 5.6 | -NH2/ lactobionic acid/ indocyanine green/DOX | Grafting | pH/NIR dual-responsive release of DOX | [60] |
| MCM-41 | Spheres | 140 | 2.7 | -COOH/ Ca2+/5-fluorouracil | Grafting | pH-responsive release of 5-fluorouracil | [61] |
| MCM-41 | Spheres | 100 | 2.7 | DOX/dopamine | Impregnation | pH-responsive release of DOX | [62] |
| MCM-41 | Spheres | 165 | 3.6 | -S-S-S-S-/-NH2/ N-acetylgalactosamine | Co- condensation | GSH- responsive release of epirubicin | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Zhang, L.; Yang, W. Synthesis of Mesoporous Silica Using the Sol–Gel Approach: Adjusting Architecture and Composition for Novel Applications. Nanomaterials 2024, 14, 903. https://doi.org/10.3390/nano14110903
Han Y, Zhang L, Yang W. Synthesis of Mesoporous Silica Using the Sol–Gel Approach: Adjusting Architecture and Composition for Novel Applications. Nanomaterials. 2024; 14(11):903. https://doi.org/10.3390/nano14110903
Chicago/Turabian StyleHan, Yandong, Lin Zhang, and Wensheng Yang. 2024. "Synthesis of Mesoporous Silica Using the Sol–Gel Approach: Adjusting Architecture and Composition for Novel Applications" Nanomaterials 14, no. 11: 903. https://doi.org/10.3390/nano14110903
APA StyleHan, Y., Zhang, L., & Yang, W. (2024). Synthesis of Mesoporous Silica Using the Sol–Gel Approach: Adjusting Architecture and Composition for Novel Applications. Nanomaterials, 14(11), 903. https://doi.org/10.3390/nano14110903







