Controlled Growth of WO3 Photoanode under Various pH Conditions for Efficient Photoelectrochemical Performance
Abstract
:1. Introduction
2. Experiments
2.1. Synthesis of WO3·nH2O and WO3 Materials
2.2. Preparation of a WO3 Photoanode on Substrate
2.3. Characterizations
2.4. Data Acquisition
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Armaroli, N.; Balzani, V. The future of energy supply: Challenges and opportunities. Angew. Chem. Int. Ed. 2007, 46, 52. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, C.; Grätzel, M.; Kostecki, R.; Mao, S.S. Nanomaterials for renewable energy production and storage. Chem. Soc. Rev. 2012, 41, 7909. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H.-W. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. 2017, 27, 34. [Google Scholar] [CrossRef]
- Kim, J.H.; Hansora, D.; Sharma, P.; Jang, J.W.; Lee, J.S. Toward practical solar hydrogen production—An artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 2019, 48, 1908. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Ding, C.; Liu, M.; Wang, A.; Huang, Q.; Li, C. Water oxidation catalysts for artificial photosynthesis. Adv. Mater. 2019, 31, 1902069. [Google Scholar] [CrossRef] [PubMed]
- Kuti, L.M.; Bhella, S.S.; Thangadurai, V. Transformation of proton-conducting perovskite-type into fluorite-type fast oxide ion electrolytes using a CO2 capture technique and their electrical properties. Inorg. Chem. 2009, 48, 6804. [Google Scholar] [CrossRef]
- Gotić, M.; Ivanda, M.; Popović, S.; Musić, S. Synthesis of tungsten trioxide hydrates and their structural properties. Mater. Sci. Eng. B 2000, 77, 193. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, J.; Ke, L.; Sun, X.W.; Demir, H.V. Morphology-tailored synthesis of tungsten trioxide (hydrate) thin films and their photocatalytic properties. ACS Appl. Mater. Interfaces 2011, 3, 229. [Google Scholar] [CrossRef]
- Amano, F.; Tian, M.; Wu, G.; Ohtani, B.; Chen, A. Facile preparation of platelike tungsten oxide thin film electrodes with high photoelectrode activity. ACS Appl. Mater. Interfaces 2011, 3, 4047. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Sohn, Y.; Pradhan, D. Facile green synthesis of WO3·H2O nanoplates and WO3 nanowires with enhanced photoelectrochemical performance. Cryst. Growth Des. 2017, 17, 4949. [Google Scholar] [CrossRef]
- Mitchell, J.B.; Lo, W.C.; Genc, A.; Lebeau, J.; Augustyn, V. Transition from Battery to Pseudocapacitor Behavior via Structural Water in Tungsten Oxide. Chem. Mater. 2017, 29, 3928. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, S.; Chen, Y.; Gaskov, A.M. Facile morphology control of WO3 nanostructure arrays with enhanced photoelectrochemical performance. Appl. Surf. Sci. 2017, 403, 274. [Google Scholar] [CrossRef]
- Nayak, A.K.; Lee, S.; Choi, Y.I.; Yoon, H.J.; Sohn, Y.; Pradhan, D. Crystal phase and size-controlled synthesis of tungsten trioxide hydrate nanoplates at room temperature: Enhanced Cr(VI) photoreduction and methylene blue adsorption properties. ACS Sustain. Chem. Eng. 2017, 5, 2741. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, J.; Liu, H.; Murugadoss, V.; Zu, G.; Che, H.; Lai, C.; Li, H.; Ding, T.; Gao, Q.; et al. Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting. Nanoscale 2019, 11, 18968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, H.; Peng, Q.; Liu, X.; Dian, L.; Ye, W.; Chang, Y.; Ma, X.; Wang, H. Controlled fabrication of WO3 nanoplate films and its photoelectrochemical properties. Mater. Res. Express. 2019, 6, 095901. [Google Scholar] [CrossRef]
- Pham, N.L.; Luu, T.L.A.; Nguyen, H.L.; Nguyen, C.T. Effects of acidity on the formation and adsorption activity of tungsten oxide nanostructures prepared via the acid precipitation method. Mater. Chem. Phys. 2021, 272, 125014. [Google Scholar] [CrossRef]
- Liang, F.; Lin, Y.; He, Z.; Chen, W.; Zhu, Y.; Chen, T.; Liang, L.; Ma, S.; Wu, Y.; Tu, B.; et al. Promising ITO-free perovskite solar cells with WO3-Ag-SnO2 as transparent conductive oxide. J. Mater. Chem. A 2018, 6, 19330. [Google Scholar] [CrossRef]
- Yadav, S.; Lohia, P.; Sahu, A. Enhanced performance of double perovskite solar cell using WO3 as an electron transport material. J. Opt. 2023, 52, 776. [Google Scholar] [CrossRef]
- Farhadian, M.; Sangpout, P.; Hosseinzadeh, G. Morphology dependent photocatalytic activity of WO3 nanostructures. J. Energy Chem. 2015, 24, 171. [Google Scholar] [CrossRef]
- Park, M.; Seo, J.H.; Song, H.; Nam, K.M. Enhanced visible light activity of single-crystalline WO3 microplates for photoelectrochemical water oxidation. J. Phys. Chem. C 2016, 120, 9192. [Google Scholar] [CrossRef]
- Ahmed, B.; Kumar, S.; Ojha, A.K.; Donfack, P.; Materny, A. Facile and controlled synthesis of aligned WO3 nanorods and nanosheets as an efficient photocatalyst material. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 175, 250. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Zhou, H.; Liu, J.; Duan, X.; Zhang, H.; Liu, S.; Wang, S. Crystal transformation of 2D tungstic acid H2WO4 to WO3 for enhanced photocatalytic water oxidation. J. Colloid Interface Sci. 2018, 514, 576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xi, X.; Liu, Y.; Ma, L.; Nie, Z. Facile synthesis of WO3 micro/nanostructures by paper-assisted calcination for visible-light-driven photocatalysis. Chem. Phys. 2020, 528, 110515. [Google Scholar] [CrossRef]
- Perfecto, T.M.; Zito, C.A.; Volanti, D.P. Room-temperature volatile organic compounds sensing based on WO3·0.33H2O, hexagonal-WO3, and their reduced graphene oxide composites. RSC Adv. 2016, 6, 105171. [Google Scholar]
- Wang, L.; Hu, H.; Xu, J.; Zhu, S.; Ding, A.; Deng, C. WO3 nanocubes: Hydrothermal synthesis, growth mechanism, and photocatalytic performance. J. Mater. Res. 2019, 34, 2955. [Google Scholar] [CrossRef]
- Ou, P.; Song, F.; Yang, Y.; Shao, J.; Hua, Y.; Yang, S.; Wang, H.; Luo, Y.; Liao, J. WO3·nH2O crystals with controllable morphology/phase and their optical absorption properties. ACS Omega 2022, 7, 8833. [Google Scholar] [CrossRef]
- Livage, J.; Guzman, G. Aqueous precursors for electrochromic tungsten oxide hydrates. Solid State Ion. 1996, 84, 205. [Google Scholar] [CrossRef]
- Hu, W.-H.; Han, G.-Q.; Dong, B.; Liu, C.-G. Facile Synthesis of Highly Dispersed WO3·H2O and WO3 Nanoplates for Electrocatalytic Hydrogen Evolution. J. Nanomater. 2015, 2015, 346086. [Google Scholar]
- Han, H.; Nayak, A.K.; Choi, H.; Ali, G.; Kwon, J.; Choi, S.; Paik, U.; Song, T. Partial dehydration in hydrated tungsten oxide nanoplates leads to excellent and robust bifunctional oxygen reduction and hydrogen evolution reactions in acidic media. ACS Sustain. Chem. Eng. 2020, 8, 9507. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, C.; Mei, Z.; Liu, X.; Qu, X.; Li, Y.; Li, S.; Qi, W.; Zhang, Y.; Ye, J.; et al. Monoclinic tungsten oxide with {100} facet orientation and tuned electronic band structure for enhanced photocatalytic oxidations. ACS Appl. Mater. Interfaces 2016, 8, 10367. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.F.; Desbat, B.; Lassegues, J.C.; Gerand, B.; Figlarz, M. Infrared and Raman study of WO3 tungsten trioxides and WO3, xH2O tungsten trioxide tydrates. J. Solid State Chem. 1987, 67, 235. [Google Scholar] [CrossRef]
- Baek, S.-H.; Jeong, Y.-M.; Kim, D.Y.; Park, I.-K. Phase transformation of NiCo hydroxides derived from carbonate anion and its effect on electrochemical pseudocapacitor performance. Chem. Eng. J. 2020, 393, 124713. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Song, G.; Hong, J.; Van, T.K.; Pawar, A.U.; Kim, D.Y.; Kim, C.W.; Haider, Z.; Kang, Y.S. Facile fabrication of WO3 nanoplates thin films with dominant crystal facet of (002) for water splitting. Cryst. Growth Des. 2014, 14, 6057. [Google Scholar] [CrossRef]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical impedance spectroscopy of metal oxide electrodes for energy applications. ACS Appl. Energy Mater. 2020, 3, 66. [Google Scholar] [CrossRef]
- Kim, J.Y.; Magesh, G.; Youn, D.H.; Jang, J.-W.; Kubata, J.; Domen, K.; Lee, J.S. Single-crystalline, wormlike hematite photoanodes for efficient solar water splitting. Sci. Rep. 2013, 3, 2681. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Sun, H.; Fan, W.; Wang, L.; Zhao, H.; Zhao, X.; Yuan, S. Enhanced photoelectrochemical performance of tungsten oxide film by bifunctional Au nanoparticles. RSC Adv. 2017, 7, 15201. [Google Scholar] [CrossRef]
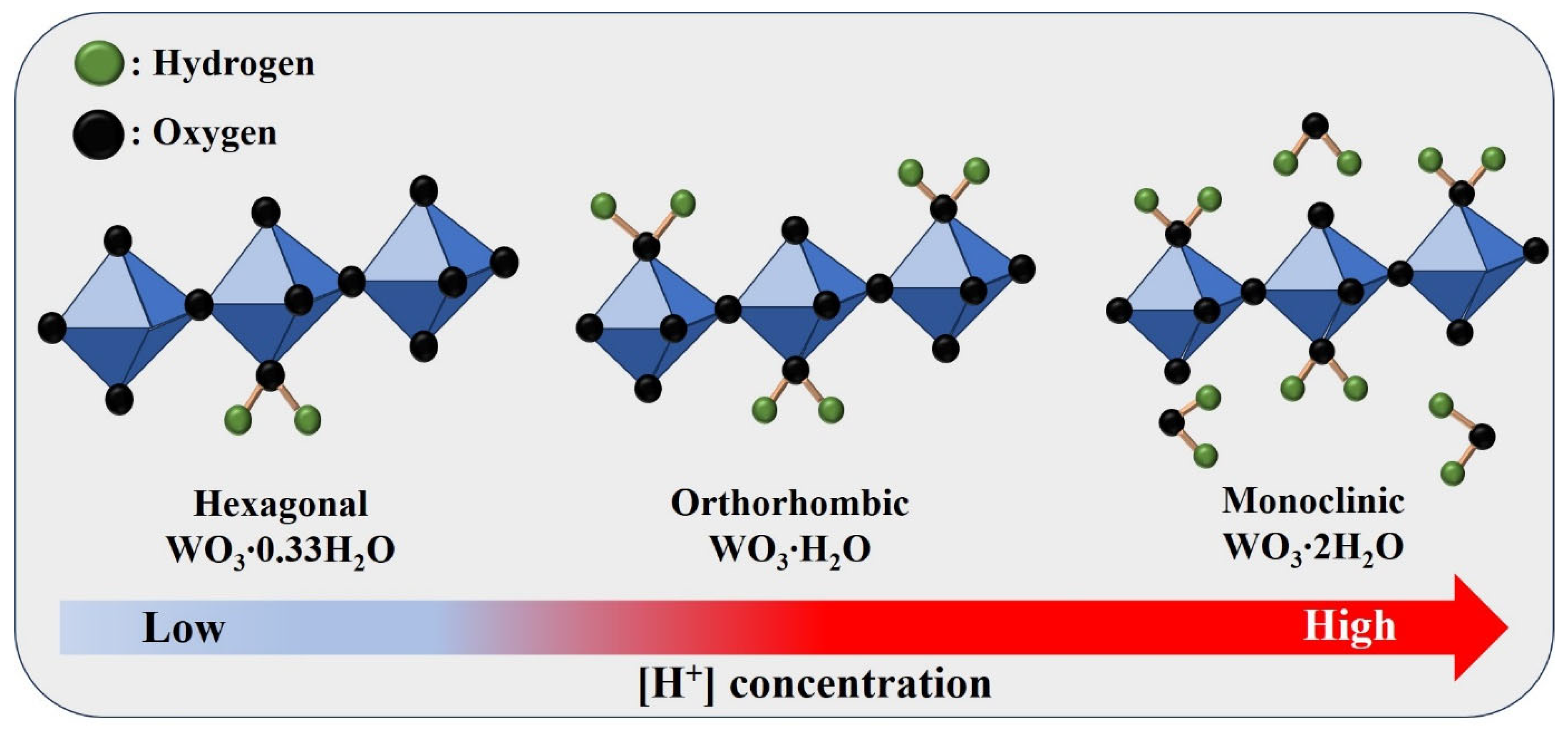
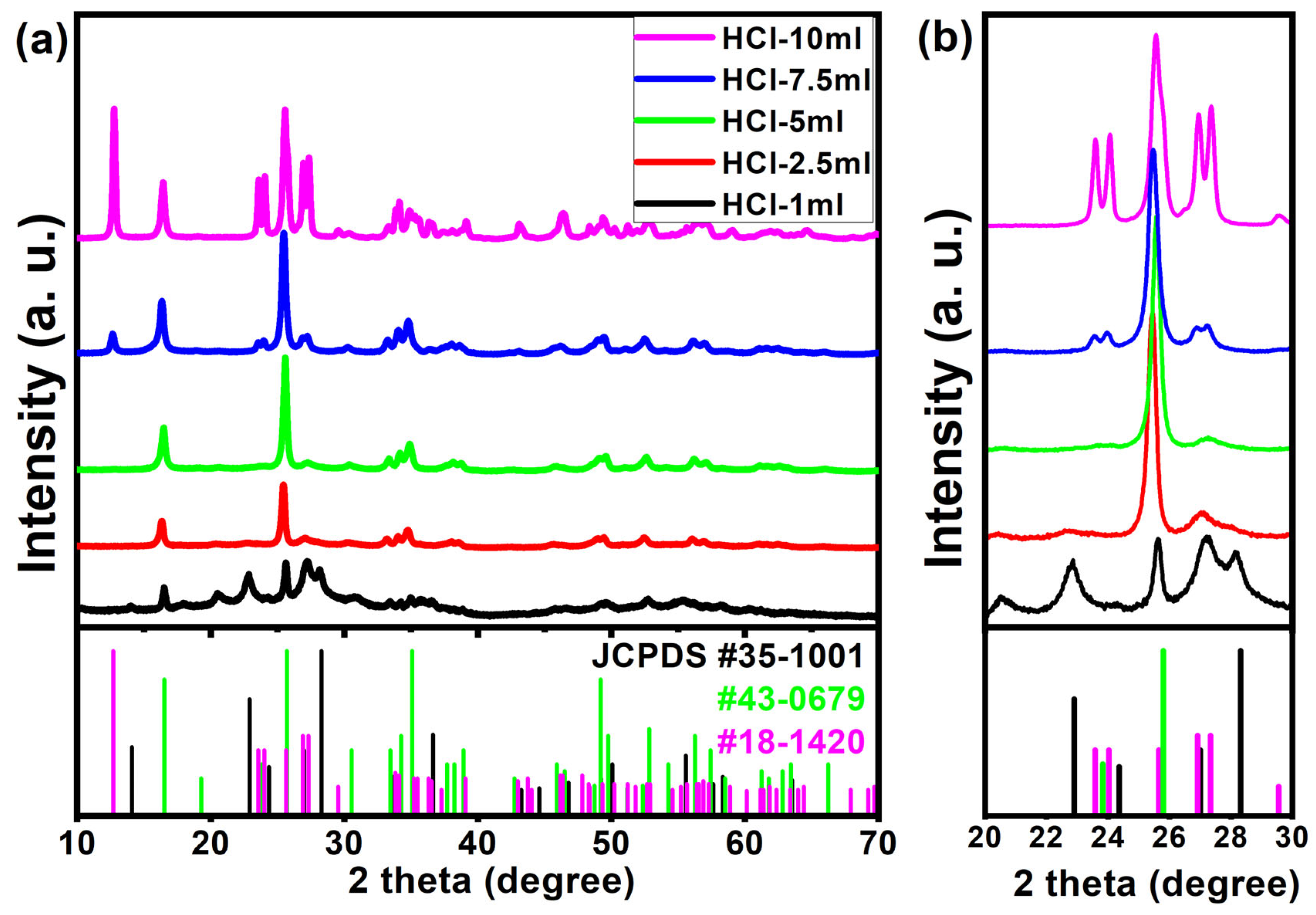
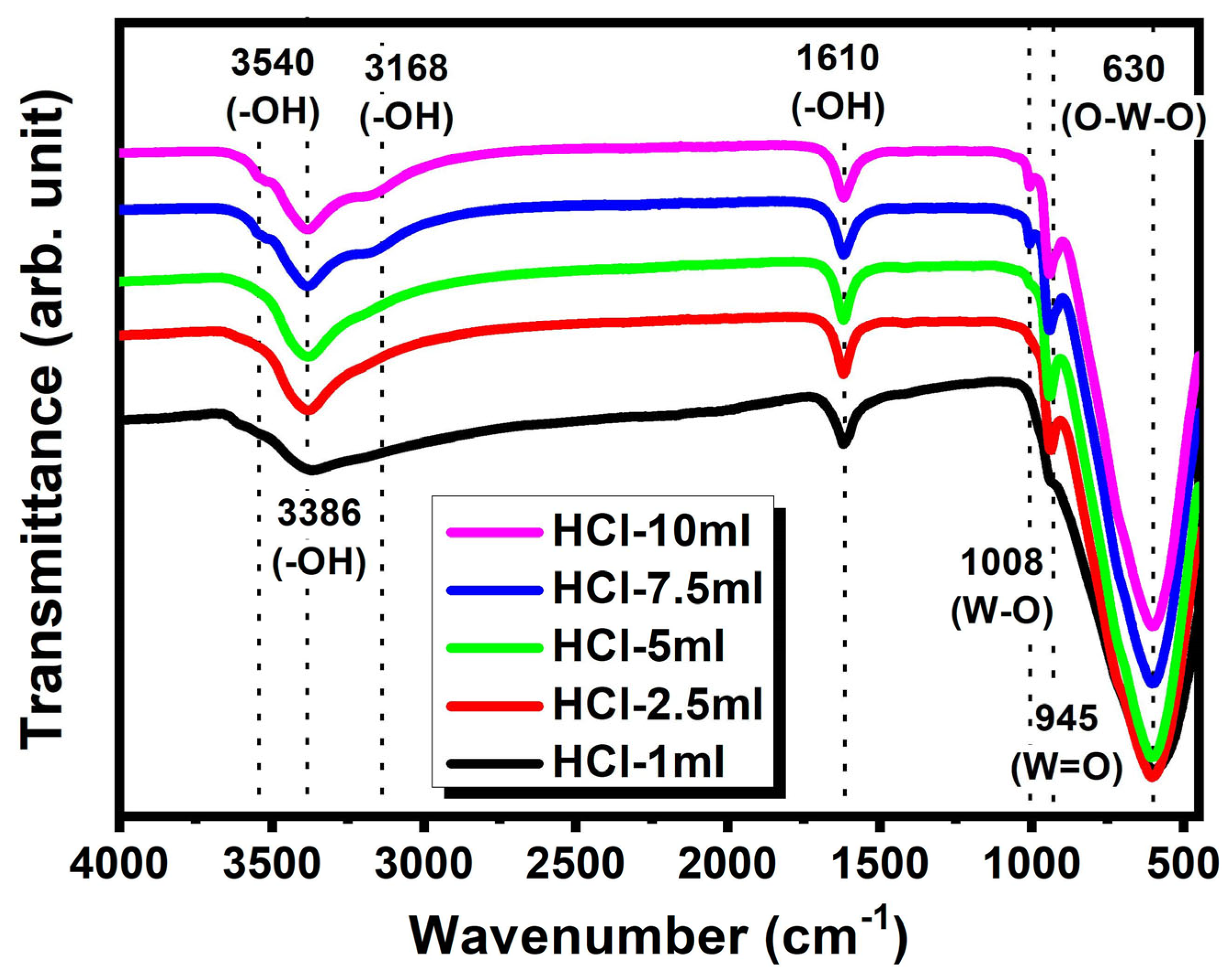
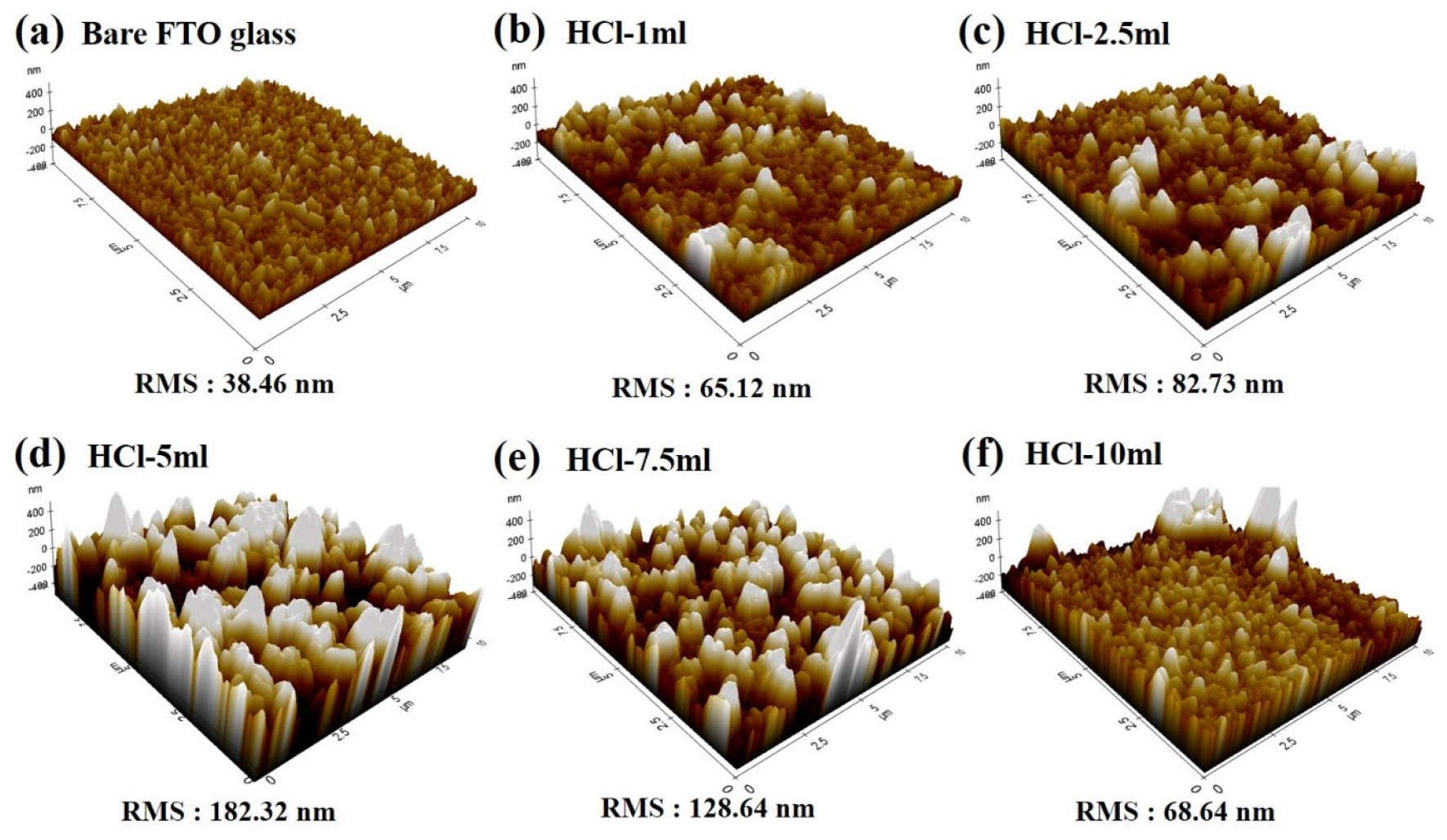
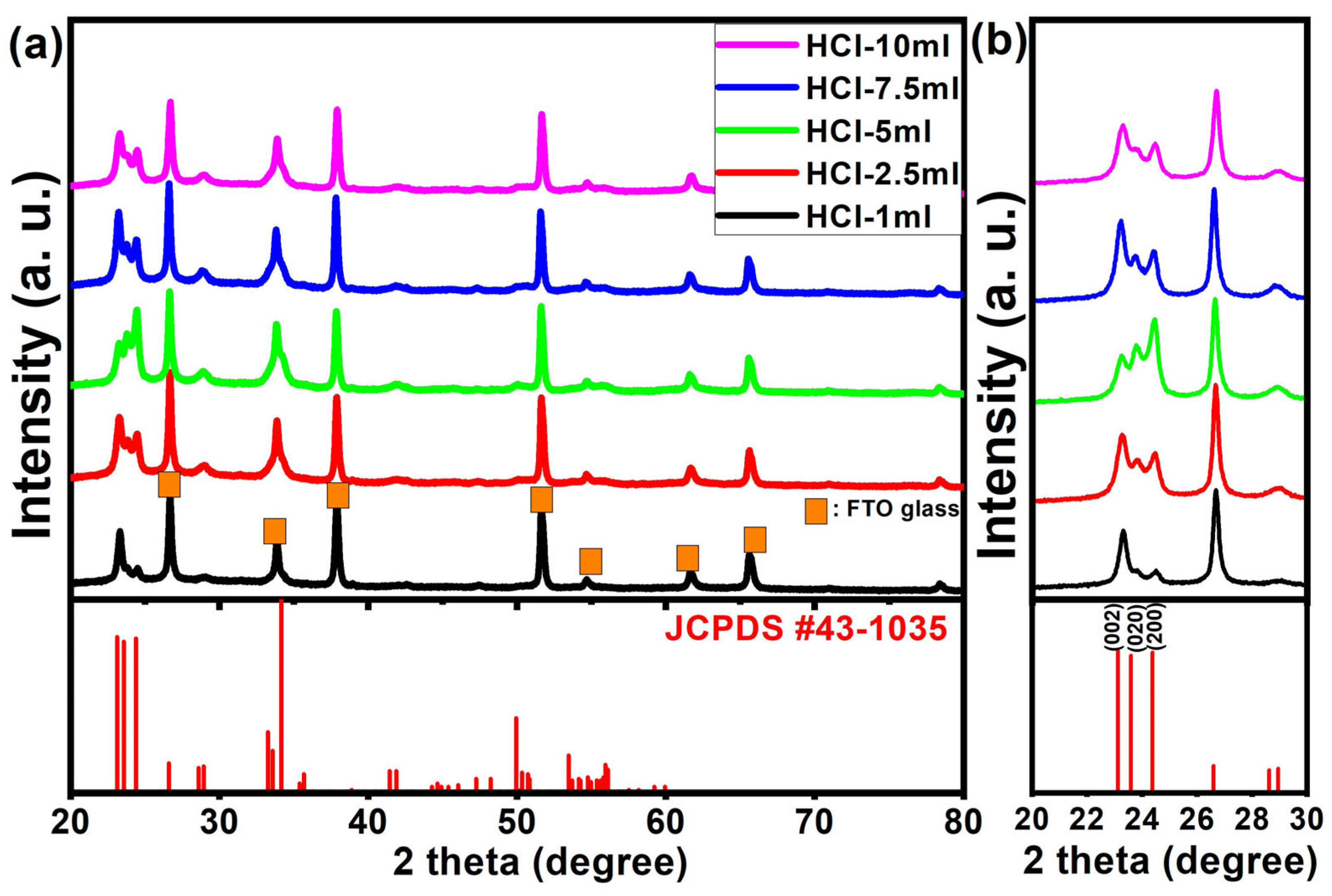
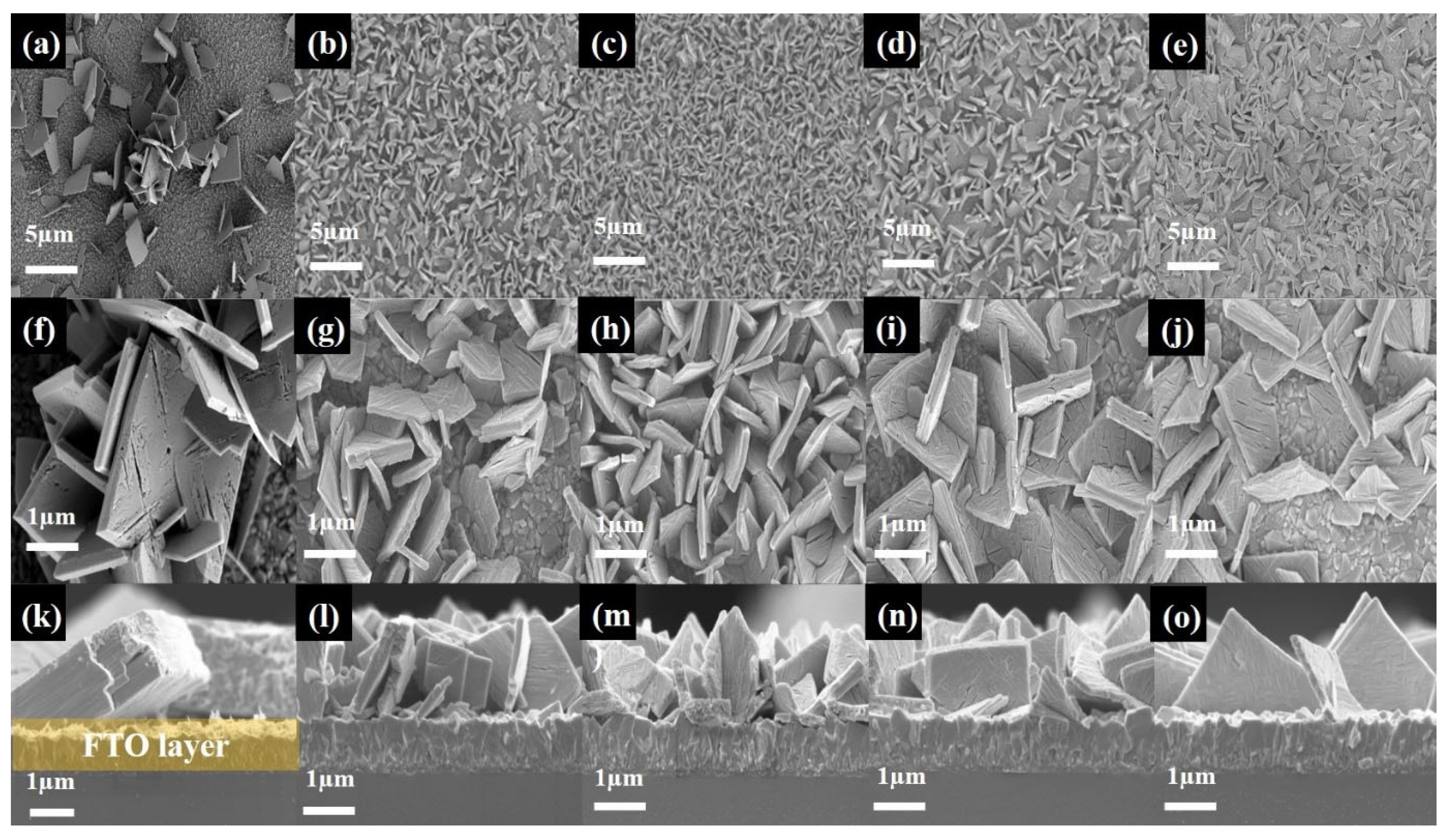
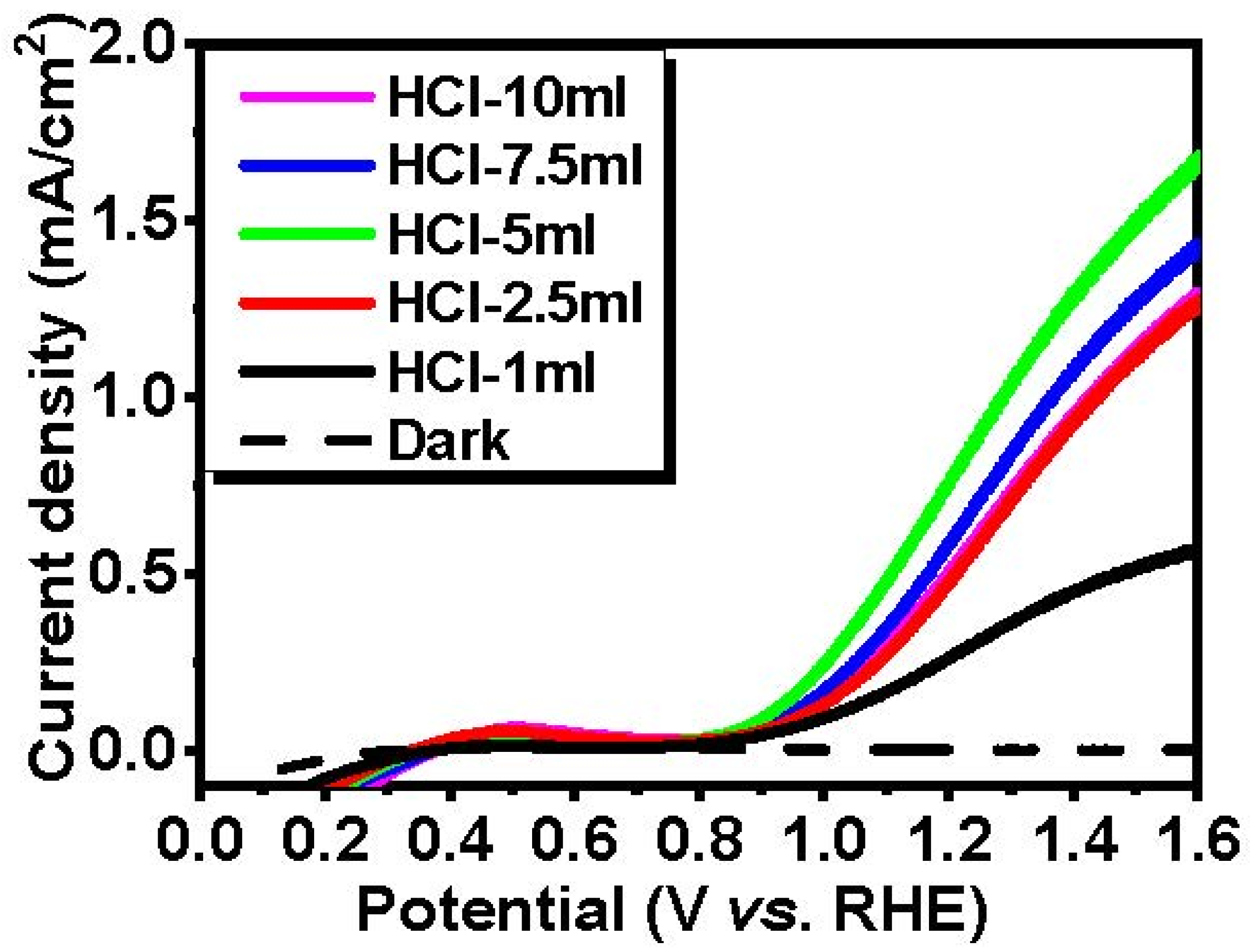
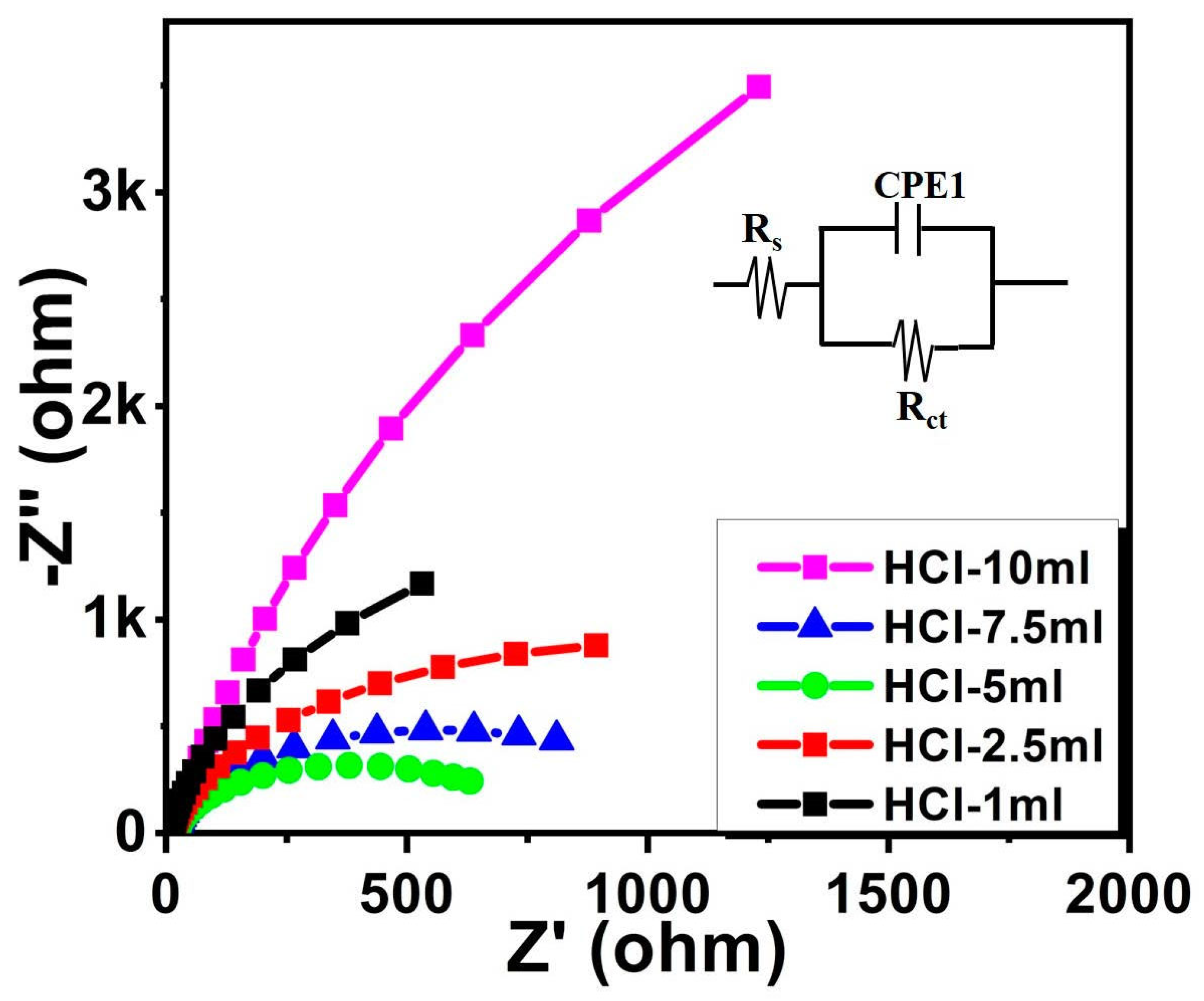
| Sample | HCl-1 mL | HCl-2.5 mL | HCl-5 mL | HCl-7.5 mL | HCl-10 mL |
|---|---|---|---|---|---|
| pH value | 1.12 0.02 | 0.86 0.02 | 0.62 0.01 | 0.34 0.01 | 0.05 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, S.-J.; Kim, D.; Baek, S.-H. Controlled Growth of WO3 Photoanode under Various pH Conditions for Efficient Photoelectrochemical Performance. Nanomaterials 2024, 14, 8. https://doi.org/10.3390/nano14010008
Yoo S-J, Kim D, Baek S-H. Controlled Growth of WO3 Photoanode under Various pH Conditions for Efficient Photoelectrochemical Performance. Nanomaterials. 2024; 14(1):8. https://doi.org/10.3390/nano14010008
Chicago/Turabian StyleYoo, Seung-Je, Dohyun Kim, and Seong-Ho Baek. 2024. "Controlled Growth of WO3 Photoanode under Various pH Conditions for Efficient Photoelectrochemical Performance" Nanomaterials 14, no. 1: 8. https://doi.org/10.3390/nano14010008
APA StyleYoo, S.-J., Kim, D., & Baek, S.-H. (2024). Controlled Growth of WO3 Photoanode under Various pH Conditions for Efficient Photoelectrochemical Performance. Nanomaterials, 14(1), 8. https://doi.org/10.3390/nano14010008






