Enhancing Sodium-Ion Energy Storage of Commercial Activated Carbon by Constructing Closed Pores via Ball Milling
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
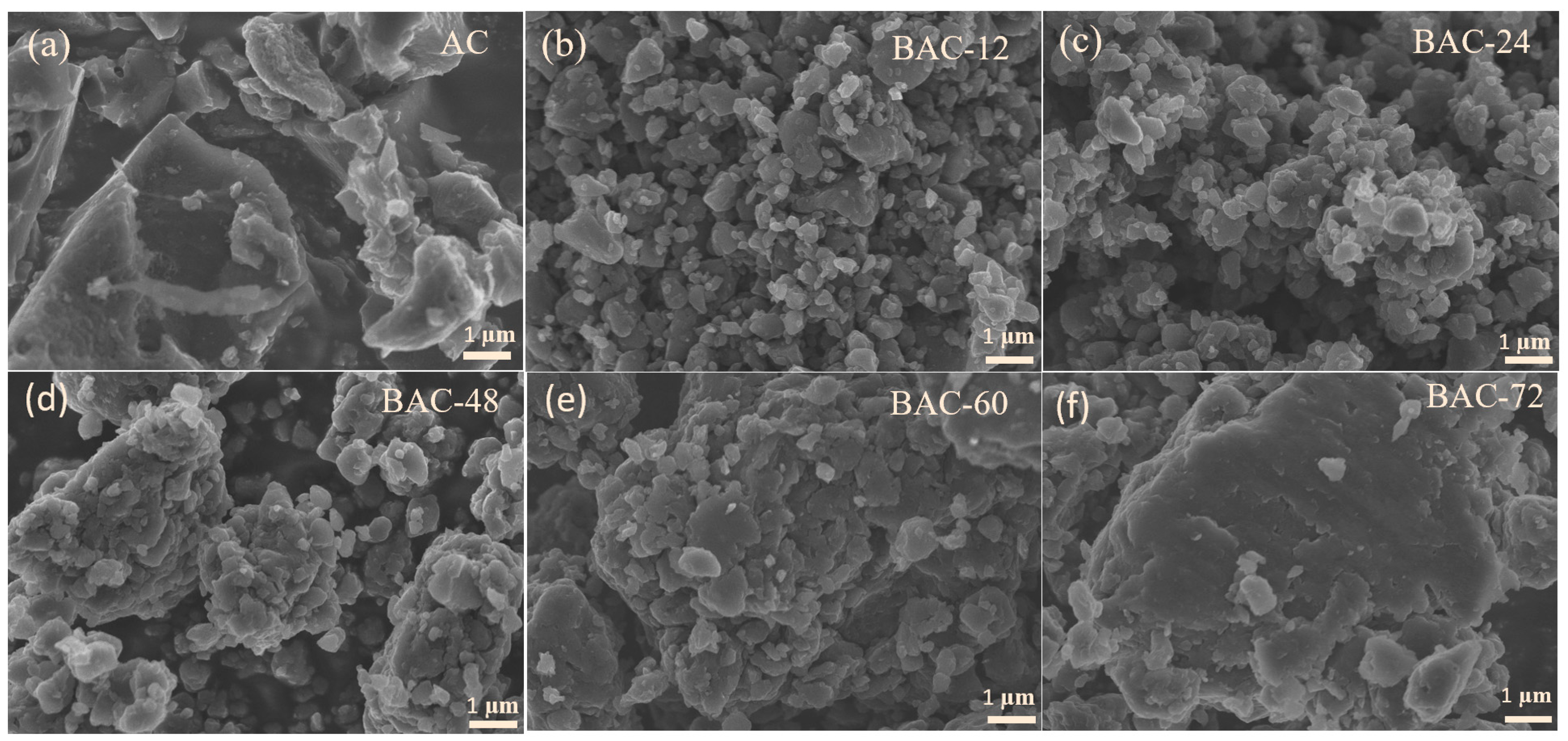
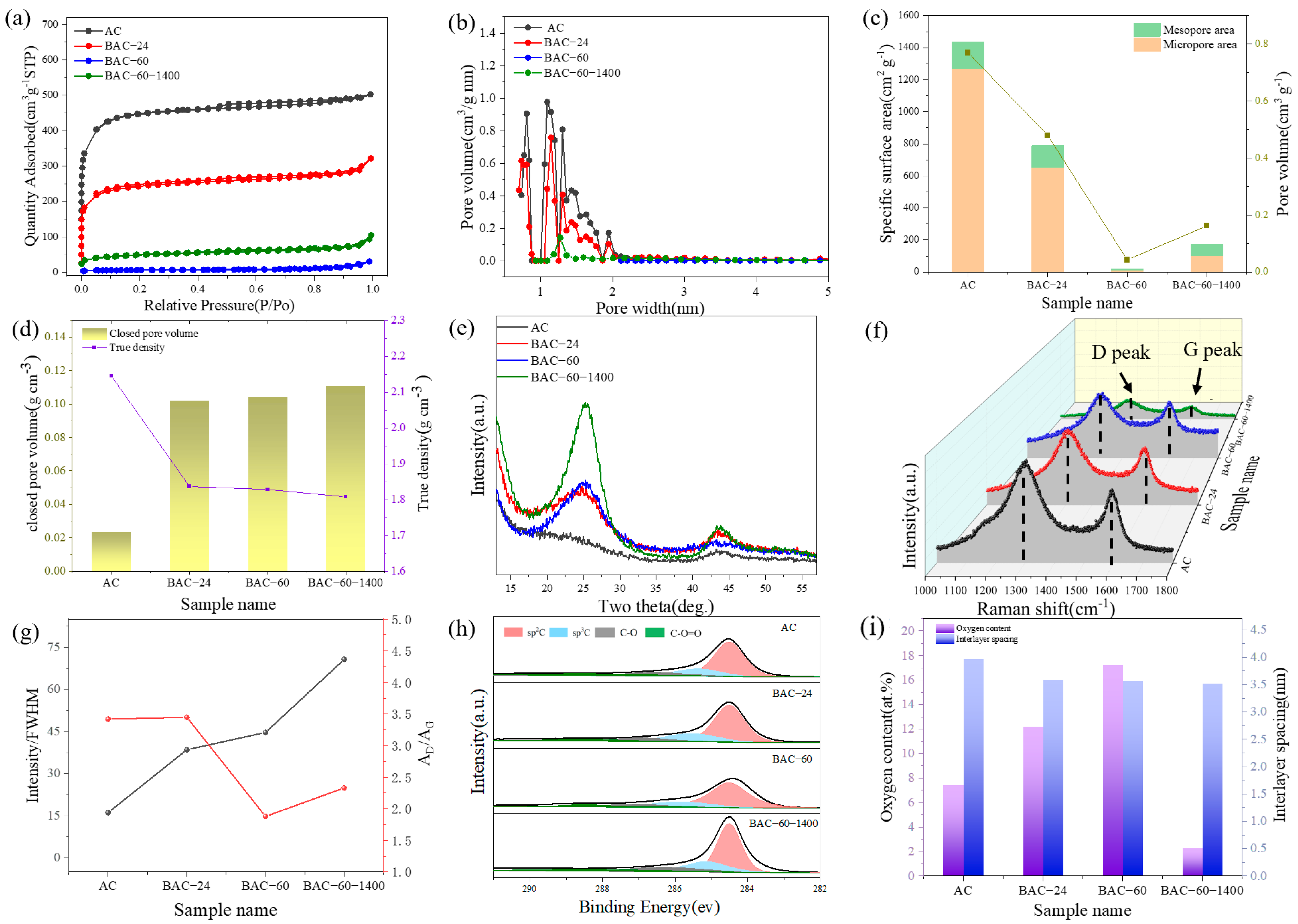
3.1. Materials Characterizations
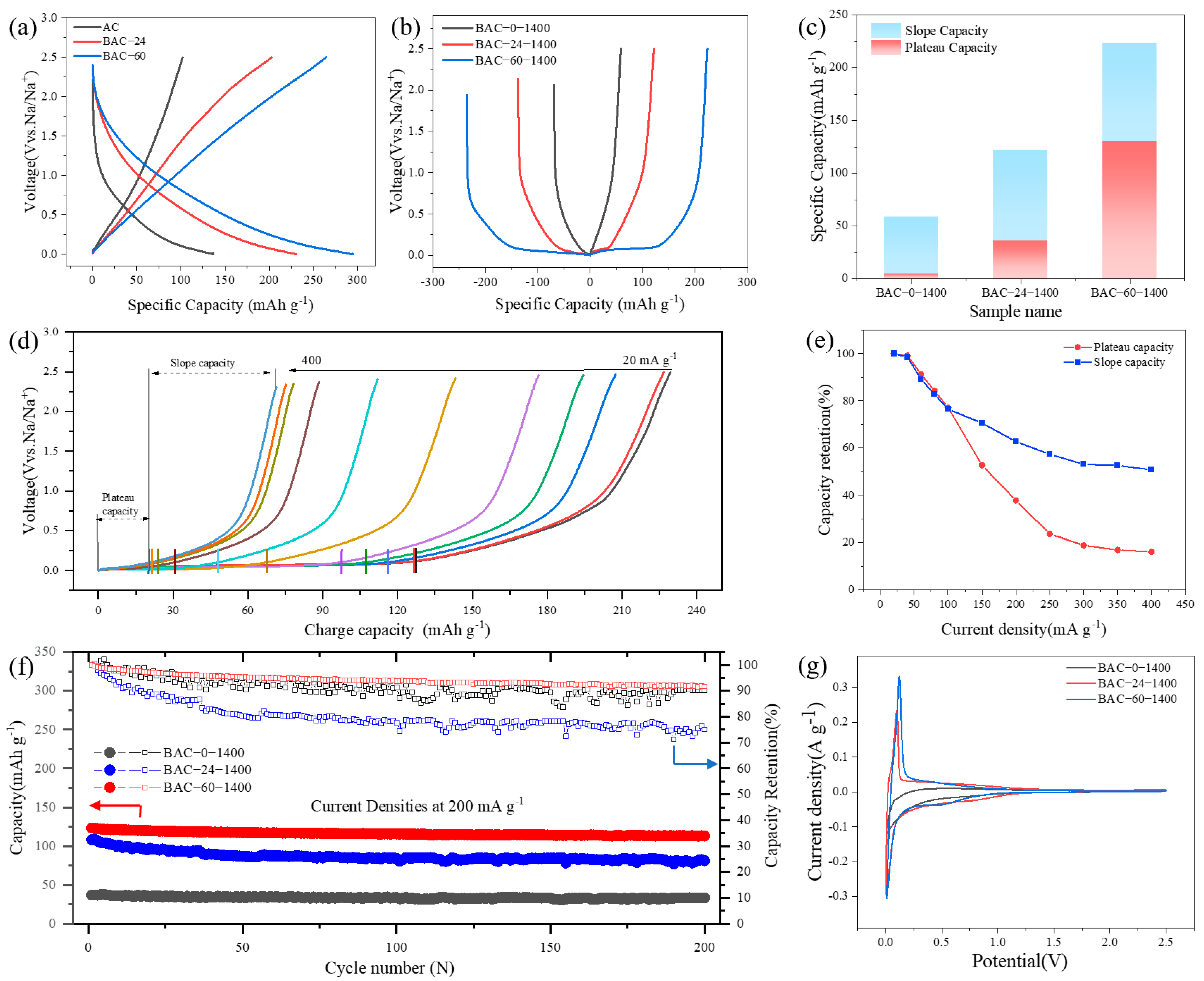
3.2. Electrochemical Performance
3.3. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Su, D.; Shanmukaraj, D.; Rojo, T.; Armand, M.; Wang, G. Electrode Materials for Sodium-Ion Batteries: Considerations on Crystal Structures and Sodium Storage Mechanisms. Electrochem. Energy Rev. 2018, 1, 200–237. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Yang, Z.; Lemmon, J.P.; Imhoff, C.; Graff, G.L.; Li, L.; Hu, J.; Wang, C.; Xiao, J.; et al. Materials Science and Materials Chemistry for Large Scale Electrochemical Energy Storage: From Transportation to Electrical Grid. Adv. Funct. Mater. 2012, 23, 929–946. [Google Scholar] [CrossRef]
- Delmas, C. Sodium and Sodium-Ion Batteries: 50 Years of Research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, J.; Zhang, Y.; Li, Q.; Jia, Y.; Dong, X.; Xiao, J.; Tao, Y.; Yang, Q. Reconfiguring Hard Carbons with Emerging Sodium-Ion Batteries: A Perspective. Adv. Mater. 2023, 35, e2212186. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Tan, X.; An, Q.; Mai, L. Nanostructured Conversion-Type Negative Electrode Materials for Low-Cost and High-Performance Sodium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1804458. [Google Scholar] [CrossRef]
- Li, Y.; Lai, X.; Qu, J.; Lai, Q.; Yi, T. Research Progress in Regulation Strategies of High-Performance Antimony-Based Anode Materials for Sodium Ion Batteries. Acta Phys. Chim. Sin. 2022, 38, 2204049. [Google Scholar] [CrossRef]
- Song, T.; Chen, H.; Li, Z.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Creating an Air-Stable Sulfur-Doped Black Phosphorus-TiO2 Composite as High-Performance Anode Material for Sodium-Ion Storage. Adv. Funct. Mater. 2019, 29, 1900535. [Google Scholar] [CrossRef]
- Igarashi, D.; Tanaka, Y.; Kubota, K.; Tatara, R.; Maejima, H.; Hosaka, T.; Komaba, S. New Template Synthesis of Anomalously Large Capacity Hard Carbon for Na- and K-Ion Batteries. Adv. Energy Mater. 2023, 13, 2302647. [Google Scholar] [CrossRef]
- Surta, T.W.; Koh, E.; Li, Z.; Fast, D.B.; Ji, X.; Greaney, P.A.; Dolgos, M.R. Combining Experimental and Theoretical Techniques to Gain an Atomic Level Understanding of the Defect Binding Mechanism in Hard Carbon Anodes for Sodium Ion Batteries. Adv. Energy Mater. 2022, 12, 2200647. [Google Scholar] [CrossRef]
- LeGe, N.; He, X.-X.; Wang, Y.-X.; Lei, Y.; Yang, Y.-X.; Xu, J.-T.; Liu, M.; Wu, X.; Lai, W.-H.; Chou, S.-L. Reappraisal of hard carbon anodes for practical lithium/sodium-ion batteries from the perspective of full-cell matters. Energy Environ. Sci. 2023, 16, 5688–5720. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, C.; He, X.X.; Zhao, J.; Yang, Z.; Li, L.; Wu, X.; Li, L.; Chou, S.L. Boosting the Development of Hard Carbon for Sodium-Ion Batteries: Strategies to Optimize the Initial Cou-lombic Efficiency. Adv. Funct. Mater. 2023, 2302277. [Google Scholar] [CrossRef]
- Wu, S.; Peng, H.; Huang, L.; Liu, Y.; Wu, Y.; Liu, L.; Ai, W.; Sun, Z. P-doped hard carbon microspheres for sodium-ion battery anodes with superior rate and cyclic performance. Inorg. Chem. Front. 2023, 10, 5908–5916. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, H.; Wu, P.F.; Zhou, S.Y.; Huang, Y.C.; Zhang, R.; Sun, D.; Tang, Y.G.; Wang, H.Y. Electrode–Electrolyte Interfacial Chemistry Modulation for Ultra-High Rate Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, 202200475. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, Z.; Yuan, H.; Yan, C.; Hao, R.; Zhang, F.; Luo, W.; Wang, H.; Cao, Y.; Gu, S.; et al. Deciphering Electrolyte Dominated Na+ Storage Mechanisms in Hard Carbon Anodes for Sodium-Ion Batteries. Adv. Sci. 2023, e2305414. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Zhou, Q.; Ma, M.; Liu, H.K.; Dou, S.X.; Chong, S. Advanced Anode Materials for Rechargeable Sodium-Ion Batteries. ACS Nano 2023, 17, 11220–11252. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, M.; Barmi, M.; Mitchell, D.R.; Barlow, A.J.; Fichtner, M. Effect of oxidizer in the synthesis of NiO anchored nanostructure nickel molybdate for sodium-ion battery. Mater. Today Energy 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Wu, J.; Wang, G.; Zhang, W.; Wang, L.; Peng, J.; Li, Q.; Liang, Z.; Fan, W.; Wang, J.; Huang, S. Universal architecture and defect engineering dual strategy for hierarchical antimony phosphate composite toward fast and durable sodium storage. J. Energy Chem. 2024, 90, 110–119. [Google Scholar] [CrossRef]
- Yang, J.; Guo, X.; Gao, H.; Wang, T.; Liu, Z.; Yang, Q.; Yao, H.; Li, J.; Wang, C.; Wang, G. A High-Performance Alloy-Based Anode Enabled by Surface and Interface Engineering for Wide-Temperature Sodium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300351. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M. Status on electrodeposited manganese dioxide and biowaste carbon for hybrid capacitors: The case of high-quality oxide composites, mechanisms, and prospects. J. Energy Storage 2022, 56, 106099. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, R.; Liu, Z.; Wan, J.; Zhang, S.; Wang, S.; Hua, K.; Liu, X.; Zhou, X.; Luo, X.; et al. Porous Organic Polymer with Hierarchical Structure and Limited Volume Expansion for Ultrafast and Highly Durable Sodium Storage. Adv. Mater. 2023, 35, e2210082. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, C.; Fang, Y.; Ai, X.; Zhong, F.; Yang, H.; Cao, Y. Understanding of the sodium storage mechanism in hard carbon anodes. Carbon Energy 2022, 4, 1133–1150. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Q.; Yuan, F.; Zhang, D.; Li, Z.; Wang, Q.; Wang, H.; Wang, B. Unraveling the effect of carbon morphology evolution in hard carbons on sodium storage performance. Inorg. Chem. Front. 2023, 10, 6547–6556. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, Z.; Xie, F.; Jiang, J.; Zheng, K.; Alabidun, S.; Crespo-Ribadeneyra, M.; Hu, Y.S.; Au, H.; Titirici, M.M. Investigating the Superior Performance of Hard Carbon Anodes in Sodium-Ion Compared With Lithium- and Potassium-Ion Batteries. Adv. Mater. 2023, 35, 2304091. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Yi, Z.; Xu, R.; Chen, J.; Cheng, J.; Wang, Z.; Liu, Q.; Guo, Q.; Xie, L.; Chen, C. Towards enhanced sodium storage of hard carbon anodes: Regulating the oxygen content in precursor by low-temperature hydrogen reduction. Energy Storage Mater. 2022, 51, 620–629. [Google Scholar] [CrossRef]
- Alvin, S.; Yoon, D.; Chandra, C.; Cahyadi, H.S.; Park, J.-H.; Chang, W.; Chung, K.Y.; Kim, J. Revealing sodium ion storage mechanism in hard carbon. Carbon 2019, 145, 67–81. [Google Scholar] [CrossRef]
- Oh, J.A.S.; Deysher, G.; Ridley, P.; Chen, Y.T.; Cheng, D.; Cronk, A.; Ham, S.Y.; Tan, D.H.; Jang, J.; Nguyen, L.H.B.; et al. High-Performing All-Solid-State Sodium-Ion Batteries Enabled by the Presodiation of Hard Carbon. Adv. Energy Mater. 2023, 13, 2300776. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- He, X.X.; Lai, W.H.; Liang, Y.; Zhao, J.H.; Yang, Z.; Peng, J.; Liu, X.H.; Wang, Y.X.; Qiao, Y.; Li, L.; et al. Achieving All-Plateau and High-Capacity Sodium Insertion in Topological Graphitized Carbon. Adv. Mater. 2023, 35, 2302613. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, R.; Wang, H.; Zhou, S.; Pan, Z.; Huang, Y.; Sun, D.; Tang, Y.; Ji, X.; Amine, K.; et al. Revealing the closed pore formation of waste wood-derived hard carbon for advanced sodium-ion battery. Nat. Commun. 2023, 14, 6024. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Tang, Z.; Pan, Z.; Huang, Y.; Zhao, L.; Zhang, X.; Sun, D.; Tang, Y.; Dhmees, A.S.; Wang, H. Regulating closed pore structure enables significantly improved sodium storage for hard carbon pyrolyzing at relatively low temperature. Susmat 2022, 2, 357–367. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, X.; Hu, X.; Li, Y.; Wang, K.; Tu, H.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Rationally Designing Closed Pore Structure by Carbon Dots to Evoke Sodium Storage Sites of Hard Carbon in Low-Potential Region. Adv. Funct. Mater. 2023, 2308392. [Google Scholar] [CrossRef]
- Meng, Q.; Lu, Y.; Ding, F.; Zhang, Q.; Chen, L.; Hu, Y.-S. Tuning the Closed Pore Structure of Hard Carbons with the Highest Na Storage Capacity. ACS Energy Lett. 2019, 4, 2608–2612. [Google Scholar] [CrossRef]
- Wang, K.; Sun, F.; Wang, H.; Wu, D.; Chao, Y.; Gao, J.; Zhao, G. Altering Thermal Transformation Pathway to Create Closed Pores in Coal-Derived Hard Carbon and Boosting of Na+ Plateau Storage for High-Performance Sodium-Ion Battery and Sodium-Ion Capacitor. Adv. Funct. Mater. 2022, 32, 2203725. [Google Scholar] [CrossRef]
- Chen, X.; Sawut, N.; Chen, K.; Li, H.; Zhang, J.; Wang, Z.; Yang, M.; Tang, G.; Ai, X.; Yang, H.X.; et al. Filling carbon: A microstructure-engineered hard carbon for efficient alkali metal ion storage. Energy Environ. Sci. 2023, 16, 4041–4053. [Google Scholar] [CrossRef]
- Burmeister, C.F.; Kwade, A. Process engineering with planetary ball mills. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Rodriguez-Padron, D.; Puente-Santiago, A.R.; Luque, R. Mechanochemistry: Toward Sustainable Design of Advanced Nanomaterials for Electrochemical Energy Storage and Catalytic Applications. ACS Sustain. Chem. Eng. 2018, 6, 9530–9544. [Google Scholar] [CrossRef]
- Gorrasi, G.; Sorrentino, A. Mechanical milling as a technology to produce structural and functional bio-nanocomposites. Green Chem. 2015, 17, 2610–2625. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Luo, J.; Wang, K.; Zhang, Q.; Qiu, Y.; Sun, S.; Liu, S.; Han, J.; Huang, Y. Enhancing Sodium-Ion Storage Behaviors in TiNb2O7 by Mechanical Ball Milling. ACS Appl. Mater. Interfaces 2017, 9, 8696–8703. [Google Scholar] [CrossRef]
- Driscoll, L.L.; Driscoll, E.H.; Dong, B.; Sayed, F.N.; Wilson, J.N.; O’keefe, C.A.; Gardner, D.J.; Grey, C.P.; Allan, P.K.; Michalchuk, A.A.L.; et al. Under pressure: Offering fundamental insight into structural changes on ball milling battery materials. Energy Environ. Sci. 2023, 16, 7893. [Google Scholar] [CrossRef]
- Lu, H.; Ai, F.; Jia, Y.; Tang, C.; Zhang, X.; Huang, Y.; Yang, H.; Cao, Y. Exploring Sodium-Ion Storage Mechanism in Hard Carbons with Different Microstructure Prepared by Ball-Milling Method. Small 2018, 14, e1802694. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, Y.; Zhao, P.; Yuan, C.; Chen, M.; Wang, C. Commercial activated carbon as a novel precursor of the amorphous carbon for high-performance sodium-ion batteries anode. Carbon 2018, 129, 85–94. [Google Scholar] [CrossRef]
- Yuan, R.; Dong, Y.; Hou, R.; Shang, L.; Zhang, J.; Zhang, S.; Chen, X.; Song, H. Structural transformation of porous and disordered carbon during ball-milling. Chem. Eng. J. 2023, 454, 140418. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Hu, X.; Fan, F.; Cai, S.; Zheng, C.; Stucky, G.D. Review on comprehending and enhancing the initial Coulombic efficiency of anode materials in lithi-um-ion/sodium-ion batteries. Nano Energy 2020, 77, 105143. [Google Scholar] [CrossRef]
- Qiu, S.; Xiao, L.; Sushko, M.L.; Han, K.S.; Shao, Y.; Yan, M.; Liang, X.; Mai, L.; Feng, J.; Cao, Y.; et al. Manipulating Adsorption–Insertion Mechanisms in Nanostructured Carbon Materials for High-Efficiency Sodium Ion Storage. Adv. Energy Mater. 2017, 7, 1700403. [Google Scholar] [CrossRef]
- Sun, D.; Luo, B.; Wang, H.; Tang, Y.; Ji, X.; Wang, L. Engineering the trap effect of residual oxygen atoms and defects in hard carbon anode towards high initial Coulombic efficiency. Nano Energy 2019, 64, 103937. [Google Scholar] [CrossRef]
- Sun, N.; Guan, Z.; Liu, Y.; Cao, Y.; Zhu, Q.; Liu, H.; Wang, Z.; Zhang, P.; Xu, B. Extended “adsorption-insertion” model: A new insight into the sodium storage mechanism of hard carbons. Adv. Energy Mater. 2019, 9, 1901351. [Google Scholar] [CrossRef]
- Sun, N.; Qiu, J.; Xu, B. Understanding of Sodium Storage Mechanism in Hard Carbons: Ongoing Development under Debate. Adv. Energy Mater. 2022, 12, 2200715. [Google Scholar] [CrossRef]
- Yi, X.; Li, X.; Zhong, J.; Wang, S.; Wang, Z.; Guo, H.; Wang, J.; Yan, G. Unraveling the Mechanism of Different Kinetics Performance between Ether and Carbonate Ester Electrolytes in Hard Carbon Electrode. Adv. Funct. Mater. 2022, 32, 2209523. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Fang, Q.; Zheng, T.; Xu, Y.; Dai, R.; Qiao, Z.; Ruan, D.; Wang, Y. Enhancing Sodium-Ion Energy Storage of Commercial Activated Carbon by Constructing Closed Pores via Ball Milling. Nanomaterials 2024, 14, 65. https://doi.org/10.3390/nano14010065
Wang X, Fang Q, Zheng T, Xu Y, Dai R, Qiao Z, Ruan D, Wang Y. Enhancing Sodium-Ion Energy Storage of Commercial Activated Carbon by Constructing Closed Pores via Ball Milling. Nanomaterials. 2024; 14(1):65. https://doi.org/10.3390/nano14010065
Chicago/Turabian StyleWang, Xiaojie, Qian Fang, Tiejun Zheng, Yanyan Xu, Rui Dai, Zhijun Qiao, Dianbo Ruan, and Yuzuo Wang. 2024. "Enhancing Sodium-Ion Energy Storage of Commercial Activated Carbon by Constructing Closed Pores via Ball Milling" Nanomaterials 14, no. 1: 65. https://doi.org/10.3390/nano14010065
APA StyleWang, X., Fang, Q., Zheng, T., Xu, Y., Dai, R., Qiao, Z., Ruan, D., & Wang, Y. (2024). Enhancing Sodium-Ion Energy Storage of Commercial Activated Carbon by Constructing Closed Pores via Ball Milling. Nanomaterials, 14(1), 65. https://doi.org/10.3390/nano14010065





