One-Dimensional La0.2Sr0.8Cu0.4Co0.6O3−δ Nanostructures for Efficient Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Experimental
2.1. Chemical Reagents
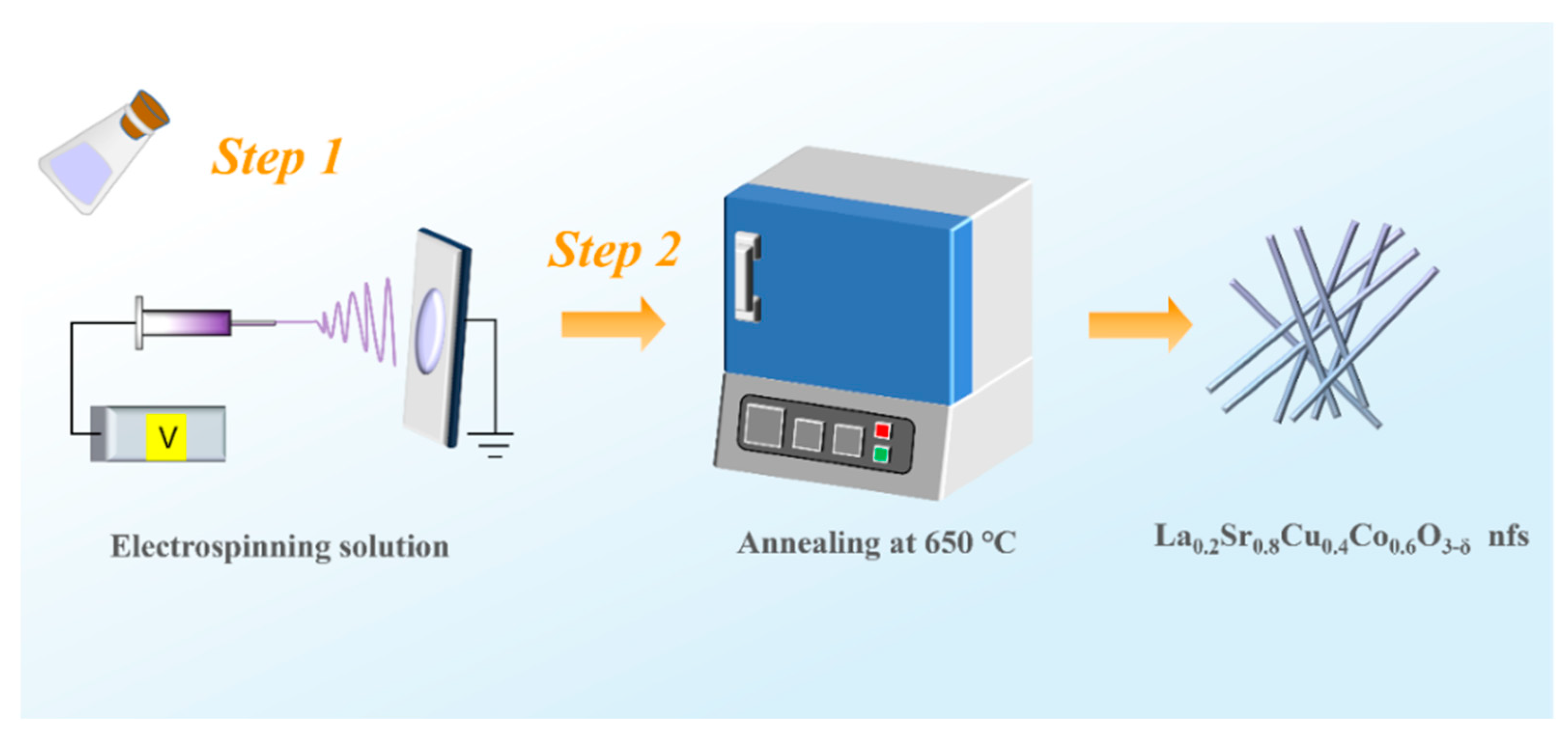
2.2. Synthesis of PAN/LSCC Nanofibers
2.3. Synthesis of LSCC Nanofibers
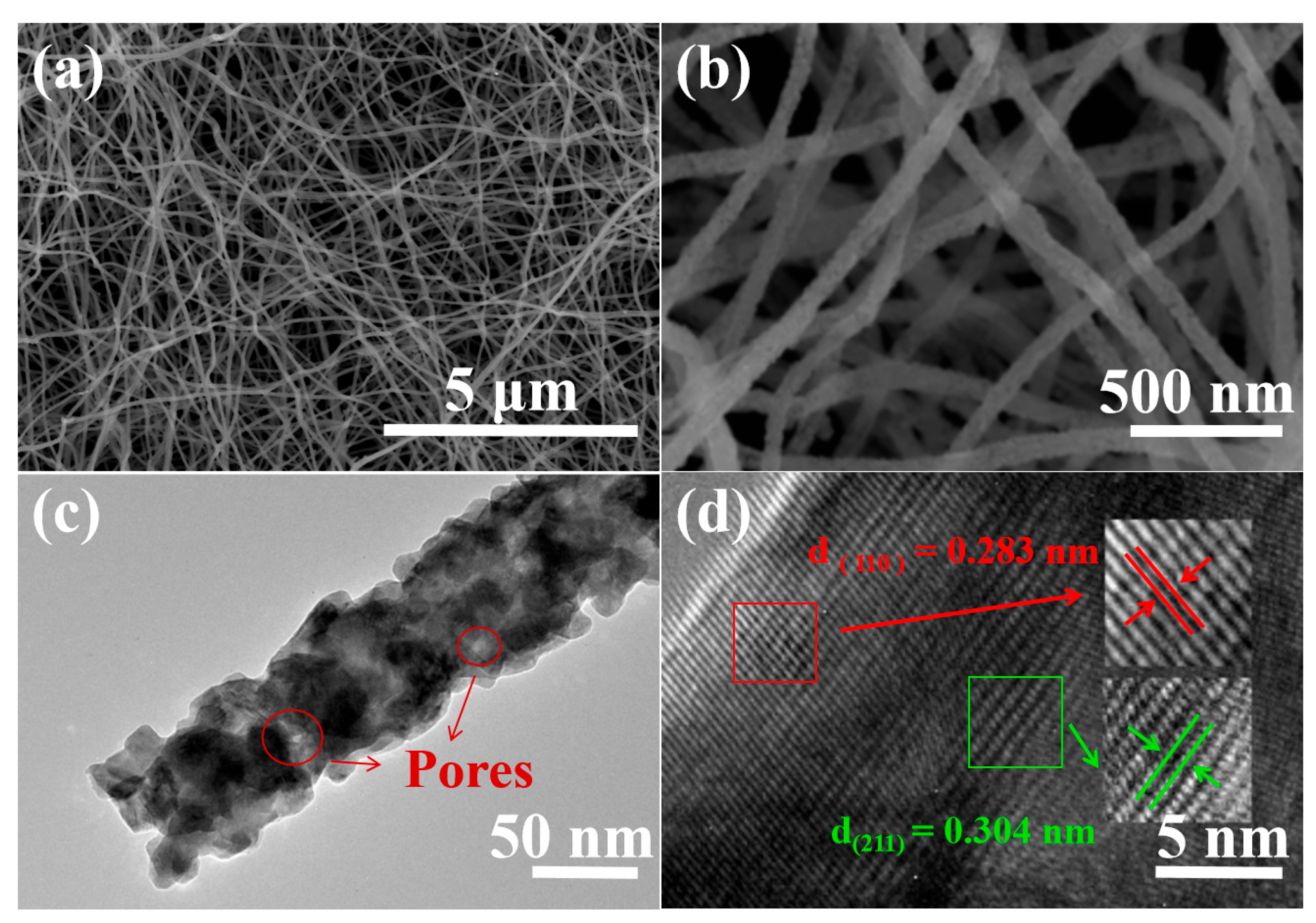
2.4. Materials Characterization
2.5. Electrochemical Measurements
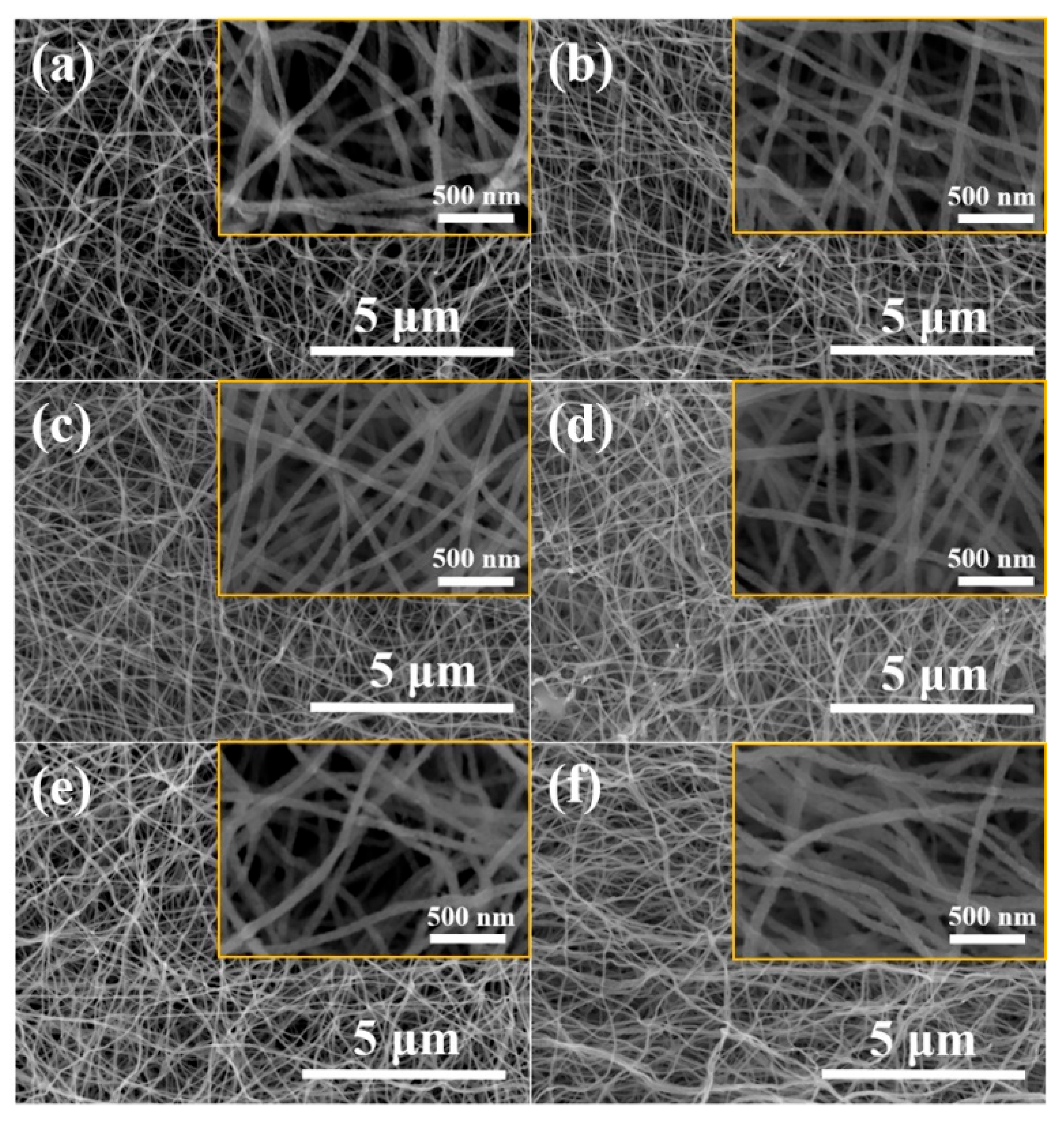
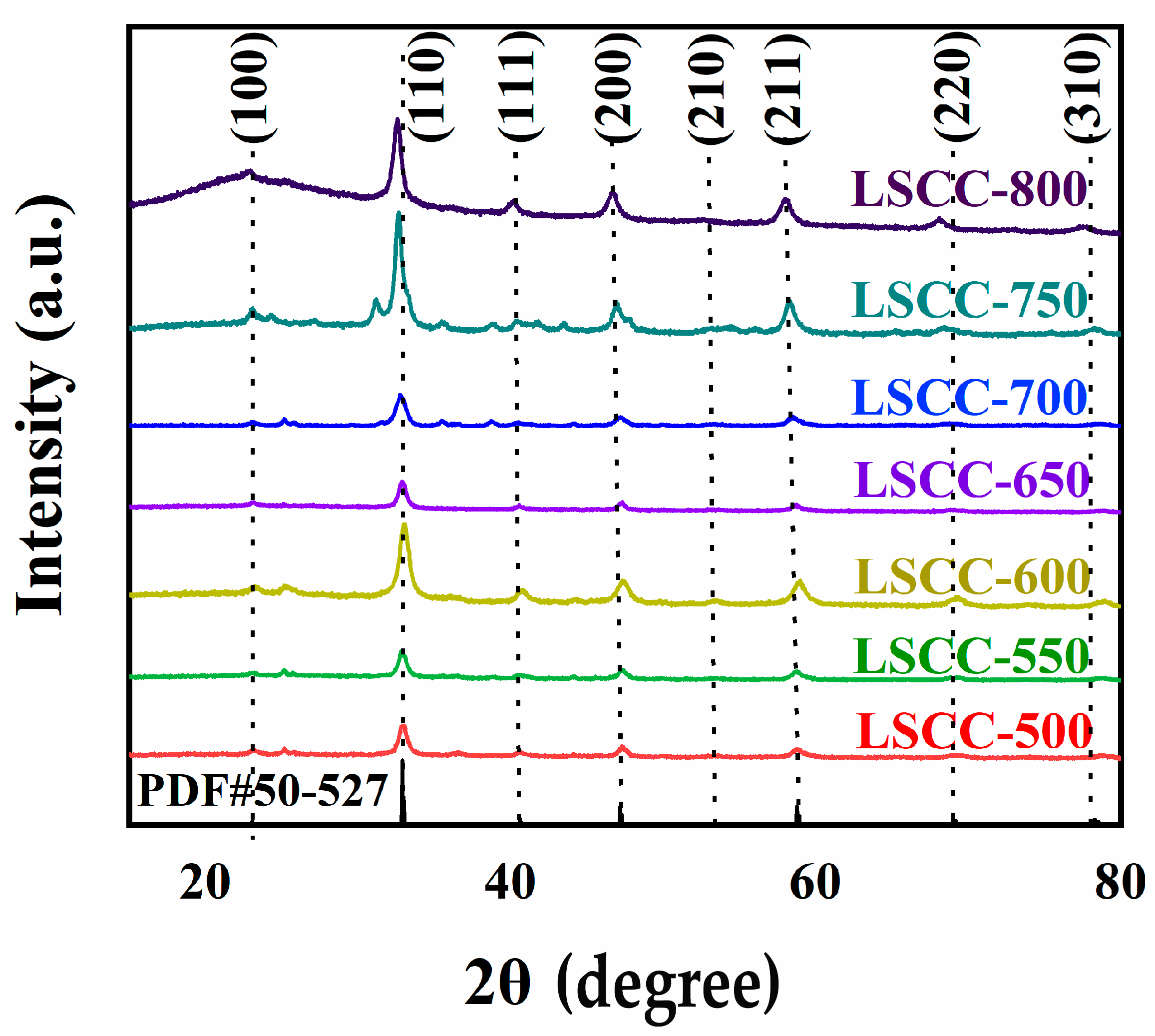
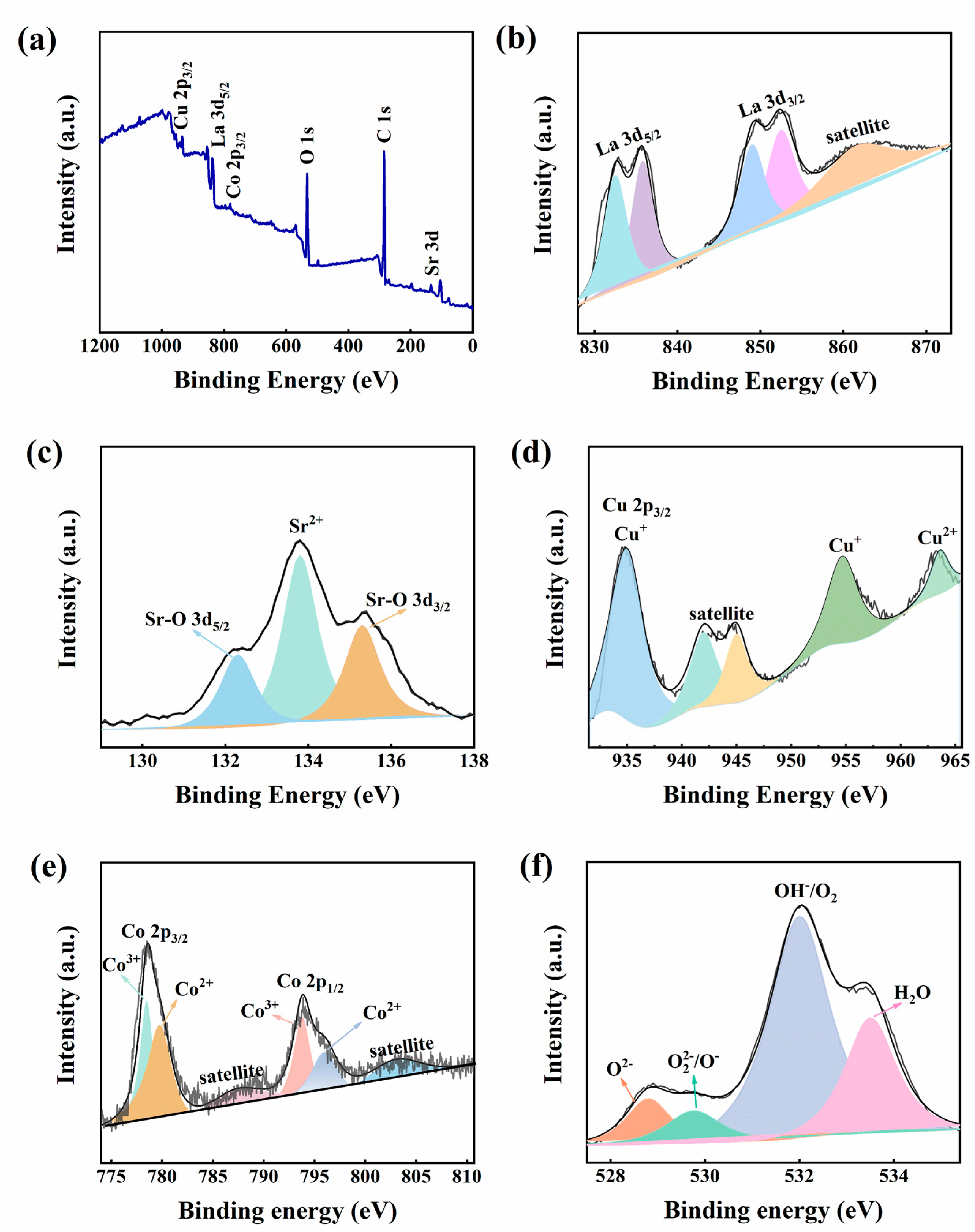
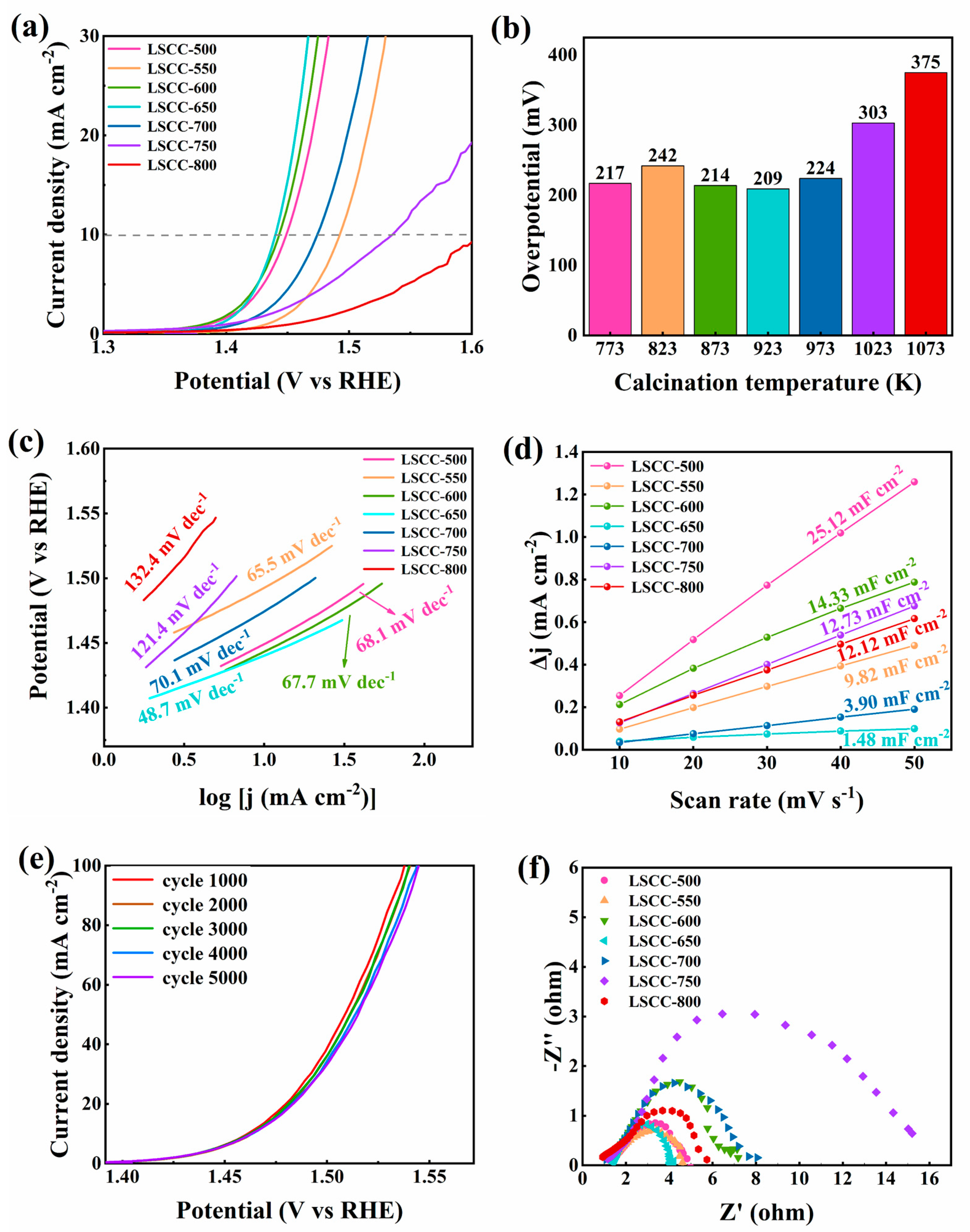
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Bi, X.; Wang, R.; Li, Y.; Jiang, G.; Li, L.; Zhong, C.; Chen, Z.; Lu, J. Relating catalysis between fuel cell and metal-air batteries. Matter 2020, 2, 32–49. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, Q.; Guan, J. Applications of metal–organic framework-derived materials in fuel cells and metal-air batteries. Coord. Chem. Rev. 2020, 409, 213214. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, M.; Zhao, X.; Cai, J.; Yan, W.; Yen, J.C.; Chen, S.; Yu, Y.; Zhang, J. Advanced noncarbon materials as catalyst supports and non-noble electrocatalysts for fuel cells and metal-air batteries. Electrochem. Energy Rev. 2021, 4, 336–381. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, Y.; Yang, R.; Kakimov, A.; Li, X. Recent advances of layered double hydroxides–based bifunctional electrocatalysts for ORR and OER. Mater. Today Chem. 2021, 21, 100488. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Z.; Duan, X.; Huang, Y. Nanoscale structure design for high-performance Pt-based ORR catalysts. Adv. Mater. 2019, 31, 1802234. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, H.; Xi, S.; Lee, W.S.V.; Xue, J. Understanding of oxygen redox in the oxygen evolution reaction. Adv. Mater. 2022, 34, 2107956. [Google Scholar] [CrossRef]
- Xie, X.; Du, L.; Yan, L.; Park, S.; Qiu, Y.; Sokolowski, J.; Wang, W.; Shao, Y. Oxygen evolution reaction in alkaline environment: Material challenges and solutions. Adv. Funct. Mater. 2022, 32, 2110036. [Google Scholar] [CrossRef]
- Gao, F.; He, J.; Wang, H.; Lin, J.; Chen, R.; Yi, K.; Huang, F.; Lin, Z.; Wang, M. Te-mediated electro-driven oxygen evolution reaction. Nano Res. Energy 2022, 1, e9120029. [Google Scholar] [CrossRef]
- Haase, F.T.; Bergmann, A.; Jones, T.E.; Timoshenko, J.; Herzog, A.; Jeon, H.S.; Rettenmaier, C.; Cuenya, B.R. Size effects and active state formation of cobalt oxide nanoparticles during the oxygen evolution reaction. Nat. Energy 2022, 7, 765–773. [Google Scholar] [CrossRef]
- Guo, T.; Li, L.; Wang, Z. Recent development and future perspectives of amorphous transition metal-based electrocatalysts for oxygen evolution reaction. Adv. Energy Mater. 2022, 12, 2200827. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, J.; He, G.; Chen, H. Current and future trends for spinel-type electrocatalysts in electrocatalytic oxygen evolution reaction. Coord. Chem. Rev. 2023, 475, 214869. [Google Scholar] [CrossRef]
- Wang, C.; Jin, L.; Shang, H.; Xu, H.; Shiraishi, Y.; Du, Y. Advances in engineering RuO2 electrocatalysts towards oxygen evolution reaction. Chin. Chem. Lett. 2021, 32, 2108–2116. [Google Scholar] [CrossRef]
- Schweinar, K.; Gault, B.; Mouton, I.; Kasian, O. Lattice oxygen exchange in rutile IrO2 during the oxygen evolution reaction. J. Phys. Chem. Lett. 2020, 11, 5008–5014. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Hao, L.; Quan, F.; Guo, R. Recent developments of Co3O4-based materials as catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2022, 12, 436–461. [Google Scholar] [CrossRef]
- Peng, X.; Feng, S.; Lai, S.; Liu, Z.; Gao, J.; Javanbakht, M.; Gao, B. Structural engineering of rare-earth-based perovskite electrocatalysts for advanced oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 39470–39485. [Google Scholar] [CrossRef]
- Zhao, J.-W.; Zhang, H.; Li, C.-F.; Zhou, X.; Wu, J.-Q.; Zeng, F.; Zhang, J.; Li, G.-R. Key roles of surface Fe sites and Sr vacancies in the perovskite for an efficient oxygen evolution reaction via lattice oxygen oxidation. Energy Environ. Sci. 2022, 15, 3912–3922. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, C.; Zhao, S.-N.; Wu, Y.; Liu, C.; Huang, J.; Xue, L.; Sun, J.; Zhang, W.; Wang, X. A dual-site doping strategy for developing efficient perovskite oxide electrocatalysts towards oxygen evolution reaction. Nano Energy 2022, 99, 107344. [Google Scholar] [CrossRef]
- Tomar, A.K.; Pan, U.N.; Kim, N.H.; Lee, J.H. Enabling Lattice Oxygen Participation in a Triple Perovskite Oxide Electrocatalyst for the Oxygen Evolution Reaction. ACS Energy Lett. 2022, 8, 565–573. [Google Scholar] [CrossRef]
- Liu, C.; Ji, D.; Shi, H.; Wu, Z.; Huang, H.; Kang, Z.; Chen, Z. An A-site management and oxygen-deficient regulation strategy with a perovskite oxide electrocatalyst for the oxygen evolution reaction. J. Mater. Chem. A 2022, 10, 1336–1342. [Google Scholar] [CrossRef]
- Xie, J.; Gao, Y.; Chen, G.; Wang, Y.; Yu, J.; Ciucci, F.; Chen, D.; Shao, Z. Simultaneously improved surface and bulk participation of evolved perovskite oxide for boosting oxygen evolution reaction activity using a dynamic cation exchange strategy. Small 2022, 18, 2204109. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Qi, S.; Su, Z.; Chen, K.; Yu, X.; Xie, A.; Luo, S. Coral-Like LaNixFe1− xO3 perovskite catalyst for high-performance oxygen evolution reaction. J. Electrochem. Soc. 2022, 169, 026508. [Google Scholar] [CrossRef]
- Jo, H.; Yang, Y.; Seong, A.; Jeong, D.; Kim, J.; Joo, S.H.; Kim, Y.J.; Zhang, L.; Liu, Z.; Wang, J.-Q. Promotion of the oxygen evolution reaction via the reconstructed active phase of perovskite oxide. J. Mater. Chem. A 2022, 10, 2271–2279. [Google Scholar] [CrossRef]
- He, X.; Qiao, T.; Li, B.; Zhang, Z.; Wang, S.; Wang, X.; Liu, H. Tuning Electronic Structure of CuCo2O4 Spinel via Mn-Doping for Enhancing Oxygen Evolution Reaction. ChemElectroChem 2023, 10, e202200933. [Google Scholar] [CrossRef]
- Olowoyo, J.O.; Kriek, R.J. Recent Progress on Bimetallic-Based Spinels as Electrocatalysts for the Oxygen Evolution Reaction. Small 2022, 18, 2203125. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Yang, N.; Li, X.; Linnemann, J.; Hagemann, U.; Ruediger, O.; Heidelmann, M.; Falk, T.; Aramini, M.; DeBeer, S. 3D atomic-scale imaging of mixed Co-Fe spinel oxide nanoparticles during oxygen evolution reaction. Nat. Commun. 2022, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Avcı, O.N.; Sementa, L.; Fortunelli, A. Mechanisms of the Oxygen Evolution reaction on NiFe2O4 and CoFe2O4 inverse-spinel oxides. ACS Catal. 2022, 12, 9058–9073. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, J.; Wang, L.; Liu, Y.; Liu, W.; Zhao, S.; Liu, Z.Q. Cation-tuning induced d-band center modulation on Co-based spinel oxide for oxygen reduction/evolution reaction. Angew. Chem. 2022, 134, e202114696. [Google Scholar] [CrossRef]
- Talluri, B.; Yoo, K.; Kim, J. High entropy spinel metal oxide (CoCrFeMnNi)3O4 nanoparticles as novel efficient electrocatalyst for methanol oxidation and oxygen evolution reactions. J. Environ. Chem. Eng. 2022, 10, 106932. [Google Scholar] [CrossRef]
- Cui, M.; Yang, C.; Li, B.; Dong, Q.; Wu, M.; Hwang, S.; Xie, H.; Wang, X.; Wang, G.; Hu, L. High-entropy metal sulfide nanoparticles promise high-performance oxygen evolution reaction. Adv. Energy Mater. 2021, 11, 2002887. [Google Scholar] [CrossRef]
- He, R.; Huang, X.; Feng, L. Recent progress in transition-metal sulfide catalyst regulation for improved oxygen evolution reaction. Energy Fuels 2022, 36, 6675–6694. [Google Scholar] [CrossRef]
- Peng, L.; Shah, S.S.A.; Wei, Z. Recent developments in metal phosphide and sulfide electrocatalysts for oxygen evolution reaction. Chin. J. Catal. 2018, 39, 1575–1593. [Google Scholar] [CrossRef]
- Sivanantham, A.; Ganesan, P.; Vinu, A.; Shanmugam, S. Surface activation and reconstruction of non-oxide-based catalysts through in situ electrochemical tuning for oxygen evolution reactions in alkaline media. Acs Catal. 2019, 10, 463–493. [Google Scholar] [CrossRef]
- Li, W.; Xiong, D.; Gao, X.; Liu, L. The oxygen evolution reaction enabled by transition metal phosphide and chalcogenide pre-catalysts with dynamic changes. Chem. Commun. 2019, 55, 8744–8763. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Bai, X.; Zhu, Y.-a.; Zhang, Y.; Lu, T.; Pan, Y.; Wang, J. Surface reconstruction induced in situ phosphorus doping in nickel oxides for an enhanced oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 6432–6441. [Google Scholar] [CrossRef]
- Gao, L.; Cui, X.; Sewell, C.D.; Li, J.; Lin, Z. Recent advances in activating surface reconstruction for the high-efficiency oxygen evolution reaction. Chem. Soc. Rev. 2021, 50, 8428–8469. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, C.; Ling, Z.; Li, J.; Qu, L.; Zhu, J.; Yang, W.; Wang, M.; Owusu, K.A.; Qin, L. Ni/Fe based bimetallic coordination complexes with rich active sites for efficient oxygen evolution reaction. Chem. Eng. J. 2021, 405, 126959. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Xue, H.; Pang, H.; Xu, Q. Electrocatalysts optimized with nitrogen coordination for high-performance oxygen evolution reaction. Coord. Chem. Rev. 2020, 422, 213468. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Li, H.; Hua, H. Simulation and experimental study on vibration and sound radiation control with piezoelectric actuators. Shock Vib. 2011, 18, 343–354. [Google Scholar] [CrossRef]
- Liu, H.; Xie, R.; Wang, Q.; Han, J.; Han, Y.; Wang, J.; Fang, H.; Qi, J.; Ding, M.; Ji, W.; et al. Enhanced OER Performance and Dynamic Transition of Surface Reconstruction in LaNiO3 Thin Films with Nanoparticles Decoration. Adv. Sci. 2023, 10, 2207128. [Google Scholar] [CrossRef]
- Li, L.; Jiang, B.; Tang, D.; Zhang, Q.; Zheng, Z. Hydrogen generation by acetic acid steam reforming over Ni-based catalysts derived from La1−xCexNiO3 perovskite. Int. J. Hydrogen Energy 2018, 43, 6795–6803. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Sanchez-Adsuar, M.-S.; Illan-Gomez, M.-J. Analyzing the role of copper in the soot oxidation performance of BaMnO3-perovskite-based catalyst obtained by modified sol-gel synthesis. Fuel 2022, 328, 125258. [Google Scholar] [CrossRef]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on sol-gel synthesis of perovskite and oxide nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef]
- Yu, L.; Xu, N.; Zhu, T.; Xu, Z.; Sun, M.; Geng, D. La0.4Sr0.6Co0.7Fe0.2Nb0.1O3−δ perovskite prepared by the sol-gel method with superior performance as a bifunctional oxygen electrocatalyst. Int. J. Hydrogen Energy 2020, 45, 30583–30591. [Google Scholar] [CrossRef]
- Karami, M.; Ghanbari, M.; Amiri, O.; Salavati-Niasari, M. Enhanced antibacterial activity and photocatalytic degradation of organic dyes under visible light using cesium lead iodide perovskite nanostructures prepared by hydrothermal method. Sep. Purif. Technol. 2020, 253, 117526. [Google Scholar] [CrossRef]
- Walton, R.I. Perovskite oxides prepared by hydrothermal and solvothermal synthesis: A review of crystallisation, chemistry, and compositions. Chem.–A Eur. J. 2020, 26, 9041–9069. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, Y.; Zhang, C.; Yao, C.; Ni, C.; Li, X. In Situ Growth of Perovskite on 2D Hydrothermal Carbonation Carbon for Photocatalytic Reduction of Nitrate to Ammonia. ACS Appl. Nano Mater. 2023, 6, 13127–13136. [Google Scholar] [CrossRef]
- Vu, T.H.Q.; Bondzior, B.; Stefańska, D.; Miniajluk, N.; Dereń, P.J. Synthesis, structure, morphology, and luminescent properties of Ba2MgWO6: Eu3+ double perovskite obtained by a novel Co-precipitation method. Materials 2020, 13, 1614. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Pham, V.; Pham, T.L.; Nguyen, L.T.T.; Mittova, I.Y.; Mittova, V.; Vo, L.N.; Nguyen, B.T.T.; Bui, V.X.; Viryutina, E. Simple synthesis of NdFeO3 nanoparticles by the co-precipitation method based on a study of thermal behaviors of Fe (III) and Nd (III) hydroxides. Crystals 2020, 10, 219. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Zhao, Y.; Ge, X.; Lu, Q.; Zhang, T.; Xie, D.; Li, M.; Bu, Y. Design strategies of perovskite nanofibers electrocatalysts for water splitting: A mini review. Chem. Eng. J. 2023, 451, 138710. [Google Scholar] [CrossRef]
- Wu, X.; Miao, H.; Hu, R.; Chen, B.; Yin, M.; Zhang, H.; Xia, L.; Zhang, C.; Yuan, J. A-site deficient perovskite nanofibers boost oxygen evolution reaction for zinc-air batteries. Appl. Surf. Sci. 2021, 536, 147806. [Google Scholar] [CrossRef]
- Bkkar, M.; Olekhnovich, R.; Kremleva, A.; Sitnikova, V.; Kovach, Y.; Zverkov, N.; Uspenskaya, M. Influence of electrospinning setup parameters on properties of polymer-perovskite nanofibers. Polymers 2023, 15, 731. [Google Scholar] [CrossRef]
- Zhao, B.; Gao, X.; Pan, K.; Deng, J. Chiral helical polymer/perovskite hybrid nanofibers with intense circularly polarized luminescence. ACS Nano 2021, 15, 7463–7471. [Google Scholar] [CrossRef]
- Li, G.; Hou, S.; Gui, L.; Feng, F.; Zhang, D.; He, B.; Zhao, L. Carbon quantum dots decorated Ba0.5Sr0.5Co0.8Fe0.2O3−δ perovskite nanofibers for boosting oxygen evolution reaction. Appl. Catal. B Environ. 2019, 257, 117919. [Google Scholar] [CrossRef]
- Chen, L.; Chuang, Y.; Yang, W.-D.; Tsai, K.-C.; Chen, C.-W.; Dong, C.-D. All-inorganic perovskite CsPbX3 electrospun nanofibers with color-tunable photoluminescence and high-performance optoelectronic applications. J. Alloys Compd. 2021, 856, 157426. [Google Scholar] [CrossRef]
- Fu, L.; Zhou, J.; Deng, Q.; Yang, J.; Li, Q.; Zhu, Z.; Wu, K. Interfacial electron transfer in heterojunction nanofibers for highly efficient oxygen evolution reaction. Nanoscale 2023, 15, 677–686. [Google Scholar] [CrossRef]
- Wei, P.; Sun, X.; Liang, Q.; Li, X.; He, Z.; Hu, X.; Huang, Y. Enhanced oxygen evolution reaction activity by encapsulating NiFe alloy nanoparticles in nitrogen-doped carbon nanofibers. ACS Appl. Mater. Interfaces 2020, 12, 31503–31513. [Google Scholar] [CrossRef]
- Lin, H.; Xie, J.; Zhang, Z.; Wang, S.; Chen, D. Perovskite nanoparticles@ N-doped carbon nanofibers as robust and efficient oxygen electrocatalysts for Zn-air batteries. J. Colloid Interface Sci. 2021, 581, 374–384. [Google Scholar] [CrossRef]
- El-Shazly, A.N.; Rezk, M.Y.; Gameel, K.M.; Allam, N.K. Electrospun lead-free all-inorganic double perovskite nanofibers for photovoltaic and optoelectronic applications. ACS Appl. Nano Mater. 2019, 2, 7085–7094. [Google Scholar] [CrossRef]
- Fu, Y.; Guo, L.; Ren, Z.; Li, X.; Zhang, Q.; Wu, J.; Li, Y.; Liu, W.; Li, P.; Ma, J. Enhanced property of flexible UV photodetectors based on electrospinning ZnO-SnO2 heterojunction nanofibers by the formation of Zn2SnO4. Ceram. Int. 2023, 49, 11402–11410. [Google Scholar] [CrossRef]
- Bathula, C.; Jana, A.; Soni, R.; Naushad, M.; Kim, H.-S. Enhanced stability of methylammonium lead bromide perovskite interlaced on cellulose nanofiber. Ceram. Int. 2023, 49, 30886–30891. [Google Scholar] [CrossRef]
- Béjar, J.; Ãlvarez-Contreras, L.; Ledesma-GarcÃa, J.; Arjona, N.; Arriaga, L. Electrocatalytic evaluation of Co3O4 and NiCo2O4 rosettes-like hierarchical spinel as bifunctional materials for oxygen evolution (OER) and reduction (ORR) reactions in alkaline media. J. Electroanal. Chem. 2019, 847, 113190. [Google Scholar] [CrossRef]
- Sun, B.; Liu, Y.; Hu, Y.; Zhou, H.; Zhang, Z.; Wang, J.; Gu, S.; Shen, Y. Hygroscopic La2O3 hybridized LaCoO3-based composite catalyst for efficient ozone decomposition under humidity conditions. Appl. Surf. Sci. 2023, 640, 158317. [Google Scholar] [CrossRef]
- Lv, C.; Chen, H.; Hu, M.; Ai, T.; Fu, H. Nano-oxides washcoat for enhanced catalytic oxidation activity toward the perovskite-based monolithic catalyst. Environ. Sci. Pollut. Res. 2021, 28, 37142–37157. [Google Scholar] [CrossRef]
- Natile, M.M.; Ugel, E.; Maccato, C.; Glisenti, A. LaCoO3: Effect of synthesis conditions on properties and reactivity. Appl. Catal. B Environ. 2007, 72, 351–362. [Google Scholar] [CrossRef]
- Mandal, R.; Mahton, Y.; Sowjanya, C.; Sanket, K.; Behera, S.K.; Pratihar, S.K. Electrocatalytic behaviour of Cu-substituted La0.5Sr0.5Co0.8Fe0.2−xCuxO3−δ (x = 0–0.2) perovskite oxides. J. Solid State Chem. 2023, 317, 123668. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, H.; Hao, J.; Wang, C.; Wen, Y.; Li, H.; Lu, S.; Duan, F.; Du, M. Integrating the cationic engineering and hollow structure engineering into perovskites oxides for efficient and stable electrocatalytic oxygen evolution. Electrochim. Acta 2019, 327, 135033. [Google Scholar] [CrossRef]
- Yang, J.; Liu, W.; Xu, M.; Liu, X.; Qi, H.; Zhang, L.; Yang, X.; Niu, S.; Zhou, D.; Liu, Y. Dynamic behavior of single-atom catalysts in electrocatalysis: Identification of Cu-N3 as an active site for the oxygen reduction reaction. J. Am. Chem. Soc. 2021, 143, 14530–14539. [Google Scholar] [CrossRef]
- Li, F.; Tang, J.; Ke, Q.; Guo, Y.; Ha, M.N.; Wan, C.; Lei, Z.; Gu, J.; Ling, Q.; Nguyen, V.N. Investigation into Enhanced Catalytic Performance for Epoxidation of Styrene over LaSrCoxFe2−xO6 Double Perovskites: The Role of Singlet Oxygen Species Promoted by the Photothermal Effect. ACS Catal. 2021, 11, 11855–11866. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, X.; Liu, J.; Li, X.; Xing, C.; Lyu, X.; Cai, W.; Wang, W.; Li, Y. Cr dopant induced breaking of scaling relations in Co Fe layered double hydroxides for improvement of oxygen evolution reaction. Small 2019, 15, 1902373. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ma, W.; Li, H. In situ growth of CoP3/carbon polyhedron/CoO/NF nanoarrays as binder†free anode for lithium†ion batteries with enhanced specific capacity. Small 2020, 16, 1907468. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yoshida, A.; Wang, P.; Yu, T.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Catalytic oxidation of volatile organic compound over cerium modified cobalt-based mixed oxide catalysts synthesized by electrodeposition method. Appl. Catal. B Environ. 2020, 271, 118941. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Liu, C.; Xu, S.; Min, S.; Wang, W.; Mitsuzaki, N.; Chen, Z. A/B-Site Management Strategy to Boost Electrocatalytic Overall Water Splitting on Perovskite Oxides in an Alkaline Medium. Inorg. Chem. 2023, 62, 12590–12599. [Google Scholar] [CrossRef]
- Zhu, K.; Shi, F.; Zhu, X.; Yang, W. The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction. Nano Energy 2020, 73, 104761. [Google Scholar] [CrossRef]
- Li, P.; Wan, X.; Su, J.; Liu, W.; Guo, Y.; Yin, H.; Wang, D. A single-phase FeCoNiMnMo high-entropy alloy oxygen evolution anode working in alkaline solution for over 1000 h. ACS Catal. 2022, 12, 11667–11674. [Google Scholar] [CrossRef]
- Yu, J.; Chen, G.; Sunarso, J.; Zhu, Y.; Ran, R.; Zhu, Z.; Shao, Z. Cobalt oxide and cobalt-graphitic carbon core–shell based catalysts with remarkably high oxygen reduction reaction activity. Adv. Sci. 2016, 3, 1600060. [Google Scholar] [CrossRef]
- Alegre, C.; Busacca, C.; Di Blasi, A.; Di Blasi, O.; Aricò, S.; Antonucci, A.V.; Baglio, V. Toward more efficient and stable bifunctional electrocatalysts for oxygen electrodes using FeCo2O4/carbon nanofiber prepared by electrospinning. Mater. Today Energy 2020, 18, 100508. [Google Scholar] [CrossRef]
- Wang, H.; Xu, W.; Richins, S.; Liaw, K.; Yan, L.; Zhou, M.; Luo, H. Polymer-assisted approach to LaCo1-xNixO3 network nanostructures as bifunctional oxygen electrocatalysts. Electrochim. Acta 2019, 296, 945–953. [Google Scholar] [CrossRef]
- He, D.; He, G.; Jiang, H.; Chen, Z.; Huang, M. Enhanced durability and activity of the perovskite electrocatalyst Pr0.5Ba0.5CoO3−δ by Ca doping for the oxygen evolution reaction at room temperature. Chem. Commun. 2017, 53, 5132–5135. [Google Scholar] [CrossRef]
- Yi, K.; Sun, L.; Li, Q.; Xia, T.; Huo, L.; Zhao, H.; Li, J.; Lü, Z.; Bassat, J.M.; Rougier, A.; et al. Effect of Nd-deficiency on electrochemical properties of NdBaCo2O6−δ cathode for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2016, 41, 10228–10238. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Z.; Wang, J.; Zhong, W.; Ju, M.; Cai, R.; Qiu, C.; Long, X.; Yang, S. The role of ceria in a hybrid catalyst toward alkaline water oxidation. ChemSusChem 2020, 13, 5273–5279. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Hu, M.; Deng, Y.; Wang, C. One-pot fabrication of a novel agar-polyacrylamide/graphene oxide nanocomposite double network hydrogel with high mechanical properties. Adv. Eng. Mater. 2016, 18, 1799–1807. [Google Scholar] [CrossRef]
- Xiao, L.; Liang, Y.; Li, Z.; Wu, S.; Luo, S.; Sun, H.; Zhu, S.; Cui, Z. Amorphous FeNiNbPC nanoprous structure for efficient and stable electrochemical oxygen evolution. J. Colloid Interface Sci. 2022, 608, 1973–1982. [Google Scholar] [CrossRef]
- Wang, C.; Wang, R.; Peng, Y.; Chen, J.; Chen, Z.; Yin, H.; Li, J. Nb-incorporated Fe(oxy) hydroxide derived from structural transformation for efficient oxygen evolution electrocatalysis. J. Mater. Chem. A 2020, 8, 24598–24607. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, G.; Liu, S.; Su, H.; Wang, Y.; Li, J.; Luo, C. Remodeling the tumor microenvironment with emerging nanotherapeutics. Trends Pharmacol. Sci. 2018, 39, 59–74. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, Y.; Ianni, E.; Lin, B. A highly active and stable La0.5Sr0.5Ni0.4Fe0.6O3-δ perovskite electrocatalyst for oxygen evolution reaction in alkaline media. Electrochim. Acta 2017, 246, 997–1003. [Google Scholar] [CrossRef]
- Retuerto, M.; Calle-Vallejo, F.; Pascual, L.; Lumbeeck, G.; Fernandez-Diaz, M.T.; Croft, M.; Gopalakrishnan, J.; Pena, M.A.; Hadermann, J.; Greenblatt, M.; et al. La1.5Sr0.5NiMn0.5Ru0.5O6 double perovskite with enhanced ORR/OER bifunctional catalytic activity. ACS Appl. Mater. Interfaces 2019, 11, 21454–21464. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, M.; Nagaiah, T.; Siruguri, V.; Rayaprol, S.; Yadav, A.; Jha, S.N.; Bhattacharyya, D.; Paul, A.K. Investigation of new B-site-disordered perovskite oxide CaLaScRuO6+δ: An efficient oxygen bifunctional electrocatalyst in a highly alkaline medium. ACS Appl. Mater. Interfaces 2020, 12, 9190–9200. [Google Scholar] [CrossRef]
- Sun, Y.; Li, R.; Chen, X.; Wu, J.; Xie, Y.; Wang, X.; Zhang, Y. A-site management prompts the dynamic reconstructed active phase of perovskite oxide OER catalysts. Adv. Energy Mater. 2021, 11, 2003755. [Google Scholar] [CrossRef]
- Duan, Y.; Yu, Y.; Hu, J.; Zheng, S.; Zhang, T.; Ding, H.; Hu, B.; Fu, Q.; Yu, L.; Zheng, X.; et al. Scaled-up synthesis of amorphous NiFeMo oxides and their rapid surface reconstruction for superior oxygen evolution catalysis. Angew. Chem. Int. Ed. 2019, 58, 15772–15777. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.; Zhang, F.; Wu, L.; Xing, X.; Wang, D.; Song, S.; Zhou, Q.; Yu, L.; Bao, J.; Chen, S.; et al. Boosting efficient alkaline fresh water and seawater electrolysis via electrochemical reconstruction. Energy Environ. Sci. 2022, 15, 3945–3957. [Google Scholar] [CrossRef]
- Karthik, N.; Atchudan, R.; Edison, T.; Choi, S. Insights of pristine stainless steel mesh oxygen evolution reaction in diverse concentrations of potassium hydroxide. Mater. Lett. 2023, 333, 133557. [Google Scholar] [CrossRef]
- Peng, Q.; Zhuang, X.; Wei, L.; Shi, L.; Isimjan, T.; Hou, R.; Yang, X. Niobium-Incorporated CoSe2 Nanothorns with Electronic Structural Alterations for Efficient Alkaline Oxygen Evolution Reaction at High Current Density. ChemSusChem 2022, 15, e202200827. [Google Scholar] [CrossRef]
- Mondal, R.; Ratnawat, H.; Mukherjee, S.; Gupta, A.; Singh, P. Investigation of the role of Sr and development of superior Sr-doped hexagonal BaCoO3−δ perovskite bifunctional OER/ORR catalysts in alkaline media. Energy Fuels 2022, 36, 3219–3228. [Google Scholar] [CrossRef]
- Zhu, C.; Nobuta, A.; Nakatsugawa, I.; Akiyama, T. Solution combustion synthesis of LaMO3 (M = Fe, Co, Mn) perovskite nanoparticles and the measurement of their electrocatalytic properties for air cathode. Int. J. Hydrogen Energy 2013, 38, 13238–13248. [Google Scholar] [CrossRef]
- Lu, Y.; Chien, Y.; Liu, C.; You, T.; Hu, C. Active site-engineered bifunctional electrocatalysts of ternary spinel oxides, M0.1Ni0.9Co2O4 (M: Mn, Fe, Cu, Zn) for the air electrode of rechargeable zinc–air batteries. J. Mater. Chem. A 2017, 5, 21016–21026. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Chen, Y.; Bai, Y.; Zhu, C.; Zhang, M. One-Dimensional La0.2Sr0.8Cu0.4Co0.6O3−δ Nanostructures for Efficient Oxygen Evolution Reaction. Nanomaterials 2024, 14, 64. https://doi.org/10.3390/nano14010064
Wu D, Chen Y, Bai Y, Zhu C, Zhang M. One-Dimensional La0.2Sr0.8Cu0.4Co0.6O3−δ Nanostructures for Efficient Oxygen Evolution Reaction. Nanomaterials. 2024; 14(1):64. https://doi.org/10.3390/nano14010064
Chicago/Turabian StyleWu, Dongshuang, Yidan Chen, Yuelei Bai, Chuncheng Zhu, and Mingyi Zhang. 2024. "One-Dimensional La0.2Sr0.8Cu0.4Co0.6O3−δ Nanostructures for Efficient Oxygen Evolution Reaction" Nanomaterials 14, no. 1: 64. https://doi.org/10.3390/nano14010064
APA StyleWu, D., Chen, Y., Bai, Y., Zhu, C., & Zhang, M. (2024). One-Dimensional La0.2Sr0.8Cu0.4Co0.6O3−δ Nanostructures for Efficient Oxygen Evolution Reaction. Nanomaterials, 14(1), 64. https://doi.org/10.3390/nano14010064






