Abstract
In this study, a novel organic–inorganic hybrid material IIGK@MnO2 (2-naphthalenemethyl-isoleucine-isoleucine-glycine-lysine@manganese dioxide) was designed as a novel adsorbent for the removal of strontium ions (Sr2+). The morphology and structure of IIGK@MnO2 were characterized using TEM, AFM, XRD, and XPS. The results indicate that the large specific surface area and abundant negative surface charges of IIGK@MnO2 make its surface rich in active adsorption sites for Sr2+ adsorption. As expected, IIGK@MnO2 exhibited excellent adsorbing performance for Sr2+. According to the adsorption results, the interaction between Sr2+ and IIGK@MnO2 can be fitted with the Langmuir isotherm and pseudo-second-order equation. Moreover, leaching and desorption experiments were conducted to assess the recycling capacity, demonstrating significant reusability of IIGK@MnO2.
1. Introduction
Nuclear energy is widely used due to its ability to reduce greenhouse effects, high energy density, and ease of storing nuclear fuel [1]. However, in nuclear disasters such as Chernobyl and Fukushima, the release of large amounts of radioactive isotopes and waste has had a catastrophic impact on ecosystems, causing significant damage. In terms of radioactive safety, strontium (90Sr), cobalt (60Co), and cesium (137Cs) are considered the main radioactive isotopes due to their relatively long half-lives, high solubility, and easy transfer [2]. Among them, 90Sr is a carcinogen and hazardous pollutant that has chemical properties similar to calcium and is easily absorbed by the human body [3]. Therefore, the development of new materials for removing strontium ions (Sr2+) from aqueous solutions has received great attention. At present, various methods have been developed to remove radioactive toxic ions from aqueous solutions, including chemical precipitation, membrane, solvent extraction, ion exchange, and adsorption [4,5,6,7,8]. Among them, the adsorption method is the most used and highly efficient technology. Up to now, a large number of nanomaterials, such as hydroxyapatite nanoparticles, metal sulfide, metallic oxide, graphene oxide (GO), and MOFs, have been employed as adsorbents for removing Sr2+ [9,10,11,12,13]. Attributed to their larger specific surface area and more active sites, MnO2 nanoparticles have been widely studied and applied as effective adsorbents for Sr2+ [14]. Moreover, MnO2 particles also exhibit selectivity for Sr2+ in the presence of competing ions, including Na+, K+, Ca2+, and Mg2+ [15]. However, MnO2 nanoparticles have drawbacks such as susceptibility to van der Waals forces, which may lead to aggregation and a decrease in their adsorption performance [16]. Therefore, it is necessary to fix MnO2 nanoparticles on the carrier to improve their dispersion stability as well as adsorption performance and reusability for Sr2+ removal.
Due to the presence of abundant functional groups such as hydroxyl groups, thiol groups, and carboxyl groups as adsorption sites, along with diverse secondary structures, a series of peptides has been studied for their binding to various metallic materials, including metal oxides, metal sulfides, and metals [17,18]. Moreover, some specifically designed peptides demonstrate a selective affinity for particular metal ions, which can serve as effective metal-binding moieties in aqueous solutions and promote selective metal ion adsorption [19]. For example, Mondal et al. confirmed the affinity of a tripeptide gel for metal ions, including lead and cadmium [20]. Besides direct metal ion adsorption, the peptides can be used to composite with other materials to achieve synergistic metal ion adsorption. At present, various peptide sequences have been used for biomimetic synthesis of ceramics, metal sulfides, metal oxides, and metal nanoparticles, and composite materials with low dispersion, good crystallinity, diverse morphology, and excellent biological functions have been constructed in mild water environments [21,22]. In addition, peptides have excellent assembly performance and can form different functional structures through covalent and noncovalent interactions (such as hydrophilicity and hydrophobicity, π-π stacking, hydrogen bonding, and electrostatic interactions). These peptide assembly structures can regulate the structure of peptide–inorganic nanoparticles by inducing the nucleation and growth process of inorganic nanoparticles and selectively binding to certain crystal planes. This makes peptides an excellent template for in situ loading and construction of inorganic nanomaterials.
As mentioned in the above text, though MnO2 nanoparticles are good candidates for Sr2+ absorption and removal, they easily experience severe agglomeration and poor dispersibility in aqueous solution, which influences the adsorption performance. To address this issue, we chose the self-assembled nanofibrils of short peptide 2-naphthalenemethyl-isoleucine-isoleucine-glycine-lysine (IIGK) (Figure 1) as templates to mediate the mineralization of MnO2 on their surface, which can greatly enhance the dispersibility of MnO2 nanoparticles. Compared with other methods for synthesizing MnO2, such as electrodeposition, demulsification, and hydrothermal methods, this method is environmentally friendly, mild, and easy to operate. The physicochemical properties of the as-prepared IIGK@MnO2 were systematically characterized first by using various techniques. Then, experiments were performed to investigate the adsorption performance of the IIGK@MnO2 nanocomposites towards Sr2+. The results show that the IIGK@MnO2 nanocomposites have a strong affinity and adsorption capacity for Sr2+, and a synergistic effect on the adsorption of Sr2+ with IIGK@MnO2 can be concluded. This study demonstrated the merits of IIGK@MnO2 as a promising adsorbent to remove Sr2+ from nuclear wastewater.
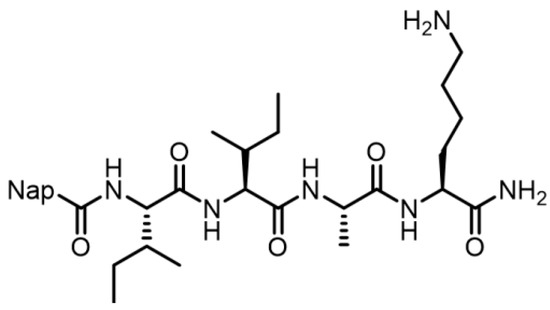
Figure 1.
Molecular structure of the short peptide IIGK.
2. Materials and Methods
2.1. Materials
Manganese chloride tetrahydrate (MnCl2·4H2O), potassium permanganate (KMnO4), sodium nitrate (NaNO3), potassium nitrate (KNO3), strontium nitrate (Sr(NO3)2), sodium hydroxide (NaOH), and potassium hydroxide (KNO3) were acquired from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All working solutions were prepared using distilled water. The pH was adjusted using NaOH and HCl as needed and monitored with a digital pH meter. All adsorption experiments were conducted at ambient temperature.
2.2. Preparation of IIGK@MnO2 Nanocomposite
First, 300 mg IIGK powder was dissolved in 250 mL ultrapure water, which was then subjected to ultrasonic dispersion for approximately 10 min. Then, the mixture was left to stand overnight, followed by adding another 225.6 mL ultrapure water. Subsequently, 9.6 mL KmnO4 (30 mM) aqueous solution and 9.6 mL MnCl2·4H2O (42 mM) aqueous solution were added to the mixture, which was then stirred for 24 h. Finally, the samples were centrifuged, washed three times with ultrapure water, and subsequently freeze-dried to obtain IIGK@MnO2 powder for further use.
2.3. Characterization
The morphology of samples was characterized using a scanning electron microscope (SEM, Hitachi S-4800, Tokyo, Japan) and transmission electron microscope (TEM, JOEL JEM1400 Plus, Tokyo, Japan). The SEM equipped with an Oxford Instruments energy-dispersive X-ray spectroscopy (EDS) system was used for analyzing elemental composition. The thickness of materials was measured using atomic force microscopy (AFM, Santa Babara Vecco Nanoscope Iva, Santa Barbara, CA, USA). The size and zeta potential of samples were determined on a Zetasizer Nano (DLS, Malvern NANO ZS, Marvin City, UK). The element analysis of the samples before and after Sr2+ adsorption was carried out using X-ray photoelectron spectroscopy (XPS, Thermo Scientific Esclab 250Xi, Waltham, MA, USA) with a monochromatic Al Kα X-ray source (15 KV). Fourier-transform infrared spectroscopy (FT-IR, Thermo Scientific Nicolet iS5, Waltham, MA, USA) was performed to investigate the interaction between MnO2 and IIGK. X-ray diffraction (XRD, PANalitical X‘Pert PRO MPD, Almelo, The Netherland) was performed to study the crystal structure of the samples using Cu-Kα radiation in the 2θ range of 10°–80° at room temperature. N2 adsorption–desorption isotherm was conducted on the gas sorption analyzer at 373.15 K (Malvern PANalitical Autosorb-6B, Marvin City, UK), and the specific area and the average pore diameters were stimulated using the Brunauer-Emmett-Teller (BET) and Barrett–Joyner–Halenda (BJH) method. Inductively coupled plasma optical emission spectroscopy (ICP-OES, HORIBA JY 2000-2, Loos, Naples, FL, USA) was employed to determine the concentration of residual metal ions in the solution.
2.4. Adsorption Performance of IIGK@MnO2
All adsorption experiments were carried out in 15 mL polyethylene centrifuge tubes. After mixing 1 mg IIGK@MnO2 with 10 mL Sr2+ aqueous solution, the pH of the mixture was adjusted to a suitable value. The mixture was left in a constant-temperature shaker for a certain time, followed by centrifugation at 12,000 rpm for 20 min to extract the supernatant. Finally, the residue Sr2+ concentration in the adsorbed solution was measured using ICP-OES. The equilibrium adsorption amount (qe, mg/g) and removal rate (R, %) of IIGK@MnO2 towards Sr2+ can be calculated by the following equations:
where c0 (mg/L) and ce (mg/L) are the initial and equilibrium concentrations of Sr2+ in the solution, respectively. V (mL) represents the volume of the adsorbed solution, and M (g) is the quantity of adsorbent.
2.5. Desorption of Strontium Ions from IIGK@MnO2
The desorption behavior of the IIGK@MnO2 was investigated by treating IIGK-MnO2-Sr composites with 1 M HCl aqueous solution. The amount of Sr2+ desorbed from the IIGK@MnO2, QD_Sr, was determined according to Equation (3), while the desorption efficiency (De, %) was obtained from Equation (4).
where CD (mg/L) is the equilibrium desorption concentrations of Sr2+, VD (mL) represents the volume of the eluent, and MD (g) is the weight of adsorbent after Sr2+ adsorption.
3. Results and Discussion
3.1. Synthesis and Characterization of IIGK@MnO2 Nanocomposite
In this work, the IIGK@MnO2 nanocomposite was synthesized by depositing MnO2 nanoparticles along peptide fibers using a green, simple, and easy-to-operate biomimetic mineralization method. For comparison, pure manganese dioxide nanoparticles without peptide involvement were also synthesized. The morphology of MnO2 nanoparticles and IIGK@MnO2 nanocomposites was characterized using SEM, TEM, and AFM. As shown in Figure 2a,b, the MnO2 nanoparticles generated without peptide show a disordered flower shape with a diameter of 400–500 nm, while the IIGK@MnO2 nanocomposites took on elongated structures with many MnO2 nanoparticles aligned in an axial direction (Figure 2c,d). The TEM result (Figure 2e) shows more clearly the one-dimensional nanowire/nanosheet composited structure. The results indicate that MnO2 is generated through biomimetic mineralization using IIGK short peptides as templates. The inner core should be the IIGK fibrils, while the outer layer sheets should be MnO2 nanostructures. The results demonstrate clearly the template role of the peptide fibrils in mediating MnO2 mineralization. The formation of fibrous IIGK@MnO2 nanocomposite can be confirmed with an AFM image (Figure 2f).
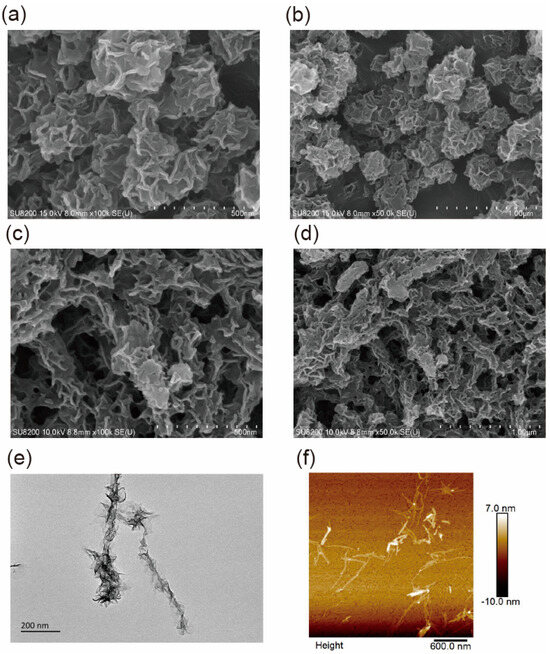
Figure 2.
(a,b) SEM images of MnO2, (c,d) SEM images of IIGK@MnO2, (e) TEM, and (f) AFM images of IIGK@MnO2 nanocomposite.
The surface zeta potential of the one-dimensional fiber-like IIGK@MnO2 nanocomposites at different pH was then measured, as shown in Figure 3a. It can be observed that the isoelectric point of IIGK@MnO2 appears at pH 1.7. While the pH value increases from 0.9 to 10.5, the zeta potential of IIGK@MnO2 decreases gradually from 10 mV to −40 mV. Notably, when the pH value is greater than 1.7, IIGK@MnO2 undergoes charge reversal, changing from a positively charged surface to a negatively charged surface. Moreover, the excess negative charges on the IIGK@MnO2 surface are beneficial for the subsequent adsorption of Sr2+. The particle size distribution of MnO2 and IIGK@MnO2 was measured using the dynamic light scattering measurement. As shown in Figure 3b, the size of IIGK-induced IIGK@MnO2 nanocomposites is about 100–400 nm, while that of MnO2 produced in the absence of peptide is above 1000 nm. This result indicates that the IIGK@MnO2 nanocomposites induced with IIGK have a higher dispersion stability in the solution and a larger specific surface area, which is beneficial for the adsorption of Sr2+.
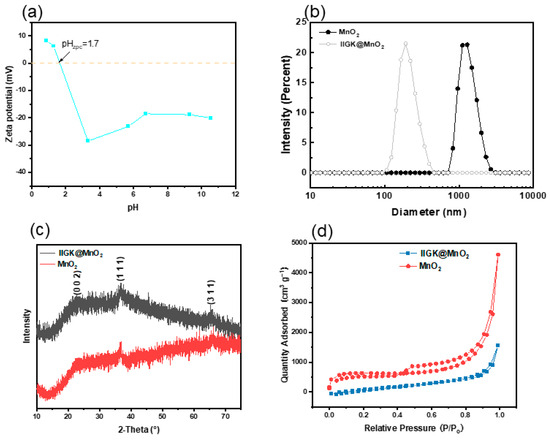
Figure 3.
(a) Zeta potential of IIGK@MnO2, (b) diameter distribution of MnO2 and IIGK@MnO2, (c) XRD spectra of IIGK@MnO2 and MnO2, and (d) N2 adsorption–desorption isotherms of IIGK@MnO2 and MnO2.
In order to investigate the crystal structure, X-ray powder diffraction (XRD) was employed to characterize IIGK@MnO2 and MnO2. The XRD patterns of the IIGK@MnO2 nanocomposites and MnO2 itself are shown in Figure 3c. For MnO2, the peaks are found at 2θ = 22.8°, 36.5°, and 65.7°. These crystalline microregions correspond to δ-standard diffraction patterns (002), (111), and (311) of MnO2 (JCPDS 80-1098), respectively. The same peaks were found in the XRD patterns of IIGK@MnO2, indicating that MnO2 was successfully loaded on the IIGK fibril’s surface. Moreover, as indicated by the weak XRD peaks, the IIGK@MnO2 nanocomposites show a low crystallinity level. The reason for the poor crystallinity may be due to the fast reaction rate during the interaction between KMnO4 and MnCl2, which leads to the rapid formation and precipitation of MnO2 [23]. This rapid process may have hindered the formation of an orderly atomic arrangement.
Subsequently, the surface area, pore size, as well as pore volume of the IIGK@MnO2 and MnO2 were determined using BET analysis and the BJH method. According to the adsorption–desorption isotherms shown in Figure 3d, the surface area of IIGK@MnO2 is approximately 46.19 m2·g−1. In addition, the pore volume and average pore size of IIGK@MnO2 are calculated to be 2.40 cm3·g−1 and 14.02 nm, respectively. The whole results also indicate that the IIGK@MnO2 nanocomposite has suitable physical properties as an adsorbent and is suitable for the adsorption of Sr2+ [24].
Furthermore, Fourier transform infrared spectroscopy (FTIR) was used to characterize IIGK@MnO2 at a molecular level (Figure S1). The broad band at about 3421.6 cm−1 belongs to the stretching vibration of -OH, which may be attributed to the adsorption of a small amount of water by IIGK@MnO2 and the presence of hydroxyl groups on the Mn surface [25]. In addition, the characteristic absorption peaks at 1628.2 cm−1, 1383.7 cm−1, and 1022.8 cm−1 can be attributed to the bending vibration of the hydroxyl group (Mn-OH) directly connected to the Mn atom. The peak appearing at 500 cm−1 indicates the presence of [MnO6] octahedra, which is caused by the stretching of Mn-O and Mn-O-Mn bonds in the octahedral structure [26]. The elements’ valence states of IIGK@MnO2 were further characterized using XPS spectroscopy. As shown in Figure 4a, the XPS spectrum of Mn 2p exhibits two peaks at 642.7 eV and 654.4 eV, corresponding to Mn 2p 3/2 and Mn 2p 1/2, respectively. The spin energy range of 11.7 eV indicates that the IIGK short peptide fibers are mainly used as templates for the deposition of MnO2 [27]. In addition, the weak peak appearing at 645.6 eV indicates the presence of a small amount of unreacted Mn2+ in the system. According to Chigane et al., the XPS spectrum of Mn 3s can split into two weaker peaks, which are located at 84.6 eV and 89.5 eV, corresponding to Mn4+ and Mn3+, respectively [28]. The difference in binding energy between these two peaks increases with the decrease in the valence state of Mn. As shown in Figure 4b, a 4.9 eV difference can be observed between the two peaks, indicating that Mn in IIGK@MnO2 is mainly composed of Mn4+, with a small amount of Mn3+ present. The O 1s peak exhibits three resolved peaks, with binding energies concentrated at 530.1 eV, 531.4 eV, and 533.12 eV (Figure 4c). These peaks are attributed to Mn-O-Mn, Mn-O, and O-H, respectively [29]. Figure 4d shows the complete XPS spectrum of IIGK@MnO2, including Mn 3s, Mn 2p, C 1s, O 1s, and N 1s signals, indicating the successful mineralization of MnO2.
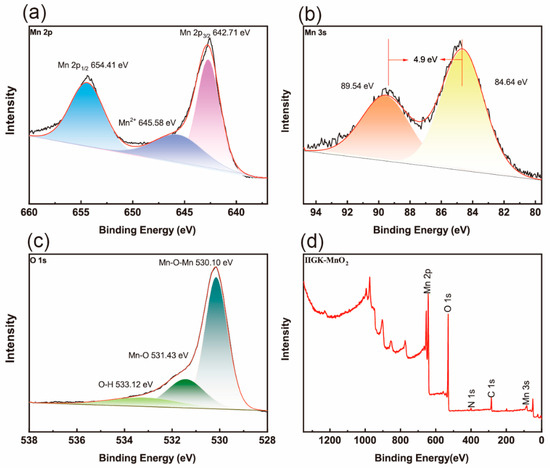
Figure 4.
XPS spectra of (a) Mn 2p, (b) Mn 3s, and (c) O 1s of IIGK@MnO2. (d) XPS full survey spectra of IIGK@MnO2. The fitting data of spectra (a–c) are shown by different colors.
3.2. Sr2+ Adsorption Studies
Previous reports have indicated that MnO2 possesses excellent adsorbing performance for Sr2+. Then, the performance of IIGK@MnO2 in adsorbing Sr2+ was studied. The ability of IIGK@MnO2 to adsorb Sr2+ at different pH values was first evaluated. As shown in Figure 5a, the adsorption efficiency of Sr2+ increases with the increase in pH value and then tends to equilibrium. The low adsorption efficiency of IIGK@MnO2 for Sr2+ in acidic pH environments may be caused by the competitive interaction between H+ and Sr2+. At higher pH conditions, the concentration of H+ is lower; thus, there are more active sites left on IIGK@MnO2 for the adsorption of Sr2+. In addition, under high pH conditions, the surface of IIGK@MnO2 carries more negative charges, which has a stronger electrostatic force on positively charged Sr2+, thereby improving the adsorption efficiency of Sr2+ [30]. Except for pH, temperature also affects the adsorption efficiency of IIGK@MnO2 for Sr2+. As shown in Figure 5b, IIGK@MnO2 has the highest adsorption efficiency for Sr2+ at room temperature. When the temperature rises above 40 °C, the adsorption efficiency sharply decreases. This phenomenon may be due to the weakening of the interaction between IIGK@MnO2 and Sr2+ at higher temperatures, resulting in the desorption of Sr2+ [31].
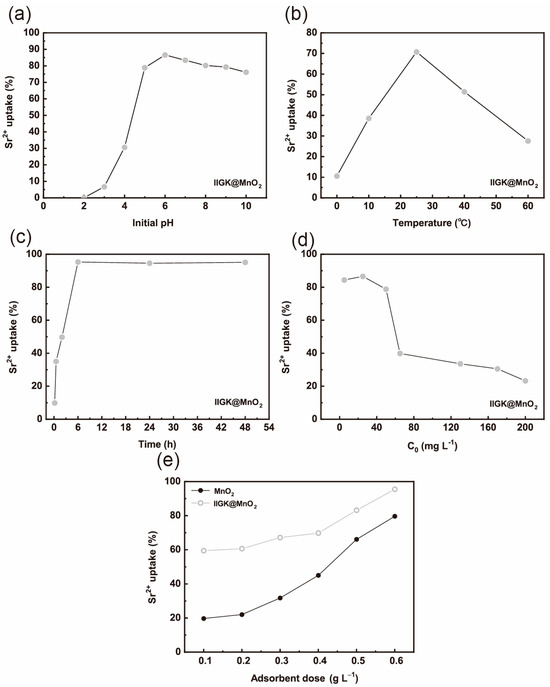
Figure 5.
Sr2+ removal efficiency of the IIGK@MnO2 nanocomposite at different (a) pH, (b) temperature, (c) contact time, (d) initial Sr2+ concentration, and (e) different adsorbent dosage.
The contact time between IIGK@MnO2 and Sr2+ would also affect the adsorption efficiency. As shown in Figure 5c, the adsorption of Sr2+ by IIGK@MnO2 rapidly reaches equilibrium at 6 h. The initial rapid adsorption may be attributed to the large number of available adsorption sites exposed on the surface of IIGK@MnO2. Then, the adsorption process reached equilibrium after 6 h, indicating that most of the available adsorption sites were occupied. The whole adsorption process was assessed using both the pseudo-first-order and pseudo-second-order kinetic models. The equations are as follows [32]:
where qe and qt represent the Sr2+ adsorption capacity (mg/g) at equilibrium or at time t (adsorption time), respectively; k1 (min−1) and k2 (g/(mg∙min)) represent the rate constants for the pseudo-first-order and pseudo-second-order models. The fitted parameters are summarized in Table 1. Comparing the R2 value of these models, the pseudo-second-order model fits better than the pseudo-first-order model [33].

Table 1.
Fitted parameters of pseudo-first-order and pseudo-second-order kinetic models.
For adsorbents, the initial concentration of the adsorbed substance often affects the adsorption rate and efficiency. Therefore, the effect of initial Sr2+ concentration on the adsorption efficiency of IIGK@MnO2 was further studied. According to Figure 5d, the adsorption efficiency of IIGK@MnO2 for Sr2+ shows a trend of first increasing and then decreasing with the increase in initial Sr2+ concentration. The maximum adsorption efficiency occurs when the Sr2+ concentration is 25 mg·L−1. When the initial Sr2+ concentration reaches 40 mg·L−1, the adsorption efficiency sharply decreases. When the Sr2+ concentration increases to 60 mg·L−1, the adsorption efficiency tends to stabilize. The adsorption isotherms of Sr2+ on IIGK@MnO2 were studied using Freundlich and Langmuir isotherm models. The model is shown as follows [34]:
where qm (mg/g) represents the maximum adsorption capacity at the isotherm temperature, b (L/mg) is the Langmuir constant associated with the free energy or net enthalpy of adsorption, Ce (mg/L) and qe (mg/g) denote the concentration of the adsorbate in the equilibrated solution and the amount adsorbed on the adsorbent, respectively, Kf and n are equilibrium constants that provide information about the adsorption capacity and intensity. Shown in Table 2 is the fitting effect of equilibrium data, and the Langmuir isotherm model (R2 = 0.991) is better than that of the Freundlich model (R2 = 0.982). Furthermore, the Sr2+ adsorption performance of the IIGK@MnO2 nanocomposite was compared with that of other reported materials (Table 3). Compared to other adsorbents, IIGK@MnO2 showed better performance in the aspects of higher adsorption capacity (748.2 mg/g), which is beneficial for the treatment of nuclear wastewater. Therefore, the adsorbent surface is considered homogeneous and monolayer in terms of adsorption energy [35]. Compared with pure MnO2, IIGK@MnO2 composites exhibit significantly stronger adsorption efficiency for Sr2+ (Figure 5e). As previously speculated, after combing IIGK peptide with MnO2, the obtained IIGK@MnO2 composites contain more adsorption active sites due to their high dispersion, large specific surface area, and high negative charge, which is beneficial for the adsorption of Sr2+.

Table 2.
The parameters calculated from the Langmuir and Freundlich for Sr2+ adsorption by the IIGK@MnO2 nanocomposite.

Table 3.
Comparison of the adsorption performance of IIGK@MnO2 nanocomposite and other adsorbents for removal of Sr2+.
3.3. Desorption and Reusability Studies
When evaluating the economy and applicability of an adsorbent, the reusability is an important indicator that plays a crucial role in the practical application of adsorbents. Herein, the adsorption/desorption process was repeated to assess the reusability of IIGK@MnO2. Firstly, IIGK@MnO2 was used to adsorb Sr2+, and then the IGK@MnO2-Sr2+ composite was treated with 1M HCl solution for Sr2+ desorption. The whole adsorption/desorption process was repeated three times. As shown in Figure 6, when the regeneration cycle is repeated three times, the adsorption efficiency of IIGK@MnO2 for Sr2+ is still higher than 64%, indicating that 1M HCl solution can effectively desorb Sr2+ from IIGK@MnO2. Moreover, the adsorption capacity of IIGK@MnO2 for Sr2+ decreased with the increase in cycling number, which may be due to H+ occupying some adsorption sites. However, it can also be noted that the adsorption efficiency of IIGK@MnO2 for Sr2+ can still reach over 60% at the end of three cycles, indicating that IIGK@MnO2 has good reusability.
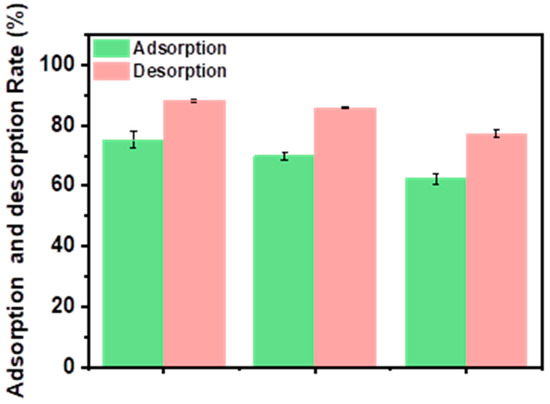
Figure 6.
Reusability of the IIGK@MnO2 nanocomposite during three cycles of Sr2+ adsorption and desorption.
3.4. Adsorption Mechanism of Sr2+ by the IIGK@MnO2 Nanocomposite
The adsorption mechanism of IIGK@MnO2 for Sr2+ was studied. Figure 7a shows the morphology and distribution of various elements of IIGK@MnO2 after the adsorption of Sr2+. After adsorbing Sr2+, there was no significant change in the morphology of IIGK@MnO2. The mapping of IIGK@MnO2-Sr elements shows that the adsorbed Sr2+ is evenly distributed on the surface of IIGK@MnO2, indicating that the adsorption sites on IIGK@MnO2 are relatively uniform. XPS data confirmed the presence of strontium on IIGK@MnO2 after adsorption (Figure 7b). The deconvolution peaks located at 133.5 eV and 135.4 eV belong to Sr 3d 5/2 and Sr 3d 3/2, respectively (Figure 7c) [10]. There is a shift in binding energy in the high-resolution spectrum of O1s in Figure 7d. The binding energy of the O1s-Mn-OH peak decreased from 531.4 eV to 531.0 eV, and the binding energy of the O 1s O-H peak decreased from 533.1 eV to 532.8 eV. This result indicates that oxygen atoms interact with Sr2+ and play a dominant role in the Sr2+ adsorption process.
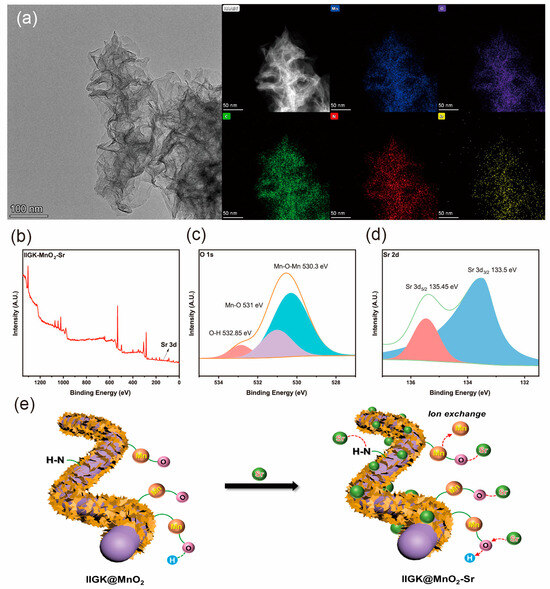
Figure 7.
(a) TEM imagine and EDS element-mapping of IIGK@MnO2-Sr composite. (b) XPS full survey spectra of the IIGK@MnO2-Sr nanocomposite. (c,d) XPS fine spectra of O 1s and Sr 3d of the IIGK@MnO2-Sr nanocomposite. (e) Schematic diagram of the adsorption mechanism of Sr2+ by IIGK@MnO2.
From the above results, the mechanism of Sr2+ adsorption by IIGK@MnO2 can be proposed, as schemed in Figure 7e. On the one hand, the peptide IIGK can bind with Sr2+ through coordination or electrostatic interactions. On the other hand, the MnO2 nanoparticles on the IIGK fibrils’ surface have many absorption sites, which can adsorb Sr2+ by either coordination interaction or ion exchange reaction. Therefore, IIGK and MnO2 work synergistically to enhance the removal efficiency of the IIGK@MnO2 nanocomposites.
4. Conclusions
In this work, the IIGK@MnO2 nanocomposites were synthesized using environmentally friendly and mild methods. Using the short peptide IIGK as a template leads to the formation of a one-dimensional fibrous structure of IIGK@MnO2 nanocomposite, which not only gives the mineralized IIGK@MnO2 complex a large specific surface area but also makes the overall IIGK@MnO2 negatively charged. These unique properties are conducive to the adsorption and removal of Sr2+ from wastewater. According to the adsorption performance of IIGK@MnO2 towards Sr2+, it can be observed that the IIGK@MnO2 hybrid adsorbents possess excellent Sr2+ adsorption effects in a large range of temperature and pH values and show much higher Sr2+ adsorption efficiency than pure MnO2 nanoparticles. In addition, IIGK@MnO2 also exhibits good reusability, achieving a Sr2+ adsorption efficiency of over 60% after three cycles of adsorption–desorption processes. By analyzing the element distribution and valence states of IIGK@MnO2-Sr after the adsorbing process, the mechanism of Sr2+ adsorption was proposed. The interaction between N-H groups on IIGK and oxygen atoms on MnO2 with Sr2+ may play an important role in the adsorption of strontium ions. In conclusion, the designed IIGK@MnO2 nanocomposite in this work exhibits excellent adsorption performance towards Sr2+ and will shed light on the construction of organic–inorganic hybrid adsorbents with multiple active adsorption sites and high adsorption efficiency for adsorbing radioactive ions in wastewater.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano14010052/s1, Figure S1. FTIR spectra of IIGK@MnO2 and MnO2.
Author Contributions
Conceptualization, M.C. and W.W.; software, X.L., Z.L., X.W. and H.M.; resources, M.C. and W.W.; writing—original draft preparation, X.L., Z.L., X.W. and H.M.; writing—review and editing, H.M. and M.C.; supervision, M.C.; project administration, M.C.; funding acquisition, W.W. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (22172194, 21972167, 21872173).
Data Availability Statement
All data generated or analyzed during this study are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davis, L.W. Prospects for nuclear power. J. Econ. Perspect. 2012, 26, 49–66. [Google Scholar] [CrossRef]
- Park, Y.; Lee, Y.C.; Shin, W.S. Removal of cobalt, strontium and cesium from radioactive laundry wastewater by ammonium molybdophosphate–polyacrylonitrile (AMP-PAN). Chem. Eng. J. 2010, 162, 685–695. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J. Removal of radionuclide Sr2+ ions from aqueous solution using synthesized magnetic chitosan beads. Nucl. Eng. Des. 2012, 242, 445–451. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaffar, J.; Ismail, A.F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Extraction and adsorption of U (VI) from aqueous solution using affinity ligand-based technologies: An overview. Rev. Environ. Sci. Biotechnol. 2019, 18, 437–452. [Google Scholar] [CrossRef]
- Rae, I.B.; Pap, S.; Svobodova, D.; Gibb, S.W. Comparison of sustainable biosorbents and ion-exchange resins to remove Sr2+ from simulant nuclear wastewater: Batch, dynamic and mechanism studies. Sci. Total Environ. 2019, 650, 2411–2422. [Google Scholar] [CrossRef]
- Mironyuk, I.; Tatarchuk, T.; Vasylyeva, H.; Gun’ko, V.M.; Mykytyn, I. Effects of chemosorbed arsenate groups on the mesoporous titania morphology and enhanced adsorption properties towards Sr (II) cations. J. Mol. Liq. 2019, 282, 587–597. [Google Scholar] [CrossRef]
- Ma, B.; Shin, W.S.; Oh, S.; Park, Y.J.; Choi, S.J. Adsorptive removal of Co and Sr ions from aqueous solution by synthetic hydroxyapatite nanoparticles. Sep. Sci. Technol. 2010, 45, 453–462. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, P.; Zhang, M.; Yan, S.; Dong, L.; Zhang, G. Synthesis of a robust layered metal sulfide for rapid and effective removal of Sr2+ from aqueous solutions. J. Chem. Eng. 2019, 372, 1205–1215. [Google Scholar] [CrossRef]
- Villard, A.; Siboulet, B.; Toquer, G.; Merceille, A.; Grandjean, A.; Dufrêche, J. Strontium selectivity in sodium nonatitanate Na4Ti9O20·xH2O. J. Hazard. Mater. 2015, 283, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, H.; Gao, Y. Removal of Sr (II) from aqueous solutions using polyacrylamide modified graphene oxide composites. J. Mol. Liq. 2015, 208, 394–401. [Google Scholar] [CrossRef]
- Asgari, P.; Mousavi, S.H.; Aghayan, H.; Ghasemi, H.; Yousefi, T. Nd-BTC metal-organic framework (MOF); synthesis, characterization and investigation on its adsorption behavior toward cesium and strontium ions. Microchem. J. 2019, 150, 104–188. [Google Scholar] [CrossRef]
- Valsala, T.P.; Joseph, A.; Sonar, N.L.; Sonavane, M.; Shah, J.; Raj, K.; Venugopal, V. Separation of strontium from low level radioactive waste solutions using hydrous manganese dioxide composite materials. J. Nucl. Mater. 2010, 404, 138–143. [Google Scholar] [CrossRef]
- Bondar, Y.V.; Alekseev, S.A. Synthesis and evaluation of manganese dioxide with layered structure as an adsorbent for selective removal of strontium ions from aqueous solution. SN Appl. Sci. 2020, 2, 1379. [Google Scholar] [CrossRef]
- Fei, J.B.; Cui, Y.; Yan, X.H.; Qi, W.; Yang, Y.; Wang, K.W.; He, Q.; Li, J.B. Controlled preparation of MnO2 hierarchical hollow nanostructures and their application in water treatment. Adv. Mater. 2008, 20, 452–456. [Google Scholar] [CrossRef]
- Nair, B.; Pradeep, T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst. Growth Des. 2002, 2, 293–298. [Google Scholar] [CrossRef]
- Xu, Y.; Yoo, I.K. Removal of lead from water solution by reusable magnetic adsorbent incorporating selective lead-binding peptide. Appl. Sci. 2020, 10, 6418. [Google Scholar]
- Chen, C.L.; Rosi, N.L. Peptide-based methods for the preparation of nanostructured inorganic materials. Angew. Chem. Int. Ed. 2010, 49, 1924–1942. [Google Scholar] [CrossRef]
- Mondal, B.; Bairagi, D.; Nandi, N. Peptide-based gel in environmental remediation: Removal of toxic organic dyes and hazardous Pb2+ and Cd2+ ions from wastewater and oil spill recovery. Langmuir 2020, 36, 12942–12953. [Google Scholar] [CrossRef]
- Sweeney, R.Y.; Mao, C.B.; Gao, X.X.; Burt, J.L.; Belcher, A.M.; Georgiou, G.; Iverson, B.L. Bacterial biosynthesis of cadmium sulfide nanocrystals. Chem. Biol. 2004, 11, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Sewell, S.L.; Wright, D.W. Biomimetic synthesis of titanium dioxide utilizing the R5 peptide derived from Cylindrotheca fusiformis. Chem. Mater. 2006, 18, 3108–3113. [Google Scholar] [CrossRef]
- Portehault, D.; Cassaignon, S.; Nassif, N.; Baudrin, E.; Jolivet, J.P. A core-corona hierarchical manganese oxide and its formation by an aqueous soft chemistry mechanism. Angew. Chem. Int. Ed. 2008, 4, 6441–6444. [Google Scholar] [CrossRef] [PubMed]
- Asim, U.; Husnain, S.M.; Abbas, N.; Shahzad, F.; Khan, A.R.; Ali, T. Morphology controlled facile synthesis of MnO2 adsorbents for rapid strontium removal. J. Ind. Eng. Chem. 2021, 98, 375–382. [Google Scholar] [CrossRef]
- Ganesan, K.; Ghosh, S.; Krishna, N.G.; Ilango, S.; Kamruddin, M.; Tyagi, A.K. A comparative study on defect estimation using XPS and Raman spectroscopy in few layer nanographitic structures. Phys. Chem. Chem. Phys. 2016, 18, 22160–22167. [Google Scholar] [CrossRef] [PubMed]
- Shaker, K.S.; AbdAlsalm, A.H. Synthesis and characterization nano structure of MnO2 via chemical method. Eng. Technol. J. 2018, 36, 946–950. [Google Scholar] [CrossRef]
- Ren, Y.; Yan, N.; Wen, Q.; Fan, Z.; Wei, T.; Zhang, M.; Ma, J. Graphene/δ-MnO2 composite as adsorbent for the removal of nickel ions from wastewater. Chem. Eng. J. 2011, 175, 1–7. [Google Scholar] [CrossRef]
- Sang, L.; Wang, T.K.; Chen, G.Y.; Liang, J.C.; Wei, Z.Y. A comparative study of the crystalline structure and mechanical properties of carbon fiber/polyamide 6 composites enhanced with/without silane treatment. Rsc. Adv. 2016, 6, 107739–107747. [Google Scholar] [CrossRef]
- Nesbitt, H.; Banerjee, W.D. Interpretation of XPS Mn (2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2 precipitation. Am. Min. 1998, 83, 305–315. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, J.; Zhao, X.; Li, F.; Jiang, F.; Zhang, M.; Cheng, X. Removal of strontium (II) and cobalt (II) from acidic solution by manganese antimonate. Chem. Eng. J. 2016, 302, 733–743. [Google Scholar] [CrossRef]
- Karaseva, O.N.; Ivanova, L.I.; Lakshtanov, L.Z. Adsorption of strontium on manganese oxide (δ-MnO2) at elevated temperatures: Experiment and modeling. Geokhimiya. Int. 2019, 64, 1091–1104. [Google Scholar] [CrossRef]
- Lei, H.Y.; Muhammad, Y.; Wang, K.T.; Yi, M.; He, C.L.; Wei, Y.Z.; Fujita, T. Facile fabrication of metakaolin/slag-based zeolite microspheres (M/SZMs) geopolymer for the efficient remediation of Cs+ and Sr2+ from aqueous media. J. Hazard. Mater. 2021, 406, 124292. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Zhang, S.J.; Pan, B.C.; Zhang, W.M.; Lv, L.; Zhang, Q.X. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manage. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, C.M.; Chen, X.J.; Fu, H.; Chen, Y.L.; Ning, S.Y.; Fujita, T.; Wei, Y.Z.; Wang, X.P. Layered ammonium vanadate nanobelt as efficient adsorbents for removal of Sr2+ and Cs+ from contaminated water. J. Colloid Interface Sci. 2022, 615, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Tsai, S.C.; Wu, M.C.; Tsai, T.L. A study on removal of Cs and Sr from aqueous solution by bentonite–alginate microcapsules. J. Radioanal. Nucl. Chem. 2018, 318, 2381–2387. [Google Scholar] [CrossRef]
- Smiciklas, I.; Coha, I.; Jovic, M.; Nodilo, M.; Sljivic-Ivanovic, M.; Smiljanic, S.; Grahek, Z. Efficient separation of strontium radionuclides from high-salinity wastewater by zeolite 4A synthesized from Bayer process liquids. Sci. Rep. 2021, 11, 1738. [Google Scholar] [CrossRef]
- Guo, Y.L.; Sun, H.Y.; Zeng, X.; Lv, T.T.; Yao, Y.X.; Zhuang, T.H.; Feng, M.L.; Huang, X.Y. Efficient removal of Sr2+ ions by a one-dimensional potassium phosphatoantimonate. Chem. Eng. J. 2023, 460, 141697. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.J.; Jiang, L.; Luo, M.B.; Chi, C.X.; Ma, J.G. Adsorption of strontium from aqueous solution by silica mesoporous SBA-15. J. Radioanal. Nucl. Chem. 2015, 303, 1671–1677. [Google Scholar] [CrossRef]
- Li, Z.Q.; Vivas, E.L.; Suh, Y.J.; Cho, K. Highly efficient and selective removal of Sr2+ from aqueous solutions using ammoniated zirconium phosphate. J. Environ. Chem. Eng. 2022, 10, 107333. [Google Scholar] [CrossRef]
- Li, G.D.; Ji, G.X.; Liu, W.; Zhang, J.R.; Song, L.P.; Cheng, L.W.; Wang, X.; Wang, Y.L.; Liu, J.J.; Chen, X.D.; et al. A hydrolytically stable anionic layered indium–organic framework for the efficient removal of 90Sr from seawater. Dalton Trans. 2019, 48, 17858–17863. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).