Novel Nanostructured Pd/Co-Alumina Materials for the Catalytic Oxidation of Atmospheric Pollutants
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Catalyst Characterization Results
3.1.1. Textural Characterization (ICP, BET)
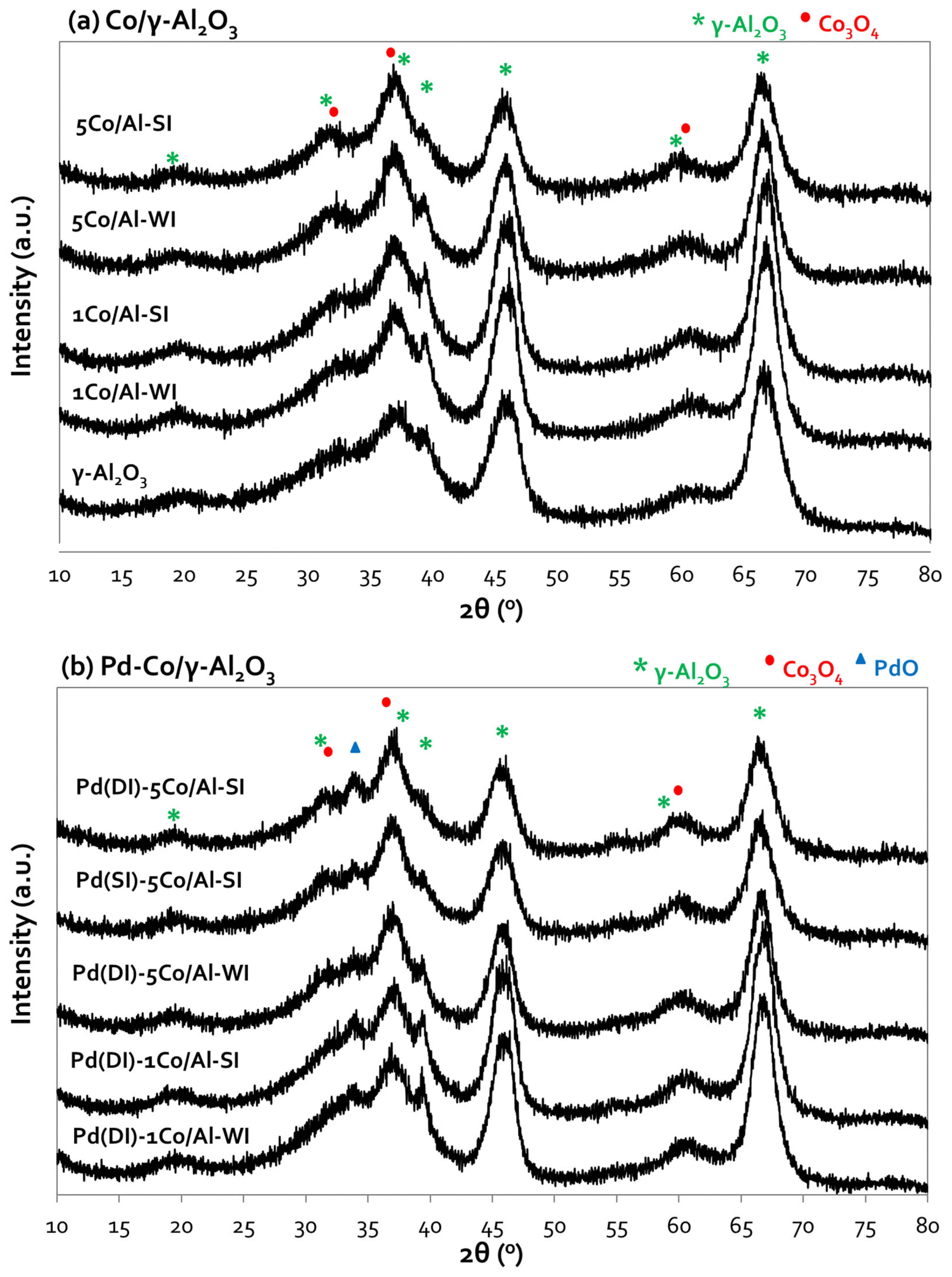
3.1.2. Structural Characterization (XRD, SEM)
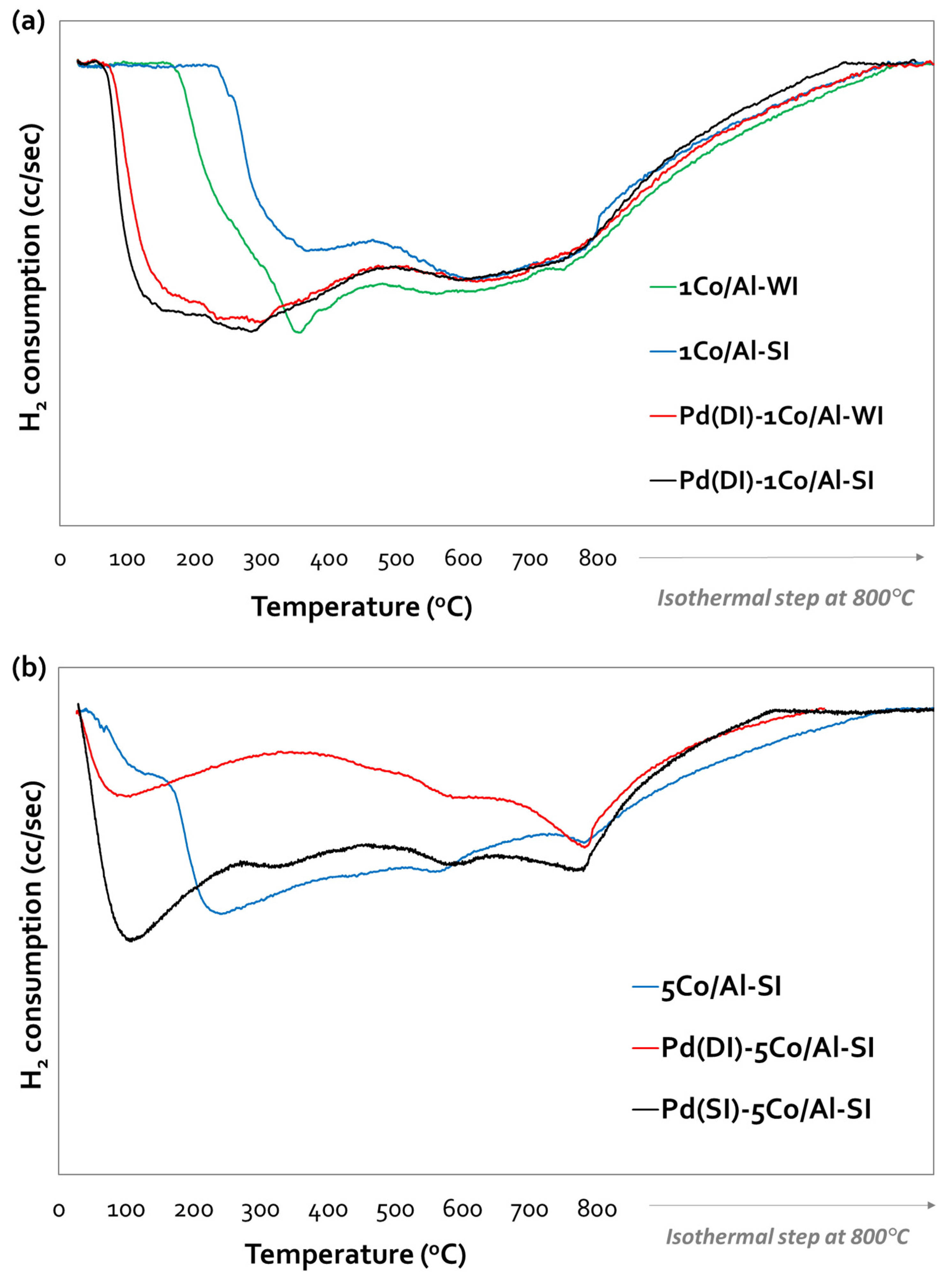
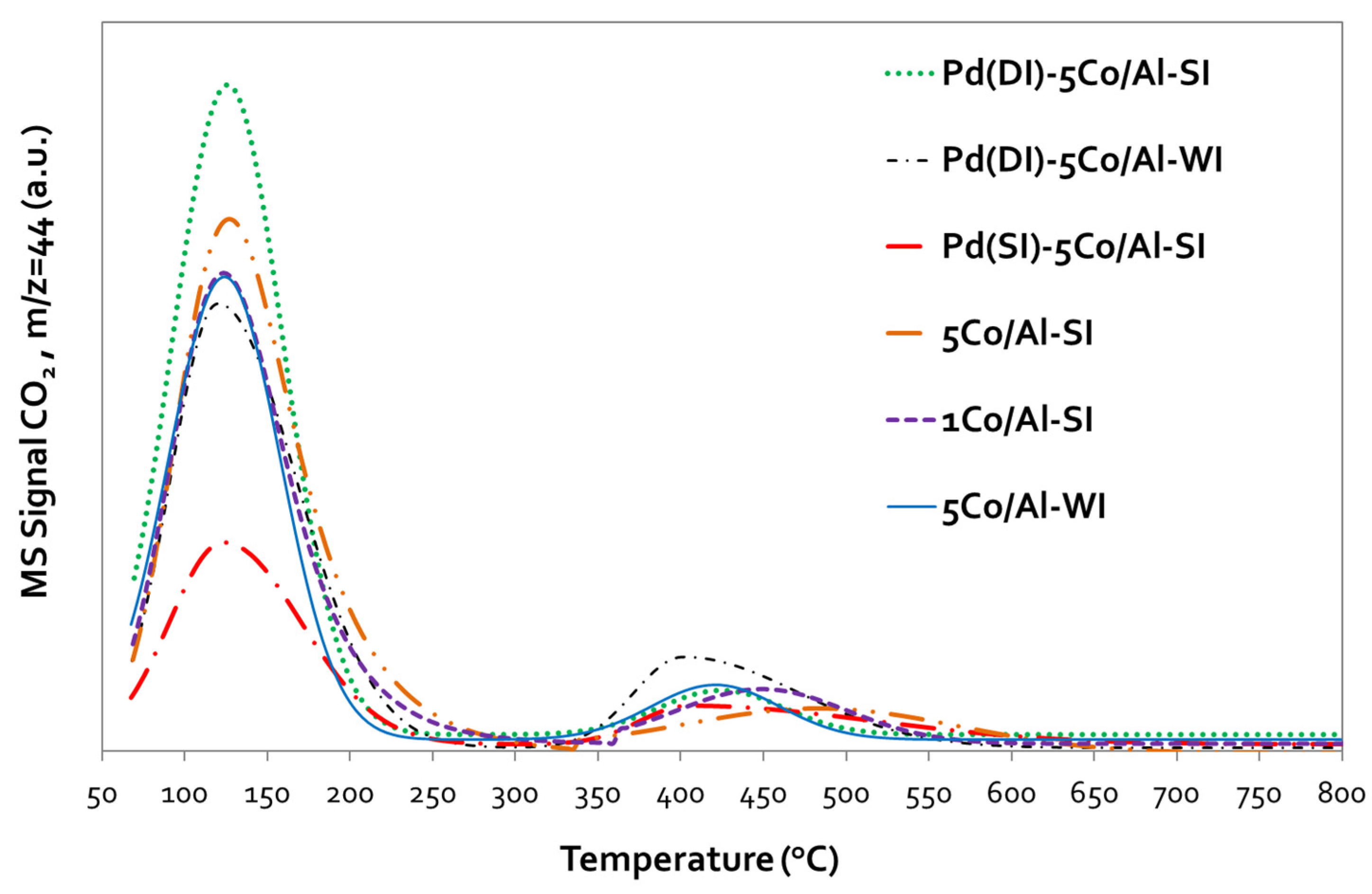
3.1.3. Catalyst Reducibility (TPR-H2)
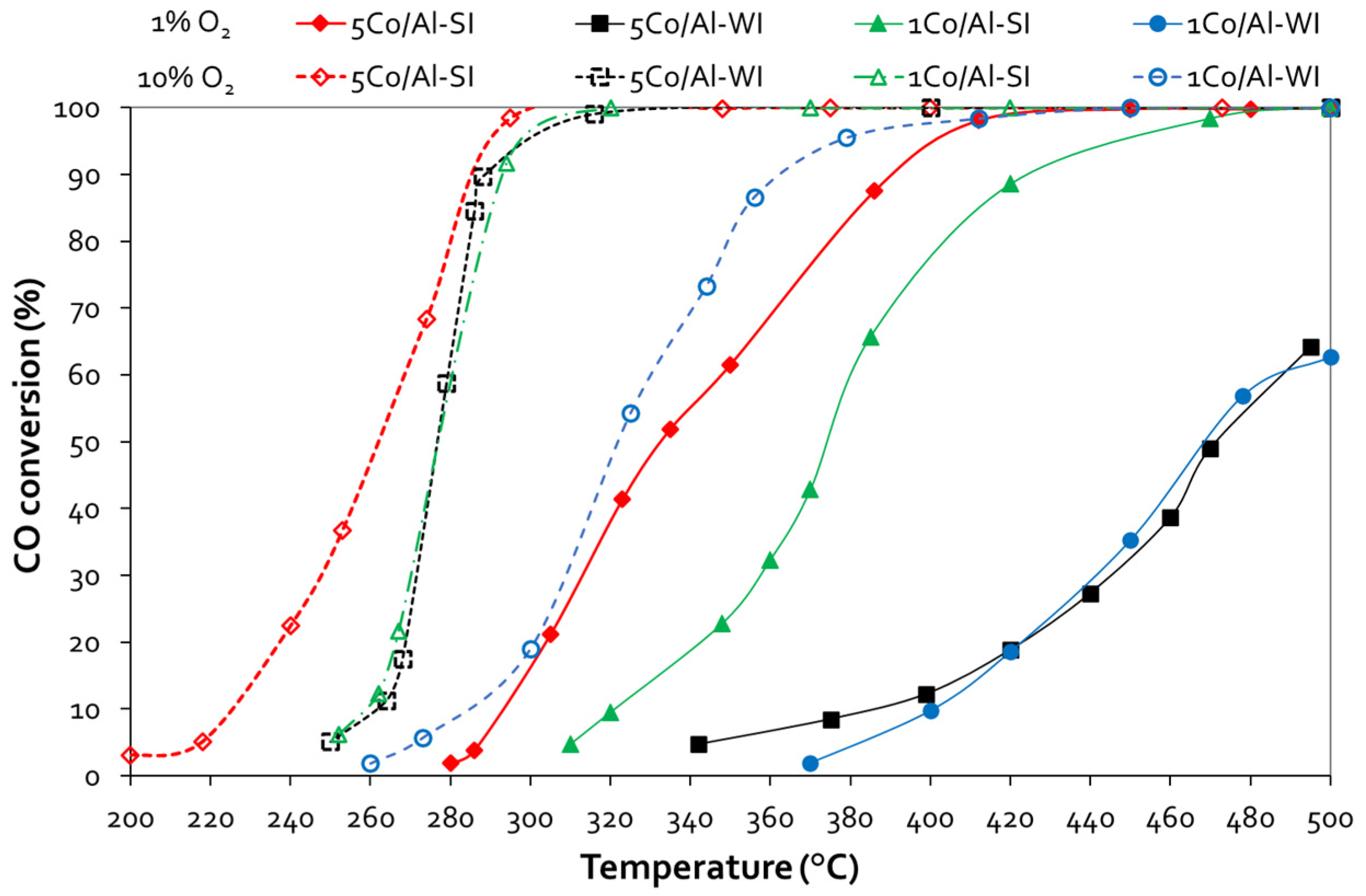
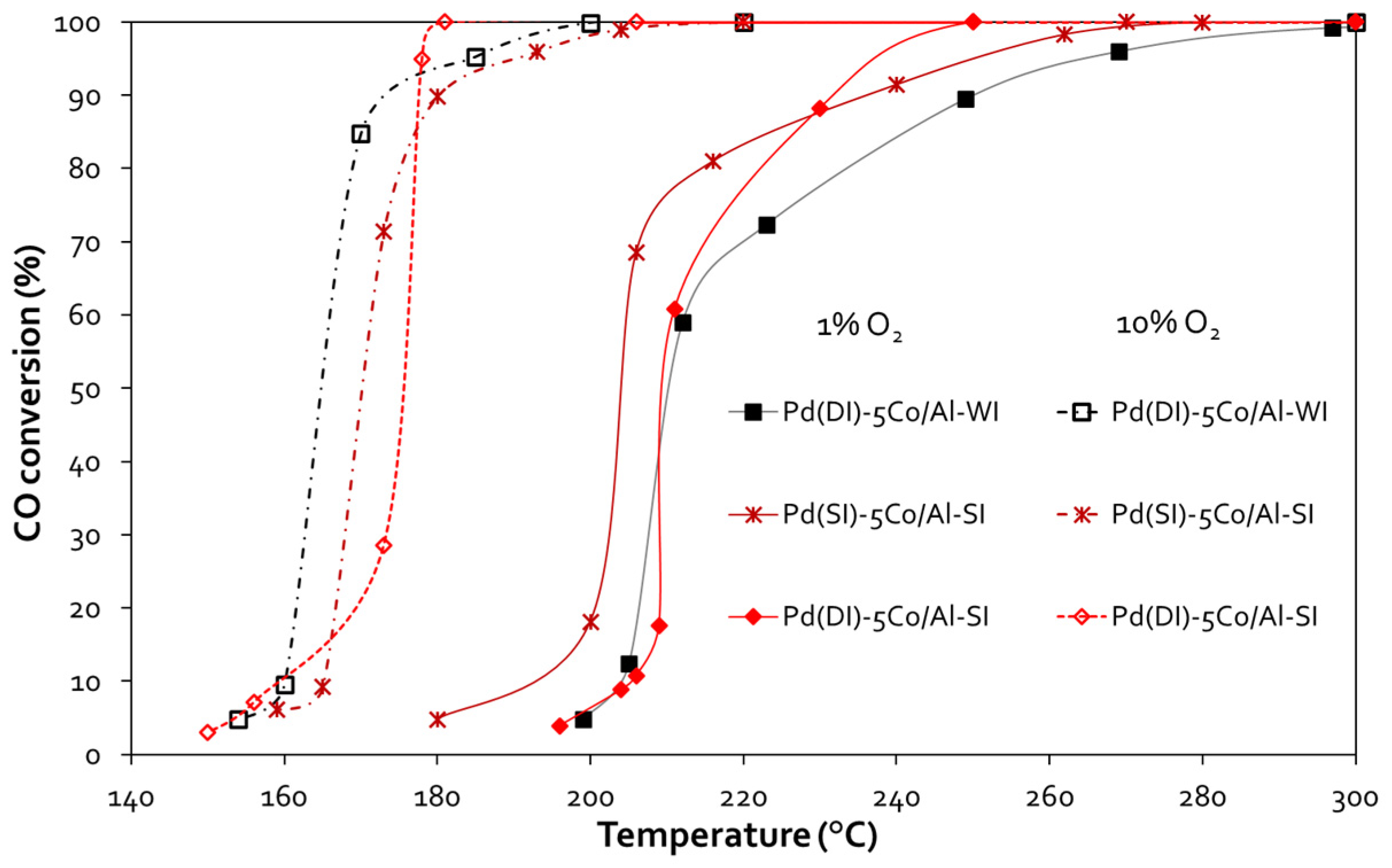
3.2. CO Oxidation
3.3. Methanol Oxidation
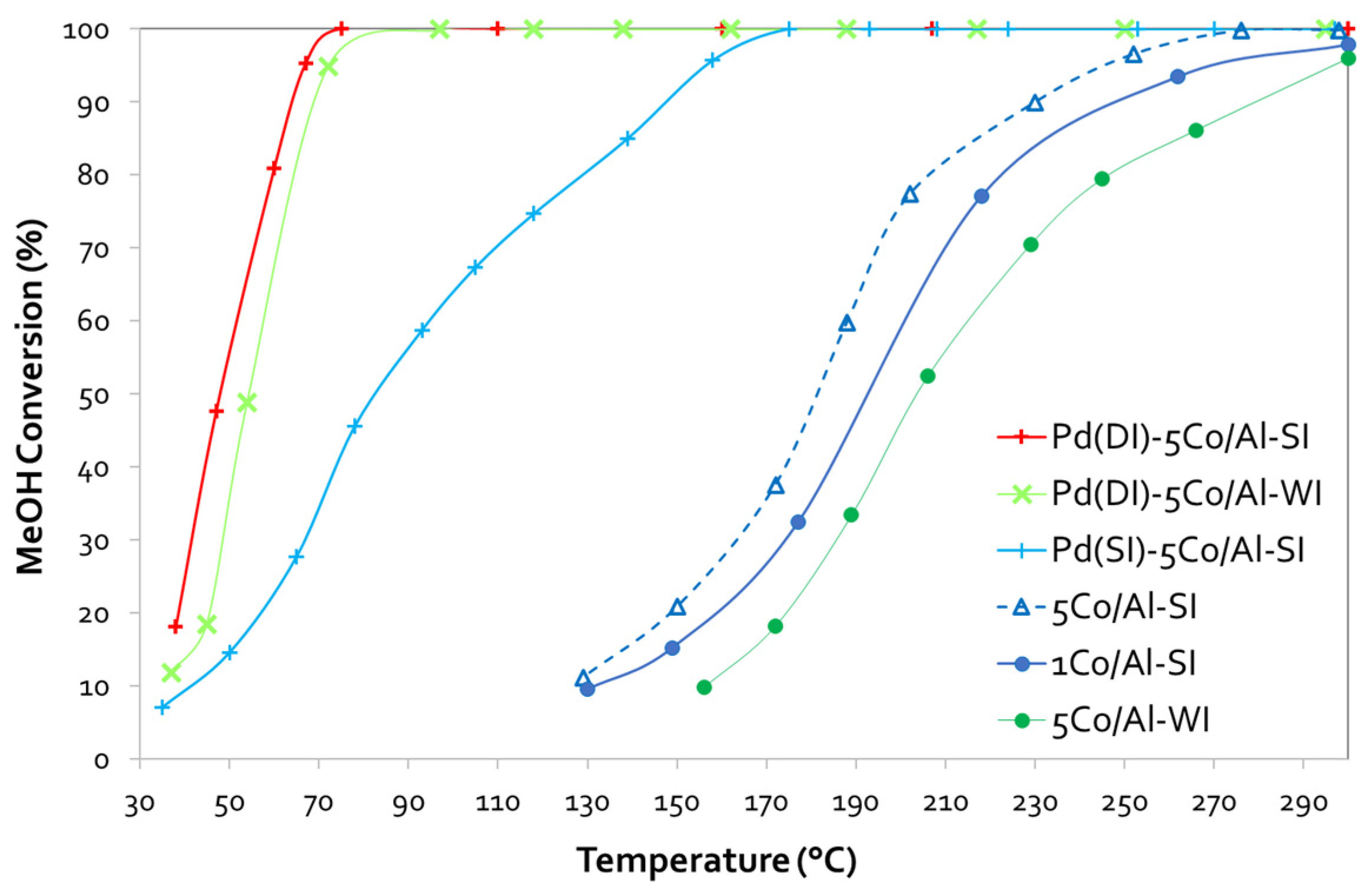
3.3.1. Catalytic Evaluation
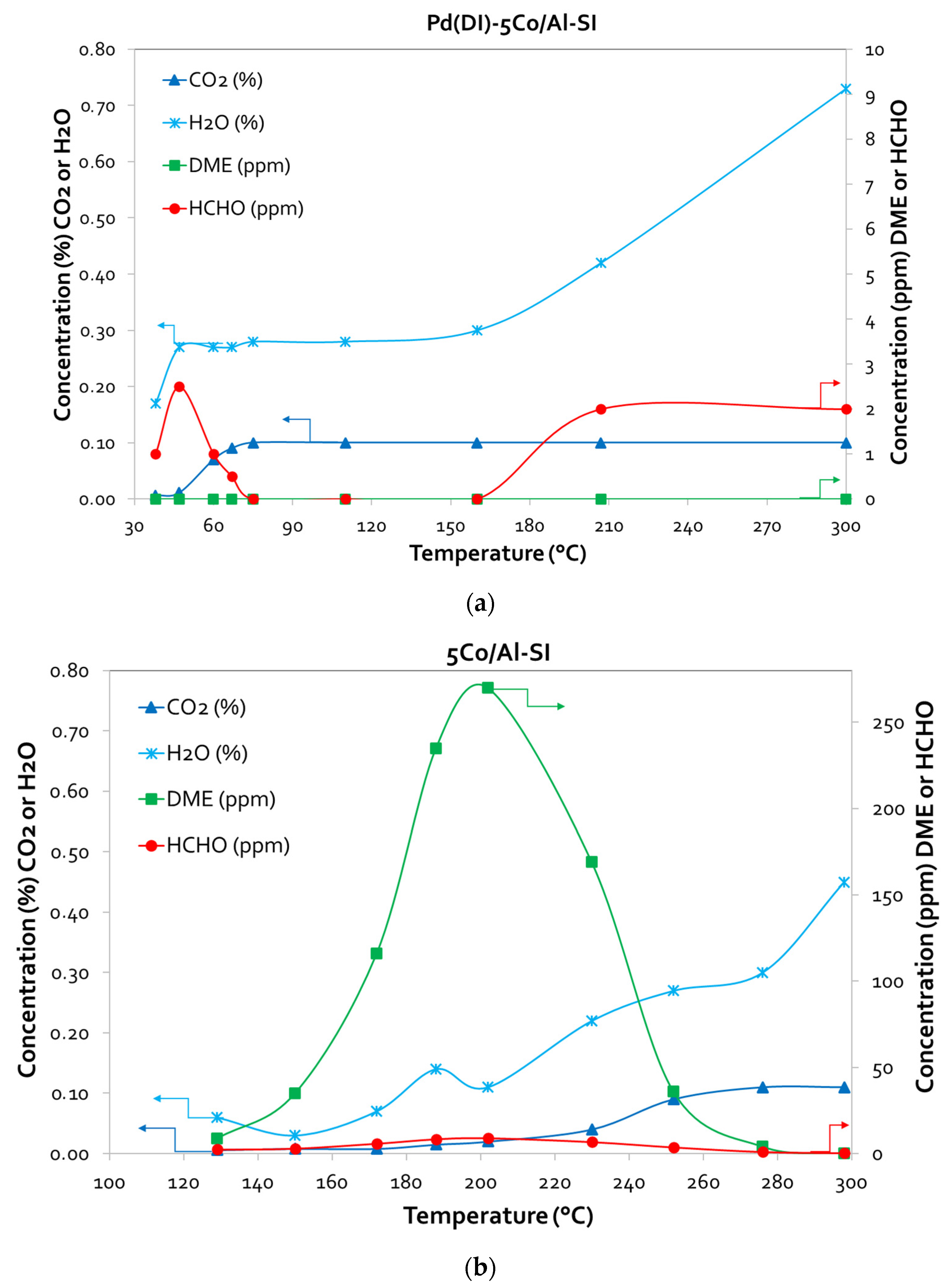
3.3.2. Methanol Desorption Studies (TPD-MeOH)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.; Ge, Y.; Hao, C.; Han, X.; Fu, M.; Yu, L.; Shah, A.N. Carbonyl compound emissions from passenger cars fueled with methanol/gasoline blends. Sci. Total Environ. 2010, 408, 3607–3613. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Ge, Y.; Wang, X.; Zhang, M.; Hao, L.; Tan, J.; Shi, F.; Guo, D.; Yang, Z. Evaluating the in-service emissions of high-mileage dedicated methanol-fueled passenger cars: Regulated and unregulated emissions. Energies 2020, 13, 2680. [Google Scholar] [CrossRef]
- Luo, Y.; Qian, Q.; Chen, Q. On the promoting effect of the addition of CexZr1−xO2 to palladium-based alumina catalysts for methanol deep oxidation. Mater. Res. Bull. 2015, 62, 65–70. [Google Scholar] [CrossRef]
- Brewer, T.F.; Abraham, M.A.; Silver, R.G. Mixture Effects and Methanol Oxidation Kinetics over a Palladium Monolith Catalyst. Ind. Eng. Chem. Res. 1994, 33, 526–533. [Google Scholar] [CrossRef]
- McCabe, R.W.; Mitchell, P.J. Exhaust-Catalyst Development for Methanol Fueled Vehicles III. Formaldehyde Oxidation. Appl. Catal. 1988, 44, 73–93. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649. [Google Scholar] [CrossRef]
- Okumura, K.; Kobayashi, T.; Tanaka, H.; Niwa, M. Toluene combustion over palladium supported on various metal oxide supports. Appl. Catal. B Environ. 2003, 44, 325–331. [Google Scholar] [CrossRef]
- Jabłonska, M.; Nocun, M.; Bidzinska, E. Silver–Alumina Catalysts for Low-Temperature Methanol Incineration. Catal. Lett. 2016, 146, 937–944. [Google Scholar] [CrossRef]
- Aguilera, D.A.; Perez, A.; Molina, R.; Moreno, S. Cu–Mn and Co–Mn catalysts synthesized from hydrotalcites and their use in the oxidation of VOCs. Appl. Catal. B Environ. 2011, 104, 144–150. [Google Scholar] [CrossRef]
- Gaálová, J.; Topka, P. Gold and Ceria as Catalysts for VOC Abatement: A Review. Catalysts 2021, 11, 789. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Deng, J.; Luo, L.; Gao, W.; Yuan, L. Effect of modified CeO2 on the performance of PdCu/Ce1−xTixO2 catalyst for methanol purification. Environ. Sci. Pollut. Res. Int. 2022, 29, 73935–73945. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, S.; Liu, W.; Gao, X.; Gao, D.; Wang, M.; Wang, S. Morphology-dependent performance of Co3O4 via facile and controllable synthesis for methane combustion. Appl. Catal. A 2016, 525, 94–102. [Google Scholar] [CrossRef]
- Hattori, M.; Nakakura, S.; Katsui, H.; Goto, T.; Ozawa, M. High CO reactivity of cobalt oxide catalyst deposited on alumina powders by rotary chemical vapor deposition. Mater. Lett. 2021, 284, 128922. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C. The catalytic activity of cobalt nanoparticles for low-temperature oxidation of carbon monoxide. Mater. Today Chem. 2019, 14, 100198. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Wang, Y.; Wang, T.; Gu, J.; Peng, L.; Xue, N.; Zhu, Y.; Guo, X.; Ding, W. Reduction-oxidation pretreatment enhanced catalytic performance of Co3O4/Al2O3 over CO oxidation. Appl. Surf. Sci. 2018, 453, 330–335. [Google Scholar] [CrossRef]
- Yan, J.; Wang, L.; Guo, Y.; Guo, Y.; Dai, Q.; Zhan, W. Comparisons on thermal and water-resistance of Ru and Pd supported on cobalt-doped alumina nanosheets for catalytic combustion of propane. Appl. Catal. A 2021, 628, 118398–118407. [Google Scholar] [CrossRef]
- Huang, R.; Kim, K.; Kim, H.J.; Jang, M.G.; Han, J.W. Size-Controlled Pd Nanoparticles Loaded on Co3O4 Nanoparticles By Calcination for Enhanced CO Oxidation. ACS Appl. Nano Mater. 2020, 3, 486–495. [Google Scholar] [CrossRef]
- Chen, Z.; He, Y.; Chen, J.; Fu, X.; Sun, R.; Chen, Y.; Wong, C. PdCu alloy lower-like nanocages with high electrocatalytic performance for methanol oxidation. J. Phys. Chem. C 2018, 16, 8976–8983. [Google Scholar] [CrossRef]
- Gholinejad, M.; Khosravi, F.; Afrasi, M.; Sansano, J.M.; Nájera, C. Applications of bimetallic PdCu catalysts. Catal. Sci. Technol. 2021, 11, 2652–2702. [Google Scholar] [CrossRef]
- Wang, J.A.; Aguilar-Rios, G.; Wang, R.G. Inhibition of carbon monoxide on methanol oxidation over g-alumina supported Ag, Pd and Ag–Pd catalysts. Appl. Surf. Sci. 1999, 147, 41–51. [Google Scholar] [CrossRef]
- Stefanov, P.; Tdorova, S.; Naydenov, A.; Tzaneva, B.; Kolev, H.; Atanasova, G.; Stoyanova, D.; Karakirova, Y.; Aleksieva, K. On the development of active and stable Pd–Co/γ-Al2O3 catalyst for complete oxidation of methane. Chem. Eng. J. 2015, 266, 329–338. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Lei, Z.; Chen, B. Pd−Co Coating onto Cordierite Monoliths as Structured Catalysts for Methane Catalytic Combustion. Energy Fuels 2012, 26, 443–450. [Google Scholar] [CrossRef]
- Srifa, A.; Viriya-empikul, N.; Assabumrungrat, S.; Faungnawakij, K. Catalytic behaviors of Ni/γ-Al2O3 and Co/γ-Al2O3 during the hydrodeoxygenation of palm oil. Catal. Sci. Technol. 2015, 5, 3693–3705. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Darda, S.; Pachatouridou, E.P.; Lappas, A.A. Exploring Synthesis Approaches of Co based Catalysts for the Efficient Oxidation of CH4 and CO. Top. Catal. 2022, 66, 999–1012. [Google Scholar] [CrossRef]
- James, O.O.; Maity, S. Temperature programme reduction (TPR) studies of cobalt phases in alumina supported cobalt catalysts. J. Petrol. Technol. Altern. Fuels 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Ma, W.; Jacobs, G.; Shafer, W.D.; Ji, Y.; Klettlinger, J.L.S.; Khalid, S.; Hopps, S.D.; Davis, B.H. Fischer-Tropsch Synthesis: Cd, In and Sn Effects on a 15%Co/Al2O3 Catalyst. Catalysts 2019, 9, 862. [Google Scholar] [CrossRef]
- Guo, S.; Niu, C.; Ma, Z.; Wang, J.; Hou, B.; Jia, L.; Li, D. A novel and facile strategy to decorate Al2O3 as an effective support for Co-based catalyst in Fischer-Tropsch synthesis. Fuel 2021, 289, 11978. [Google Scholar] [CrossRef]
- Hossain, M.M. Co–Pd/γ-Al2O3 Catalyst for Heavy Oil Upgrading: Desorption Kinetics, Reducibility and Catalytic Activity. Can. J. Chem. 2011, 90, 946–955. [Google Scholar] [CrossRef]
- Xu, D.; Li, W.; Duan, H.; Ge, Q.; Xu, H. Reaction performance and characterization of Co/Al2O3 Fischer–Tropsch catalysts promoted with Pt, Pd and Ru. Catal. Lett. 2005, 102, 229–235. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Liu, F.; He, H. Well-dispersed palladium supported on ordered mesoporous Co3O4 for catalytic oxidation of o-xylene Applied. Catal. B Environ. 2013, 142–143, 72–79. [Google Scholar] [CrossRef]
- Scire, S.; Crisafulli, C.; Maggiore, R.; Minico, S.; Galvagno, S. Effect of the acid–base properties of Pd–Ca/Al2O3 catalysts on the selective hydrogenation of phenol to cyclohexanone: FT-IR and TPD characterization. Appl. Surf. Sci. 1998, 136, 311–320. [Google Scholar] [CrossRef]
- Badlani, M.; Wachs, I.E. Methanol: A “smart” chemical probe molecule. Catal. Lett. 2001, 75, 137–149. [Google Scholar] [CrossRef]
- Zafeiratos, S.; Dintzer, T.; Teschner, D.; Blume, R.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R. Methanol oxidation over model cobalt catalysts: Influence of the cobalt oxidation state on the reactivity. J. Catal. 2010, 269, 309–317. [Google Scholar] [CrossRef]
- Akarmazyana, S.S.; Panagiotopouloua, P.; Kambolis, A.; Papadopoulou, C.; Kondarides, D.I. Methanol dehydration to dimethylether over Al2O3 catalysts. Appl. Catal. B Environ. 2014, 145, 136–148. [Google Scholar] [CrossRef]




| Sample | Co, wt.% | Pd, wt.% | Surface Area, m2/g | Pore Volume, cm3/g | Pore Size, nm |
|---|---|---|---|---|---|
| γ-Al2O3 | - | - | 226.0 | 0.65 | 11.5 |
| 1Co/Al-WI | 1.06 | - | 182.2 | 0.64 | 13.0 |
| 1Co/Al-SI | 1.05 | - | 170.0 | 0.64 | 13.2 |
| 5Co/Al-WI | 5.46 | - | 148.6 | 0.59 | 13.0 |
| 5Co/Al-SI | 5.14 | - | 158.5 | 0.57 | 11.6 |
| Pd(DI)-1Co/Al-WI | 1.06 | 0.58 | 159.7 | 0.63 | 13.5 |
| Pd(DI)-1Co/Al-SI | 1.05 | 0.60 | 152.9 | 0.63 | 13.6 |
| Pd(DI)-5Co/Al-WI | 5.46 | 0.58 | 140.1 | 0.60 | 13.4 |
| Pd(DI)-5Co/Al-SI | 5.46 | 0.60 | 157.7 | 0.54 | 10.9 |
| Pd(SI)-5Co/Al-SI 1 | 5.14 | 0.50 | 144.0 | 0.56 | 11.9 |
| Sample | CO Oxidation | |||
|---|---|---|---|---|
| 1 vol.% O2 | 10 vol.% O2 | |||
| T50, °C | T90, °C | T50, °C | T90, °C | |
| 1Co/Al-WI | 469 | - | 322 | 365 |
| 1Co/Al-SI | 375 | 427 | 278 | 293 |
| 5Co/Al-WI | 472 | - | 277 | - |
| 5Co/Al-SI | 333 | 392 | 262 | 289 |
| Pd(DI)-5Co/Al-WI | 211 | 250 | 165 | 177 |
| Pd(DI)-5Co/Al-SI | 211 | 233 | 175 | 178 |
| Pd(SI)-5Co/Al-SI | 204 | 237 | 170 | 180 |
| Sample | MeOH Oxidation | |
|---|---|---|
| T50, °C | T90, °C | |
| 1Co/Al-SI | 193 | 252 |
| 5Co/Al-WI | 204 | 279 |
| 5Co/Al-SI | 181 | 230 |
| Pd(DI)-5Co/Al-WI | 54 | 70 |
| Pd(DI)-5Co/Al-SI | 48 | 64 |
| Pd(SI)-5Co/Al-SI | 83 | 148 |
| Sample | Basicity, μmol CO2/g Catalyst | ||
|---|---|---|---|
| Weak/Medium Sites | Strong Sites | Total | |
| 1Co/Al-SI | 15.2 | 2.5 | 17.7 |
| 5Co/Al-WI | 15.7 | 2.2 | 17.9 |
| 5Co/Al-SI | 16.9 | 2.1 | 19.1 |
| Pd(DI)-5Co/Al-WI | 14.9 | 3.6 | 18.5 |
| Pd(DI)-5Co/Al-SI | 21.2 | 2.1 | 23.3 |
| Pd(SI)-5Co/Al-SI | 7.0 | 2.4 | 9.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iliopoulou, E.F.; Pachatouridou, E.; Lappas, A.A. Novel Nanostructured Pd/Co-Alumina Materials for the Catalytic Oxidation of Atmospheric Pollutants. Nanomaterials 2024, 14, 124. https://doi.org/10.3390/nano14010124
Iliopoulou EF, Pachatouridou E, Lappas AA. Novel Nanostructured Pd/Co-Alumina Materials for the Catalytic Oxidation of Atmospheric Pollutants. Nanomaterials. 2024; 14(1):124. https://doi.org/10.3390/nano14010124
Chicago/Turabian StyleIliopoulou, Eleni F., Eleni Pachatouridou, and Angelos A. Lappas. 2024. "Novel Nanostructured Pd/Co-Alumina Materials for the Catalytic Oxidation of Atmospheric Pollutants" Nanomaterials 14, no. 1: 124. https://doi.org/10.3390/nano14010124
APA StyleIliopoulou, E. F., Pachatouridou, E., & Lappas, A. A. (2024). Novel Nanostructured Pd/Co-Alumina Materials for the Catalytic Oxidation of Atmospheric Pollutants. Nanomaterials, 14(1), 124. https://doi.org/10.3390/nano14010124






