Comparison of Toxicity and Cellular Uptake of CdSe/ZnS and Carbon Quantum Dots for Molecular Tracking Using Saccharomyces cerevisiae as a Fungal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Quantum Dots (QDs), Conjugation of Cysteine to QDs, and Physicochemical Characterization
2.2. Saccharomyces Cerevisiae BY4741 and Cultivation Conditions
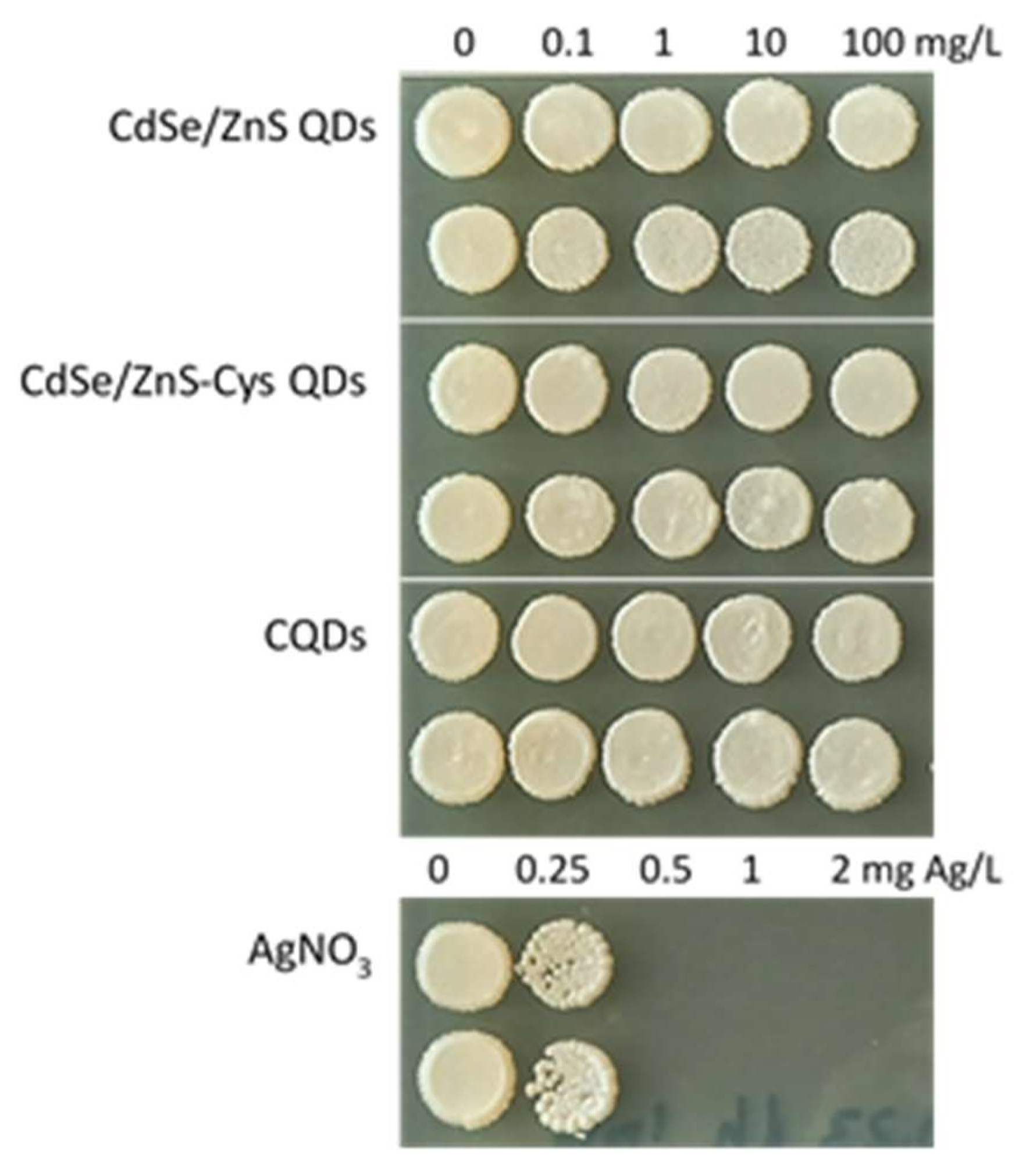
2.3. Toxicity Testing of QDs (Spot Test)
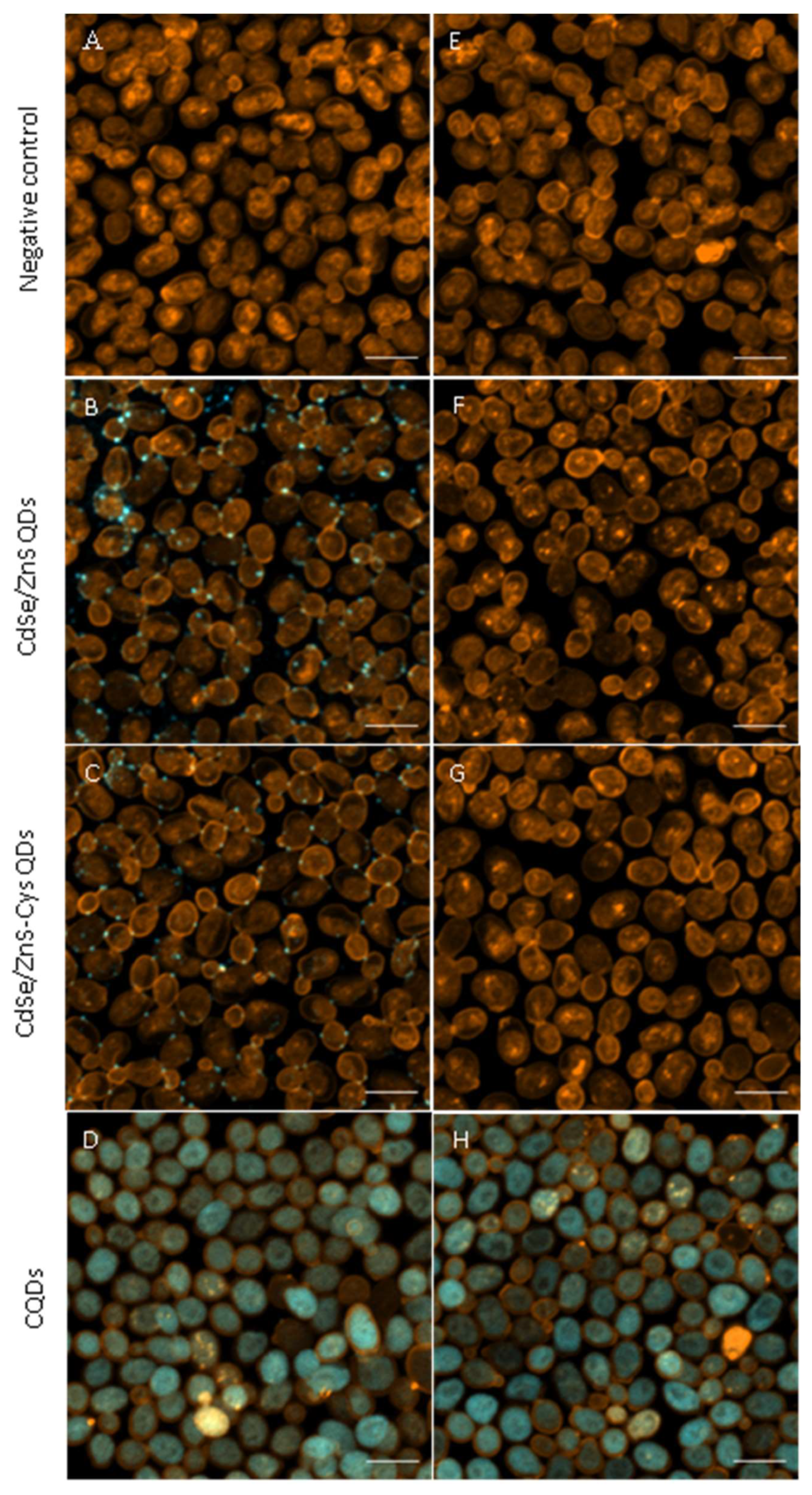
2.4. Evaluation of the QDs Cellular Interactions by Confocal Laser Scanning Microscopy
3. Results
3.1. Physico-Chemical Characteristics of Quantum Dots
3.2. QDs Were Not Toxic to Yeast S. cerevisiae
3.3. CQDs Readily Entered Yeast Cells, While CdSe/ZnS QDs Did Not
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, D.G.; Ammer, C.; Polle, A.; Bauhus, J.; Aloni, R.; Annighöfer, P.; Baskin, T.I.; Blatt, M.R.; Bolte, A.; Bugmann, H.; et al. Mother Trees, Altruistic Fungi, and the Perils of Plant Personification. Trends Plant Sci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, N.; Marshall, J.; Högberg, M.N.; Högberg, P.; Polle, A.; Franklin, O.; Näsholm, T. Re-Examining the Evidence for the Mother Tree Hypothesis—Resource Sharing among Trees via Ectomycorrhizal Networks. New Phytol. 2023, 239, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hevesy, G. Application of Isotopes in Biology. J. Chem. Soc. 1939, 1213–1223. [Google Scholar] [CrossRef]
- Newsome, S.D.; Martinez del Rio, C.; Bearhop, S.; Phillips, D.L. A Niche for Isotopic Ecology. Front. Ecol. Environ. 2007, 5, 429–436. [Google Scholar] [CrossRef]
- Karst, J.; Jones, M.D.; Hoeksema, J.D. Positive Citation Bias and Overinterpreted Results Lead to Misinformation on Common Mycorrhizal Networks in Forests. Nat. Ecol. Evol. 2023, 7, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, M.D.; Werner, G.D.A.; Caldas, V.E.A.; van’t Padje, A.; Dupin, S.E.; Elbers, B.; Bakker, M.; Wyatt, G.A.K.; Klein, M.; Hink, M.A.; et al. Mycorrhizal Fungi Respond to Resource Inequality by Moving Phosphorus from Rich to Poor Patches across Networks. Curr. Biol. 2019, 29, 2043–2050. [Google Scholar] [CrossRef]
- van’t Padje, A.; Bonfante, P.; Ciampi, L.T.; Kiers, E.T. Quantifying Nutrient Trade in the Arbuscular Mycorrhizal Symbiosis Under Extreme Weather Events Using Quantum-Dot Tagged Phosphorus. Front. Ecol. Evol. 2021, 9, 153. [Google Scholar] [CrossRef]
- Whiteside, M.D.; Treseder, K.K.; Atsatt, P.R. The Brighter Side of Soils: Quantum Dots Track Organic Nitrogen through Fungi and Plants. Ecology 2009, 90, 100–108. [Google Scholar] [CrossRef]
- Whiteside, M.D.; Digman, M.A.; Gratton, E.; Treseder, K.K. Organic Nitrogen Uptake by Arbuscular Mycorrhizal Fungi in a Boreal Forest. Soil Biol. Biochem. 2012, 55, 7–13. [Google Scholar] [CrossRef]
- Hardman, R. A Toxicologic Review of Quantum Dots: Toxicity Depends on Physicochemical and Environmental Factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M. Review of in Vitro Toxicological Research of Quantum Dot and Potentially Involved Mechanisms. Sci. Total Environ. 2018, 625, 940–962. [Google Scholar] [CrossRef] [PubMed]
- Mo, D.; Hu, L.; Zeng, G.; Chen, G.; Wan, J.; Yu, Z.; Huang, Z.; He, K.; Zhang, C.; Cheng, M. Cadmium-Containing Quantum Dots: Properties, Applications, and Toxicity. Appl. Microbiol. Biotechnol. 2017, 101, 2713–2733. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-Analysis of Cellular Toxicity for Cadmium-Containing Quantum Dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.K.; Pettine, M.; Zboril, R. Assessment of Toxicity of Selenium and Cadmium Selenium Quantum Dots: A Review. Chemosphere 2017, 188, 403–413. [Google Scholar] [CrossRef]
- Chen, N.; He, Y.; Su, Y.; Li, X.; Huang, Q.; Wang, H.; Zhang, X.; Tai, R.; Fan, C. The Cytotoxicity of Cadmium-Based Quantum Dots. Biomaterials 2012, 33, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Tang, M. Research Advances on Cytotoxicity of Cadmium-Containing Quantum Dots. J. Nanosci. Nanotechnol. 2019, 19, 5375–5387. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zeng, S.; Zhang, B.; Swihart, M.T.; Yong, K.T.; Prasad, P.N. New Generation Cadmium-Free Quantum Dots for Biophotonics and Nanomedicine. Chem. Rev. 2016, 116, 12234–12327. [Google Scholar] [CrossRef]
- Rosenthal, S.J.; Chang, J.C.; Kovtun, O.; McBride, J.R.; Tomlinson, I.D. Biocompatible Quantum Dots for Biological Applications. Chem. Biol. 2011, 18, 10–24. [Google Scholar] [CrossRef]
- Reshma, V.G.; Mohanan, P.V. Quantum Dots: Applications and Safety Consequences. J. Lumin. 2019, 205, 287–298. [Google Scholar] [CrossRef]
- Reiss, P.; Carrière, M.; Lincheneau, C.; Vaure, L.; Tamang, S. Synthesis of Semiconductor Nanocrystals, Focusing on Nontoxic and Earth-Abundant Materials. Chem. Rev. 2016, 116, 10731–10819. [Google Scholar] [CrossRef]
- Winnik, F.M.; Maysinger, D. Quantum Dot Cytotoxicity and Ways to Reduce It. Acc. Chem. Res. 2013, 46, 672–680. [Google Scholar] [CrossRef]
- Mortimer, M.; Kahru, A.; Slaveykova, V.I. Uptake, Localization and Clearance of Quantum Dots in Ciliated Protozoa Tetrahymena thermophila. Environ. Pollut. 2014, 190, 58–64. [Google Scholar] [CrossRef]
- Färkkilä, S.M.A.; Kiers, E.T.; Jaaniso, R.; Mäeorg, U.; Leblanc, R.M.; Treseder, K.K.; Kang, Z.; Tedersoo, L. Fluorescent Nanoparticles as Tools in Ecology and Physiology. Biol. Rev. 2021, 96, 2392–2424. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How Mycorrhizal Associations Drive Plant Population and Community Biology: Review. Science 2020, 367, 867. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global Diversity and Geography of Soil Fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Whiteside, M.D.; Garcia, M.O.; Treseder, K.K. Amino Acid Uptake in Arbuscular Mycorrhizal Plants. PLoS ONE 2012, 7, 8–11. [Google Scholar] [CrossRef]
- Kasemets, K.; Ivask, A.; Dubourguier, H.C.; Kahru, A. Toxicity of Nanoparticles of ZnO, CuO and TiO2 to Yeast Saccharomyces cerevisiae. Toxicol. Vitr. 2009, 23, 1116–1122. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E.; Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces cerevisiae and Its Industrial Applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Kasemets, K.; Käosaar, S.; Vija, H.; Fascio, U.; Mantecca, P. Toxicity of Differently Sized and Charged Silver Nanoparticles to Yeast Saccharomyces cerevisiae BY4741: A Nano-Biointeraction Perspective. Nanotoxicology 2019, 13, 1041–1059. [Google Scholar] [CrossRef]
- Lu, R.; Drubin, D.G.; Sun, Y. Clathrin-Mediated Endocytosis in Budding Yeast at a Glance. J. Cell Sci. 2016, 129, 1531–1536. [Google Scholar] [CrossRef]
- Nomura, T.; Miyazaki, J.; Miyamoto, A.; Kuriyama, Y.; Tokumoto, H.; Konishi, Y. Exposure of the Yeast Saccharomyces cerevisiae to Functionalized Polystyrene Latex Nanoparticles: Influence of Surface Charge on Toxicity. Environ. Sci. Technol. 2013, 47, 3417–3423. [Google Scholar] [CrossRef]
- Käosaar, S.; Kahru, A.; Mantecca, P.; Kasemets, K. Profiling of the Toxicity Mechanisms of Coated and Uncoated Silver Nanoparticles to Yeast Saccharomyces cerevisiae BY4741 Using a Set of Its 9 Single-Gene Deletion Mutants Defective in Oxidative Stress Response, Cell Wall or Membrane Integrity and Endocytosis. Toxicol. Vitr. 2016, 35, 149–162. [Google Scholar] [CrossRef]
- Prescianotto-Baschong, C.; Riezman, H. Morphology of the Yeast Endocytic Pathway. Mol. Biol. Cell 1998, 9, 173–189. [Google Scholar] [CrossRef]
- Suner, S.S.; Sahiner, M.; Ayyala, R.S.; Bhethanabotla, V.R.; Sahiner, N. Versatile Fluorescent Carbon Dots from Citric Acid and Cysteine with Antimicrobial, Anti-Biofilm, Antioxidant, and AChE Enzyme Inhibition Capabilities. J. Fluoresc. 2021, 31, 1705–1717. [Google Scholar] [CrossRef]
- Suppi, S.; Kasemets, K.; Ivask, A.; Künnis-Beres, K.; Sihtmäe, M.; Kurvet, I.; Aruoja, V.; Kahru, A. A Novel Method for Comparison of Biocidal Properties of Nanomaterials to Bacteria, Yeasts and Algae. J. Hazard. Mater. 2015, 286, 75–84. [Google Scholar] [CrossRef]
- Wang, W.; Li, S.; Yin, P.; Li, J.; Tang, Y.; Yang, M. Response surface methodology optimization for the synthesis of N, S-codoped carbon dots and its application for tetracyclines detection. Chemosphere 2022, 303, 135145. [Google Scholar] [CrossRef]
- Tong, Y.J.; Yu, L.D.; Huang, Y.; Li, Y.; Li, N.; Fu, Q.; Ouyang, G. High-quality full-color carbon quantum dots synthesized under an unprecedentedly mild condition. iScience 2022, 25, 104421. [Google Scholar] [CrossRef]
- Alam, A.M.; Park, B.Y.; Ghouri, Z.K.; Park, M.; Kim, H.Y. Synthesis of carbon quantum dots from cabbage with down-and up-conversion photoluminescence properties: Excellent imaging agent for biomedical applications. Green Chem. 2015, 17, 3791–3797. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for Photoluminescence Quantum Yield Measurements in Solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Zhang, L.; Yang, X.; Yin, Z.; Sun, L. A Review on Carbon Quantum Dots: Synthesis, Photoluminescence Mechanisms and Applications. Luminescence 2022, 37, 1612–1638. [Google Scholar] [CrossRef]
- Mishra, S.; Bhowmick, D. Asymmetric Magnetoelectrochemistry: An Efficient Method to Grow Enantiopure Self-Assemble Monolayer. Magnetochemistry 2020, 6, 37. [Google Scholar] [CrossRef]
- Donaldson, L. Autofluorescence in Plants. Molecules 2020, 25, 2393. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon Quantum Dots and Their Applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J. A Review of Carbon Dots in Biological Applications. J. Mater. Sci. 2016, 51, 4728–4738. [Google Scholar] [CrossRef]
- Yang, S.T.; Wang, X.; Wang, H.; Lu, F.; Luo, P.G.; Cao, L.; Meziani, M.J.; Liu, J.H.; Liu, Y.; Chen, M.; et al. Carbon Dots as Nontoxic and High-Performance Fluorescence Imaging Agents. J. Phys. Chem. C 2009, 113, 18110–18114. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Song, Y.; Li, H.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. Carbon Dots Promote the Growth and Photosynthesis of Mung Bean Sprouts. Carbon 2018, 136, 94–102. [Google Scholar] [CrossRef]
- Su, L.X.; Ma, X.L.; Zhao, K.K.; Shen, C.L.; Lou, Q.; Yin, D.M.; Shan, C.X. Carbon Nanodots for Enhancing the Stress Resistance of Peanut Plants. ACS Omega 2018, 3, 17770–17777. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Zhang, M.; Song, Y.; Huang, J.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. Carbon Dots Enhance the Nitrogen Fixation Activity of Azotobacter Chroococcum. ACS Appl. Mater. Interfaces 2018, 10, 16308–16314. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Liu, Y.; Lu, F.; Zhong, J.; Wang, Y.; Li, S.; Lifshitz, Y.; Lee, S.T.; Kang, Z. Enhanced RuBisCO Activity and Promoted Dicotyledons Growth with Degradable Carbon Dots. Nano Res. 2019, 12, 1585–1593. [Google Scholar] [CrossRef]
- Navarro-Ruiz, M.C.; Cayuela, A.; Soriano, M.L.; Guzm, R.; Malag, M.M.; Valc, M. A Systematic Comparative Study of the Toxicity of Semiconductor and Graphitic Carbon-Based Quantum Dots Using In Vitro Cell Models. Appl. Sci. 2020, 10, 8845. [Google Scholar] [CrossRef]
- Xiao, A.; Wang, C.; Chen, J.; Guo, R.; Yan, Z.; Chen, J. Carbon and Metal Quantum Dots Toxicity on the Microalgae Chlorella Pyrenoidosa. Ecotoxicol. Environ. Saf. 2016, 133, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Cailotto, S.; Amadio, E.; Facchin, M.; Selva, M.; Pontoglio, E.; Rizzolio, F.; Riello, P.; Toffoli, G.; Benedetti, A.; Perosa, A. Carbon Dots from Sugars and Ascorbic Acid: Role of the Precursors on Morphology, Properties, Toxicity, and Drug Uptake. ACS Med. Chem. Lett. 2018, 9, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Claudel, M.; Ronzani, C.; Arezki, Y.; Lebeau, L.; Pons, F. Physicochemical Characteristics That Affect Carbon Dot Safety: Lessons from a Comprehensive Study on a Nanoparticle Library. Int. J. Pharm. 2019, 569, 118521. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Carrillo-Carrión, C.; Valcárcel, M. Semiconductor and Carbon-Based Fluorescent Nanodots: The Need for Consistency. Chem. Commun. 2016, 52, 1311–1326. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The Photoluminescence Mechanism in Carbon Dots (Graphene Quantum Dots, Carbon Nanodots, and Polymer Dots): Current State and Future Perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Amornwachirabodee, K.; Khramchantuk, S.; Pienpinijtham, P.; Israsena, N.; Palaga, T.; Wanichwecharungruang, S. Enhancing Passive Transport of Micro/Nano Particles into Cells by Oxidized Carbon Black. ACS Omega 2018, 3, 6833–6840. [Google Scholar] [CrossRef]
- Chae, Y.; Kim, S.W.; An, Y.J. In Vivo Visual Evaluation of Nanoparticle Transfer in a Three-Species Terrestrial Food Chain. Chemosphere 2016, 151, 101–107. [Google Scholar] [CrossRef]
- Strtak, A.; Sathiamoorthy, S.; Tang, P.S.; Tsoi, K.M.; Song, F.; Anderson, J.B.; Chan, W.C.W.; Shin, J.A. Yeast Populations Evolve to Resist CdSe Quantum Dot Toxicity. Bioconjugate Chem. 2017, 28, 1205–1213. [Google Scholar] [CrossRef]
- Gustafsson, F.S.; Whiteside, M.D.; Jiranek, V.; Durall, D.M. Development and Use of a Quantum Dot Probe to Track Multiple Yeast Strains in Mixed Culture. Sci. Rep. 2014, 4, 6971. [Google Scholar] [CrossRef]
- Han, X.; Lei, J.; Chen, K.; Li, Q.; Hao, H.; Zhou, T.; Jiang, F.L.; Li, M.; Liu, Y. Cytotoxicity of CdTe Quantum Dots with Different Surface Coatings against Yeast Saccharomyces cerevisiae. Ecotoxicol. Environ. Saf. 2019, 174, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lai, L.; Tian, F.; Jiang, F.L.; Xiao, Q.; Li, Y.; Yu, Q.; Li, D.; Wang, J.; Zhang, Q.; et al. Toxicity of CdTe Quantum Dots on Yeast Saccharomyces cerevisiae. Small 2012, 8, 2680–2689. [Google Scholar] [CrossRef] [PubMed]
- De Farias, P.M.A.; Santos, B.S.; Menezes, F.D.; Brasil, A.G.; Ferreira, R.; Motta, M.A.; Castro-Neto, A.G.; Vieira, A.A.S.; Silva, D.C.N.; Fontes, A.; et al. Highly Fluorescent Semiconductor Core-Shell CdTe-CdS Nanocrystals for Monitoring Living Yeast Cells Activity. Appl. Phys. A Mater. Sci. Process. 2007, 89, 957–961. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Zhu, H.; Braam, J.; Schnoor, J.L.; Alvarez, P.J.J. Uptake, Translocation, and Transformation of Quantum Dots with Cationic versus Anionic Coatings by Populus deltoides × Nigra Cuttings. Environ. Sci. Technol. 2014, 48, 6754–6762. [Google Scholar] [CrossRef]
- Sun, H.; Wang, M.; Lei, C.; Li, R. Cell Wall: An Important Medium Regulating the Aggregation of Quantum Dots in Maize (Zea mays L.) Seedlings. J. Hazard. Mater. 2021, 403, 123960. [Google Scholar] [CrossRef]
- Koo, Y.; Wang, J.; Zhang, Q.; Zhu, H.; Chehab, E.W.; Colvin, V.L.; Alvarez, P.J.J.; Braam, J. Fluorescence Reports Intact Quantum Dot Uptake into Roots and Translocation to Leaves of Arabidopsis thaliana and Subsequent Ingestion by Insect Herbivores. Environ. Sci. Technol. 2015, 49, 626–632. [Google Scholar] [CrossRef]
- Agarwal, R.; Domowicz, M.S.; Schwartz, N.B.; Henry, J.; Medintz, I.; Delehanty, J.B.; Stewart, M.H.; Susumu, K.; Huston, A.L.; Deschamps, J.R.; et al. Delivery and Tracking of Quantum Dot Peptide Bioconjugates in an Intact Developing Avian Brain. ACS Chem. Neurosci. 2015, 6, 494–504. [Google Scholar] [CrossRef]
- Zrazhevskiy, P.; Sena, M.; Gao, X. Designing Multifunctional Quantum Dots for Bioimaging, Detection, and Drug Delivery. Chem. Soc. Rev. 2010, 39, 4326–4354. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum Dots in Biomedical Applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef]
- Marmiroli, M.; Pagano, L.; Pasquali, F.; Zappettini, A.; Tosato, V.; Bruschi, C.V.; Marmiroli, N. A Genome-Wide Nanotoxicology Screen of Saccharomyces cerevisiae Mutants Reveals the Basis for Cadmium Sulphide Quantum Dot Tolerance and Sensitivity. Nanotoxicology 2016, 10, 84–93. [Google Scholar] [CrossRef]
- Pagano, L.; Caldara, M.; Villani, M.; Zappettini, A.; Marmiroli, N.; Marmiroli, M. In Vivo-In Vitro Comparative Toxicology of Cadmium Sulphide Quantum Dots in the Model Organism Saccharomyces cerevisiae. Nanomaterials 2019, 9, 512. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, F.; Agrimonti, C.; Pagano, L.; Zappettini, A.; Villani, M.; Marmiroli, M.; White, J.C.; Marmiroli, N. Nucleo-Mitochondrial Interaction of Yeast in Response to Cadmium Sulfide Quantum Dot Exposure. J. Hazard. Mater. 2017, 324, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Kawai-Noma, S.; Pack, C.G.; Terajima, H.; Yajima, J.; Nishizaka, T.; Kinjo, M.; Taguchi, H. Single-Particle Tracking of Quantum Dot-Conjugated Prion Proteins inside Yeast Cells. Biochem. Biophys. Res. Commun. 2011, 405, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Bachhawat, A.K. Yct1p, a Novel, High-Affinity, Cysteine-Specific Transporter From the Yeast Saccharomyces cerevisiae. Genetics 2007, 176, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.; Gardner, R.C. The IRC7 Gene Encodes Cysteine Desulphydrase Activity and Confers on Yeast the Ability to Grow on Cysteine as a Nitrogen Source. Yeast 2015, 32, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Oh, E.; Liu, R.; Breger, J.C.; Medintz, I.L.; Cohen, Y. Bayesian Network Resource for Meta-Analysis: Cellular Toxicity of Quantum Dots. Small 2019, 15, 1900510. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, M.; Wu, H.F. Probing the Fungicidal Property of CdS Quantum Dots on Saccharomyces cerevisiae and Candida utilis Using MALDI-MS. J. Nanoparticle Res. 2013, 15, 1–13. [Google Scholar] [CrossRef]
- Marmiroli, M.; Birarda, G.; Gallo, V.; Villani, M.; Zappettini, A.; Vaccari, L.; Marmiroli, N.; Pagano, L. Cadmium Sulfide Quantum Dots, Mitochondrial Function and Environmental Stress: A Mechanistic Reconstruction through In Vivo Cellular Approaches in Saccharomyces cerevisiae. Nanomaterials 2023, 13, 1944. [Google Scholar] [CrossRef]
- Rossi, R.; Ruotolo, R.; De Giorgio, G.; Marmiroli, M.; Villani, M.; Zappettini, A.; Marmiroli, N. Cadmium Sulfide Quantum Dots Adversely Affect Gametogenesis in Saccharomyces cerevisiae. Nanomaterials 2022, 12, 2208. [Google Scholar] [CrossRef]
- Ruotolo, R.; Pira, G.; Villani, M.; Zappettini, A.; Marmiroli, N. Ring-Shaped Corona Proteins Influence the Toxicity of Engineered Nanoparticles to Yeast. Environ. Sci. Nano 2018, 5, 1428–1440. [Google Scholar] [CrossRef]
- Kloepfer, J.A.; Mielke, R.E.; Wong, M.S.; Nealson, K.H.; Stucky, G.; Nadeau, J.L. Quantum Dots as Strain- and Metabolism-Specific Microbiological Labels. Appl. Environ. Microbiol. 2003, 69, 4205–4213. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yu, Q.; Liu, M.; Chen, X.; Liu, Z.; Zhou, H.; Yuan, Y.; Liu, L.; Li, M.; Zhang, C. A Novel Toxicity Mechanism of CdSe Nanoparticles to Saccharomyces cerevisiae: Enhancement of Vacuolar Membrane Permeabilization (VMP). Chem. Biol. Interact. 2014, 220, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, R.S.; Qureshi, A.; Niazi, J.H. Determining the Fate of Fluorescent Quantum Dots on Surface of Engineered Budding S. cerevisiae Cell Molecular Landscape. Biosens. Bioelectron. 2015, 69, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, C.; Kim, K. Comparing Transcriptome Profiles of Saccharomyces cerevisiae Cells Exposed to Cadmium Selenide/Zinc Sulfide and Indium Phosphide/Zinc Sulfide. Genes 2021, 12, 428. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, C.; Kim, D.S.; Campbell, C.; Kim, K. Transcriptome Profile Alteration with Cadmium Selenide/Zinc Sulfide Quantum Dots in Saccharomyces cerevisiae. Biomolecules 2019, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Routh, J.; Kirk, C.; Wu, Q.; Patel, R.; Keyes, C.; Kim, K. Red CdSe/ZnS QDs’ Intracellular Trafficking and Its Impact on Yeast Polarization and Actin Filament. Cells 2023, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Yang, L.Y.; Lai, L.; Xu, Z.Q.; Wang, C.; Zhao, J.; Jin, J.C.; Jiang, F.L.; Liu, Y. The Interactions between CdSe Quantum Dots and Yeast Saccharomyces cerevisiae: Adhesion of Quantum Dots to the Cell Surface and the Protection Effect of ZnS Shell. Chemosphere 2014, 112, 92–99. [Google Scholar] [CrossRef]
- Coulon, J.; Thouvenin, I.; Aldeek, F.; Balan, L.; Schneider, R. Glycosylated Quantum Dots for the Selective Labelling of Kluyveromyces bulgaricus and Saccharomyces cerevisiae Yeast Strains. J. Fluoresc. 2010, 20, 591–597. [Google Scholar] [CrossRef]
- Cunha, C.R.A.; Andrade, C.G.; Pereira, M.I.A.; Cabral Filho, P.E.; Carvalho, L.B.; Coelho, L.C.B.B.; Santos, B.S.; Fontes, A.; Correia, M.T.S. Quantum Dot–Cramoll Lectin as Novel Conjugates to Glycobiology. J. Photochem. Photobiol. B 2018, 178, 85–91. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Cabrera, M.P.; Santos, N.R.M.; Napoleão, T.H.; Paiva, P.M.G.; Neves, R.P.; Silva, M.V.; Santos, B.S.; Coelho, L.C.B.B.; Cabral Filho, P.E.; et al. Evaluating Glucose and Mannose Profiles in Candida Species Using Quantum Dots Conjugated with Cramoll Lectin as Fluorescent Nanoprobes. Microbiol. Res. 2020, 230, 126330. [Google Scholar] [CrossRef]
- Tenório, D.P.L.A.; Andrade, C.G.; Cabral Filho, P.E.; Sabino, C.P.; Kato, I.T.; Carvalho, L.B.; Alves, S.; Ribeiro, M.S.; Fontes, A.; Santos, B.S. CdTe Quantum Dots Conjugated to Concanavalin A as Potential Fluorescent Molecular Probes for Saccharides Detection in Candida albicans. J. Photochem. Photobiol. B 2015, 142, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Xie, Q.; Huang, Z.; Yang, A.; Duan, Y. Induction of Autophagy and Endoplasmic Reticulum Autophagy Caused by Cadmium Telluride Quantum Dots Are Protective Mechanisms of Yeast Cell. J. Appl. Toxicol. 2022, 42, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Shao, M.; Lai, L.; Liu, Y.; Xie, Z. Inhibition of Autophagy Contributes to the Toxicity of Cadmium Telluride Quantum Dots in Saccharomyces cerevisiae. Int. J. Nanomed. 2016, 11, 3371–3383. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Chakradhar, D.; Perumal, S.; Shim, J.J.; Lee, Y.R. Facile Green Synthesis of Nitrogen-Doped Carbon Dots Using Chionanthus retusus Fruit Extract and Investigation of Their Suitability for Metal Ion Sensing and Biological Applications. Sens. Actuators B Chem. 2017, 246, 497–509. [Google Scholar] [CrossRef]
- Gunjal, D.B.; Gore, A.H.; Bhosale, A.R.; Naik, V.M.; Anbhule, P.V.; Shejwal, R.V.; Kolekar, G.B. Waste Derived Sustainable Carbon Nanodots as a New Approach for Sensitive Quantification of Ethionamide and Cell Imaging. J. Photochem. Photobiol. A Chem. 2019, 376, 54–62. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Rawat, K.; Prasad, T.; Bohidar, H.B. Antifungal Efficacy of Au@ Carbon Dots Nanoconjugates against Opportunistic Fungal Pathogen, Candida albicans. Colloids Surf. B Biointerfaces 2018, 163, 355–361. [Google Scholar] [CrossRef]
- Bagheri, Z.; Ehtesabi, H.; Hallaji, Z.; Latifi, H.; Behroodi, E. Investigation the Cytotoxicity and Photo-Induced Toxicity of Carbon Dot on Yeast Cell. Ecotoxicol. Environ. Saf. 2018, 161, 245–250. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Z.; Huo, X.; Gu, M.; Ma, S.; Jing, Y.; Wang, Y.; Yue, Y.; Feng, Z.; Tian, B. Efficient Imaging of Saccharomyces cerevisiae Based on B- A Nd N-Doped Carbon Dots. J. Agric. Food Chem. 2020, 68, 10223–10231. [Google Scholar] [CrossRef]
- Tian, B.; Fu, T.; Wan, Y.; Ma, Y.; Wang, Y.; Feng, Z.; Jiang, Z. B- and N-Doped Carbon Dots by One-Step Microwave Hydrothermal Synthesis: Tracking Yeast Status and Imaging Mechanism. J. Nanobiotechnology 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Mehta, V.N.; Jha, S.; Kailasa, S.K. One-Pot Green Synthesis of Carbon Dots by Using Saccharum officinarum Juice for Fluorescent Imaging of Bacteria (Escherichia coli) and Yeast (Saccharomyces cerevisiae) Cells. Mater. Sci. Eng. C 2014, 38, 20–27. [Google Scholar] [CrossRef]
- De Falco, A.; Santa-Helena, E.; Toloza, C.A.T.; Almeida, J.M.S.; Larrude, D.G.; Meirelles, F.V.P.; Gioda, C.R.; Aucelio, R.Q.; Gioda, A. Luminescence Imaging and Toxicity Assessment of Graphene Quantum Dots Using in Vitro Models. Fuller. Nanotub. Carbon Nanostructures 2022, 30, 657–666. [Google Scholar] [CrossRef]
- Jain, R.; Vithalani, R.; Patel, D.; Lad, U.; Modi, C.K.; Suthar, D.; Solanki, J.D.; Surati, K.R. Highly Fluorescent Nitrogen-Doped Graphene Quantum Dots (N-GQDs) as an Efficient Nanoprobe for Imaging of Microbial Cells. Fuller. Nanotub. Carbon Nanostructures 2021, 29, 588–595. [Google Scholar] [CrossRef]
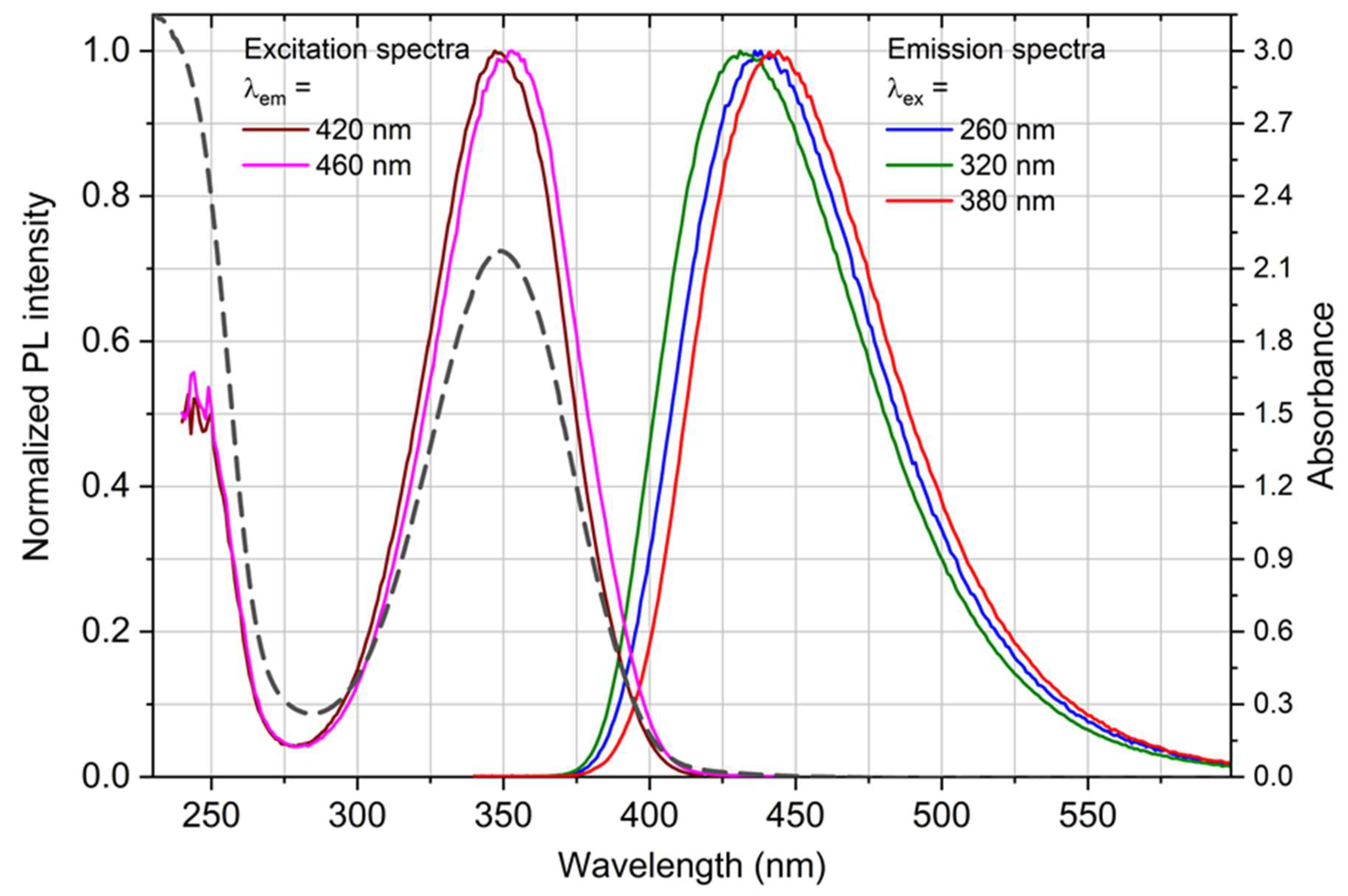
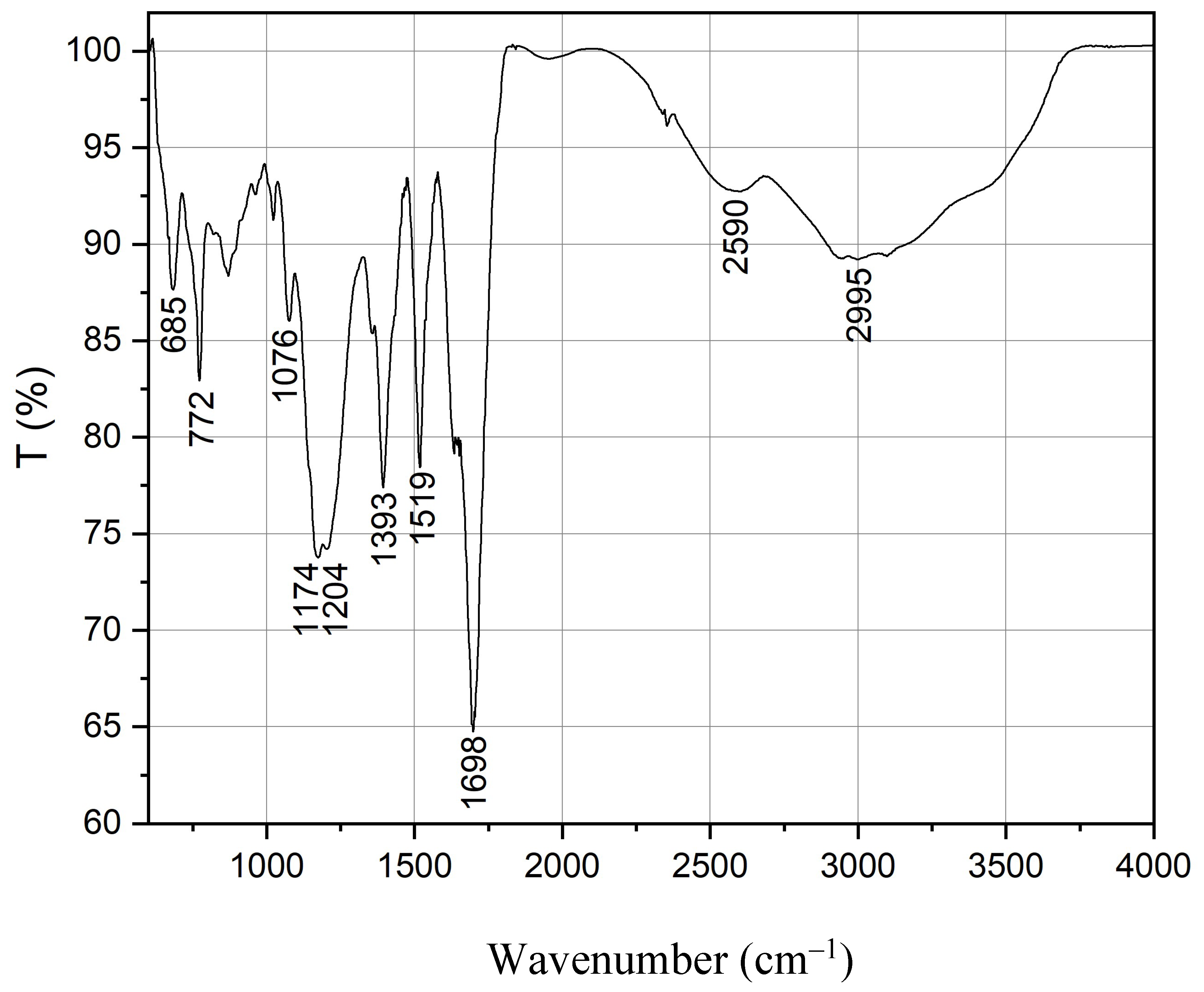

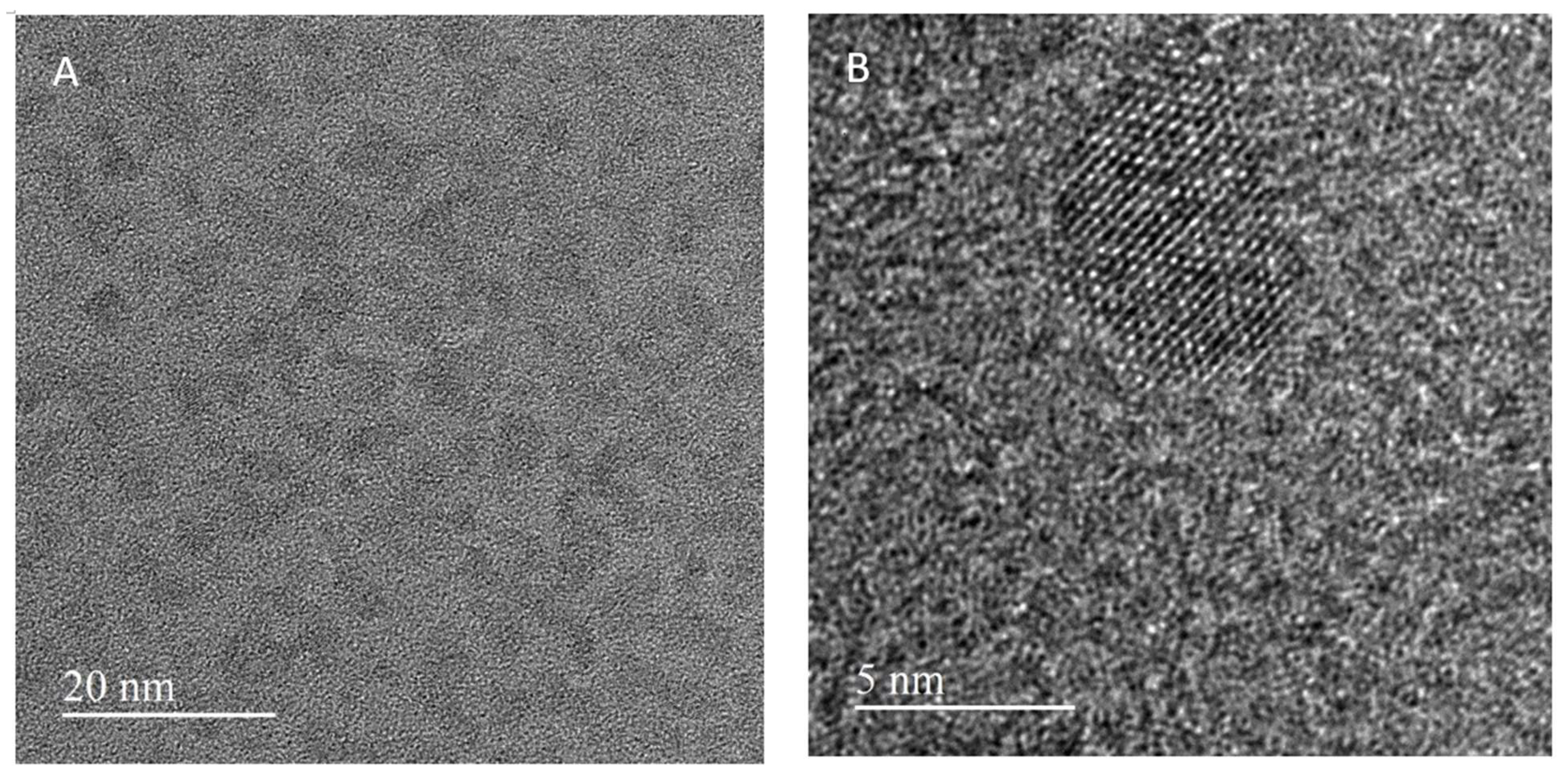

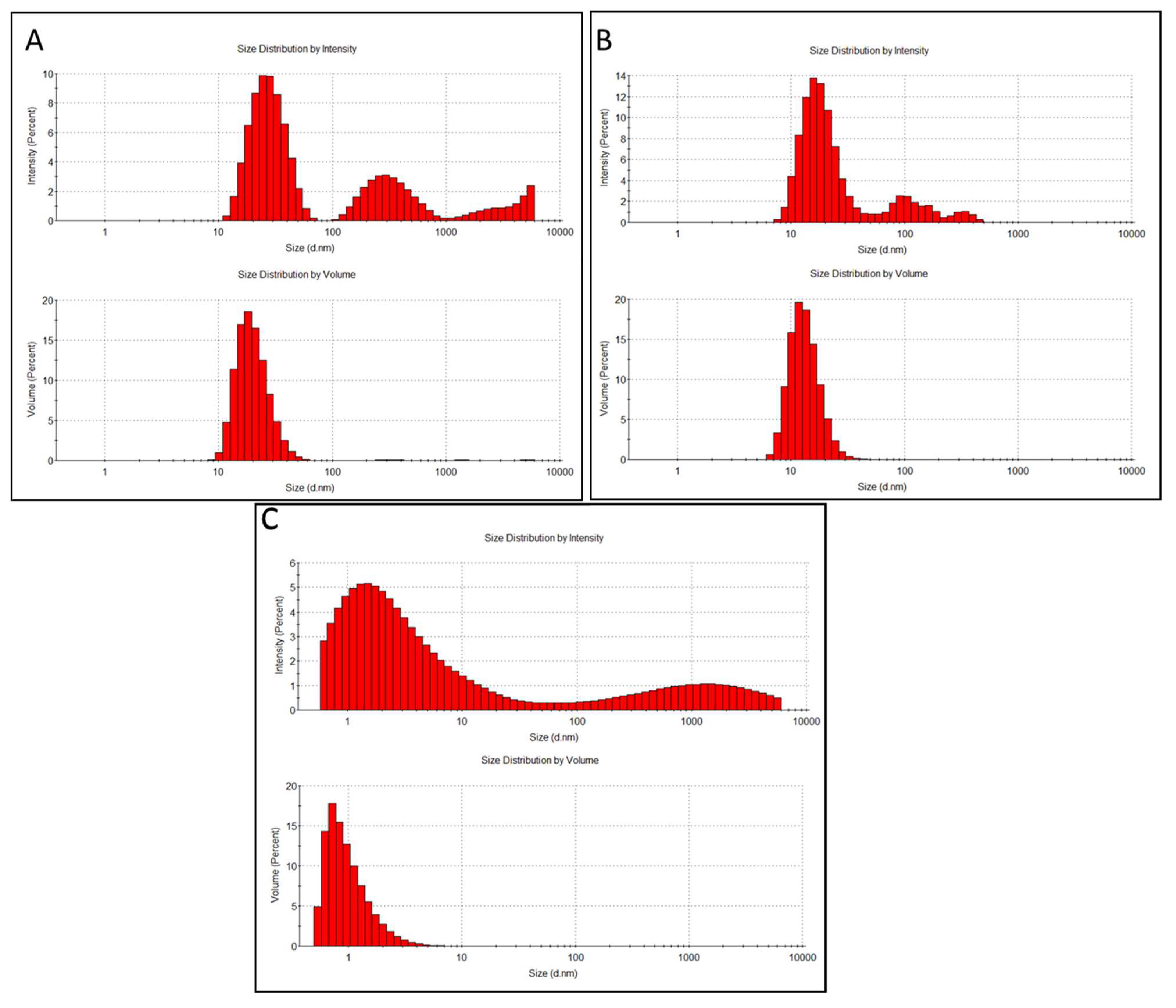
| Sample | Pristine Diameter, nm | Hydrodynamic Diameter, nm ** | Average Sizes of Main Peaks, nm (% Intensity) *** | PdI | Zeta Potential, mV |
|---|---|---|---|---|---|
| CdSe/ZnS QDs 100 mg/L | 10 * | 47.4 ± 2.4 | Peak1 28.3 ± 9.7 (66%) Peak2 347 ± 140 (31%) Peak3 5245 ± 447 (3.8%) | 0.50 ± 0.05 | −70.0 ± 2.4 |
| CdSe/ZnS—Cys QDs 100 mg/L | 15–20 * | 308 ± 150 | Peak1 17.4 ± 5.9 (77%) Peak2 67.7 ± 6.5 (6.4%) Peak3 368 ± 94 (9.1%) | 0.43 ± 0.05 | −64.7 ± 4.0 |
| CQDs 500 mg/L | 2–3 | 6.08 ± 3.35 | Peak1 1.98 ± 1.16 (59%) Peak2 1425 ± 1359 (41%) | 0.43 ± 0.16 | −19.4 ± 12.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Färkkilä, S.M.A.; Mortimer, M.; Jaaniso, R.; Kahru, A.; Kiisk, V.; Kikas, A.; Kozlova, J.; Kurvet, I.; Mäeorg, U.; Otsus, M.; et al. Comparison of Toxicity and Cellular Uptake of CdSe/ZnS and Carbon Quantum Dots for Molecular Tracking Using Saccharomyces cerevisiae as a Fungal Model. Nanomaterials 2024, 14, 10. https://doi.org/10.3390/nano14010010
Färkkilä SMA, Mortimer M, Jaaniso R, Kahru A, Kiisk V, Kikas A, Kozlova J, Kurvet I, Mäeorg U, Otsus M, et al. Comparison of Toxicity and Cellular Uptake of CdSe/ZnS and Carbon Quantum Dots for Molecular Tracking Using Saccharomyces cerevisiae as a Fungal Model. Nanomaterials. 2024; 14(1):10. https://doi.org/10.3390/nano14010010
Chicago/Turabian StyleFärkkilä, Sanni M. A., Monika Mortimer, Raivo Jaaniso, Anne Kahru, Valter Kiisk, Arvo Kikas, Jekaterina Kozlova, Imbi Kurvet, Uno Mäeorg, Maarja Otsus, and et al. 2024. "Comparison of Toxicity and Cellular Uptake of CdSe/ZnS and Carbon Quantum Dots for Molecular Tracking Using Saccharomyces cerevisiae as a Fungal Model" Nanomaterials 14, no. 1: 10. https://doi.org/10.3390/nano14010010
APA StyleFärkkilä, S. M. A., Mortimer, M., Jaaniso, R., Kahru, A., Kiisk, V., Kikas, A., Kozlova, J., Kurvet, I., Mäeorg, U., Otsus, M., & Kasemets, K. (2024). Comparison of Toxicity and Cellular Uptake of CdSe/ZnS and Carbon Quantum Dots for Molecular Tracking Using Saccharomyces cerevisiae as a Fungal Model. Nanomaterials, 14(1), 10. https://doi.org/10.3390/nano14010010








