Experiments on the Electrical Conductivity of PEG 400 Nanocolloids Enhanced with Two Oxide Nanoparticles
Abstract
1. Introduction
2. Chemicals and Experimental Procedure
3. Results and Discussion
3.1. pH
3.2. Electrical Conductivity Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| R² | R-squared value, - |
| T | Temperature, K |
| w | Mass fraction, - |
| Greek Symbols | |
| φ | Volume fraction of nanoparticles, - |
| ϕ | Mass concentration of nanoparticles, % |
| ρ | Density, kg/m3 |
| σ | Electrical conductivity, µS/cm |
| Subscripts | |
| bf | Denotes to base fluid |
| nf | Denotes to nanocolloid |
| p | Denotes to nanoparticles |
| Abbreviations | |
| EC | Electrical conductivity |
| EDL | Electrical double layer |
| EG | Ethylene glycol |
| NF | Nanofluids |
| NP | Nanoparticle |
| PEG | Polyethylene glycol |
References
- Siegel, R.; Ramasamy, S.; Hahn, H.; Zongquan, L.; Ting, L.; Gronsky, R. Synthesis, characterization, and properties of nanophase TiO2. J. Mater. Res. 1988, 3, 1367–1372. [Google Scholar] [CrossRef]
- Tang, H.; Berger, H.; Schmid, P.; Levy, F. Optical properties of anatase (TiO2). Solid State Commun. 1994, 92, 267–271. [Google Scholar] [CrossRef]
- Angayarkanni, S.; Philip, J. Effect of nanoparticles aggregation on thermal and electrical conductivities of nanofluids. J. Nanofluid 2014, 3, 17–25. [Google Scholar] [CrossRef]
- Sikdar, S.; Basu, S.; Ganguly, S. Investigation of electrical conductivity of titanium dioxide nanofluids. Int. J. Nanoparticles 2011, 4, 336–349. [Google Scholar] [CrossRef]
- Islam, R.; Shabani, B.; Andrews, J.; Rosengarten, G. Experimental investigation of using ZnO nanofluids as coolants in a pem fuel cell. Int. J. Hydrogen Energy 2017, 42, 19272–19286. [Google Scholar] [CrossRef]
- Islam, M.R.; Shabani, B.; Rosengarten, G. Electrical and thermal conductivities of 50/50 water-ethylene glycol based TiO2 nanofluids to be used as coolants in pemfuel cells. Energy Procedia 2017, 110, 101–108. [Google Scholar] [CrossRef]
- Islam, R.; Shabani, B. Prediction of electrical conductivity of TiO2 water and ethylene glycol-based nanofluids for cooling application in low temperature pem fuel cells. Energy Procedia 2019, 160, 550–557. [Google Scholar] [CrossRef]
- Kumar, R.S.; Goswami, R.; Chaturvedi, K.R.; Sharma, T. Effect of nanoparticle on rheological properties of surfactant-based nanofluid for effective carbon utilization: Capturing and storage prospects. Environ. Sci. Pollut. Res. 2021, 28, 53578–53593. [Google Scholar] [CrossRef]
- Cieśliński, J.T.; Ronewicz, K.; Smoleń, S. Measurement of temperature-dependent viscosity and thermal conductivity of alumina and titania thermal oil nanofluids. Arch. Thermodyn. 2022, 36, 35–47. [Google Scholar] [CrossRef]
- Shoghl, S.N.; Jamali, J.; Moraveji, M.K. Electrical conductivity, viscosity, and density of different nanofluids: An experimental study. Exp. Therm. Fluid Sci. 2016, 74, 339–346. [Google Scholar] [CrossRef]
- Wang, H.; Zou, C. β-Cyclodextrin modified TiO2 nanofluids to improve the stability and thermal conductivity of oil-based drilling fluids. Colloids Surf. A Physicochem. Eng. Asp. 2023, 661, 130957. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, C.; Li, W.; Zou, Y.; Huang, H. Improving stability and thermal properties of TiO2 nanofluids by supramolecular modification: High energy efficiency heat transfer medium for data center cooling system. Int. J. Heat Mass Transf. 2020, 156, 119735. [Google Scholar] [CrossRef]
- Mehrabi, M.; Sharifpur, M.; Meyer, J.P. Electrical conductivity and pH modelling of magnesium oxide–ethylene glycol nanofluids. Bull. Mater. Sci. 2019, 42, 108. [Google Scholar] [CrossRef]
- Adio, S.A.; Sharifpur, M.; Meyer, J.P. Factors affecting the pH and electrical conductivity of MgO–ethylene glycol nanofluids. Bull. Mater. Sci. 2015, 38, 1345–1357. [Google Scholar] [CrossRef]
- Posner, J.D. Properties and electrokinetic behavior of non-dilute colloidal suspensions. Mech. Res. Commun. 2009, 36, 22. [Google Scholar] [CrossRef]
- Adio, S.A.; Sharifpur, M.; Meyer, J.P. Investigation into the pH and electrical conductivity enhancement of MgO—Ethylene glycol nanofluids. In Proceedings of the 15th International Heat Transfer Conference, IHTC-15, Kyoto, Japan, 10–15 August 2014. [Google Scholar]
- Chereches, M.; Bejan, D.; Chereches, E.I.; Alexandru, A.; Minea, A.A. An Experimental Study on Electrical Conductivity of Several Oxide Nanoparticle Enhanced PEG 400 Fluid. Int. J. Thermophys. 2021, 42, 104. [Google Scholar] [CrossRef]
- Maxwell, J.C. A Treatise of Electricity and Magnetism, 3rd ed.; Oxford University Press: London, UK, 1892; pp. 435–449. [Google Scholar]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 24, 639–664. [Google Scholar]
- Fricke, H. A Mathematical Treatment of the Electric Conductivity and Capacity of Disperse Systems I. The Electric Conductivity of a Suspension of Homogeneous Spheroids. Phys. Rev. 1924, 24, 575–585. [Google Scholar] [CrossRef]
- Wamkam, C.T.; Opoku, M.K.; Hong, H.; Smith, P. Effects of 𝑝H on heat transfer nanofluids containing ZrO2 and TiO2 nanoparticles. Int. J. Appl. Phys. 2011, 109, 024305. [Google Scholar] [CrossRef]
- Wang, X.-J.; Li, H.; Li, X.-F.; Wang, Z.-F.; Lin, F. Stability of TiO2 and Al2O3 Nanofluids. Chin. Phys. Lett. 2011, 28, 086601. [Google Scholar] [CrossRef]
- Menbari, A.; Alemrajabi, A.A.; Ghayeb, Y. Investigation on the stability, viscosity and extinction coefficient of CuO–Al2O3/Water binary mixture nanofluid. Exp. Therm. Fluid Sci. 2016, 74, 122–129. [Google Scholar] [CrossRef]
- Babita, S.K.; Sharma, S.M. Gupta, Preparation and evaluation of stable nanofluids for heat transfer application: A review. Exp. Therm. Fluid Sci. 2016, 79, 202–212. [Google Scholar] [CrossRef]
- Liu, W.; Sun, W.; Borthwick, A.G.L.; Ni, J. Comparison on aggregation and sedimentation of titanium dioxide, titanate nanotubes and titanate nanotubes-TiO2: Influence of pH, ionic strength and natural organic matter. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 319–328. [Google Scholar] [CrossRef]
- Fal, J.; Wanic, M.; Malick, M.; Oleksy, M.; Zyła, G. Experimental investigation of electrical conductivity of ethylene glycol containing indium oxide nanoparticles. Acta Phys. Pol. A 2019, 135, 1237–1239. [Google Scholar] [CrossRef]
- Zyła, G.; Vallejo, J.P.; Fal, J.; Lugo, L. Nanodiamonds–Ethylene Glycol nanofluids: Experimental investigation of fundamental physical properties. Int. J. Heat Mass Transf. 2018, 121, 1201–1213. [Google Scholar] [CrossRef]
- Minea, A.A. A Review on Electrical Conductivity of Nanoparticle-Enhanced Fluids. Nanomaterials 2019, 9, 1592. [Google Scholar] [CrossRef]
- Fal, J.; Sobczakb, J.; Stagraczyński, R.; Estellé, P.; Żyła, G. Electrical conductivity of titanium dioxide ethylene glycol-based nanofluids: Impact of nanoparticles phase and concentration. Powder Technol. 2022, 404, 117423. [Google Scholar] [CrossRef]
- Chereches, M.; Bejan, D.; Chereches, E.I.; Minea, A.A. Experimental studies on several properties of PEG 400 and MWCNT nano-enhanced PEG 400 fluids. J. Mol. Liq. 2022, 356, 119049. [Google Scholar] [CrossRef]
- Minea, A.A. State of the Art in PEG-Based Heat Transfer Fluids and Their Suspensions with Nanoparticles. Nanomaterials 2021, 11, 86. [Google Scholar] [CrossRef]
- Awin, E.; Sajith, V.; Sobhan, C.; Peterson, G. Electrical and thermal conductivities of dilute nanofluids—Experimental determination and parametric studies. J. Nanofluid 2016, 5, 653–660. [Google Scholar] [CrossRef]
- Chereches, E.I.; Minea, A.A. Electrical conductivity of new nanoparticle enhanced fluids: An experimental study. Nanomaterials 2019, 9, 1228. [Google Scholar] [CrossRef]
- CurveExpert Pro 2.7.3. Software, Version 2.7.3; Hyams Development: Madison, AL, USA, 2023.




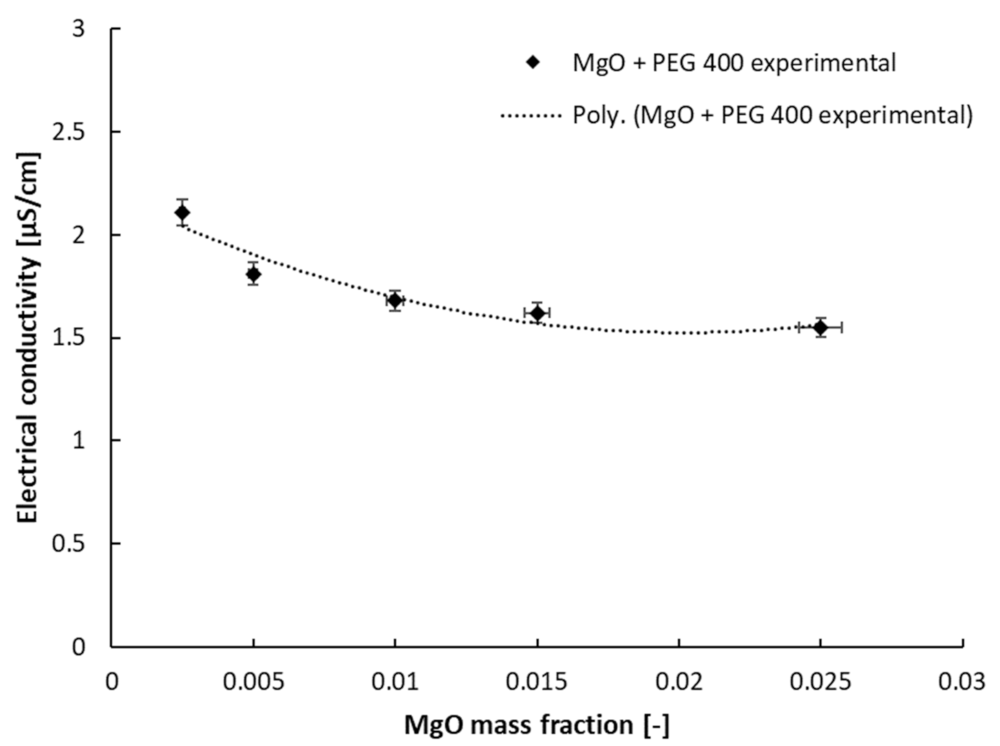

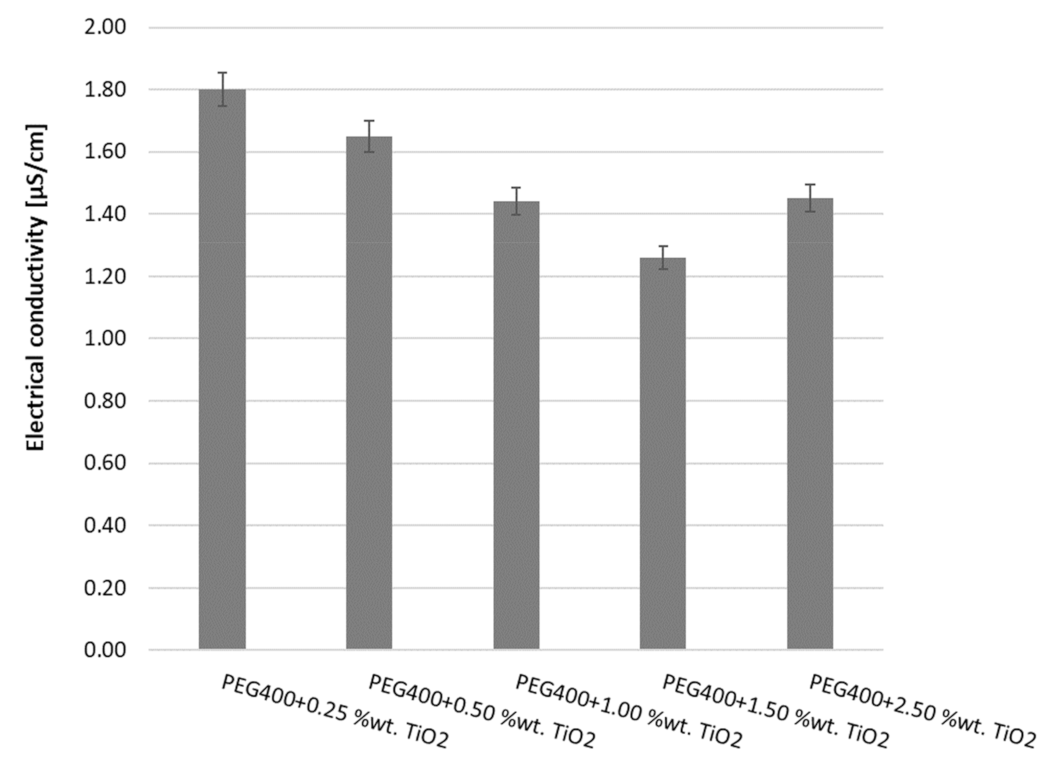
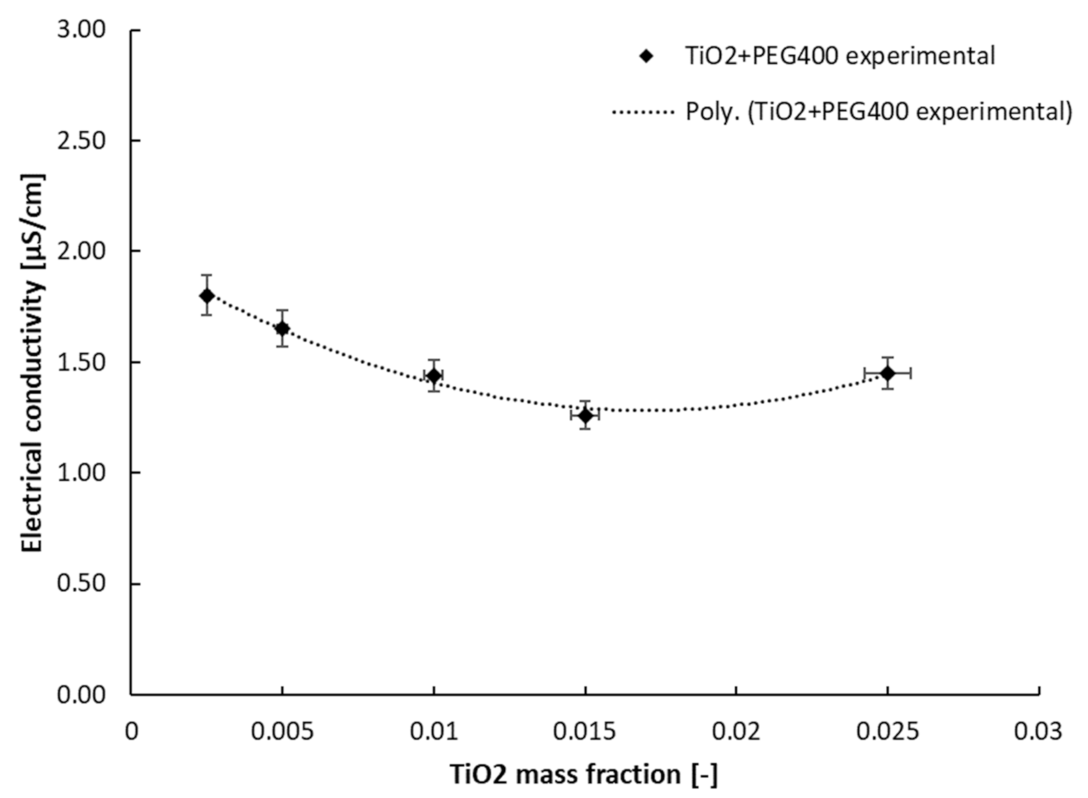
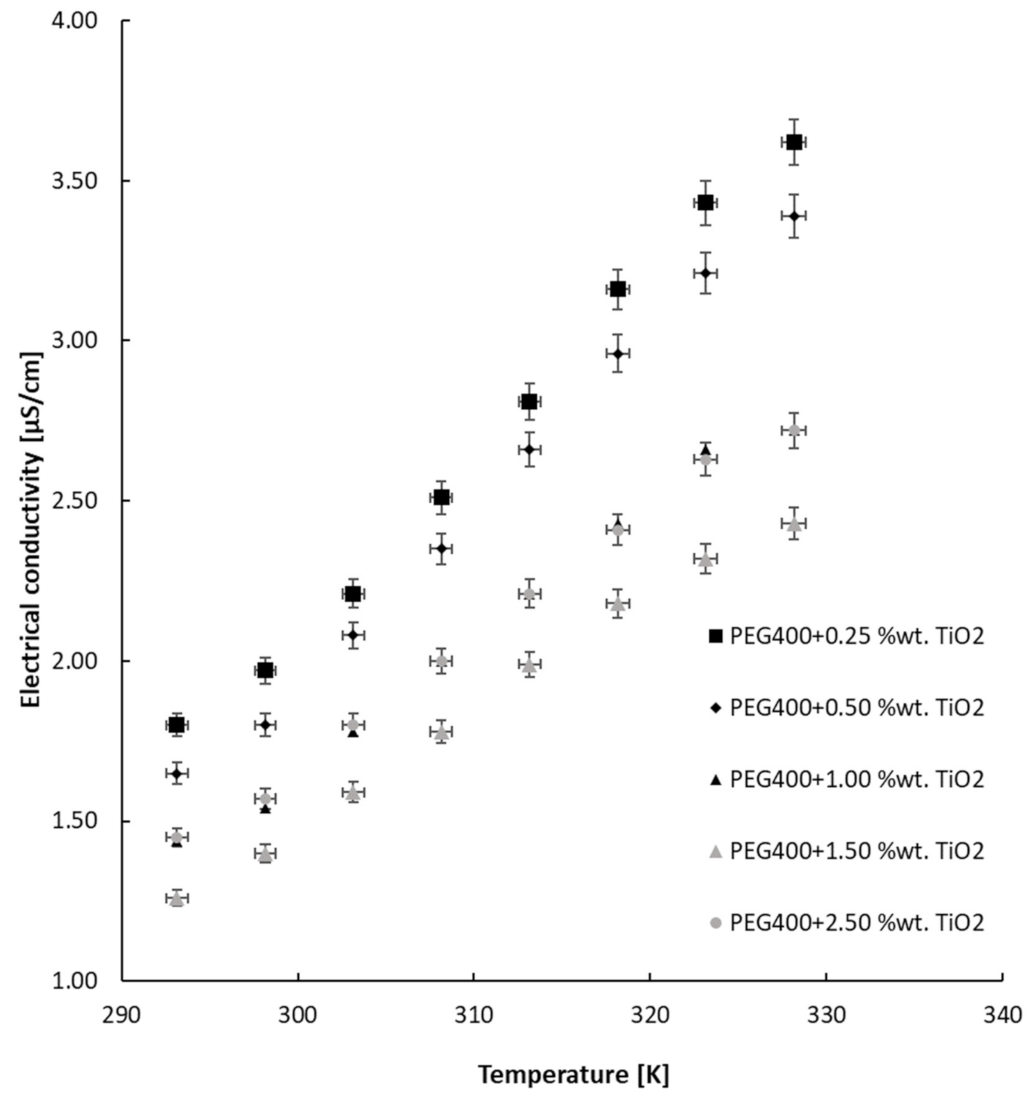
| Sample | a | b | R-Squared Value |
|---|---|---|---|
| PEG 400 + 0.25 %wt. MgO | 0.0461 | −11.472 | 0.99 |
| PEG 400 + 0.50 %wt. MgO | 0.0453 | −11.594 | 0.97 |
| PEG 400 + 1.00 %wt. MgO | 0.0495 | −12.782 | 0.95 |
| PEG 400 + 1.50 %wt. MgO | 0.0486 | −12.66 | 0.99 |
| PEG 400 + 2.50 %wt. MgO | 0.0475 | −12.367 | 0.98 |
| Sample | a | b | R-Squared Value |
|---|---|---|---|
| PEG 400 + 0.25 %wt. TiO2 | 0.0524 | −13.524 | 0.99 |
| PEG 400 + 0.50 %wt. TiO2 | 0.0528 | −13.893 | 0.99 |
| PEG 400 + 1.00 %wt. TiO2 | 0.0398 | −10.269 | 0.99 |
| PEG 400 + 1.50 %wt. TiO2 | 0.0352 | −9.0558 | 0.99 |
| PEG 400 + 2.50 %wt. TiO2 | 0.0386 | −9.9057 | 0.99 |
| Sample | a | b | c | Standard Error |
|---|---|---|---|---|
| PEG 400 + TiO2 | 0.55 | −0.79 | −0.14 | 0.06 |
| PEG 400 + MgO | 0.91 | −1.21 | −0.08 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chereches, E.I.; Minea, A.A. Experiments on the Electrical Conductivity of PEG 400 Nanocolloids Enhanced with Two Oxide Nanoparticles. Nanomaterials 2023, 13, 1555. https://doi.org/10.3390/nano13091555
Chereches EI, Minea AA. Experiments on the Electrical Conductivity of PEG 400 Nanocolloids Enhanced with Two Oxide Nanoparticles. Nanomaterials. 2023; 13(9):1555. https://doi.org/10.3390/nano13091555
Chicago/Turabian StyleChereches, Elena Ionela, and Alina Adriana Minea. 2023. "Experiments on the Electrical Conductivity of PEG 400 Nanocolloids Enhanced with Two Oxide Nanoparticles" Nanomaterials 13, no. 9: 1555. https://doi.org/10.3390/nano13091555
APA StyleChereches, E. I., & Minea, A. A. (2023). Experiments on the Electrical Conductivity of PEG 400 Nanocolloids Enhanced with Two Oxide Nanoparticles. Nanomaterials, 13(9), 1555. https://doi.org/10.3390/nano13091555







