Monolayer AsC5 as the Promising Hydrogen Storage Material for Clean Energy Applications
Abstract
1. Introduction
2. Method
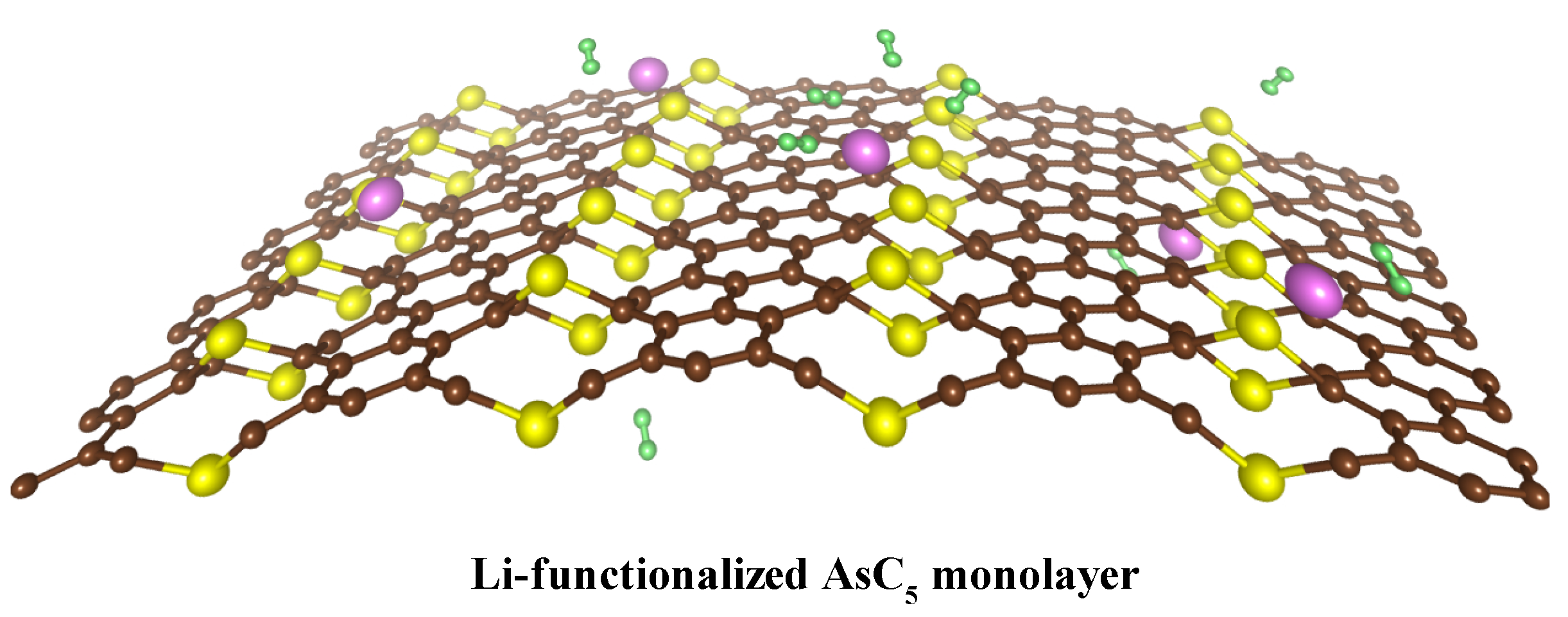
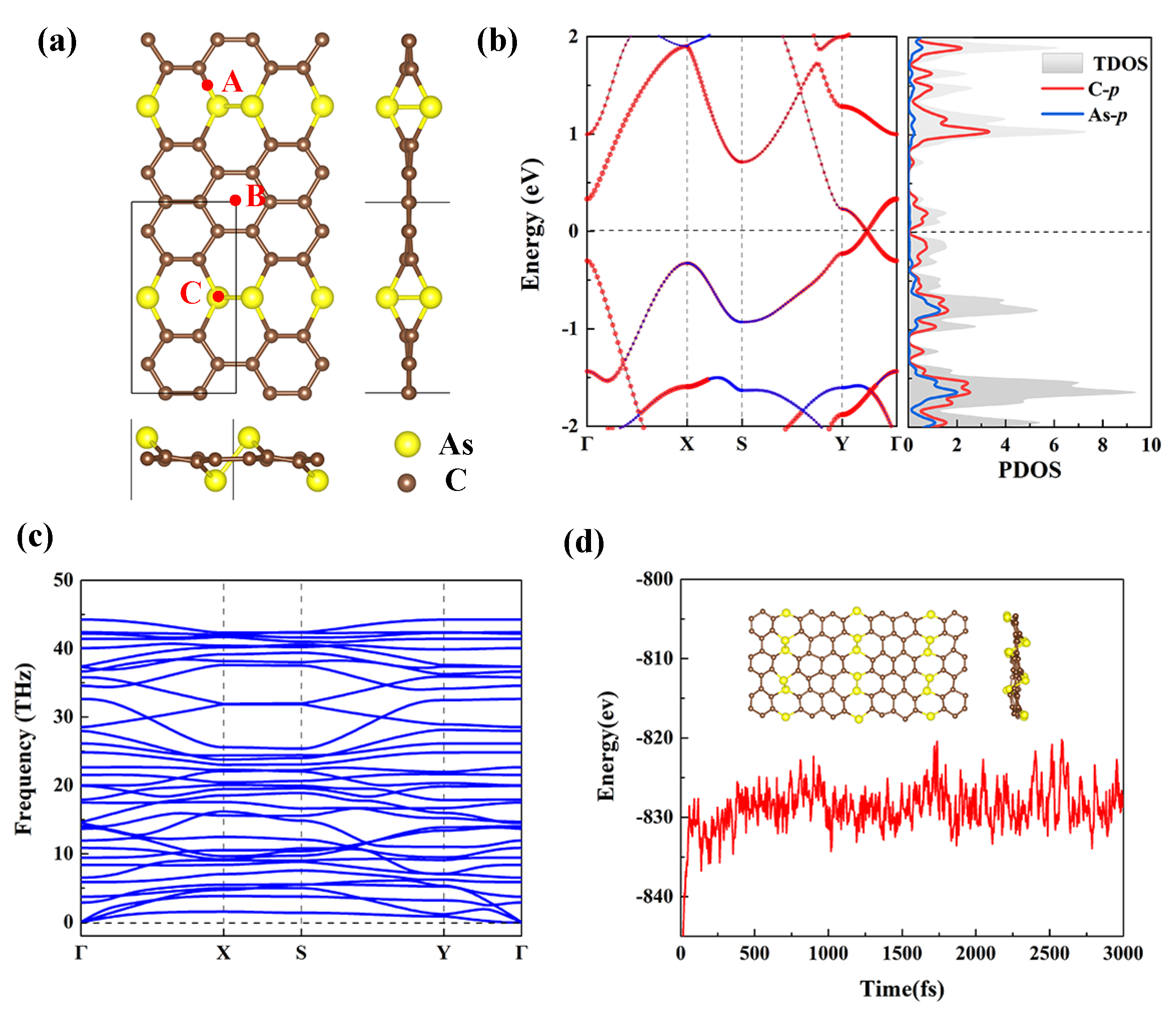
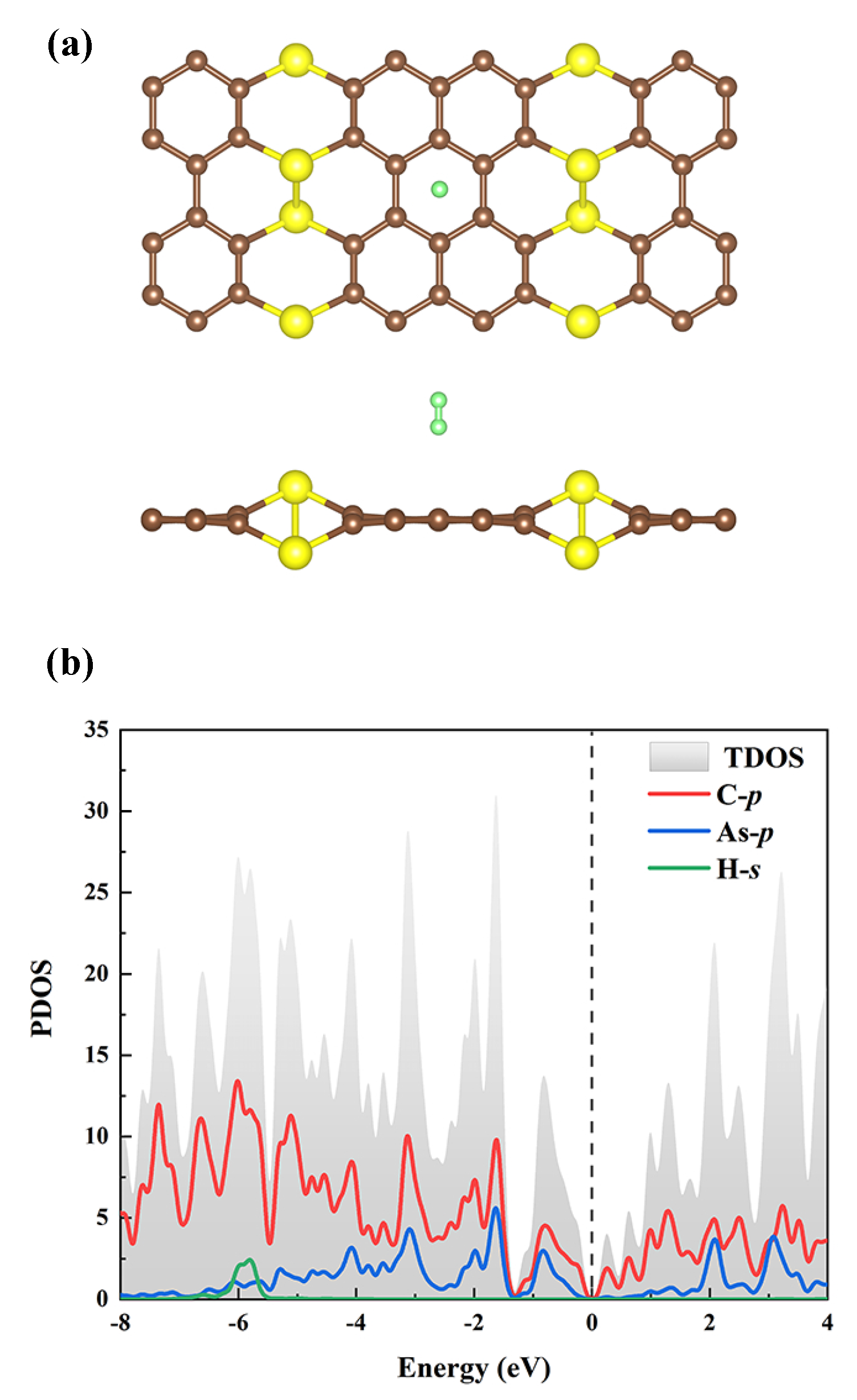
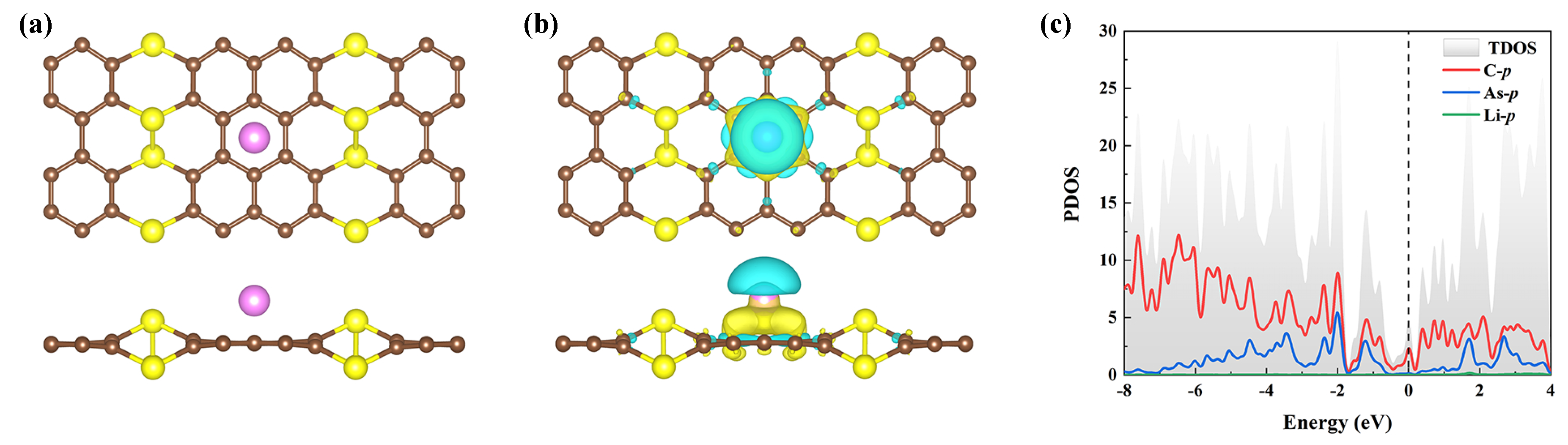
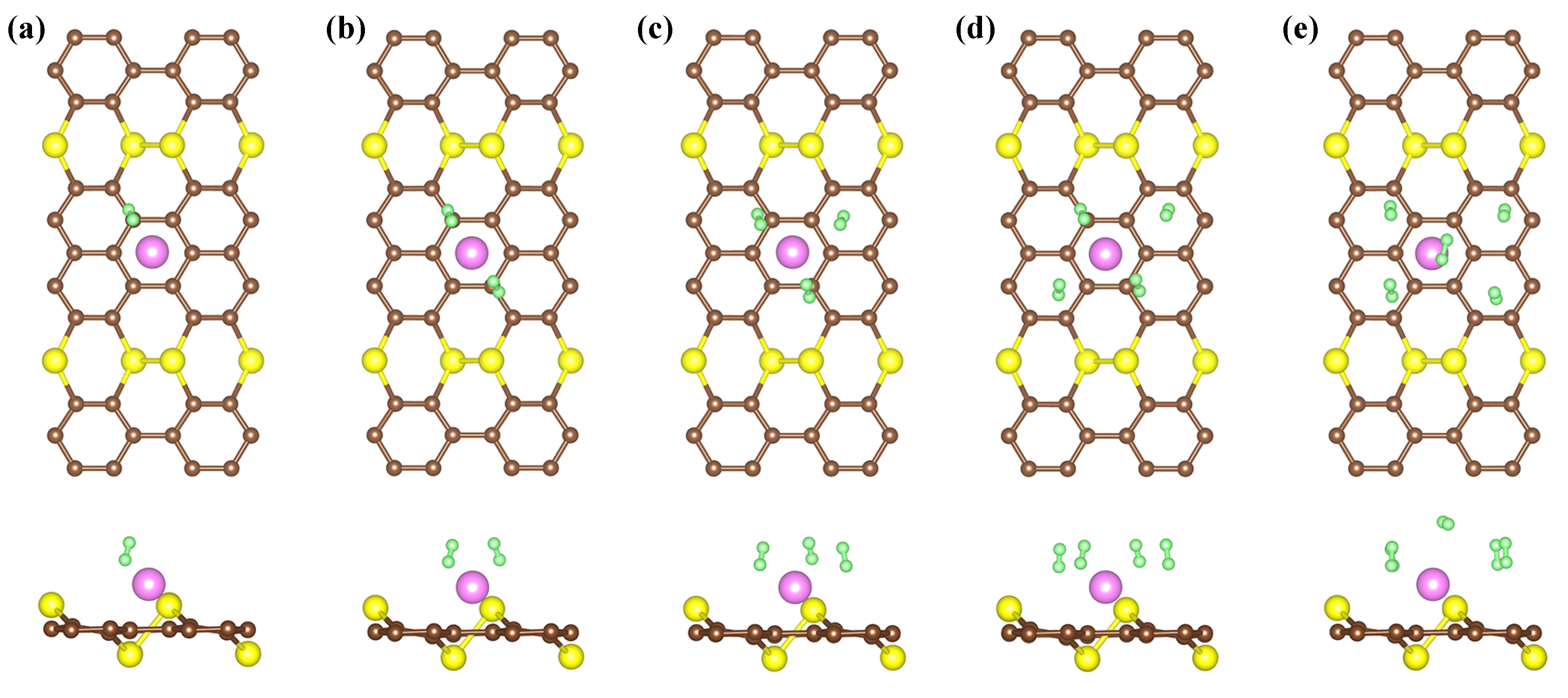
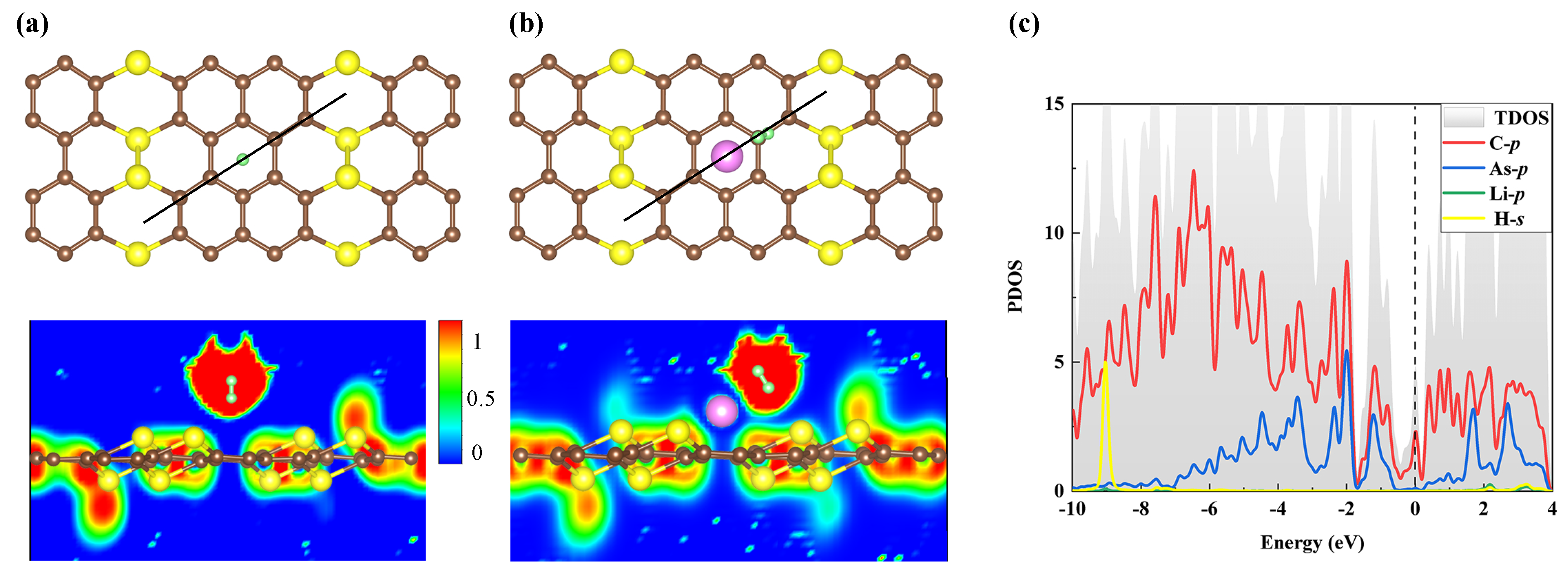
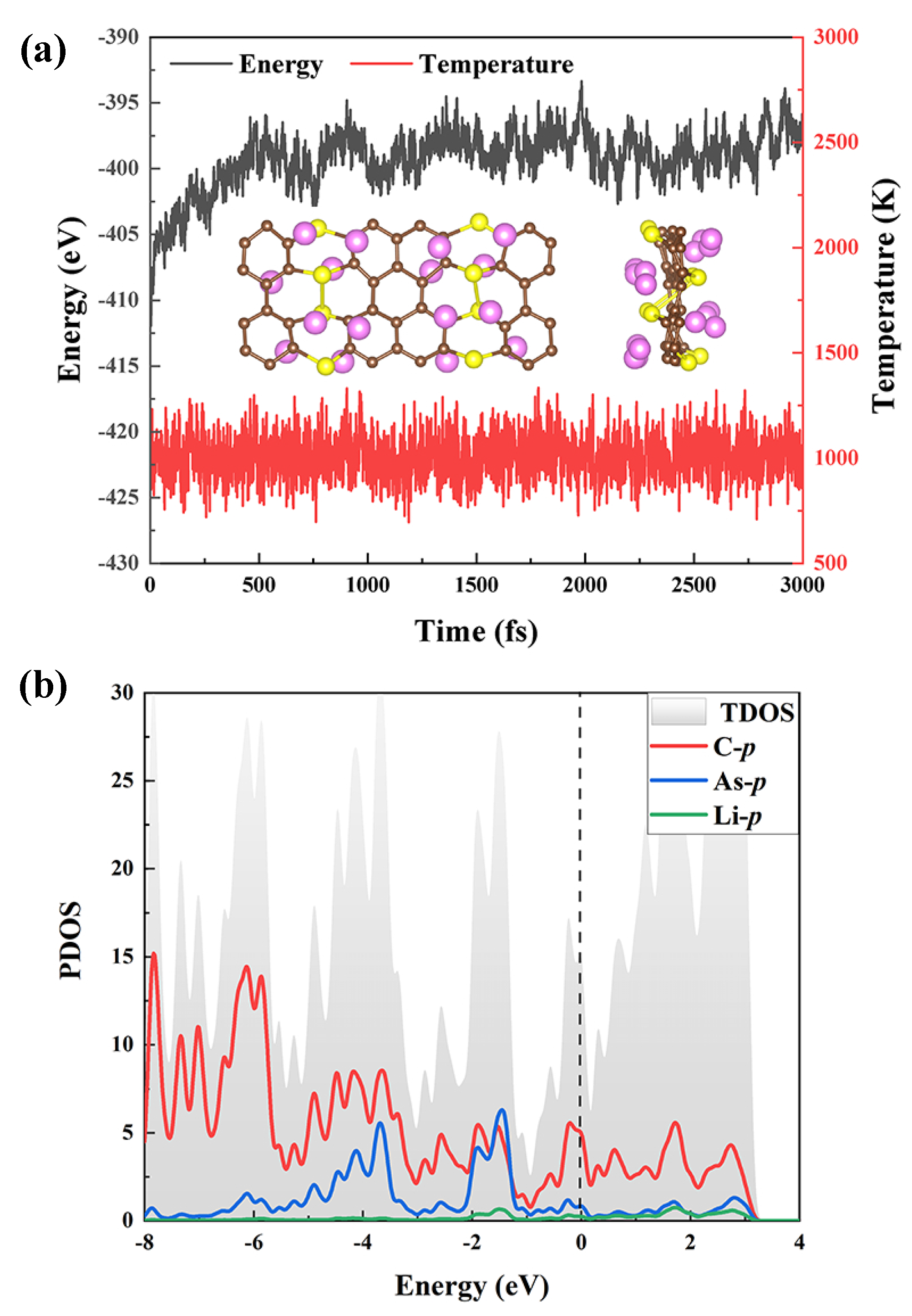
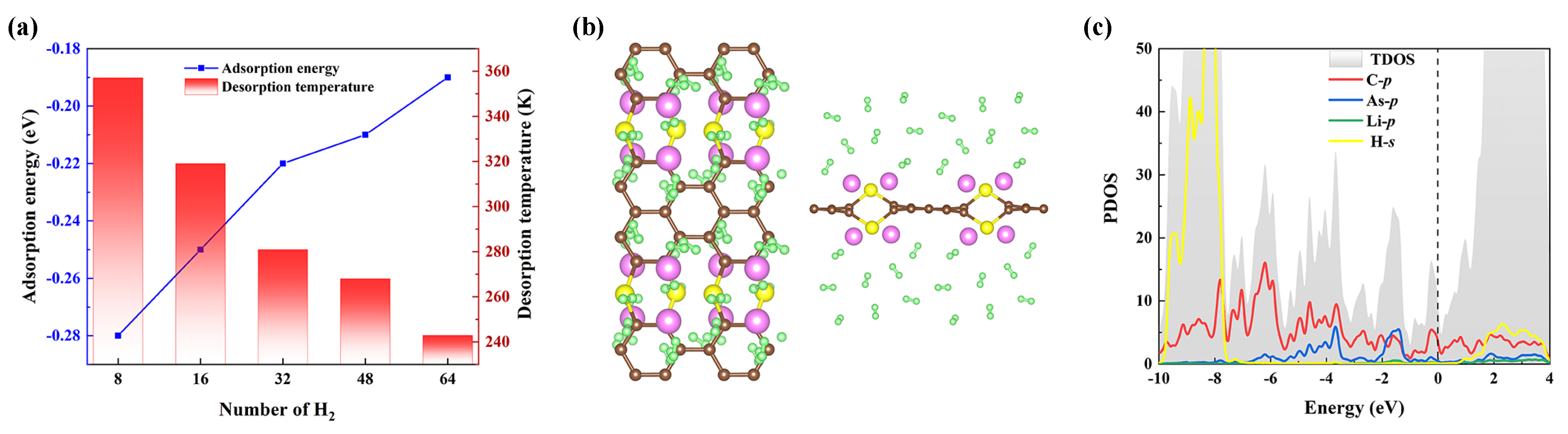
3. Results and Discussion
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jacobson, M.Z. Review of solutions to global warming, air pollution, and energy security. Energy Environ. Sci. 2009, 2, 148–173. [Google Scholar] [CrossRef]
- Qu, Y.; Duan, X. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef]
- Konda, R.; Deshmukh, A.; Kalamse, V.; Chaudhari, A. Functionalized tetrahedral silsesquioxane cages for hydrogen storage. Int. J. Hydrogen Energy 2020, 45, 32157–32167. [Google Scholar] [CrossRef]
- Sherif, S.A.; Barbir, F.; Veziroglu, T.N. Wind energy and the hydrogen economy—Review of the technology. Sol. Energy 2005, 78, 647–660. [Google Scholar] [CrossRef]
- Jacobson, M.Z.; Colella, W.G.; Golden, D.M. Cleaning the air and improving health with hydrogen fuel cell vehicles. Science 2005, 308, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Kittner, N.; Lill, F.; Kammen, D.M. Energy storage deployment and innovation for the clean energy transition. Nat. Energy 2017, 2, 17125. [Google Scholar] [CrossRef]
- Cheng, H.M.; Yang, Q.H.; Liu, C. Hydrogen storage in carbon nanotubes. Carbon 2001, 39, 1447–1454. [Google Scholar] [CrossRef]
- Glenk, G.; Reichelstein, S. Economics of converting renewable power to hydrogen. Nat. Energy 2019, 4, 216–222. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Colbe, J.M.B.V.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P. Materials for hydrogen-based energy storage-past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Midilli, A.; Ay, M.; Dincer, I.; Rosen, M.A. On hydrogen and hydrogen energy strategies I: Current status and needs. Renew. Sust. Energy Rev. 2005, 9, 255–271. [Google Scholar] [CrossRef]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997, 386, 377. [Google Scholar] [CrossRef]
- Sathe, R.Y.; Bae, H.; Lee, H.; Kumar, T.J.D. Hydrogen storage capacity of low-lying isomer of C24 functionalized with Ti. Int. J. Hydrogen Energy 2020, 45, 9936–9945. [Google Scholar] [CrossRef]
- Liu, Z.; Hussain, T.; Karton, A.; Er, S. Empowering hydrogen storage properties of haeckelite monolayers via metal atom functionalization. Appl. Surf. Sci. 2021, 556, 149709. [Google Scholar] [CrossRef]
- Panigrahi, P.; Desai, M.; Talari, M.K.; Bae, H.; Lee, H.; Ahuja, R. Selective decoration of nitrogenated holey graphene (C2N) with titanium clusters for enhanced hydrogen storage application. Int. J. Hydrogen Energy 2021, 46, 7371–7380. [Google Scholar] [CrossRef]
- Mou, Z.; Dong, Y.; Li, S.; Du, Y.; Wang, X.; Yang, P.; Wang, S. Eosin Y functionalized graphene for photocatalytic hydrogen production from water. Int. J. Hydrogen Energy 2011, 36, 8885–8893. [Google Scholar] [CrossRef]
- Zheng, S.; Fang, F.; Zhou, G.; Chen, G.; Ouyang, L.; Zhu, M.; Sun, D. Hydrogen storage properties of space-confined NaAlH4 nanoparticles in ordered mesoporous silica. Chem. Mater. 2008, 20, 3954–3958. [Google Scholar] [CrossRef]
- Jin, X.; Qi, P.; Yang, H.; Zhang, Y.; Li, J.; Chen, H. Enhanced hydrogen adsorption on Li-coated B12C6N6. J. Chem. Phys. 2016, 145, 164301. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, C.; Jiang, Q. Electric field induced enhancement of hydrogen storage capacity for Li atom decorated graphene with Stone-Wales defects. Int. J. Hydrogen Energy 2016, 41, 10776–10785. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Yu, L.; Pan, X.; Cao, X.; Hu, P.; Bao, X. Oxygen reduction reaction mechanism on nitrogen-doped graphene: A density functional theory study. J. Catal. 2011, 282, 183–190. [Google Scholar] [CrossRef]
- Dai, C.L.; Sun, G.Q.; Hu, L.Y.; Xiao, Y.K.; Zhang, Z.P.; Qu, L.T. Recent progress in graphene-based electrodes for flexible batteries. InfoMat 2020, 2, 509–526. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, N.; Li, J.; Liu, E.; He, C.; Shi, C. Hydrogen spillover storage on Ca-decorated graphene. Int. J. Hydrogen Energy 2012, 37, 11835–11841. [Google Scholar] [CrossRef]
- Jian, N.; Xue, P.; Diao, D. Thermally induced atomic and electronic structure evolution in nanostructured carbon film by in situ TEM/EELS analysis. Appl. Surf. Sci. 2019, 498, 143831. [Google Scholar] [CrossRef]
- Ye, Y.; Dai, L. Graphene-based Schottky junction solar cells. J. Mater. Chem. 2012, 22, 24224–24229. [Google Scholar] [CrossRef]
- Yin, Z.; Zhu, J.; He, Q.; Cao, X.; Tan, C.; Chen, H.; Yan, Q.; Hua, Z. Graphene-Based Materials for Solar Cell Applications. Adv. Energy Mater. 2014, 4, 1300574. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, L.L.; Cui, W.B.; Zhang, S.F.; Gong, W.J. Ab initio investigation of physical properties of the graphene/As-F hetero-bilayer. Appl. Surf. Sci. 2021, 563, 150339. [Google Scholar] [CrossRef]
- Zhang, H.P.; Luo, X.G.; Lin, X.Y.; Lu, X.; Leng, Y. Density functional theory calculations of hydrogen adsorption on Ti-, Zn-, Zr-, Al-, and N-doped and intrinsic graphene sheets. Int. J. Hydrogen Energy 2013, 38, 14269–14275. [Google Scholar] [CrossRef]
- Labroussea, J.; Belasfar, K.; Aziz, O.; Kenz, A.E.; Benyoussef, A. Two-dimensional GeC3: A reversible, high-capacity hydrogen molecule storage material predicted by first-principles calculations. Surf. Interfaces 2022, 31, 101984. [Google Scholar] [CrossRef]
- Sosa, A.N.; Cid, B.J.; Miranda, L. Light metal functionalized two-dimensional siligene for high capacity hydrogen storage: DFT study. Int. J. Hydrogen Energy 2021, 46, 29348–29360. [Google Scholar] [CrossRef]
- Varunaa, R.; Ponniah, R. Potential hydrogen storage materials from metal decorated 2D-C2N: An ab-initio study. Phys. Chem. Chem. Phys. 2019, 21, 25311–25322. [Google Scholar] [CrossRef]
- Chan, K.T.; Neaton, J.B.; Cohen, M.L. First-principles study of metal adatom adsorption on graphene. Phy. Rev. B 2008, 77, 235430. [Google Scholar] [CrossRef]
- Yang, S.L.; Wang, X.T.; Lei, G.; Xu, H.X.; Wang, Z.; Xiong, J.; Gu, H.S. A DFT study on the outstanding hydrogen storage performance of the Ti-decorated MoS2 monolayer. Surf. Interfaces 2021, 26, 101329. [Google Scholar] [CrossRef]
- Yamamoto, H.; Miyaoka, H.; Hino, S.; Nakanishi, H. Recyclable hydrogen storage system composed of ammonia and alkali metal hydride. Int. J. Hydrogen Energy 2009, 34, 9760–9764. [Google Scholar] [CrossRef]
- Zuttel, A.; Borgschulte, A.; Orimo, S.I. Tetrahydroborates as new hydrogen storage materials. Scr. Mater. 2007, 56, 823–828. [Google Scholar] [CrossRef]
- Cao, H.J.; Richter, T.M.M.; Pistidda, C.; Chaudhary, A.L. Ternary Amides Containing Transition Metals for Hydrogen Storage: A Case Study with Alkali Metal Amidozincates. ChemSusChem 2015, 8, 3777–3782. [Google Scholar] [CrossRef] [PubMed]
- Seenithurai, S.; Pandyan, R.K.; Kumar, S.V.; Saranya, C.; Mahendran, M. Li-decorated double vacancy graphene for hydrogen storage application: A first principles study. Int. J. Hydrogen Energy 2014, 39, 11016. [Google Scholar] [CrossRef]
- Chen, Y.D.; Yu, S.; Zhao, W.H. A potential material for hydrogen storage: A Li decorated graphitic-CN monolayer. Phys. Chem. Chem. Phys. 2018, 20, 13473. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Liu, S.; Er, S. Hydrogen storage properties of Li-decorated B2S monolayers: A DFT study. Int. J. Hydrogen Energy 2019, 44, 16803–16810. [Google Scholar] [CrossRef]
- Wang, Y.; Lü, J.; Zhu, L.; Ma, Y. Crystal Structure Prediction Via Particle-Swarm Optimization. Phys. Rev. B 2010, 82, 94116. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, J.; Zhu, L.; Ma, Y. CALYPSO: A Method for Crystal Structure Prediction. Comput. Phys. Commun. 2012, 183, 2063. [Google Scholar] [CrossRef]
- Benzidi, H.; Lakhal, M.; Garara, M.; Mounkachi, O. Arsenene monolayer as an outstanding anode material for (Li/Na/Mg)-ion batteries: Density functional theory. Phys. Chem. Chem. Phys. 2019, 21, 19951. [Google Scholar] [CrossRef]
- Ding, W.; Zhu, J.; Wang, Z.; Gao, Y.; Xiao, D.; Zhu, W. Prediction of Intrinsic Two-Dimensional Ferroelectrics inIn2Se3 and Other III2-VI3 van Der Waals Materials. Nat. Commun. 2017, 8, 14956. [Google Scholar] [CrossRef]
- Wei, D.; Lin, Z.G.; Hu, C.W. Two-step fabrication of a porous γ-In2Se3 tetragonal photocatalyst for water splitting. Chem. Comm. 2013, 49, 9609–9611. [Google Scholar] [CrossRef] [PubMed]
- Sim, Y.; Kim, J.; Seong, M.J. Simple synthesis of ultra-high quality In2S3 thin films on InAs substrates. J. Alloys Compd. 2016, 685, 518–522. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2010, 27, 1787–1799. [Google Scholar] [CrossRef]
- Ambrosetti, A.; Ferri, N.; Distasio, R.A. Wavelike charge density fluctuations and van der Waals interactions at the nanoscale. Science 2016, 351, 1171–1176. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Nose, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Z.; Li, Y. Atomically Thin Arsenene and Antimonene: Semimetal-Semiconductor and Indirect-Direct Band-Gap Transitions. Angew. Chem. 2015, 127, 3112–3115. [Google Scholar] [CrossRef] [PubMed]
- Rajput, K.; He, J.; Frauenheim, T.; Roy, D.R. Monolayer PC3: A promising material for environmentally toxic nitrogen-containing multi gases. J. Hazard 2022, 422, 126761. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xu, S.; Ma, K.; Wu, C.; Ding, X. Geometric structures, electronic characteristics, stabilities, catalytic activities, and descriptors of graphene-based single-atom catalysts. Nano Mater. Sci. 2020, 2, 120–131. [Google Scholar] [CrossRef]
- Tavhare, P.; Titus, E.; Chaudhari, A. Boron substitution effect on adsorption of H2 molecules on organometallic complexes. Int. J. Hydrogen Energy 2019, 44, 345–353. [Google Scholar] [CrossRef]
| E ( eV) | h (Å) | l (Å) | E ( eV) | Q | |
|---|---|---|---|---|---|
| A | −0.037 | 2.30 | 0.75 | −2.33 | 0.57 |
| B | −0.043 | 1.71 | 0.75 | −2.36 | 0.77 |
| C | −0.020 | 2.52 | 0.75 | −2.34 | 0.63 |
| n | d (Å) | l (Å) | E | E |
|---|---|---|---|---|
| 1 | 2.04 | 0.76 | −0.31 | 0.31 |
| 2 | 2.07 | 0.76 | −0.29 | 0.29 |
| 3 | 2.49 | 0.76 | −0.28 | 0.24 |
| 4 | 2.43 | 0.76 | −0.27 | 0.23 |
| 5 | 2.70 | 0.75 | −0.24 | 0.12 |
| Structure | wt% |
|---|---|
| B@r57-Li4 | 10 |
| Li-decorated T4,4,4-graphyne | 10.46 |
| Ti-decorated MoS | 5.93 |
| Pd-decorated SiBN | 6 |
| Ti-decorated graphene | 7.8 |
| GeC | 7.25 |
| Li-decorated BeC | 10.21 |
| Li-decorated AsC (This work) | 9.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Zhang, B.; Zhang, L.; Zhu, Y.; Gong, W. Monolayer AsC5 as the Promising Hydrogen Storage Material for Clean Energy Applications. Nanomaterials 2023, 13, 1553. https://doi.org/10.3390/nano13091553
Lu Q, Zhang B, Zhang L, Zhu Y, Gong W. Monolayer AsC5 as the Promising Hydrogen Storage Material for Clean Energy Applications. Nanomaterials. 2023; 13(9):1553. https://doi.org/10.3390/nano13091553
Chicago/Turabian StyleLu, Qiang, Binyuan Zhang, Lianlian Zhang, Yulian Zhu, and Weijiang Gong. 2023. "Monolayer AsC5 as the Promising Hydrogen Storage Material for Clean Energy Applications" Nanomaterials 13, no. 9: 1553. https://doi.org/10.3390/nano13091553
APA StyleLu, Q., Zhang, B., Zhang, L., Zhu, Y., & Gong, W. (2023). Monolayer AsC5 as the Promising Hydrogen Storage Material for Clean Energy Applications. Nanomaterials, 13(9), 1553. https://doi.org/10.3390/nano13091553






