2.2. Materials Characterization
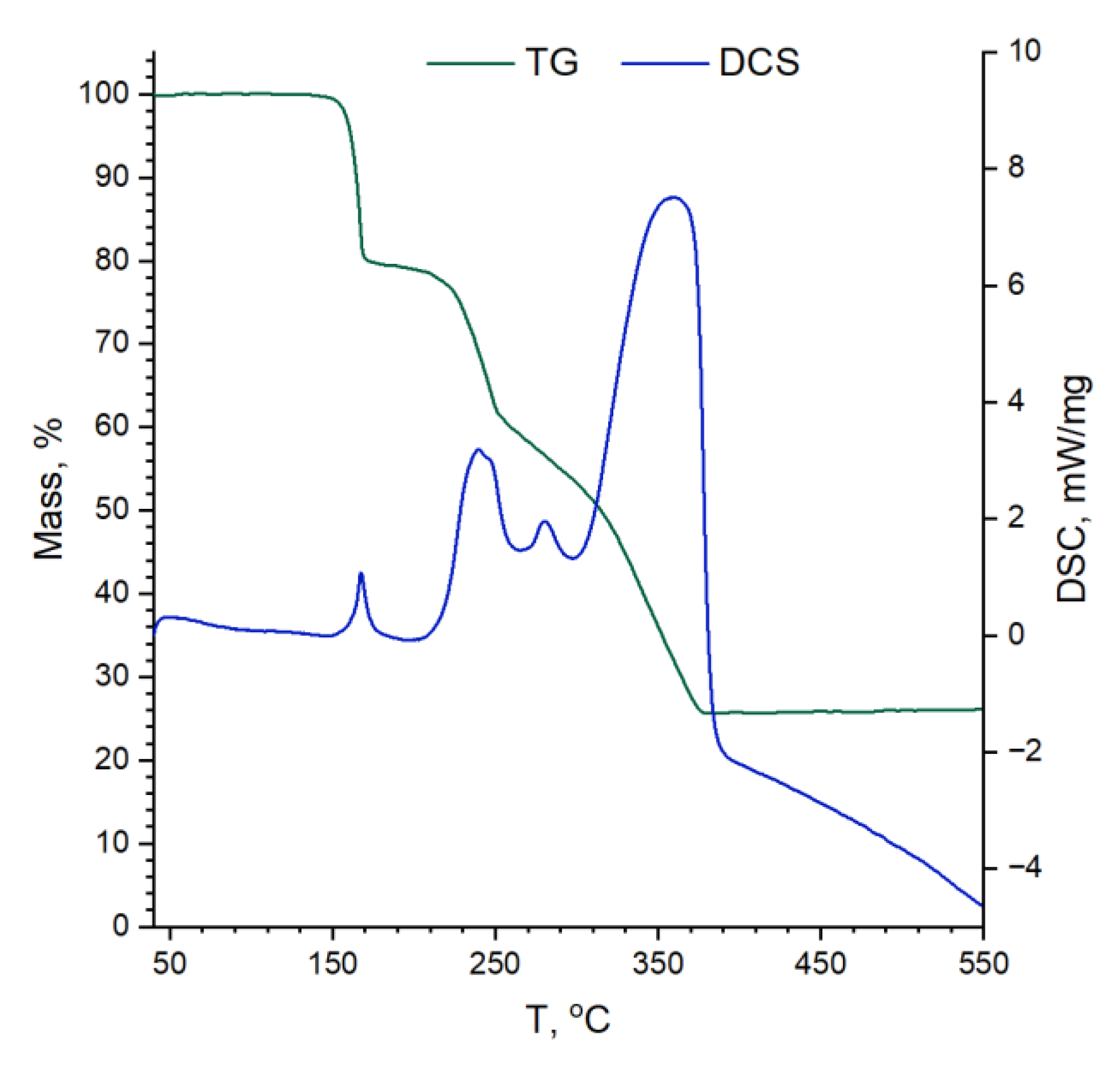
The thermal stability of manganese (III) acetylacetonate used for materials preparation was studied by thermogravimetry (TG) combined with differential scanning calorimetry (DSC) and mass-spectrometry (MS) using NETZSCH STA 409 PC thermobalance with NETZSCH QMS 403C MS system (NETZSCH-Gerätebau GmbH, Selb, Germany). Samples were heated in a corundum crucible from RT to 550 °C (5 °C/min) in the airflow (30 mL/min).
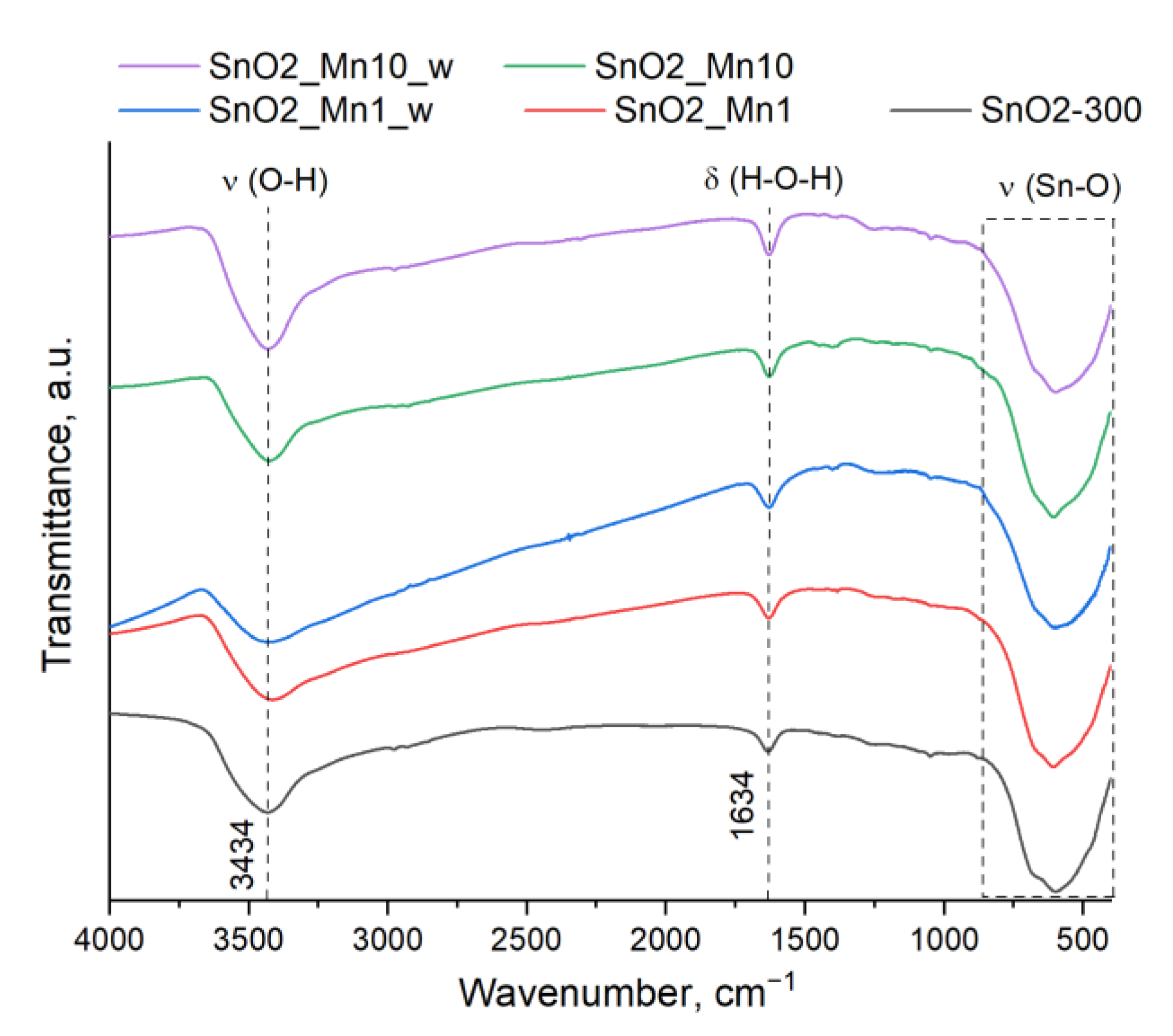
Fourier transforms infrared (FTIR) spectroscopy measurements were performed to check the completeness of Mn(acac)3 thermolysis on Frontier (Perkin Elmer Inc., Beaconsfield, UK) spectrometer in the transmission mode in the wavenumber range of 400–4000 cm−1 with a step of 1 cm−1. Samples (about 5 mg) were ground with 100 mg of potassium bromide (Aldrich, for FTIR analysis) and pressed into tablets 6 mm in diameter.
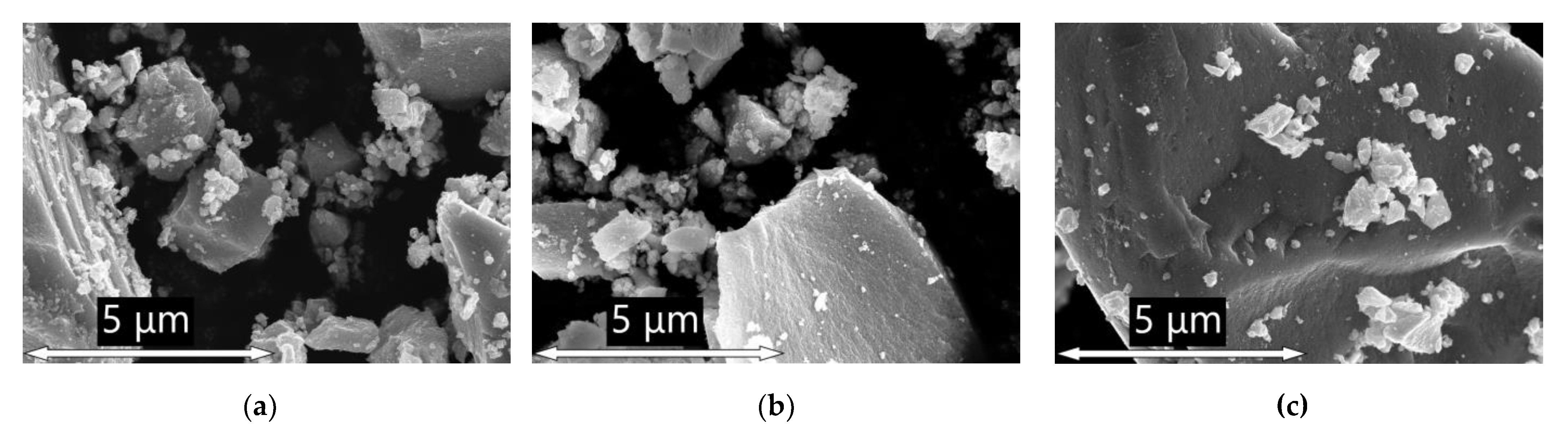
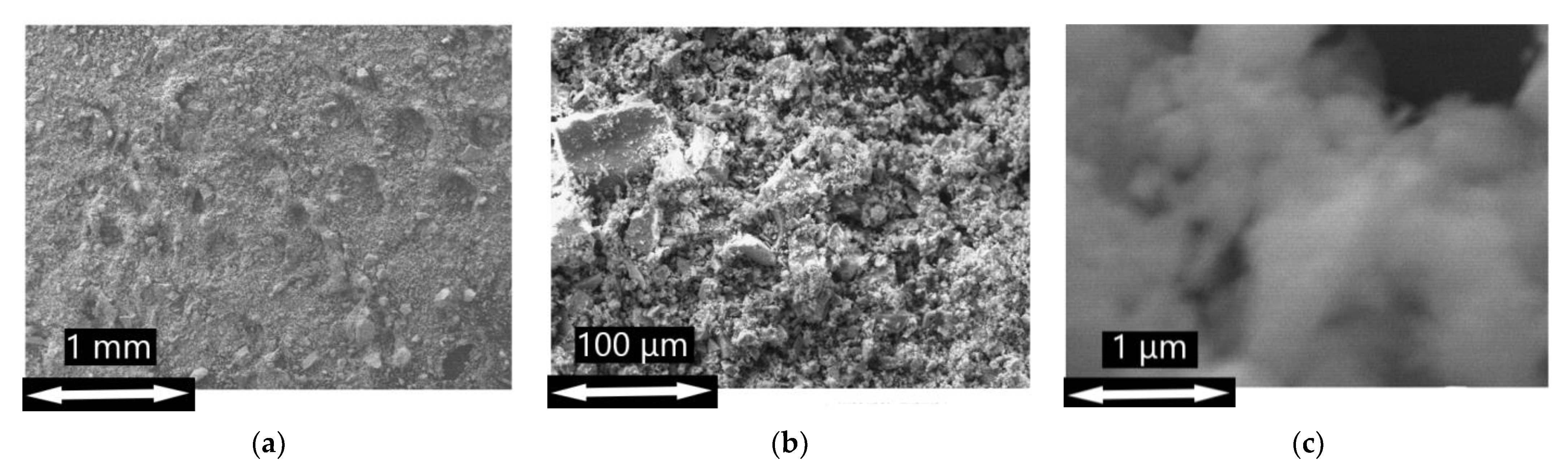
The Microstructure and morphology of prepared materials were investigated by scanning electron microscopy (SEM) with Zeiss Supra 40 FE-SEM microscope (Carl Zeiss, Inc., Oberkochen, Germany) with an in-lens detector. The accelerating voltage was set to 10 kV, and the aperture size was 30 microns.
The specific surface area (S
surf) of nanocrystalline oxides was measured by low-temperature nitrogen adsorption using a Chemisorb 2750 instrument (Micromeritics, Norcross, GA, USA). The surface area is available for adsorption was calculated using the BET model (Brunauer, Emmett, Teller). The size of aggregates whose surface is available for gas adsorption (d
BET) was calculated for each material in the approach of uniform spherical shape:
where ρ is the density of material (accepted equal to the blank SnO
2—6.95 g/cm
3).
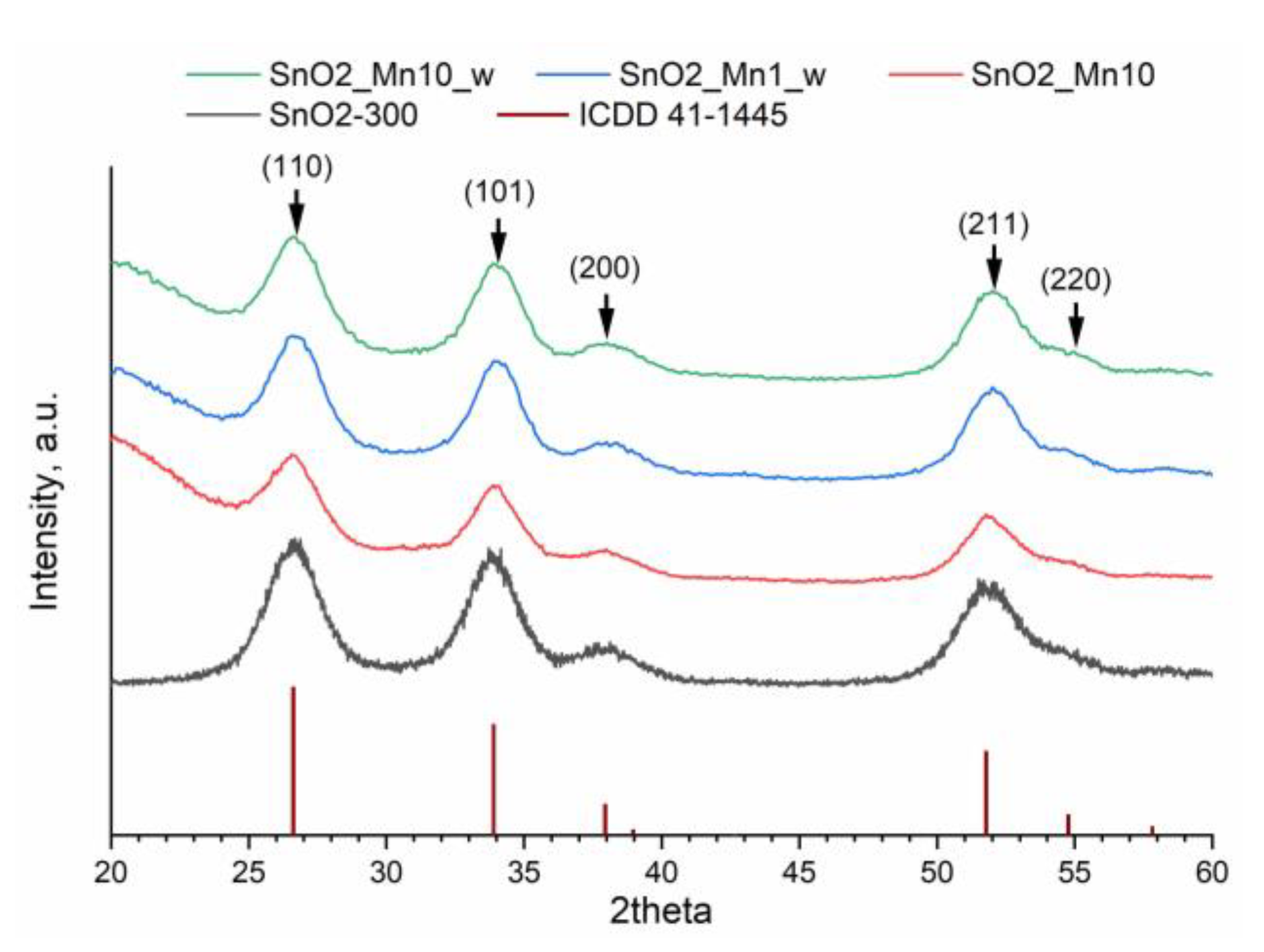
The phase composition of the obtained materials was investigated by X-ray diffraction (XRD) with a DRON-4-07 (Burevestnik, St. Petersburg, Russia) and Rigaku D/MAX 2500 (Rigaku, Japan, Tokyo) diffractometers equipped with CuKα (λ = 1.5406 Ǻ) radiation source. Obtained diffraction patterns were processed using the STOE WinXPOW software (v. 1.06). The ICDD PDF2 database was used to identify the crystal phases. The SnO2 crystalline grain size (dXRD) was calculated using the Scherrer formula for (110), (101) and (111) diffraction maxima.
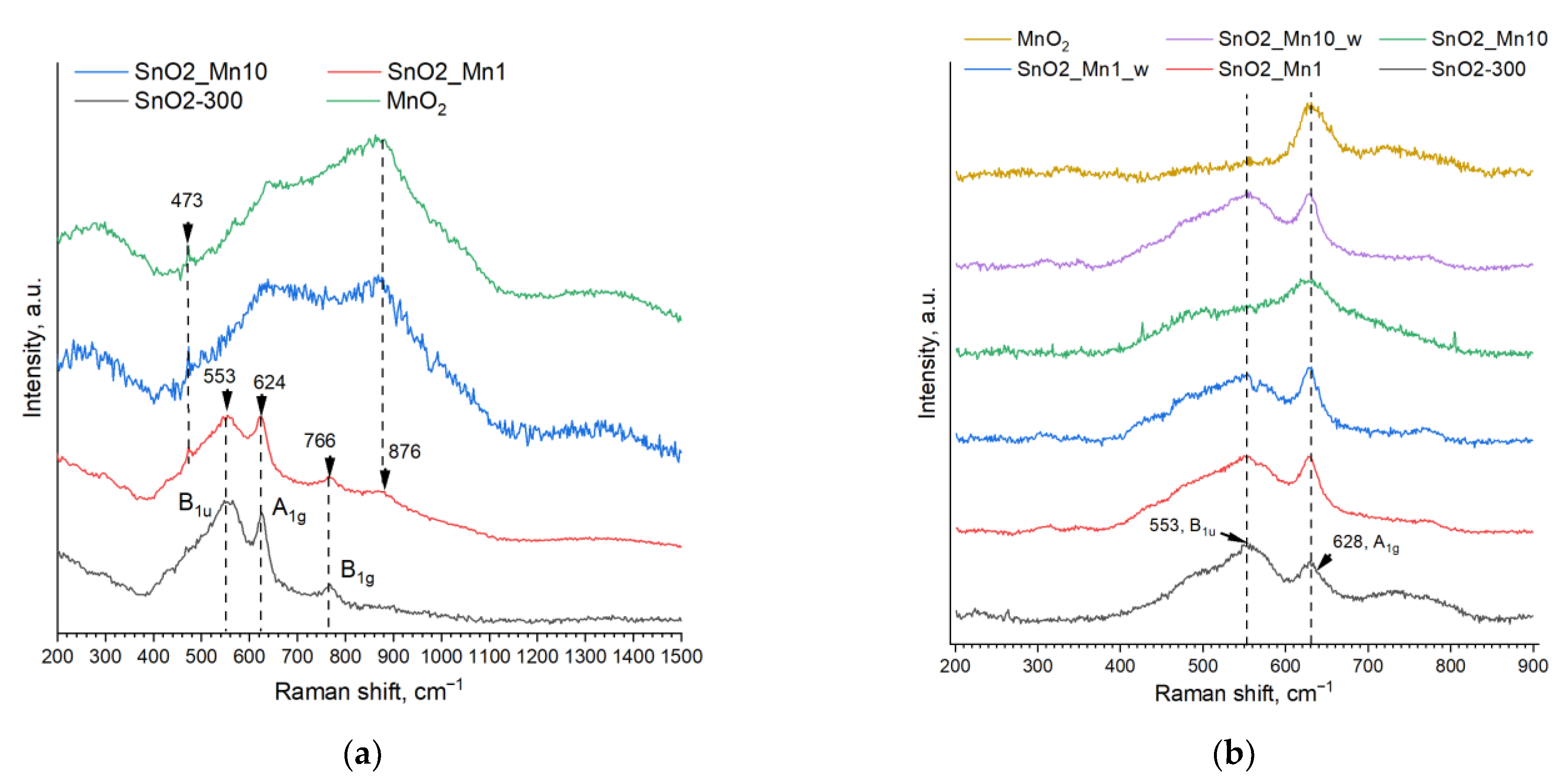
To clarify the manganese state in SnO2/MnOx materials, the phase composition and crystal structure of the synthesized samples were also studied by Raman spectroscopy. The studies were carried out without specific sample preparation on the i-Raman Plus spectrometer (B&W Tek, Plainsboro, NJ, USA) equipped with a BAC 151C microscope and 532 nm laser and Horiba LabRam Evolution Raman spectrometer (Horiba Ltd., Minami-ku, Kyoto, Japan) equipped with a single-mode CW-laser (633 nm, 170 mW) in the confocal microscope (Olympus, Shinjuku, Tokyo, Japan) scheme with a spatial amplification of ×50 and numeric aperture 0.25. During the measurements, only 25% of the maximum possible laser power was used. Spectra processing (baseline correction, peak fitting, and integration) was implemented with Origin Pro 2021 v. 9.8.0.200 software package.
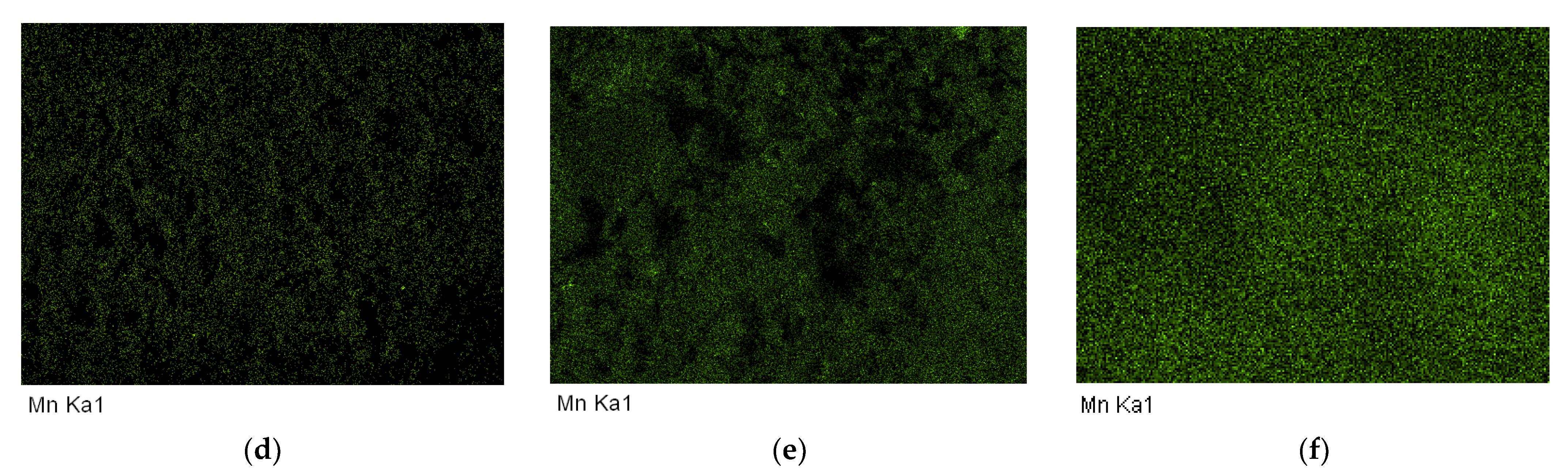
Elemental composition mapping was performed using NVision 40 microscope (Carl Zeiss, Oberkochen, Germany) with an energy dispersive X-ray analyzer (EDX) X-MAX (Oxford Instruments, Abingdon, Oxfordshire, UK) at an accelerating voltage of 20 kV and aperture of 60 microns.
Total reflection X-ray fluorescence analysis (TXRF) was used to determine the elemental composition of SnO2_Mn1 and SnO2_Mn10. Sample SnO2-300 was taken as a control. The analysis was carried out with Bruker S2 Picofox TXRF spectrometer (Bruker Corporation, Billerica, MA, USA). Special techniques of sample preparation were used. To determine the total manganese content, the sample (6 mg) was dispersed in 0.100 mL of polyvinyl alcohol 0.3 g/L solution, 0.300 mL deionized water and 0.100 mL standard Co2+ solution with a concentration of 100 mg/L. A 0.005 mL probe of stirred suspension was taken, placed on a quartz support and dried before the measurements. An attempt to study the manganese distribution between the SnO2 surface and lattice was also made: first, weighed material (12 mg) was treated with 1.00 mL of saturated (at RT) oxalic acid solution in an ultrasonic bath for 1 h at 70 °C with followed addition of 0.100 mL of HCl (conc.) and kept for one night at RT. Second, the 0.400 mL probe was taken from the supernatant solution, and 0.100 mL of standard (Co2+ solution, 100 mg/L) was added to the specimen. A dried 0.005 mL droplet of this mixture was used to determine the amount of manganese in the MnOx segregation state.
The remaining manganese content in SnO2_Mn1_w and SnO2_Mn10_w samples (which appeared to contain manganese only in the SnO2 crystal structure) was studied by inductively coupled plasma mass spectrometry (ICP MS) on a quadrupole ICP mass spectrometer (Agilent 7500c: Agilent Technologies, Santa Clara, CA, USA), which was controlled with a PC using the ChemStation (version G1834B) software package (Agilent Technologies, Santa Clara, CA, USA). Sample SnO2-300 was taken as a blank. Measurements were performed for the 55Mn isotope. Before analysis, pre-weighed powders (10 mg) were entirely dissolved in the mixture of HF (conc.) and HCl (conc.) at a ratio of 1:2, respectively, using a two-steps program in closed-type microwave system (Milestone ETHOS Advanced Microwave Labstation, Milestone Srl, Sorisole (BG), Italy) with temperature and pressure control options. The working frequency of the system was 2455 MHz, and the radiated power was 800 W. In the first stage, samples were heated from room temperature to 200 °C for 30 min. Then in the second stage, the reached temperature was kept for 30 min else. The resulting solutions were diluted to a volume of 6 mL by adding deionized water. After that, the 0.100 mL sample probes were diluted 100 times with deionized water. The ICP MS single element standards (Mn) were prepared from the standard solution (High-Purity Standards, Charleston, SC, USA) with a 10 mg/L concentration. The solution of the control sample was used to measure the background signal.
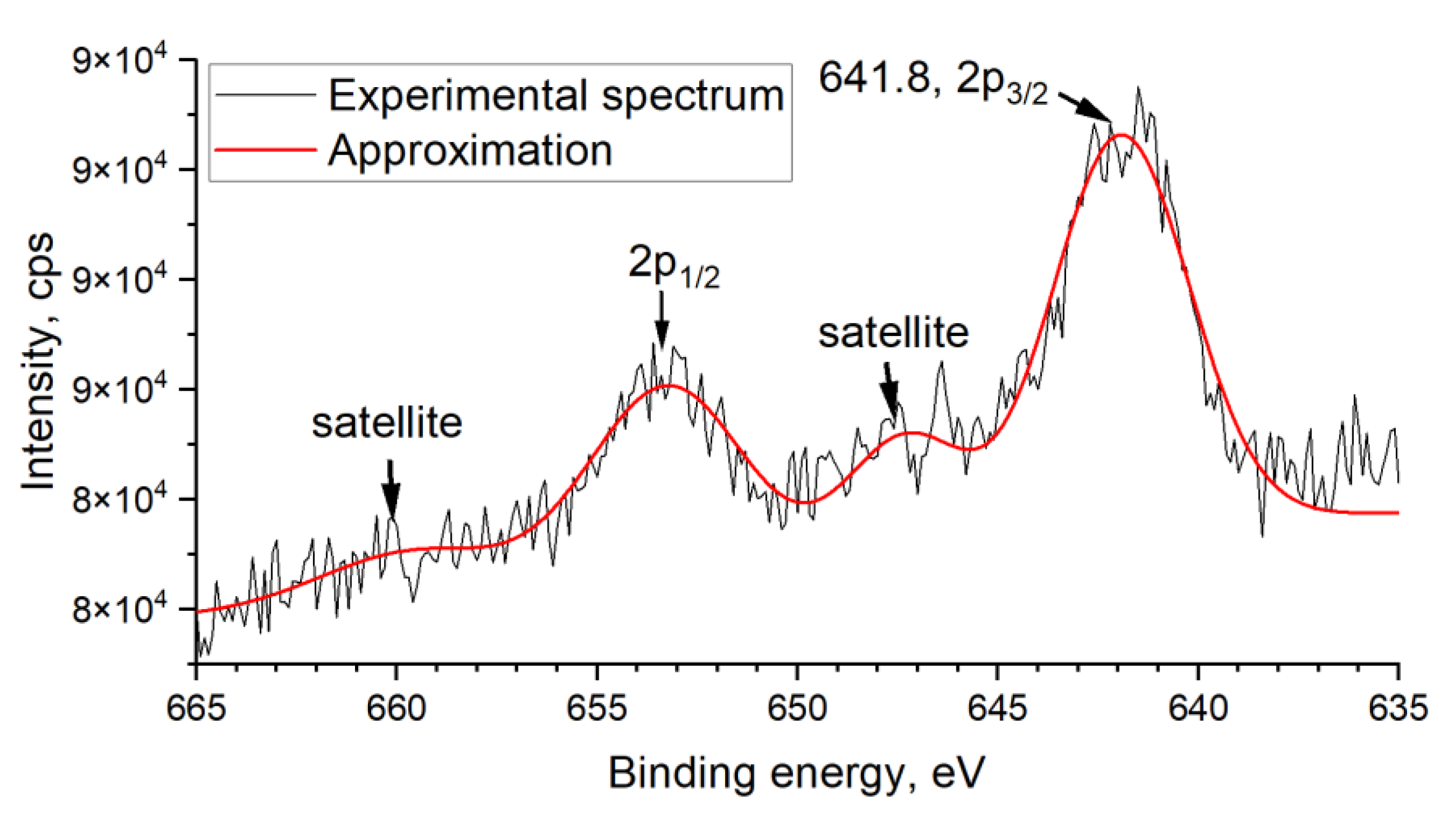
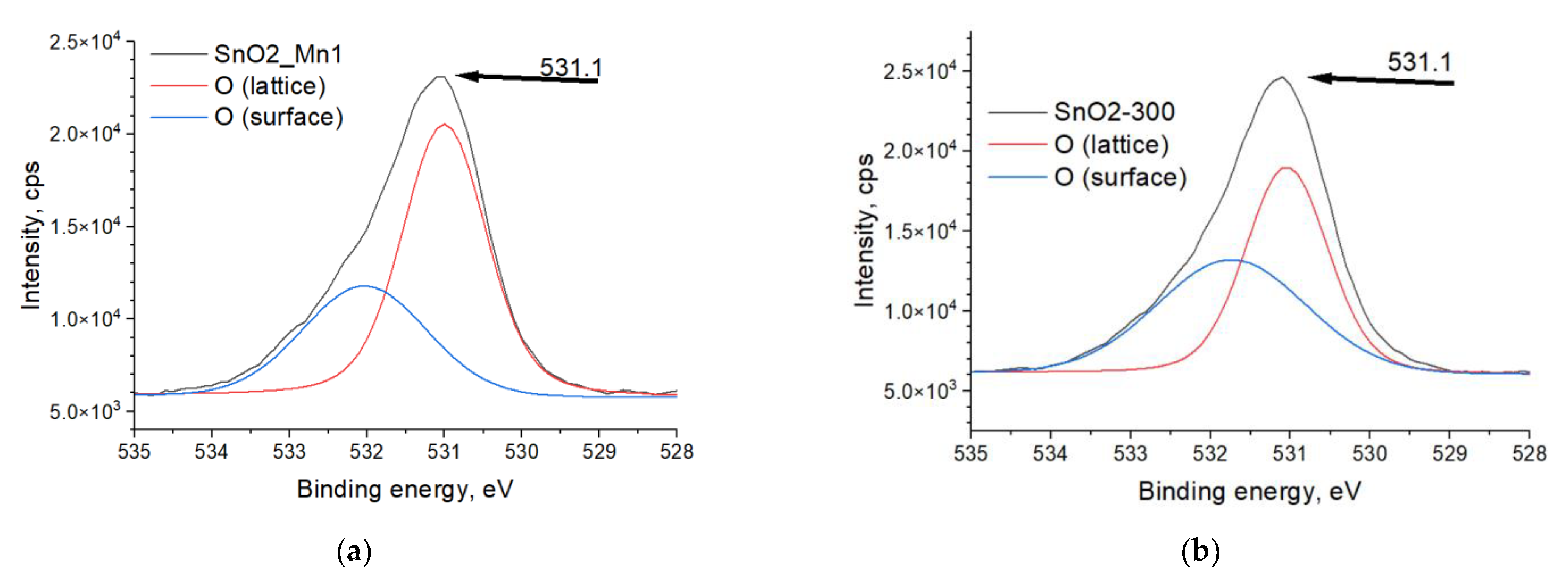
The chemical state of elements (Sn, O, Mn) in SnO2-300 and SnO2_Mn1 was studied by X-ray photoelectron spectroscopy (XPS) using Omicron ESCA+ (Scienta Omicron, Uppsala, Sweden) spectrometer with monochromatized aluminum anode Al Kα (1486.6 eV) with neutralizer. The binding energy step was 0.1 eV/s. Transmittance energy was 20 eV. Spectra of C 1s (trace carbon), O 1s, Sn 3d, Mn 3s, and Mn 2p were recorded. Unifit v. 2006 was used for spectra processing by approximating Gaussian and Lorentzian peak functions combination and background fitting. No special sample preparation procedure was used before analysis.
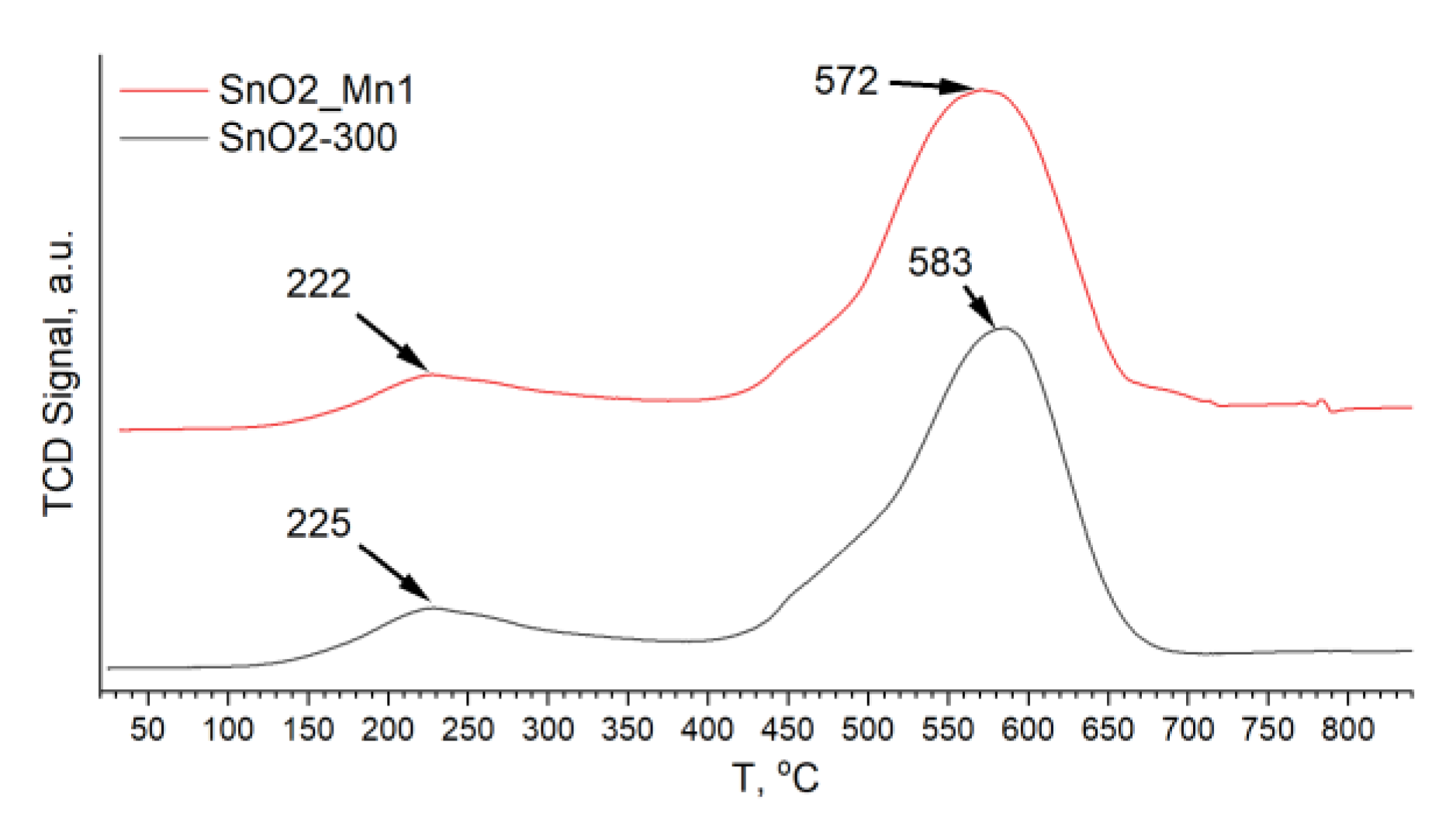
Oxidative active centers on the surface of prepared materials were studied by thermo-programmed reduction with hydrogen (TPR-H
2) on Chemisorb 2750 instrument (Micromeritics, Norcross, GA, USA) without specific sample preparation. Pre-weighed specimen (15–20 mg) was placed into a quartz flow test tube equipped with a thermocouple located in close proximity to the sample. The flow value 50 mL/min of Ar-H
2 mixture (8% vol. H
2) was set, and the temperature was raised by 10 °C/min till 800 °C. Hydrogen consumption during sample reduction was detected by a thermal conductivity detector (TCD) and recorded in arbitrary units. The volume of consumed hydrogen (V
H2) was calculated by the following equation:
The proportion quotient k = 0.28 mL/(g·a.u.) was determined by calibration measurements of a standard Ag2O sample with H2 absorption of 96.55 mL/g at normal conditions. The ideal gas approximation was used to recalculate the amount of consumed H2 at real temperature and pressure.
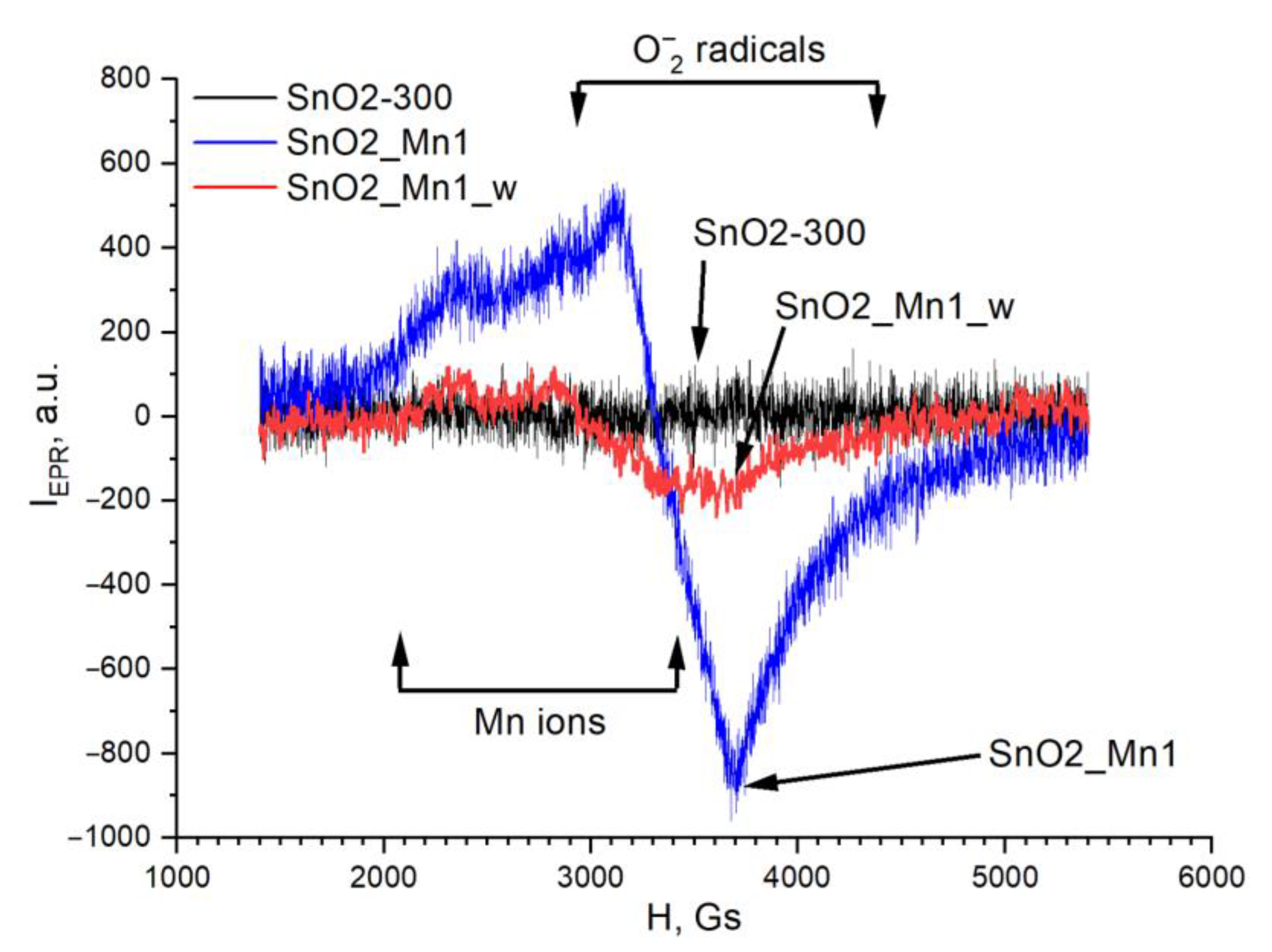
Paramagnetic centers on the surface of the synthesized materials were investigated by electron paramagnetic resonance spectroscopy (EPR) with Bruker ELEXSYS-580 spectrometer (Bruker Corporation, Billerica, MA, USA) at working frequency 9.5 GHz and apparatus sensitivity 5·1010 spin/Gs. A standard sample with Mn2+ ions was used to determine the g-factor values. A reference sample of CuCl2·2H2O was used to calculate the concentrations of paramagnetic centers in the material. Theoretical spectra simulated in Easyspin software were used to prove the material’s qualitative and quantitative composition of spin centers. Experimental spectra were obtained at 298 K. Samples were analysed and diluted 100 times with blank SnO2 (SnO2-300).
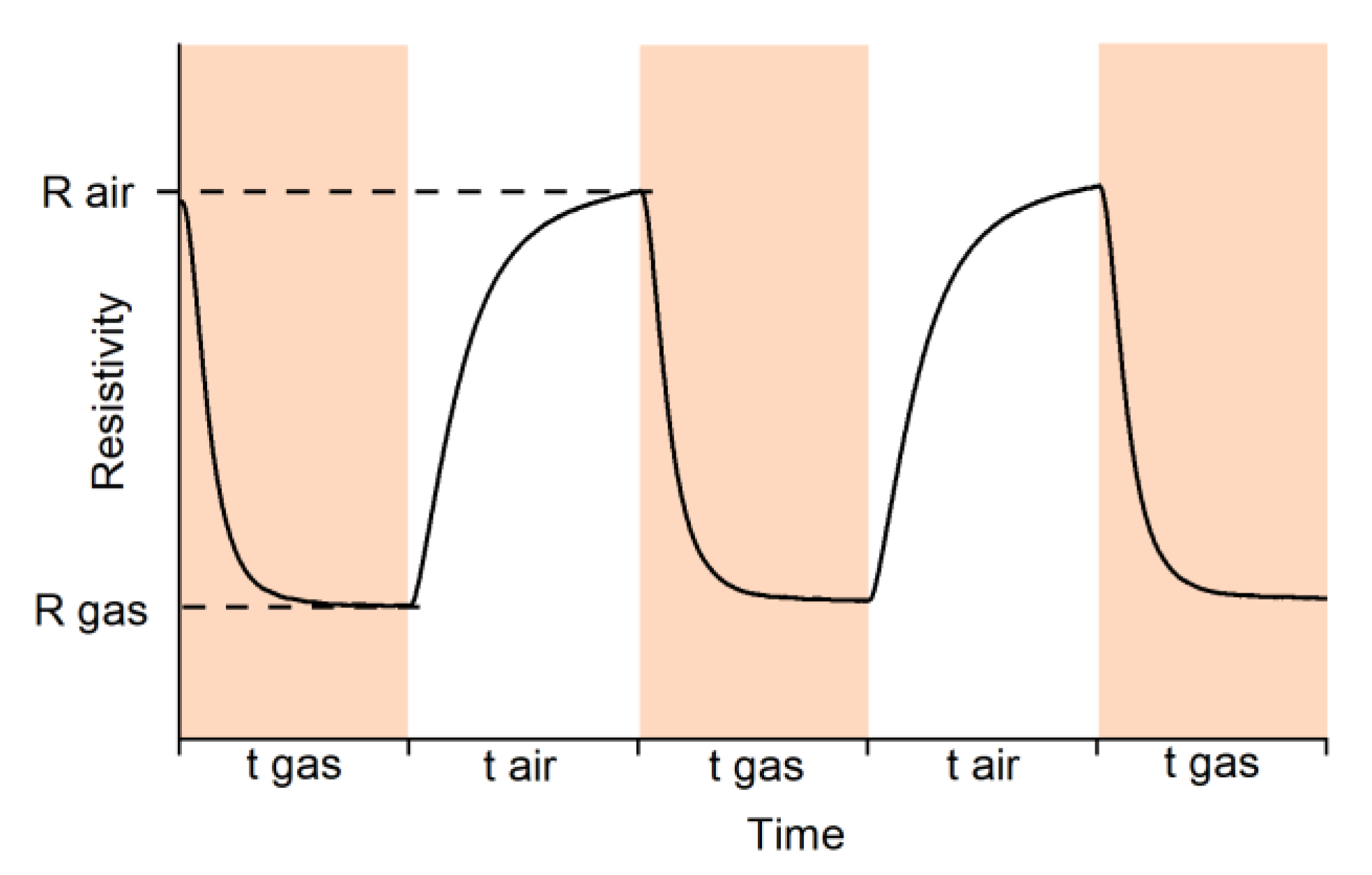
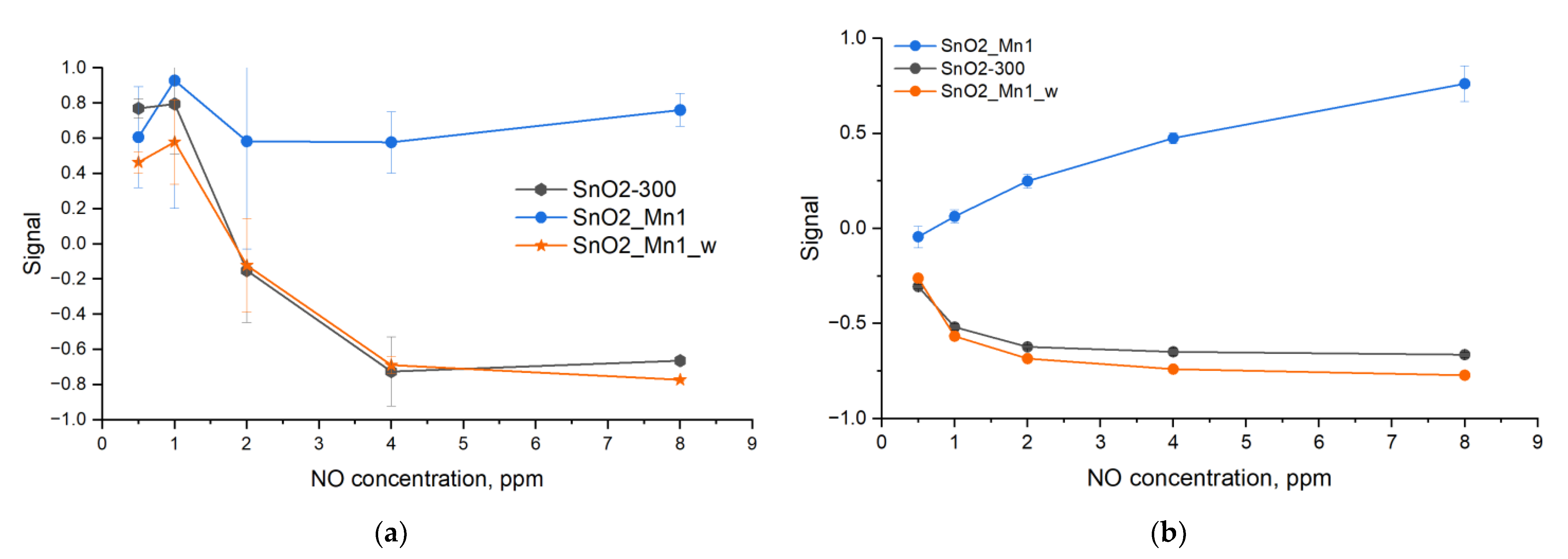
Gas sensor properties of prepared materials (excepting SnO2_Mn10_w) toward CO and NO were investigated by measuring the resistance of thick films in situ in a flow cell under a controlled gas flow of 100 ± 0.1 mL/min. The gas mixture for measurements was prepared by dilution of certified gas mixtures (CO 2530 ppm in N
2, NO 246 ppm in N
2) with dry synthetic air (relative humidity RH about 1%) using a pure air generator (GChV-1,2-3,5; Chimelectronika, Moscow, Russia) and RRG-12 electron mass-flow controllers (Eltochpribor, Zelenograd, Moscow, Russia). The concentrations of NO in gas mixtures were additionally verified with a Teledyne API N500 CAPS NOX Analyzer (Teledyne API, Inc., San Diego, CA, USA). The sensor signal values were calculated as follows:
where R
air is sample resistance in pure air, and R
gas is sample resistance in air, containing a preassigned concentration of target gas.
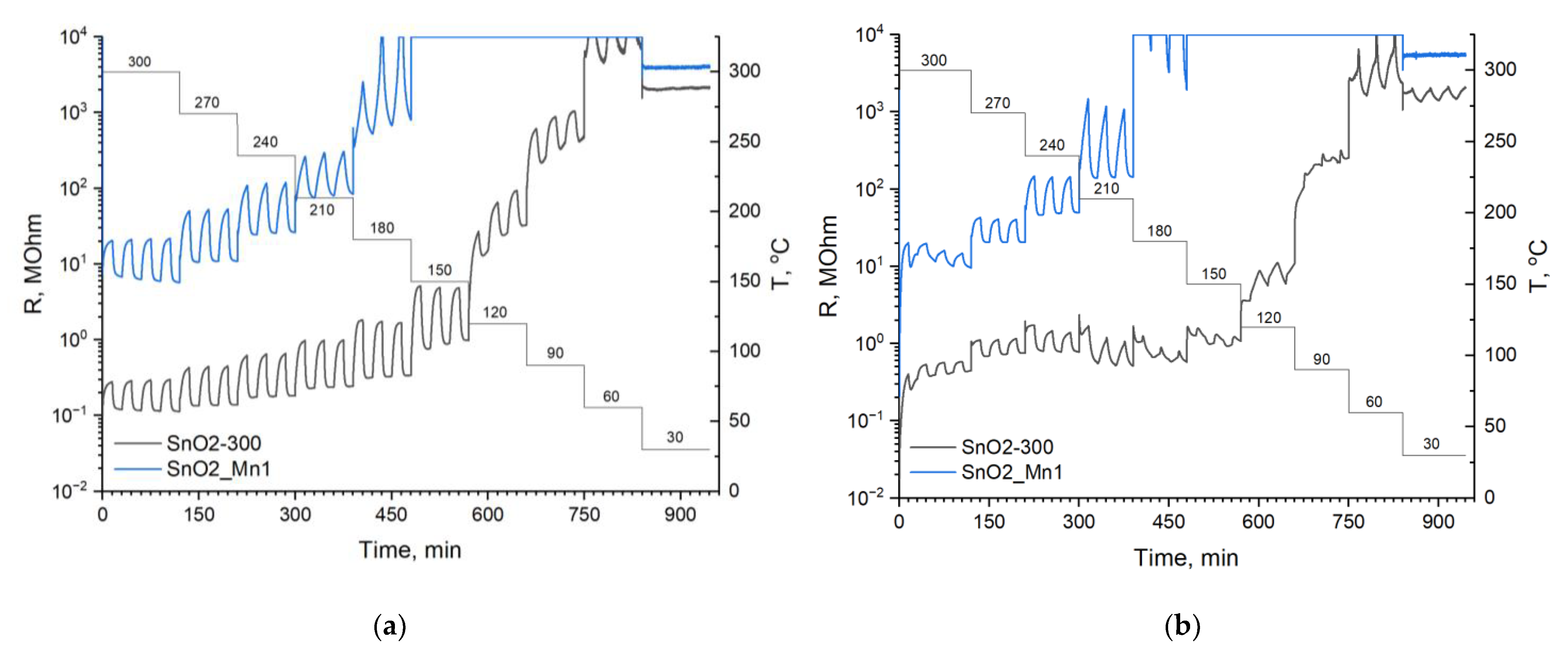
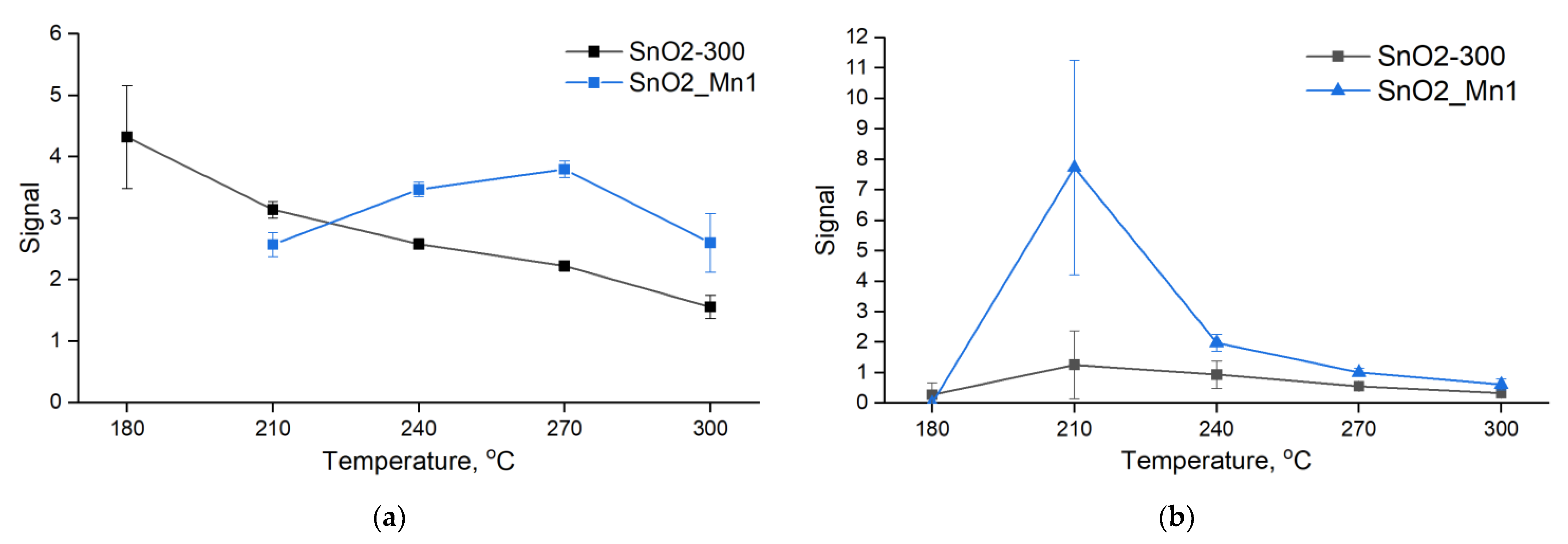
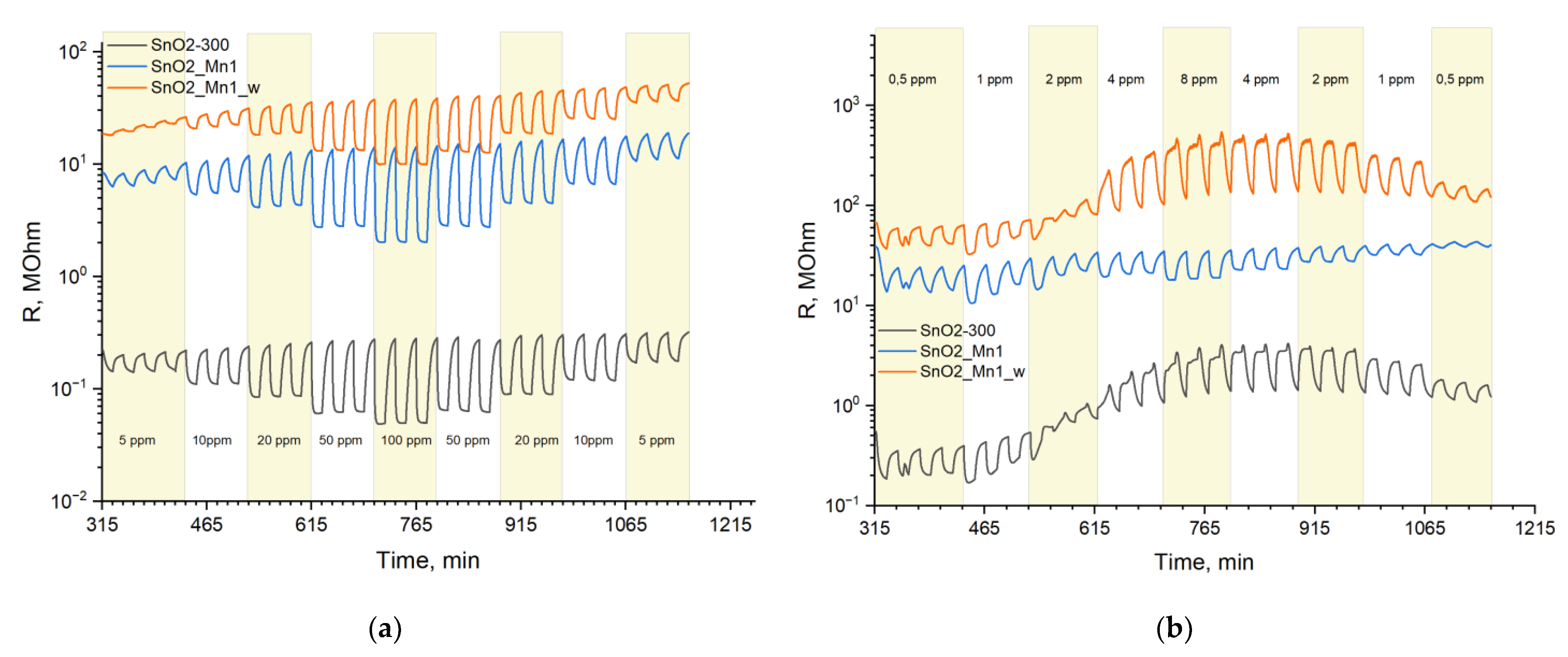
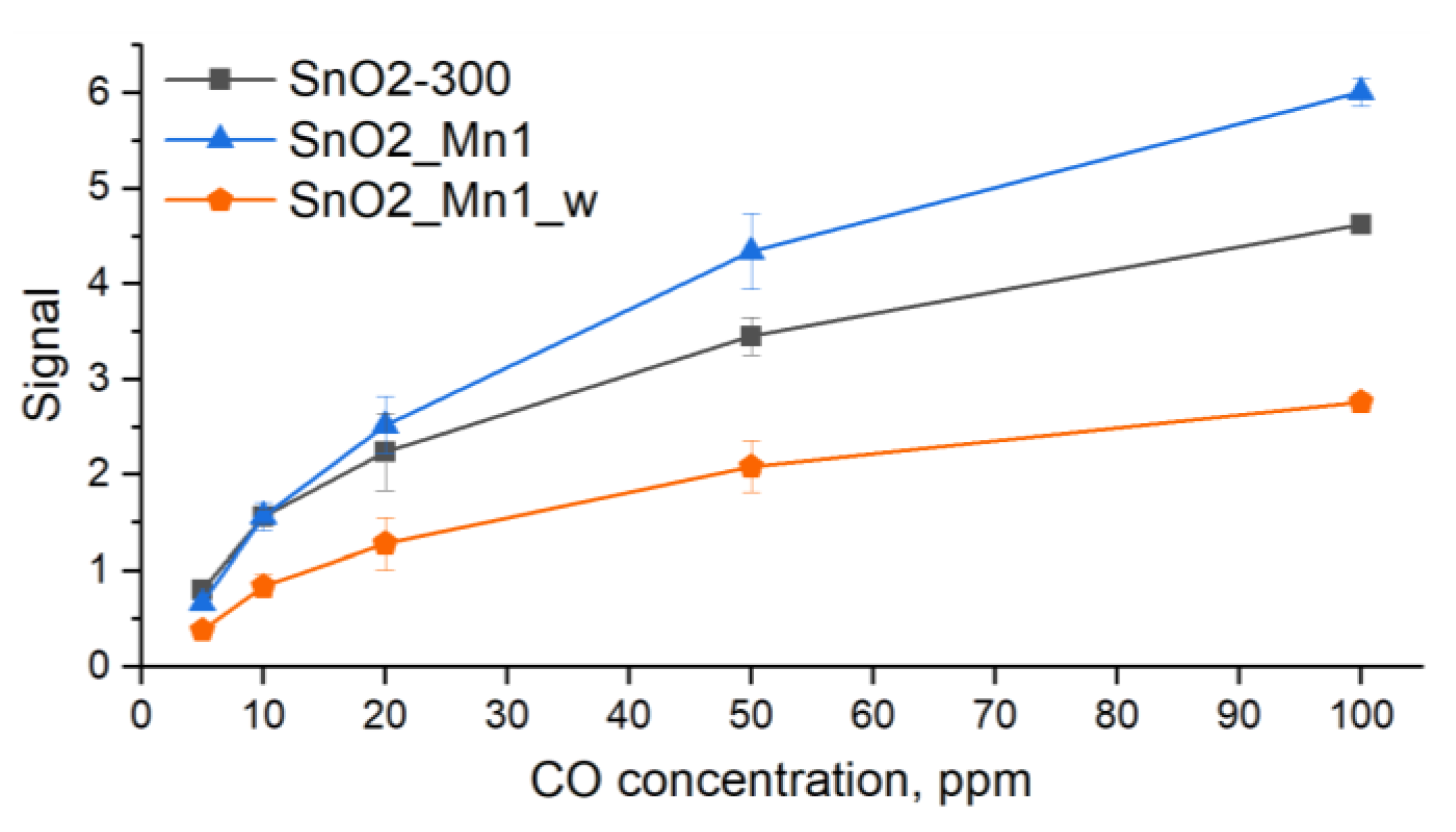
Sensitive layers were deposited on chips with platinum microheaters in the form of mushy dispersion in α-terpineol, which was evaporated into the air by heating the layer at 300 °C for 1 h. Heating control and resistance values reading were carried out automatically by a 4-channel analyzer with a measuring range from 1 to 1012 Ω at 1.3 V. Temperature and concentration signal dependencies were investigated. In the first case test gas mixture with constant concentration (CO 20 ppm; NO 4 ppm) was supplied to the flow cell alternatively with air (exposure time 15 min) at a fixed temperature for 1.5 h. The temperature was varied with a 30 °C step in the 30–300 °C. In the second case, the data were recorded at the constant pre-determined temperature, corresponding to maximum and stable sensor signal under various test gas concentrations (5, 10, 20, 50 and 100 ppm at 270 °C for CO; 0.5, 1, 2, 4 and 8 ppm at 270 °C for NO).