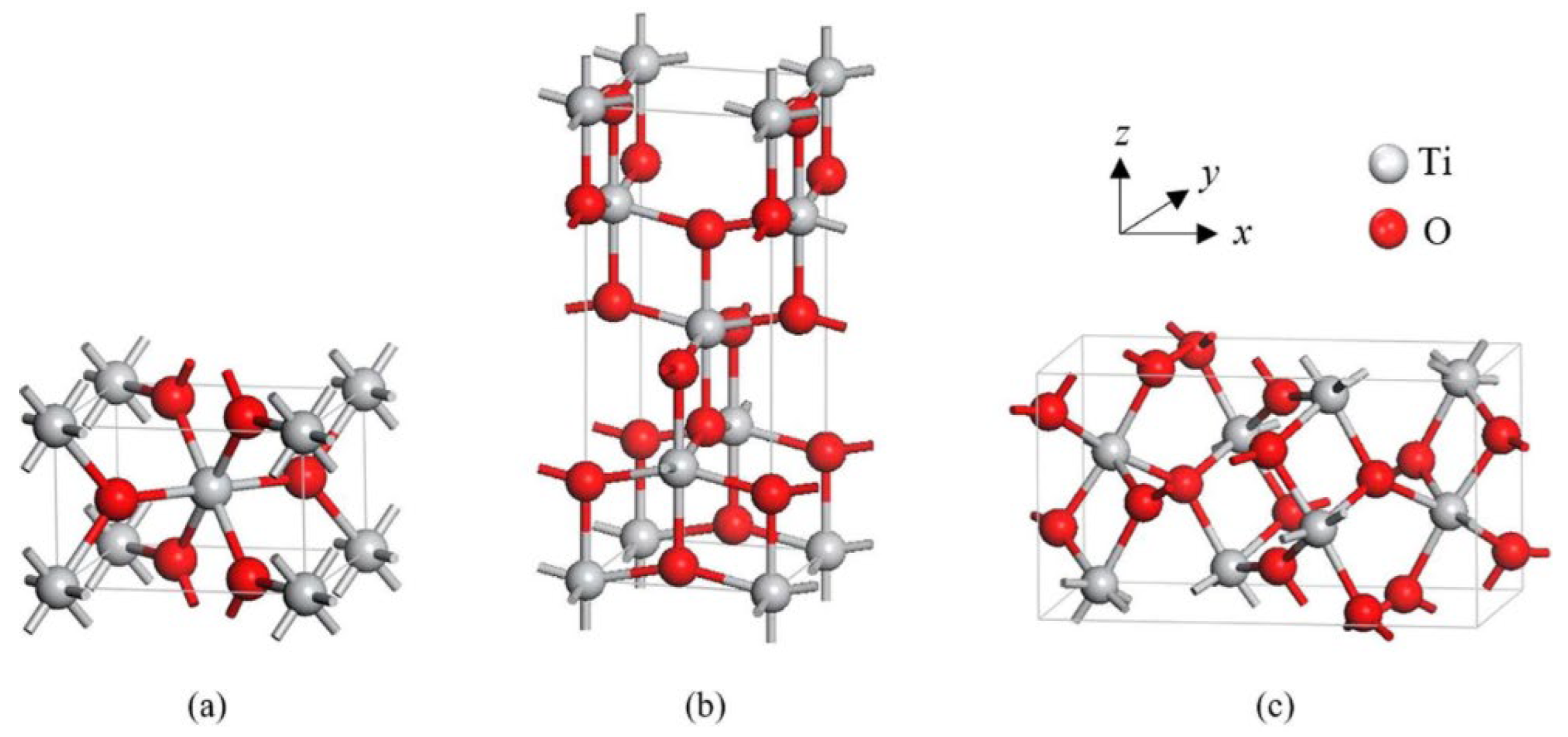
Recent Advancements in TiO2 Nanostructures: Sustainable Synthesis and Gas Sensing
Abstract
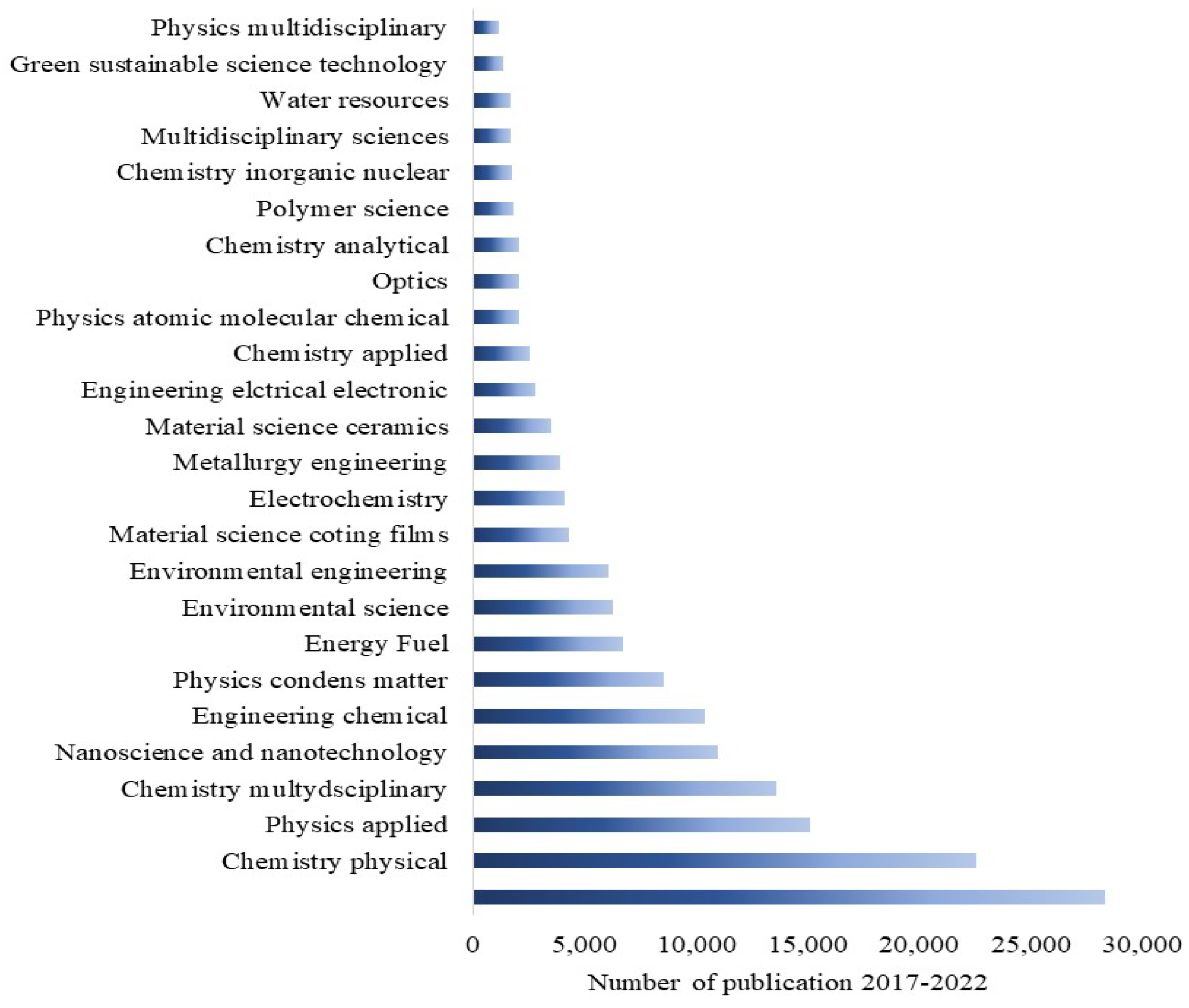
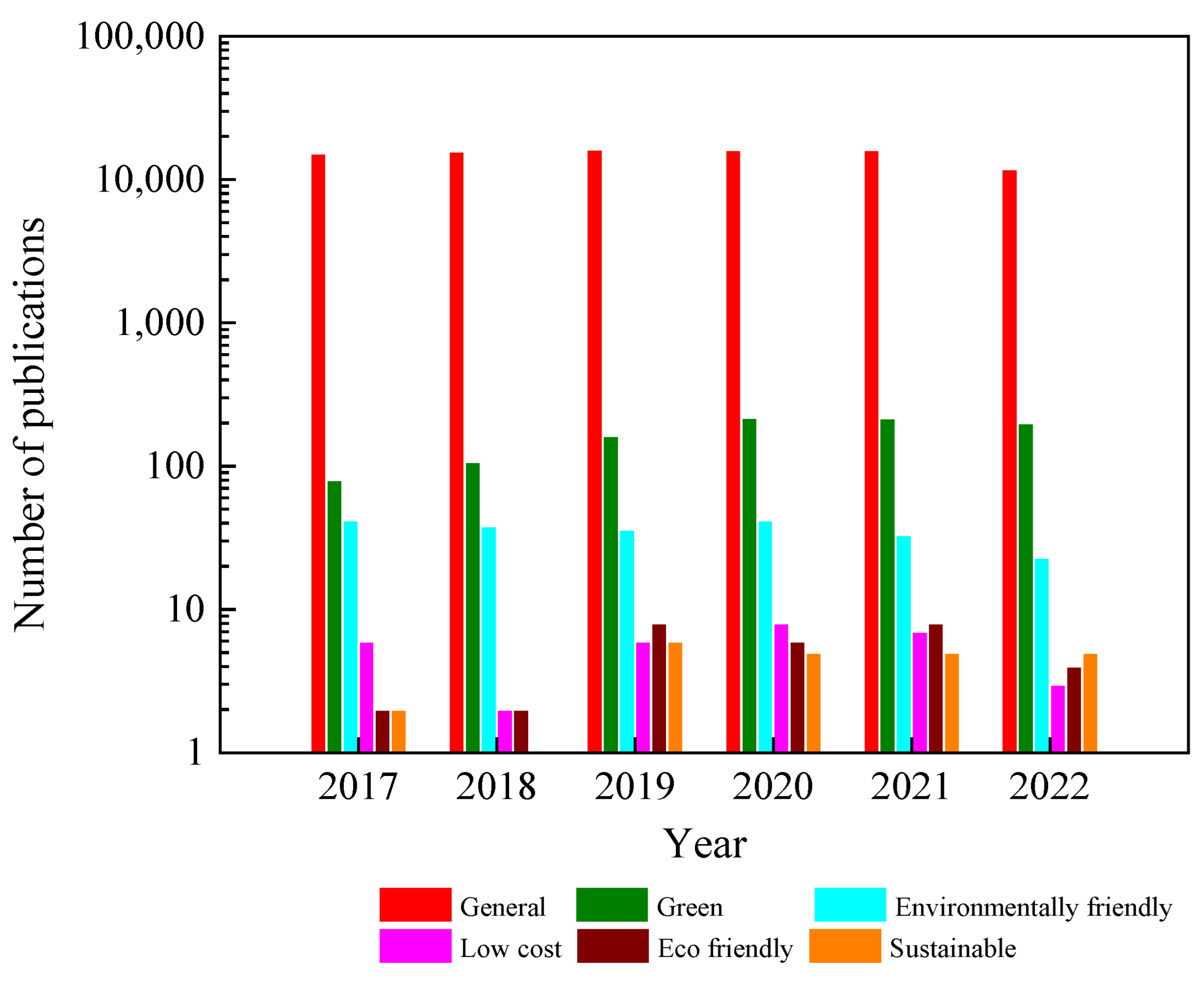
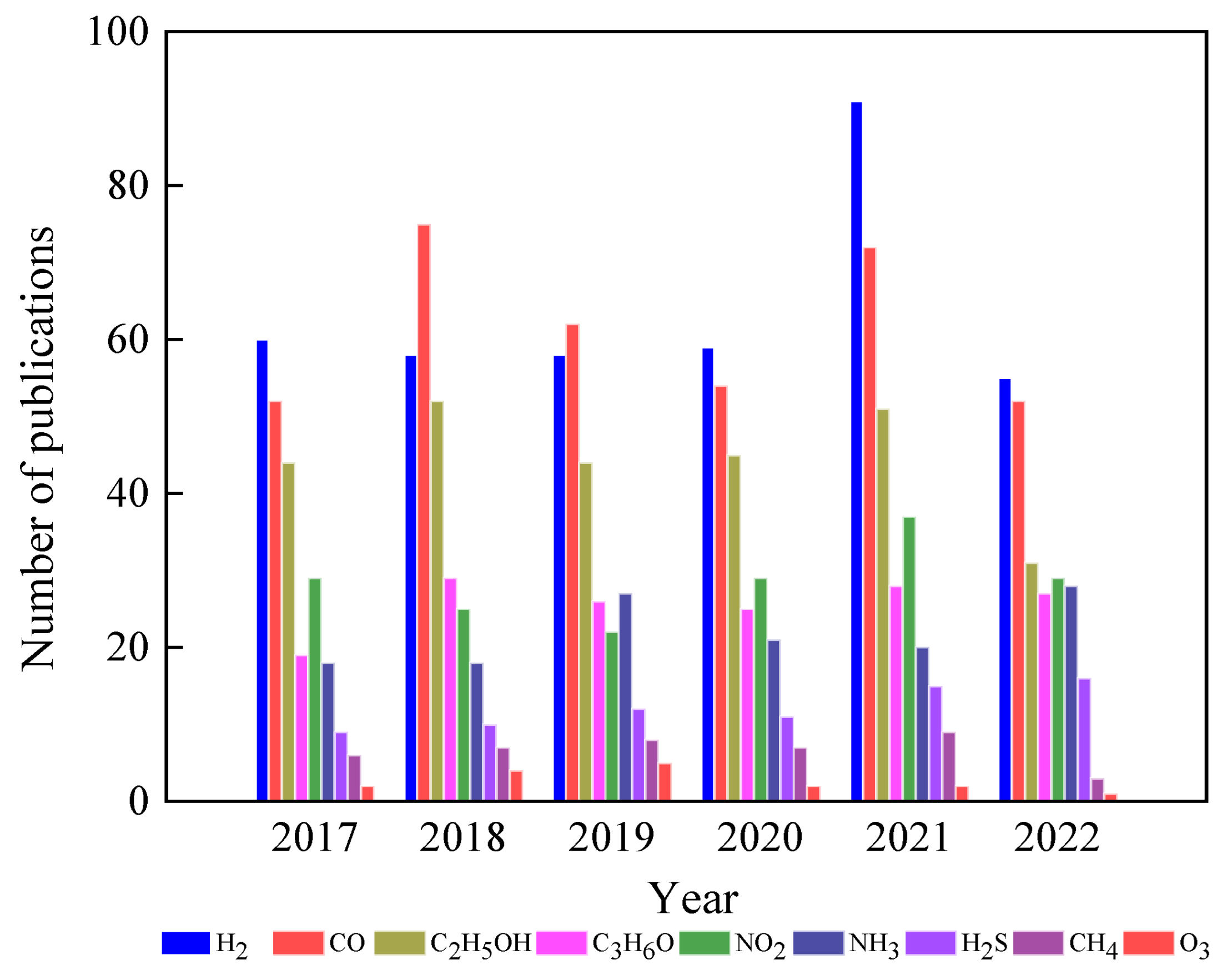
1. Introduction
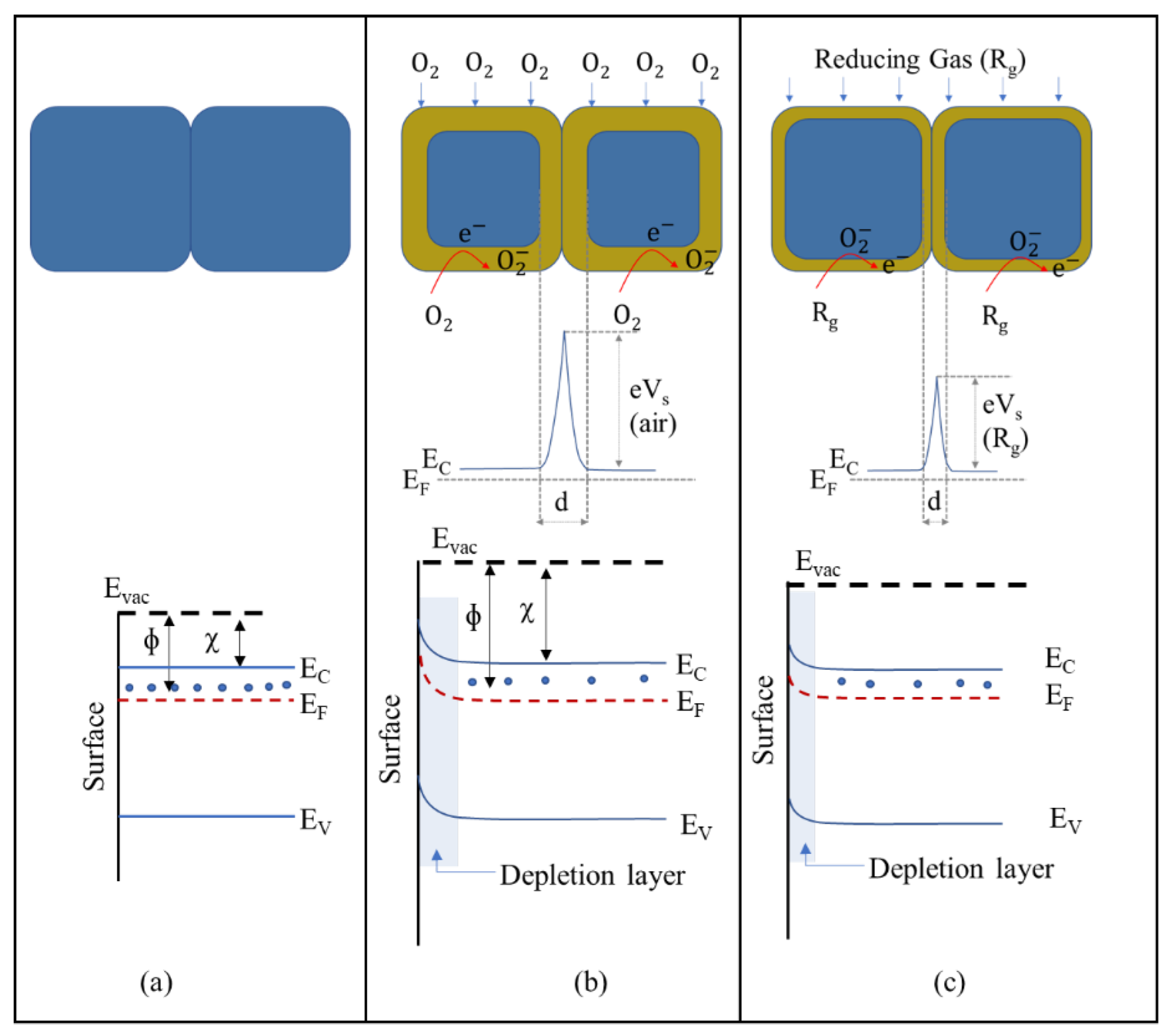
2. Gas-Sensing Mechanisms
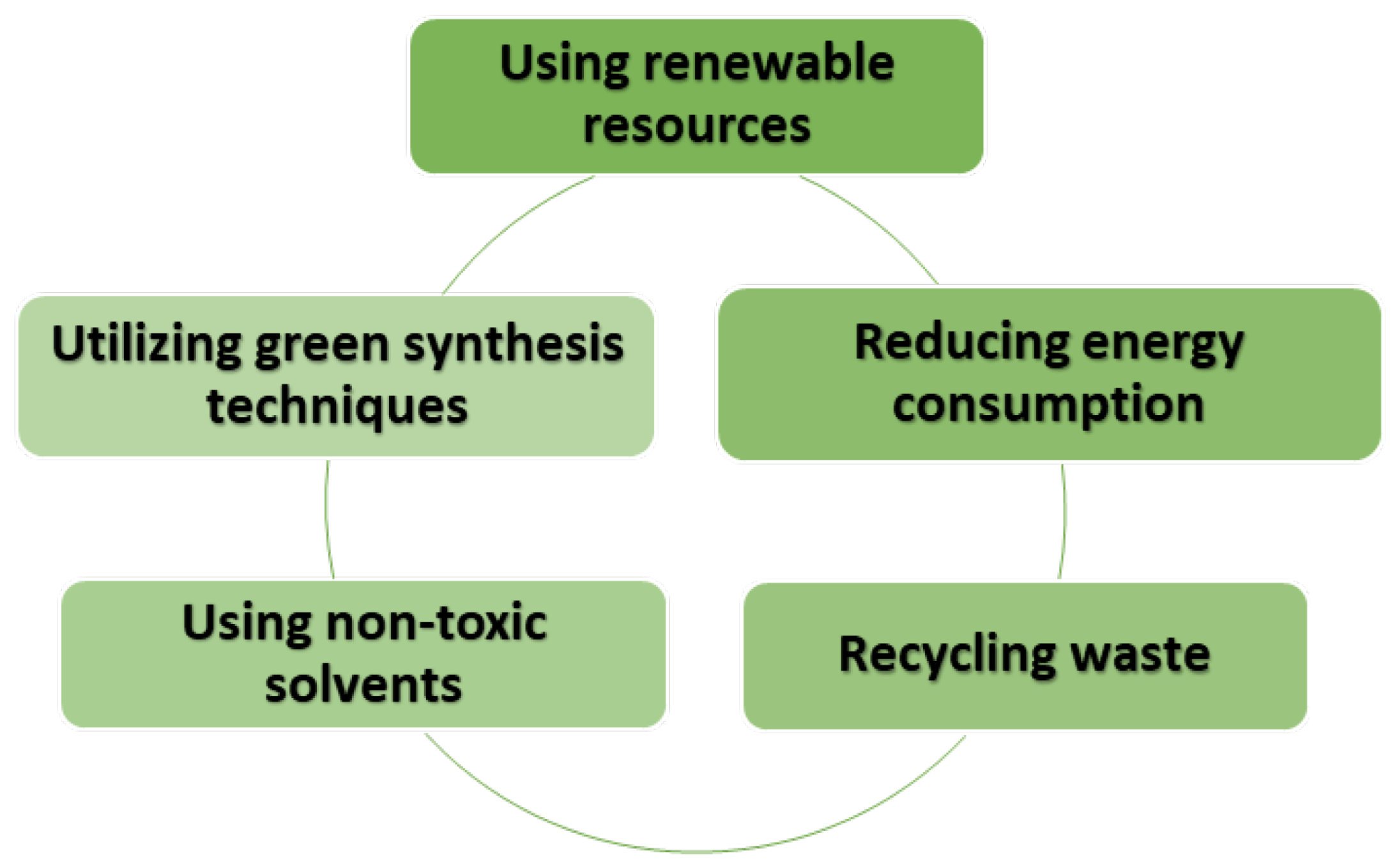
3. Sustainable Synthesis Methods of TiO2 Nanostructures
3.1. Sustainable and Green Sol–Gel Synthesis of TiO2 Nanostructures
3.2. Sustainable Precipitation and Green Synthesis of TiO2 Nanostructures
3.3. Sustainable and Green Hydro/Solvo-Thermal Synthesis of TiO2 Nanostructures
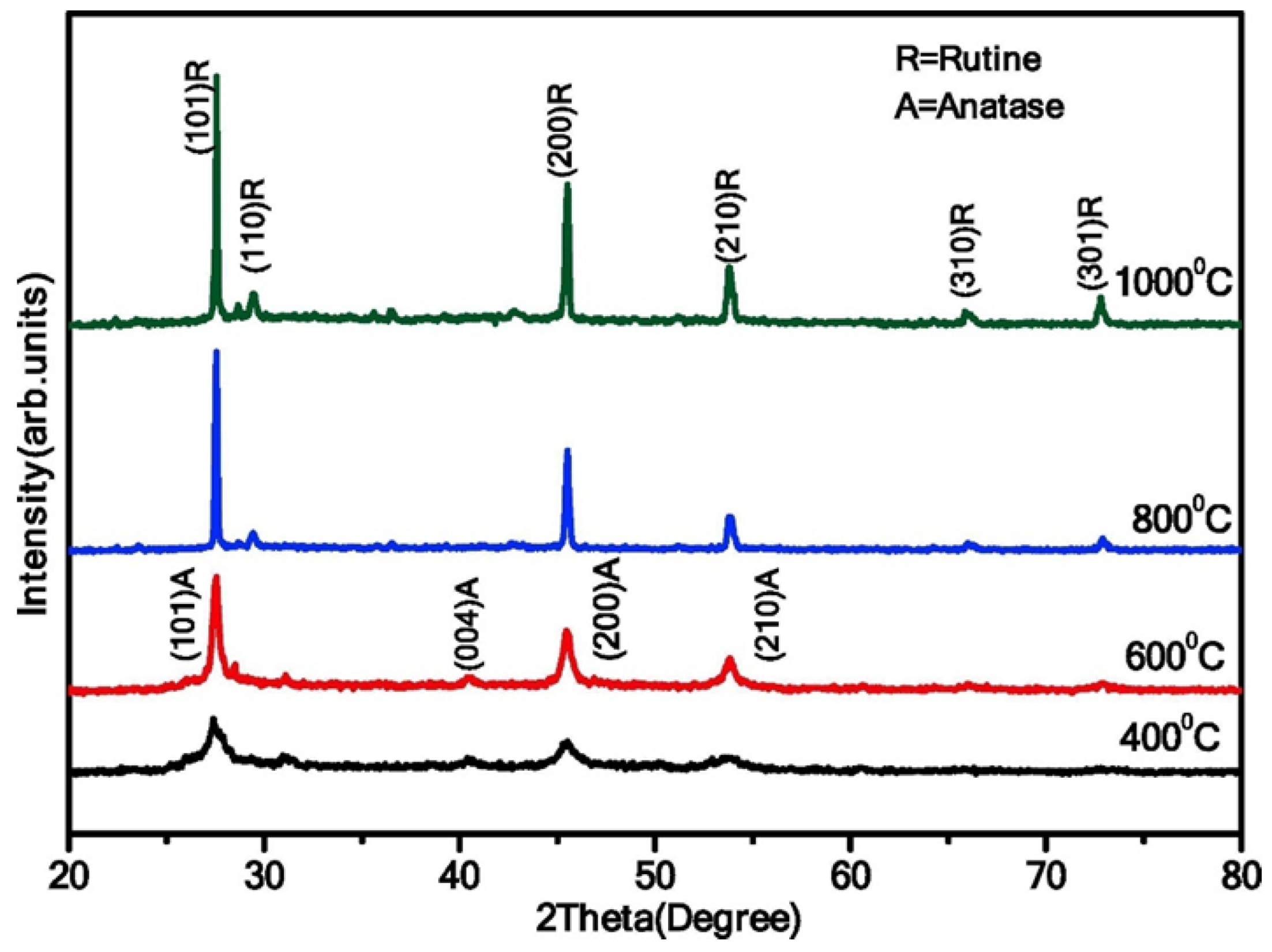
3.3.1. Hydrothermal Synthesis
| Chemicals | Synthesis Conditions | Structural and Morphological Properties | Ref. |
|---|---|---|---|
| Titanium glycolate Distilled water | Autoclave temp: 180 °C—Time: 6 h Washing: distilled water Drying- Temp: 60 °C—Time: 2 h | Phase: tetragonal anatase Morphology: aggregates, composed of NPs | [81] |
| Titanium butoxide Silver nitrate Trini-nitrophenol nitric Hydrochloric acids DD water Leucas aspera plant | Plant Stirring: plant + DD water Time: 30 min—Stored at temp: 5 °C TiO2 preparation Stirring time: 2 h—Temp: RT Autoclave temp: 160 °C—Time: 15 h Drying temp: 110 °C—Time: 10 h | Morphology: spherical shape Particle size: 5 nm | [84] |
| Deionized water Aloe Vera gel TTIP | Plant ectract Stirring: aloe vera gel + DI water Time: 1 h—Temp: 20 °C TiO2 preparation Autoclave temp: 180 °C—Time: 4 h Drying temp: 80 °C Calcination temp: 500 °C—Time: 5 h | Phase: anatase Morphology: nanoparticles Particle size: Around 6 to 13 | [85] |
| Algal powder Graphite powder TTIP Ethanol DI water | Plant extract Stirring: algal powder + DI water Temp: 70 °C—Time: 30 min—Then filtered TiO2 nanoparticle preparation Stirring: algal extract + TTIP Temp: 60 °C—Time: 4 h Washing: DI water Drying temp: 60 °C—Time: 12 h Annealing temp: 600 °C—Time: 2 h TiO2-GO nanocomposite preparation Ultrasonic treatment: graphene oxide + DI water + ethanol Time: 1 h Stirring: TiO2 NPs + GO—Time: 2 h Autoclave temp: 120 °C—Time: 5 h Washing: DI water + ethanol Drying temp: 60 °C—Time: 15 h | TiO2 Phase: tetragonal anatase Morphology: spherical nanoparticles Diameter: 50 nm TiO2-GO Morphology: TiO2 nanoparticles + GO sheets | [86] |
| Tetrabutyl orthotitanate Acetylacetone Millipore water Ammonia solution | Autoclavev temp: 170 °C—Time: 24 h Washing: HCl, 2-propanol, and Millipore water Drying temp: 120 °C—Time: 12 h Calcination temp: 450 °C—Time: 1 h | Phase: anatase Morphology: nanorods (NRs) Length of NRs: 100 nm Diameter of NRs: 30 nm | [87] |
| Titanium tetraisopropoxide Deionized water NaOH | Stirring time: 3 h Autoclave temp: 180 °C—Time: 24 h Washing: distilled water and ethanol Drying temp: 80 °C—Time: 3 h Annealing temp: 400 °C—Time: 5 h | Phase: anatase Crystallite size: 14 nm (pH = 7) and 16 nm (pH = 9) Morphology: nanorods with rod-like morphology (pH = 7) nanoplatelet-like structure (pH = 9) | [88] |
| TTIP HNO3 NaOH | Stirring temp: 80 °C—Time: 6 h Autoclave temp: 150 °C—Time: 60 min Washing: water (Acidic medium) Washing: HCl (alkaline medium) Drying temp: 60 °C Note: Teflon autoclave placed inside a modified domestic microwave oven | Acidic medium Phase: anatase + brookite + rutile Crystallite size: 6.0 nm Morphology: NPs Particle size: 10 and 15 nm Alkaline medium Phase: anatase + brookite Morphology: irregular spheres | [89] |
| TTIP Ethanol Distilled water | Stirring time: 30 min Ultrasonic bath time: 20 min Autoclave temp 150 °C—Time: 3 h Washing: deionized water Drying temp: 110 °C—Time: 5 h Calcination temp: 500 °C—Time: 2 h | Phase: tetragonal crystal structure Crystallite size: range of 31–42 nm Morphology: spherical nanoparticles Particle size: range of 32–48 nm | [90] |
| C12 H28 O4 Ti C2 H7 NO3 NaOH | Autoclave temp: 150 °C—Time: 20 h Washing: distilled water Drying temp: 60 °C—Time: 24 h—Treated with HCl for 1 h Washing: distilled water and ethanol Drying temp: 100 °C—Time: 1 h Calcination temp: 500 °C—Time: 1 h | Phase: anatase + rutile Morphology: nanorod-like structure Diameters: 30–50 nm Lengths: ≈1825 nm | [91] |
| Titanium powder Distilled water | Autoclave temp: 75 °C—Time: 4 h Washing: ethanol and water Calcination temp: 500 °C—Time: 3 h | Phase: tetragonal Morphology: semi-spherical NPs Particle size: ≥50 nm | [92] |
| TTIP Cobalt nitrate Glacial acetic acid Ethanol Distilled water | Autoclave temp: 120 °C—Time: 5 h Washing: ethanol Drying temp: 60 °C Calcination temp: 350 °C—Time: 2 h | Morphology: nanoparticles | [93] |
| M. citrifolia extract Ethanol TiCl4 | Plant extract Stirring: leaves of M. citrifolia + ethanol Time: 20 min—Temp: 50 °C—Stored at temp of 4 °C TiO2 preparation Autoclave temp: 120 °C—Time: 8 h Drying temp: 100 °C—Time: 4 h | Phase: tetragonal crystal structure Crystallite size: ≈10 nm Morphology: quasi-spherical NPs Particle size: from 10 to 20 nm | [94] |
| TTIP Distilled water Aloe vera gel Silver nitrate | Plant extract Stirring: aloe vera gel + DI water Time: 2 h—Temp: 90 °C—Stored at temp of 4 °C TiO2 preparation Stirring time: 1 h Autoclave temp: 180 °C—Time: 24 h Drying temp: 120 °C—Time: 2 h Calcination temp: 500 °C—Time: 5 h | Morphology: nanorods and nanoparticles | [95] |
3.3.2. Solvothermal Synthesis
4. Chemical/Gas-Sensing Applications
4.1. Pristine Nanostructures
- I.
- The large surface area and high surface-to-volume ratio enable a greater number of atoms to interact with the atmosphere.
- II.
- Higher mechanical stability.
- III.
- With a relatively larger lateral size, 2D nanostructured materials provide a more conformal contact with electrodes compared to other low-dimensional nanostructures.
- IV.
- The possibility of assembling into three-dimensional (3D) architectures.
4.2. Advanced Techniques to Enhance Gas-Sensing Functionality
4.2.1. Doping
4.2.2. Loading/Decoration/Functionalization
4.2.3. Heterojunctions/Heterostructures
4.3. Carbon Hybrids
5. Future Perspectives and Challenges
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ancient Meditations. Available online: https://visuddhimagga.info/ (accessed on 21 February 2023).
- Laurent, S.; Boutry, S.; Muller, R.N. Metal Oxide Particles and Their Prospects for Applications. In Iron Oxide Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–42. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Estrella, L.L.; Alemaida, I.M.A.; Lisin, A.; Moiseev, N.; Ahmadi, M.; Nazari, M.; Wali, M.; Zaheb, H.; Senjyu, T. Photocatalyt-ic Applications of Metal Oxides for Sustainable Environmental Remediation. Metals 2021, 1, 80. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal Oxides Nanoparticles via Sol-Gel Method: A Review on Synthesis, Characterization and Applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Roch, T.; Dobročka, E.; Mikula, M.; Pidík, A.; Durina, P.; Haidry, A.A.; Plecenik, T.; Truchlý, M.; Grancic, B.; Plecenik, A.; et al. Strong Biaxial Texture and Polymorph Nature in TiO2 Thin Film Formed by Ex-Situ Annealing on c-Plane Al2O3 Surface. J. Cryst. Growth 2012, 338, 118–124. [Google Scholar] [CrossRef]
- Samat, M.H.; Ali, A.M.M.; Taib, M.F.M.; Hassan, O.H.; Yahya, M.Z.A. Hubbard U Calculations on Optical Properties of 3D Transition Metal Oxide TiO2. Results Phys. 2016, 6, 891–896. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor Metal Oxide Gas Sensors: A Review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Kumarage, G.W.C.; Comini, E. Conductometric Gas Sensors. Encycl. Mater. Electron. 2023, 1, 564–580. [Google Scholar] [CrossRef]
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide Materials for Development of Integrated Gas Sensors—A Comprehensive Review. Crit. Rev. Solid State Mater. Sci. 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Insights in the Application of Stoichiometric and Non-Stoichiometric Titanium Oxides for the Design of Sensors for the Determination of Gases and Vocs (TiO2−X and TinO2n−1 vs. TiO2). Sensors 2020, 20, 6833. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Gas Sensors Based on Titanium Oxides (Review). Coatings 2022, 12, 699. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Tereshchenko, A.; Karpicz, R.; Ratautaite, V.; Bubniene, U.; Maneikis, A.; Jagminas, A.; Ramanavicius, A. TiO2−X/TiO2-Structure Based ‘Self-Heated’ Sensor for the Determination of Some Reducing Gases. Sensors 2019, 20, 74. [Google Scholar] [CrossRef]
- Gurlo, A.; Riedel, R. In Situ and Operando Spectroscopy for Assessing Mechanisms of Gas Sensing. Angew. Chem. Int. Ed. 2007, 46, 3826–3848. [Google Scholar] [CrossRef]
- Haidry, A.A.; Kind, N.; Saruhan, B. Investigating the Influence of Al-Doping and Background Humidity on NO2 Sensing Characteristics of Magnetron-Sputtered SnO2 Sensors. J. Sens. Sens. Syst. 2015, 4, 271–280. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Z.; Haidry, A.A.; Plecenik, T.; Xie, L.; Sun, L.; Fatima, Q. Resistive-Type Hydrogen Gas Sensor Based on TiO2: A Review. Int. J. Hydrog. Energy 2018, 43, 21114–21132. [Google Scholar] [CrossRef]
- Galstyan, V.; Moumen, A.; Kumarage, G.W.; Comini, E. Progress towards Chemical Gas Sensors: Nanowires and 2D Semiconductors. Sens. Actuators B Chem. 2022, 357, 131466. [Google Scholar] [CrossRef]
- Kumarage, G.W.; Comini, E. Low-Dimensional Nanostructures Based on Cobalt Oxide (CO3O4) in Chemical-Gas Sensing. Chemosensors 2021, 9, 197. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, S.; Deng, T.; Ding, H.; Chen, W.; Chen, Y. The Preparation of TiO2 Film by the Sol-Gel Method and Evaluation of Its Self-Cleaning Property. Materials 2018, 11, 450. [Google Scholar] [CrossRef]
- Haque, F.Z.; Nandanwar, R.; Singh, P. Evaluating Photodegradation Properties of Anatase and Rutile TiO2 Nanoparticles for Organic Compounds. Optik 2017, 128, 191–200. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The Evolution of ‘Sol-Gel’ Chemistry as a Technique for Materials Synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Thiagarajan, S.; Sanmugam, A.; Vikraman, D. Facile Methodology of Sol-Gel Synthesis for Metal Oxide Nanostructures. In Recent Applications in Sol-Gel Synthesis; IntechOpen Limited: London, UK, 2017. [Google Scholar] [CrossRef]
- Abel, S.; Jule, L.T.; Belay, F.; Shanmugam, R.; Dwarampudi, L.P.; Nagaprasad, N.; Krishnaraj, R. Application of Titanium Dioxide Nanoparticles Synthesized by Sol-Gel Methods in Wastewater Treatment. J. Nanomater. 2021, 2021, 3039761. [Google Scholar] [CrossRef]
- Kang, O.L.; Ahmad, A.; Rana, U.A.; Hassan, N.H. Sol-Gel Titanium Diox-ide Nanoparticles: Preparation and Structural Characterization. J. Nanotechnol. 2016, 2016, 5375939. [Google Scholar] [CrossRef]
- Nachit, W.; Ait Ahsaine, H.; Ramzi, Z.; Touhtouh, S.; Goncharova, I.; Benkhouja, K. Photocatalytic Activity of Anatase-Brookite TiO2 Nanoparticles Synthesized by Sol Gel Method at Low Temperature. Opt. Mater. 2022, 129, 112256. [Google Scholar] [CrossRef]
- Johari, N.D.; Rosli, Z.M.; Juoi, J.M.; Yazid, S.A. Comparison on the TiO2 Crystalline Phases Deposited via Dip and Spin Coating Using Green Sol-Gel Route. J. Mater. Res. Technol. 2019, 8, 2350–2358. [Google Scholar] [CrossRef]
- Yang, M.; Liu, W.; Jiang, C.; He, S.; Xie, Y.; Wang, Z. Fabrication of Super-hydrophobic Cotton Fabric with Fluorinated TiO2 Sol by a Green and One-Step Sol-Gel Process. Carbohydr. Polym. 2018, 197, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.A.; Nawaz, R.; ur Rehman, M.Z.; Imran, M.; Ahmad, J.; Ahmad, S.; Inam, A.; Razzaq, A.; Rizwan, M.; Ali, S. Synthesis and Characterization of Titanium Dioxide Nanoparticles by Chemical and Green Methods and Their Antifungal Activities against Wheat Rust. Chemosphere 2020, 258, 127352. [Google Scholar] [CrossRef]
- Yazid, S.A.; Rosli, Z.M.; Juoi, J.M. Effect of Titanium (IV) Isopropox-ide Molarity on the Crystallinity and Photocatalytic Activity of Titanium Dioxide Thin Film Deposited via Green Sol-Gel Route. J. Mater. Res. Technol. 2019, 8, 1434–1439. [Google Scholar] [CrossRef]
- Zou, L. Multilevel Resistive Switching Performance of TiO2-Based Flexible Memory Prepared by Low-Temperature Sol-Gel Method with UV Irradiation. Curr. Appl. Phys. 2021, 24, 32–38. [Google Scholar] [CrossRef]
- Maurya, I.C.; Singh, S.; Senapati, S.; Srivastava, P.; Bahadur, L. Green Synthesis of TiO2 Nanoparticles Using Bixa Orellana Seed Extract and Its Application for Solar Cells. Sol. Energy 2019, 194, 952–958. [Google Scholar] [CrossRef]
- Quintero, Y.; Mosquera, E.; Diosa, J.; García, A. Ultrasonic-Assisted Sol-Gel Synthesis of TiO2 Nanostructures: Influence of Synthesis Parameters on Morphology, Crystallinity, and Photocatalytic Performance. J. Sol Gel Sci. Technol. 2020, 94, 477–485. [Google Scholar] [CrossRef]
- Lal, M.; Sharma, P.; Ram, C. Calcination Temperature Effect on Titanium Oxide (TiO2) Nanoparticles Synthesis. Optik 2021, 241, 166934. [Google Scholar] [CrossRef]
- Jule, L.T.; Ramaswamy, K.; Bekele, B.; Saka, A.; Nagaprasad, N. Experimental Investigation on the Impacts of Annealing Temperatures on Titanium Dioxide Nanoparticles Structure, Size and Optical Properties Synthesized through Sol-Gel Methods. Mater. Today Proc. 2021, 45, 5752–5758. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Dal Poggetto, G.; Pasquali, M.; Dell’Era, A.; Vecchio Ciprioti, S. Influence of the Heat Treatment on the Particles Size and on the Crystalline Phase of TiO2 Synthesized by the Sol-Gel Method. Materials 2018, 11, 2364. [Google Scholar] [CrossRef]
- Rodríguez, P.A.O.; Pecchi, G.A.; Casuscelli, S.G.; Elias, V.R.; Eimer, G.A. A Simple Synthesis Way to Obtain Iron-Doped TiO2 Nanoparticles as Photocatalytic Surfaces. Chem. Phys. Lett. 2019, 732, 136643. [Google Scholar] [CrossRef]
- Zedek, R.; Djedjiga, H.; Megherbi, M.; Belkaid, M.S.; Ntsoenzok, E. Effects of Slight Fe (Iii)-Doping on Structural and Optical Properties of TiO2 Nanoparticles. J. Sol Gel Sci. Technol. 2021, 100, 44–54. [Google Scholar] [CrossRef]
- Nithyaa, N.; Victor Jaya, N. Structural, Optical, and Magnetic Properties of Gd-Doped TiO2 Nano-particles. J. Supercond. Nov. Magn. 2018, 31, 4117–4126. [Google Scholar] [CrossRef]
- Hajizadeh-Oghaz, M. Synthesis and Characterization of Nb-La Co-Doped TiO2 Nanoparticles by Sol-Gel Process for Dye-Sensitized Solar Cells. Ceram. Int. 2019, 45, 6994–7000. [Google Scholar] [CrossRef]
- Helmy, E.T.; Abouellef, E.M.; Soliman, U.A.; Pan, J.H. Novel Green Synthesis of S-Doped TiO2 Nanoparticles Using Malva Parviflora Plant Extract and Their Photocatalytic, Antimicrobial and Antioxidant Activities under Sunlight Illumination. Chemosphere 2021, 271, 129524. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.M.; Mushtaq, S.; Al Qahtani, H.S.; Sedky, A.; Alam, M.W. Investigation of TiO2 Nanoparticles Synthesized by Sol-Gel Method for Effectual Photodegradation, Oxidation and Reduction Reaction. Crystals 2021, 11, 1456. [Google Scholar] [CrossRef]
- Panneerselvam, A.; Velayutham, J.; Ramasamy, S. Green Synthesis of TiO2 Na-noparticles Prepared from Phyllanthus Niruri Leaf Extract for Dye Adsorption and Their Isotherm and Kinetic Studies. IET Nanobiotechnol. 2021, 15, 164–172. [Google Scholar] [CrossRef]
- Bundele, M.; Rane, N.; Lande, V.; Dani, A.; Shende, S. Green Synthe-sis of TiO2 Nanoparticle from Plumeria rubra L. Leaves for Anticorrosive Application. Mater. Today Proc. 2023, 72, 1685–1691. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Naydenov, A.; Stoyanova, A.; Georgieva, N.; Nemska, V.; Foteva, T. Sol-Gel Obtaining of TiO2/Eeo2 Nanopowders with Biocidal and Environmental Applications. Catalysts 2023, 13, 257. [Google Scholar] [CrossRef]
- Sun, M.; Xu, J.; Ma, J.; Xu, G. Facile Sol-Gel Preparation of Amorphous TiO2 Nano-particles under Ph of 8 for Synergistic Adsorption-Photocatalytic Degradation of Tetracycline. J. Alloy. Compd. 2023, 937, 168254. [Google Scholar] [CrossRef]
- Essam, Y.; El-Nowihy, G.H. N-Co-Cd-Doped TiO2 Nanocomposite for Efficient Dye-Synthesized Solar Cells. Ain Shams Eng. J. 2023, 14, 102180. [Google Scholar] [CrossRef]
- Tamarani, A.; Zainul, R.; Dewata, I. Preparation and Characterization of XRD Nano Cu-TiO2 Using Sol-Gel Method. J. Phys. Conf. Ser. 2019, 1185, 012020. [Google Scholar] [CrossRef]
- Elbushra, H.; Ahmed, M.; Wardi, H.; Eassa, N. Synthesis and Characterization of TiO2 Using Sol-Gel Method at Different Annealing Temperatures. MRS Adv. 2018, 3, 2527–2535. [Google Scholar] [CrossRef]
- Buraso, W.; Lachom, V.; Siriya, P.; Laokul, P. Synthesis of TiO2 Nanoparticles via a Simple Precipitation Method and Photocatalytic Performance. Mater. Res. Express 2018, 5, 115003. [Google Scholar] [CrossRef]
- Kalaivani, T.; Anilkumar, P. Role of Temperature on the Phase Modification of TiO2 Nanoparticles Synthesized by the Precipitation Method. Silicon 2017, 10, 1679–1686. [Google Scholar] [CrossRef]
- Fischer, K.; Gawel, A.; Rosen, D.; Krause, M.; Abdul Latif, A.; Griebel, J.; Prager, A.; Schulze, A. Low-Temperature Synthesis of Anatase/Rutile/Brookite TiO2 Nanoparticles on a Polymer Membrane for Photocatalysis. Catalysts 2017, 7, 209. [Google Scholar] [CrossRef]
- Lontio Fomekong, R.; Saruhan, B. Synthesis of Co3+ Doped TiO2 by Co-Precipitation Route and Its Gas Sensing Properties. Front. Mater. 2019, 6, 252. [Google Scholar] [CrossRef]
- Fomekong, R.L.; Kelm, K.; Saruhan, B. High-Temperature Hydrogen Sensing Performance of Ni-Doped TiO2 Prepared by Co-Precipitation Method. Sensors 2020, 20, 5992. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, M.; Senthil, T.S.; Senthilkumar, N.; Kang, M. Solar-Light-Induced Photocatalyst Based on Bi–B Co-Doped TiO2 Prepared via Co-Precipitation Method. J. Mater. Sci. Mater. Electron. 2022, 33, 16550–16563. [Google Scholar] [CrossRef]
- Sharmila, K.; Kamath, V.A.; Swaroop, K.; Somashekarappa, H.M. Thermoluminescence Properties of Li Doped TiO2 Nanoparticles Synthesized Using Co-Precipitation Method. AIP Conf. Proc. 2020, 2244, 090003. [Google Scholar] [CrossRef]
- Rilda, Y.; Damara, D.; Putri, Y.E.; Agustien, A. Synthesis of Zno-TiO2/Chitosan Nano-rods by Using Precipitation Methods and Studying Their Structures and Optics Properties at Different Precursor Molar Compositions. IOP Conf. Ser. Earth Environ. Sci. 2019, 217, 012015. [Google Scholar] [CrossRef]
- Ismael, M. Enhanced Photocatalytic Hydrogen Production and Degradation of Organic Pollutants from Fe (III) Doped TiO2 Nanoparticles. J. Environ. Chem. Eng. 2020, 8, 103676. [Google Scholar] [CrossRef]
- Lassoued, M.S.; Lassoued, A.; Ammar, S.; Gadri, A.; Salah, A.B.; García-Granda, S. Synthesis and Characterization of Co-Doped Nano-TiO2 through Co-Precipitation Method for Photocatalytic Activity. J. Mater. Sci. Mater. Electron. 2018, 29, 8914–8922. [Google Scholar] [CrossRef]
- Ansari, A.; Siddiqui, V.U.; Rehman, W.U.; Akram, M.K.; Siddiqi, W.A.; Alosaimi, A.M.; Hussein, M.A.; Rafatullah, M. Green Synthesis of TiO2 Nanoparti-cles Using Acorus Calamus Leaf Extract and Evaluating Its Photocatalytic and in Vitro Antimicrobial Activity. Catalysts 2022, 12, 181. [Google Scholar] [CrossRef]
- Subhapriya, S.; Gomathipriya, P. Green Synthesis of Titanium Dioxide (TiO2) Nanoparticles by Trigonella Foenum-Graecum Extract and Its Antimicrobial Properties. Microb. Pathog. 2018, 116, 215–220. [Google Scholar] [CrossRef]
- Nabi, G.; Majid, A.; Riaz, A.; Alharbi, T.; Kamran, M.A.; Al-Habardi, M. Green Synthesis of Spherical TiO2 Nanoparticles Using Citrus Limetta Extract: Excellent Photocatalytic Water Decontamination Agent for Rhb Dye. Inorg. Chem. Commun. 2021, 129, 108618. [Google Scholar] [CrossRef]
- Reddy, P.N.K.; Shaik, D.P.; Ganesh, V.; Nagamalleswari, D.; Thyagarajan, K.; Prasanth, P.V. Structural, Optical and Electrochemical Properties of TiO2 Nanoparticles Synthesized Using Medicinal Plant Leaf Extract. Ceram. Int. 2019, 45, 16251–16260. [Google Scholar] [CrossRef]
- Reddy, P.N.K.; Shaik, D.P.M.D.; Nagamalleswari, D.; Thyagarajan, K.; Prasanth, P.V. Electro-chemical Activity of TiO2 Nanoparticles in NaOH Electrolyte via Green Synthesis Using Calotropis Gigantea Plant Leaf Extract. Indian J. Sci. Technol. 2021, 14, 2766–2772. [Google Scholar] [CrossRef]
- Prashanth, V.; Priyanka, K.; Remya, N. Solar Photocatalytic Degradation of Metformin by TiO2 Synthesized Using Calotropis Gigantea Leaf Extract. Water Sci. Technol. 2021, 83, 1072–1084. [Google Scholar] [CrossRef]
- Hariharan, D.; Srinivasan, K.; Nehru, L.C. Synthesis and Characterization of TiO2 Nanoparticles Using Cynodon Dactylon Leaf Extract for Antibacterial and Anticancer (A549 Cell Lines) Activity. J. Nanomed. Res. 2017, 5, 00138. [Google Scholar] [CrossRef]
- Rajkumari, J.; Magdalane, C.M.; Siddhardha, B.; Madhavan, J.; Ramalingam, G.; Al-Dhabi, N.A.; Arasu, M.V.; Ghilan, A.K.M.; Duraipandiayan, V.; Kaviyarasu, K. Synthesis of Titanium Oxide Nanoparticles Using Aloe Barbadensis Mill and Evaluation of Its Antibiofilm Potential against Pseudomonas Aeruginosa PAO1. J. Photochem. Photobiol. B Biol. 2019, 201, 111667. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, M.; Obodo, R.M.; Shahzad, M.I.; Mukhtar, S.; Ilyas, S.Z.; Mahmood, T. Green Syn-thesis of Cu@TiO2 via Cedrus Deodara Leaf Extract: A Novel Composite with High Photocatalytic and Antibacterial Activity. Curr. Res. Green Sustain. Chem. 2021, 4, 100137. [Google Scholar] [CrossRef]
- Pushpamalini, T.; Keerthana, M.; Sangavi, R.; Nagaraj, A.; Kamaraj, P. Comparative Analysis of Green Synthesis of TiO2 Nanoparticles Using Four Different Leaf Extract. Mater. Today Proc. 2021, 40, S180–S184. [Google Scholar] [CrossRef]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green Synthesis of TiO2 Nanoparticles from Syzygium Cumini Extract for Photo-Catalytic Removal of Lead (PB) in Explosive Industrial Wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green Synthesis of TiO2 Nanoparticles Using Leaf Extract of Jatropha curcas L. for Photocatalytic Degradation of Tannery Wastewater. Chem. Eng. J. 2018, 336, 386–396. [Google Scholar] [CrossRef]
- Aswini, R.; Murugesan, S.; Kannan, K. Bio-Engineered TiO2 Nanoparticles Using Ledebouria Revoluta Extract: Larvicidal, Histopathological, Antibacterial and Anticancer Activity. Int. J. Environ. Anal. Chem. 2020, 101, 2926–2936. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Alasiri, A.S.; Ahmad, J.; Alqahtani, A.A.; Abdullah, M.M.; Abdel-Wahab, B.A.; Pathak, K.; Saikia, R.; Das, A.; Sarma, H.; et al. Green Synthesis of Titanium Dioxide Nanoparticles Using Ocimum Sanctum Leaf Extract: In Vitro Characterization and Its Healing Efficacy in Diabetic Wounds. Molecules 2022, 27, 7712. [Google Scholar] [CrossRef]
- Wu, Z.G.; Ren, Z.M.; Li, L.; Lv, L.; Chen, Z. Hydrothermal Synthesis of TiO2 Quantum Dots with Mixed Titanium Precursors. Sep. Purif. Technol. 2020, 251, 117328. [Google Scholar] [CrossRef]
- Jaggessar, A.; Mathew, A.; Wang, H.; Tesfamichael, T.; Yan, C.; Yarlagadda, P.K. Mechanical, Bactericidal and Osteogenic Behaviours of Hydrothermally Synthesised TiO2 Nanowire Arrays. J. Mech. Behav. Biomed. Mater. 2018, 80, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Vats, T.; Sharma, S.N.; Kumar, J. Investigation of Annealing Effects on TiO2 Nanotubes Synthesized by a Hydrothermal Method for Hybrid Solar Cells. Optik 2018, 171, 492–500. [Google Scholar] [CrossRef]
- Lan, K.; Liu, Y.; Zhang, W.; Liu, Y.; Elzatahry, A.; Wang, R.; Xia, Y.; Al-Dhayan, D.; Zheng, N.; Zhao, D. Uniform Ordered Two-Dimensional Mesoporous TiO2 Nanosheets from Hydrothermal-Induced Solvent-Confined Monomicelle Assembly. J. Am. Chem. Soc. 2018, 140, 4135–4143. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal Technology for Nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Gupta, T.; Cho, J.; Prakash, J. Hydrothermal Synthesis of TiO2 Nanorods: Formation Chemistry, Growth Mechanism, and Tailoring of Surface Properties for Photocatalytic Activities. Mater. Today Chem. 2021, 20, 100428. [Google Scholar] [CrossRef]
- Rajakumaran, R.; Abinaya, M.; Chen, S.M.; Balamurugan, K.; Muthuraj, V. Ultrasonication and Hydrothermal Assisted Synthesis of Cloud-like Zinc Molybdate Nanospheres for Enhanced Detection of Flutamide. Ultrason. Sonochem. 2020, 61, 104823. [Google Scholar] [CrossRef] [PubMed]
- Sofyan, N.; Ridhova, A.; Yuwono, A.H.; Udhiarto, A. Preparation of Anatase TiO2 Nanoparticles Using Low Hydrothermal Temperature for Dye-Sensitized Solar Cell. IOP Conf. Ser. Mater. Sci. Eng. 2018, 316, 012055. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Lv, K.; Zhou, G.; Wang, Z.S. Low Temperature Preparation of TiO2 Nanoparticle Chains without Hydrothermal Treatment for Highly Efficient Dye-Sensitized Solar Cells. J. Mater. Chem. A 2015, 3, 4477–4483. [Google Scholar] [CrossRef]
- Navale, S.T.; Yang, Z.B.; Liu, C.; Cao, P.J.; Patil, V.B.; Ramgir, N.S.; Mane, R.S.; Stadler, F.J. Enhanced Acetone Sensing Properties of Titanium Dioxide Nanoparticles with a Sub-Ppm Detection Limit. Sens. Actuators B Chem. 2018, 255, 1701–1710. [Google Scholar] [CrossRef]
- Sagadevan, S.; Imteyaz, S.; Murugan, B.; Anita Lett, J.; Sridewi, N.; Weldegebrieal, G.K.; Fatimah, I.; Oh, W.C. A Comprehensive Review on Green Synthesis of Titanium Dioxide Nanoparticles and Their Diverse Biomedical Applications. Green Process. Synth. 2022, 11, 44–63. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Chen, Q.; Ren, B.; Duan, R.; Guan, R. Synchronous Regulation of Morphology and Crystal Phase of TiO2 via a Facile Green Hydrothermal Approach and Their Photocatalytic Activity. Mater. Res. Bull. 2019, 109, 90–97. [Google Scholar] [CrossRef]
- Nagaraj, K.; Thankamuniyandi, P.; Kamalesu, S.; Lokhandwala, S.; Parekh, N.M.; Sakthinathan, S.; Chiu, T.-W.; Karuppiah, C. Green Synthesis, Characterization and Efficient Photocatalytic Study of Hydrothermal-Assisted Ag@TiO2 Nanocomposites. Inorg. Chem. Commun. 2023, 148, 110362. [Google Scholar] [CrossRef]
- Hariharan, D.; Christy, A.J.; Mayandi, J.; Nehru, L.C. Visible Light Active Photo-catalyst: Hydrothermal Green Synthesized TiO2 NPS for Degradation of Picric Acid. Mater. Lett. 2018, 222, 45–49. [Google Scholar] [CrossRef]
- Sharma, M.; Behl, K.; Nigam, S.; Joshi, M. TiO2-Go Nanocomposite for Photocatalysis and Environmental Applications: A Green Synthesis Approach. Vacuum 2018, 156, 434–439. [Google Scholar] [CrossRef]
- Govindaraj, R.; Santhosh, N.; Senthil Pandian, M.; Ramasamy, P. Synthesis of Nanocrystalline TiO2 Nanorods via Hydrothermal Method: An Efficient Photoanode Material for Dye Sensitized Solar Cells. J. Cryst. Growth 2017, 468, 125–128. [Google Scholar] [CrossRef]
- Santhi, K.; Navaneethan, M.; Harish, S.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and Characterization of TiO2 Nanorods by Hydrothermal Method with Different Ph Conditions and Their Photo-catalytic Activity. Appl. Surf. Sci. 2020, 500, 144058. [Google Scholar] [CrossRef]
- Bregadiolli, B.A.; Fernandes, S.L.; Graeff, C.F.D.O. Easy and Fast Preparation of TiO2-Based Nanostructures Using Microwave Assisted Hydrothermal Synthesis. Mater. Res. 2017, 20, 912–919. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Sony Mary, M. Synthesis of TiO2 Nanoparticles by Chemical and Green Synthesis Methods and Their Multifaceted Properties. SN Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Zhu, Z.; Lin, S.J.; Wu, C.H.; Wu, R.J. Synthesis of TiO2 Nanowires for Rapid no2 Detection. Sens. Actuators A Phys. 2018, 272, 288–294. [Google Scholar] [CrossRef]
- Shahat, A.M.; El-Hossary, F.M.; Ghitas, A.; Abd El-Rahman, A.M.; Ebnalwaled, A.A. Low-Temperature Hydrothermal Synthesis of Titanium Dioxide Nanoparticles for Photocatalytic Applications. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1171, p. 012008. [Google Scholar] [CrossRef]
- Kamble, R.J.; Gaikwad, P.V.; Garadkar, K.M.; Sabale, S.R.; Puri, V.R.; Mahajan, S.S. Photocatalytic Degradation of Malachite Green Using Hydrothermally Synthesized Cobalt-Doped TiO2 Nanoparticles. J. Iran. Chem. Soc. 2021, 19, 303–312. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Bama, K.; Bhavani, M.; Jegatheeswaran, S.; Ambika, S.; Sangili, A.; Nithya, P.; Sumathi, R. Obtaining Titanium Dioxide Nanoparticles with Spherical Shape and Antimicrobial Properties Using M. Citrifolia Leaves Extract by Hydrothermal Method. J. Photochem. Photobiol. B Biol. 2017, 171, 117–124. [Google Scholar] [CrossRef]
- Hariharan, D.; Thangamuniyandi, P.; Christy, A.J.; Vasantharaja, R.; Selvakumar, P.; Sagadevan, S.; Pugazhendhi, A.; Nehru, L.C. Enhanced Photocatalysis and Anticancer Activity of Green Hydrothermal Synthesized Ag@TiO2 Nanoparticles. J. Photochem. Photobiol. B Biol. 2020, 202, 111636. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, M.; Hoang, S.; Suib, S.L.; Gao, P.X. Solvent Effects on the Heterogeneous Growth of TiO2 Nanostructure Arrays by Solvothermal Synthesis. Catal. Today 2021, 360, 275–283. [Google Scholar] [CrossRef]
- Alosfur, F.K.M.; Ouda, A.A.; Ridha, N.J.; Abud, S.H. High Photocatalytic Activity of TiO2 Nanorods Prepared by Simple Method. Mater. Res. Express 2019, 6, 065028. [Google Scholar] [CrossRef]
- Huang, J.; Liu, H.; Li, Z.; Zhong, J.; Wang, T.; Li, J.; Li, M. Photocata-lytic Activity of TiO2 Prepared by Different Solvents through a Solvothermal Approach. Solid State Sci. 2019, 98, 106024. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Sufian, S.; Mansor, N.; Rabat, N.E. Synthesis and Characterization of TiO2-Based Nanostructures via Fluorine-Free Solvothermal Method for Enhancing Visible Light Photocatalytic Activity: Experimental and Theoretical Approach. J. Photochem. Photobiol. A Chem. 2021, 404, 112834. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas Sensors Based on TiO2 Nanostructured Materials for the Detection of Hazardous Gases: A Review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Sugahara, T.; Alipour, L.; Hirose, Y.; Ekubaru, Y.; Nakamura, J.I.; Ono, H.; Harada, N.; Suganuma, K. Formation of Metal-Organic Decomposition Derived Nano-crystalline Structure Titanium Dioxide by Heat Sintering and Photosintering Methods for Advanced Coating Process, and Its Volatile Organic Compounds’ Gas-Sensing Properties. ACS Appl. Electron. Mater. 2020, 2, 1670–1678. [Google Scholar] [CrossRef]
- Chen, N.; Deng, D.; Li, Y.; Liu, X.; Xing, X.; Xiao, X.; Wang, Y. TiO2 Na-noparticles Functionalized by Pd Nanoparticles for Gas-Sensing Application with Enhanced Butane Response Performances. Sci. Rep. 2017, 7, 7692. [Google Scholar] [CrossRef]
- Gakhar, T.; Hazra, A. C60-Encapsulated TiO2 Nanoparticles for Selective and Ultrahigh Sensitive Detection of Formaldehyde. Nanotechnology 2021, 32, 505505. [Google Scholar] [CrossRef]
- Mintcheva, N.; Srinivasan, P.; Rayappan, J.B.B.; Kuchmizhak, A.A.; Gurbatov, S.; Kulinich, S.A. Room-Temperature Gas Sensing of Laser-Modified Anatase TiO2 Decorated with Au Nanoparticles. Appl. Surf. Sci. 2020, 507, 145169. [Google Scholar] [CrossRef]
- Fernández-Ramos, M.D.; Capitan-Vallvey, L.F.; Pastrana-Martínez, L.M.; Morales-Torres, S.; Maldonado-Hodar, F.J. Chemoresistive NH3 Gas Sensor at Room Temperature Based on the Carbon Gel-TiO2 Nanocomposites. Sens. Actuators B Chem. 2022, 368, 132103. [Google Scholar] [CrossRef]
- Giampiccolo, A.; Tobaldi, D.M.; Leonardi, S.G.; Murdoch, B.J.; Seabra, M.P.; Ansell, M.P.; Neri, G.; Ball, R.J. Sol Gel Graphene/TiO2 Nanoparti-cles for the Photocatalytic-Assisted Sensing and Abatement of No2. Appl. Catal. B Environ. 2019, 243, 183–194. [Google Scholar] [CrossRef]
- Wang, Z.; Haidry, A.A.; Xie, L.; Zavabeti, A.; Li, Z.; Yin, W.; Fomekong, R.L.; Saruhan, B. Acetone Sensing Applications of Ag Modified TiO2 Porous Nanoparticles Synthesized via Facile Hydrothermal Method. Appl. Surf. Sci. 2020, 533, 147383. [Google Scholar] [CrossRef]
- Tshabalala, Z.P.; Shingange, K.; Cummings, F.R.; Ntwaeaborwa, O.M.; Mhlongo, G.H.; Motaung, D.E. Ultra-Sensitive and Selective NH3 Room Temperature Gas Sensing Induced by Manganese-Doped Titanium Dioxide Nanoparticles. J. Colloid Interface Sci. 2017, 504, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, X.; Sui, L.; Wang, W.; Zhou, X.; Cheng, X.; Gao, S.; Xu, Y.; Huo, L. C-Doped TiO2 Nanoparticles to Detect Alcohols with Different Carbon Chains and Their Sensing Mechanism Analysis. Sens. Actuators B Chem. 2020, 312, 127942. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Xu, Z.; Shen, W.; Wu, Y.; Corriou, J.P. Computational Assist-ed Tuning of Co-Doped TiO2 Nanoparticles for Ammonia Detection at Room Temperatures. Appl. Surf. Sci. 2022, 601, 154214. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.Y.; Mirzaei, A.; Nam, M.S.; Kim, H.W.; Kim, S.S. Room-Temperature Detection of Acetone Gas by Pani/Nio-Loaded TiO2 Nanoparticles under UV Irradiation. Sens. Actuators B Chem. 2023, 374, 132850. [Google Scholar] [CrossRef]
- Comini, E. Metal Oxides Nanowires Chemical/Gas Sensors: Recent Advances. Mater. Today Adv. 2020, 7, 100099. [Google Scholar] [CrossRef]
- Avansi, W., Jr.; Catto, A.C.; da Silva, L.F.; Fiorido, T.; Bernardini, S.; Mastelaro, V.R.; Aguir, K.; Arenal, R. One-Dimensional V2O5/TiO2 Heterostructures for Chemiresistive Ozone Sensors. ACS Appl. Nano Mater. 2019, 2, 4756–4764. [Google Scholar] [CrossRef]
- Munasinghe Arachchige, H.M.; Zappa, D.; Poli, N.; Gunawardhana, N.; Attanayake, N.H.; Comini, E. Seed-Assisted Growth of TiO2 Nanowires by Thermal Oxidation for Chemical Gas Sensing. Nanomaterials 2020, 10, 935. [Google Scholar] [CrossRef]
- Li, A.; Zhao, S.; Bai, J.; Gao, S.; Wei, D.; Shen, Y.; Yuan, Z.; Meng, F. Optimal Construction and Gas Sensing Properties of SnO2@TiO2 Heterostructured Nanorods. Sens. Actuators B Chem. 2022, 355, 131261. [Google Scholar] [CrossRef]
- Ng, S.; Kuberský, P.; Krbal, M.; Prikryl, J.; Gärtnerová, V.; Moravcová, D.; Sopha, H.; Zazpe, R.; Yam, F.K.; Jäger, A.; et al. ZnO Coated Anodic 1d TiO2 Nanotube Layers: Efficient Photo-Electrochemical and Gas Sensing Heterojunction. Adv. Eng. Mater. 2017, 20, 1700589. [Google Scholar] [CrossRef]
- Jin, Q.; Wen, W.; Zheng, S.; Jiang, R.; Wu, J.M. Branching TiO2 Nanowire Arrays for Enhanced Ethanol Sensing. Nanotechnology 2021, 32, 295501. [Google Scholar] [CrossRef]
- Tshabalala, Z.P.; Swart, H.C.; Motaung, D.E. Fabrication of TiO2 Nanofibers Based Sensors for Enhanced CH4 Performance Induced by Notable Surface Area and Acid Treatment. Vacuum 2021, 187, 110102. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Lu, H.; Yan, C.; Chen, K.; Lu, H.; Gao, J.; Yang, Z.; Zhu, G.; Wang, C.; et al. Ethanol Sensing Properties and Reduced Sensor Resistance Using Porous NB2O5-TiO2 n-n Junction Nanofibers. Sens. Actuators B Chem. 2019, 283, 602–612. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, J.; Zhou, L.; Liu, D.; Liu, F.; Liang, X.; Gao, Y.; Liu, F.; Yan, X.; Lu, G. Preparation of Silver-Loaded Titanium Dioxide Hedgehog-like Architecture Composed of Hundreds of Nanorods and Its Fast Response to Xylene. J. Colloid Interface Sci. 2019, 536, 215–223. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Y.; Wu, B.; Zhang, X. Different Crystalline Phases of Aligned TiO2 Nanowires and Their Ethanol Gas Sensing Properties. Phys. E Low Dimens. Syst. Nanostructures 2019, 114, 113601. [Google Scholar] [CrossRef]
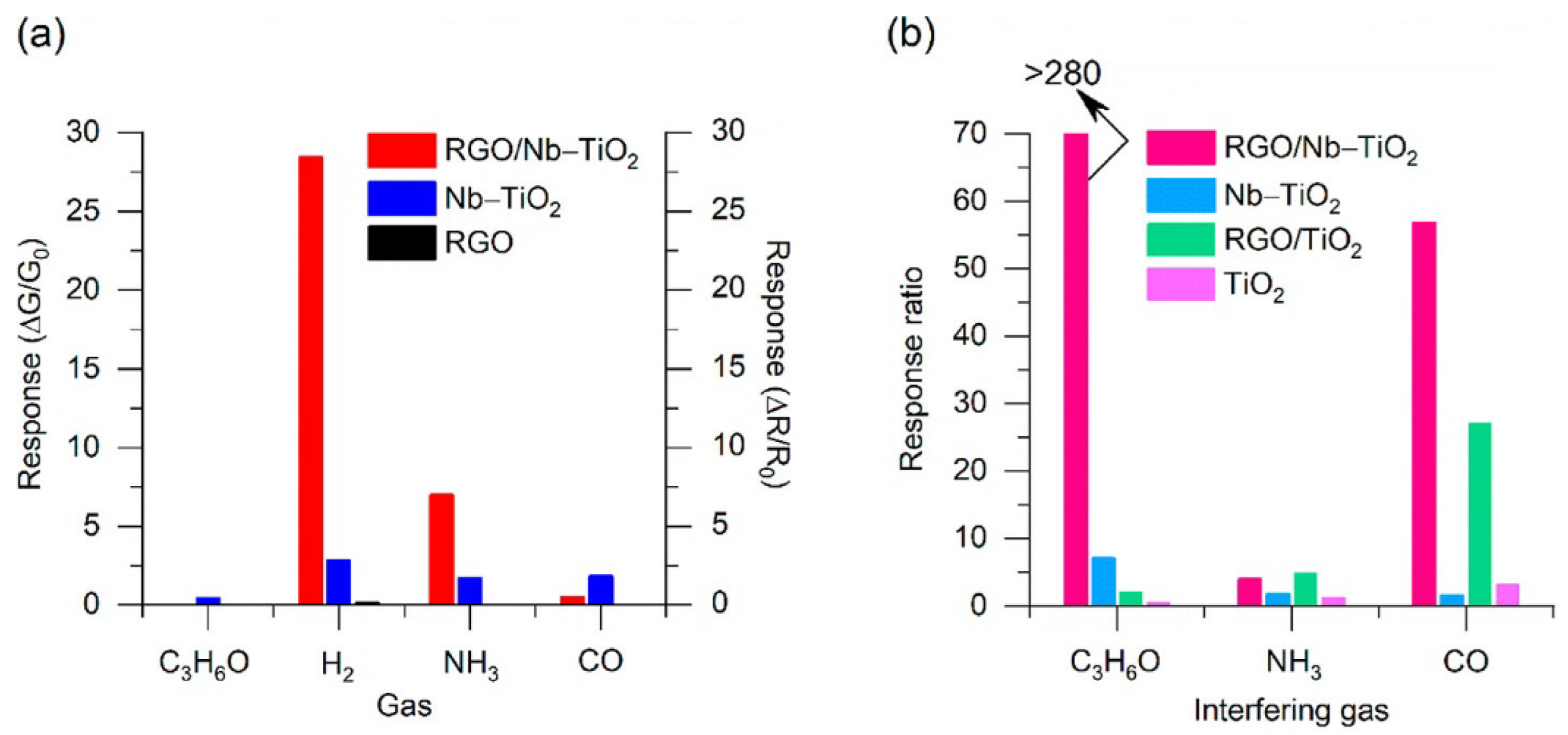
- Galstyan, V.; Ponzoni, A.; Kholmanov, I.; Natile, M.M.; Comini, E.; Nematov, S.; Sberveglieri, G. Investigation of Reduced Graphene Oxide and a Nb-Doped TiO2 Nanotube Hybrid Structure to Improve the Gas-Sensing Response and Selectivity. ACS Sens. 2019, 4, 2094–2100. [Google Scholar] [CrossRef]
- Alev, O.; Şennik, E.; Öztürk, Z.Z. Improved Gas Sensing Performance of P-Copper Ox-ide Thin Film/n-TiO2 Nanotubes Heterostructure. J. Alloy. Compd. 2018, 749, 221–228. [Google Scholar] [CrossRef]
- He, H.; Liu, J.; Liu, H.; Pan, Q.; Zhang, G. The Development of High-Performance Room Temperature NOx One-Dimensional NA0.23TiO2/TiO2 Compound Gas Sensor. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129444. [Google Scholar] [CrossRef]
- Gakhar, T.; Rosenwaks, Y.; Hazra, A. Fullerene (C60) Functionalized TiO2 Nanotubes for Conductometric Sensing of Formaldehyde. Sens. Actuators B Chem. 2022, 364, 131892. [Google Scholar] [CrossRef]
- Tong, X.; Shen, W.; Chen, X.; Corriou, J.P. A Fast Response and Recovery H2S Gas Sensor Based on Free-Standing TiO2 Nanotube Array Films Prepared by One-Step Anodization Method. Ceram. Int. 2017, 43, 14200–14209. [Google Scholar] [CrossRef]
- Tshabalala, Z.P.; Shingange, K.; Dhonge, B.P.; Ntwaeaborwa, O.M.; Mhlongo, G.H.; Motaung, D.E. Fabrication of Ultra-High Sensitive and Selective CH4 Room Temperature Gas Sensing of TiO2 Nanorods: Detailed Study on the Annealing Temperature. Sens. Actuators B Chem. 2017, 238, 402–419. [Google Scholar] [CrossRef]
- Prakash, C.; Dixit, A. Catalyst Free Rutile Phase TiO2 Nanorods as Efficient Hydrogen Sensor with Enhanced Sensitivity and Selectivity. Curr. Appl. Phys. 2022, 41, 183–190. [Google Scholar] [CrossRef]
- Cai, Z.; Park, S. Highly Selective Acetone Sensor Based on CO3O4-Decorated Porous TiO2 Nanofibers. J. Alloy. Compd. 2022, 919, 165875. [Google Scholar] [CrossRef]
- Cao, S.; Sui, N.; Zhang, P.; Zhou, T.; Tu, J.; Zhang, T. TiO2 Nanostructures with Different Crystal Phases for Sensitive Acetone Gas Sensors. J. Colloid Interface Sci. 2022, 607, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, A.; Alev, O.; Özdemir, O.; Arslan, L.Ç.; Büyükköse, S.; Öztürk, Z.Z. The Effect of AG Loading on Gas Sensor Properties of TiO2 Nanorods. Thin Solid Film. 2021, 726, 138662. [Google Scholar] [CrossRef]
- Chen, K.; Chen, S.; Pi, M.; Zhang, D. Sno2 Nanoparticles/TiO2 Nanofibers Heterostructures: In Situ Fabrication and Enhanced Gas Sensing Performance. Solid State Electron. 2019, 157, 42–47. [Google Scholar] [CrossRef]
- Xun, H.; Zhang, Z.; Yu, A.; Yi, J. Remarkably Enhanced Hydrogen Sensing of Highly-Ordered SnO2-Decorated TiO2 Nanotubes. Sens. Actuators B Chem. 2018, 273, 983–990. [Google Scholar] [CrossRef]
- Ghosal, S.; Bhattacharyya, P. A Potential Gas Sensor Device Based on PD/RGO/TiO2 Nanotube Ternary Hybrid Junction. Microelectron. Reliab. 2017, 78, 299–306. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Cheng, P.; Xu, L.; Dang, F.; Wang, T.; Lei, Z. In-Situ Generated TiO2/α-FE2O3 Heterojunction Arrays for Batch Manufacturing of Conductometric Acetone Gas Sensors. Sens. Actuators B Chem. 2021, 340, 129926. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Guo, C.; Cheng, X.; Zhu, C.; Xu, Y.; Major, Z.; Huo, L. Resistive Room Temperature DMA Gas Sensor Based on the Forest-like Unusual N-Type Pani/TiO2 Nanocomposites. Sens. Actuators B Chem. 2021, 342, 130067. [Google Scholar] [CrossRef]
- Safe, A.M.; Nikfarjam, A.; Hajghassem, H. UV Enhanced Ammonia Gas Sensing Properties of Pani/TiO2 Core-Shell Nanofibers. Sens. Actuators B Chem. 2019, 298, 126906. [Google Scholar] [CrossRef]
- Korotcenkov, G. Current Trends in Nanomaterials for Metal Oxide-Based Conductometric Gas Sensors: Advantages and Limitations. Part 1: 1D and 2D Nanostructures. Nanomaterials 2020, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, H.; Zhang, J.; Gao, J.; Zhu, G.; Yang, Z.; Yin, F.; Wang, C. Crystal Facet-Dependent P-Type and N-Type Sensing Responses of TiO2 Nanocrystals. Sens. Actuators B Chem. 2018, 263, 557–567. [Google Scholar] [CrossRef]
- Liu, C.; Lu, H.; Zhang, J.; Yang, Z.; Zhu, G.; Yin, F.; Gao, J.; Chen, C.; Xin, X. Abnormal P-Type Sensing Response of TiO2 Nanosheets with Exposed {001} Facets. J. Alloy. Compd. 2017, 705, 112–117. [Google Scholar] [CrossRef]
- Wang, W.; Liu, F.; Wang, B.; Wang, Y. Effect of Defects in TiO2 Nanoplates with Exposed {001} Facets on the Gas Sensing Properties. Chin. Chem. Lett. 2019, 30, 1261–1265. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Lu, H.; Leng, D.; Li, G.; Liu, Y.; Liang, Q.; Gao, J.; Wang, C.; Zhu, B. Construction of Anatase@Rutile Core@Shell TiO2 Nanosheets with Controllable Shell Layer Thicknesses for Enhanced Ethanol Sensing. Sens. Actuators B Chem. 2020, 325, 128815. [Google Scholar] [CrossRef]
- Ge, W.; Jiao, S.; Chang, Z.; He, X.; Li, Y. Ultrafast Response and High Selectivity toward Acetone Vapor Using Hierarchical Structured TiO2 Nanosheets. ACS Appl. Mater. Interfaces 2020, 12, 13200–13207. [Google Scholar] [CrossRef]
- Wang, P.; Song, T.; Gao, G.; Matras-Postolek, K.; Yang, P. SnO2 Clusters Embedded in TiO2 Nanosheets: Heterostructures and Gas Sensing Performance. Sens. Actuators B Chem. 2022, 357, 131433. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, L.; Du, H.; Hu, C.; Liu, N.; Ma, S.; Jia, Y.; Fan, X. Hier-archical Flower-like TiO2 Microspheres for High-Selective NH3 Detection: A Density Functional Theory Study. Sens. Actuators B Chem. 2021, 345, 130303. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, L.; Chen, W.; Peng, Z.; Li, Y. A Strategy for Supportless Sensors: Fluorine Doped TiO2 Nanosheets Directly Grown onto TI Foam Enabling Highly Sensitive Detection toward Acetone. Sens. Actuators B Chem. 2020, 322, 128633. [Google Scholar] [CrossRef]
- Wen, J.; Song, Z.; Ding, J.; Wang, F.; Li, H.; Xu, J.; Zhang, C. Mxene-Derived TiO2 Nanosheets Decorated with Ag Nanoparticles for Highly Sensitive Detection of Ammonia at Room Temperature. J. Mater. Sci. Technol. 2022, 114, 233–239. [Google Scholar] [CrossRef]
- Wang, D.; Yang, J.; Bao, L.; Cheng, Y.; Tian, L.; Ma, Q.; Xu, J.; Li, H.J.; Wang, X. Pd Nanocrystal Sensitization Two-Dimension Porous TiO2 for Instantaneous and High Efficient H2 Detection. J. Colloid Interface Sci. 2021, 597, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Murali, G.; Reddeppa, M.; Seshendra Reddy, C.; Park, S.; Chandrakalavathi, T.; Kim, M.D.; In, I. Enhancing the Charge Carrier Separation and Transport via Nitrogen-Doped Graphene Quantum Dot-TiO2 Nanoplate Hybrid Structure for an Efficient No Gas Sensor. ACS Appl. Mater. Interfaces 2020, 12, 13428–13436. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Haidry, A.A.; Liu, Y.; Sun, L.; Xie, L.; Fatima, Q.; Yao, Z. Strongly Coupled AG/TiO2 Heterojunction: From One-Step Facile Synthesis to Effective and Stable Ethanol Sensing Performances. J. Mater. Sci. Mater. Electron. 2018, 29, 19219–19227. [Google Scholar] [CrossRef]
- Li, Z.; Ding, D.; Liu, Q.; Ning, C. Hydrogen Sensing with Ni-Doped TiO2 Nanotubes. Sensors 2013, 13, 8393–8402. [Google Scholar] [CrossRef]
- Mariammal, R.N.; Ramachandran, K. Increasing the Reactive Sites of Zno Nanoparticles by Li Doping for Ethanol Sensing. Mater. Res. Express 2018, 6, 015024. [Google Scholar] [CrossRef]
- Varpula, A.; Novikov, S.; Haarahiltunen, A.; Kuivalainen, P. Transient Characterization Techniques for Resistive Metal-Oxide Gas Sensors. Sens. Actuators B Chem. 2011, 159, 12–26. [Google Scholar] [CrossRef]
- Sarf, F. Metal Oxide Gas Sensors by Nanostructures. In Gas Sensors; IntechOpen Limited: London, UK, 2020. [Google Scholar] [CrossRef]
- Mathew, S.; John, B.K.; Abraham, T.; Mathew, B. Metal-Doped Titanium Dioxide for Environmental Remediation, Hydrogen Evolution and Sensing: A Review. ChemistrySelect 2021, 6, 12742–12751. [Google Scholar] [CrossRef]
- Li, Z.; Haidry, A.A.; Wang, T.; Yao, Z.J. Low-Cost Fabrication of Highly Sensitive Room Temperature Hydrogen Sensor Based on Ordered Mesoporous Co-Doped TiO2 Structure. Appl. Phys. Lett. 2017, 111, 032104. [Google Scholar] [CrossRef]
- Wu, K.; Debliquy, M.; Zhang, C. Room Temperature Gas Sensors Based on CE Doped TiO2 Nanocrystals for Highly Sensitive NH3 Detection. Chem. Eng. J. 2022, 444, 136449. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, X.; Cui, H.; Zhang, J. A First Principle Simulation of Competitive Adsorption of SF6 Decomposition Components on Nitrogen-Doped Anatase TiO2 (101) Surface. Appl. Surf. Sci. 2017, 422, 331–338. [Google Scholar] [CrossRef]
- Gao, L.; Gan, W.; Qiu, Z.; Cao, G.; Zhan, X.; Qiang, T.; Li, J. Biomorphic Carbon-Doped TiO2 for Photocatalytic Gas Sensing with Continuous Detection of Persistent Volatile Organic Compounds. ACS Appl. Nano Mater. 2018, 1, 1766–1775. [Google Scholar] [CrossRef]
- Wang, K.; Peng, T.; Wang, Z.; Wang, H.; Chen, X.; Dai, W.; Fu, X. Correlation between the H2 Response and Its Oxidation over TiO2 and n Doped TiO2 under UV Irradiation Induced by Fermi Level. Appl. Catal. B Environ. 2019, 250, 89–98. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Cui, H. Adsorption Mechanism of SF6 Decomposition Components onto N, F-Co-Doped TiO2: A DFT Study. J. Fluor. Chem. 2018, 213, 18–23. [Google Scholar] [CrossRef]
- Zhong, Z.-C.; Jing, Z.-J.; Liu, K.-Y.; Liu, T. Acetylene Sensing by Zno/TiO2 Nano-particles. J. Nanoelectron. Optoelectron. 2020, 15, 41–45. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.Y.; Nam, M.S.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Synergistic Effect between UV Light and Pani/CO3O4 Content on TiO2 Composite Nanoparticles for Room-Temperature Acetone Sensing. Sens. Actuators B Chem. 2023, 375, 132868. [Google Scholar] [CrossRef]
- Xie, L.; Li, Z.; Sun, L.; Dong, B.; Fatima, Q.; Wang, Z.; Yao, Z.; Haidry, A.A. Sol-Gel Synthesis of TiO2 with P-Type Response to Hydrogen Gas at Elevated Temperature. Front. Mater. 2019, 6, 96. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, D.; Mi, Q. A High-Performance Room Temperature Benzene Gas Sensor Based on COTIO3 Covered TiO2 Nanospheres Decorated with Pd Nanoparticles. Sens. Actuators B Chem. 2022, 350, 130830. [Google Scholar] [CrossRef]
- Tong, X.; Shen, W.; Chen, X. Enhanced H2S Sensing Performance of Cobalt Doped Free-Standing TiO2 Nanotube Array Film and Theoretical Simulation Based on Density Functional Theory. Appl. Surf. Sci. 2019, 469, 414–422. [Google Scholar] [CrossRef]
- Moumen, A.; Kumarage, G.C.; Comini, E. P-Type Metal Oxide Semiconductor Thin Films: Synthesis and Chemical Sensor Applications. Sensors 2022, 22, 1359. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Luo, Y.; Gao, L.; Li, T.; Duan, G. Au-Decorated Znfe2o4 Yolk–Shell Spheres for Trace Sensing of Chlorobenzene. ACS Appl. Mater. Interfaces 2020, 12, 16792–16804. [Google Scholar] [CrossRef] [PubMed]
- Şennik, E.; Alev, O.; Öztürk, Z.Z. The Effect of PD on the H 2 and VOC Sensing Properties of TiO2 Nanorods. Sens. Actuators B Chem. 2016, 229, 692–700. [Google Scholar] [CrossRef]
- Zhao, P.X.; Tang, Y.; Mao, J.; Chen, Y.X.; Song, H.; Wang, J.W.; Song, Y.; Liang, Y.Q.; Zhang, X.M. One-Dimensional mos2-Decorated TiO2 Nanotube Gas Sensors for Efficient Alcohol Sensing. J. Alloy. Compd. 2016, 674, 252–258. [Google Scholar] [CrossRef]
- Moumen, A.; Zappa, D.; Poli, N.; Comini, E. Catalyst–Assisted Vapor Liquid Solid Growth of α-Bi2O3 Nanowires for Acetone and Ethanol Detection. Sens. Actuators B Chem. 2021, 346, 130432. [Google Scholar] [CrossRef]
- Zappa, D.; Galstyan, V.; Kaur, N.; Arachchige, H.M.M.; Sisman, O.; Comini, E. Metal Oxide-Based Heterostructures for Gas Sensors’—A Review. Anal. Chim. Acta 2018, 1039, 1–23. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, W.; Tao, T.; Li, X.; Xia, X.; Bao, Y.; Lourenço, M.; Homewood, K.; Huang, Z.; Gao, Y. Hierarchical Nio/TiO2 Heterojuntion-Based Conduc-tometric Hydrogen Sensor with Anti-CO-Interference. Sens. Actuators B Chem. 2023, 380, 133321. [Google Scholar] [CrossRef]
- Alev, O.; Kılıç, A.; Çakırlar, Ç.; Büyükköse, S.; Öztürk, Z.Z. Gas Sensing Properties of P-Co3O4/N-TiO2 Nanotube Heterostructures. Sensors 2018, 18, 956. [Google Scholar] [CrossRef]
- Wang, X.; Sang, Y.; Wang, D.; Ji, S.; Liu, H. Enhanced Gas Sensing Property of Sno2 Nanoparticles by Constructing the SNO2–TiO2 Nanobelt Heterostructure. J. Alloy. Compd. 2015, 639, 571–576. [Google Scholar] [CrossRef]
- Sun, D.; Luo, Y.; Debliquy, M.; Zhang, C. Graphene-Enhanced Metal Oxide Gas Sensors at Room Temperature: A Review. Beilstein J. Nanotechnol. 2018, 9, 2832–2844. [Google Scholar] [CrossRef]
- Jian, Y.; Hu, W.; Zhao, Z.; Cheng, P.; Haick, H.; Yao, M.; Wu, W. Gas Sensors Based on Chemi-Resistive Hybrid Functional Nanomaterials. Nano-Micro Lett. 2020, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Karthik, P.; Gowthaman, P.; Venkatachalam, M.; Rajamanickam, A.T. Propose of High Performance Resistive Type H2S and CO2 Gas Sensing Response of Reduced Graphene Oxide/Titanium Oxide (RGO/TiO2) Hybrid Sensors. J. Mater. Sci. Mater. Electron. 2020, 31, 3695–3705. [Google Scholar] [CrossRef]
- Seekaew, Y.; Wisitsoraat, A.; Phokharatkul, D.; Wongchoosuk, C. Room Temperature Toluene Gas Sensor Based on TiO2 Nanoparticles Decorated 3D Graphene-Carbon Nanotube Nanostructures. Sens. Actuators B Chem. 2019, 279, 69–78. [Google Scholar] [CrossRef]
- Long, H.; Harley-Trochimczyk, A.; Pham, T.; Tang, Z.; Shi, T.; Zettl, A.; Carraro, C.; Worsley, M.A.; Maboudian, R. High Surface Area MoS2/Graphene Hybrid Aerogel for Ultrasensitive NO2 Detection. Adv. Funct. Mater. 2016, 26, 5158–5165. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, T.; Chen, F.; Sun, X.; Li, X.; Yu, Z.; Wan, P.; Chen, X. Hierarchical Graphene–Polyaniline Nanocomposite Films for High-Performance Flexible Electronic Gas Sensors. Nanoscale 2016, 8, 12073–12080. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Zhu, X.; Zhou, X.; Chi, L. An Ultrasensitive Organic Semicon-ductor NO2 Sensor Based on Crystalline Tips-Pentacene Films. Adv. Mater. 2017, 29, 1703192. [Google Scholar] [CrossRef]
- Jalil, A.R.; Chang, H.; Bandari, V.K.; Robaschik, P.; Zhang, J.; Siles, P.F.; Li, G.; Bürger, D.; Grimm, D.; Liu, X.; et al. Fully Integrated Organic Nanocrystal Diode as High Performance Room Temperature NO2 Sensor. Adv. Mater. 2016, 28, 2971–2977. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale Metal Oxide-Based Heterojunctions for Gas Sensing: A Review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, W.; Ye, H.; Li, Y. Enhanced Carbon Monoxide Sensing Properties of TiO2 with Exposed (0 0 1) Facet: A Combined First-Principle and Experimental Study. Appl. Surf. Sci. 2018, 442, 507–516. [Google Scholar] [CrossRef]
- Wang, B.; Deng, L.; Sun, L.; Lei, Y.; Wu, N.; Wang, Y. Growth of TiO2 Nanostructures Exposed {001} and {110} Facets on Sic Ultrafine Fibers for Enhanced Gas Sensing Performance. Sens. Actuators B Chem. 2018, 276, 57–64. [Google Scholar] [CrossRef]





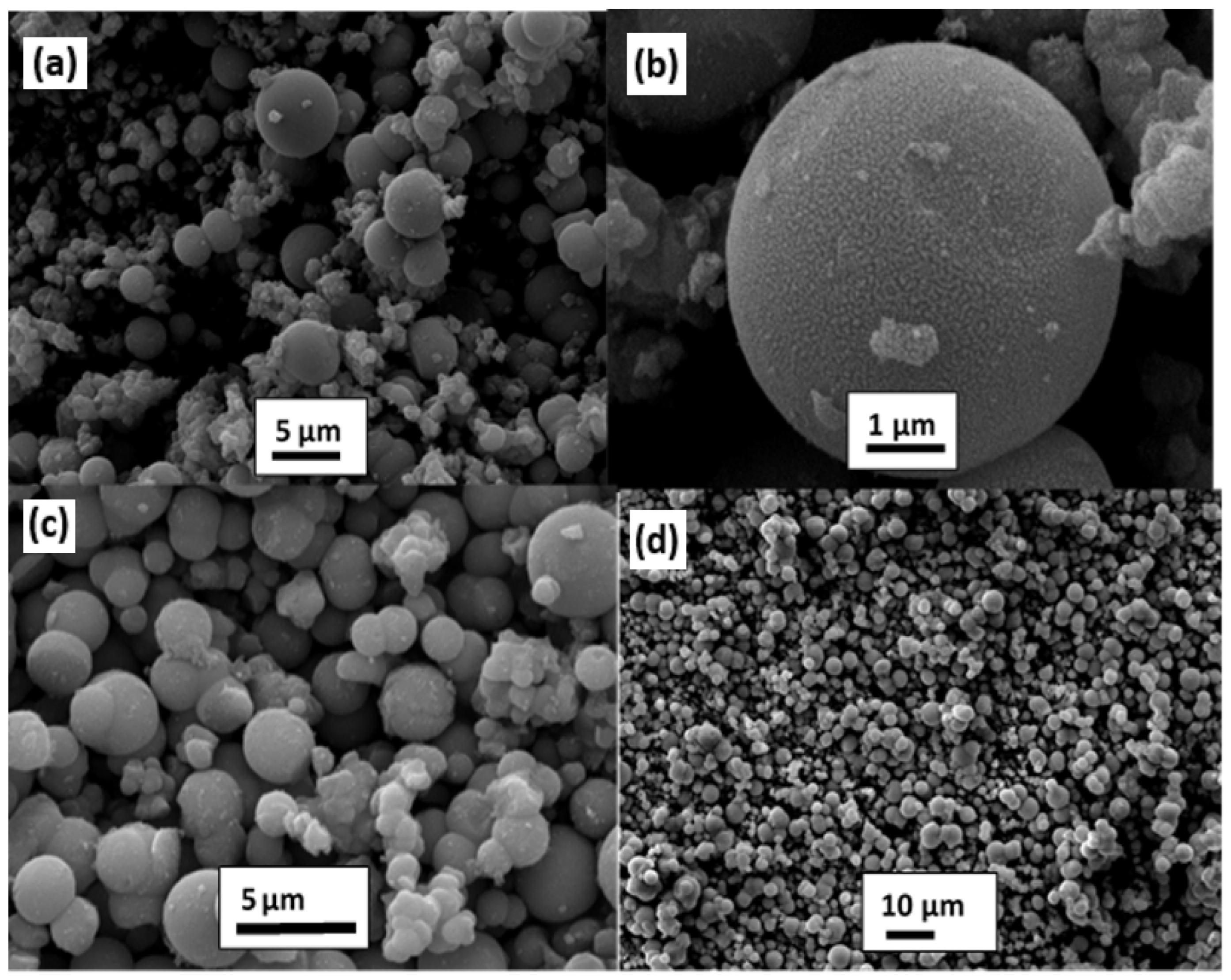
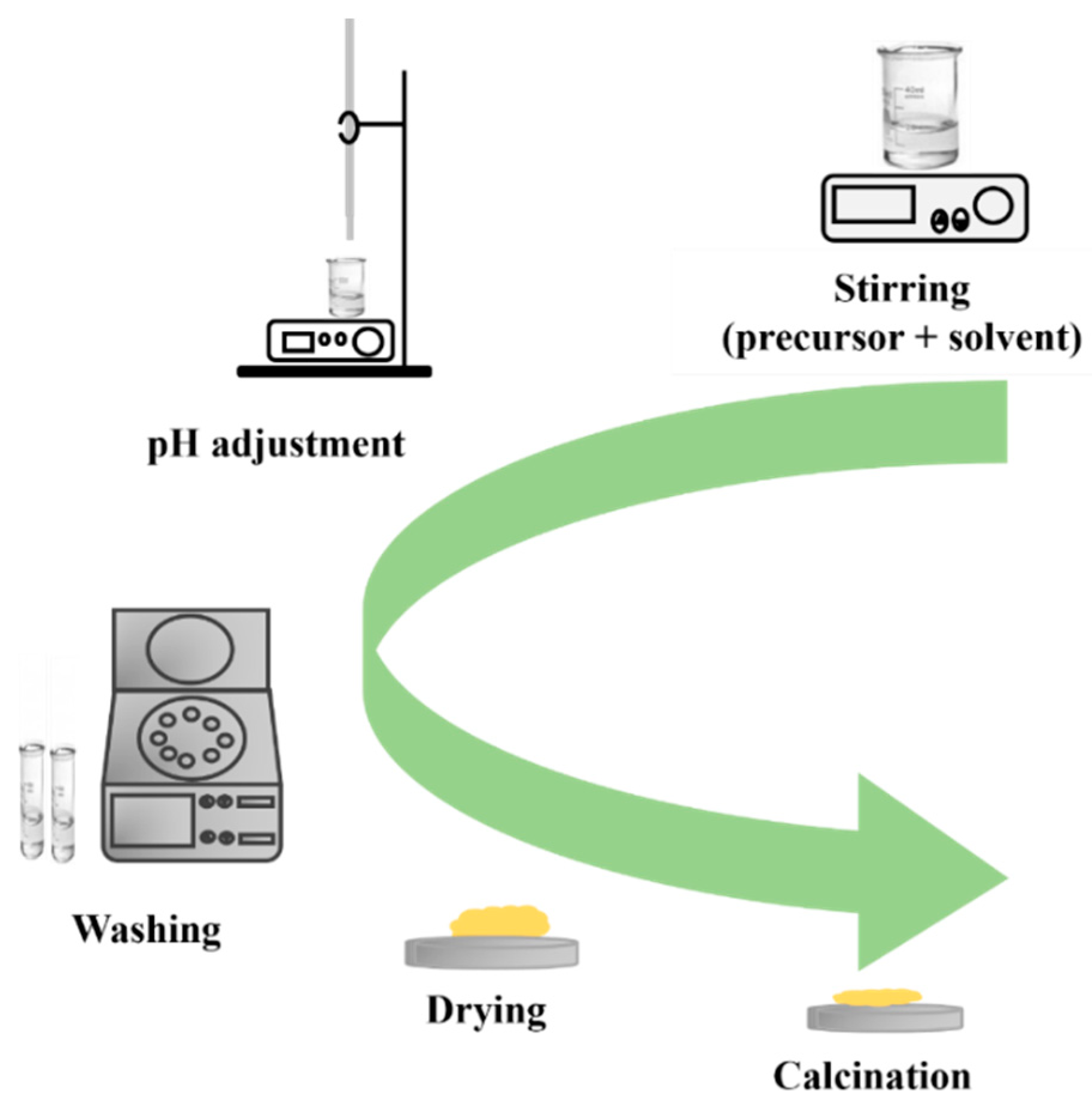
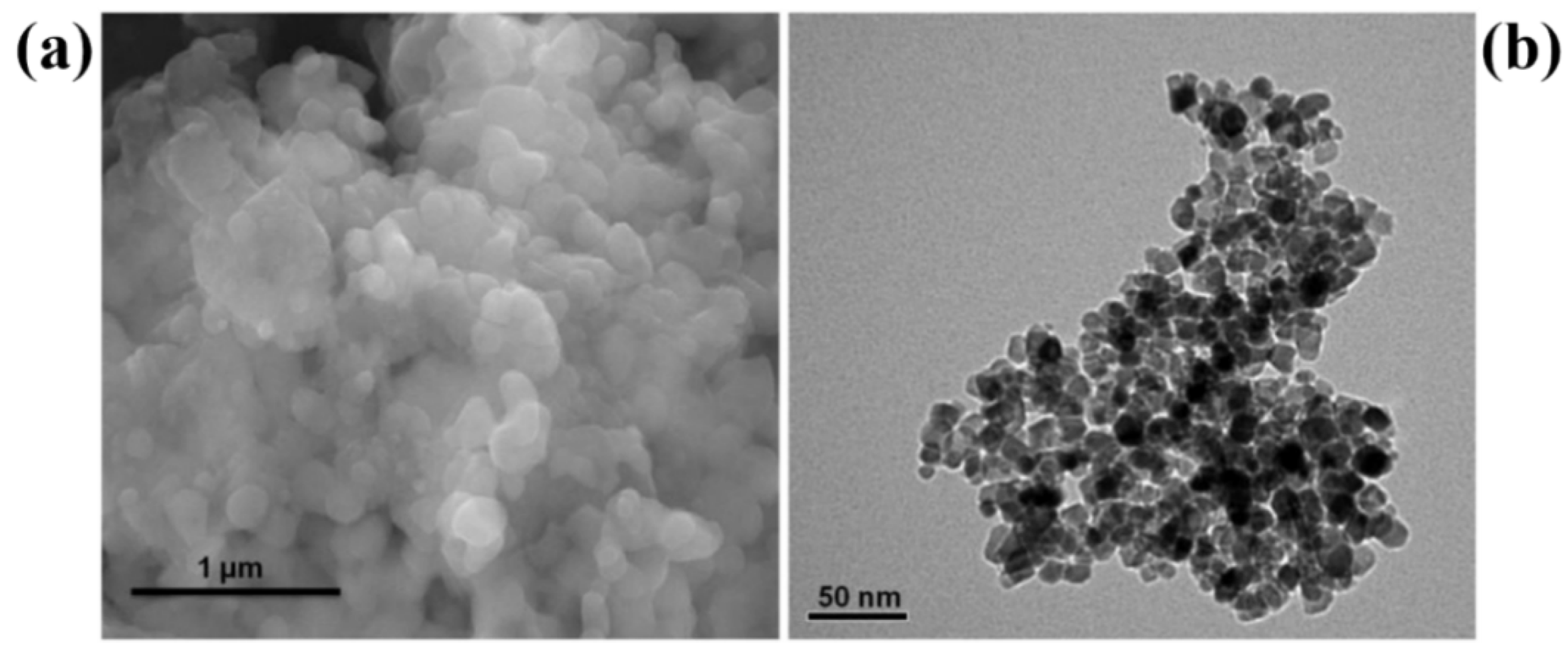
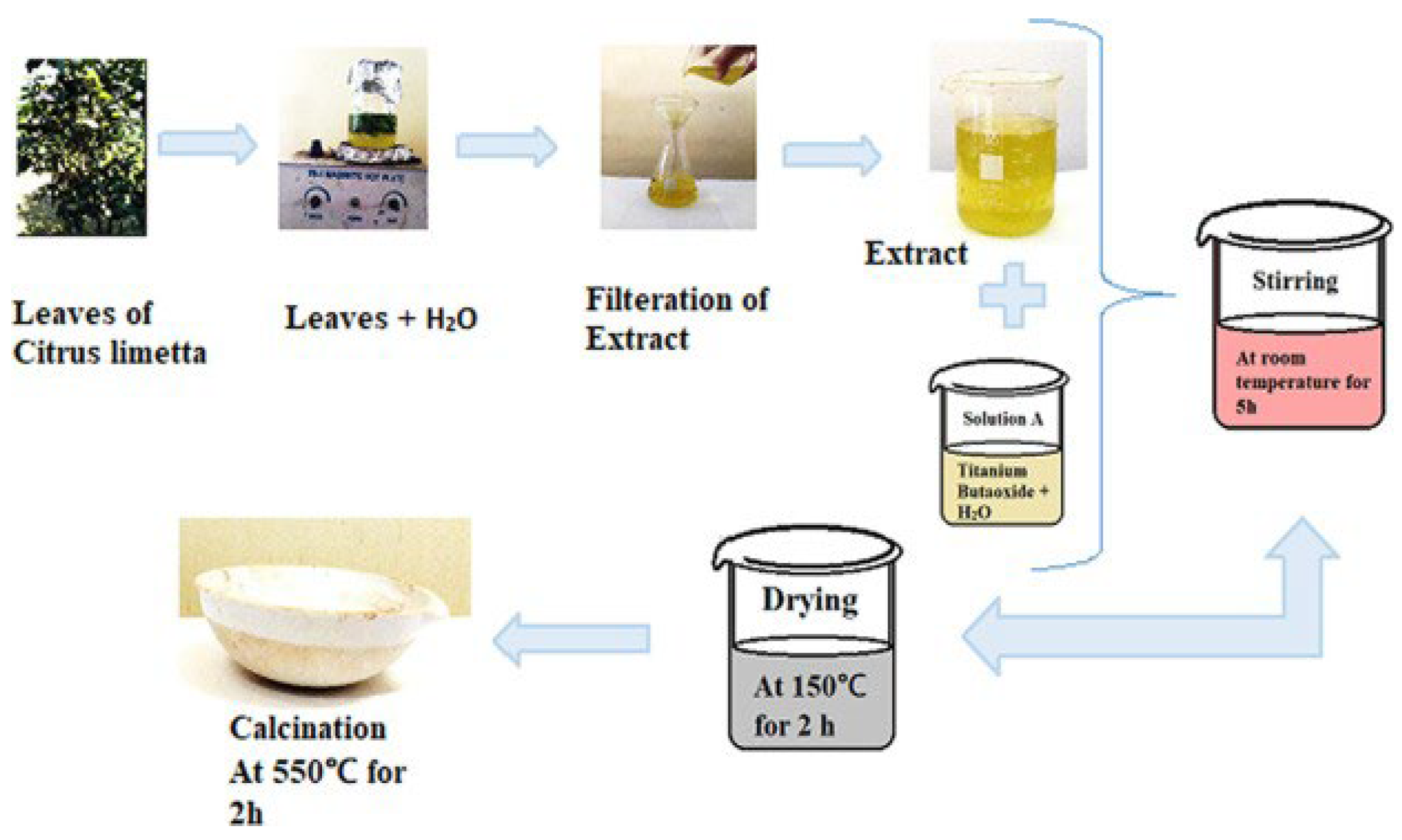
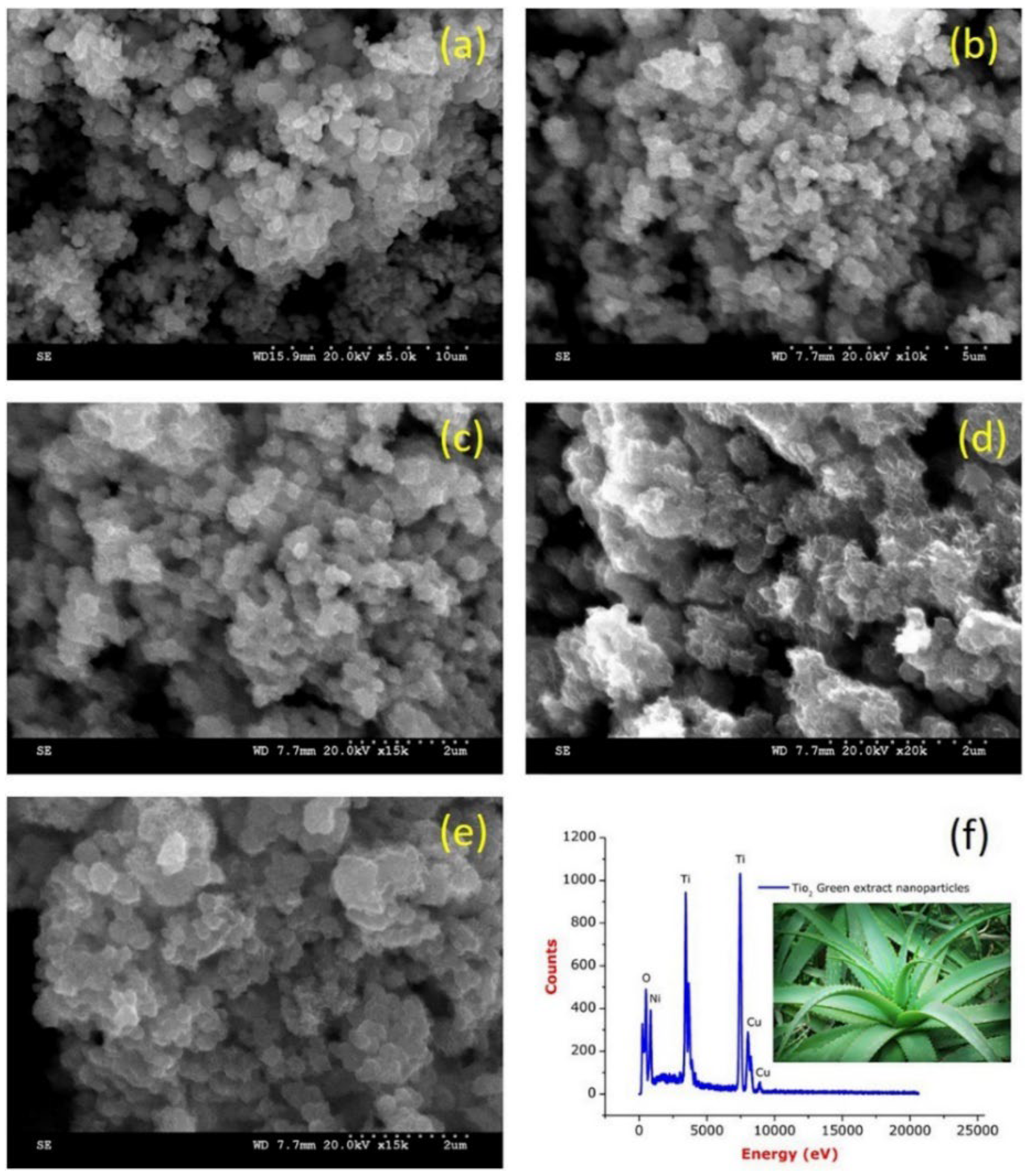
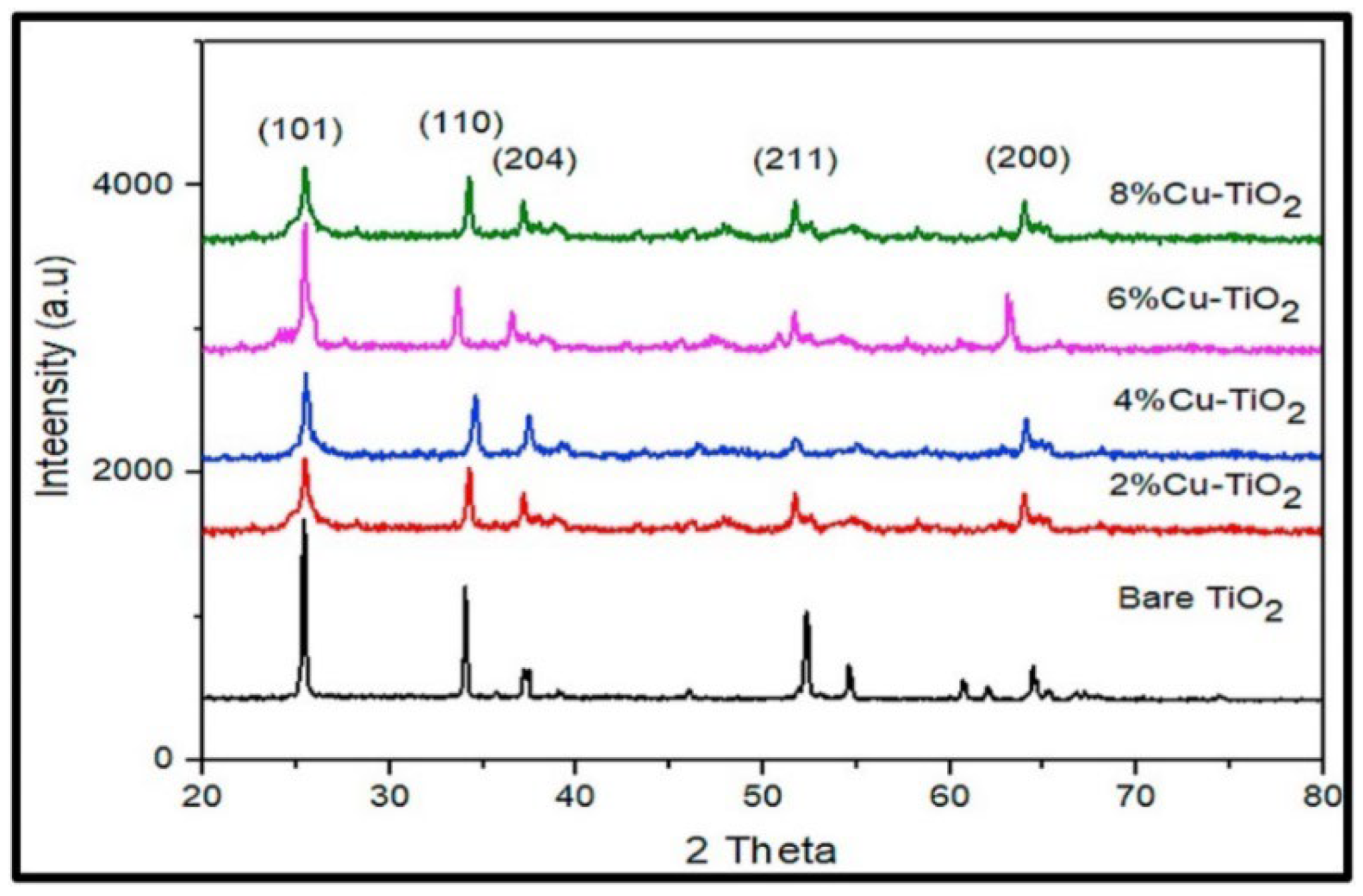

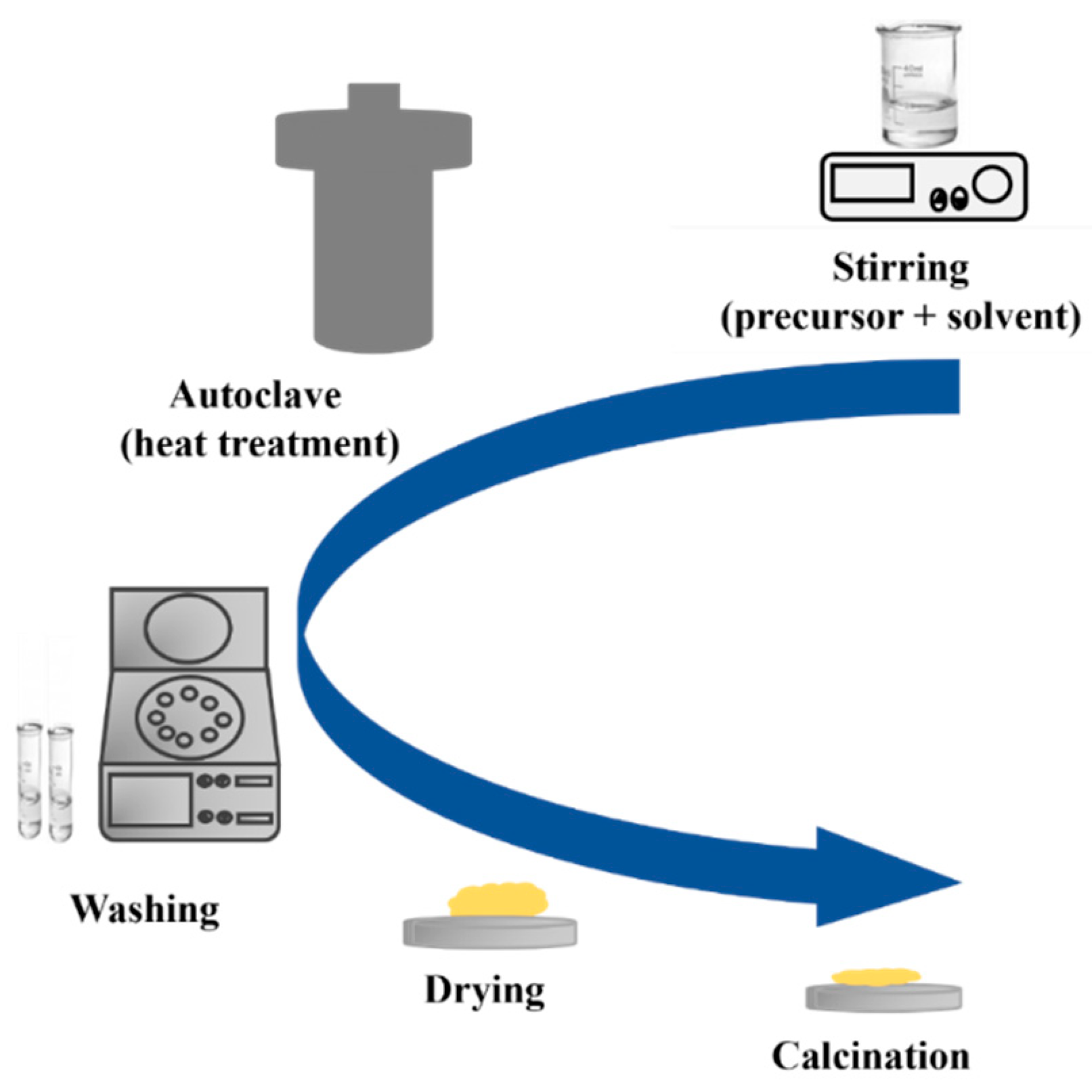


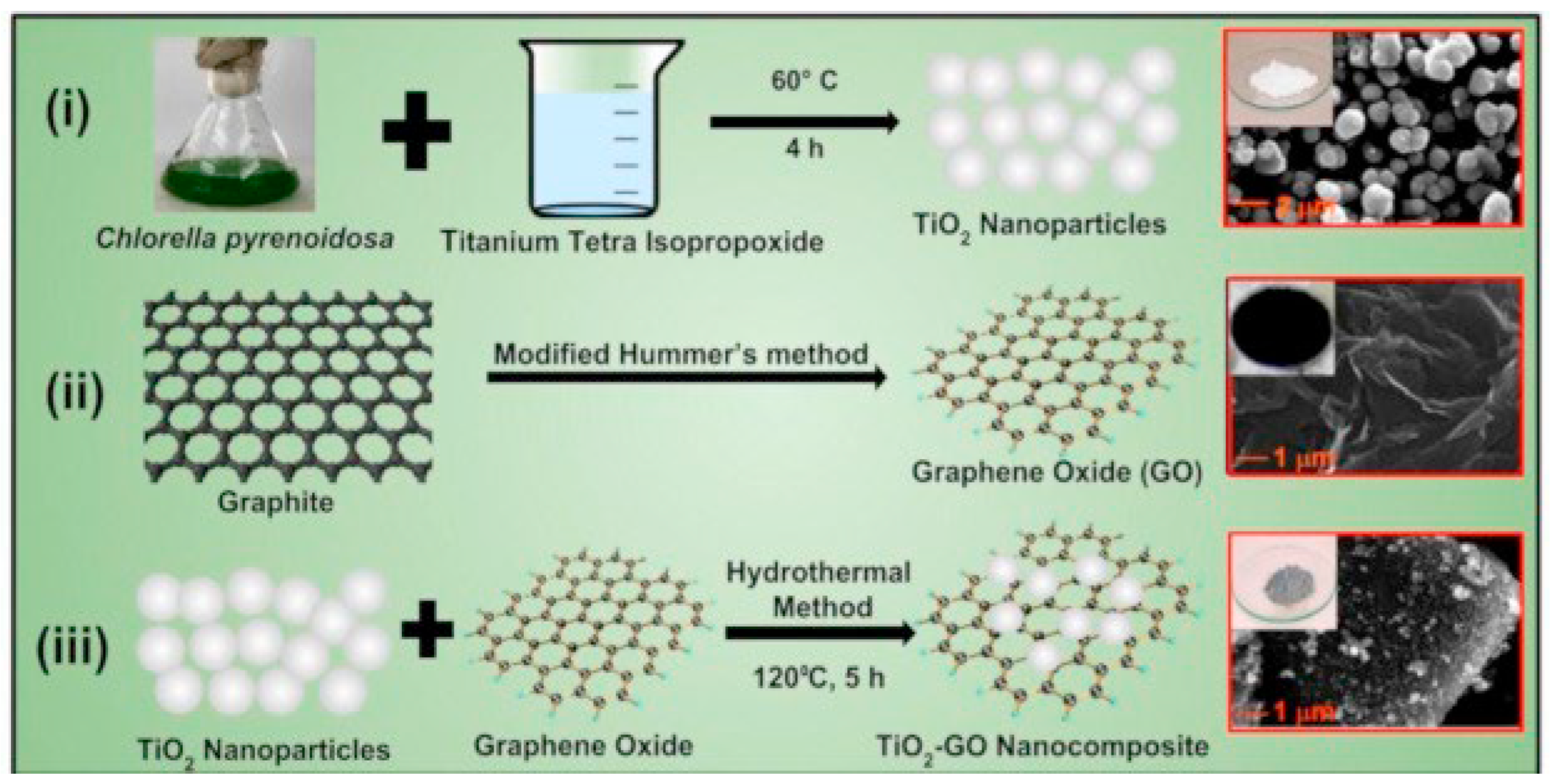
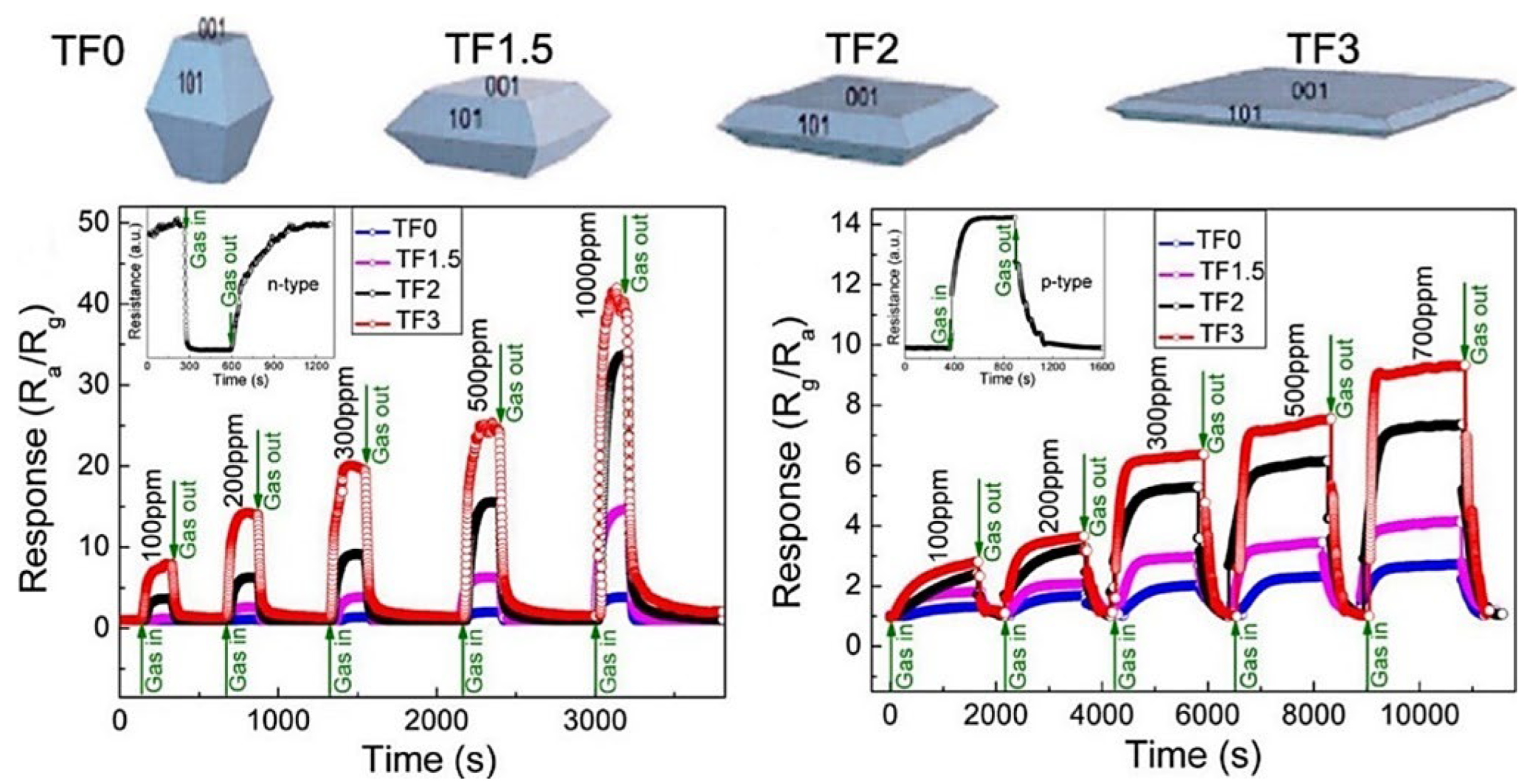

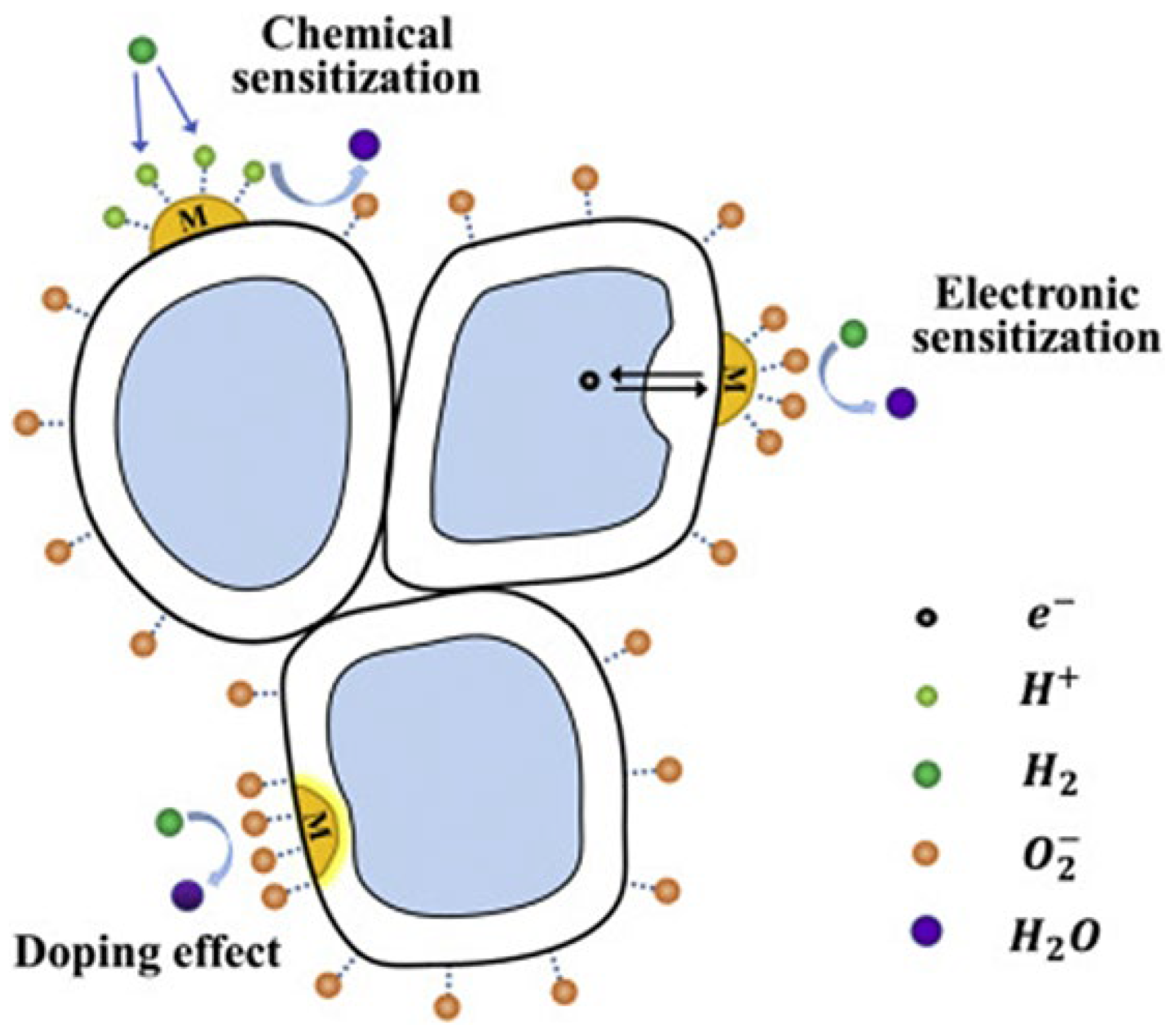
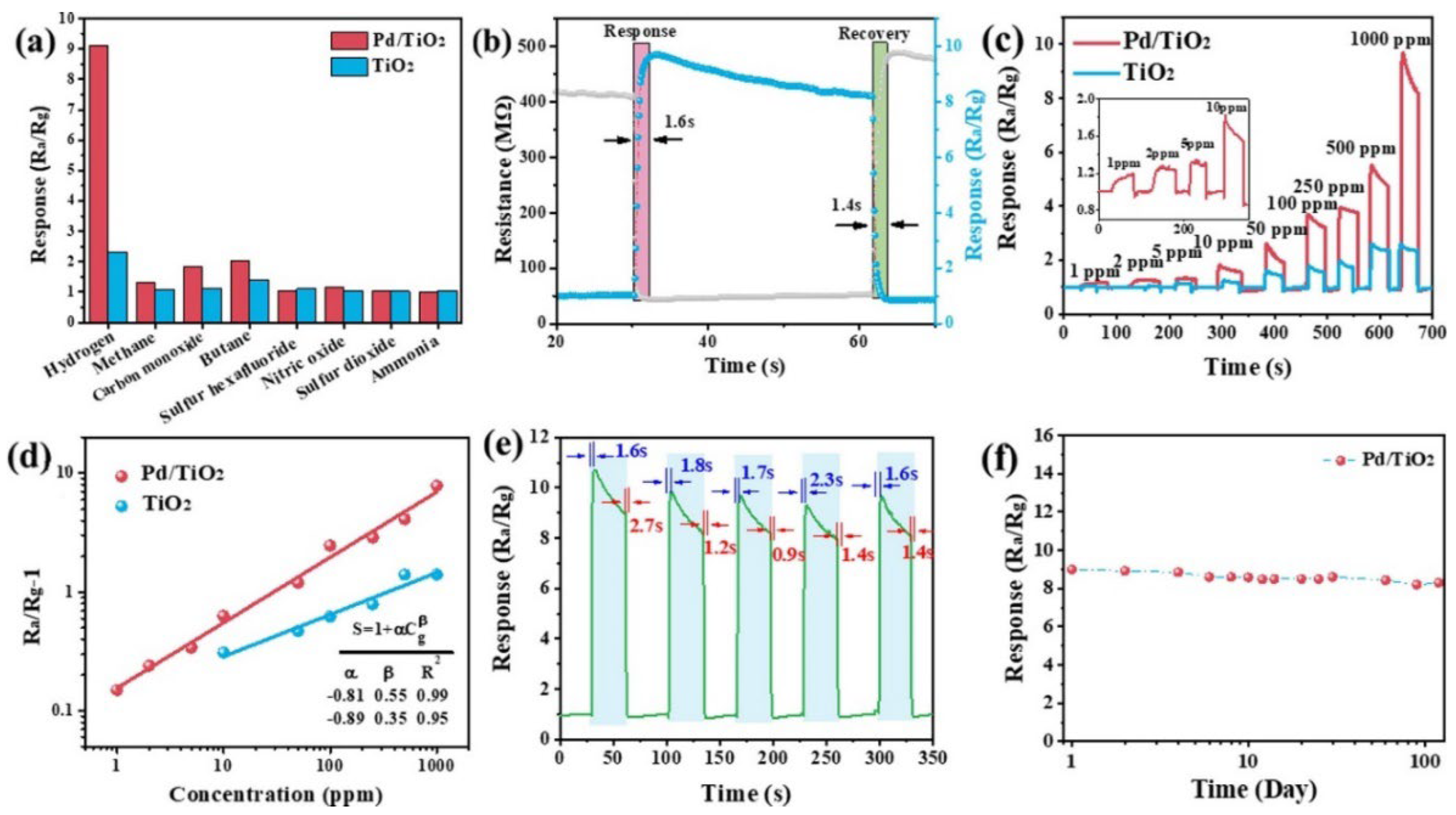
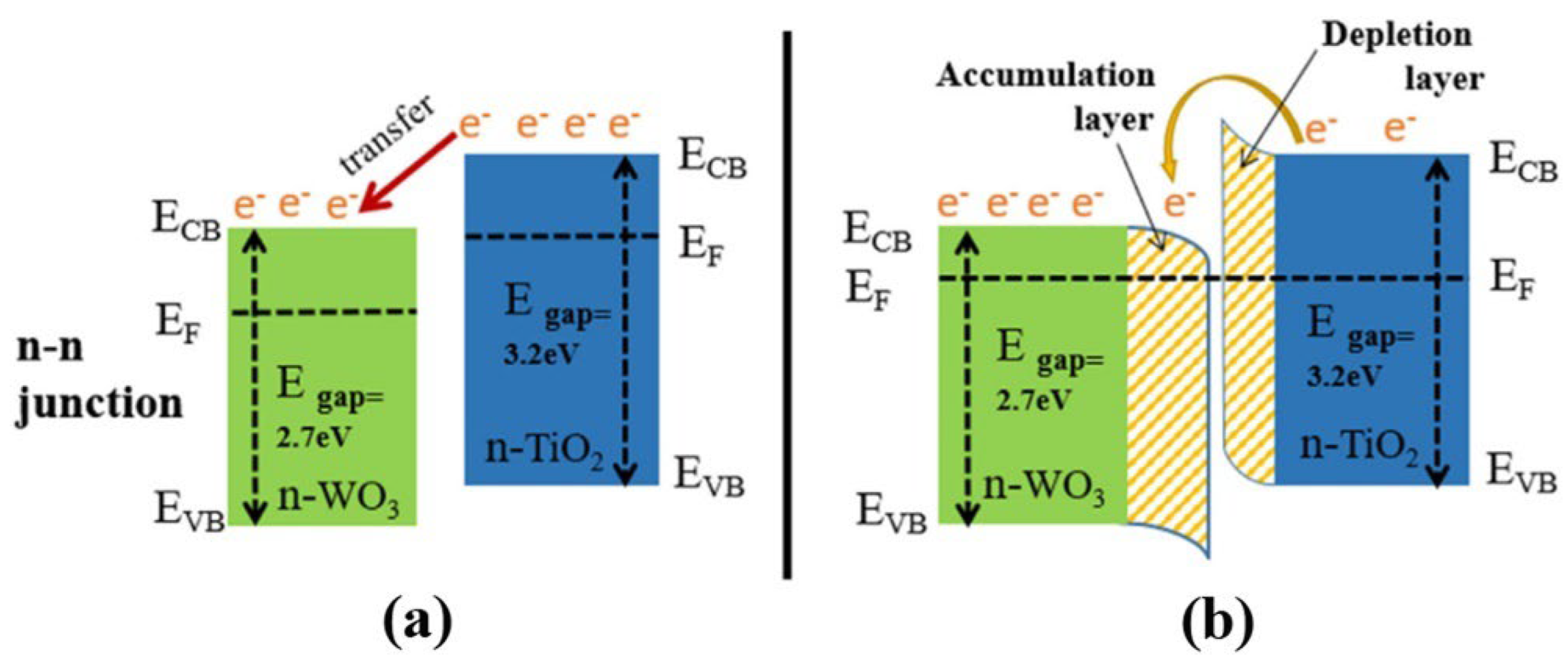
| Chemical Compounds | Synthesis Conditions | Structural and Morphological Properties | Ref. |
|---|---|---|---|
| Titanium isopropoxide (TTIP) Deionized water (DI) Isopropanol | Stirring: TTIP + isopropanol—Time: 30 min Stirring: TTIP + isopropanol + DI water—Time: 2 h Ultrasonic treatment: Temp: 80 °C—Time: 1 h Aging temp: RT—Time: 22 h Washing: DI water and ethanol Drying temp: 70 °C—Time: 2 h Calcination—300–800 °C | Phase: anatase + rutile Crystallite size: increases with increase in calcination temperate from 8.45 to 38.55 nm Calcination (300 °C) Morphology: nanoparticles with roughly spherical and spongy shape Particle size: ~35 nm Calcination (800 °C) Morphology: non-uniform particles | [32] |
| Citric acid Ethanol TTIP Ethylene glycol (EG) | Stirring: citric acid + ethanol + TTIP Temp: RT—Time: 1 h Doping: NbOEt5 and La (NO3) 3·6H2O added to the solution Stirring temp: RT—Time: 1 h Stirring: solution + EG Calcination time: 2 h—Temp: 400 °C | Phase: anatase Morphology: semi-spherical nanoparticles Crystallite size: ~30 nm | [38] |
| Titanium chloride (TiCl4) Malva parviflora extract (MPE) Deionized water | Stirring: TiCl4 + MPE + DI water Time: 3 h Doping: Thiourea added to have S2− Aging time: 24 h—Temp: 60 °C Calcination temp: 400 °C—Time: 3 h | Phase: Anatase related to TiO2 Morphology: S-doped TiO2 nanoparticles Particle size: 20.3 nm | [39] |
| TTIP Glacial acetic acid Double-distilled water | Stirring: TTIP + glacial acetic acid + double-distilled water—Time: 5 h Ultrasonic treatment: Time 30 min Aging time: 24 h in dark Thermal treatment: Temp: 80 °C—Time: 10 h Drying temp: 100 °C Calcination temp: 600 °C—Time: 5 h | Phase: anatase Crystallite size: ~10.88 nm Morphology: spherical and rod Particle size: ~121 nm | [40] |
| Phyllanthus niruri leaf extract Distilled water TTIP | Plant Stirring: Phyllanthus niruri leaf + distilled water Time: 2 h—Temp: 50 °C TiO2 preparation Stirring: TTIP + plant extract Time: 4 h—Temp: RT Centrifuging time: 20 min Drying temp: 80 °C—Time: 24 h Calcination temp: 600 °C—Time: 3 h | Phase: anatase Crystallite size: ~20.90 nm Morphology: nanoparticles | [41] |
| Plumeria leaves TTIP Hexane Ethanol | Stirring: TTIP + hexane + plant extract Washing: ethanol Drying temp: 100 °C—120 °C Calcination temp: 500 °C—Time: 4 h | Phase: anatase Morphology: nanoparticles Particle size: 77.6 nm | [42] |
| Te (VI) acid Ti (IV) butoxide EG | Stirring A: Ti (IV) butoxide + EG Stirring B: Te (VI) acid + EG Stirring: A + B pH: 7.5–8 Heat treatment temp: 200 °C Calcination temp: 300 °C to 700 °C—Time: 2 h | Phase: anatase Crystallite size: ~60 nm Morphology: spherical nanoparticles Particle size: 50 to 100 nm | [43] |
| TBOT Ethanol | Stirring: TBOT + ethanol Time: 2 h—Temp: RT Washing: ethanol Drying time: 10 h—Temp: 60 °C Calcination temp: 200 °C—Time: 2 h | Phase: anatase Morphology: spherical particles Particle size: 30–40 nm | [44] |
| TTIP Ethanol Cobalt acetate Cadmium acetate Urea | N-Co-Cd-doped TiO2 nanocomposite Stirring: TTIP + ethanol—Time: 30 min Doping: 1% Co, Cd, and N Drying temp: 100 °C—Time: 24 h Calcination temp: 550 °C—Time: 4 h | Phase: anatase + β-Cadmium nitrate-cobalt titanium + titanium nitride Morphology: mesoporous nanocomposite | [45] |
| TTIP Isopropanol CuCl2.2H2O | Stirring solution 1: TTIP + isopropanol Temp: RT—Time: 2 h Stirring solution 2: CuCl2.2H2O + isopropanol Doping: Solution 1 added to solution 2 Calcination time: 2 h—Temp: 400–500–600 °C | Doping concentration: 5% Calcination temp: 400 °C Phase: anatase Crystallite size: 4.63 nm Calcination temp: 500 °C Phase: rutile Crystallite size: 8.7 nm | [46] |
| TiCl4 Distilled water Ethanol | Stirring: TiCl4 + distilled water + ethanol Time: 30 min—Temp: RT pH: from 1 to 10 Aging time: 12 h Washing: distilled water Drying temp: 120 °C—Time: 1 h Calcination temp: 500, 600, 700, and 800 °C Time: 4 h | Phase: anatase Morphology: non-uniform cluster of TiO2 | [47] |
| Plant | Synthesis Conditions | Structural and Morphological Properties | Ref. |
|---|---|---|---|
| Leaf of Trigonella foenum | Plant extract Stirring: leaves + distilled water Time: 20 min TiO2 preparation Stirring: titanium oxysulphate + plant extract + NaOH Calcination temp: 700 °C—Time: 3 h | Phase: anatase Morphology: spherical NPs Particle size: ≈25 nm | [59] |
| Citrus limetta leaves | Plant extract Boiling: leaves + distilled water Temp: 100 °C—Time: 15 min TiO2 preparation Stirring: titanium butoxide + distilled water + citrus limetta extract Time: 5 h Aging time: 24 h—Temp: RT Drying temp: 150 °C—Time: 2 h Calcination temp: 550 °C—Time: 2 h | Phase: anatase Gain size: 45 nm Morphology: NPs with spherical shape Particle size: 80–100 nm | [60] |
| Leaf of piper betel | Plant extract Stirring: leaves + distilled water Time: 30 min—Temp: 50 °C TiO2 preparation Stirring: TTIP + distilled water + plant extract Time: 3 h—Temp: 70 °C Calcination temp: 400 °C—Time: 3 h | Phase: anatase Morphology: rounded shape and formed in clusters Particle size: 6.4 nm | [67] |
| Leaf of Ocimum tenuiflorum | Phase: anatase Morphology: rounded shape and formed in clusters Particle size: 7 nm | ||
| Leaf of Moringa oleifera | Phase: anatase Morphology: rounded shape and formed in clusters, with many aggregated particles Particle size: 6.6 nm | ||
| Leaf of Coriandrum sativum | Phase: anatase Morphology: rounded shape and formed in clusters Particle size: 6.8 nm | ||
| Syzygium cumini (jamun) leaves | Plant extract stirring: leaf powder + distilled water Temp: 80 °C—Time: 60 min TiO2 preparation Stirring: TTIP + Syzygium cumini extract Temp: RT—Time: 8 h Drying temp: 100 °C—Time: one night Calcination temp: 570 °C—Time: 3 h | Phase: anatase Crystallite size: 10 nm Morphology: Spherical Particle size: ≈22 nm | [68] |
| Jatropha curcas leaves | Plant extract Stirring: leaves + DD water Temp: 80 °C—Time: 40 min TiO2 preparation Stirring: TiCl4 + leaf extract Temp: RT—Time: 2 h Ammonia was added to the solution Washing: ethyl alcohol Calcination temp: 450 °C—Time: 3 h | Phase: anatase Crystallite size: ≈ 13 nm Surface area: 27.038 m2/g Pore size diameter: 19.1 nm Pore volume: 0.1291 cm3/g Morphology: spherical | [69] |
| Ledebouria revoluta (African hyacinth) | Plant extract Stirring: leaves + ethanol Time: 20 min TiO2 preparation Stirring: titanium dioxide + distilled water + extract Temp: 50 °C—Time: 4 h | Phase: tetragonal Crystallite size: 47 nm Morphology: spherical | [70] |
| Leaves of Ocimum sanctum | Plant extract Stirring: leaves + distilled water Time: 10 min—Temp: 80 °C—Stored at 4 °C TiO2 preparation Stirring: TiO2 + plant extract Temp: RT—Time: 6 h | Phase: anatase Morphology: spherical and polygonal Particle size: 75–123 nm | [71] |
| Material | Method | Temp (°C) | Gas Conc. (ppm) | Resp. | Resp./Reco. Time (s) | LOD (ppb) | Stab. Repe. | Ref. |
|---|---|---|---|---|---|---|---|---|
| TiO2 | hydrothermal | 270 | 500 C3H6O | 9.19 a | 10/9 | 500 | 10 days and 4 cycles | [101] |
| 7.5 mol% Pd-TiO2 | hydrothermal | 400 | 3000 C4H10 | 39.63 a | 13/8 | N/A | 30 days and 7 cycles | [102] |
| C60–TiO2 | sol–gel | 150 | 100 CH2O | 1.20 b | 12/331 | 1000 | N/A 3 cycles | [103] |
| Au@TiO2 | laser-irradiated | 29 | 200 NH3 | 64 a | 28/24 | 5000 | 90 days N/A | [104] |
| 200 C2H4O | 115 a | 59/78 | 40,000 | |||||
| 200 C6H6 | 19.76 a | 39/26 | 50,000 | |||||
| Carbon gel–TiO2 | sol–gel | RT | 100 NH3 | 18.8 b | 50/70 | 1550 | 150 days and 5 cycles | [105] |
| TiO2/GO | sol–gel | RT/UV | 1.75 NO2 | 1.17 a | 35/90 | 50 | N/A N/A | [106] |
| Au@TiO2 | hydrothermal | 275 | 100 C3H6O | 13.9 a | 11/14 | N/A | 30 days N/A | [107] |
| Mn@TiO2 | hydrothermal | RT | 20 NH3 | 127.39 a | 21/24 | 440 | N/A N/A | [108] |
| C-TiO2 | hydrothermal | 170 | 100 C5H12O | 11.12 a | 100/675 | 500 | 120 days and 10 cycles | [109] |
| Co@TiO2 | wet chemical | RT | 50 NH3 | 14 a | 25/48 | 1000 | 30 days N/A | [110] |
| 0.01 wt% PANI- 0.1 NiO–0.9 TiO2 | sol–gel | 25 | 50 C3H6O | 10.3 b | 150/290 | 176.2 | 6 months and 4 cycles | [111] |
| Material | Method | Temp. (°C) | Gas Conc. (ppm) | Resp. | Resp./Reco. Time (s) | LOD (ppb) | Stab. Repe. | Ref. |
|---|---|---|---|---|---|---|---|---|
| TiO2 NWs | Hydrothermal | RT | 100 NO2 | 3.1 a | 10/19 | N/A | 20 days N/A | [81] |
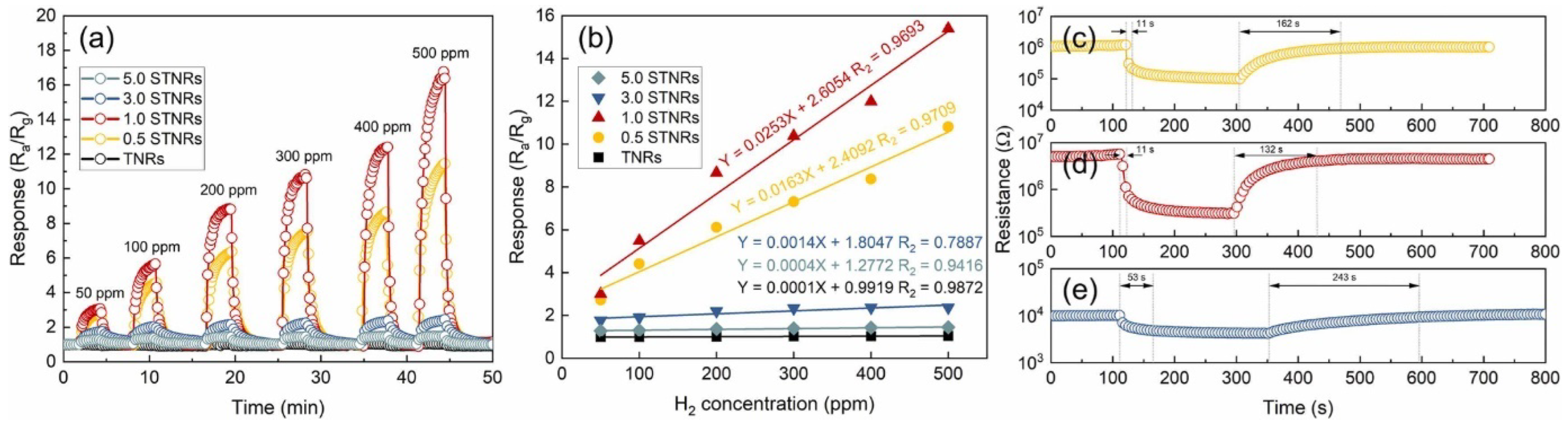
| SnO2@TiO2 NRs | Hydrothermal | 100 | 500 H2 | 15.4 a | 11/132 | N/A | N/A 4 cycles | [115] |
| TiO2 NWs | Sol−gel dip coating | 340 | 100 C2H5OH | 9.2 a | 13/46 | N/A | 50 days and 5 cycles | [117] |
| TiO2 NFs | Hydrothermal | 23 | 60 CH4 | 57 a | 66/162 | 250 | N/A N/A | [118] |
| 6 mol% Nb2O5-TiO2 NFs | Electrospinning | 250 | 500 C2H5OH | 21.6 a | N/A | 41 | 20 days N/A | [119] |
| 0.5 at% Ag/TiO2 NRs | Facile hydrothermal | 375 | 100 C8H10 | 6.5 a | 5/2 | N/A | 15 days and 3 cycles | [120] |
| TiO2 NWs | Electrospinning | 400 | 500 C2H5OH | 11.9 a | 8/1 | N/A | N/A N/A | [121] |
| Nb @ TiO2 NTs | Anodic oxidation | 200 | 120 H2 | 28.4 b | 930/300 | N/A | N/A N/A | [122] |
| CuO-TiO2 NTs | Anodization | 200 | 1000 H2 | 2 d | 444/408 | N/A | N/A N/A | [123] |
| Na0.23TiO2/TiO2 NTs | Hydrothermal | RT | 10 NOX | 5.9 a | 7/N/A | 1.8 | 60 days and 4 cycles | [124] |
| C(60) 0.02 w% @ TiO2 NTs | Anodization | 150 | 100 CH2O | 0.9 a | 4/7 | 100 | 23 days and 3 cycles | [125] |
| TiO2 NTs | Anodization | 300 | 50 H2S | 26 a | 22/6 | N/A | 70 days N/A | [126] |
| TiO2 NRs | Hydrothermal | RT | 60 CH4 | 6028 a | <70/<70 | 329 | 30 days and 3 cycles | [127] |
| TiO2 NRs | Hydrothermal | 100 | 150 H2 | 0.53%b | 85/620 | N/A | N/A N/A | [128] |
| Co3O4@TiO2 porous NFs | Hydrothermal | 250 | 100 C3H6O | 71.88 a | 122/351 | N/A | 30 days and 20 cycles | [129] |
| TiO2 NRs | Hydrothermal | 320 | 100 C3H6O | 12.3 a | 3/292 | N/A | 25 days and 6 cycles | [130] |
| Ag@TiO2 NRs | Hydrothermal | 200 | 3.8 C3H6O | 7.31 d | 1380/2280 | 65 | N/A N/A | [131] |
| SnO2@TiO2 NFs | Facile electrospinning | 240 | 100 C2H5OH | 9.53 a | 8/10 | N/A | 40 days N/A | [132] |
| SnO2@TiO2 NTs | Anodization | 250 | 1000 H2 | 1410 a | 2/~400 | 120,000 | 9 days and 5 cycles | [133] |
| Pd/RGO/TiO2 NTs | Anodization | 50 | 700 CH3OH | 0.96 b | 11/15 | N/A | 6 days and 3 cycles | [134] |
| TiO2/α-Fe2O3 NRs | Hydrothermal | 225 | 100 C3H6O | 21.9 b | 12/9 | 36 | 30 days and 5 cycles | [135] |
| PANI/TiO2 NRs | Hydrothermal | RT | 30 C4H9NO | 6.36 a | 100/349 | 50 | 30 days and 5 cycles | [136] |
| PANI/TiO2 NFs | Electrospining | RT/UV assisted | 1 NH3 | 1.09 c | 63/37 | 50 | 60 days and 4 cycles | [137] |
| Material | Method | Temp. (°C) | Gas Conc. (ppm) | Resp. | Resp./Reco. Time (s) | LOD (ppb) | Stab. Repe. | Ref. |
|---|---|---|---|---|---|---|---|---|
| TiO2 (NSs) | Hydrothermal | 310 | 500 C2H5OH | 25.4 a | N/A/N/A | 1.4 | N/A N/A | [139] |
| Core–shell TiO2 (NSs) | Hydrothermal | 270 | 500 C2H5OH | 43.9 a | N/A/N/A | 5.7 | 30 days N/A | [142] |
| Porous TiO2 (NSs) | Hydrothermal | 400 | 200 C3H6O | 21.6 a | 0.75/0.5 | 500 | 150 days N/A | [143] |
| SnO2/TiO2 (NSs) | Solvothermal | 300 | 100 C6H15N | 19.8 a | 8/N/A | N/A | 60 days N/A | [144] |
| Hierarchical TiO2 (NSs) | Hydrothermal | 25 | 50 NH3 | 122.6 a | 53/23 | 500 | N/A 5 cycles | [145] |
| Fluorine-doped TiO2 (NSs) | Hydrothermal | 25 | 100 C3H6O | 5.95 b | 160/220 | N/A | 35 days and 5 cycles | [146] |
| Au5%@TiO2 (NSs) | Facile and in situ process | 25 | 50 NH3 | 71.8 a | 38/168 | 5000 | 16 days and 5 cycles | [147] |
| Porous Pd@TiO2 (NSs) | Template assisted | 230 | 1000 H2 | 9 a | 1.6/1.4 | 1000 | 100 days and 5 cycles | [148] |
| TiO2@NGQDs (NPTs) | Hydrothermal | 250 | 100 NO | 2.23 b | 235/285 | N/A | N/A N/A | [149] |
| Ag@TiO2 (NSs) | Hydrothermal | 300 | 100 C2H5OH | 8.5 a | 9/10 | N/A | 30 days and 5 cycles | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumarage, G.W.C.; Hakkoum, H.; Comini, E. Recent Advancements in TiO2 Nanostructures: Sustainable Synthesis and Gas Sensing. Nanomaterials 2023, 13, 1424. https://doi.org/10.3390/nano13081424
Kumarage GWC, Hakkoum H, Comini E. Recent Advancements in TiO2 Nanostructures: Sustainable Synthesis and Gas Sensing. Nanomaterials. 2023; 13(8):1424. https://doi.org/10.3390/nano13081424
Chicago/Turabian StyleKumarage, Gayan W. C., Hadjer Hakkoum, and Elisabetta Comini. 2023. "Recent Advancements in TiO2 Nanostructures: Sustainable Synthesis and Gas Sensing" Nanomaterials 13, no. 8: 1424. https://doi.org/10.3390/nano13081424
APA StyleKumarage, G. W. C., Hakkoum, H., & Comini, E. (2023). Recent Advancements in TiO2 Nanostructures: Sustainable Synthesis and Gas Sensing. Nanomaterials, 13(8), 1424. https://doi.org/10.3390/nano13081424






