High-Performance Thin-Film Transistors with ZnO:H/ZnO Double Active Layers Fabricated at Room Temperature
Abstract
1. Introduction
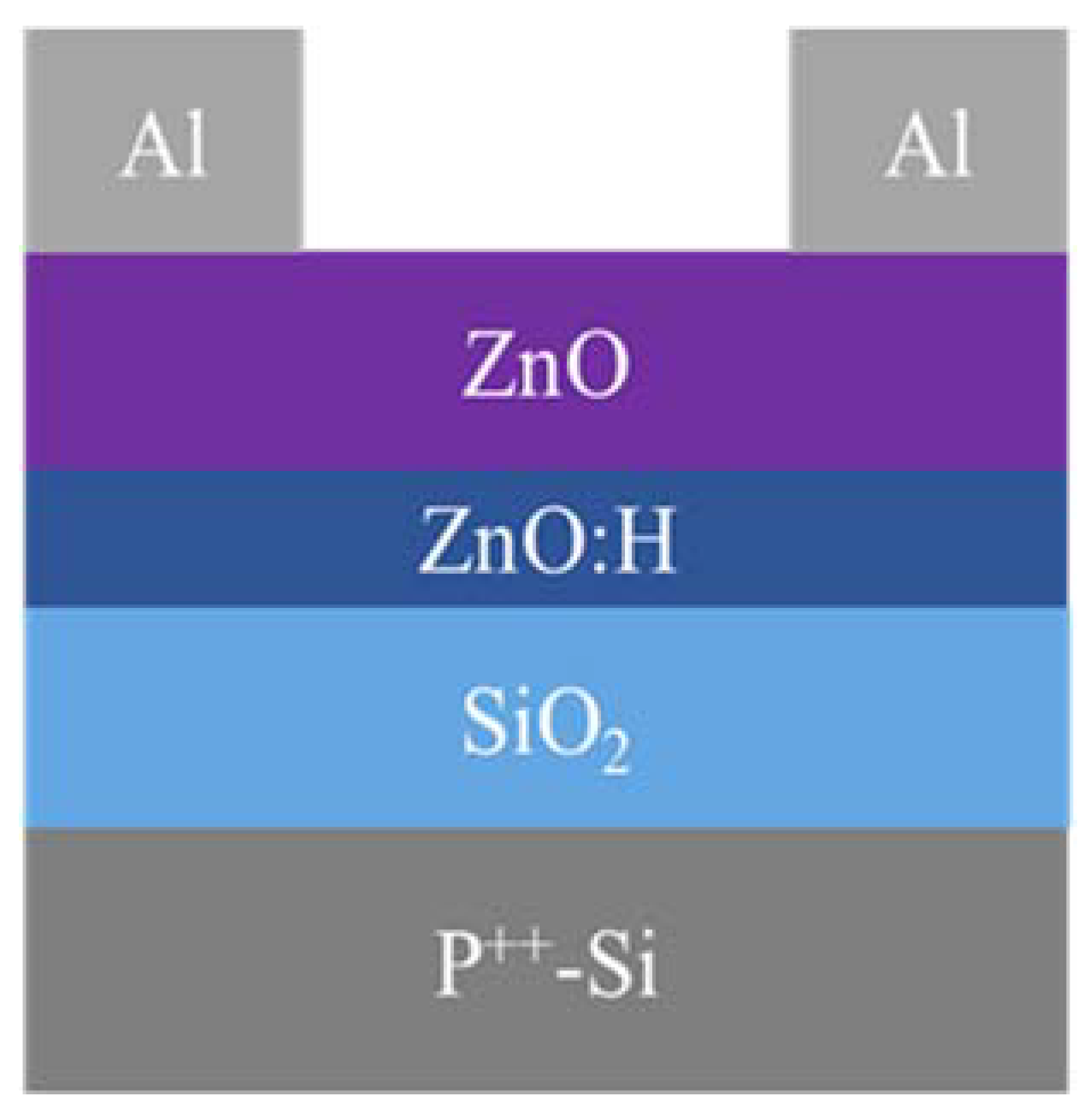
2. Experimental Section
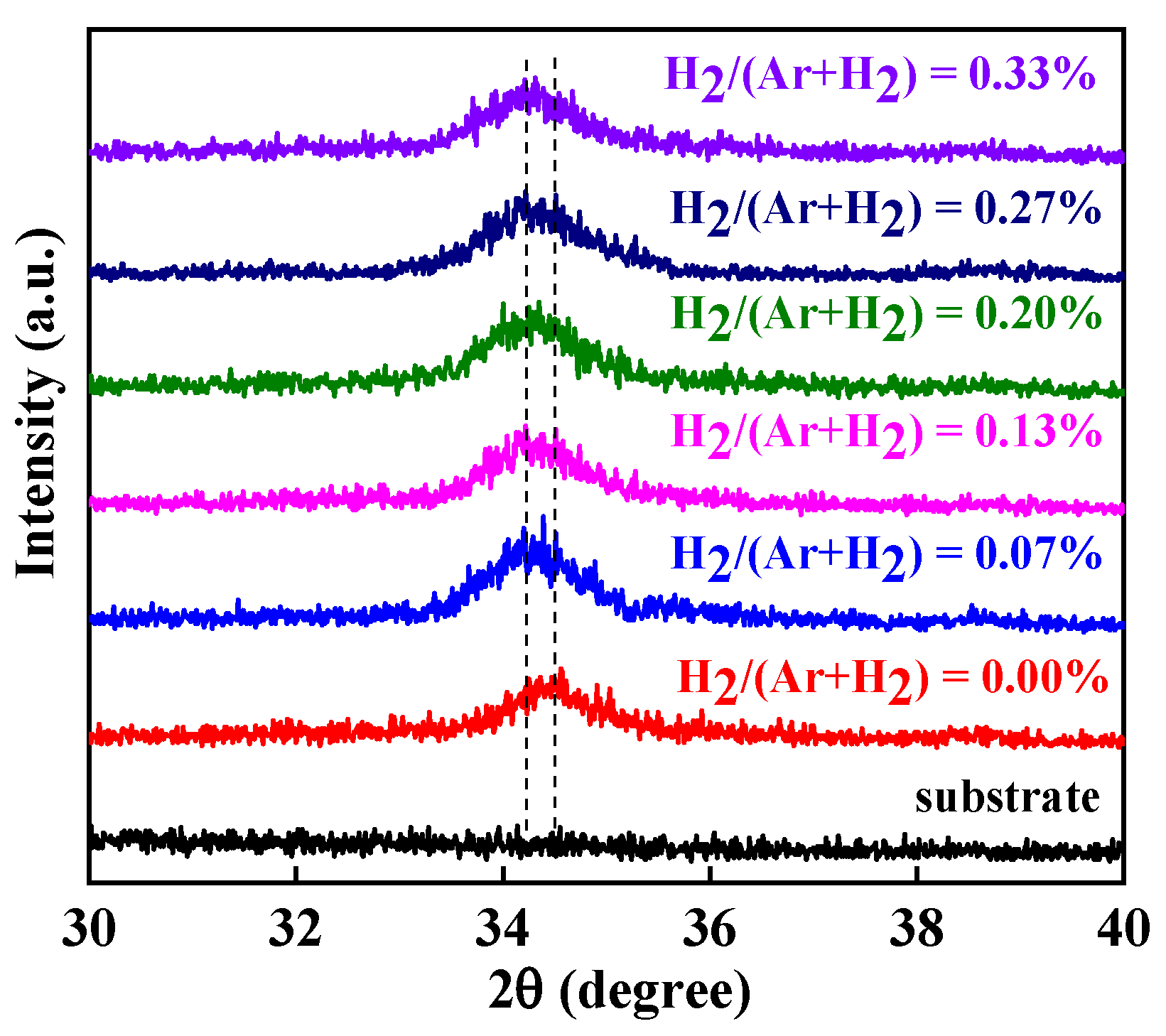
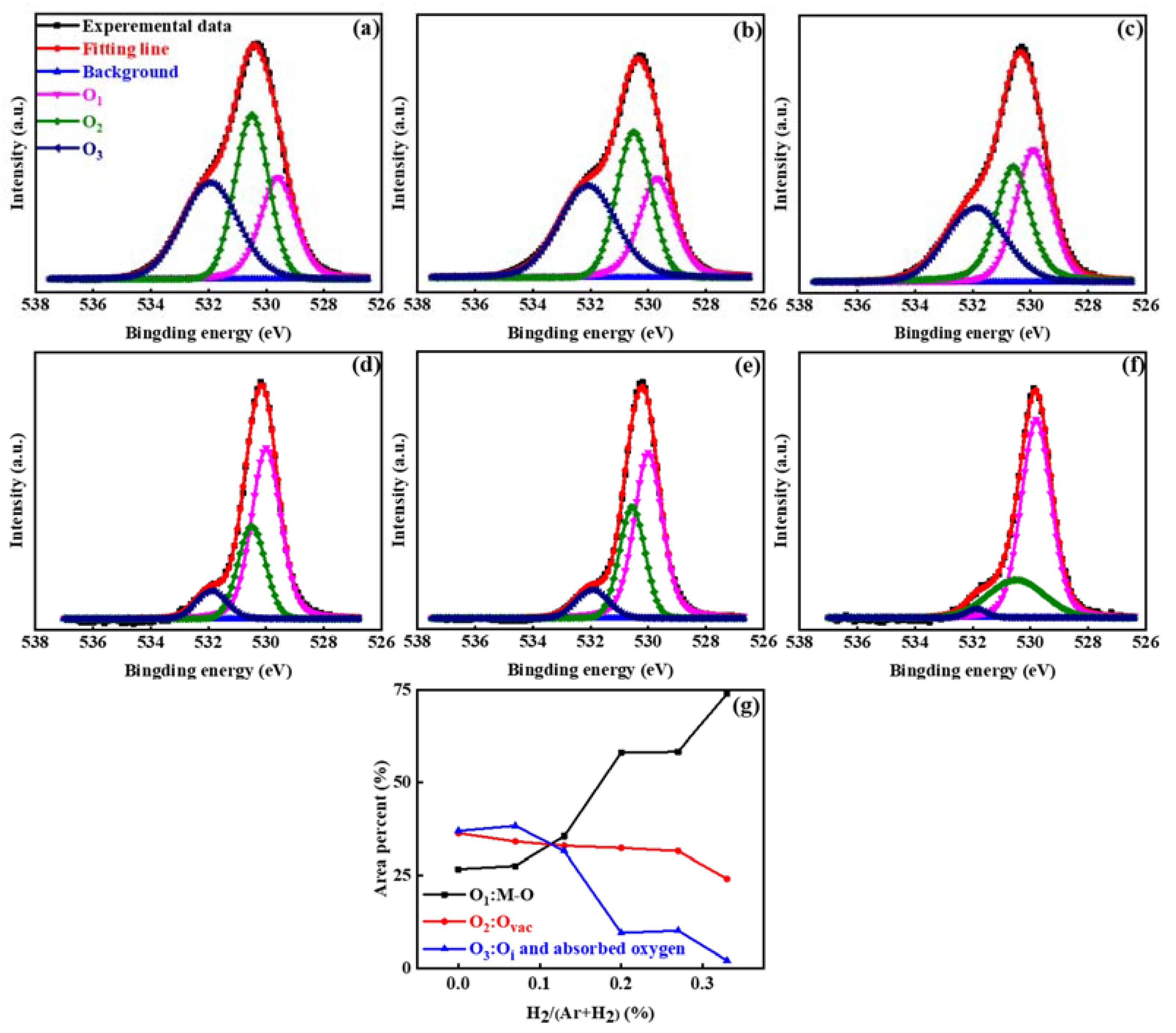
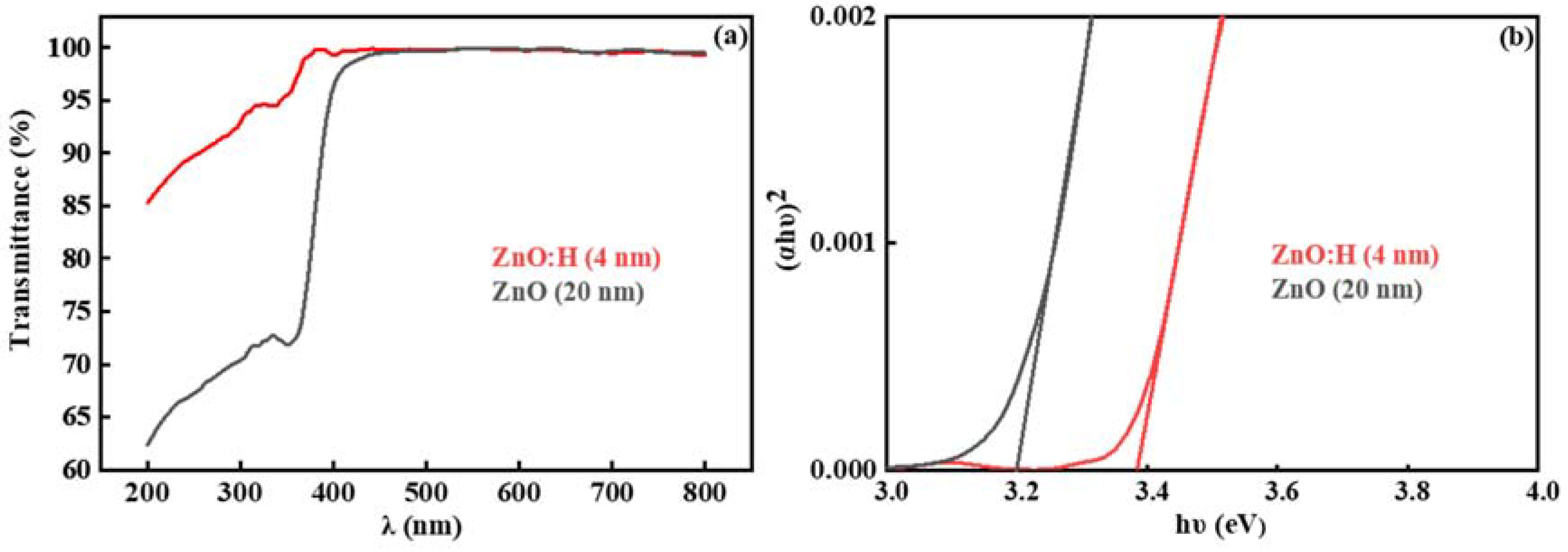
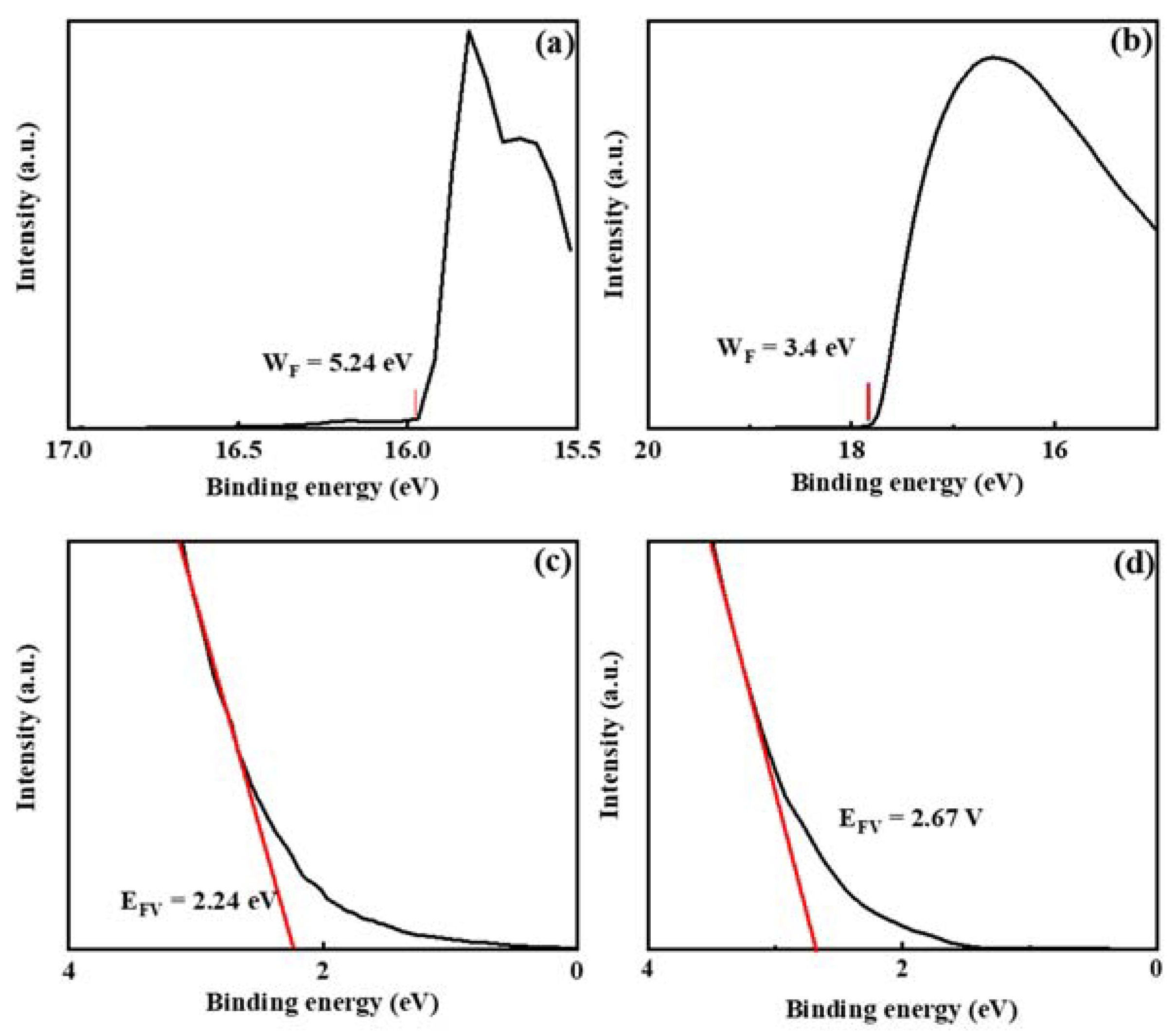
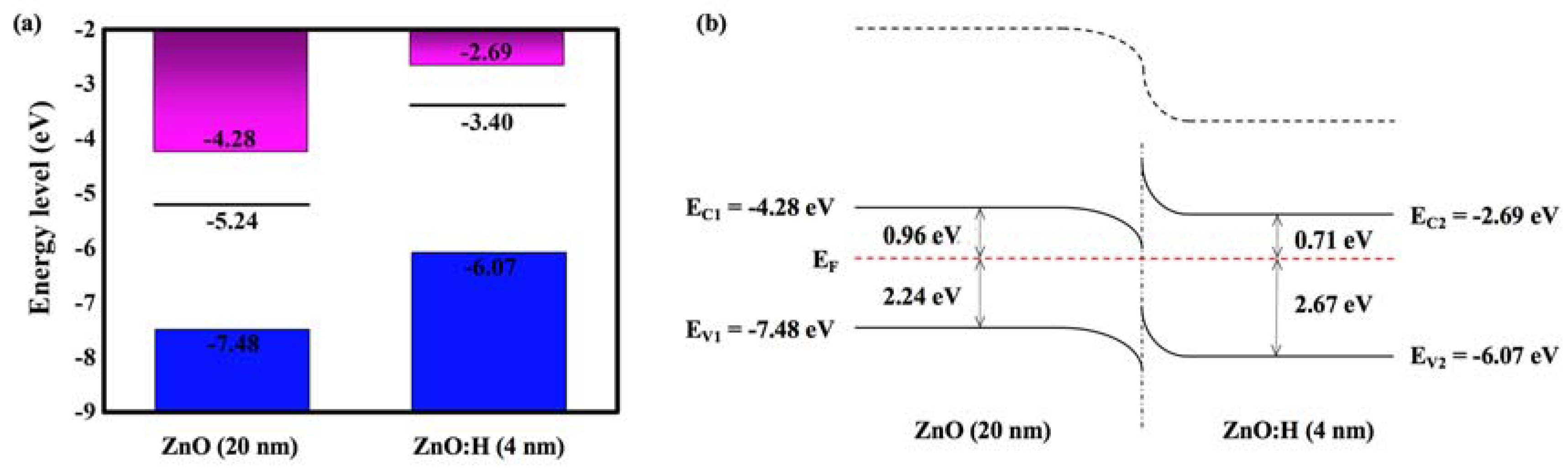
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, E.; Barquinha, P.; Martins, R. Oxide semiconductor thin-film transistors: A review of recent advances. Adv. Mater. 2012, 24, 2945–2986. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhou, N.; Smith, J.; Lin, H.; Stallings, K.; Yu, J.; Marks, T.J.; Facchetti, A. Synergistic approach to high-performance oxide thin film transistors using a bilayer channel architecture. ACS Appl. Mater. Interfaces 2013, 5, 7983–7988. [Google Scholar] [CrossRef]
- Park, C. Improvement of the performance and stability of oxide semiconductor thin-film transistors using double-stacked active layers. IEEE Electron. Device Lett. 2012, 33, 818–820. [Google Scholar] [CrossRef]
- Presley, E.; Munsee, L.; Park, H.; Hong, D.; Wager, F. Tin oxide transparent thin-film transistors. J. Phys. D Appl. Phys. 2004, 37, 2810–2813. [Google Scholar] [CrossRef]
- Noh, H.; Ryu, Y.; Jo, J.; Kim, J. Indium oxide thin-film transistors fabricated by RF sputtering at room temperature. IEEE Electron. Device Lett. 2010, 31, 567–569. [Google Scholar] [CrossRef]
- Prins, M.; Grosse, O.; Müller, G.; Cillessen, M. A ferroelectric transparent thin-film transistor. Appl. Phy. Lett. 1996, 68, 3650–3652. [Google Scholar] [CrossRef]
- Cai, G.; Qiang, L.; Yang, P. High-sensitivity pH sensor based on electrolyte-gated In2O3 TFT. IEEE Electron. Device Lett. 2018, 39, 1409–1412. [Google Scholar] [CrossRef]
- Kim, W.; Moon, Y.; Kim, K.; Shin, Y.; Park, W. Improvement in the bias stability of tin oxide thin-film transistors by hafnium doping. Thin Solid Films 2012, 520, 2220–2223. [Google Scholar] [CrossRef]
- Huh, S.; Yang, B.; Oh, S.; Lee, J.; Yoon, K.; Jeong, K.; Hwang, C.; Kim, H. Improvement in the performance of tin oxide thin-film transistors by alumina doping. Electrochem. Solid-State Lett. 2009, 12, H385–H387. [Google Scholar] [CrossRef]
- Chin, A.; Cheng, C.; Li, K.; Wu, R.; Chen, Z.; Lu, S.; Lee, L. Electrical properties of modulation doped rf-sputtered polycrystalline MgZnO/ZnO heterostructures. J. Phys. D Appl. Phys. 2011, 44, 455101. [Google Scholar] [CrossRef]
- Huang, C.; Chin, H.; Wu, Y.; Cheng, I.; Chen, J.; Chiu, K.; Lin, T. Mobility enhancement of polycrystalline MgZnO/ZnO thin-film layers with modulation doping and polarization effects. IEEE Trans. Electron. Devices 2010, 57, 696–703. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Cheng, C. Mobility enhancement in RF-sputtered MgZnO/ZnO heterostructure thin-film transistors. IEEE Trans. Electron. Devices 2016, 63, 1545–1549. [Google Scholar] [CrossRef]
- Magari, Y.; Kataoka, T.; Yeh, W.; Furuta, M. High-mobility hydrogenated polycrystalline In2O3 (In2O3:H) thin-film transistors. Nat. Commun. 2022, 13, 1078. [Google Scholar] [CrossRef]
- Kim, Y.; Park, H.; Kim, G. Effect of photochemical hydrogen doping on the electrical properties of ZnO thin-film transistors. J. Alloys Compd. 2018, 732, 300–305. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Q.; Liao, L.; Liu, X.; Chang, T.; Chang, K.; Tsai, T.; Jiang, C.; Wang, J.; Li, J. Rational hydrogenation for enhanced mobility and high reliability on ZnO-based thin film transistors: From simulation to experiment. ACS Appl. Mater. Interfaces 2016, 8, 5408–5415. [Google Scholar] [CrossRef]
- Zhu, D.; Jiang, Z.; Zhang, W.; Yin, D.; Xu, W.; Han, S.; Fang, M.; Liu, W.; Cao, P.; Lu, Y. Room-temperature fabrication of high-performance H doped ZnO thin-film transistors. Mater. Chem. Phys. 2021, 261, 124248. [Google Scholar] [CrossRef]
- Faber, H.; Das, S.; Lin, H.; Pliatsikas, N.; Zhao, K.; Kehagias, T.; Dimitrakopulos, G.; Amassian, A.; Patsalas, A.; Anthopoulos, D. Heterojunction oxide thin-film transistors with unprecedented electron mobility grown from solution. Sci. Adv. 2017, 3, 1602640. [Google Scholar] [CrossRef]
- Cong, Y.; Han, D.; Dong, C.; Zhang, D.; Zhang, X. Fully transparent high performance thin film transistors with bilayer ITO/Al-Sn-Zn-O channel structures fabricated on glass substrate. Sci. Rep. 2017, 7, 1497. [Google Scholar] [CrossRef]
- Khim, D.; Lin, H.; Nam, S.; Faber, H.; Tetzner, K.; Zhang, Q.; Zhang, X.; Anthopoulos, D. Modulation-Doped In2O3/ZnO heterojunction transistors processed from Solution. Adv. Mater. 2017, 29, 1605837. [Google Scholar] [CrossRef]
- Abliz, A.; Huang, C.; Wang, J.; Xu, L.; Liao, L.; Xiao, X.; Wu, W.; Fan, Z.; Jiang, C.; Li, J.; et al. Rational design of ZnO:H/ZnO bilayer structure for high-performance thin-film transistors. ACS Appl. Mater. Interfaces 2016, 8, 7862–7868. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jo, W.; Kim, J.; Choi, S.; Kwon, M.; Jeon, P.; Facchetti, A. Corrugated heterojunction metal-oxide thin-film transistors with high electron mobility via vertical interface manipulation. Adv. Mater. 2018, 30, 1804120. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, W.; Wang, J.; Hong, F.; Su, Y. Hydrogen-doped high conductivity ZnO films deposited by radio-frequency magnetron sputtering. Appl. Phys. Lett. 2004, 85, 5628–5630. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, J.; Roh, Y. Effects of controlling the interface trap densities in InGaZnO thin-film transistors on their threshold voltage shifts. J. Korean Phys. Soc. 2014, 65, 1919–1924. [Google Scholar] [CrossRef]
- Komaru, T.; Shimizu, S.; Kanbe, H.; Maeda, Y.; Kamiya, T.; Fortmann, C.; Shimizu, I. Optimization of transparent conductive oxide for improved resistance to reactive and/or high temperature optoelectronic device processing. Jpn. J. Appl. Phys. 1999, 38, 5796–5804. [Google Scholar] [CrossRef]
- Chen, M.; Pei, L.; Sun, C.; Wen, L.; Wang, X. Surface characterization of transparent conductive oxide Al-doped ZnO films. J. Cryst. Growth 2000, 220, 254–262. [Google Scholar] [CrossRef]
- Trinh, T.; Nguyen, D.; Ryu, K.; Jang, K.; Lee, W.; Baek, S.; Raja, J.; Yi, J. Improvement in the performance of an InGaZnO thin-film transistor by controlling interface trap densities between the insulator and active layer. Semicond. Sci. Technol. 2011, 26, 085012. [Google Scholar] [CrossRef]
- Peng, C.; Lin, C.; Tseng, A.; Lee, L. Characterization of the single and double films consisting of Al, Sc-co-doped ZnO/Al-doped ZnO and Al-doped ZnO/Al, Sc-co-doped ZnO. Surf. Coat. Technol. 2008, 202, 5425–5430. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, G. Hydrogen multicentre bonds. Nat. Mater. 2007, 6, 44–47. [Google Scholar] [CrossRef]
- Selim, A.; Weber, H.; Solodovnikov, D.; Lynn, G. Nature of native defects in ZnO. Phys. Rev. Lett. 2007, 99, 085502. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Singh, A.; Chaudhary, S.; Pandya, K. Hydrogen incorporation induced metal-semiconductor transition in ZnO:H thin films sputtered at room temperature. Appl. Phys. Lett. 2013, 102, 172106. [Google Scholar] [CrossRef]
- Zhu, D.; Xiang, H.; Cao, P.; Jia, F.; Han, S.; Liu, W.; Ma, X.; Lu, Y. Influence of H2 introduction on properties in Al-doped ZnO thin films prepared by RF magnetron sputtering at room temperature. J. Mater. Sci. Mater. Electron. 2013, 24, 1966–1969. [Google Scholar] [CrossRef]

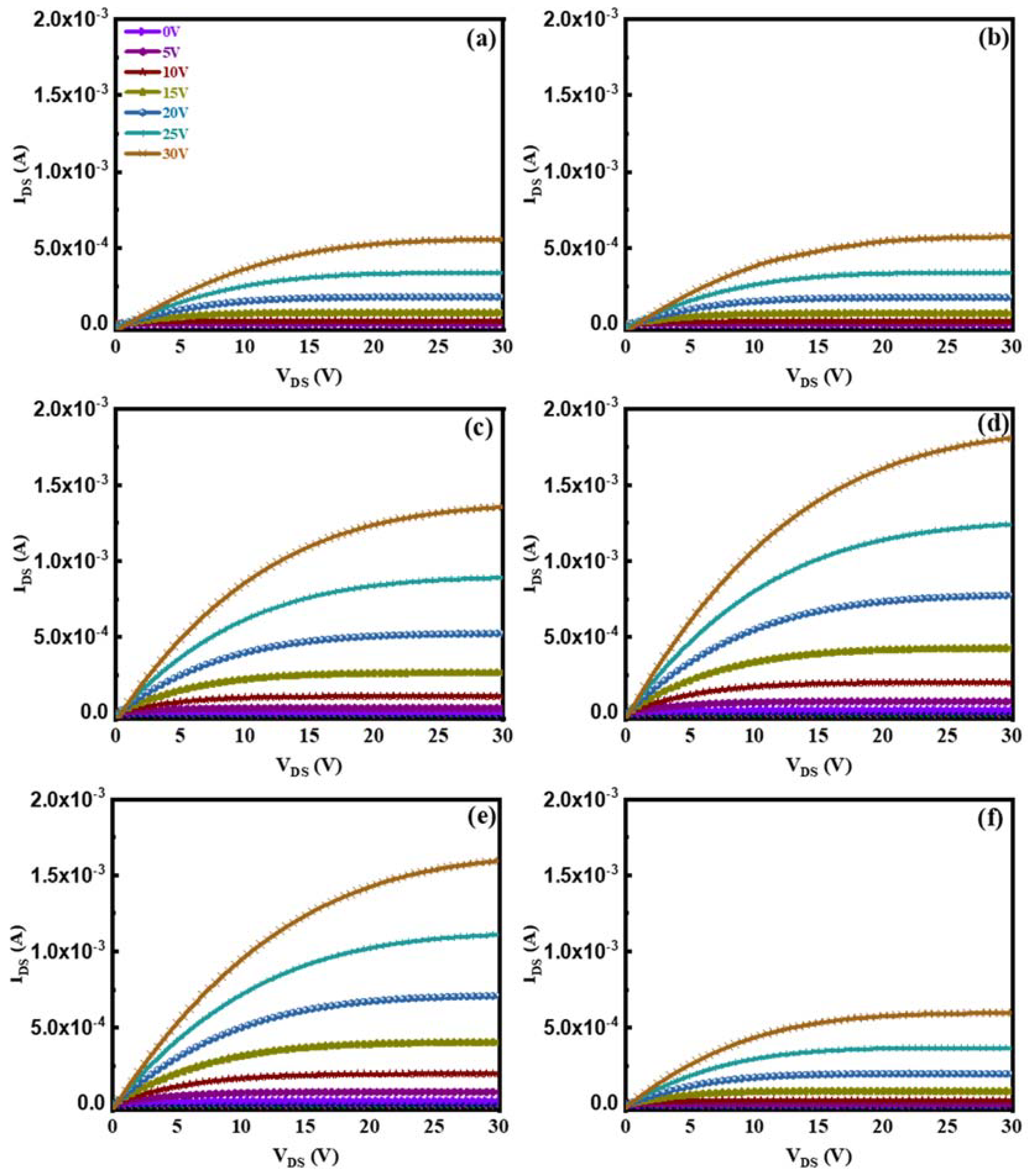

| H2/(Ar + H2) (%) | μsat (cm2/Vs) | Vth (V) | Ion/Ioff | SS (V/Dec) |
|---|---|---|---|---|
| 0 | 6.52 | 5.68 | 2.19 × 106 | 0.78 |
| 0.07 | 6.53 | 5.41 | 7.65 × 106 | 0.68 |
| 0.13 | 12.10 | 1.68 | 2.32 × 107 | 0.67 |
| 0.20 | 9.88 | 0.26 | 1.76 × 107 | 0.99 |
| 0.27 | 9.04 | 1.24 | 1.47 × 107 | 1.07 |
| 0.33 | 5.47 | 5.35 | 7.52 × 106 | 0.84 |
| ZnO:H-TFT [17] | 5.17 | 6.03 | 4.98 × 106 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Jiang, Z.; Li, L.; Zhu, D.; Wang, C.; Han, S.; Fang, M.; Liu, X.; Liu, W.; Cao, P.; et al. High-Performance Thin-Film Transistors with ZnO:H/ZnO Double Active Layers Fabricated at Room Temperature. Nanomaterials 2023, 13, 1422. https://doi.org/10.3390/nano13081422
Wang D, Jiang Z, Li L, Zhu D, Wang C, Han S, Fang M, Liu X, Liu W, Cao P, et al. High-Performance Thin-Film Transistors with ZnO:H/ZnO Double Active Layers Fabricated at Room Temperature. Nanomaterials. 2023; 13(8):1422. https://doi.org/10.3390/nano13081422
Chicago/Turabian StyleWang, Daoqin, Zongjin Jiang, Linhan Li, Deliang Zhu, Chunfeng Wang, Shun Han, Ming Fang, Xinke Liu, Wenjun Liu, Peijiang Cao, and et al. 2023. "High-Performance Thin-Film Transistors with ZnO:H/ZnO Double Active Layers Fabricated at Room Temperature" Nanomaterials 13, no. 8: 1422. https://doi.org/10.3390/nano13081422
APA StyleWang, D., Jiang, Z., Li, L., Zhu, D., Wang, C., Han, S., Fang, M., Liu, X., Liu, W., Cao, P., & Lu, Y. (2023). High-Performance Thin-Film Transistors with ZnO:H/ZnO Double Active Layers Fabricated at Room Temperature. Nanomaterials, 13(8), 1422. https://doi.org/10.3390/nano13081422






