1. Introduction
The awareness that certain human activities can adversely affect the environment makes it necessary to monitor environmental matrices such as air, water, and soil to detect any anomalies due to pollution [
1]. The monitoring process is effective when it leads to an understanding of the sources of pollution and, as a result, how to limit it. The first step is to map the substances that may be hazardous to humans and the environment, such as heavy metals [
2]. The World Health Organization has identified a ranking of the top 10 dangerous chemicals of public health concern, and one of the metals mentioned is lead [
3]. Lead, if ingested or inhaled, can cause several illnesses. Therefore, the application of systems to capture it during, after, or even before its release into the environment is crucial to avoid damages in the case of its uncontrolled diffusion. A material that can be suitable for this purpose is titanium dioxide. TiO
2 is one of the most popular nanomaterials that has numerous applications in medicine [
4], the food industry [
5], green energy [
6], and many more. In the medical field, in particular, TiO
2 can be used for more effective delivery of anticancer drugs [
4]. Moreover, TiO
2 can be used to extend the storage life of food and to avoid waste [
5]. TiO
2 is also used for photocatalysis under UV light irradiation to transform dangerous species into harmless elements to protect humans and the environment [
7,
8,
9,
10].
In the renewable energy field, TiO
2 is applied to boost the performance of Perovskite Solar Cells (PSCs) [
6]. In particular, TiO
2 is used as an Electron Transporting Layer (ETL), allowing the achievement of high-efficiency values primarily thanks to the convenient band alignment of the anatase polymorphism with the perovskite material.
The production of TiO
2 layers is feasible through techniques based on chemical methods, such as spin coating and doctor blading [
11], or based on physical methods, such as sputtering [
12]. Among the others, gig-lox sputtering deposition [
13], based on the Grazing Incidence Geometry of the solid titanium source coupled with the Local Oxidation of the species at the sample side, represents a recent and innovative physical approach for the deposition of TiO
2. This process allows growing, at room temperature and without solvents, spongy layers with interlinked mesopores and nanopores. This multi-scale porosity, gained along a bottom-up oxidation process of spatially separated TiO
2 seeds, is a huge benefit for the infiltration capacity to capture species of different sizes and natures. On the other hand, TiO
2 layers deposited by standard parallel plate geometries are more compact [
14]. Infiltration by soaking with N-719 [
15] molecules was successfully tested in gig-lox TiO
2 layers. N-719 is a photoactive dye molecule whose diameter is comparable to the gig-lox nanopore size and, indeed, an octopus configuration can be achieved.
Gig-lox application was also extended to Perovskites. Sanzaro et al. [
16] demonstrated that MAPbI
3 can be infiltrated into layers of gig-lox TiO
2 with the empty volume decreasing from 42% to 18%. Lead atoms were found to decorate the surfaces of the sponge deeply into the gig-lox layer to reach the inner interface.
Gig-lox TiO
2 has been recently implemented to capture Pb atoms to prevent its release into the environment from PSC devices [
17]. The possibility of trapping lead into the gig-lox TiO
2 layer has been demonstrated by achieving up to 94% of Pb sequestration after 4 h of dipping in aqueous solution of lead iodide (PbI
2) using a 340 nm-thick layer.
To complement consolidated methods, such as the inductively coupled plasma mass spectrometry ICP-MS [
18], that detect lead in liquid environments, we propose a new approach based on X-ray reflectometry that has been applied to a solid gig-lox sponge after lead sequestration in a liquid environment for ex-situ investigations in dry conditions. This technique is based on the change of the critical angle for total external reflection of the X-rays from a solid material and has been applied to quantify the lead captured by a 340 nm-thick TiO
2 sponge with an area 1.25 × 1.25 cm
2 immersed in an aqueous solution of 10 mL with increasing concentrations of Pb, from 0.4 to 6.4 ppm. The method quantifies the captured lead, and it could be extended to other capturing spongy oxides and toxic species.
2. Materials and Methods
Gig-lox TiO2 sputtering deposition. The instrument used for the deposition of gig-lox TiO2 layers is a customized DC-pulsed Magnetron Sputtering equipment. Produced by Kenosistec s.r.l., it consists of a Titanium target at an incident angle of 12.7° with respect to the sample surface, coupled with the local oxidation of the species at the substrate side. The deposition is carried out at room temperature, and it is always preceded by a pre-sputtering process to clean the target and eliminate residual oxide layers. The gasses introduced into the system are (1) oxygen, which is the reactive gas, with a flow rate of 2 sccm, and (2) argon, which is the carrier gas, with flow rate of 69 sccm. A rotation of 20 rpm is applied to the substrate during deposition to increase layer uniformity. The samples were deposited on glass with a power of 140 W, a voltage of 330 V, a current of 424 mA, and a deposition rate of 3.4 nm/min. The thickness of the implemented samples is 340 nm.
Pb quantification in a liquid matrix by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) analyses. The process for the lead adsorption in gig-lox TiO2 consists of the immersion of samples 1.25 × 1.25 cm2 made of porous material into aqueous solutions with different concentrations of PbI2 (Sigma–Aldrich, 99.99% purity) for a period of 96 h to reach the maximum Pb uptake. The aqueous solution used as solvent was 10 mL of deionized water with a pH of 5.8. After immersion, the gig-lox TiO2 samples are shaken at 200 rpm with a flat orbital shaker (IKA KS 260 basic). Pb concentrations were assessed with a Nexion 300X ICP/MS using the kinetic energy discrimination mode (KED) for interference suppression. Before analysis, the sampled solution was diluted, acidified with nitric acid, and added to the internal standards required for quantifying lead ions. Each process was repeated 3 times for higher accuracy, which was validated by comparing it with a standard reference material, SRM 1643f—Trace Elements in Water.
Pb quantification in a solid matrix by X-ray Reflectivity (XRR) analyses. The instrument used for XRR analyses is a D8Discover Bruker AXS diffractometer with a Cu-ka source, a Goebel mirror, a 0.1 mm slit at the primary beam, a 0.2 mm slit, and a scintillator as a detector. The critical angle for total external reflection was measured correspondingly to a penetration depth of ~20 nm in the pure TiO2 material. The penetration depth, also known as attenuation length or absorption length, corresponds to an intensity loss of the primary intensity by 1/e (~40%). The critical angle is a parameter related to the electron density of the material. The XRR profiles were acquired with a step size of 0.002° and a time per step of 2 s in the 2θ range 0.35–0.70°.
Density Functional Theory Calculations. All calculations have been carried out at density functional theory level using the BigDFT software [
19,
20]. The interface with water is modelled by the soft sphere implicit solvation [
21,
22,
23,
24]. Core electrons and exchange correlation are described by soft norm-conserving pseudo-potentials along with the Perdew Burke Ernzerhol functional as implemented in the Libxc library [
25]. A six trilayers 4 × 1 supercell has been employed to model the anatase TiO
2 (101) surface (288 atoms). We built the supercell starting from the optimized bulk lattice parameters. Atoms at the bottom (non-hydrated) trilayer of the slab were fixed at their crystal bulk coordinates. Surface boundary conditions have been set for all surface calculations. The wavelet basis functions were distributed on an adaptive uniform mesh with a resolution of hgrid: = hx = hy = hz = 0.40 Bohr for all calculations. All geometry optimizations have been carried out at Γ point.
STEM analyses. They were performed with a Cs-corrected JEOL ARM200C at 200 keV, equipped with 100 mm2 JEOL energy dispersive X-ray (EDX) detector. A HAADF detector was used to acquire STEM images in scanning mode.
3. Results and Discussion
We investigated and quantified the lead amount captured by a multi-scale-porosity gig-lox sponge in a liquid environment with the application of an ex-situ dry method based on the X-ray interaction with the solid matter. In particular, we exploited the relationship between the critical angle for total external reflection and the electronic density of the materials viewed by the X-ray probe. We thus collected the X-ray beam reflected by the gig-lox sponge close to the total external reflection condition. The resulting intensity profile is characterized by a critical angle, by a slope featuring the intensity reduction vs. the increasing incidence angle, and (where applicable) by interference fringes that depend on the electron density, roughness, and thickness of the analyzed layer, respectively. The introduction of lead into the TiO2 matrix causes a change in the electron density of the material, resulting in a critical angle shift. Interference fringes are not visible in our specific case due to the layer thickness that is above a threshold value of ~200 nm. All data hereafter discussed refers to a gig-lox sponge with a thickness of 340 nm, but similar results are achieved by changing the thickness in a range up to 1000 nm. We further noted that lead capturing is a process that takes place from the sample surface towards the inner part of the layer, with the saturation of sequestered lead achieved at high concentrations. The saturation level can be extended by increasing the layer thickness.
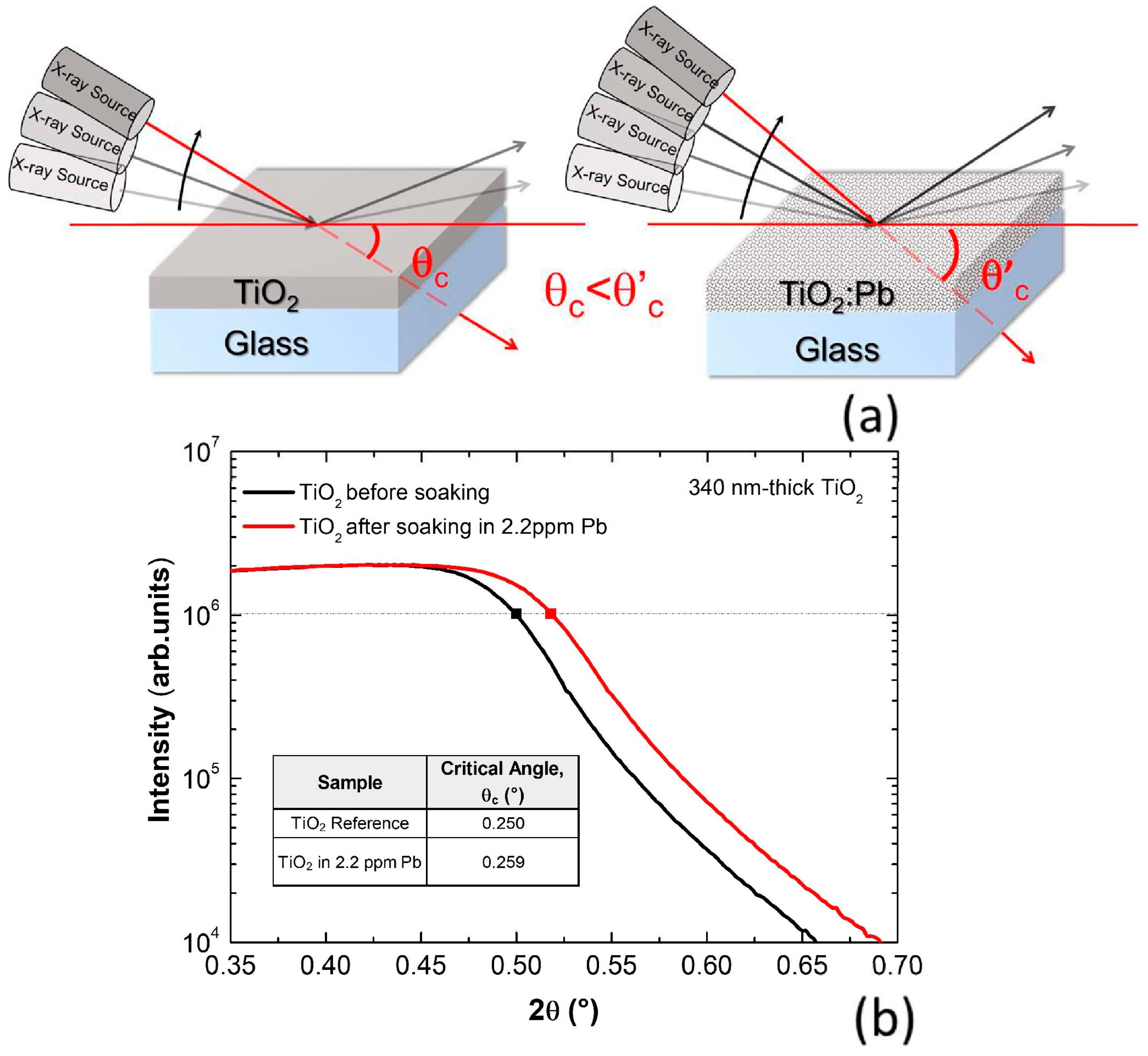
Figure 1a shows a schematic of the XRR method applied to samples with different film densities, namely a pure gig-lox TiO
2 and a gig-lox TiO
2 containing Pb atoms. The schematic represents that the critical angle of the film with lead is higher than the one of a pure empty oxide due to the higher film density caused by the presence of Pb inside the film. To give an idea, the electronic density in lead is 2.6% higher than in a TiO
2 moiety. Given the direct relationship between the square root of the electronic density and critical angle [
26], an increase in θ
c occurs by lead incorporation into the TiO
2 layer.
The XRR curves acquired before and after TiO
2 soaking into a Pb aqueous solution at a concentration of 2.2 ppm of Pb for 96 h are shown in
Figure 1b. The Pb capture and the consequent increase of the film density are translated into a shift of critical angle at higher 2θ values, as shown by the marked squares taken at half of the upper plateau, which univocally identifies the critical angle for total external reflection of the beam.
According to our results, a film of pure gig-lox TiO2 has a critical angle of θc = 0.250°. After immersion of the sample and consequent infiltration of the metal in the branches of gig-lox TiO2, the density of the film increases to θc = 0.259°.
The gig-lox TiO
2 immersion was performed in five different solutions with increasing concentrations of Pb, from 0.4 to 6.4 ppm. We conducted the XRR measurements in all samples, including the pure TiO
2 used as a reference, and the resulting curves are shown in
Figure 2a. According to what is shown in
Figure 1b, the critical angle monotonically increases by increasing the concentration of lead in the solution, from 0 to 6.4 ppm.
We measured the critical angles for all the samples as listed in
Table 1. Those values were plotted as a function of the Pb amount that was captured by the film (
Figure 2b) as experimentally measured by ICP-mass spectrometry. This method quantifies the residual amount of lead into the mother solution that has hosted the gig-lox layer. A high correlation between the captured lead quantity and the critical angle of the film was found, with a linear relationship established between them. This is further corroborated by a value of R-square equal to 0.99. The finding demonstrates that a progressively increasing amount of lead is captured by the sponge that is proportional to the concentration of Pb in the solution. Differently viewed, the curve in
Figure 2b also represents a reference curve to quantitatively predict any amount of lead captured by the gig-lox layer based on the measured value of the critical angles. The mainstay of the method resides in the analyses that are done under dry conditions directly on the sponge instead of measuring the remaining lead amount in the mother solution after the immersion of the sponge. Our method can be easily extended to other porous oxides provided a reference curve is tailored. A universal curve over different materials requires specific evaluations and would be based on a similar integrated surface area available for lead capture with the same reactivity, and the same degree of pore filling that depends on the pore size and distribution.
To go deeper into the capture mechanism, we investigated the distribution of Pb atoms into the TiO
2 branches.
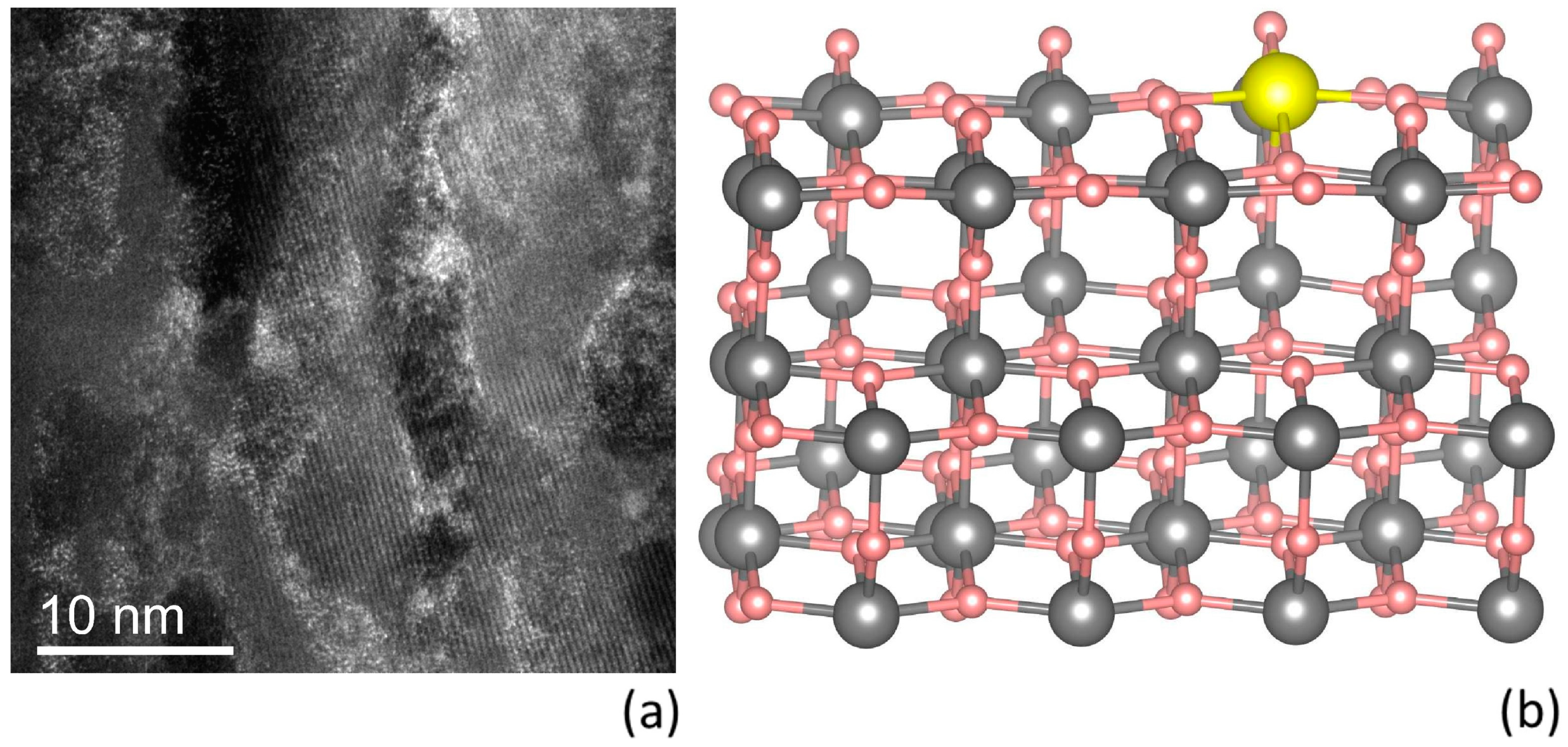
Figure 3a is a cross-sectional STEM image of the soaked gig-lox TiO
2 layer at 6.4 ppm of Pb taken in the bottom part of the layer, characterized by its spongy ramification. Since the analytical method is sensitive to atomic mass contrast, lead is well visible in the image, even in very small amounts. The white spots are, in fact, Pb atoms. They are uniformly distributed over the porous layer and are well linked to the TiO
2 surfaces. The number of lead atoms per surface unit was quantified as ~680 Pb atoms/nm
2 for the entire thickness of 340 nm.
Different bonding configurations between Pb and the TiO
2 layer could occur, especially considering that under-stochiometric surfaces are expected [
16].
Density functional theory calculations have been employed to study the adsorption behaviour of a lead atom at the interior surfaces of the gig-lox TiO
2 sponge, in contact with both vacuum and water. The gig-lox TiO
2 is known to locally arrange in the anatase phase with main terminations along the (101) surfaces [
13]. Previous calculations suggest that the anatase TiO
2 (101) surface (named A
101 hereafter) is the most stable among low-index surfaces [
27]. As a consequence, we studied Pb adsorption at an A
101 termination [
24].
According to the preferential oxidation states of lead, equal to +2 and +4, and the relative electronegativity of the implicated elements, which decreases going from O to Pb and Ti, the Pb-O bonds are more stable than the Pb-Ti. At the perfect A101, both in contact with vacuum and water, a Pb atom forms three O-Pb bonds binding to three outermost oxygens. The adsorption energy of 3.40 eV at the A101/vacuum interface and 2.49 eV at the A101/water interface testifies to the intense interaction between lead and A101.
Although the synthesized TiO
2 sponge is mainly characterized by (101) terminations [
13], we expect that the interior cavities show a variety of superficial local atomic environments. As a consequence, the local superficial stoichiometry can vary from the perfect 1:2 ratio for Ti:O of the anatase TiO
2. Pb adsorption on an A
101 surface with an oxygen vacancy, with Pb replacing one of the eight nonequivalent oxygen atoms belonging to the first two O-Ti-O layers, is highly unstable with strong local atomic adjustments. In this case, when Pb replaces the outermost superficial site of oxygen, coordinated with only two Ti, is the more favorable adsorption configuration. Instead, the adsorption of Pb on an A
101 surface with a deficit of titanium does not entail relevant rearrangements.
Figure 3b shows the most stable configuration in vacuum among the four nonequivalent replacements of Pb with a Ti atom of the first two O-Ti-O layers. Differences in energy among the various oxygen adsorption sites are on the range of 1–2 eV, whereas the various titanium adsorption sites are below 1 eV, with such differences even below 100 meV in the case of the A
101/water interface. Our study highlights a strong adsorption interaction of Pb both at the stoichiometric and defective termination in contact with vacuum and a water environment. Lead atoms can compensate and replace Ti sites in nonstoichiometric terminations with a deficit of Ti, preferring superficial sites lying at the vacuum or water interface of TiO
2. The calculations prove the strong behavior of Pb atoms towards segregation on TiO
2 surfaces where steady configurations with oxygen are obtained due to permanent loading during and after the diving in solutions.
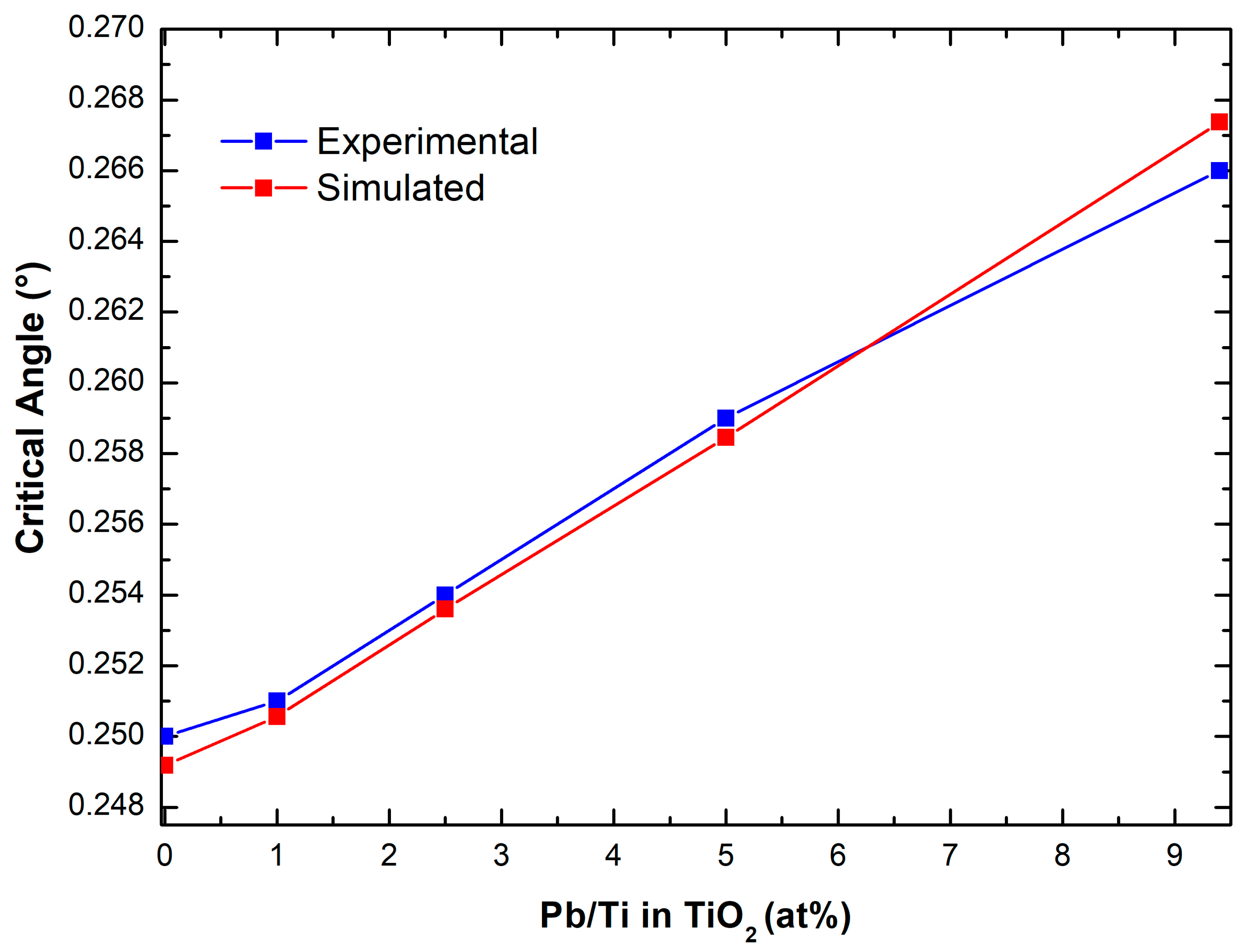
To further investigate the configurational arrangement of lead into the TiO
2 sponge, the measured critical angles have been related to the measured Pb/Ti ratio, as shown in
Figure 4. The sequence of points is almost linear, from the TiO
2 reference sample to the maximum concentration of lead in the solution, which is 6.4 ppm. In the same graph, the measured critical angles are then compared to the values that were, instead, calculated by simulating a progressively higher Pb introduction into the gig-lox structure, as reported in
Table 2. We considered a generic formula unit Pb
X Ti
(1−x)O
2, where x is the Pb index varying in the range 0-0.1, which represents the amount of Pb substitutional to Ti, as corroborated by the findings in
Figure 3. An increase of x represents a progressive incorporation of Pb that is translated into an increasing electronic density of the overall material. The agreement between the two curves in
Figure 4 supports the model to be applied to describe the process of lead capturing wherein the surfaces play a major role. We further observe that the experimental values of the critical angles are slightly and systematically above the simulated ones (except for the last point), representing that the pore filling must play another important role in the capturing procedure. We additionally calculated the critical angle by assuming a volume expansion of the TiO
2 lattice in the range 0.1–1%. We found that a volume expansion under a threshold of 0.3% would be negligible on the calculation of the critical angle, supporting that the dominant effect is the lead atom introduction into the gig-lox structure.