Form-Dependent Toxicity of Silver Nanomaterials in Rainbow Trout Gills
Abstract
1. Introduction
2. Materials and Methods
2.1. Exposure of Rainbow Trout Juveniles
2.2. Stability of Ag in the Exposure Media and Detection Gill Tissues
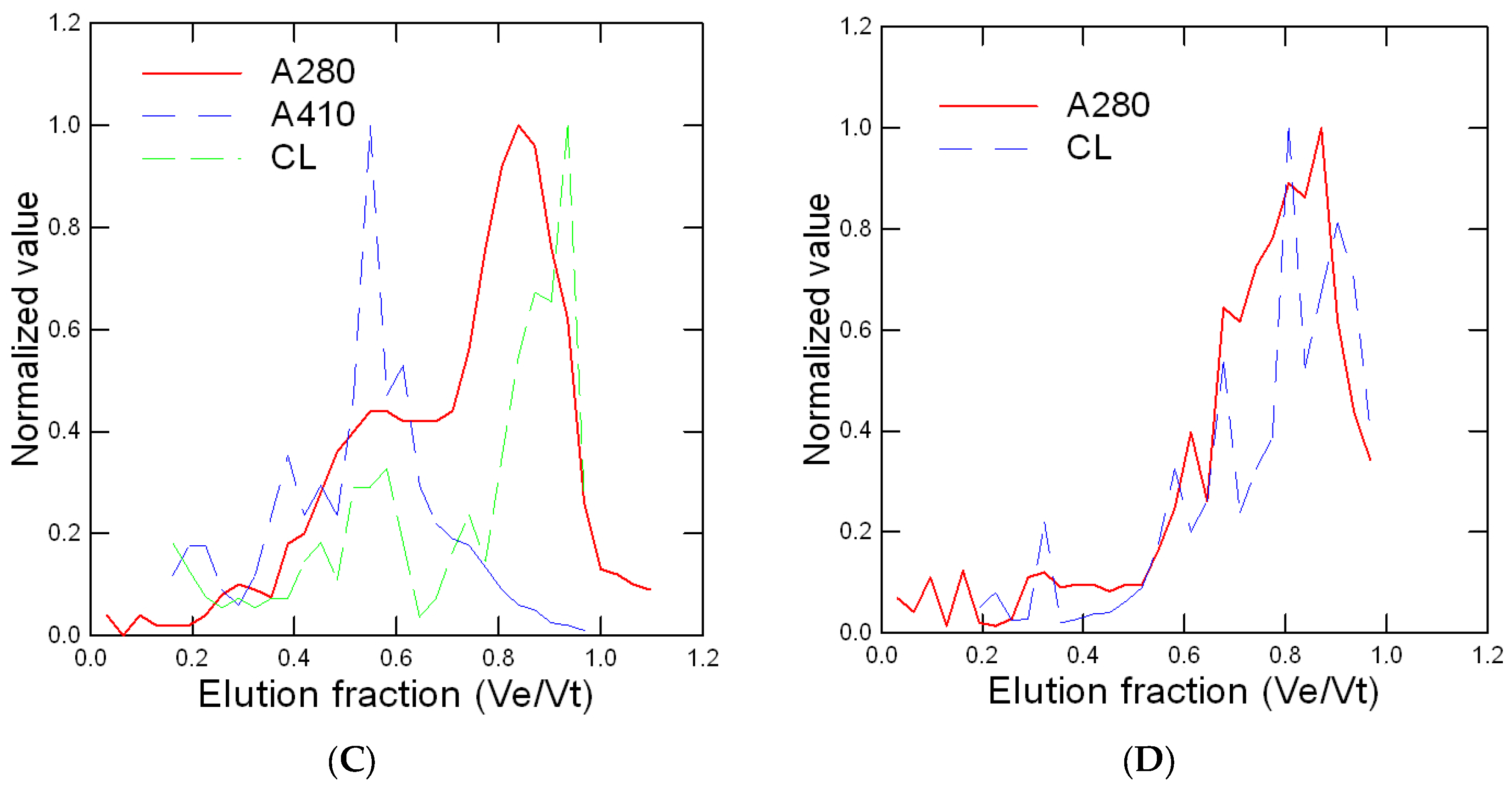
2.3. Size-Exclusion Chromatography
2.4. Biochemical Assessments
3. Data Analysis
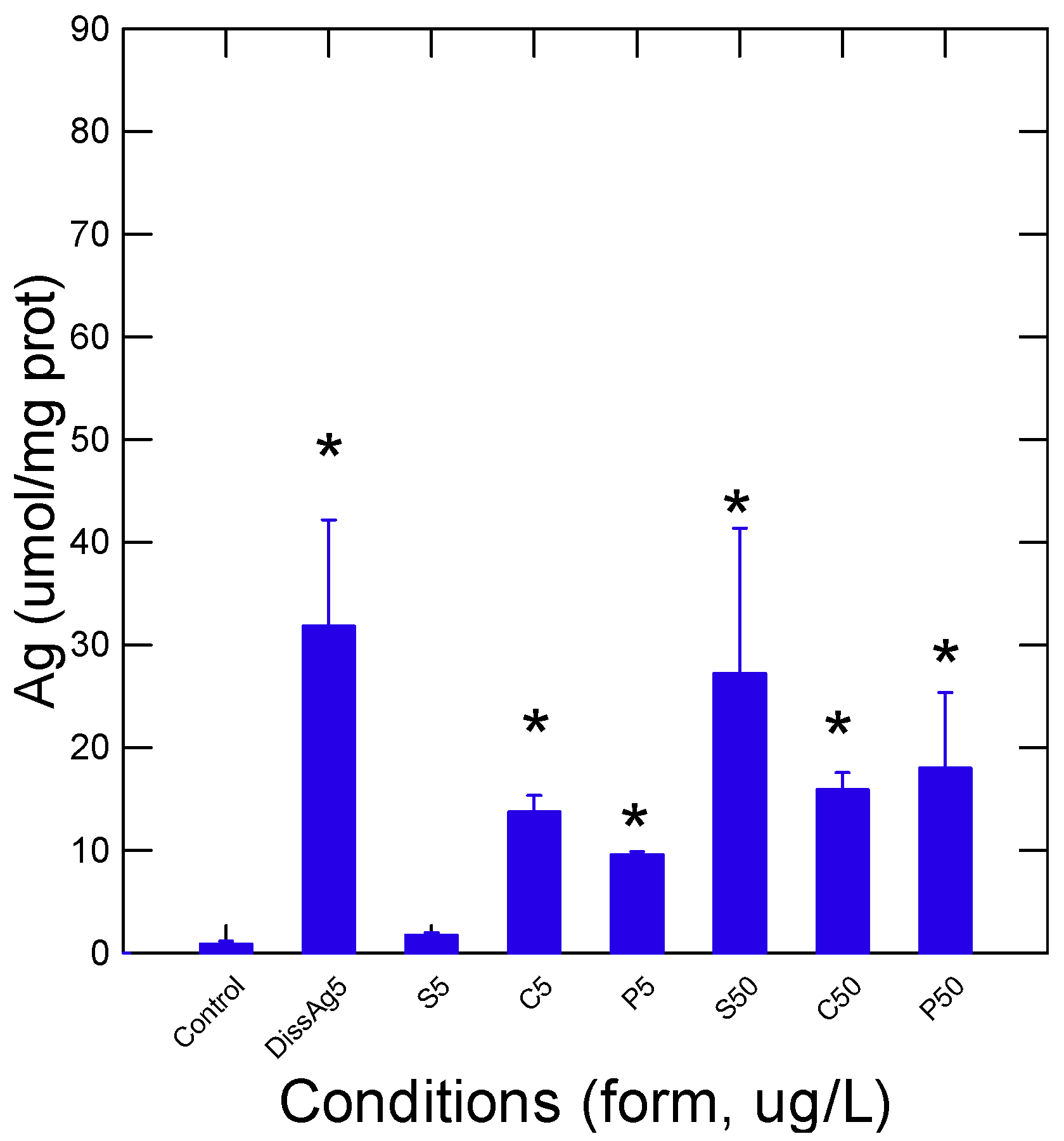
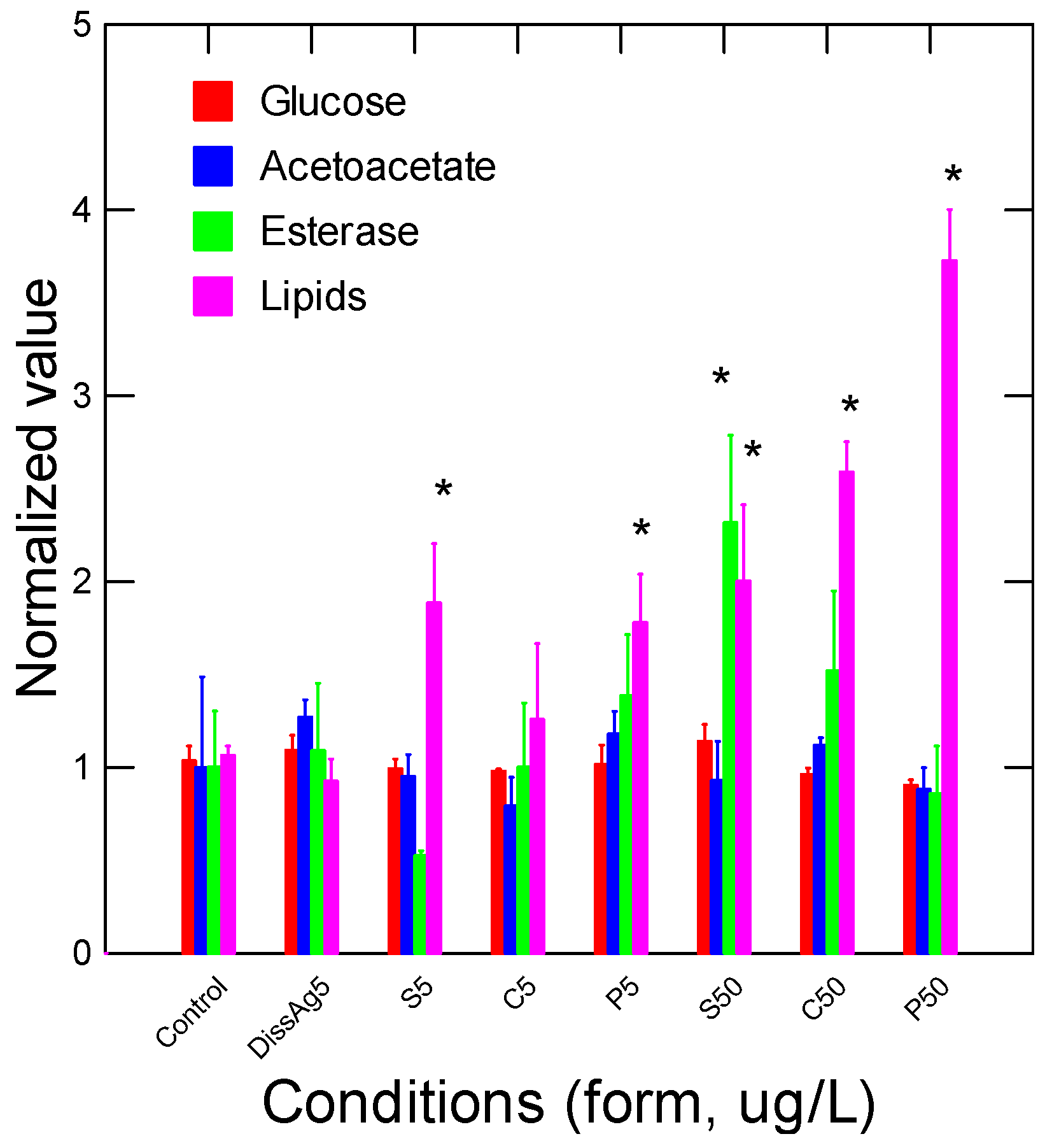
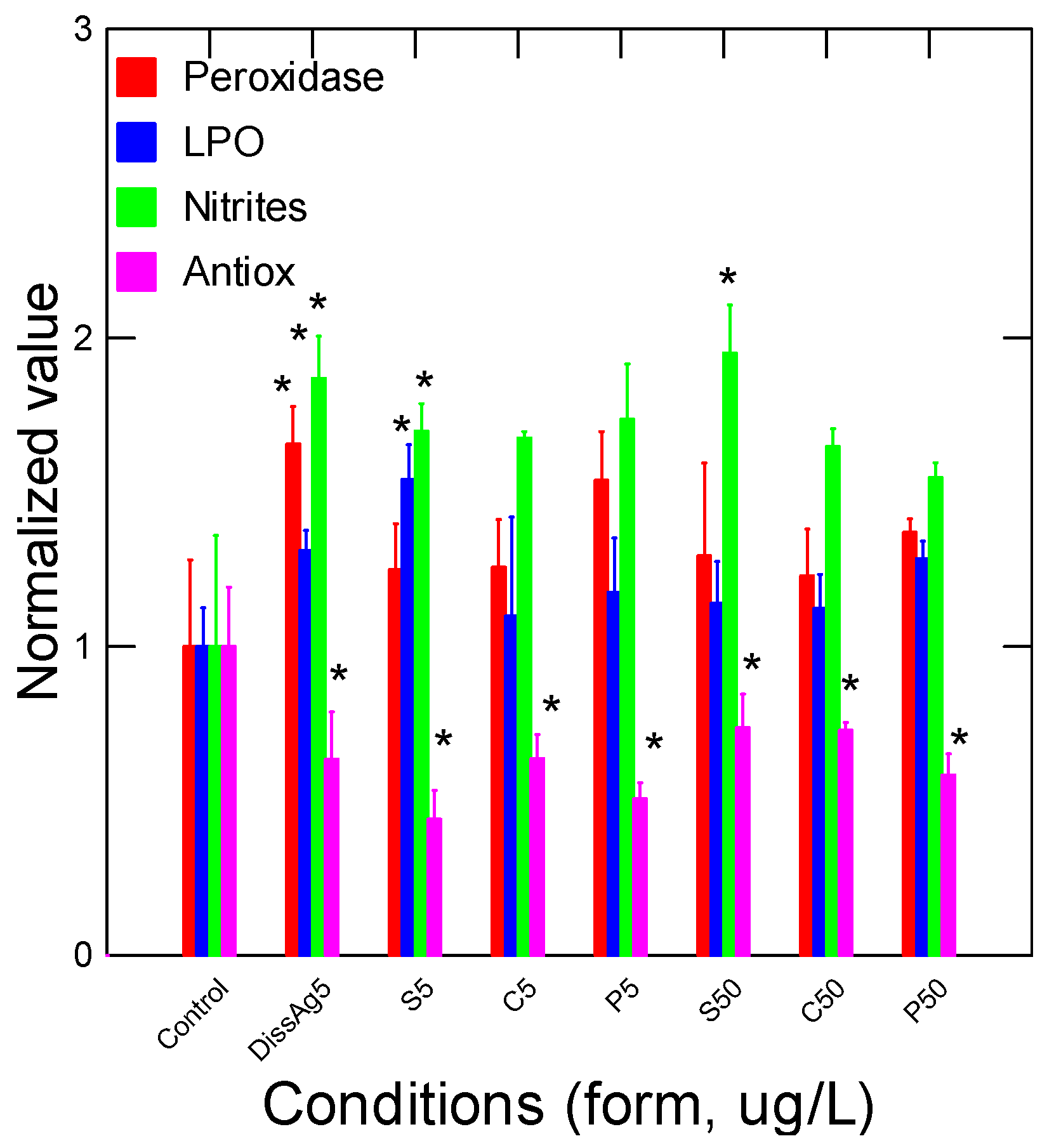
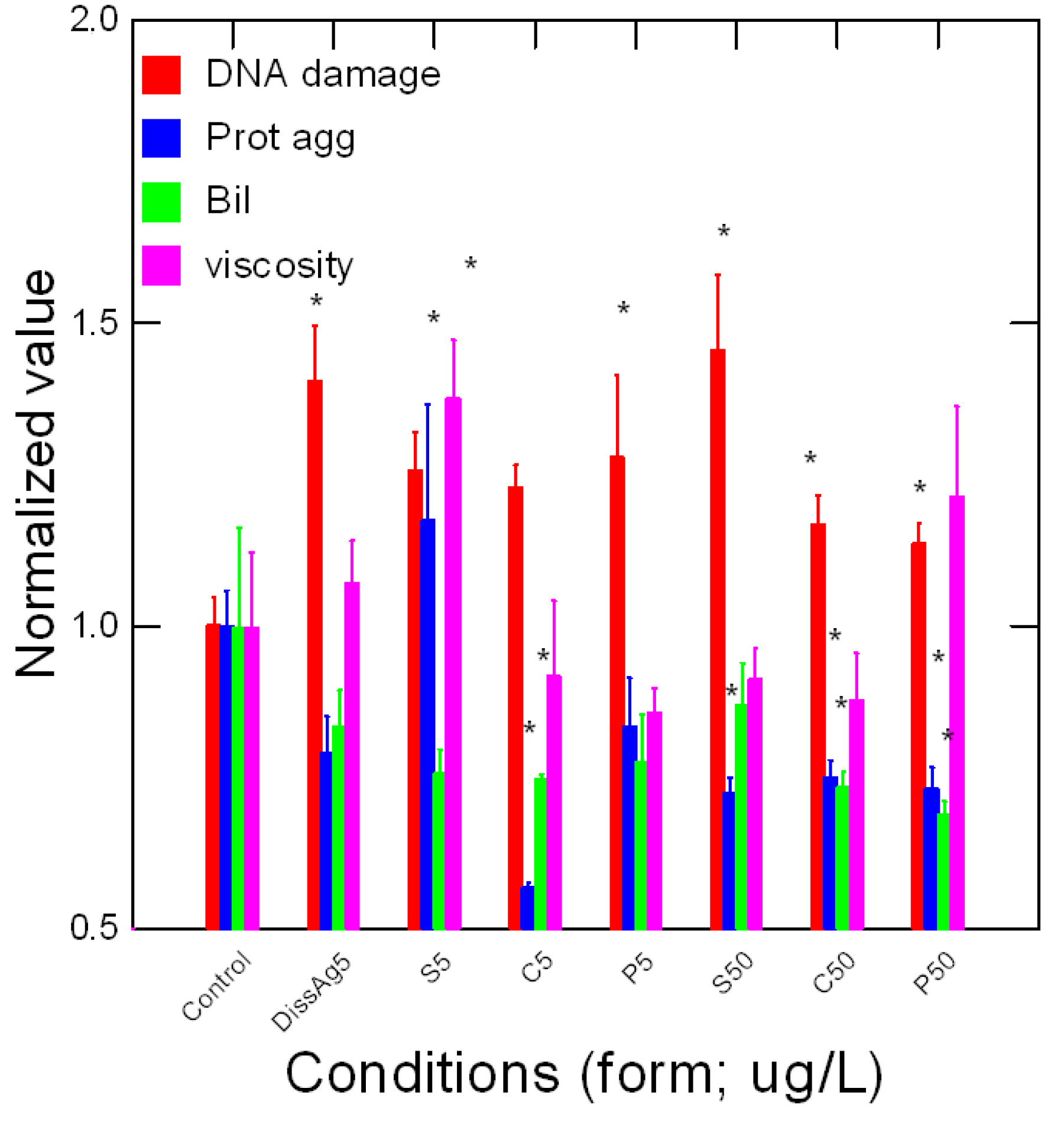
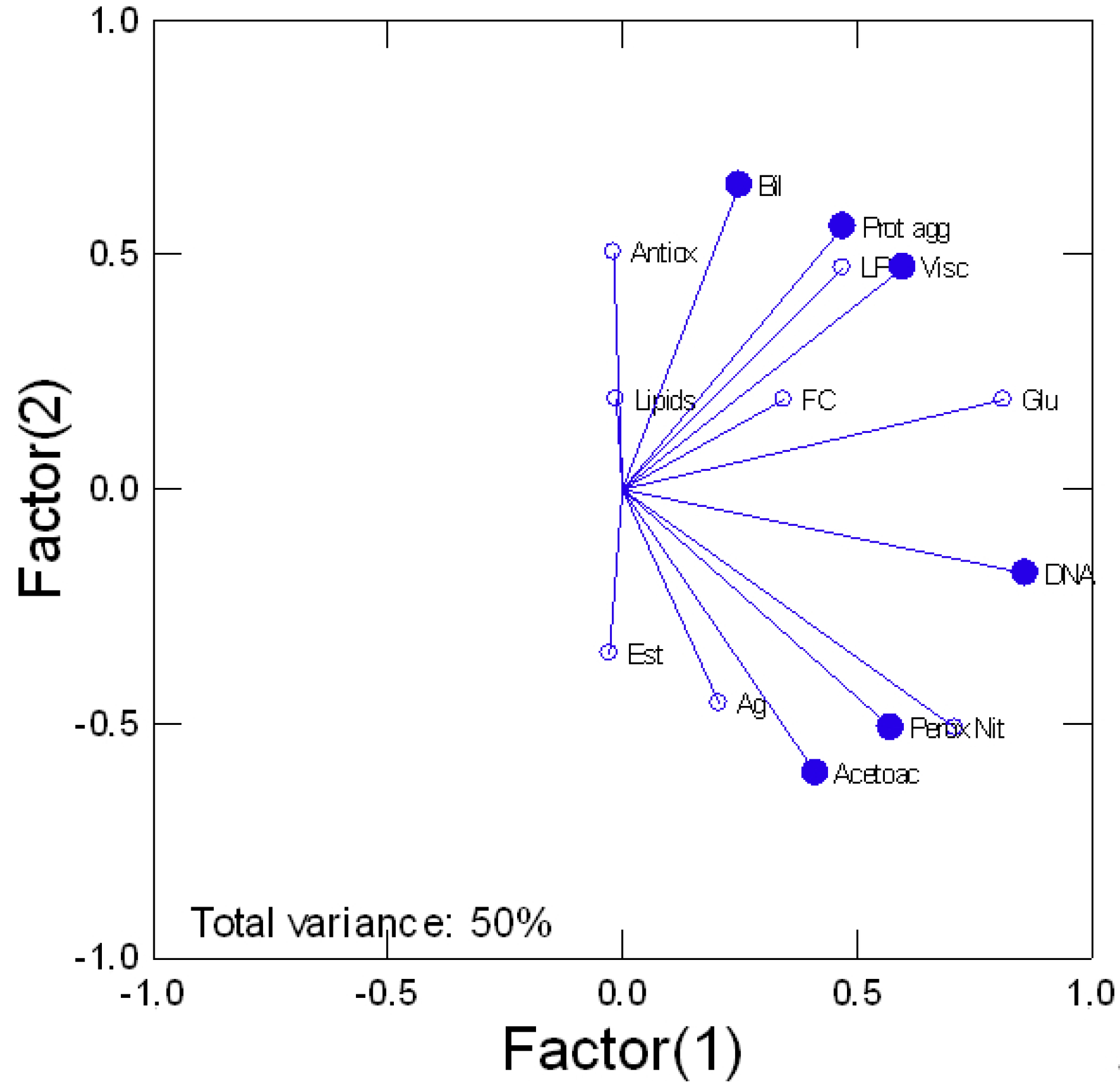
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gottardo, S.; Mech, A.; Drbohlavová, J.; Małyska, A.; Bøwadt, S.; Riego Sintes, J.; Rauscher, H. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef]
- McGillicuddy, E.; Murray, I.; Kavanagh, S.; Morrison, L.; Fogarty, A.; Cormican, M.; Dockery, P.; Prendergast, M.; Rowan, N.; Morris, D. Silver nanoparticles in the environment: Sources, detection and ecotoxicology. Sci. Total Environ. 2017, 575, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, Z.; Deng, Z. Silver nanoparticles in aquatic environments: Physicochemical behavior and antimicrobial mechanisms. Water Res. 2016, 88, 403–427. [Google Scholar] [CrossRef] [PubMed]
- Bolaños-Benítez, V.; McDermott, F.; Gill, L.; Knappe, J. Engineered silver nanoparticle (Ag-NP) behaviour in domestic on-site wastewater treatment plants and in sewage sludge amended-soils. Sci. Total Environ. 2020, 722, 137794. [Google Scholar] [CrossRef]
- Gagnon, C.; Turcotte, P.; Gagné, F.; Smyth, S.A. Occurrence and size distribution of silver nanoparticles in wastewater effluents from various treatment processes in Canada. Environ. Sci. Pollut. Res. Int. 2021, 28, 65952–65959. [Google Scholar] [CrossRef]
- Formo, E.V.; Potterf, C.B.; Yang, M.; Unocic, R.R.; Leonard, D.N.; Pawel, M. How a Nanostructure’s Shape Affects its Lifetime in the Environment: Comparing a Silver Nanocube to a Nanoparticle When Dispersed in Aqueous Media. Environ. Sci. Technol. 2016, 50, 7082–7089. [Google Scholar] [CrossRef]
- Da Silva, A.G.; Rodrigues, T.S.; Wang, J.; Yamada, L.K.; Alves, T.V.; Ornellas, F.R.; Ando, R.A.; Camargo, P.H. The Fault in Their Shapes: Investigating the Surface-Plasmon-Resonance-Mediated Catalytic Activities of Silver Quasi-Spheres, Cubes, Triangular Prisms, and Wires. Langmuir 2015, 31, 10272–10278. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Ghavami, M.; Aghaverdi, H.; Stroeve, P.; Mahmoudi, M. Bacterial Effects and Protein Corona Evaluations: Crucial Ignored Factors in the Prediction of Bio-Efficacy of Various Forms of Silver Nanoparticles. Chem. Res. Toxicol. 2012, 25, 1231–1242. [Google Scholar] [CrossRef]
- Auclair, J.; Peyrot, C.; Wilkinson, K.J.; Gagné, F. The geometry of the toxicity of silver nanoparticles to freshwater mussels. Comp. Biochem. Physiol. C Toxicol. Pharm. 2021, 239, 108841. [Google Scholar] [CrossRef]
- SIbiya, A.; Gopi, N.; Jeyavani, J.; Mahboob, S.; Al-Ghanim, K.A.; Sultana, S.; Mustafa, A.; Govindarajan, M.; Vaseeharan, B. Comparative toxicity of silver nanoparticles and silver nitrate in freshwater fish Oreochromis mossambicus: A multi-biomarker approach. Comp. Biochem. Physiol. 2022, 259, 109391. [Google Scholar] [CrossRef]
- Abdel-Khalek, A.A.; Al-Quraishy, S.; Abdel-Gaber, R. Silver Nanoparticles Induce Time- and Tissue-Specific Genotoxicity in Oreochromis niloticus: Utilizing the Adsorptive Capacities of Fruit Peels to Minimize Genotoxicity. Bull. Environ. Contam. Toxicol. 2022, 108, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Gagné, F. Isolation and quantification of polystyrene nanoplastics in tissues by low pressure size exclusion chromatography. J. Xenobiot. 2022, 12, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Biological test method: Acute lethality test using rainbow trout. In Environmental Protection Series; Report EPS 1/RM/9; Method Development and Applications Section, Environmental Technology Centre, Environment Canada: Ottawa, ON, Canada, 1990.
- Polesel, F.; Farkas, J.; Kjos, M.; Carvalho, P.A.; Flores-Alsina, X.; Gernaey, K.V.; Hansen, S.F.; Plósz, B.G.; Booth, A.M. Occurrence, characterisation and fate of (nano)particulate Ti and Ag in two Norwegian wastewater treatment plants. Water Res. 2018, 141, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Azodi, M.; Sultan, Y.; Ghoshal, S. Dissolution Behavior of Silver Nanoparticles Formation of Secondary Silver Nanoparticles in Municipal Wastewater by Single-Particle, I.C.P.-M.S. Environ. Sci. Technol. 2016, 50, 13318–13327. [Google Scholar] [CrossRef]
- Auclair, J.; Peyrot, C.; Wilkinson, K.J.; Gagné, F. The influence of silver nanoparticle form on the toxicity in freshwater mussels. Appl. Sci. 2022, 12, 1429. [Google Scholar] [CrossRef]
- Auclair, A.; Turcotte, P.; Gagnon, C.; Peyrot, C.; Wilkinson, K.J.; Gagné, F. Toxicological effects of inorganic nanoparticle mixtures in freshwater mussels. Environments 2021, 7, 109. [Google Scholar] [CrossRef]
- Li, S.; Sun, H.; Wang, D.; Hong, J.; Tao, S.; Yu, H.; Wang, X.; Wei, X. Enhanced chemiluminescence of the luminol–AgNO3 system by Ag nanoparticles. Luminescence 2012, 27, 211–216. [Google Scholar] [CrossRef]
- Zielinska, A.; Skwarek, E.; Zaleska, A.; Gazdac, M.; Hupka, J. Preparation of silver nanoparticles with controlled particle size. Procedia. Chem. 2009, 1, 1560–1566. [Google Scholar] [CrossRef]
- Wills, E.D. Evaluation oflipid peroxidation in lipids and biological membranes. In Biochemical Toxicology. A Practical Approach; Snell, K., Mullock, B., Eds.; IRL Press: Oxford, UK, 1987; pp. 127–152. [Google Scholar]
- Olive, P.L. DNA precipitation assay: A rapid and simple method for detecting DNA damage in mammalian cells. Environ. Mol. Mutagen. 1988, 11, 487–495. [Google Scholar] [CrossRef]
- West, D.C.; Sattar, A.; Kumar, S. A simplified in situ solubilization procedure for the determination of DNA and cell number in tissue cultured mammalian cells. Anal. Biochem. 1985, 147, 289–295. [Google Scholar] [CrossRef]
- Bester, M.J.; Potgieter, H.C.; Vermaak, W.J.H. Cholate and pH reduce interference by SDS in the determination of DNA with Hoescht. Anal. Biochem. 1994, 223, 299–305. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile Red: A selective fluorescent stain for intracellular lipid droplets. J. Cell. Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef]
- Cervantes-Avilés, P.; Huang, Y.; Keller, A.A. Incidence and persistence of silver nanoparticles throughout the wastewater treatment process. Water Res. 2019, 156, 188–198. [Google Scholar] [CrossRef]
- Jang, M.-H.; Bae, S.-J.; Lee, S.-K.; Lee, Y.-J.; Hwang, Y.S. Effect of material properties on stability of silver nanoparticles in water. J. Nanosci. Nanotechnol. 2014, 14, 9665–9669. [Google Scholar] [CrossRef]
- Graf, C.; Nordmeyer, D.; Sengstock, C.; Ahlberg, S.; Diendorf, J.; Raabe, J.; Epple, M.; Köller, M.; Lademann, J.; Vogt, A.; et al. Shape-dependent dissolution and cellular uptake of silver nanoparticles. Langmuir 2018, 34, 1506–1519. [Google Scholar] [CrossRef]
- Liu, W.; Worms, I.A.M.; Herlin-Boime, N.; Truffier-Boutry, D.; Michaud-Soret, I.; Mintz, E.; Vidaud, C.; Rollin-Genetet, F. Interaction of silver nanoparticles with metallothionein and ceruloplasmin: Impact on metal substitution by Ag(I), corona formation and enzymatic activity. Nanoscale 2017, 9, 6581–6594. [Google Scholar] [CrossRef]
- George, S.; Lin, S.; Ji, Z.; Thomas, C.R.; Li, L.J.; Mecklenburg, M.; Meng, H.; Wang, X.; Zhang, H.; Xia, T.; et al. Surface Defects on Plate-Shaped Silver Nanoparticles Contribute to Its Hazard Potential in Fish Gill Cell Line and Zebrafish Embryos. ACS Nano. 2012, 6, 3745–3759. [Google Scholar] [CrossRef]
- Moon, J.; Kwak, J.I.I.; An, Y.-J. The effects of silver nanomaterial shape and size on toxicity to Caenorhabditis elegans in soil media. Chemosphere 2019, 215, 50–56. [Google Scholar] [CrossRef]
- Holmes, A.M.; Lim, J.; Studier, H.; Roberts, M.S. Varying the morphology of silver nanoparticles results in differential toxicity against micro-organisms, HaCaT keratinocytes and affects skin deposition. Nanotoxicology 2016, 10, 1503–1514. [Google Scholar] [CrossRef]
- Abramenko, N.B.; Demidova, T.B.; Abkhalimov, E.V.; Ershov, B.G.; Krysanov, E.Y.; Kustov, L.M. Ecotoxicity of different-shaped silver nanoparticles: Case of zebrafish embryos. J. Hazard. Mater. 2018, 347, 89–94. [Google Scholar] [CrossRef]
- Campolo, N.; Issoglio, F.M.; Estrin, D.A.; Bartesaghi, S.; Radi, R. 3-Nitrotyrosine and related derivatives in proteins: Precursors, radical intermediates and impact in function. Essays Biochem. 2020, 64, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Auclair, J.; Turcotte, P.; Gagnon, C.; Peyrot, C.; Wilkinson, K.J.; Gagné, F. The influence of surface coatings on the toxicity of silver nanoparticle in rainbow trout. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 226, 108623. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, F.A.; Logothetis, P.; Meseguer, J.; Esteban, M.A. Evaluation of silver nanospheres on viability and innate cellular parameters of gilthead seabream (Sparus aurata L.) head-kidney leucocytes. Fish Shellfish. Immunol. 2017, 69, 99–107. [Google Scholar] [CrossRef] [PubMed]

| Ag Form (5 µg/L) | Dissolved Ag 1 h (µg/L) | Dissolved Ag 96 h (µg/L) | Decreasing Rate (h−1) /Half Life (h) |
|---|---|---|---|
| Sphere | 4.9 ± 0.4 | 3.1 ± 0.3 | 0.018/126 |
| Cube | 5.2 ± 0.2 | 2.9 ± 0.2 | 0.024/114 |
| Prismatic | 6.5 ± 0.5 | 3.4 ± 0.3 | 0.033/150 |
| Dissolved | 4.7 ± 0.4 | 4.9 ± 0.4 | ~0 |
| Ag | CF | HIS | GI |
|---|---|---|---|
| Controls | 1.02 ± 0.01 | 0.98 ± 0.07 | 0.05 ± 0.02 |
| Dissolved | 1 ± 0.01 | 1.19 ± 0.07 * | 0045 ± 0.017 |
| Sphere | |||
| 5 | 0.98 ± 0.02 | 0.9 ± 0.07 * | 0.048 ± 0.017 |
| 50 | 0.97 ± 0.01 | 1.3 ± 0.08 * | 0.052 ± 0.018 |
| Cube | |||
| 5 | 0.93 ± 0.009 | 0.93 ± 0.05 | 0.05 ± 0.018 |
| 50 | 1.09 ± 0.03 | 1.2 ± 0.1 | 0.048 ± 0.017 |
| Prism | |||
| 5 | 1 ± 0.02 | 0.76 ± 0.02 * | 0.051 ± 0.02 |
| 50 | 1 ± 0.02 | 1.1 ± 0.09 | 0.054 ± 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auclair, J.; Turcotte, P.; Gagnon, C.; Peyrot, C.; Wilkinson, K.J.; Gagné, F. Form-Dependent Toxicity of Silver Nanomaterials in Rainbow Trout Gills. Nanomaterials 2023, 13, 1356. https://doi.org/10.3390/nano13081356
Auclair J, Turcotte P, Gagnon C, Peyrot C, Wilkinson KJ, Gagné F. Form-Dependent Toxicity of Silver Nanomaterials in Rainbow Trout Gills. Nanomaterials. 2023; 13(8):1356. https://doi.org/10.3390/nano13081356
Chicago/Turabian StyleAuclair, Joëlle, Patrice Turcotte, Christian Gagnon, Caroline Peyrot, Kevin James Wilkinson, and François Gagné. 2023. "Form-Dependent Toxicity of Silver Nanomaterials in Rainbow Trout Gills" Nanomaterials 13, no. 8: 1356. https://doi.org/10.3390/nano13081356
APA StyleAuclair, J., Turcotte, P., Gagnon, C., Peyrot, C., Wilkinson, K. J., & Gagné, F. (2023). Form-Dependent Toxicity of Silver Nanomaterials in Rainbow Trout Gills. Nanomaterials, 13(8), 1356. https://doi.org/10.3390/nano13081356










