Titanium Dioxide Nanoparticles: Effects on Development and Male Reproductive System
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Work Solutions
2.2. Breeding of Zebrafish and Experiment Design
2.3. Acute Toxicity Experiment of Zebrafish Embryo
2.4. Evaluation of Toxicological Endpoints and DanioScope™ Analysis
2.5. Immunohistochemical Analysis on Zebrafish Larvae
2.6. Adult Zebrafish Exposure Experiment
2.7. TiO2-NPs Accumulation
2.8. Histological Examination
2.9. Immunohistochemical Analysis
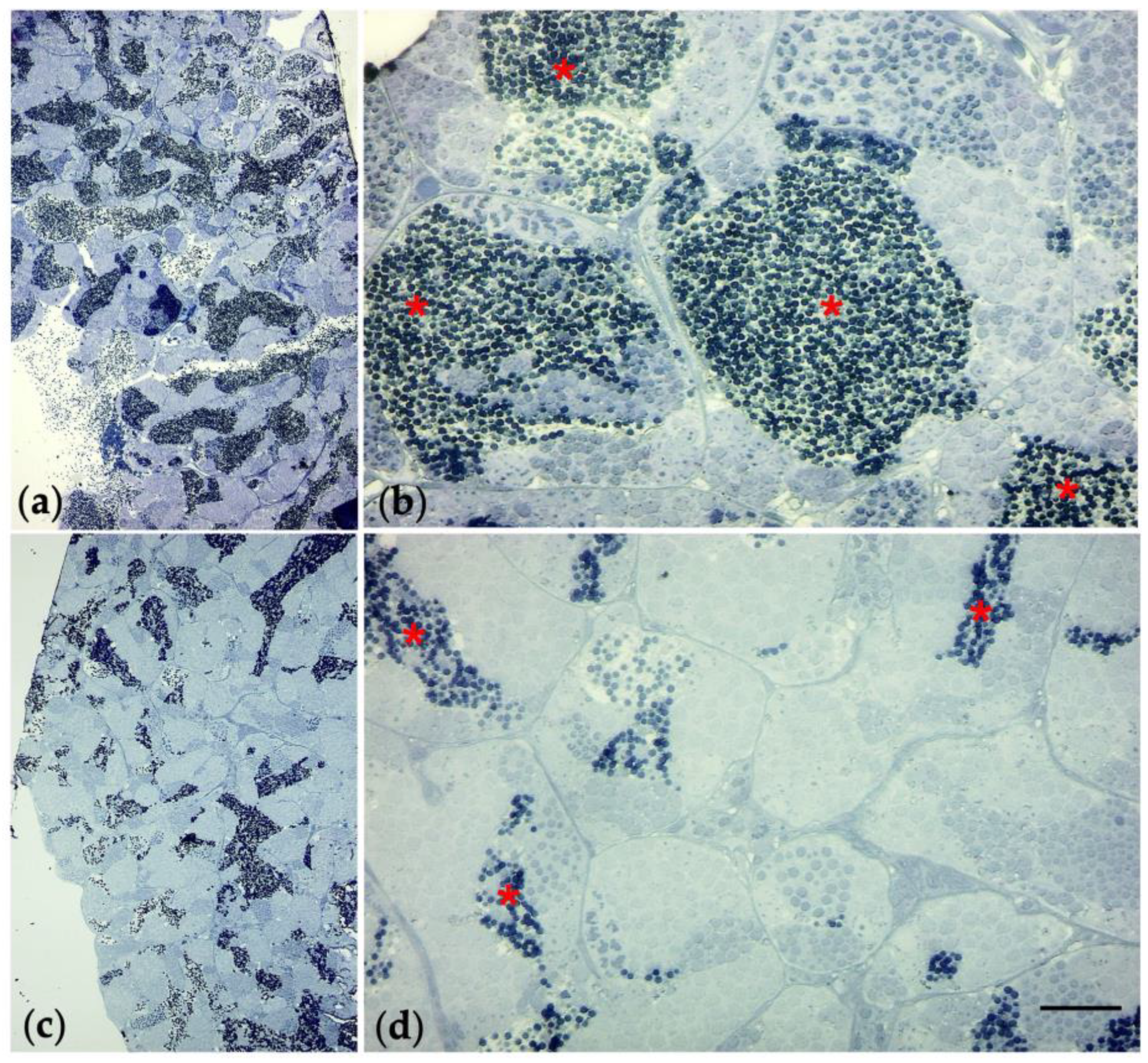
2.10. Preparation of Semithin Sections and Electron Microscopy
2.11. RNA Extraction and qRT-PCR
2.12. Crystal Structure of Sex Hormone-Binding Globulin (SHBG)
2.13. Statistical Analysis
3. Results
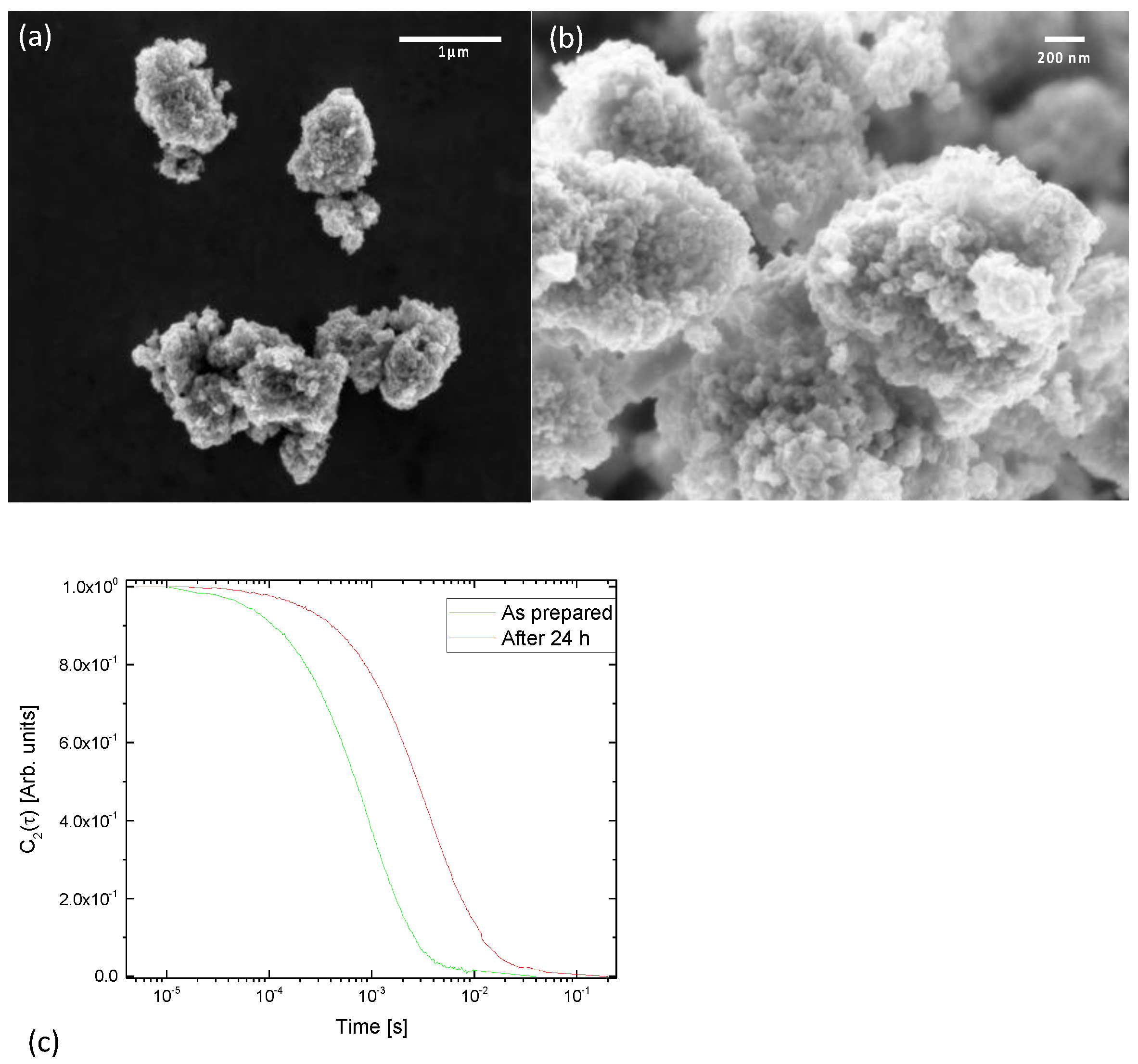
3.1. Nanoparticles Characterization
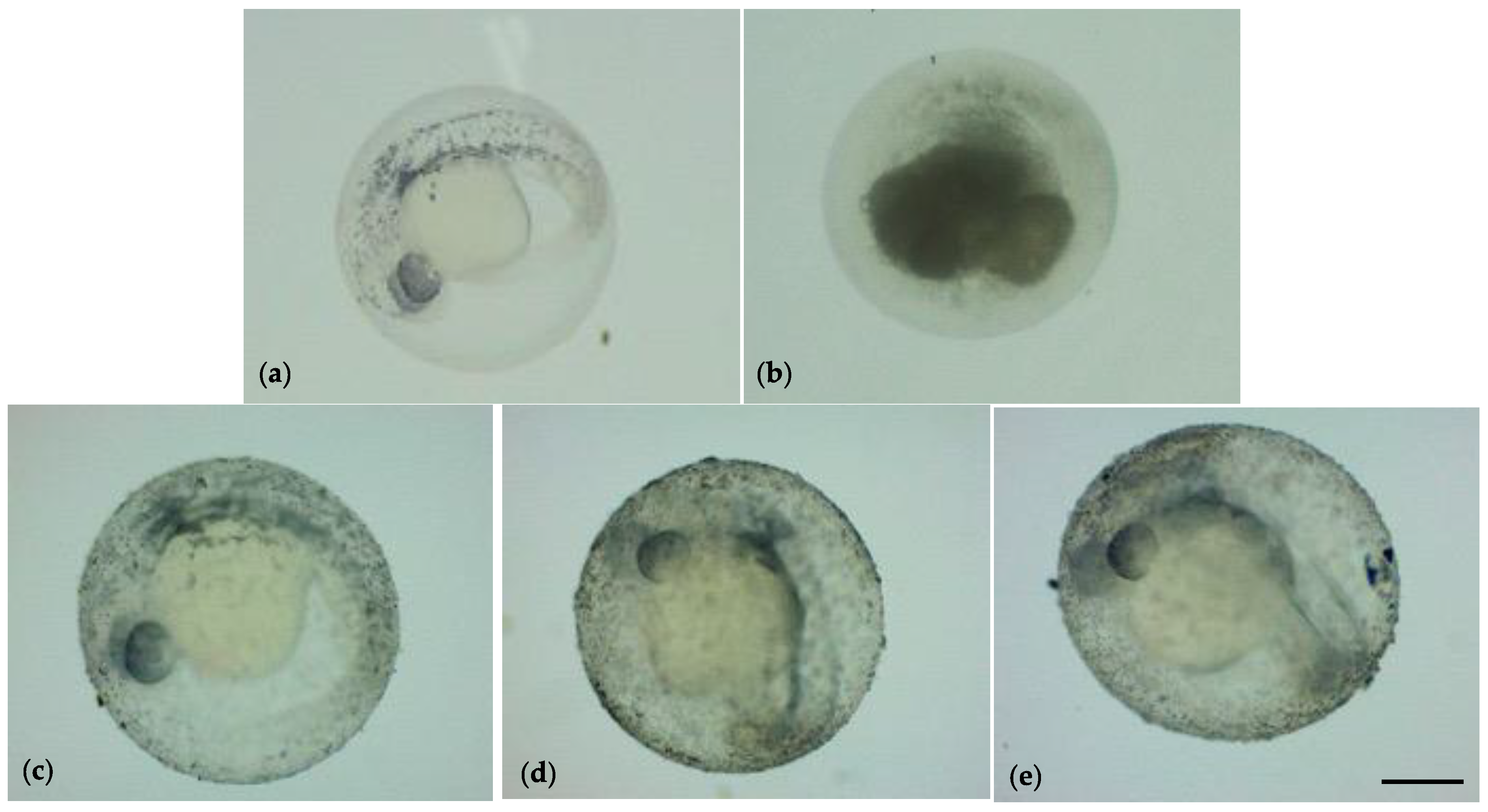
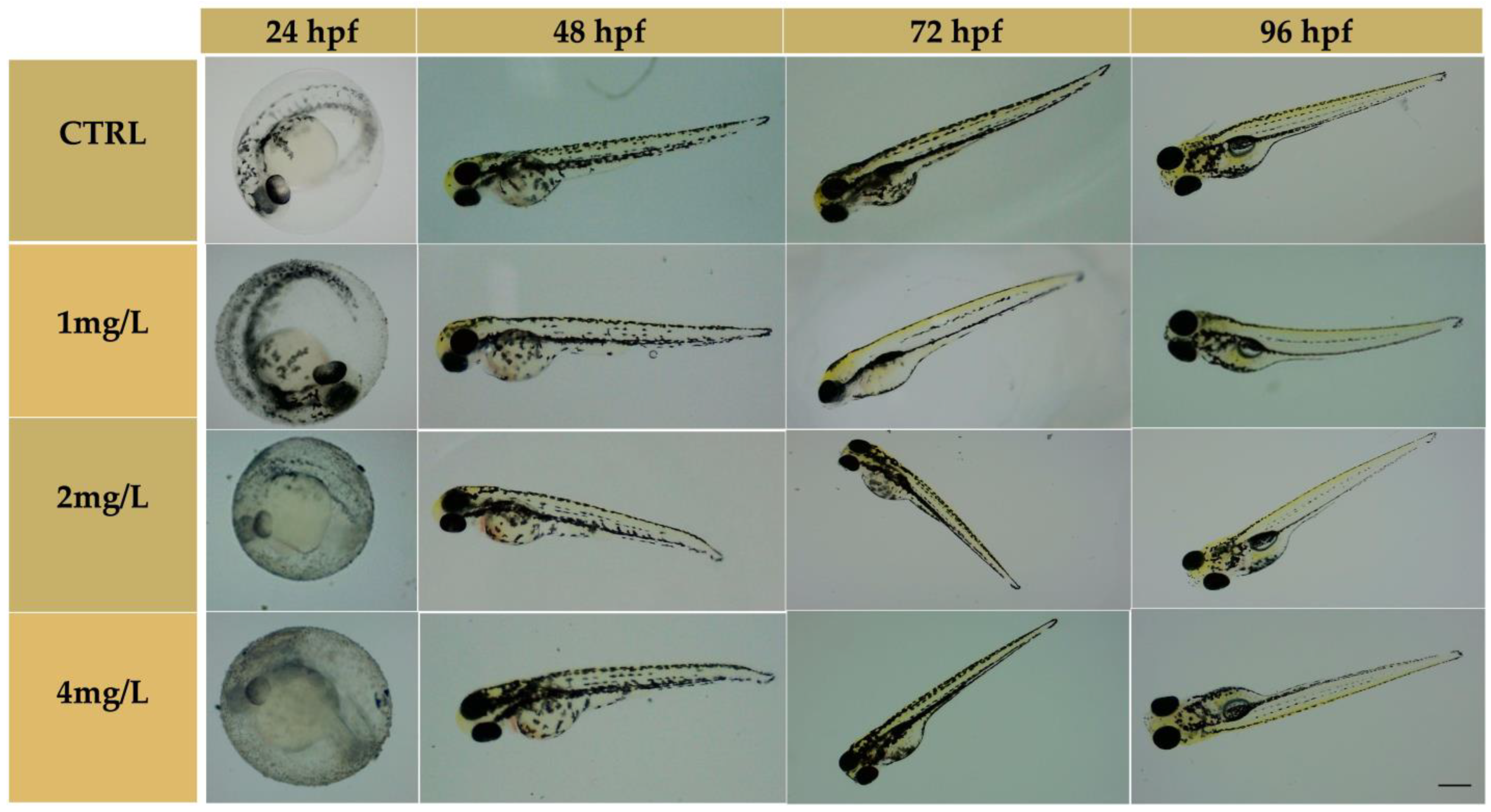
3.2. Embryonic Development of Zebrafish
3.3. DanioScope Analysis
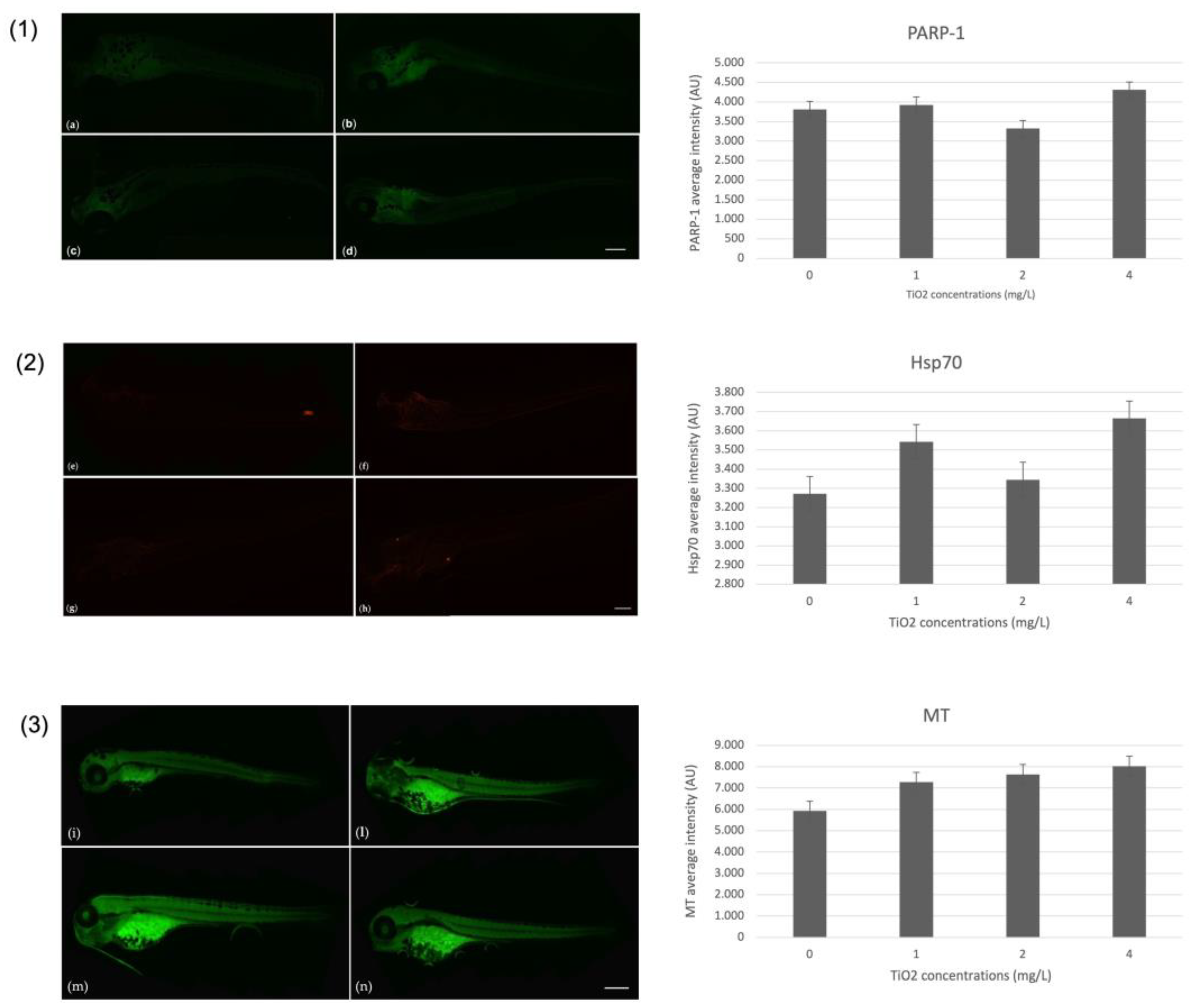
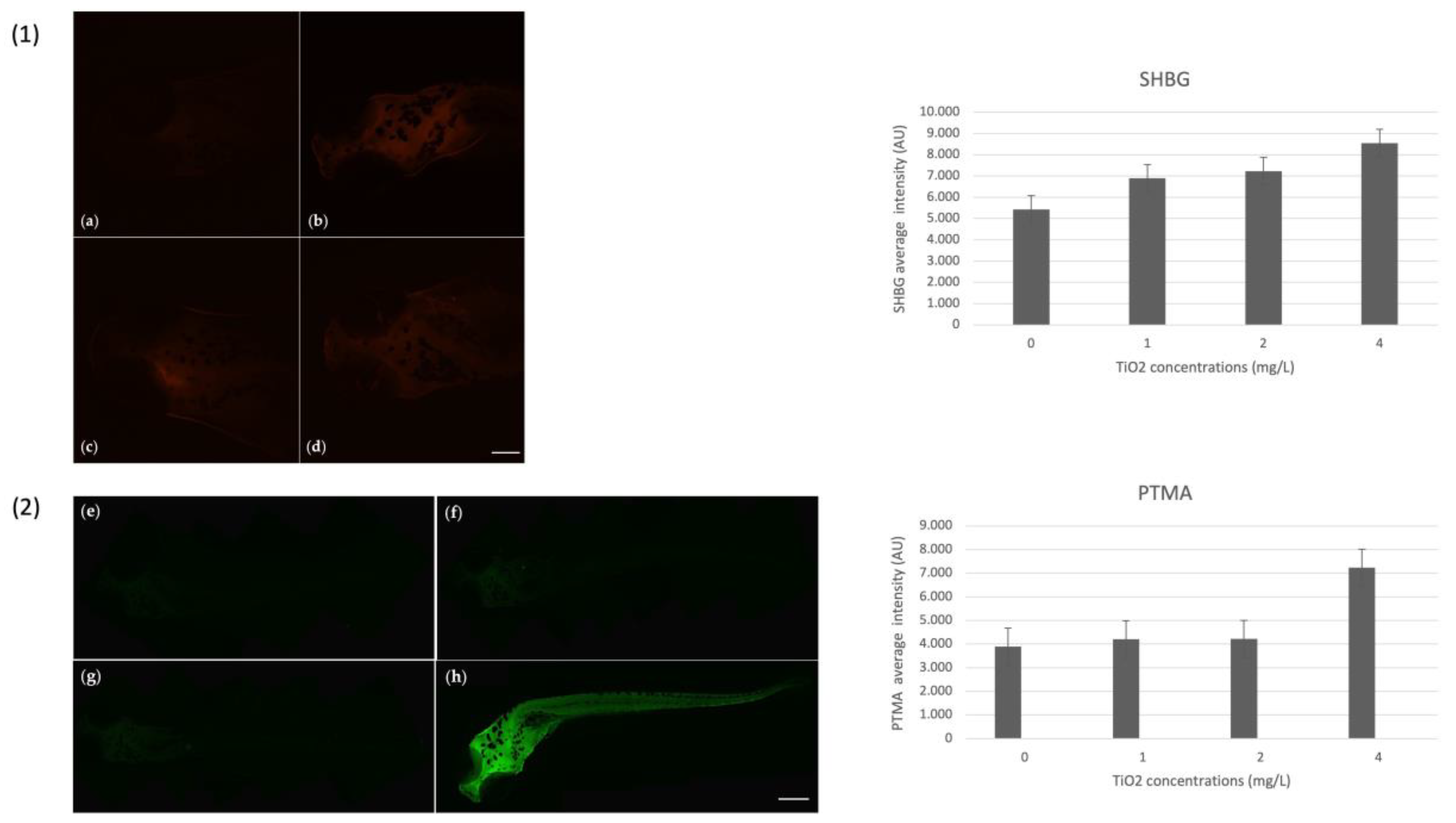
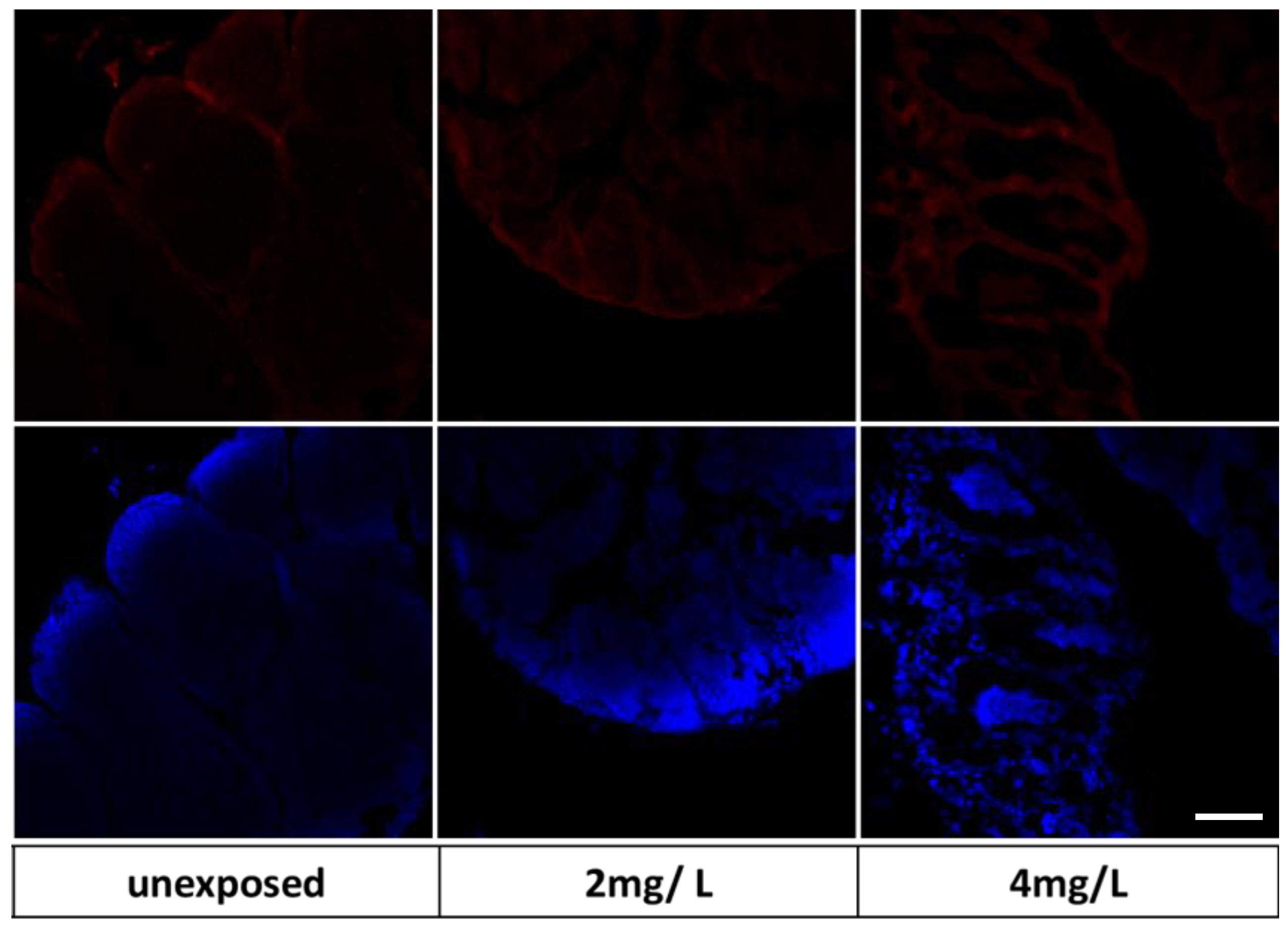
3.4. Immunohistochemical Markers on Zebrafish Larvae
3.5. Adult Exposure
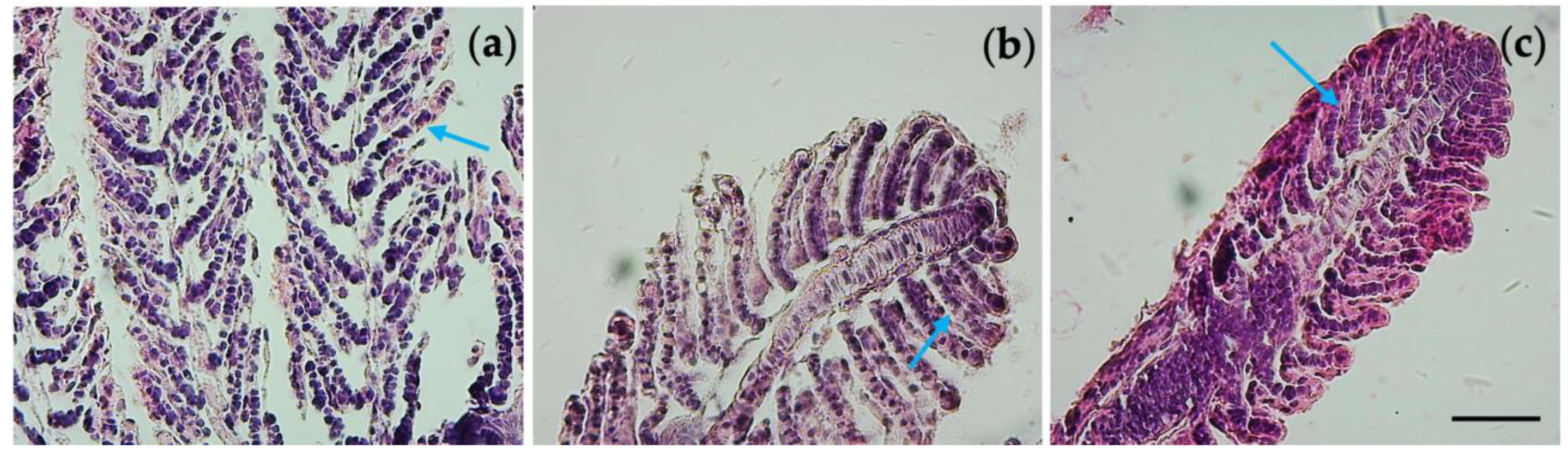
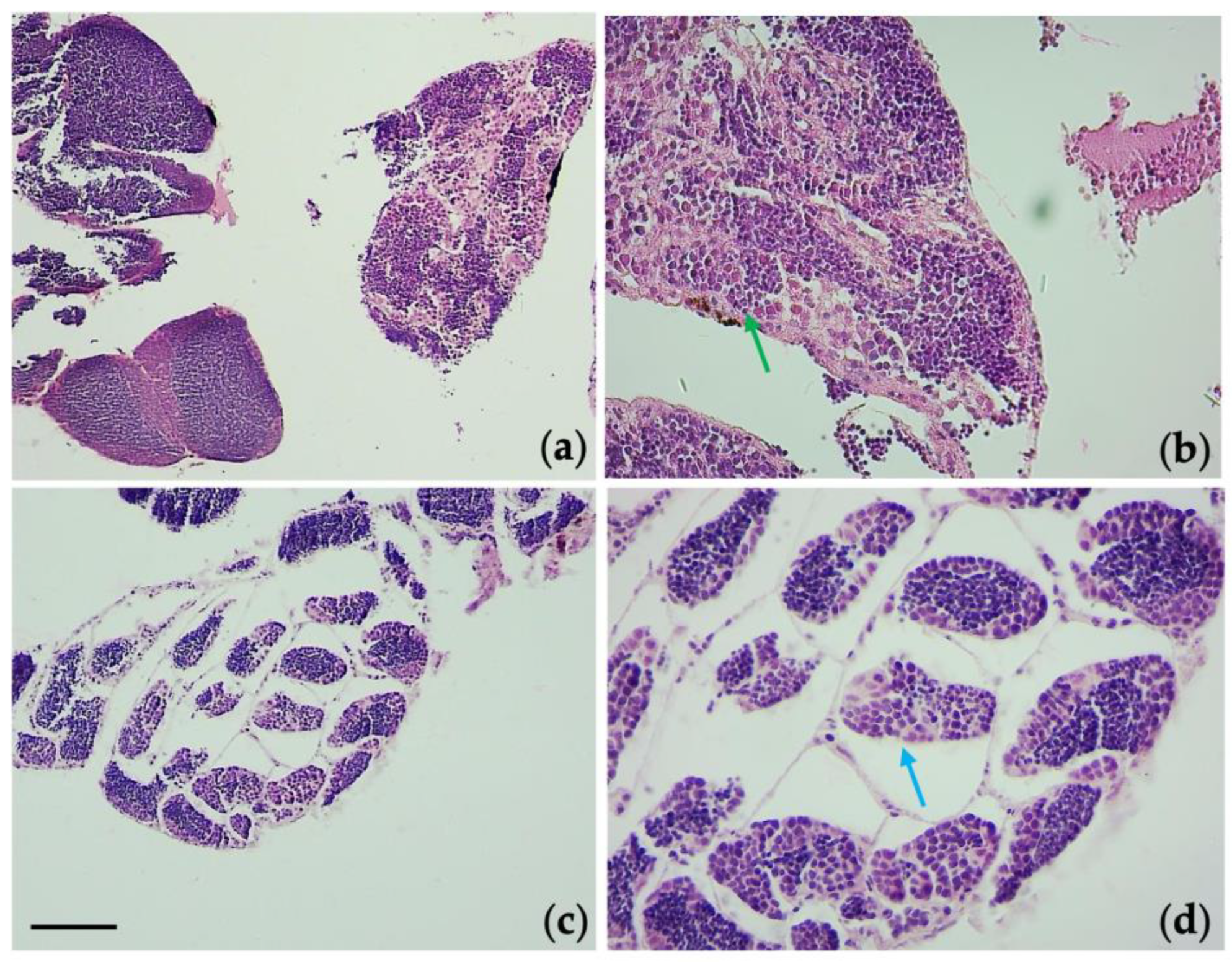
3.6. Histological Observations
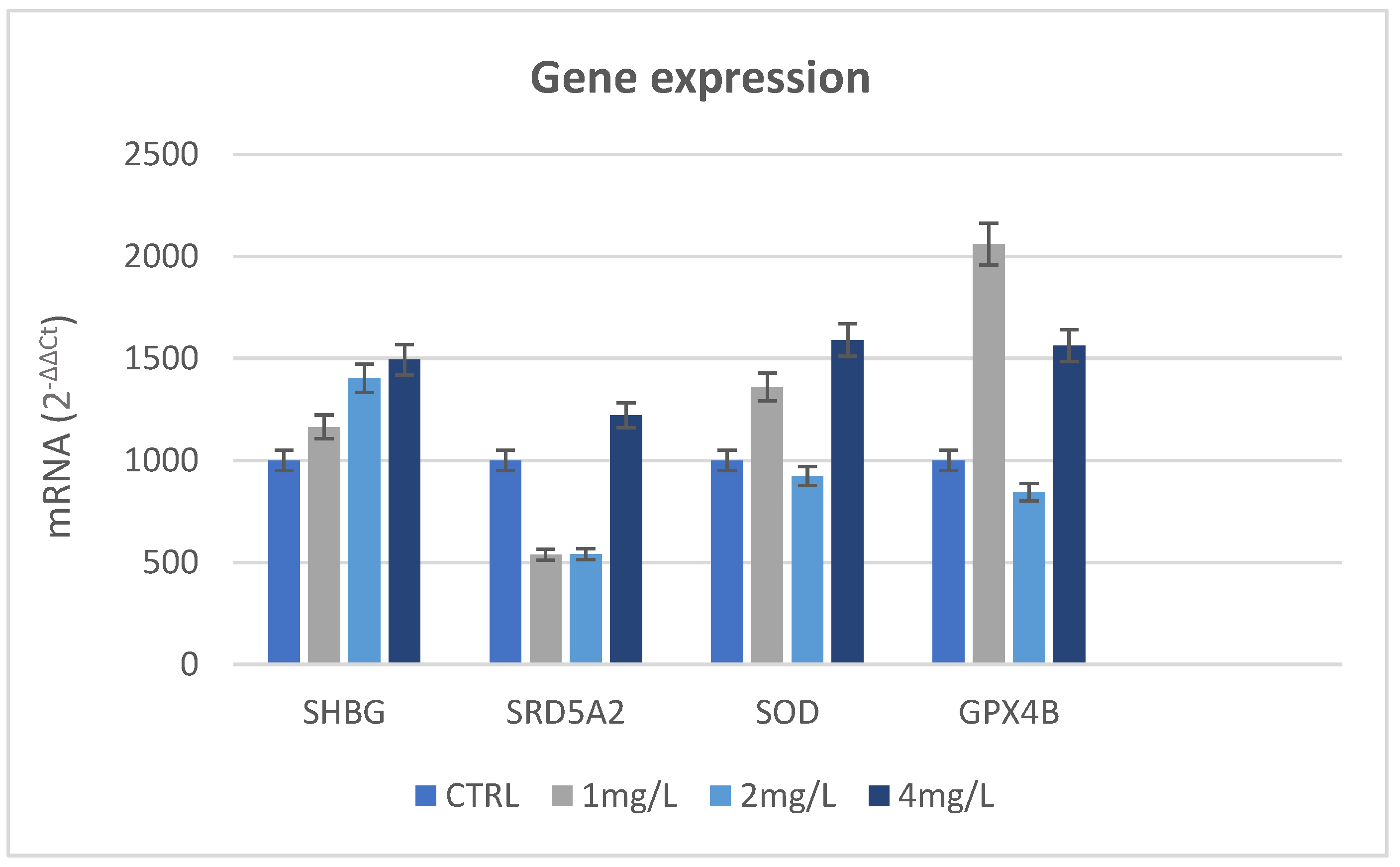
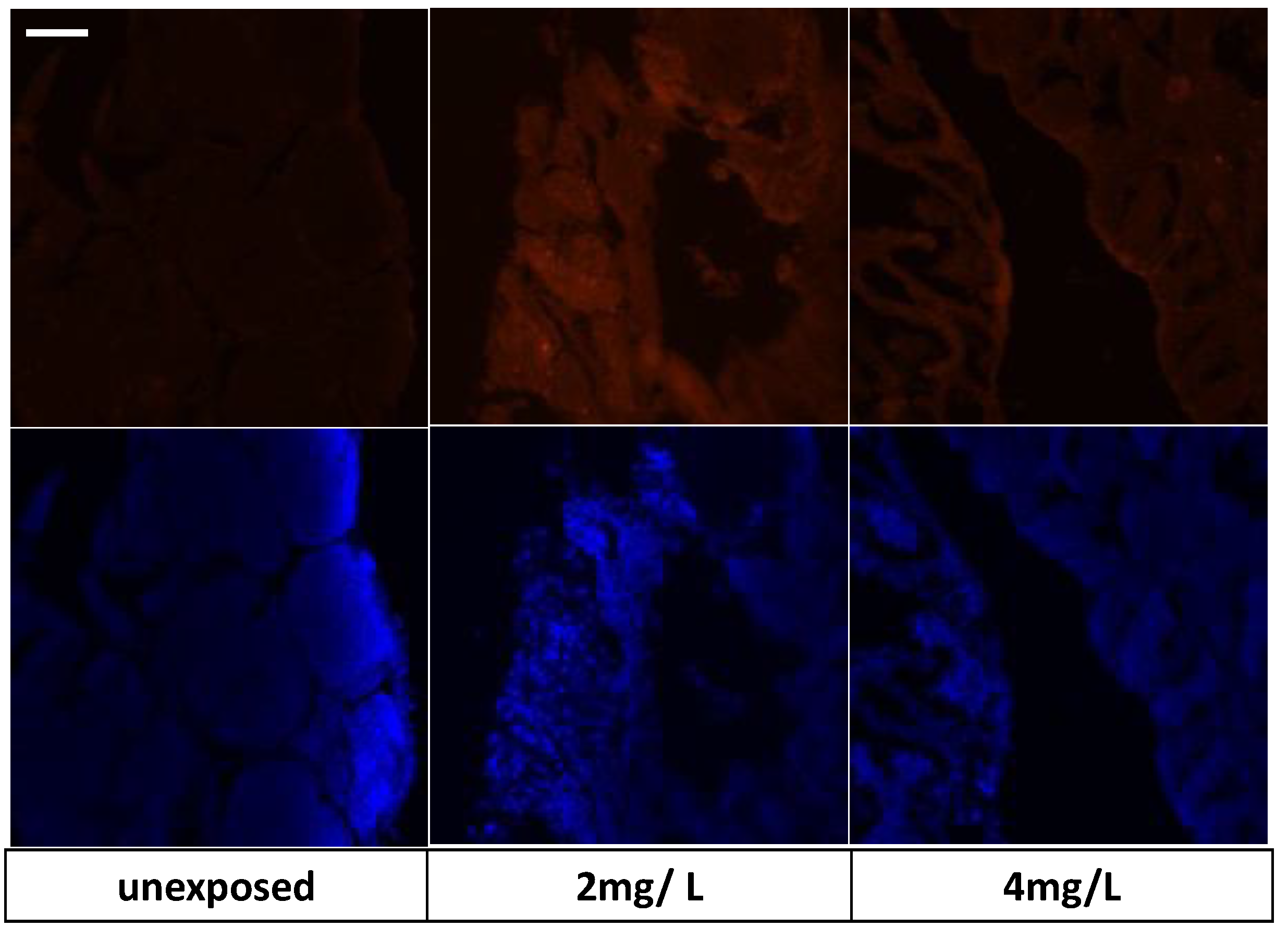
3.7. Immunohistochemical Analysis and Gene Expression
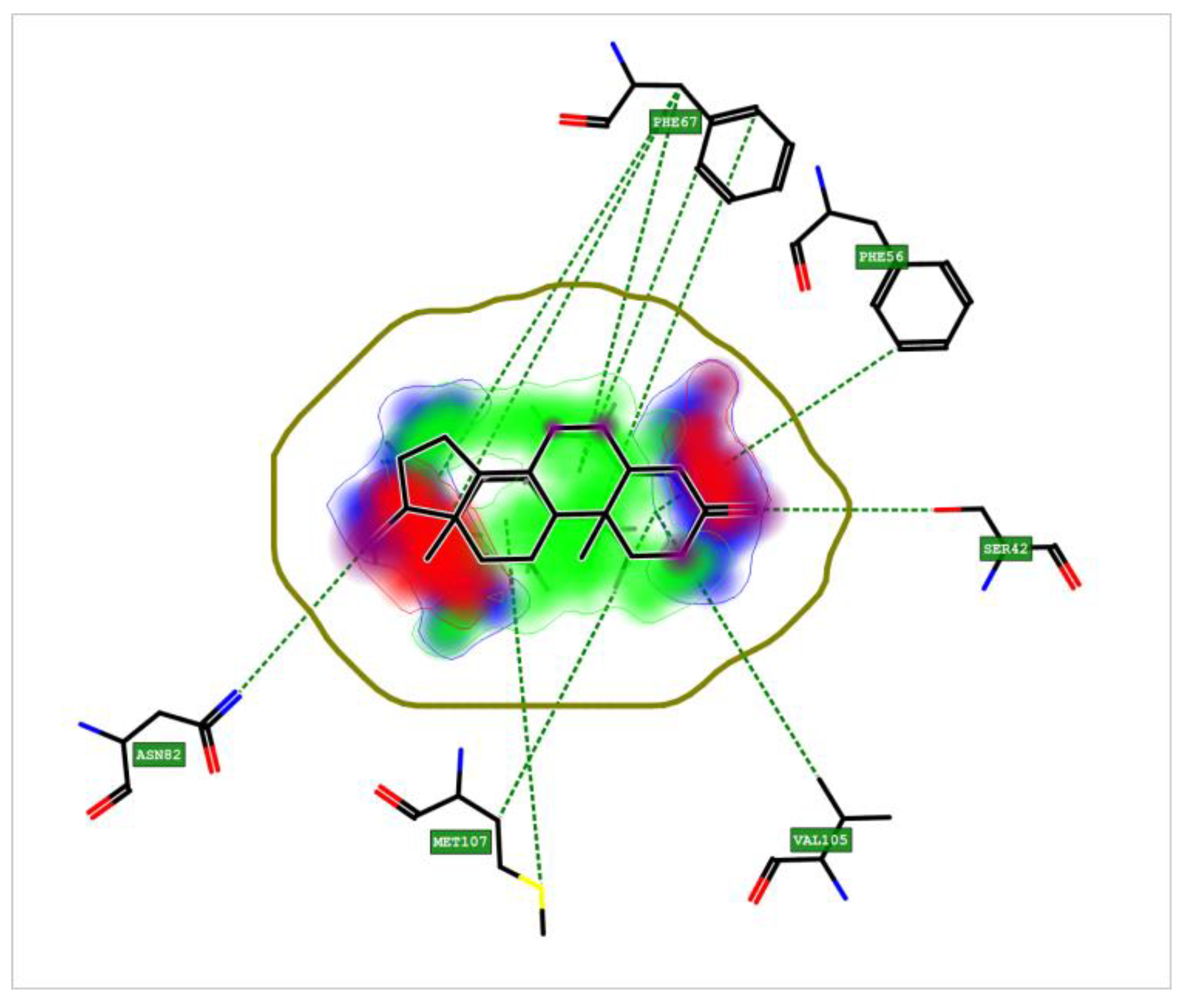
3.8. Crystal Structure of Sex Hormone-Binding Globulin (SHBG)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, N. Nanoparticles: Environmental problems or problem solvers? BioScience 2018, 68, 241–246. [Google Scholar] [CrossRef]
- Chiavola, A.; Amato, E.D.; Stoller, M.; Chianese, A.; Boni, M.R. Application of iron based nanoparticles as adsorbents for arsenic removal from water. Chem. Eng. Trans. 2016, 47, 325–330. [Google Scholar]
- Vilardi, G.; Ochando-Pulido, J.M.; Stoller, M.; Verdone, N.; Di Palma, L. Fenton oxidation and chromium recovery from tannery wastewater by means of iron-based coated biomass as heterogeneous catalyst in fixed-bed columns. Chem. Eng. J. 2018, 351, 1–11. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanoparticle Res. 2012, 14, 1–11. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, R.K.; Gaur, K.; Cátala Torres, J.F.; Loza-Rosas, S.A.; Torres, A.; Saxena, M.; Julin, M.; Tinoco, A.D. Fueling a hot debate on the application of TiO2 nanoparticles in sunscreen. Materials 2019, 12, 2317. [Google Scholar] [CrossRef]
- Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of titanium dioxide nanoparticles with soil components and plants: Current knowledge and future research needs-A critical review. Environ. Sci. Nano 2018, 5, 257–278. [Google Scholar] [CrossRef]
- Scuderi, V.; Impellizzeri, G.; Romano, L.; Scuderi, M.; Nicotra, G.; Bergum, K.; Irrera, A.; Svensson, B.; Privitera, V. TiO2-coated nanostructures for dye photo-degradation in water. Nanoscale Res. Lett. 2014, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zimbone, M.; Cacciato, G.; Spitaleri, L.; Egdell, R.G.; Grimaldi, M.G.; Gulino, A. Sb-doped titanium oxide: A rationale for its photocatalytic activity for environmental remediation. ACS Omega 2018, 3, 11270–11277. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Wang, T.; Li, Y.; Wang, X.; Du, X. Phenyl-functionalization of titanium dioxide-nanosheets coating fabricated on a titanium wire for selective solid-phase microextraction of polycyclic aromatic hydrocarbons from environment water samples. Talanta 2015, 144, 998–1006. [Google Scholar] [CrossRef]
- Ohsaka, T.; Shinozaki, K.; Tsuruta, K.; Hirano, K. Photo-electrochemical degradation of some chlorinated organic compounds on n-TiO2 electrode. Chemosphere 2008, 73, 1279–1283. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, S.J.; Venkateswaran, P.; Jang, J.S.; Kim, H.; Kim, J.G. Anion co-doped Titania for solar photocatalytic degradation of dyes. Carbon Lett. 2008, 9, 131–136. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.W.; Lee, G.M.; Lee, B.T.; Yun, S.T.; Kim, S.O. Monitoring of TiO2-catalytic UV-LED photo-oxidation of cyanide contained in mine wastewater and leachate. Chemosphere 2016, 143, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Hsieh, C.T.; Juang, R.S. Substituent effects on photodegradation of phenols in binary mixtures by hybrid H2O2 and TiO2 suspensions under UV irradiation. J. Taiwan Inst. Chem. Eng. 2016, 62, 68–75. [Google Scholar] [CrossRef]
- Moon, G.H.; Kim, D.H.; Kim, H.I.; Bokare, A.D.; Choi, W. Platinum-like behavior of reduced graphene oxide as a cocatalyst on TiO2 for the efficient photocatalytic oxidation of arsenite. Environ. Sci. Technol. Lett. 2014, 1, 185–190. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Guo, M.; Xu, F.; Wang, P.; Du, Y.; Na, P. One-pot synthesis of Mn-doped TiO2 grown on graphene and the mechanism for removal of Cr (VI) and Cr (III). J. Hazard. Mater. 2016, 310, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; Von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Hicks, A.L. Estimating human exposure to titanium dioxide from personal care products through a social survey approach. Integr. Environ. Assess. Manag. 2020, 16, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Larue, C.; Castillo-Michel, H.; Sobanska, S.; Trcera, N.; Sorieul, S.; Cecillon, L.; Ouerdane, L.; Legros, S.; Sarret, G. Fate of pristine TiO2 nanoparticles and aged paint-containing TiO2 nanoparticles in lettuce crop after foliar exposure. J. Harzard. Mater. 2014, 273, 17–26. [Google Scholar] [CrossRef]
- Klaine, S.J.; Alvarez, P.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef]
- Handy, R.D.; Owen, R.; Valsami-Jones, E. The ecotoxicology of nanoparticles and nanomaterials: Current status, knowledge gaps, challenges, and future needs. Ecotoxicology 2008, 17, 315–325. [Google Scholar] [CrossRef]
- Wu, T.; Tang, M. Review of the effects of manufactured nanoparticles on mammalian target organs. J. Appl. Toxicol. 2018, 38, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Hoet, P.M.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.F.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of inhaled particles into the blood circulation in humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical basis of interactions between engineered nanoparticles and biological systems. Chem. Rev. 2014, 114, 7740–7781. [Google Scholar] [CrossRef]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. Res. 2015, 22, 4122–4143. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Fontana, L.; Leso, V.; Bergamaschi, A. The effects of nanomaterials as endocrine disruptors. Int. J. Mol. Sci. 2013, 14, 16732–16801. [Google Scholar] [CrossRef]
- Matthiessen, P.; Johnson, I. Implications of research on endocrine disruption for the environmental risk assessment, regulation and monitoring of chemicals in the European. Union. Environ. Pollut. 2007, 146, 9–18. [Google Scholar] [CrossRef]
- Jeong, T.Y.; Simpson, M.J. Endocrine disruptor exposure causes infochemical dysregulation and an ecological cascade from zooplankton to algae. Environ. Sci. Technol. 2021, 55, 3845–3854. [Google Scholar] [CrossRef]
- Iavicoli, I.; Fontana, L.; Bergamaschi, A. The effects of metals as endocrine disruptors. J. Toxicol. Environ. Health B 2009, 12, 206–223. [Google Scholar] [CrossRef]
- Fang, Q.; Shi, Q.; Guo, Y.; Hua, J.; Wang, X.; Zhou, B. Enhanced bioconcentration of bisphenol A in the presence of nano-TiO2 can lead to adverse reproductive outcomes in zebrafish. Environ. Sci. Technol. 2016, 50, 1005–1013. [Google Scholar] [CrossRef]
- Kotil, T.; Akbulut, C.; Yön, N.D. The effects of titanium dioxide nanoparticles on ultrastructure of zebrafish testis (Danio rerio). Micron 2017, 100, 38–44. [Google Scholar] [CrossRef]
- Nüsselin-Volhard, C.; Dahm, R. Zebrafish a Practial Approch; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Pecoraro, R.; Marino, F.; Salvaggio, A.; Capparucci, F.; Di Caro, G.; Iaria, C.; Salvo, A.; Rotondo, A.; Tibullo, D.; Guerriero, G.; et al. Evaluation of Chronic Nanosilver Toxicity to Adult Zebrafish. Front. Physiol. 2017, 8, 1011. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test, OECD Guidelines for the Testing of Chemicals; Section 2; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Sobanska, M.; Scholz, S.; Nyman, A.M.; Cesnaitis, R.; Gutierrez, A.S.; Klüver, N.; De Coen, W. Applicability of the fish embryo acute toxicity (FET) test (OECD 236) in the regulatory context of registration, evaluation, authorisation, and restriction of chemicals (REACH). Environ. Toxicol. Chem. 2018, 37, 657–670. [Google Scholar] [CrossRef]
- Pecoraro, R.; Salvaggio, A.; Marino, F.; Di Caro, G.; Capparucci, F.; Lombardo, B.M.; Messina, G.; Scalisi, E.M.; Tummino, M.; Loreto, F.; et al. Metallic nano-composite toxicity evaluation by zebrafish embryo toxicity test with identification of specific exposure biomarkers. Curr. Protoc. Toxicol. 2017, 74, 1–14. [Google Scholar]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dynam. 1995, 203, 253e310. [Google Scholar] [CrossRef]
- Giannaccini, M.; Cushieri, A.; Dente, L.; Raffa, V. Non-mammalian vertebrate embryos as models in nanomedicine. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Zimbone, M.; Calcagno, L.; Messina, G.; Baeri, P.; Compagnini, G. Dynamic light scattering and UV–vis spectroscopy of gold nanoparticles solution. Mater. Lett. 2011, 65, 2906–2909. [Google Scholar] [CrossRef]
- Zimbone, M.; Musumeci, P.; Baeri, P.; Messina, E.; Boninelli, S.; Compagnini, G.; Calcagno, L. Rotational dynamics of gold nanoparticle chains in water solution. J. Nanoparticle Res. 2012, 14, 1308. [Google Scholar] [CrossRef]
- Kristofco, L.A.; Haddad, S.P.; Chambliss, C.K.; Brooks, B.W. Differential uptake of and sensitivity to diphenhydramine in embryonic and larval zebrafish. Environ. Toxicol. Chem. 2018, 37, 1175–1181. [Google Scholar] [CrossRef]
- Rawson, D.M.; Zhang, T.; Kalicharan, D.; Jogebloed, W.L. Field emission scanning electron microscopy and transmission electron microscopy studies of the chorion, plasma membrane and syncytial layers of the gastrula-stage embryo of the zebrafish Brachy Danio rerio: A consideration of the structural and functional relationships with respect to cryoprotectant penetration. Aquacult. Res. 2001, 31, 325–336. [Google Scholar]
- Fent, K.; Weisbrod, C.J.; Wirth-Heller, A.; Pieles, U. Assessment of uptake and toxicity of fluorescent silica nanoparticles in zebrafish (Danio rerio) early lifestages. Aquat. Toxicol. 2010, 100, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Flahaut, E.; Cheng, S.H. Effect of carbon nanotubes on developing zebrafish (Danio rerio) embryos. Environ. Toxicol. Chem. 2007, 26, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Gomes, T.; Machado, M.R.F.; Rocha, T.L. The zebrafish embryotoxicity test (ZET) for nanotoxicity assessment: From morphological to molecular approach. Environ. Pollut. 2019, 252, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Federici, G.; Shaw, B.J.; Handy, R.D. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquat. Toxicol. 2007, 84, 415–430. [Google Scholar] [CrossRef]
- Chen, T.H.; Lin, C.Y.; Tseng, M.C. Behavioral effects of titanium dioxide nanoparticles on larval zebrafish (Danio rerio). Mar. Pollut. Bull. 2011, 63, 303–308. [Google Scholar] [CrossRef]
- Wang, Y.J.; He, Z.Z.; Fang, Y.W.; Xu, Y.; Chen, Y.N.; Wang, G.Q.; Yang, Y.Q.; Yang, Z.; Li, Y.H. Effect of titanium dioxide nanoparticles on zebrafish embryos and developing retina. Int. J. Ophthalmol. 2014, 7, 917. [Google Scholar]
- Faria, M.; Navas, J.M.; Soares, A.M.; Barata, C. Oxidative stress effects of titanium dioxide nanoparticle aggregates in zebrafish embryos. Sci. Total Environ. 2014, 470, 379–389. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, Z.; Tian, W.; He, X.; Ma, Y.; Zhao, Y.; Chai, Z. Toxicity of zinc oxide nanoparticles to zebrafish embryo: A physicochemical study of toxicity mechanism. J. Nanopart. Res. 2010, 12, 1645–1654. [Google Scholar] [CrossRef]
- Johnson, A.; Carew, E.; Sloman, K.A. The effects of copper on the morphological and functional development of zebrafish embryos. Aquat. Toxicol. 2007, 84, 431–438. [Google Scholar] [CrossRef]
- Asharani, P.V.; Lianwu, Y.I.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2011, 5, 43–54. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 1–13. [Google Scholar] [CrossRef]
- Cerbinskaite, A.; Mukhopadhyay, A.; Plummer, E.R.; Curtin, N.J.; Edmondson, R.J. Defective homologous recombination in human cancers. Cancer Treat. Rev. 2012, 38, 89–100. [Google Scholar] [CrossRef] [PubMed]
- De Murcia, J.M.; Niedergang, C.; Trucco, C.; Ricoul, M.; Dutrillaux, B.; Mark, M.; Oliver, F.J.; Masson, M.; Dierich, A.; LeMeur, M.; et al. Requirement of poly (ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc. Natl. Acad. Sci. USA 1997, 94, 7303–7307. [Google Scholar] [CrossRef] [PubMed]
- Heacock, M.L.; Stefanick, D.F.; Horton, J.K.; Wilson, S.H. Alkylation DNA damage in combination with PARP inhibition results in formation of S-phase-dependent double-strand breaks. DNA Repair 2010, 9, 929–936. [Google Scholar] [CrossRef]
- Kondo, N.; Takahashi, A.; Ono, K.; Ohnishi, T. DNA damage induced by alkylating agents and repair pathways. J. Nucleic Acids. 2010, 21, 1–7. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Ann. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; Qi, W.; Han, F.; Shao, J.Z.; Gao, J.Q. Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int. J. Nanomed. 2011, 6, 3351. [Google Scholar]
- Lin, S.; Zhao, Y.; Xia, T.; Meng, H.; Ji, Z.; Liu, R.; George, S.; Xiong, S.; Wang, X.; Zhang, H.; et al. High content screening in zebrafish speeds up hazard ranking of transition metal oxide nanoparticles. ACS Nano 2011, 5, 7284–7295. [Google Scholar] [CrossRef] [PubMed]
- Dziegiel, P. Expression of metallothioneins in tumor cells. Pol. J. Pathol. 2004, 55, 3–12. [Google Scholar] [PubMed]
- Brundo, M.V.; Pecoraro, R.; Marino, F.; Salvaggio, A.; Tibullo, D.; Saccone, S.; Bramanti, V.; Buccheri, M.A.; Impellizzeri, G.; Scuderi, V.; et al. Toxicity evaluation of new engineered nanomaterials in zebrafish. Front. Physiol. 2016, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Muller, Y.A.; Hammond, G.L. Molecular and structural basis of steroid hormone binding and release from corticosteroid-binding globulin. Mol. Cell. Endocrinol. 2010, 316, 3–12. [Google Scholar] [CrossRef]
- Hammond, G.L. Diverse roles for sex hormone-binding globulin in reproduction. Biol. Reprod. 2011, 85, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Queralt, S.; Hammond, G.L. Sex hormone-binding globulin in fish gills is a portal for sex steroids breached by xenobiotics. Endocrinology 2008, 149, 4269–4275. [Google Scholar] [CrossRef] [PubMed]
- Bobe, J.; Guiguen, Y.; Fostier, A. Diversity and biological significance of sexhormone-binding globulin in fish, an evolutionary perspective. Mol. Cell. Endocrinol. 2010, 316, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Queralt, S.; Knowlton, M.; Avvakumov, G.V.; Al-Nouno, R.; Kelly, G.M.; Hammond, G.L. Molecular and functional characterization of sex hormone binding globulin in zebrafish. Endocrinology 2004, 145, 5221–5230. [Google Scholar] [CrossRef]
- Dechaud, H.; Ravard, C.; Claustrat, F.; de la Perrière, A.B.; Pugeat, M. Xenoestrogen interaction with human sex hormone-binding globulin (hSHBG). Steroids 1999, 64, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Hodgert Jury, H.; Zacharewski, T.R.; Hammond, G.L. Interactions between human plasma sex hormone-binding globulin and xenobiotic ligands. J. Steroid Biochem. Mol. Biol. 2000, 75, 167–176. [Google Scholar] [CrossRef]
- Hong, H.; Branham, W.S.; Ng, H.W.; Moland, C.L.; Dial, S.L.; Fang, H.; Perkins, R.; Sheehan, D.; Tong, W. Human sex hormone-binding globulin binding affinities of 125 structurally diverse chemicals and comparison with their binding to androgen receptor, estrogen receptor, and α-fetoprotein. Toxicol. Sci. 2015, 143, 333–348. [Google Scholar] [CrossRef]
- Desvergne, B.; Feige, J.N.; Casals-Casas, C. PPAR-mediated activity of phthalates: A link to the obesity epidemic? Mol. Cell. Endocrinol. 2009, 304, 43–48. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Q.; Chen, M.; Yang, J.; Wang, R.; Zhong, W.; Zhu, L.; Yang, L. Antagonistic estrogenic effects displayed by bisphenol AF and perfluorooctanoic acid on zebrafish (Danio rerio) at an early developmental stage. Environ. Sci. Technol. Lett. 2018, 5, 655–661. [Google Scholar] [CrossRef]
- Haritos, A.A.; Goodall, G.J.; Horecker, B.L. Prothymosin alpha: Isolation and properties of the major immunoreactive form of thymosin alpha 1 in rat thymus. Proc. Natl. Acad. Sci. USA 1984, 81, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Dosil, M.; Freire, M.; Gomez-Marquez, J. Tissue-specific and differential expression of prothymosin alpha gene during rat development. FEBS Lett. 1990, 269, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ono, N.; Tsukue, N.; Oshio, S.; Umeda, T.; Takano, H.; Takeda, K. In utero exposure to diesel exhaust increased accessory reproductive gland weight and serum testosterone concentration in male mice. Environ. Sci. 2006, 13, 139–147. [Google Scholar]
- Asare, N.; Instanesa, C.; Sandberga, W.J.; Refsnesa, M.; Schwarzea, P.; Kruszewskib, M.; Brunborg, G. Cytotoxic and genotoxic effects of silver nanoparticles in testicular cells. Toxicology 2012, 291, 65–72. [Google Scholar] [CrossRef]
- Gao, G.; Ze, Y.; Zhao, X.; Sang, X.; Zheng, L.; Ze, X.; Gui, S.; Sheng, L.; Sun, Q.; Hong, J.; et al. Titanium dioxide nanoparticle-induced testicular damage, spermatogenesis suppression, and gene expression alterations in male mice. J. Hazard. Mater. 2013, 258, 133–143. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef]
- Sadauskas, E.; Jacobsen, N.R.; Danscher, G.; Stoltenberg, M.; Vogel, U.; Larsen, A.; Kreyling, W.; Wallin, H. Biodistribution of gold nanoparticles in mouse lung following intratracheal instillation. Chem. Cent. J. 2009, 3, 16. [Google Scholar] [CrossRef]
- Lankveld, D.P.; Oomen, A.G.; Krystek, P.; Neigh, A.; Troostde Jong, A.; Noorlander, C.W.; Van Eijkeren, J.C.; Geertsma, R.E.; De Jong, W.H. The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials 2010, 31, 8350–8361. [Google Scholar] [CrossRef]
- Lankveld, D.P.; Rayavarapu, R.G.; Krystek, P.; Oomen, A.G.; Verharen, H.W.; van Leeuwen, T.G.; De Jong, W.H.; Manohar, S. Blood clearance and tissue distribution of PEGylated and non-PEGylated gold nanorods after intravenous administration in rats. Nanomedicine 2011, 6, 339–349. [Google Scholar] [CrossRef]
- Lead, J.R.; Batley, G.E.; Alvarez, P.J.J.; Croteau, M.N.; Handy, R.D.; Mclaughlin, M.J.; Judy, J.D.; Schrimer, K. Nanomaterials in the environment: Behavior, bioavailability, and effects—An updated review. Environ. Toxicol. Chem. 2018, 37, 2029–2063. [Google Scholar] [CrossRef] [PubMed]
- Griffitt, R.J.; Lavelle, C.M.; Kane, A.S.; Denslow, N.D.; Barber, D.S. Chronic nanoparticulate silver exposure results in tissue accumulation and transcriptomic changes in zebrafish. Aquat. Toxicol. 2013, 130, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of nano-scale TiO2, zno and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci. Total Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Hyndman, K.; Denslow, N.D.; Barber, D.S. Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles. Toxicol. Sci. 2009, 107, 404–415. [Google Scholar] [CrossRef]
- Song, G.; Lin, L.; Liu, L.; Wang, K.; Ding, Y.; Niu, Q.; Mu, L.; Wang, H.; Shen, H.; Guo, S. Toxic effects of anatase titanium dioxide nanoparticles on spermatogenesis and testicles in male mice. Pol. J. Environ. Stud. 2017, 26, 2739–2745. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.B.; Yee, J.S.; Meyer, D.N.; Yang, D.; Baker, T.R. Histological and transcriptomic changes in male zebrafish testes due to early life exposure to low level 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Zebrafish 2016, 13, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Nittoli, V.; Colella, M.; Porciello, A.; Reale, C.; Roberto, L.; Russo, F.; Russo, N.A.; Porreca, I.; De Felice, M.; Mallardo, M.; et al. Multi Species Analyses Reveal Testicular T3 Metabolism and Signalling as a Target of Environmental Pesticides. Cells 2021, 10, 2187. [Google Scholar] [CrossRef]
- Lora, A.J.; Molina, A.M.; Bellido, C.; Blanco, A.; Monterde, J.G.; Moyano, M.R. Adverse effects of bisphenol A on the testicular parenchyma of zebrafish revealed using histomorphological methods. Vet. Med. 2016, 61, 577–589. [Google Scholar] [CrossRef]
- Siiteri, P.K.; Murai, J.T.; Raymoure, W.J.; Kuhn, R.W.; Hammond, G.L.; Nisker, J.A. The Serum Transport of Steroid Hormones. In Proceedings of the 1981 Laurentian Hormone Conference; Academic Press: Cambridge, MA, USA, 1982; pp. 457–510. [Google Scholar]
- Joseph, D.R. Structure, function, and regulation of androgen-binding protein/sex hormone-binding globulin. Vitam. Horm. 1994, 49, 197–280. [Google Scholar]
- Mak, P.; Callard, G.V. A novel steroid-binding protein in the testis of the dogfish Squalus acanthias. Gen. Comp. Endocrinol. 1987, 68, 104–112. [Google Scholar] [CrossRef]
- Ovrevik, J.; Stenersen, J.; Nilssen, K.; Tollefsen, K.E. Partial characterization of a sex steroid-binding protein in plasma from arctic charr (Salvelinus alpinus L.). Gen. Comp. Endocrinol. 2001, 122, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Milligan, S.R.; Khan, O.; Nash, M. Competitive binding of xenobiotic oestrogens to rat fetoprotein and to sex steroid binding proteins in human and rainbow trout (Oncorhynchus mykiss) plasma. Gen. Comp. Endocrinol. 1998, 112, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Tollefsen, K.E. Interaction of estrogen mimics, singly and in combination, with plasma sex steroid-binding proteins in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2002, 56, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Pryce-Hobby, A.C.; McMaster, M.E.; Hewitt, L.M.; Van Der Kraak, G. The effects of pulp mill effluent on the sex steroid binding protein in white sucker (Catostomus commersoni) and longnose sucker (C. catostomus). Comp. Biochem. Physiol. 2003, 134, 241–250. [Google Scholar] [CrossRef]
- Foucher, J.L.; Le Bail, P.Y.; Le Gac, F. Influence of hypophysectomy, castration, fasting, and spermiation on SBP concentration in male rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 1992, 85, 101–110. [Google Scholar] [CrossRef]
- Hobby, A.C.; Geraghty, D.P.; Pankhurst, N.W. Differences in binding characteristics of sex steroid binding protein in reproductive and non reproductive female rainbow trout (Oncorhynchus mykiss), black bream (Acanthopagrus butcheri), and greenback flounder (Rhombosolea tapirina). Gen. Comp. Endocrinol. 2000, 120, 249–259. [Google Scholar] [CrossRef]
- Bobe, J.; Mahé, S.; Nguyen, T.; Rime, H.; Vizziano, D.; Fostier, A.; Guiguen, Y. A novel, functional, and highly divergent sex hormone-binding globulin that may participate in the local control of ovarian functions in salmonids. Endocrinology 2008, 149, 2980–2989. [Google Scholar] [CrossRef]
- Miguel-Queralt, S.; Underhill, C.; Devlin, R.H.; Hammond, G.L. Characterization and Measurement of the Plasma α-and β-Sex Hormone-Binding Globulin Paralogs in Salmon. Endocrinology 2009, 150, 366–375. [Google Scholar] [CrossRef]
- Hryb, D.J.; Nakhla, A.M.; Kahn, S.M.; St George, J.; Levy, N.C.; Romas, N.A.; Rosner, W. Sex hormone-binding globulin in the human prostate is locally synthesized and may act as an autocrine/paracrine effector. J. Biol. Chem. 2002, 277, 26618–26622. [Google Scholar] [CrossRef]
- Tokarz, J.; Möller, G.; de Angelis, M.H.; Adamski, J. Zebrafish and steroids: What do we know and what do we need to know? J. Steroid Biochem. Mol. Biol. 2013, 137, 165–173. [Google Scholar] [CrossRef]
- Hering, D.M.; Olenski, K.; Kaminski, S. Genome-wide association study for poor sperm motility in Holstein-Friesian bulls. Anim. Reprod. Sci. 2014, 146, 89–97. [Google Scholar] [CrossRef]
- Urbatzka, R.; Watermann, B.; Lutz, I.; Kloas, W. Exposure of Xenopus laevis tadpoles to finasteride, an inhibitor of 5-alpha reductase activity, impairs spermatogenesis and alters hypophyseal feedback mechanisms. J. Mol. Endocrinol. 2009, 43, 209–219. [Google Scholar] [CrossRef]
- Kang, H.J.; Imperato-McGinley, J.; Zhu, Y.S.; Rosenwaks, Z. The effect of 5α-reductase-2 deficiency on human fertility. Fertil. Steril. 2014, 101, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Margiotta-Casaluci, L.; Hannah, R.E.; Sumpter, J.P. Mode of action of human pharmaceuticals in fish: The effects of the 5-alpha-reductase inhibitor, dutasteride, on reproduction as a case study. Aquat. Toxicol. 2013, 128, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Garcìa-Garcìa, M.; Sànchez-Hernàndez, M.; Garcìa-Hernàndez, M.P.; Garcìa-Ayala, A.; Chaves-Pozo, E. Role of 5α-dihydrotestosterone in testicular development of gilthead seabream following finasteride administration. J. Steroid Biochem. Mol. Biol. 2017, 174, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Garcia, T.X.; Costa, G.M.; França, L.R.; Hofmann, M.C. Sub-acute intravenous administration of silver nanoparticles in male mice alters Leydig cell function and testosterone levels. Reprod. Toxicol. 2014, 45, 59–70. [Google Scholar] [CrossRef]
- Adebayo, O.A.; Akinloye, O.; Adaramoye, O.A. Cerium oxide nanoparticle elicits oxidative stress, endocrine imbalance and lowers sperm characteristics in testes of balb/c mice. Andrologia 2018, 50, e12920. [Google Scholar] [CrossRef]
- Goldstone, J.V.; McArthur, A.G.; Kubota, A.; Zanette, J.; Parente, T.; Jönsson, M.E.; Nelson, D.R.; Stegeman, J.J. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genom. 2010, 11, 1–21. [Google Scholar] [CrossRef]
- Vallyathan, V.; Shi, X. The role of oxygen free radicals in occupational and environmental lung diseases. Environ. Health Perspect. 1997, 105, 165–177. [Google Scholar]
- Bar-Ilan, O.; Louis, K.M.; Yang, S.P.; Pedersen, J.A.; Hamers, R.J.; Peterson, R.E.; Heideman, W. Titanium dioxide nanoparticles produce phototoxicity in the developing zebrafish. Nanotoxicology 2012, 6, 670–679. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Sun, X.; Yu, H. Phototoxicity and environmental transformation of polycyclic aromatic hydrocarbons (PAHs)-light-induced reactive oxygen species, lipid peroxidation, and DNA damage. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2012, 30, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Filho, D.W. Fish antioxidant defenses—A comparative approach. Braz. J. Med. Biol. Res. 1996, 29, 1735–1742. [Google Scholar]
- Pandey, S.; Parvez, S.; Sayeed1, I.; Haque, R.; Bin-Hafeez, B.; Raisuddin, S. Biomarkers of oxidative stress: A comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci. Total Environ. 2003, 309, 105–115. [Google Scholar]
- Zhu, X.; Zhou, J.; Cai, Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar. Pollut. Bull. 2011, 63, 334–338. [Google Scholar] [CrossRef]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, H.; Wang, X.; Wu, J.; Xue, Y. Effects of chronic exposure of 2, 4-dichlorophenol on the antioxidant system in liver of freshwater fish Carassius auratus. Chemosphere 2004, 55, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhou, D.; Dong, J.; Jiang, F.; Chen, W. Acute toxicity of dichloroacetonitrile (DCAN), a typical nitrogenous disinfection by-product (N-DBP), on zebrafish (Danio rerio). Ecotox. Environ. Safe 2016, 133, 97–104. [Google Scholar] [CrossRef]
- Asakura, H.; Kitahora, T. Antioxidants and Polyphenols in Inflammatory Bowel Disease: Ulcerative Colitis and Crohn Disease. In Polyphenols: Prevention and Treatment of Human Disease; Ronald, R.W., Victor, R.P., Sherma, Z., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 279–292. [Google Scholar]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef]
- Nash, K.M.; Ahmed, S. Nanomedicine in the ROS-mediated pathophysiology: Applications and clinical advances. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 2033–2040. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalisi, E.M.; Pecoraro, R.; Salvaggio, A.; Capparucci, F.; Fortuna, C.G.; Zimbone, M.; Impellizzeri, G.; Brundo, M.V. Titanium Dioxide Nanoparticles: Effects on Development and Male Reproductive System. Nanomaterials 2023, 13, 1783. https://doi.org/10.3390/nano13111783
Scalisi EM, Pecoraro R, Salvaggio A, Capparucci F, Fortuna CG, Zimbone M, Impellizzeri G, Brundo MV. Titanium Dioxide Nanoparticles: Effects on Development and Male Reproductive System. Nanomaterials. 2023; 13(11):1783. https://doi.org/10.3390/nano13111783
Chicago/Turabian StyleScalisi, Elena Maria, Roberta Pecoraro, Antonio Salvaggio, Fabiano Capparucci, Cosimo Gianluca Fortuna, Massimo Zimbone, Giuliana Impellizzeri, and Maria Violetta Brundo. 2023. "Titanium Dioxide Nanoparticles: Effects on Development and Male Reproductive System" Nanomaterials 13, no. 11: 1783. https://doi.org/10.3390/nano13111783
APA StyleScalisi, E. M., Pecoraro, R., Salvaggio, A., Capparucci, F., Fortuna, C. G., Zimbone, M., Impellizzeri, G., & Brundo, M. V. (2023). Titanium Dioxide Nanoparticles: Effects on Development and Male Reproductive System. Nanomaterials, 13(11), 1783. https://doi.org/10.3390/nano13111783











