Green Synthesis: The Antibacterial and Photocatalytic Potential of Silver Nanoparticles Using Extract of Teucrium stocksianum
Abstract
1. Introduction
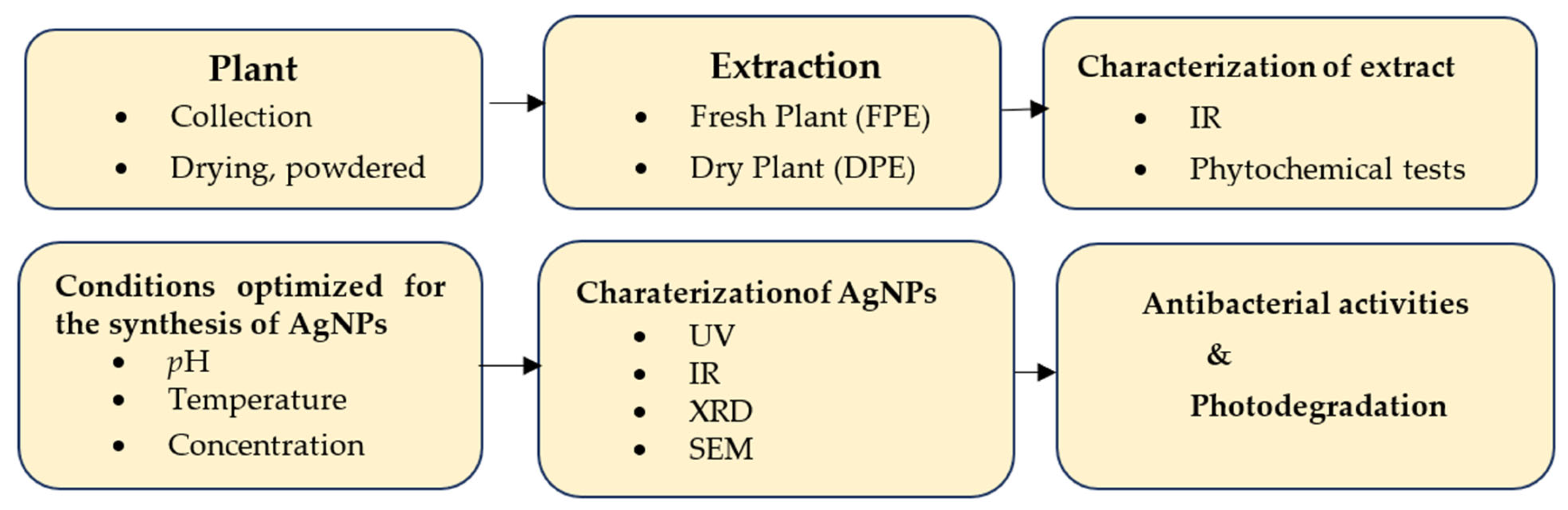
2. Materials and Methods
2.1. Preparation of the Plant Extract
2.2. Preliminary Phytochemical Screening of Teucrium stocksianum
2.3. General Procedure for the Preparation of Silver Nanoparticles Using Teucrium stocksianum
2.3.1. Effect of the Extract Concentration
2.3.2. Effect of pH
2.3.3. Effect of Temperature
2.4. Antibacterial Activity of the Synthesized SNPs
2.5. Photocatalytic Degradation Activity of the Biosynthesized SNPs
3. Results and Discussion
3.1. Preliminary Phytochemical Screening of the Teucrium stocksianum
3.2. Synthesis of the Silver Nanoparticles Using Teucrium stocksianum
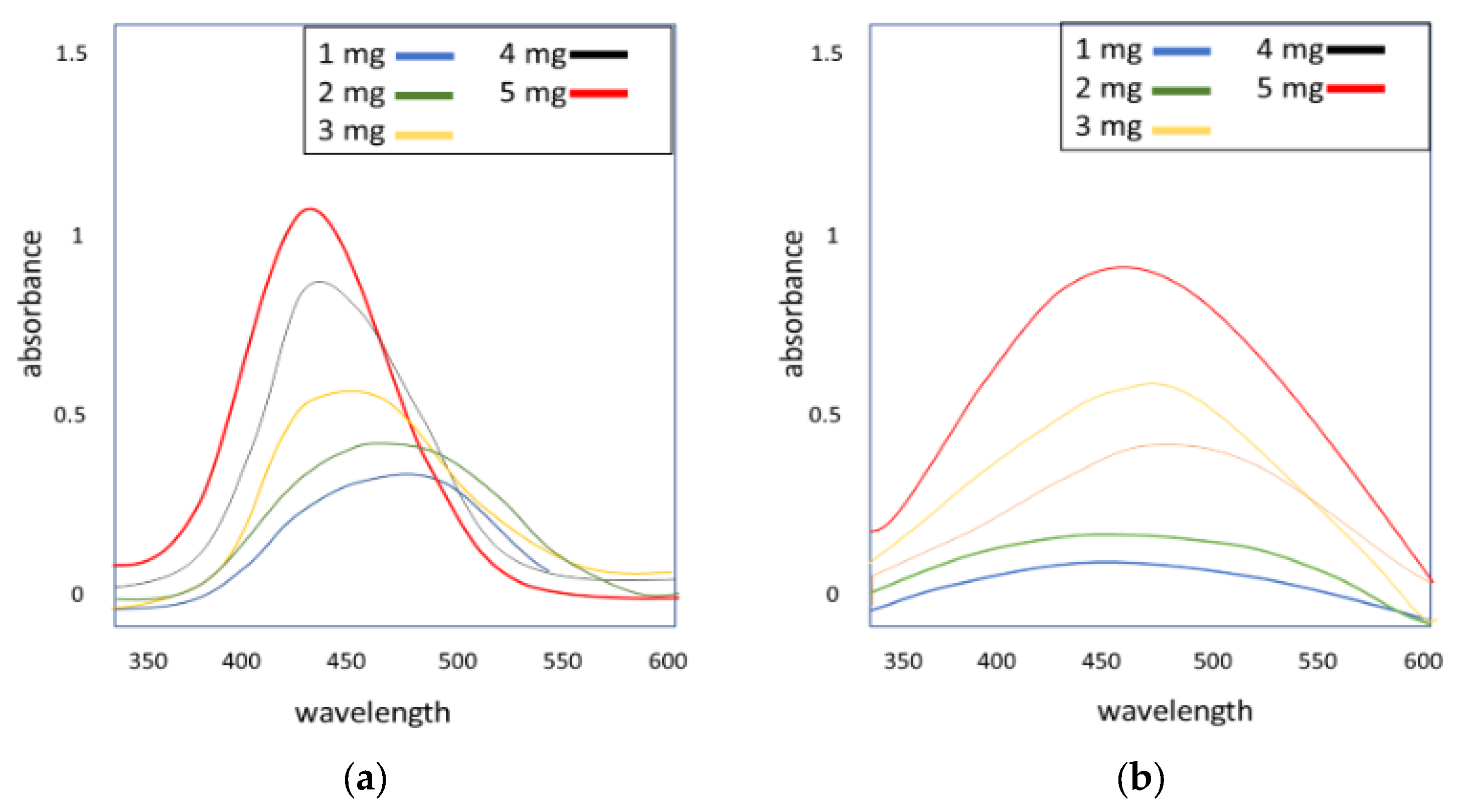
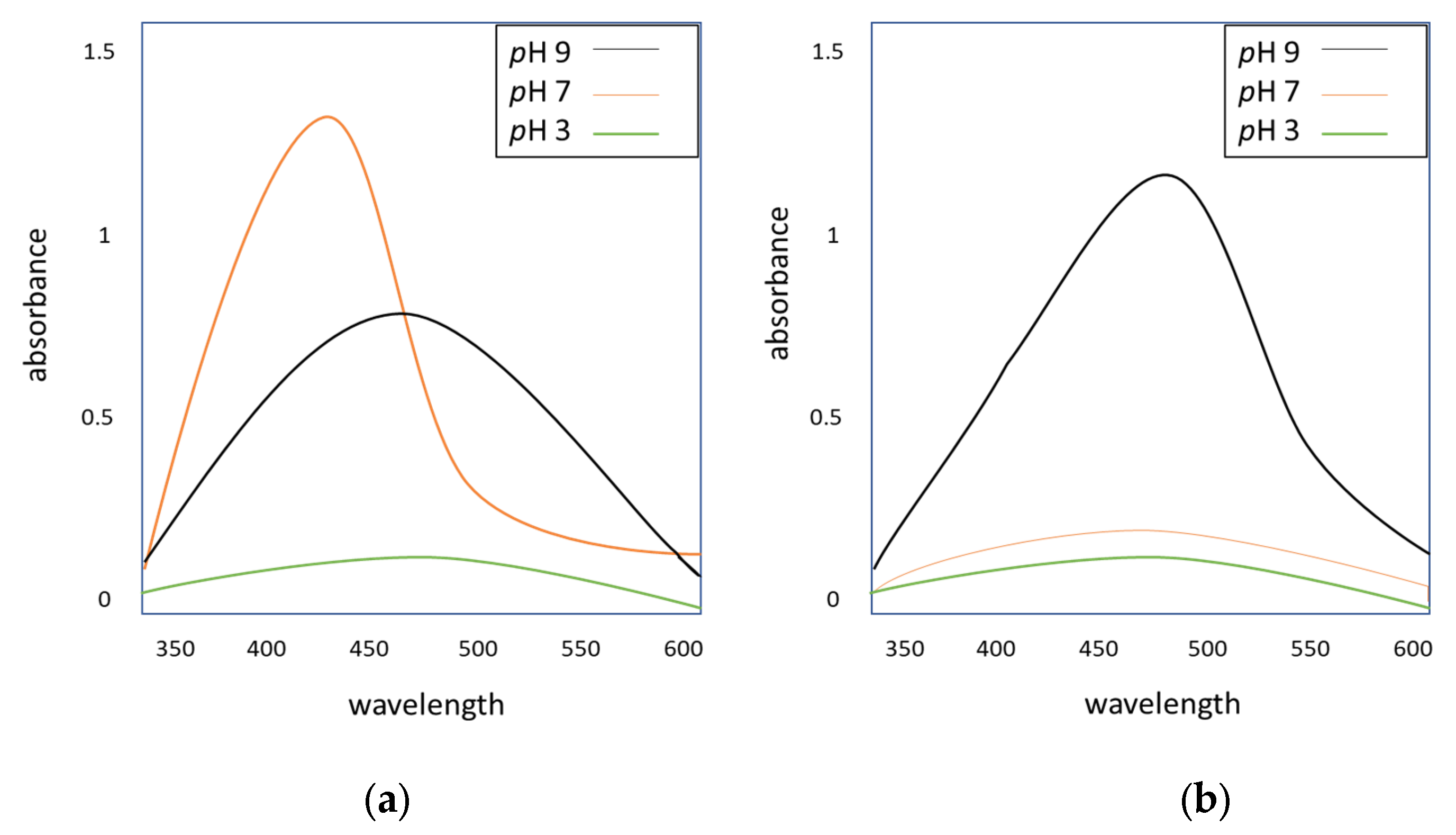
3.3. UV–Vis Analysis of Silver Nanoparticles
3.3.1. Effect of Concentration
3.3.2. Effect of pH
3.3.3. Effect of Temperature
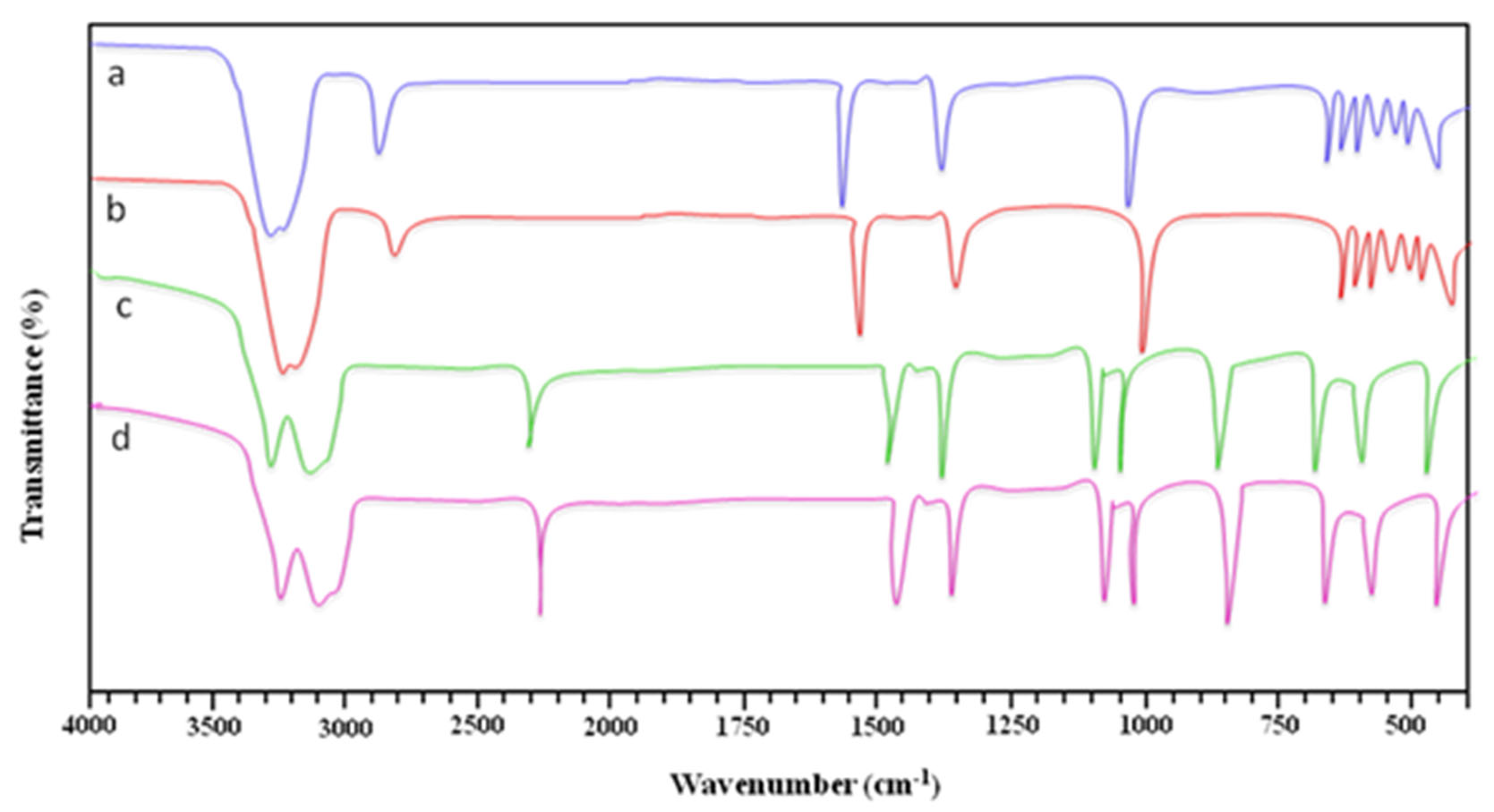
3.4. Characterization of the Synthesized Silver Nanoparticles
3.4.1. FT-IR Analysis of the Plant Extracts and Silver NPs
3.4.2. SEM Analysis of the Synthesized Silver NPs
3.4.3. Powder X-ray Diffraction (PXRD) Analysis of the SNPs
3.4.4. DLS Analysis of the Synthesized SNPs
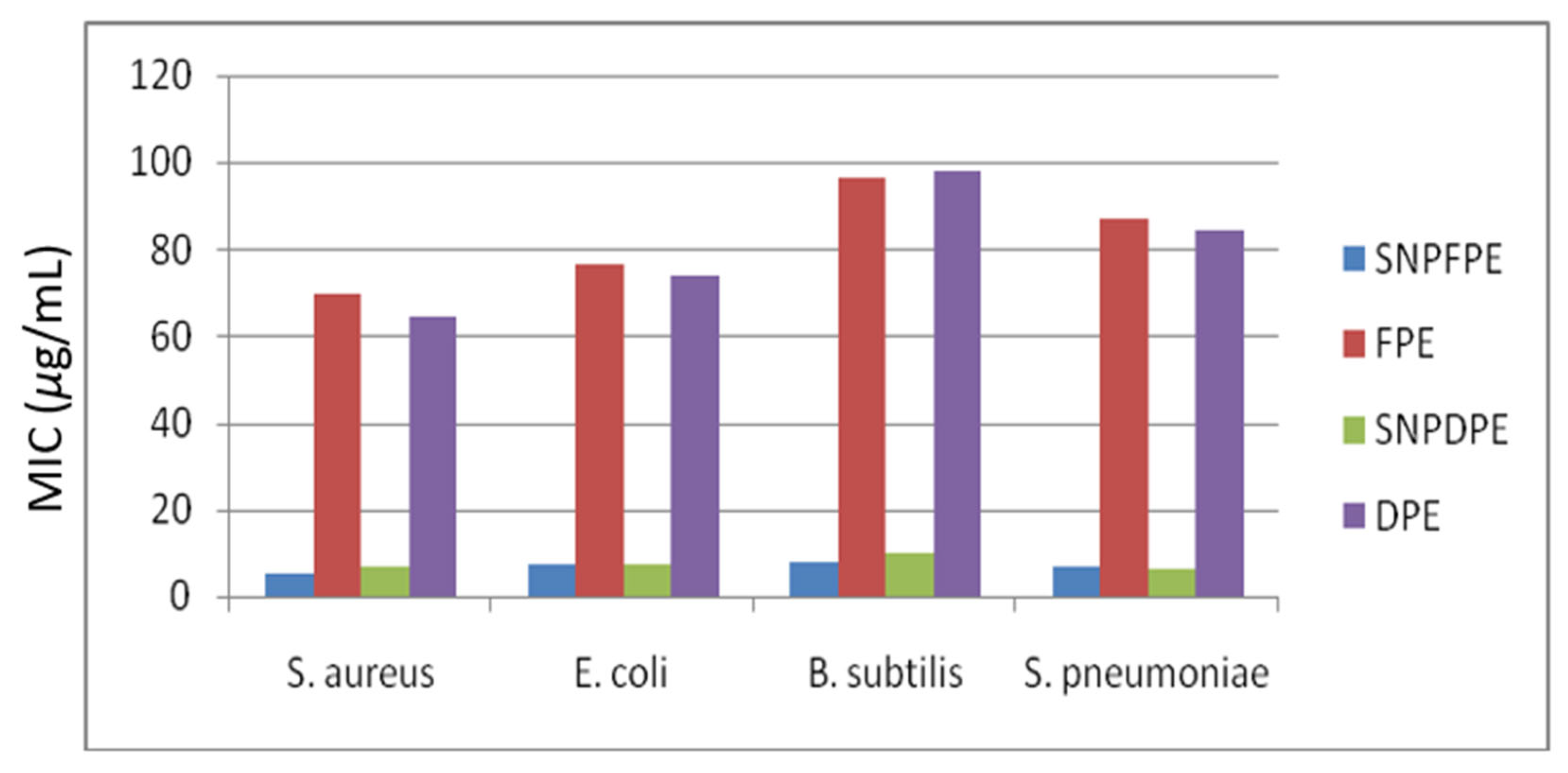
3.5. Antimicrobial Activity of the Synthesized Silver NPs
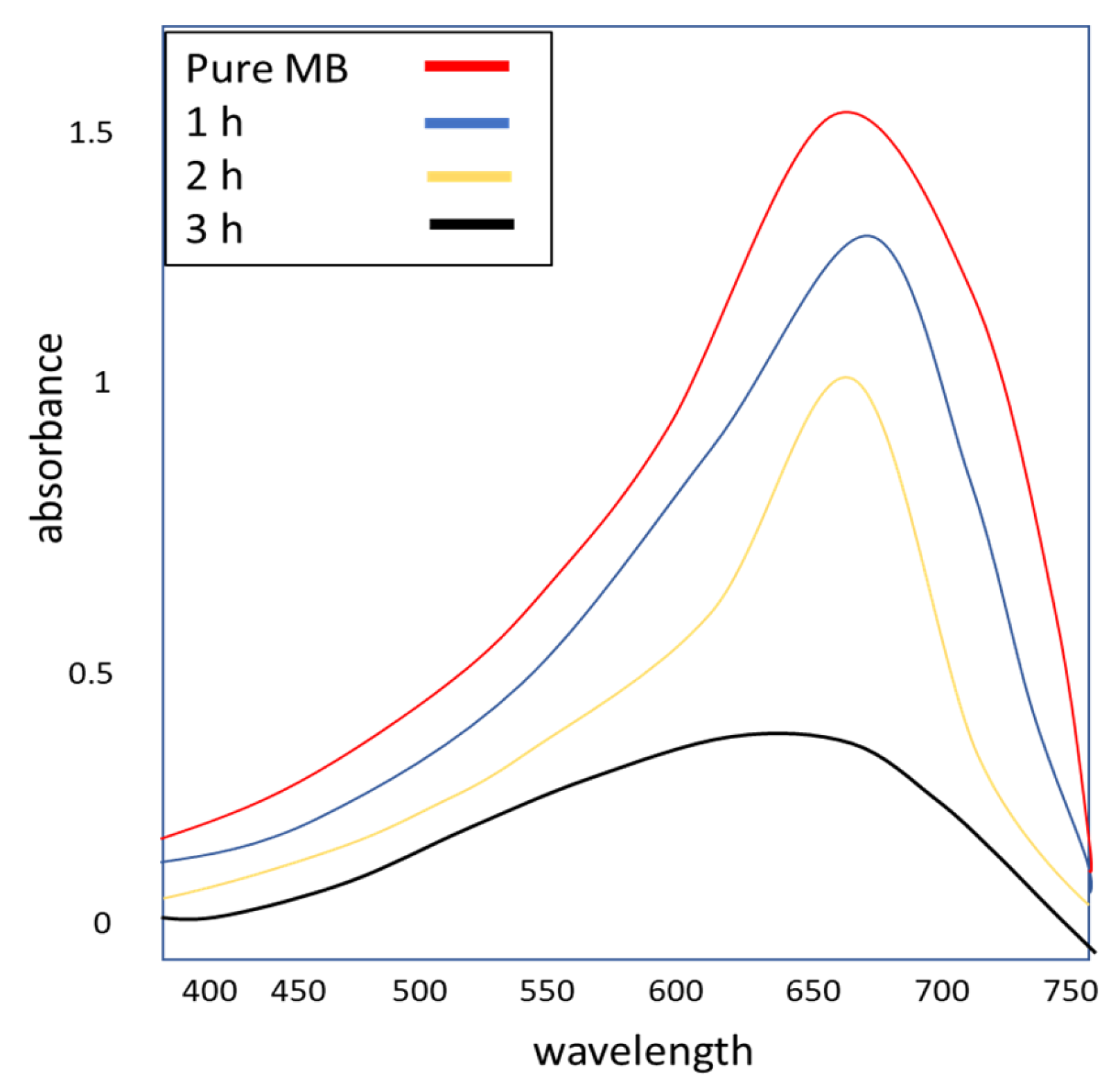
3.6. Photocatalytic Activity of the Synthesized Silver NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hulla, J.; Sahu, S.; Hayes, A. Nanotechnology: History and future. Hum. Exp. Toxicol. 2015, 34, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Yadav, S.S.; Chauhan, V.; Shukla, S.; Vishnolia, K.K. Applications of Nanoparticles in Various Fields. In Diagnostic Applications of Health Intelligence and Surveillance Systems; IGI Global: Hershey, PA, USA, 2021; pp. 221–236. [Google Scholar]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Vaseashta, A.; Dimova-Malinovska, D. Nanostructured and nanoscale devices, sensors and detectors. Sci. Technol. Adv. Mater. 2005, 6, 312–318. [Google Scholar] [CrossRef]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef]
- Roy, K.; Mao, H.-Q.; Huang, S.-K.; Leong, K.W. Oral gene delivery with chitosan–DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat. Med. 1999, 5, 387–391. [Google Scholar] [CrossRef]
- Langer, R. Drug delivery. Drugs on target. Science 2001, 293, 58–59. [Google Scholar] [CrossRef]
- da Silva, P.B.; Araújo, V.H.; Fonseca-Santos, B.; Solcia, M.C.; Ribeiro, C.M.; da Silva, I.C.; Alves, R.C.; Pironi, A.M.; Silva, A.C.L.; Victorelli, F.D. Highlights regarding the use of metallic nanoparticles against pathogens considered a priority by the world health organization. Curr. Med. Chem. 2021, 28, 1906–1956. [Google Scholar] [CrossRef]
- Tinajero-Díaz, E.; Salado-Leza, D.; Gonzalez, C.; Martínez Velázquez, M.; López, Z.; Bravo-Madrigal, J.; Knauth, P.; Flores-Hernández, F.Y.; Herrera-Rodríguez, S.E.; Navarro, R.E. Green metallic nanoparticles for cancer therapy: Evaluation models and cancer applications. Pharmaceutics 2021, 13, 1719. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Riley, M.K.; Vermerris, W. Recent advances in nanomaterials for gene delivery—A review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef]
- AMMAR, H.O.; TADROS, M.I.; SALAMA, N.M.; Mohsen, A. Development of vardenafil hydrochloride-loaded silica nanoparticles with enhanced transdermal delivery. J. Res. Pharm. 2022, 26, 451–459. [Google Scholar] [CrossRef]
- Yuliarto, B.; Septiani, N.L.W.; Kaneti, Y.V.; Iqbal, M.; Gumilar, G.; Kim, M.; Na, J.; Wu, K.C.-W.; Yamauchi, Y. Green synthesis of metal oxide nanostructures using naturally occurring compounds for energy, environmental, and bio-related applications. New J. Chem. 2019, 43, 15846–15856. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385. [Google Scholar] [PubMed]
- Pantidos, N.; Horsfall, L.E. Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. J. Nanomed. Nanotechnol. 2014, 5, 18. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.; Hussain, I.; Singh, H.; Singh, S. Plant-nanoparticle interaction: An approach to improve agricultural practices and plant productivity. Int. J. Pharm. Sci. Invent. 2015, 4, 25–40. [Google Scholar]
- Khandel, P.; Yadaw, R.K.; Soni, D.K.; Kanwar, L.; Shahi, S.K. Biogenesis of metal nanoparticles and their pharmacological applications: Present status and application prospects. J. Nanostructure Chem. 2018, 8, 217–254. [Google Scholar] [CrossRef]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Different inactivation behaviors of MS-2 phage and Escherichia coli in TiO2 photocatalytic disinfection. Appl. Environ. Microbiol. 2005, 71, 270–275. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef]
- Radetić, M. Functionalization of textile materials with silver nanoparticles. J. Mater. Sci. 2013, 48, 95–107. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.; Mullani, S.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Baker, C.; Pradhan, A.; Pakstis, L.; Pochan, D.J.; Shah, S.I. Synthesis and antibacterial properties of silver nanoparticles. J. Nanosci. Nanotechnol. 2005, 5, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Fayaz, A.; Ao, Z.; Girilal, M.; Chen, L.; Xiao, X.; Kalaichelvan, P.; Yao, X. Inactivation of microbial infectiousness by silver nanoparticles-coated condom: A new approach to inhibit HIV-and HSV-transmitted infection. Int. J. Nanomed. 2012, 7, 5007–5018. [Google Scholar] [CrossRef]
- Niakan, S.; Niakan, M.; Hesaraki, S.; Nejadmoghaddam, M.R.; Moradi, M.; Hanafiabdar, M.; Allamezadeh, R.; Sabouri, M. Comparison of the antibacterial effects of nanosilver with 18 antibiotics on multidrug resistance clinical isolates of Acinetobacter baumannii. Jundishapur J. Microbiol. 2013, 6. [Google Scholar] [CrossRef]
- Petrus, E.; Tinakumari, S.; Chai, L.; Ubong, A.; Tunung, R.; Elexson, N.; Chai, L.; Son, R. A study on the minimum inhibitory concentration and minimum bactericidal concentration of Nano Colloidal Silver on food-borne pathogens. Int. Food Res. J. 2011, 18. [Google Scholar]
- Niakan, M.; Azimi, H.R.; Jafarian, Z.; Mohammadtaghi, G.; Niakan, S.; Mostafavizade, S.M. Evaluation of nanosilver solution stability against Streptococcus mutans, Staphylococcus aureus and Pseudomonas aeruginosa. Jundishapur J. Microbiol. 2013, 6. [Google Scholar] [CrossRef]
- Emtiazi, G.; Shahrokh, S. Unusual biological behavior of staphylococci with nano silver assayed by microtiter-plate. In Proceedings of the 2009 9th Ieee Conference on Nanotechnology (Ieee-Nano), Genoa, Italy, 26–30 July 2009; pp. 394–396. [Google Scholar]
- Lotfi, M.; Vosoughhosseini, S.; Ranjkesh, B.; Khani, S.; Saghiri, M.; Zand, V. Antimicrobial efficacy of nanosilver, sodium hypochlorite and chlorhexidine gluconate against Enterococcus faecalis. Afr. J. Biotechnol. 2011, 10, 6799–6803. [Google Scholar]
- Cheng, L.; Zhang, K.; Weir, M.D.; Liu, H.; Zhou, X.; Xu, H.H. Effects of antibacterial primers with quaternary ammonium and nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dent. Mater. 2013, 29, 462–472. [Google Scholar] [CrossRef]
- Raja, K.; Saravanakumar, A.; Vijayakumar, R.; Spectroscopy, B. Efficient synthesis of silver nanoparticles from Prosopis juliflora leaf extract and its antimicrobial activity using sewage. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 490–494. [Google Scholar] [CrossRef]
- Sharma, R. Synthesis of Terminalia bellirica fruit extract mediated silver nanoparticles and application in photocatalytic degradation of wastewater from textile industries. Mater. Today Proc. 2021, 44, 1995–1998. [Google Scholar] [CrossRef]
- Khatoon, N.; Mazumder, J.A.; Sardar, M. Biotechnological applications of green synthesized silver nanoparticles. J. Nanosci. Curr. Res. 2017, 2, 2572-0813. [Google Scholar] [CrossRef]
- Kumar, P.; Govindaraju, M.; Senthamilselvi, S.; Premkumar, K. Photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Ulva lactuca. Colloids Surf. B Biointerfaces 2013, 103, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, S.; Ravi, S.; Velmurugan, S. Retracted: Green Synthesis of Silver Nanoparticles from Gloriosa Superba L. Leaf Extract and Their Catalytic Activity; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Firdhouse, M.J.; Lalitha, P. Fabrication of antimicrobial perspiration pads and cotton cloth using Amaranthus dubius mediated silver nanoparticles. J. Chem. 2013, 2013, 741743. [Google Scholar] [CrossRef]
- Verma, P.; Maheshwari, S.K. Applications of Silver nanoparticles in diverse sectors. J. Nano Dimens. 2019, 10, 18–36. [Google Scholar]
- Kumawat, M.; Madhyastha, H.; Singh, M.; Revaprasadu, N.; Srinivas, S.P.; Daima, H. Double functionalized haemocompatible silver nanoparticles control cell inflammatory homeostasis. PLoS ONE 2022, 17, e0276296. [Google Scholar] [CrossRef]
- Madhyastha, H.; Madhyastha, R.; Chakraborty, E.; Banerjee, K.; Shah, K.; Nakajima, Y.; Chauhan, N.S.; Sudhakaran, S.L.; Ohe, K.; Muthukaliannan, G.K. Fluro-Protein C-Phycocyanin Docked Silver Nanocomposite Accelerates Cell Migration through NFĸB Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 3184. [Google Scholar] [CrossRef]
- Madhyastha, H.; Madhyastha, R.; Thakur, A.; Kentaro, S.; Dev, A.; Singh, S.; Kumar, H.; Acevedo, O.; Nakajima, Y.; Daima, H.K.J.C.; et al. c-Phycocyanin primed silver nano conjugates: Studies on red blood cell stress resilience mechanism. Colloids Surf. B Biointerfaces 2020, 194, 111211. [Google Scholar] [CrossRef]
- Shah, S.M.M.; Ullah, F.; Shah, S.M.H.; Zahoor, M.; Sadiq, A. Analysis of chemical constituents and antinociceptive potential of essential oil of Teucrium Stocksianum bioss collected from the North West of Pakistan. BMC Complement. Altern. Med. 2012, 12, 244. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Zakaria, M.; Islam, M.; Kamil, M.; Ismail, A.; Chan, K.; Al-Attas, A. Analgesic and anti-inflammatory activities of Teucrium stocksianum. Pharm. Biol. 2001, 39, 455–459. [Google Scholar] [CrossRef]
- Ali, N.; Shah, S.W.A.; Shah, I.; Ahmed, G.; Ghias, M.; Khan, I. Cytotoxic and anthelmintic potential of crude saponins isolated from Achillea Wilhelmsii C. Koch and Teucrium Stocksianum boiss. BMC Complement. Altern. Med. 2011, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Rahim, G.; Qureshi, R.; Gulfraz, M.; Arshad, M.; Rahim, S. Preliminary phytochemical screening and ethnomedicinal uses of Teucrium stocksianum from Malakand Division. J. Med. Plants Res. 2012, 6, 704–707. [Google Scholar]
- Rahim, G.; Qureshi, R.; Arshad, M.; Gulfraz, M. Phytochemical analysis and antioxidant properties of Teucrium stocksianum flower from Malakand Division, Pakistan. Int. J. Agric. Biol. 2013, 15, 277–381. [Google Scholar]
- Perez, C. Antibiotic assay by agar-well diffusion method. Acta Biol. Med. Exp. 1990, 15, 113–115. [Google Scholar]
- bakht Dalir, S.J.; Djahaniani, H.; Nabati, F.; Hekmati, M. Characterization and the evaluation of antimicrobial activities of silver nanoparticles biosynthesized from Carya illinoinensis leaf extract. Heliyon 2020, 6, e03624. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; Pyne, S.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Physicochem. Eng. Asp. 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Zahir, A.A.; Chauhan, I.S.; Bagavan, A.; Kamaraj, C.; Elango, G.; Shankar, J.; Arjaria, N.; Roopan, S.M.; Rahuman, A.A.; Singh, N. Green synthesis of silver and titanium dioxide nanoparticles using Euphorbia prostrata extract shows shift from apoptosis to G0/G1 arrest followed by necrotic cell death in Leishmania donovani. Antimicrob. Agents Chemother. 2015, 59, 4782–4799. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; Pyne, S.; Misra, A. Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Physicochem. Eng. Asp. 2009, 348, 212–216. [Google Scholar] [CrossRef]
- Vanaja, M.; Annadurai, G. Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl. Nanosci. 2013, 3, 217–223. [Google Scholar] [CrossRef]




| No. | Class | Test | Reagent | Observation | Result |
|---|---|---|---|---|---|
| 1 | Alkaloids | Hager’s Reagent | A saturated solution of picric acid | Yellow precipitates | Positive |
| 2 | Tannins | Braymer’s reagent | 5% FeCl3 | Dark green color | Positive |
| 3 | Sterols | Salkowski’s reagent | Conc. H2SO4 | Red-colored ring formation | Positive |
| 4 | Flavonoids | Alkaline reagent | 2% NaOH + drops of HCl | The yellow color disappears with the addition of HCl | Positive |
| 5 | Saponins | Foam Test | Distilled water | Formation of foam | Positive |
| 6 | Waxes | Saponification | Methanolic NaOH | No saponification | Negative |
| MIC (µg/mL) | ||||
|---|---|---|---|---|
| Microbes | SNPFPE | FPE | SNPDPE | DPE |
| Staphylococcus aureus | 5.3 | 69.5 | 6.8 | 64.7 |
| Escherichia coli | 7.4 | 76.7 | 7.1 | 73.9 |
| Bacillus subtilis | 8.1 | 96.5 | 9.7 | 98.2 |
| Streptococcus pneumoniae | 6.7 | 86.9 | 6.3 | 84.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, I.; Gondal, H.Y.; Zamir, R.; Al-Hussain, S.A.; Batool, F.; Irfan, A.; Noreen, S.; Roheen, T.; Nisar, M.; Zaki, M.E.A. Green Synthesis: The Antibacterial and Photocatalytic Potential of Silver Nanoparticles Using Extract of Teucrium stocksianum. Nanomaterials 2023, 13, 1343. https://doi.org/10.3390/nano13081343
Rehman I, Gondal HY, Zamir R, Al-Hussain SA, Batool F, Irfan A, Noreen S, Roheen T, Nisar M, Zaki MEA. Green Synthesis: The Antibacterial and Photocatalytic Potential of Silver Nanoparticles Using Extract of Teucrium stocksianum. Nanomaterials. 2023; 13(8):1343. https://doi.org/10.3390/nano13081343
Chicago/Turabian StyleRehman, Iqra, Humaira Yasmeen Gondal, Roshan Zamir, Sami A. Al-Hussain, Fozia Batool, Ali Irfan, Sobia Noreen, Taleeha Roheen, Muhammad Nisar, and Magdi E. A. Zaki. 2023. "Green Synthesis: The Antibacterial and Photocatalytic Potential of Silver Nanoparticles Using Extract of Teucrium stocksianum" Nanomaterials 13, no. 8: 1343. https://doi.org/10.3390/nano13081343
APA StyleRehman, I., Gondal, H. Y., Zamir, R., Al-Hussain, S. A., Batool, F., Irfan, A., Noreen, S., Roheen, T., Nisar, M., & Zaki, M. E. A. (2023). Green Synthesis: The Antibacterial and Photocatalytic Potential of Silver Nanoparticles Using Extract of Teucrium stocksianum. Nanomaterials, 13(8), 1343. https://doi.org/10.3390/nano13081343








