Shaping Silver Nanoparticles’ Size through the Carrier Composition: Synthesis and Antimicrobial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. X-ray Diffraction (XRD)
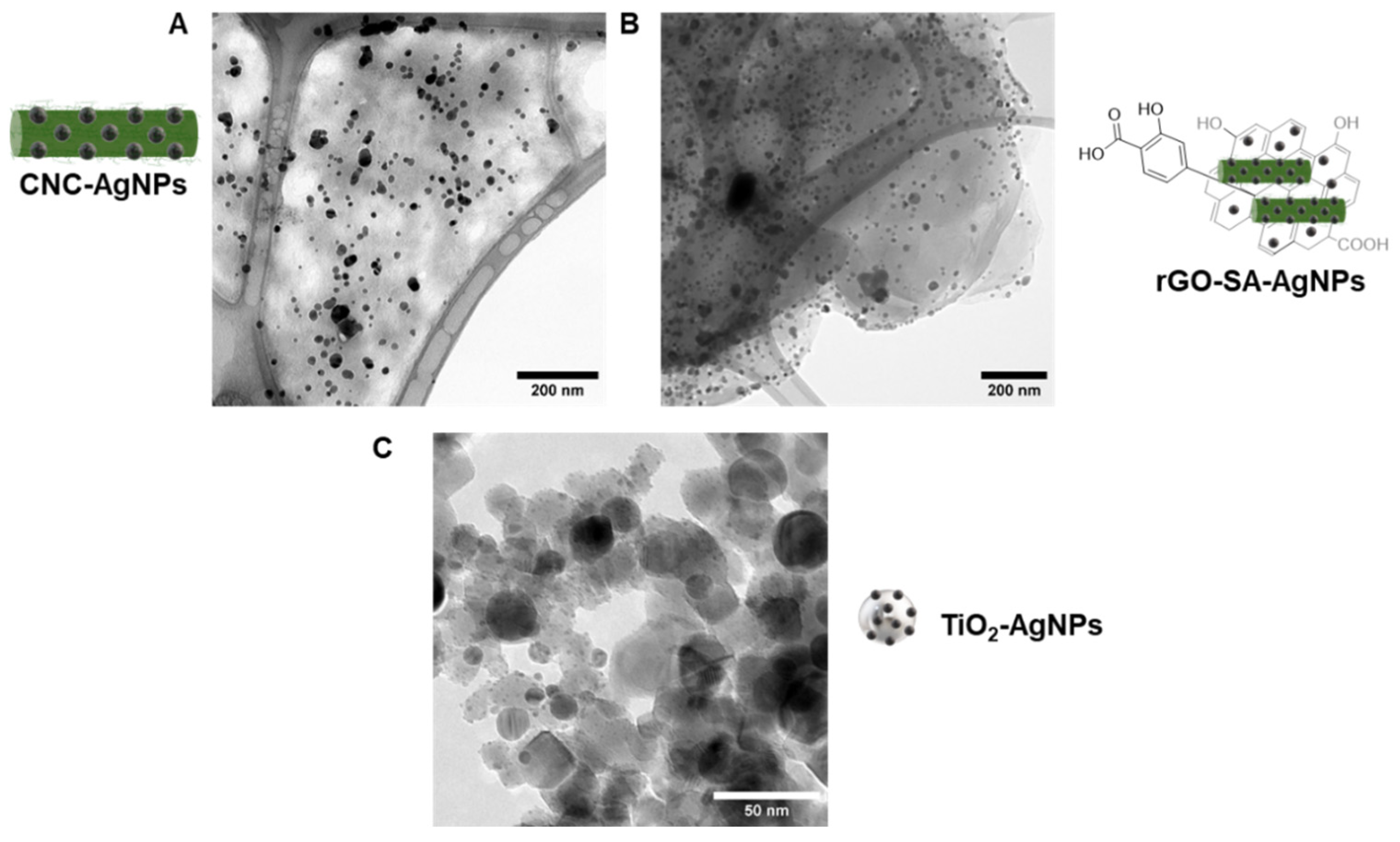
2.2. Transmission Electron Microscopy (TEM)
2.3. Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES)
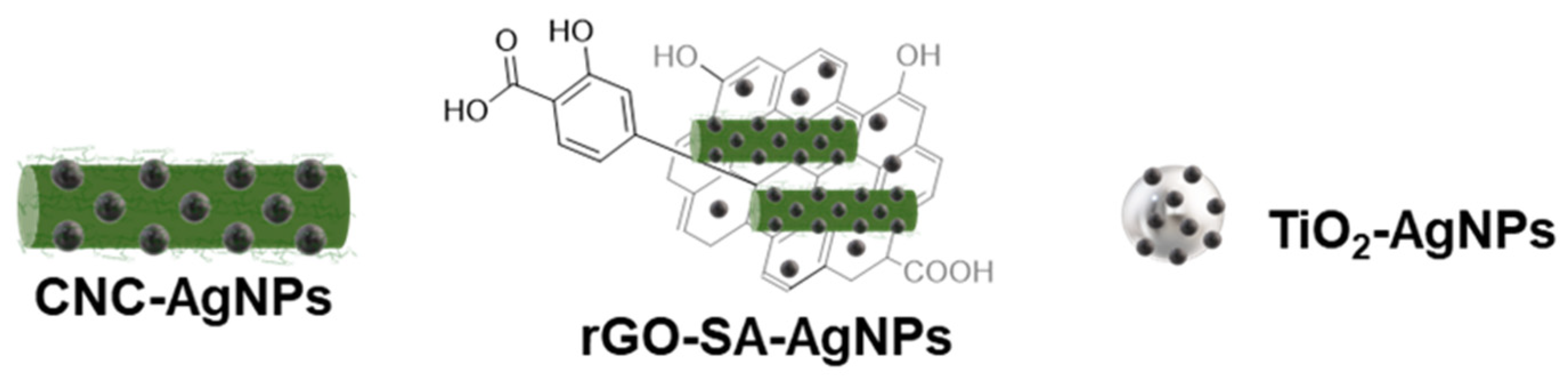
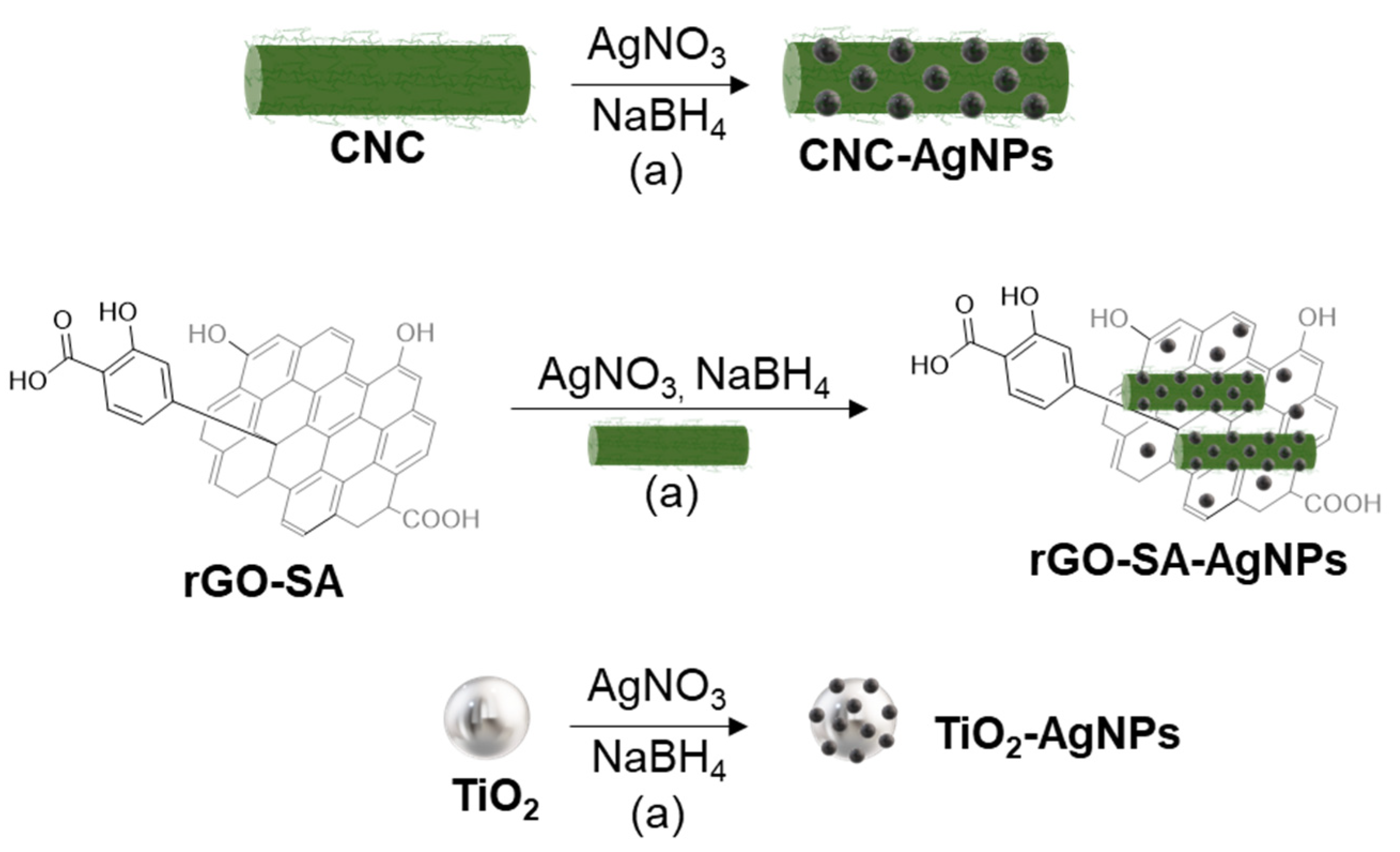
2.4. Synthesis of CNC-AgNPs
2.5. Synthesis of rGO-SA-AgNPs
2.6. Synthesis of TiO2-AgNPs
2.7. Microbial Strains, Media, and Culture Conditions
2.8. MCC Evaluation
2.9. MTS Cytotoxicity Test
3. Results
3.1. Preparation of CNC-AgNPs, rGO-SA-AgNPs, and TiO2-AgNPs
3.2. Antimicrobial Activity
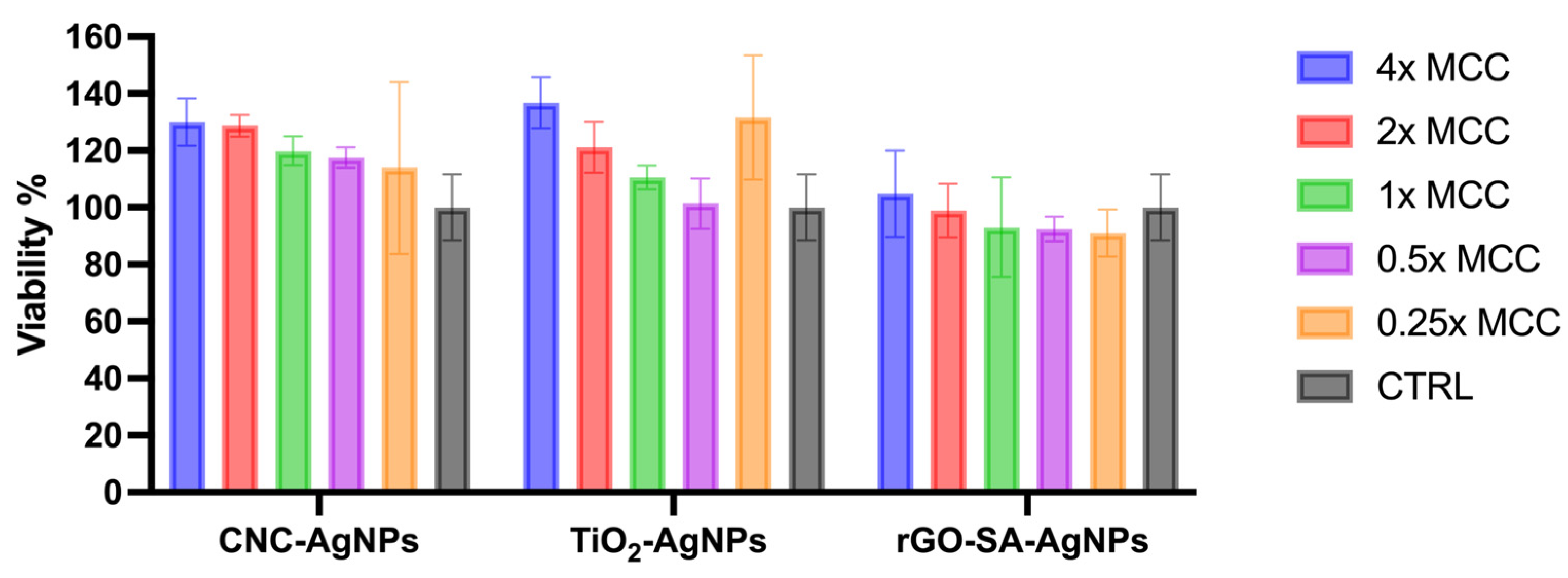
3.3. Cytotoxicity Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, O.; Puiu, R.A.; Bîrcă, A.C.; Burdușel, A.-C.; Grumezescu, A.M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver Nanoparticles: Various Methods of Synthesis, Size Affecting Factors and Their Potential Applications—A Review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A Review on Biosynthesis of Silver Nanoparticles and Their Biocidal Properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M.V. A Review of the Antibacterial Effects of Silver Nanomaterials and Potential Implications for Human Health and the Environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Betts, J.W.; Hornsey, M.; La Ragione, R.M. Chapter Four—Novel Antibacterials: Alternatives to Traditional Antibiotics. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 73, pp. 123–169. [Google Scholar]
- Natan, M.; Banin, E. From Nano to Micro: Using Nanotechnology to Combat Microorganisms and Their Multidrug Resistance. FEMS Microbiol. Rev. 2017, 41, 302–322. [Google Scholar] [CrossRef]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef]
- Seong, M.; Lee, D.G. Silver Nanoparticles Against Salmonella Enterica Serotype Typhimurium: Role of Inner Membrane Dysfunction. Curr. Microbiol. 2017, 74, 661–670. [Google Scholar] [CrossRef]
- You, C.; Han, C.; Wang, X.; Zheng, Y.; Li, Q.; Hu, X.; Sun, H. The Progress of Silver Nanoparticles in the Antibacterial Mechanism, Clinical Application and Cytotoxicity. Mol. Biol. Rep. 2012, 39, 9193–9201. [Google Scholar] [CrossRef]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Román-García, M.F.; Ramírez-Díaz, C.A.; Ulloa-Ramírez, M.; Morones-Ramírez, J.R. Nanomaterials-Based Combinatorial Therapy as a Strategy to Combat Antibiotic Resistance. Antibiotics 2022, 11, 794. [Google Scholar] [CrossRef]
- Ipe, D.S.; Kumar, P.T.S.; Love, R.M.; Hamlet, S.M. Silver Nanoparticles at Biocompatible Dosage Synergistically Increases Bacterial Susceptibility to Antibiotics. Front. Microbiol. 2020, 11, 1074. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of Antibiotics Antimicrobial Activity Due to the Silver Nanoparticles Impact on the Cell Membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kumar, R. Enhanced Bactericidal Efficacy of Polymer Stabilized Silver Nanoparticles in Conjugation with Different Classes of Antibiotics. RSC Adv. 2019, 9, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Proteomic Analysis of the Mode of Antibacterial Action of Silver Nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Das, N.C. Advances on Catalytic Reduction of 4-Nitrophenol by Nanostructured Materials as Benchmark Reaction. Int. Nano Lett. 2022, 12, 223–242. [Google Scholar] [CrossRef]
- Das, T.K.; Ganguly, S.; Remanan, S.; Ghosh, S.; Das, N.C. Mussel-Inspired Ag/Poly(Norepinephrine)/MnO2 Heterogeneous Nanocatalyst for Efficient Reduction of 4-Nitrophenol and 4-Nitroaniline: An Alternative Approach. Res. Chem. Intermed. 2020, 46, 3629–3650. [Google Scholar] [CrossRef]
- Kukushkina, E.A.; Hossain, S.I.; Sportelli, M.C.; Ditaranto, N.; Picca, R.A.; Cioffi, N. Ag-Based Synergistic Antimicrobial Composites. A Critical Review. Nanomaterials 2021, 11, 1687. [Google Scholar] [CrossRef]
- Biagiotti, G.; Salvatore, A.; Toniolo, G.; Caselli, L.; Di Vito, M.; Cacaci, M.; Contiero, L.; Gori, T.; Maggini, M.; Sanguinetti, M.; et al. Metal-Free Antibacterial Additives Based on Graphene Materials and Salicylic Acid: From the Bench to Fabric Applications. ACS Appl. Mater. Interfaces 2021, 13, 26288–26298. [Google Scholar] [CrossRef]
- Delepierre, G.; Vanderfleet, O.M.; Niinivaara, E.; Zakani, B.; Cranston, E.D. Benchmarking Cellulose Nanocrystals Part II: New Industrially Produced Materials. Langmuir 2021, 37, 8393–8409. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Nurazzi, N.M.; Jenol, M.A.; Farid, M.A.A.; Janudin, N.; Ujang, F.A.; Yasim-Anuar, T.A.T.; Syed Najmuddin, S.U.F.; Ilyas, R.A. Emerging Development of Nanocellulose as an Antimicrobial Material: An Overview. Mater. Adv. 2021, 2, 3538–3551. [Google Scholar] [CrossRef]
- Biagiotti, G.; Toniolo, G.; Albino, M.; Severi, M.; Andreozzi, P.; Marelli, M.; Kokot, H.; Tria, G.; Guerri, A.; Sangregorio, C.; et al. Simple Engineering of Hybrid Cellulose Nanocrystal–Gold Nanoparticles Results in a Functional Glyconanomaterial with Biomolecular Recognition Properties. Nanoscale Horiz. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef]
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, J.; Shang, S.; Song, Z.; Wang, D. Cellulose Nanocrystal/Silver Nanoparticle Composites as Bifunctional Nanofillers within Waterborne Polyurethane. ACS Appl. Mater. Interfaces 2012, 4, 2413–2419. [Google Scholar] [CrossRef]
- Yang, S.; Xue, B.; Li, Y.; Li, X.; Xie, L.; Qin, S.; Xu, K.; Zheng, Q. Controllable Ag-RGO Heterostructure for Highly Thermal Conductivity in Layer-by-Layer Nanocellulose Hybrid Films. Chem. Eng. J. 2020, 383, 123072. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Aldalbahi, A.; Al-Hajji, A.B.; Chaudhary, A.A.; In Het Panhuis, M.; Alhokbany, N.; Ahamad, T. Development of Carboxymethyl Cellulose-Based Hydrogel and Nanosilver Composite as Antimicrobial Agents for UTI Pathogens. Carbohydr. Polym. 2016, 138, 229–236. [Google Scholar] [CrossRef]
- Xiong, R.; Lu, C.; Zhang, W.; Zhou, Z.; Zhang, X. Facile Synthesis of Tunable Silver Nanostructures for Antibacterial Application Using Cellulose Nanocrystals. Carbohydr. Polym. 2013, 95, 214–219. [Google Scholar] [CrossRef]
- Martakov, I.S.; Torlopov, M.A.; Mikhaylov, V.I.; Krivoshapkina, E.F.; Silant’ev, V.E.; Krivoshapkin, P.V. Interaction of Cellulose Nanocrystals with Titanium Dioxide and Peculiarities of Hybrid Structures Formation. J. Sol-Gel Sci. Technol. 2018, 88, 13–21. [Google Scholar] [CrossRef]
- Ayobami, O.; Brinkwirth, S.; Eckmanns, T.; Markwart, R. Antibiotic Resistance in Hospital-Acquired ESKAPE-E Infections in Low- and Lower-Middle-Income Countries: A Systematic Review and Meta-Analysis. Emerg. Microbes Infect. 2022, 11, 443–451. [Google Scholar] [CrossRef]

| MCC (Minimal Cytocidal Concentration) µg/mL | |||||
|---|---|---|---|---|---|
| AgNP Composites | K. pneumoniae | E. coli | P. aeruginosa | S. aureus | C. albicans |
| CNC-AgNPs | 2 | 2 | 2 | 2 | 4 |
| rGO-SA-AgNPs | 2 | 2 | 2 | 2 | 2 |
| TiO2-AgNPs | 1 | 1 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacaci, M.; Biagiotti, G.; Toniolo, G.; Albino, M.; Sangregorio, C.; Severi, M.; Di Vito, M.; Squitieri, D.; Contiero, L.; Paggi, M.; et al. Shaping Silver Nanoparticles’ Size through the Carrier Composition: Synthesis and Antimicrobial Activity. Nanomaterials 2023, 13, 1585. https://doi.org/10.3390/nano13101585
Cacaci M, Biagiotti G, Toniolo G, Albino M, Sangregorio C, Severi M, Di Vito M, Squitieri D, Contiero L, Paggi M, et al. Shaping Silver Nanoparticles’ Size through the Carrier Composition: Synthesis and Antimicrobial Activity. Nanomaterials. 2023; 13(10):1585. https://doi.org/10.3390/nano13101585
Chicago/Turabian StyleCacaci, Margherita, Giacomo Biagiotti, Gianluca Toniolo, Martin Albino, Claudio Sangregorio, Mirko Severi, Maura Di Vito, Damiano Squitieri, Luca Contiero, Marco Paggi, and et al. 2023. "Shaping Silver Nanoparticles’ Size through the Carrier Composition: Synthesis and Antimicrobial Activity" Nanomaterials 13, no. 10: 1585. https://doi.org/10.3390/nano13101585
APA StyleCacaci, M., Biagiotti, G., Toniolo, G., Albino, M., Sangregorio, C., Severi, M., Di Vito, M., Squitieri, D., Contiero, L., Paggi, M., Marelli, M., Cicchi, S., Bugli, F., & Richichi, B. (2023). Shaping Silver Nanoparticles’ Size through the Carrier Composition: Synthesis and Antimicrobial Activity. Nanomaterials, 13(10), 1585. https://doi.org/10.3390/nano13101585











