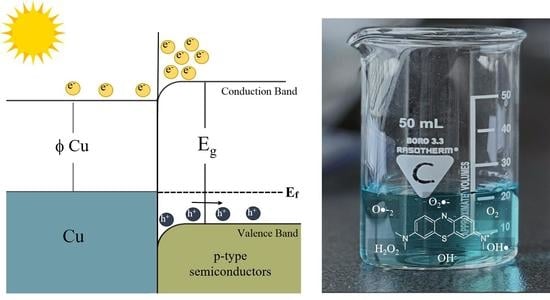
Sunlight-Driven Photocatalytic Degradation of Methylene Blue with Facile One-Step Synthesized Cu-Cu2O-Cu3N Nanoparticle Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cu-Based Nanocomposite
2.3. Characterization
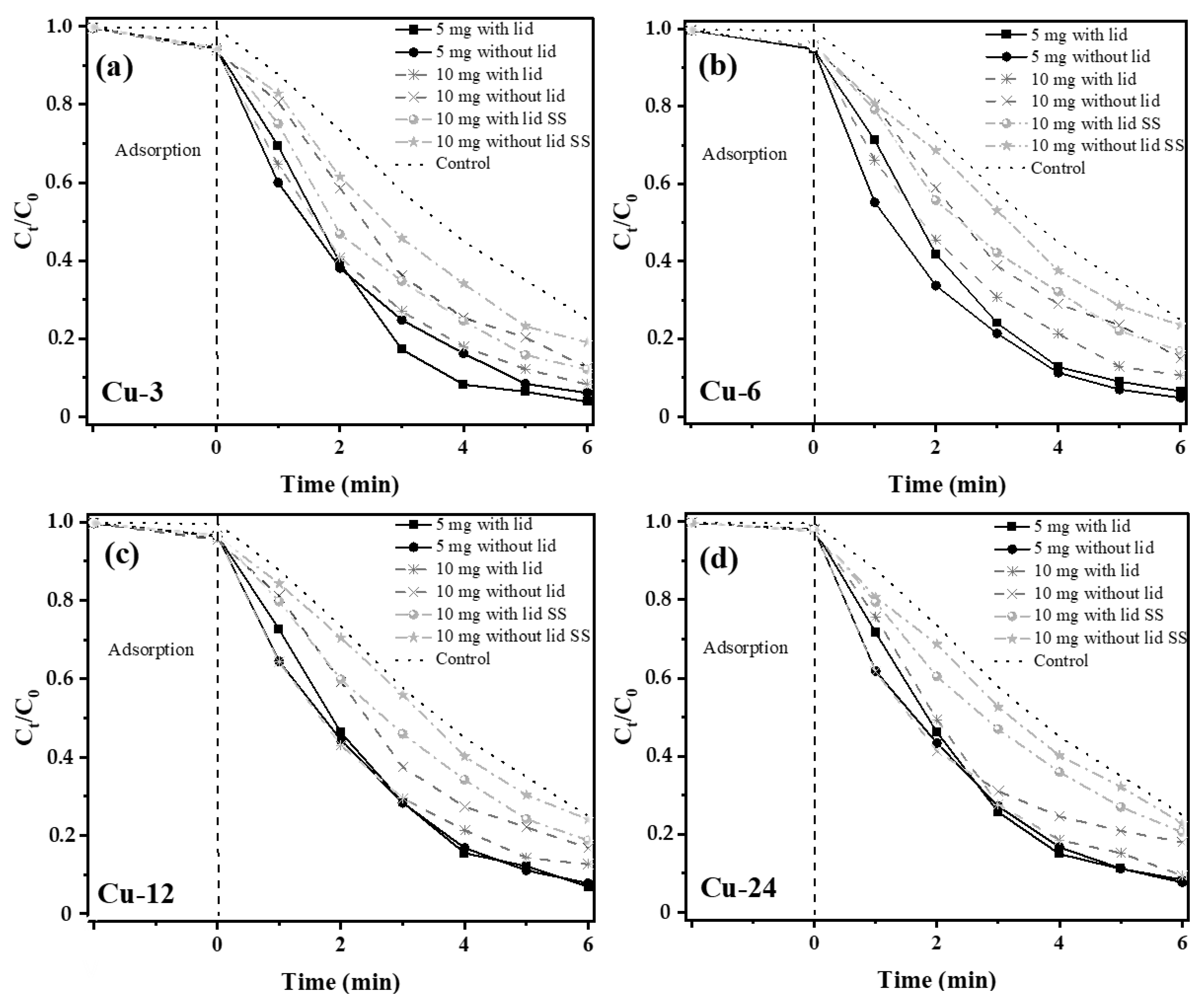
2.4. Adsorption Experiments
2.5. Photoctalytic Experiments
3. Results and Discussion
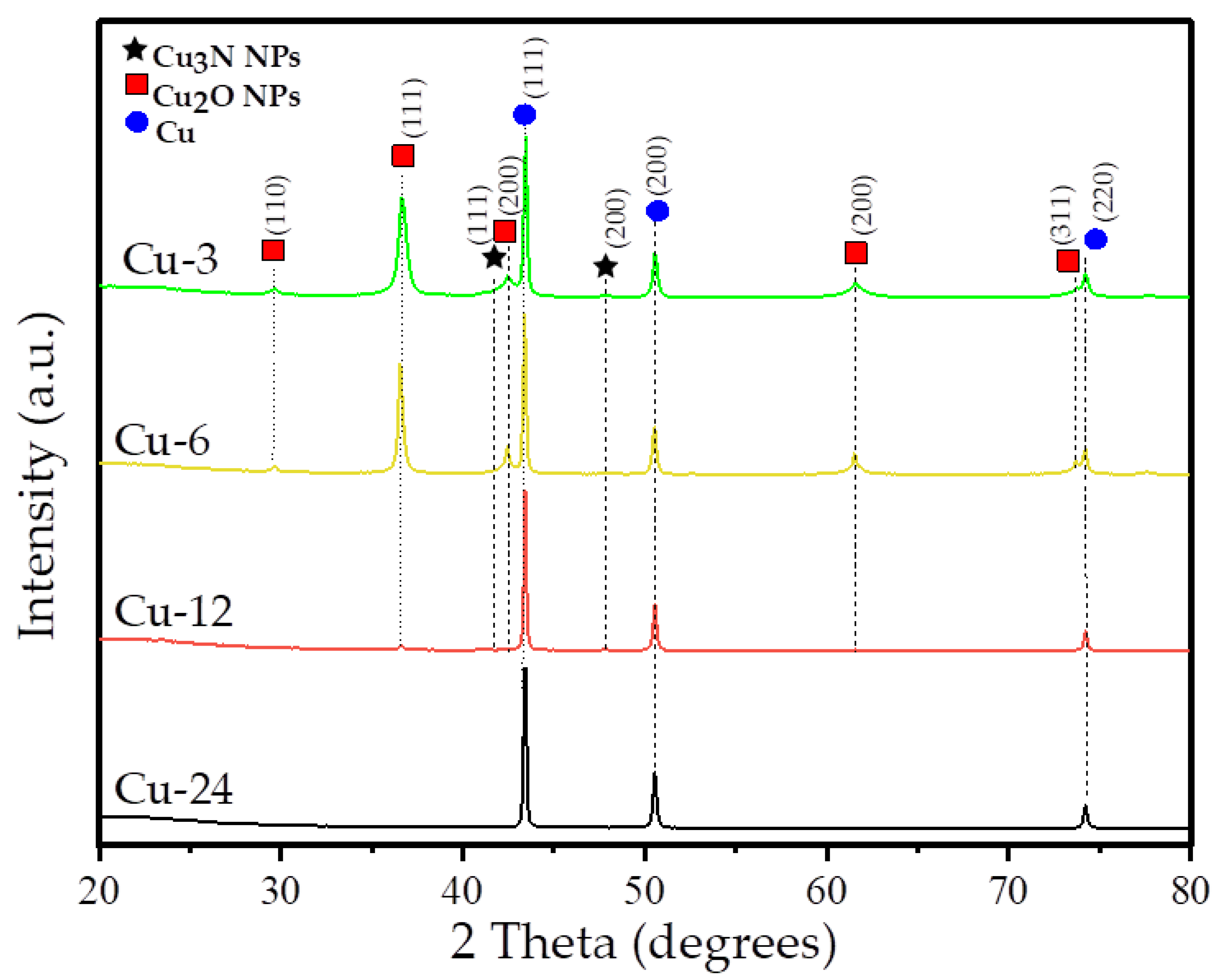
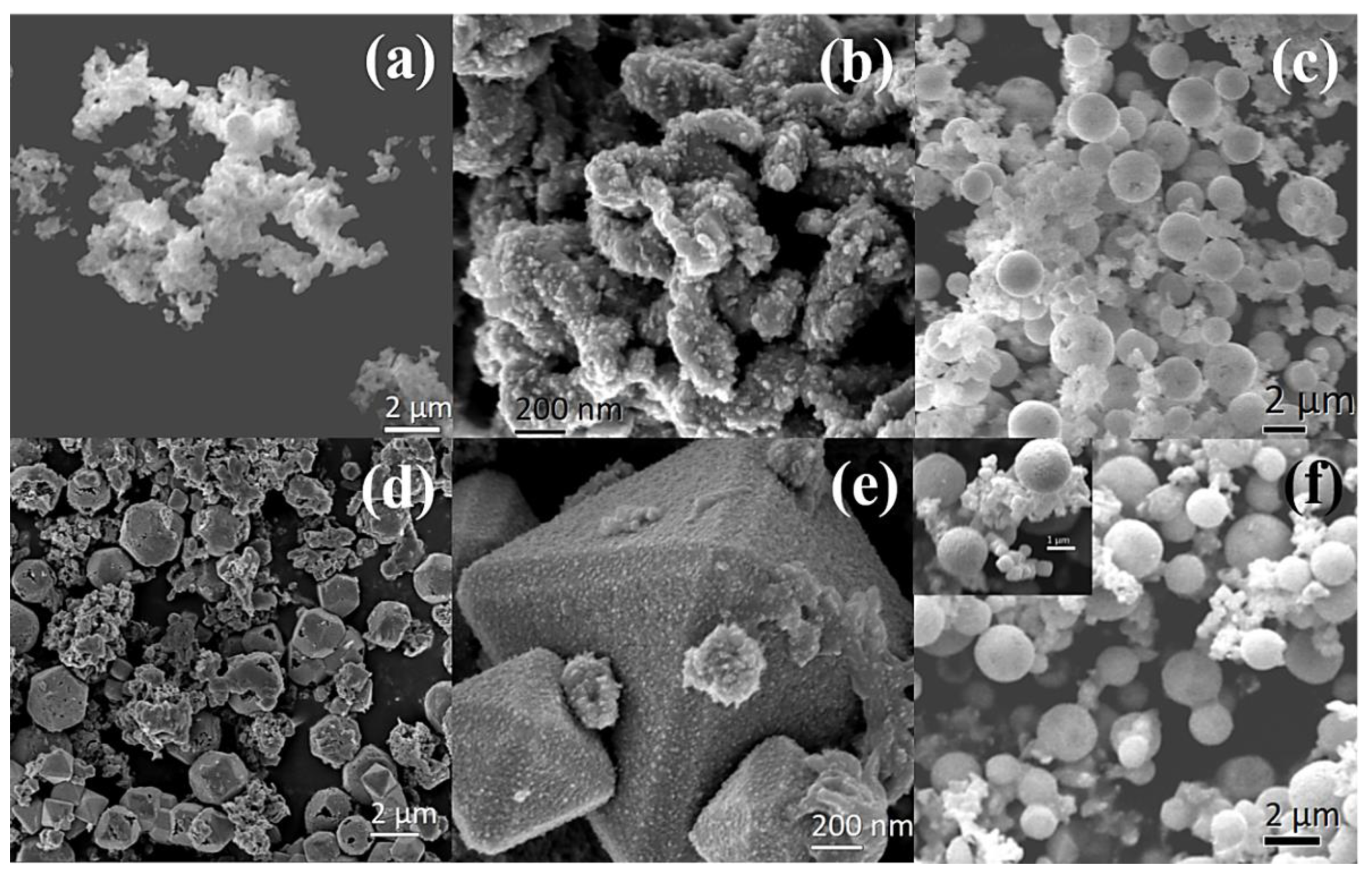
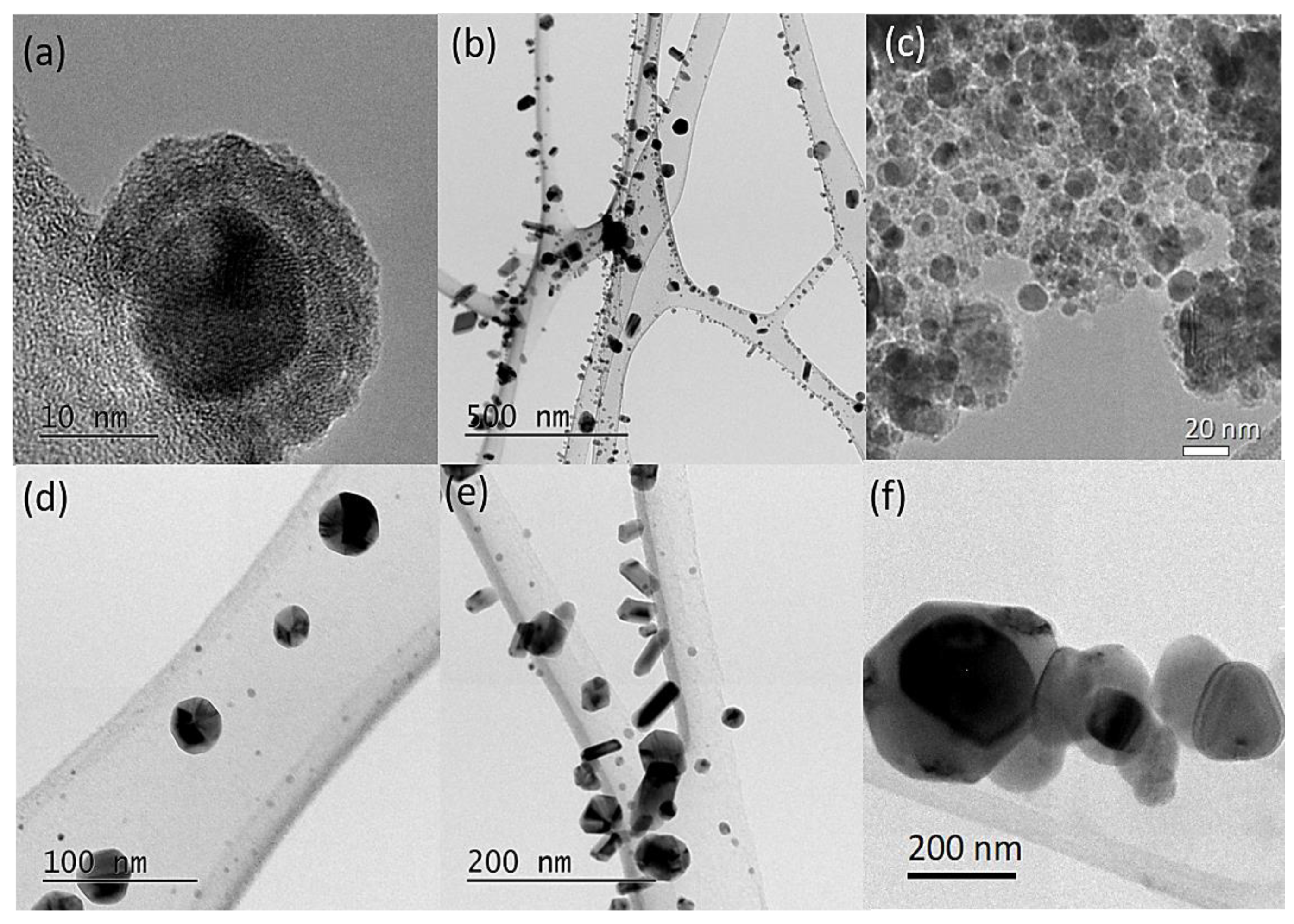
3.1. Structure and Morphology
3.2. Elemental Characterization
3.3. Optical Properties
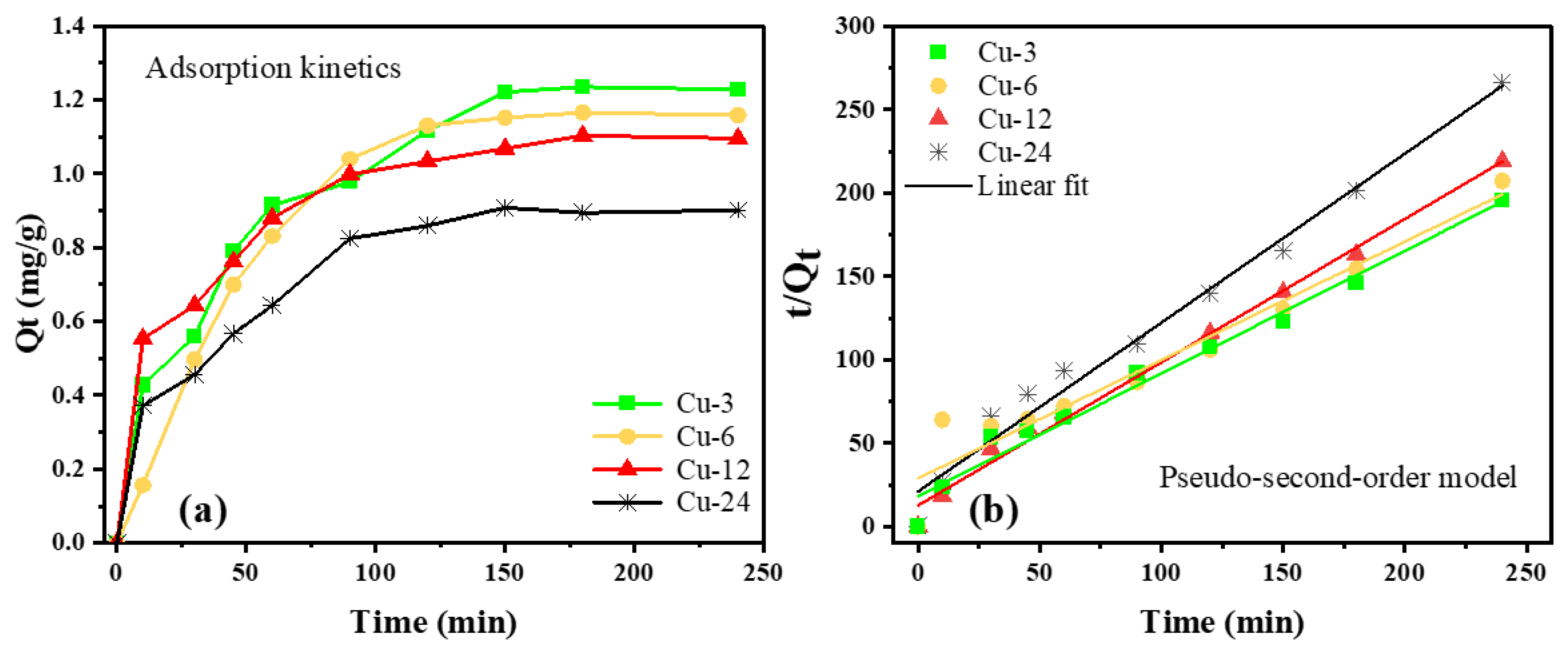
4. Adsorption Kinetics
5. Sunlight-Driven Photocatalysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Sultana, K.A.; Islam, M.T.; Silva, J.A.; Turley, R.S.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L.; Noveron, J.C. Sustainable synthesis of zinc oxide nanoparticles for photocatalytic degradation of organic pollutant and generation of hydroxyl radical. J. Mol. Liq. 2020, 307, 112931. [Google Scholar] [CrossRef]
- Gutierrez-Mata, A.G.; Velazquez-Martínez, S.; Álvarez-Gallegos, A.; Ahmadi, M.; Hernández-Pérez, J.A.; Ghanbari, F.; Silva-Martínez, S. Recent Overview of Solar Photocatalysis and Solar Photo-Fenton Processes for Wastewater Treatment. Int. J. Photoenergy 2017, 2017, 8528063. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and Prospects for Wastewater Treatment by UV and Visible-Light-Active Heterogeneous Photocatalysis: A Critical Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 225–264. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Zhang, Y.; Huang, S.; Lv, H.; Chen, J.; Zhai, Y.; Cheng, J.; Pan, J. Cu-based heterostructure photocatalysts derived from Cu sludge and municipal sewage sludge for efficient degradation of 2,4-dichlorophenol. Chem. Eng. J. 2022, 429, 132140. [Google Scholar] [CrossRef]
- Dasineh Khiavi, N.; Katal, R.; Kholghi Eshkalak, S.; Masudy-Panah, S.; Ramakrishna, S.; Jiangyong, H. Visible Light Driven Heterojunction Photocatalyst of CuO–Cu2O Thin Films for Photocatalytic Degradation of Organic Pollutants. Nanomaterials 2019, 9, 1011. [Google Scholar] [CrossRef]
- Bayat, F.; Sheibani, S. Enhancement of photocatalytic activity of CuO-Cu2O heterostructures through the controlled content of Cu2O. Mater. Res. Bull. 2022, 145, 111561. [Google Scholar] [CrossRef]
- Mkhalid, I.A.; Shawky, A. Cu-supported Cu2O nanoparticles: Optimized photodeposition enhances the visible light photodestruction of atrazine. J. Alloys Compd. 2021, 853, 157040. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, H.; Li, D.; Hu, L.; Geng, W.; Wei, P.; Ouyang, P. Improved photocatalytic activity and mechanism of Cu2O/N–TiO2 prepared by a two-step method. RSC Adv. 2014, 4, 17797–17804. [Google Scholar] [CrossRef]
- Paredes, P.; Rauwel, E.; Rauwel, P. Surveying the Synthesis, Optical Properties and Photocatalytic Activity of Cu3N Nanomaterials. Nanomaterials 2022, 12, 2218. [Google Scholar] [CrossRef]
- Norzaee, S.; Djahed, B.; Khaksefidi, R.; Mostafapour, F.K. Photocatalytic degradation of aniline in water using CuO nanoparticles. J. Water Supply Res. Technol.-Aqua 2017, 66, 178–185. [Google Scholar] [CrossRef]
- Yu, X.; Kou, S.; Zhang, J.; Tang, X.; Yang, Q.; Yao, B. Preparation and characterization of Cu2O nano-particles and their photocatalytic degradation of fluroxypyr. Environ. Technol. 2018, 39, 2967–2976. [Google Scholar] [CrossRef]
- Sithole, R.K.; Machogo, L.F.E.; Moloto, M.J.; Gqoba, S.S.; Mubiayi, K.P.; Van Wyk, J.; Moloto, N. One-step synthesis of Cu3N, Cu2S and Cu9S5 and photocatalytic degradation of methyl orange and methylene blue. J. Photochem. Photobiol. A Chem. 2020, 397, 112577. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, H.; Li, X. Photo-reduction synthesis of Cu nanoparticles as plasmon-driven non-semiconductor photocatalyst for overall water splitting. Appl. Surf. Sci. 2021, 535, 147720. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Z.; Zhang, S.; Cui, Y.; Gao, S.; Wang, Y. Hydrothermal deposition of Cu2O-Ag nanoparticles co-sensitized TiO2 nanotube arrays and their enhanced photoelectrochemical performance. Sep. Purif. Technol. 2019, 211, 866–872. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, J.; Weng, B.; Lai, F.; Zhang, M.; Hofkens, J.; Roeffaers, M.B.J.; Steele, J.A.; Long, J. Site-Sensitive Selective CO2 Photoreduction to CO over Gold Nanoparticles. Angew. Chem. Int. Ed. 2022, 61, e202204563. [Google Scholar] [CrossRef]
- Ben Saber, N.; Mezni, A.; Alrooqi, A.; Altalhi, T. A review of ternary nanostructures based noble metal/semiconductor for environmental and renewable energy applications. J. Mater. Res. Technol. 2020, 9, 15233–15262. [Google Scholar] [CrossRef]
- Rauwel, E.; Galeckas, A.; Soares, M.R.; Rauwel, P. Influence of the Interface on the Photoluminescence Properties in ZnO Carbon-Based Nanohybrids. J. Phys. Chem. C 2017, 121, 14879–14887. [Google Scholar] [CrossRef]
- Rauwel, E.; Galeckas, A.; Rauwel, P.; Fjellvåg, H. Unusual Photoluminescence of CaHfO3 and SrHfO3 Nanoparticles. Adv. Funct. Mater. 2012, 22, 1174–1179. [Google Scholar] [CrossRef]
- Grazulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quiros, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Coelho, A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Primc, D.; Indrizzi, L.; Tervoort, E.; Xie, F.; Niederberger, M. Synthesis of Cu3N and Cu3N–Cu2O multicomponent mesocrystals: Non-classical crystallization and nanoscale Kirkendall effect. Nanoscale 2021, 13, 17521–17529. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Liz-Marzán, L.M. Oleylamine in Nanoparticle Synthesis. Chem. Mater. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Barman, D.; Paul, S.; Ghosh, S.; De, S.K. Cu3N Nanocrystals Decorated with Au Nanoparticles for Photocatalytic Degradation of Organic Dyes. ACS Appl. Nano Mater. 2019, 2, 5009–5019. [Google Scholar] [CrossRef]
- Won, Y.-H.; Stanciu, L.A. Cu2O and Au/Cu2O Particles: Surface Properties and Applications in Glucose Sensing. Sensors 2012, 12, 13019–13033. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Chou, K.-H.; Kuo, Y.-Y.; Wu, C.-Y.; Hsiao, P.-W.; Chen, P.-W.; Yuan, S.-H.; Wuu, D.-S. Formation of ZnO/Zn0.5Cd0.5Se Alloy Quantum Dots in the Presence of High Oleylamine Contents. Nanomaterials 2019, 9, 999. [Google Scholar] [CrossRef]
- Mbewana-Ntshanka, N.G.; Moloto, M.J.; Mubiayi, P.K. Role of the amine and phosphine groups in oleylamine and trioctylphosphine in the synthesis of copper chalcogenide nanoparticles. Heliyon 2020, 6, e05130. [Google Scholar] [CrossRef]
- Sahai, A.; Goswami, N.; Kaushik, S.D.; Tripathi, S. Cu/Cu2O/CuO nanoparticles: Novel synthesis by exploding wire technique and extensive characterization. Appl. Surf. Sci. 2016, 390, 974–983. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, F.; Shen, S.; Fu, Y.; Chen, C.; Liu, C.; Xing, Z.; Liu, D.; Xiao, X.; Wu, W.; et al. Efficient enhancement of hydrogen production by Ag/Cu2O/ZnO tandem triple-junction photoelectrochemical cell. Appl. Phys. Lett. 2015, 106, 123901. [Google Scholar] [CrossRef]
- Wei, W.; Xu, B.; Huang, Q. Controllable Synthesis and Catalytic Property of Novel Copper Oxides (CuO and Cu2O) Nanostructures. Int. J. Mater. Sci. Appl. 2016, 5, 18–22. [Google Scholar] [CrossRef]
- Sajeev, A.; Paul, A.M.; Nivetha, R.; Gothandapani, K.; Gopal, T.S.; Jacob, G.; Muthuramamoorty, M.; Pandiaraj, S.; Alodhayb, A.; Kim, S.Y.; et al. Development of Cu3N electrocatalyst for hydrogen evolution reaction in alkaline medium. Sci. Rep. 2022, 12, 2004. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Alessi, A.; Sciortino, L.; Agnello, S.; Cannas, M.; Gelardi, F.M.; Buscarino, G. Investigation by Raman Spectroscopy of the Decomposition Process of HKUST-1 upon Exposure to Air. J. Spectrosc. 2016, 2016, 8074297. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, M.; Zhang, J.; Wang, Q.; Li, H. A molecularly imprinted nanoprobe incorporating Cu2O@Ag nanoparticles with different morphologies for selective SERS based detection of chlorophenols. Microchim. Acta 2019, 187, 59. [Google Scholar] [CrossRef] [PubMed]
- Zayyoun, N.; Bahmad, L.; Laânab, L.; Jaber, B. The effect of pH on the synthesis of stable Cu2O/CuO nanoparticles by sol–gel method in a glycolic medium. Appl. Phys. A 2016, 122, 488. [Google Scholar] [CrossRef]
- Ullah, N.; Ullah, A.; Rasheed, S. Green synthesis of copper nanoparticles using extract of Dicliptera Roxburghiana, their characterization and photocatalytic activity against methylene blue degradation. Lett. Appl. NanoBioSci. 2020, 9, 897–901. [Google Scholar] [CrossRef]
- Mikami, K.; Kido, Y.; Akaishi, Y.; Quitain, A.; Kida, T. Synthesis of Cu2O/CuO Nanocrystals and Their Application to H2S Sensing. Sensors 2019, 19, 211. [Google Scholar] [CrossRef]
- Nakamura, T.; Hayashi, H.; Hanaoka, T.A.; Ebina, T. Preparation of copper nitride (Cu3N) nanoparticles in long-chain alcohols at 130–200 °C and nitridation mechanism. Inorg. Chem. 2014, 53, 710–715. [Google Scholar] [CrossRef]
- Mott, D.; Galkowski, J.; Wang, L.; Luo, J.; Zhong, C.-J. Synthesis of Size-Controlled and Shaped Copper Nanoparticles. Langmuir 2007, 23, 5740–5745. [Google Scholar] [CrossRef]
- Atarod, M.; Nasrollahzadeh, M.; Sajadi, S.M. Green synthesis of a Cu/reduced graphene oxide/Fe3O4 nanocomposite using Euphorbia wallichii leaf extract and its application as a recyclable and heterogeneous catalyst for the reduction of 4-nitrophenol and rhodamine B. RSC Adv. 2015, 5, 91532–91543. [Google Scholar] [CrossRef]
- Valodkar, M.; Modi, S.; Pal, A.; Thakore, S. Synthesis and anti-bacterial activity of Cu, Ag and Cu–Ag alloy nanoparticles: A green approach. Mater. Res. Bull. 2011, 46, 384–389. [Google Scholar] [CrossRef]
- Mallick, S.; Sanpui, P.; Ghosh, S.S.; Chattopadhyay, A.; Paul, A. Synthesis, characterization and enhanced bactericidal action of a chitosan supported core–shell copper–silver nanoparticle composite. RSC Adv. 2015, 5, 12268–12276. [Google Scholar] [CrossRef]
- Dorranian, D.; Dejam, L.; Sari, A.H.; Hojabri, A. Effect of nitrogen content on optical constants of copper nitride thin films prepared by DC magnetron reactive sputtering. J. Theor. Appl. Phys. 2009, 3, 37–41. [Google Scholar]
- Sahoo, G.; Meher, S.R.; Jain, M.K. Band gap variation in copper nitride thin films. In Proceedings of the International Conference on Advanced Nanomaterials & Emerging Engineering Technologies, Chennai, India, 24–26 July 2013; pp. 540–542. [Google Scholar]
- Chinnaiah, K.; Maik, V.; Kannan, K.; Potemkin, V.; Grishina, M.; Gohulkumar, M.; Tiwari, R.; Gurushankar, K. Experimental and Theoretical Studies of Green Synthesized Cu2O Nanoparticles Using Datura Metel L. J. Fluoresc. 2022, 32, 559–568. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Bondarchuk, I.S.; Losytskyy, M.Y. Surface plasmon enhanced photoluminescence from copper nanoparticles: Influence of temperature. J. Appl. Phys. 2014, 116, 054309. [Google Scholar] [CrossRef]
- Li, J.; Mei, Z.; Liu, L.; Liang, H.; Azarov, A.; Kuznetsov, A.; Liu, Y.; Ji, A.; Meng, Q.; Du, X. Probing Defects in Nitrogen-Doped Cu2O. Sci. Rep. 2014, 4, 7240. [Google Scholar] [CrossRef]
- Basavalingaiah, K.R.; Udayabhanu; Harishkumar, S.; Nagaraju, G. Synthesis of Cu2O/Ag Composite Nanocubes with Promising Photoluminescence and Photodegradation Activity over Methylene Blue Dye. Adv. Mater. Lett. 2019, 10, 832–838. [Google Scholar] [CrossRef]
- Cheng, Z.; Qi, W.; Pang, C.H.; Thomas, T.; Wu, T.; Liu, S.; Yang, M. Recent Advances in Transition Metal Nitride-Based Materials for Photocatalytic Applications. Adv. Funct. Mater. 2021, 31, 2100553. [Google Scholar] [CrossRef]
- Li, H.-B.; Xie, X.; Wang, W.; Cheng, Y.; Wang, W.-H.; Li, L.; Liu, H.; Wen, G.; Zheng, R. Room-temperature ferromagnetism in nanocrystalline Cu/Cu2O core-shell structures prepared by magnetron sputtering. APL Mater. 2013, 1, 042106. [Google Scholar] [CrossRef]
- Wang, L.-C.; Liu, B.-H.; Su, C.-Y.; Liu, W.-S.; Kei, C.-C.; Wang, K.-W.; Perng, T.-P. Electronic Band Structure and Electrocatalytic Performance of Cu3N Nanocrystals. ACS Appl. Nano Mater. 2018, 1, 3673–3681. [Google Scholar] [CrossRef]
- Deuermeier, J.; Liu, H.; Rapenne, L.; Calmeiro, T.; Renou, G.; Martins, R.; Muñoz-Rojas, D.; Fortunato, E. Visualization of nanocrystalline CuO in the grain boundaries of Cu2O thin films and effect on band bending and film resistivity. APL Mater. 2018, 6, 096103. [Google Scholar] [CrossRef]
- Kumar, V.; O’Donnell, S.C.; Sang, D.L.; Maggard, P.A.; Wang, G. Harnessing Plasmon-Induced Hot Carriers at the Interfaces With Ferroelectrics. Front Chem 2019, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.; Zhu, S.-S.; Liu, Y.-Y.; Zhang, X.; Wang, J.-J.; Braun, A. Covalent S-O Bonding Enables Enhanced Photoelectrochemical Performance of Cu2S/Fe2O3 Heterojunction for Water Splitting. Small 2021, 17, 2100320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, S.-Y.; Zou, Y.; Li, T.-T.; Liu, H.; Wang, J.-J. Enhanced charge separation and conductivity of hematite enabled by versatile NiSe2 nanoparticles for improved photoelectrochemical water oxidation. Appl. Mater. Today 2022, 28, 101552. [Google Scholar] [CrossRef]
- Yuan, S.-Y.; Jiang, L.-W.; Hu, J.-S.; Liu, H.; Wang, J.-J. Fully Dispersed IrOx Atomic Clusters Enable Record Photoelectrochemical Water Oxidation of Hematite in Acidic Media. Nano Lett. 2023, 23, 2354–2361. [Google Scholar] [CrossRef]
- Mrunal, V.K.; Vishnu, A.K.; Momin, N.; Manjanna, J. Cu2O nanoparticles for adsorption and photocatalytic degradation of methylene blue dye from aqueous medium. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100265. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Shanker, V. Surface plasmon resonance-induced photocatalysis by Au nanoparticles decorated mesoporous g-C3N4 nanosheets under direct sunlight irradiation. Mater. Res. Bull. 2016, 75, 51–58. [Google Scholar] [CrossRef]
- Yunus, N.N.; Hamzah, F.; So’aib, M.S.; Krishnan, J. Effect of Catalyst Loading on Photocatalytic Degradation of Phenol by Using N, S Co-doped TiO2. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012092. [Google Scholar] [CrossRef]
- Khataee, A.; Darvishi Cheshmeh Soltani, R.; Hanifehpour, Y.; Safarpour, M.; Gholipour Ranjbar, H.; Joo, S.W. Synthesis and Characterization of Dysprosium-Doped ZnO Nanoparticles for Photocatalysis of a Textile Dye under Visible Light Irradiation. Ind. Eng. Chem. Res. 2014, 53, 1924–1932. [Google Scholar] [CrossRef]
- Soltani, N.; Saion, E.; Hussein, M.Z.; Erfani, M.; Abedini, A.; Bahmanrokh, G.; Navasery, M.; Vaziri, P. Visible Light-Induced Degradation of Methylene Blue in the Presence of Photocatalytic ZnS and CdS Nanoparticles. Int. J. Mol. Sci. 2012, 13, 12242–12258. [Google Scholar] [CrossRef]
- Uma, B.; Anantharaju, K.S.; Malini, S.S.; More, S.; Vidya, Y.S.; Meena, S.; Surendra, B.S. Synthesis of novel heterostructured Fe-doped Cu2O/CuO/Cu nanocomposite: Enhanced sunlight driven photocatalytic activity, antibacterial and supercapacitor properties. Ceram. Int. 2022, 48, 35834–35847. [Google Scholar] [CrossRef]
- Karthik, K.V.; Raghu, A.V.; Reddy, K.R.; Ravishankar, R.; Sangeeta, M.; Shetti, N.P.; Reddy, C.V. Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere 2022, 287, 132081. [Google Scholar] [CrossRef]
- Schöttle, C.; Bockstaller, P.; Gerthsen, D.; Feldmann, C. Tungsten nanoparticles from liquid-ammonia-based synthesis. Chem. Commun. 2014, 50, 4547–4550. [Google Scholar] [CrossRef]
- Kibria, M.G.; Chowdhury, F.A.; Zhao, S.; AlOtaibi, B.; Trudeau, M.L.; Guo, H.; Mi, Z. Visible light-driven efficient overall water splitting using p-type metal-nitride nanowire arrays. Nat. Commun. 2015, 6, 6797. [Google Scholar] [CrossRef]
- Stevanovic, A.; Büttner, M.; Zhang, Z.; Yates, J.T., Jr. Photoluminescence of TiO2: Effect of UV Light and Adsorbed Molecules on Surface Band Structure. J. Am. Chem. Soc. 2012, 134, 324–332. [Google Scholar] [CrossRef]


| Cu-3 | Cu-6 | Cu-12 | Cu-24 | |||||
|---|---|---|---|---|---|---|---|---|
| Crystalline Phase | Crystallite Size (nm) | Crystalline Phase (%) | Crystallite Size (nm) | Crystalline Phase (%) | Crystallite Size (nm) | Crystalline Phase (%) | Crystallite Size (nm) | Crystalline Phase (%) |
| Cu | 50 | 32.1 | 55.7 | 44.6 | 54 | 90.3 | 71 | 100 |
| Cu2O | 14.8 | 49.5 | 26.7 | 54.3 | 30 | 5.5 | - | - |
| Cu3N | 2.8 | 18 | 30 | 1.1 | 29 | 4.2 | - | - |
| Sample | Pseudo-Second-Order | ||
|---|---|---|---|
| Qe,fitted (mg/g) | K2 (g/mg min) | R2 | |
| Cu-3 | 1.3089 | 0.0296 | 0.9783 |
| Cu-6 | 1.4080 | 0.0174 | 0.9361 |
| Cu-12 | 1.1629 | 0.0588 | 0.9821 |
| Cu-24 | 0.9862 | 0.0431 | 0.9920 |
| Catalyst | Dye | Light Source | Initial Concentration | Catalyst Dosage | Degradation Rate (%) | Ref. |
|---|---|---|---|---|---|---|
| CuO-Cu2O | MB Methylene Orange | 150 W metal halide lamp | 5 mg/L | 0.2 g | 80 | [8] |
| 50 | ||||||
| CuO | Aniline | UV-C lamp | 50 mg/L | 0.01–0.1 g | 90 | [12] |
| Cu2O | Fluroxypyr | 500 W metal halide lamp | 11.17 mg/L | 0.1 g | 83 | [13] |
| Cu3N | MB | Solar simulator | 20 mg/L | 0.1 g | 61 | [14] |
| Methylene Orange | 89 | |||||
| Au-Cu3N | MB | 250 W UV lamp | 25 mg/L | 15 mg | 84 | [25] |
| Cu2O/CuO/Cu | MB | Sun light | 20 mg/L | 40 mg | 65 | [62] |
| Rhodamine B | 60 | |||||
| Cu-ZnO | Methylene Orange | 30 W UV-light lamp | 10 mg/L | 25 mg | 91 | [63] |
| Indigo Carmine | 92 | |||||
| Rhodamine B | 90 | |||||
| Cu-3 | MB | Sun light | 5 mg/L | 5 mg | 96 | Present study |
| Cu-6 | 95 | |||||
| Cu-12 | 93 | |||||
| Cu-24 | 92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paredes, P.; Rauwel, E.; Wragg, D.S.; Rapenne, L.; Estephan, E.; Volobujeva, O.; Rauwel, P. Sunlight-Driven Photocatalytic Degradation of Methylene Blue with Facile One-Step Synthesized Cu-Cu2O-Cu3N Nanoparticle Mixtures. Nanomaterials 2023, 13, 1311. https://doi.org/10.3390/nano13081311
Paredes P, Rauwel E, Wragg DS, Rapenne L, Estephan E, Volobujeva O, Rauwel P. Sunlight-Driven Photocatalytic Degradation of Methylene Blue with Facile One-Step Synthesized Cu-Cu2O-Cu3N Nanoparticle Mixtures. Nanomaterials. 2023; 13(8):1311. https://doi.org/10.3390/nano13081311
Chicago/Turabian StyleParedes, Patricio, Erwan Rauwel, David S. Wragg, Laetitia Rapenne, Elias Estephan, Olga Volobujeva, and Protima Rauwel. 2023. "Sunlight-Driven Photocatalytic Degradation of Methylene Blue with Facile One-Step Synthesized Cu-Cu2O-Cu3N Nanoparticle Mixtures" Nanomaterials 13, no. 8: 1311. https://doi.org/10.3390/nano13081311
APA StyleParedes, P., Rauwel, E., Wragg, D. S., Rapenne, L., Estephan, E., Volobujeva, O., & Rauwel, P. (2023). Sunlight-Driven Photocatalytic Degradation of Methylene Blue with Facile One-Step Synthesized Cu-Cu2O-Cu3N Nanoparticle Mixtures. Nanomaterials, 13(8), 1311. https://doi.org/10.3390/nano13081311