Synthesis of Ni-Doped Tremolite Fibers to Help Clarify the Aetiology of the Cytotoxic Outcome of Asbestos
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrothermal Synthesis of Ni-Doped Tremolite
2.2. Characterization of Ni-Doped Tremolite Fibers
3. Results and Discussion
3.1. PXRD Characterization
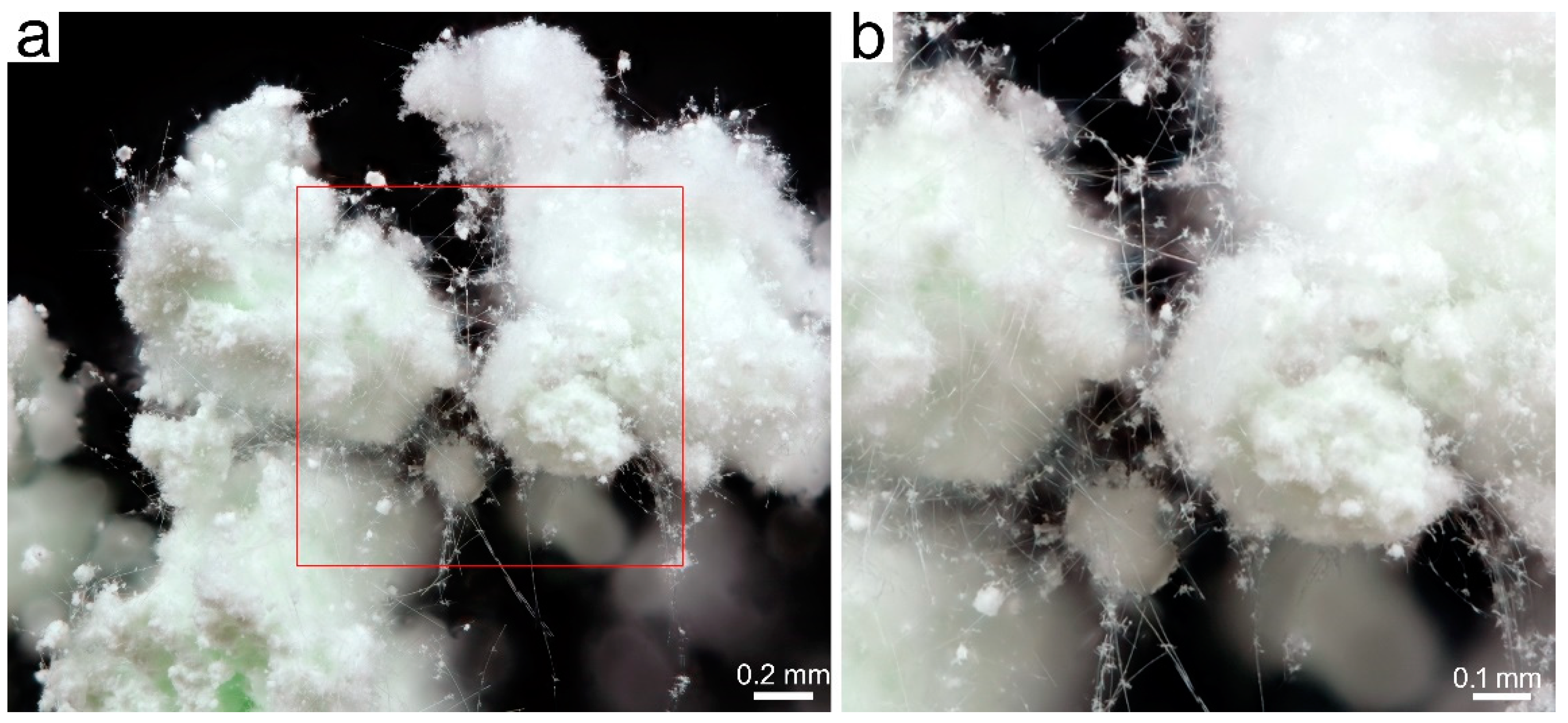
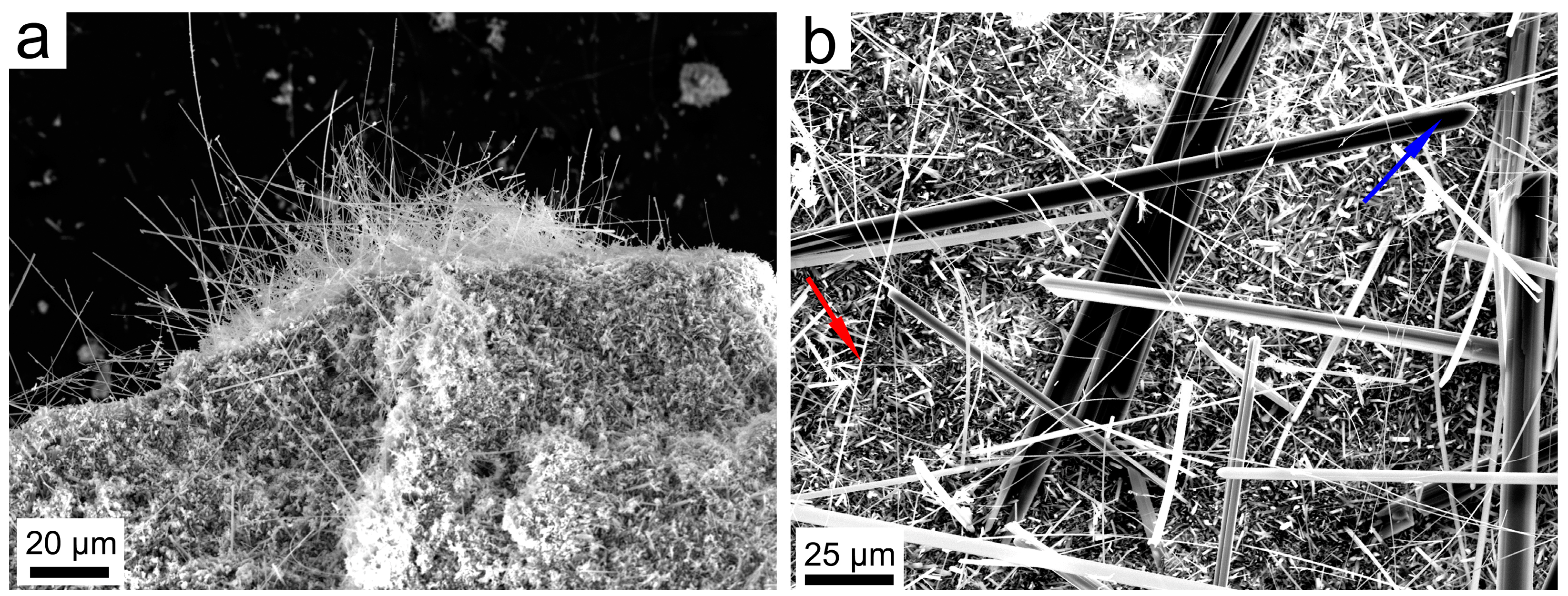
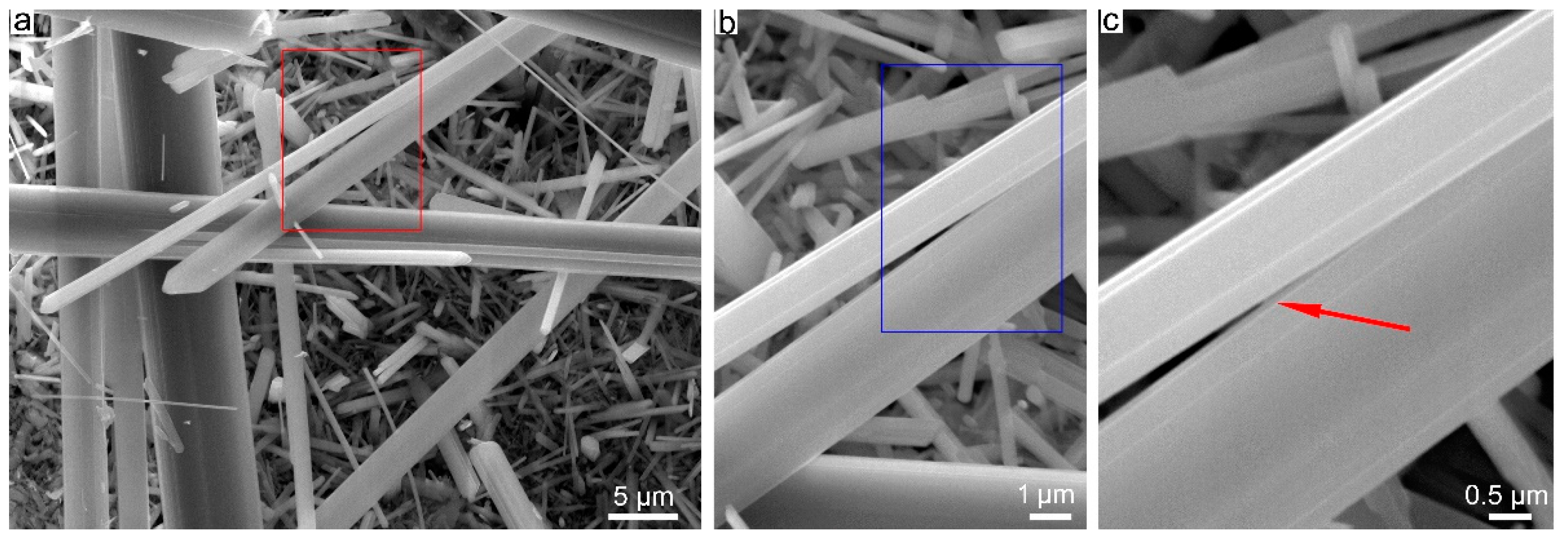
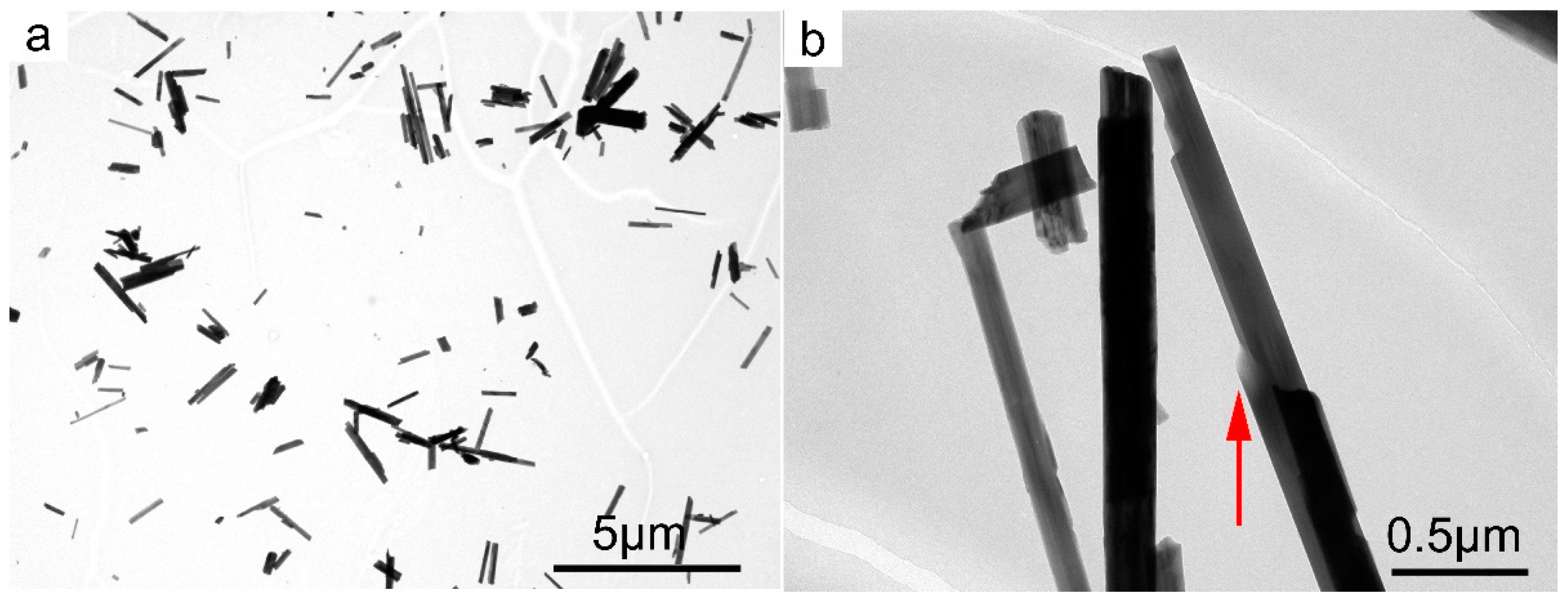
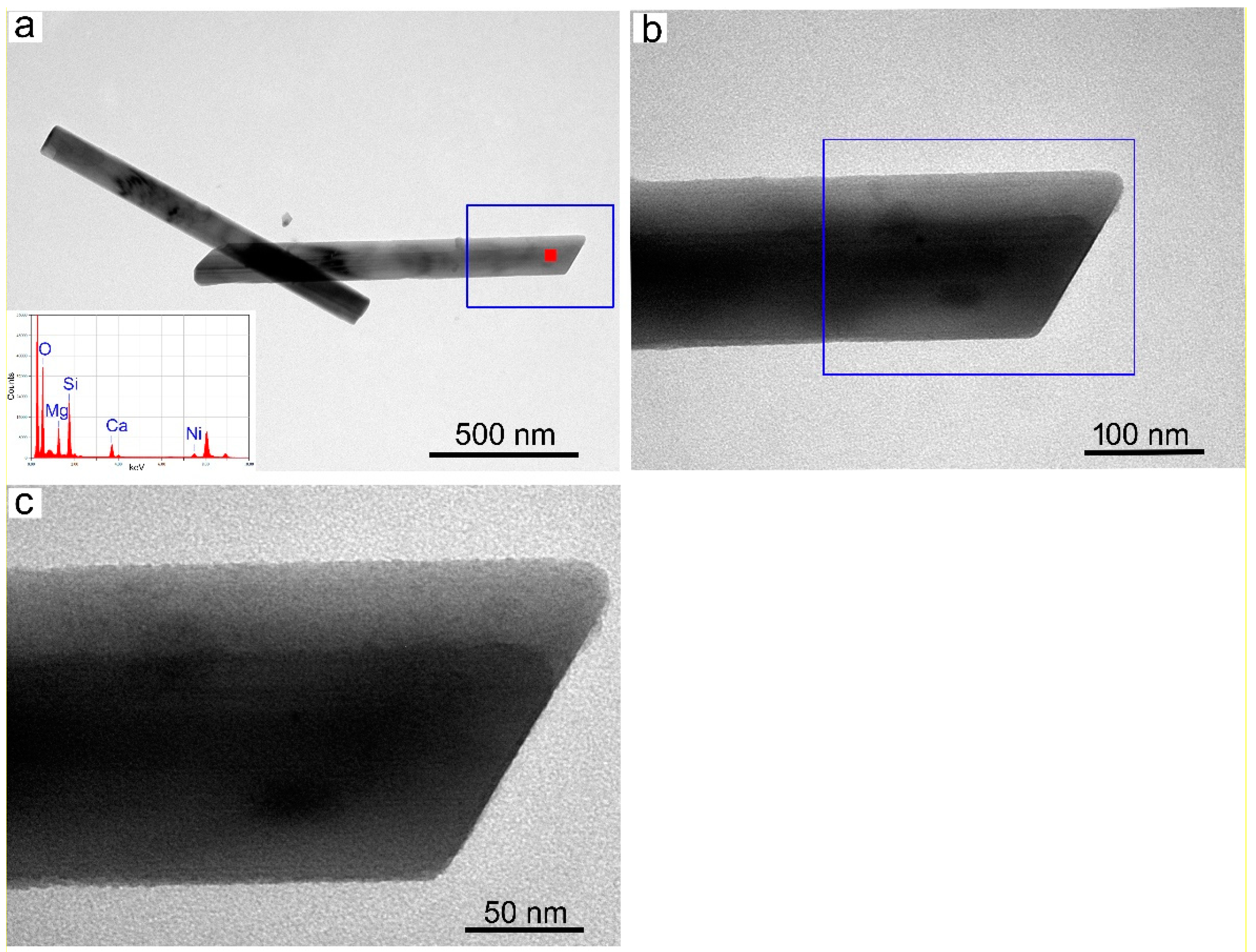
3.2. SBM/MOCF/SEM/TEM/EDS Characterization
3.3. EPMA Characterization
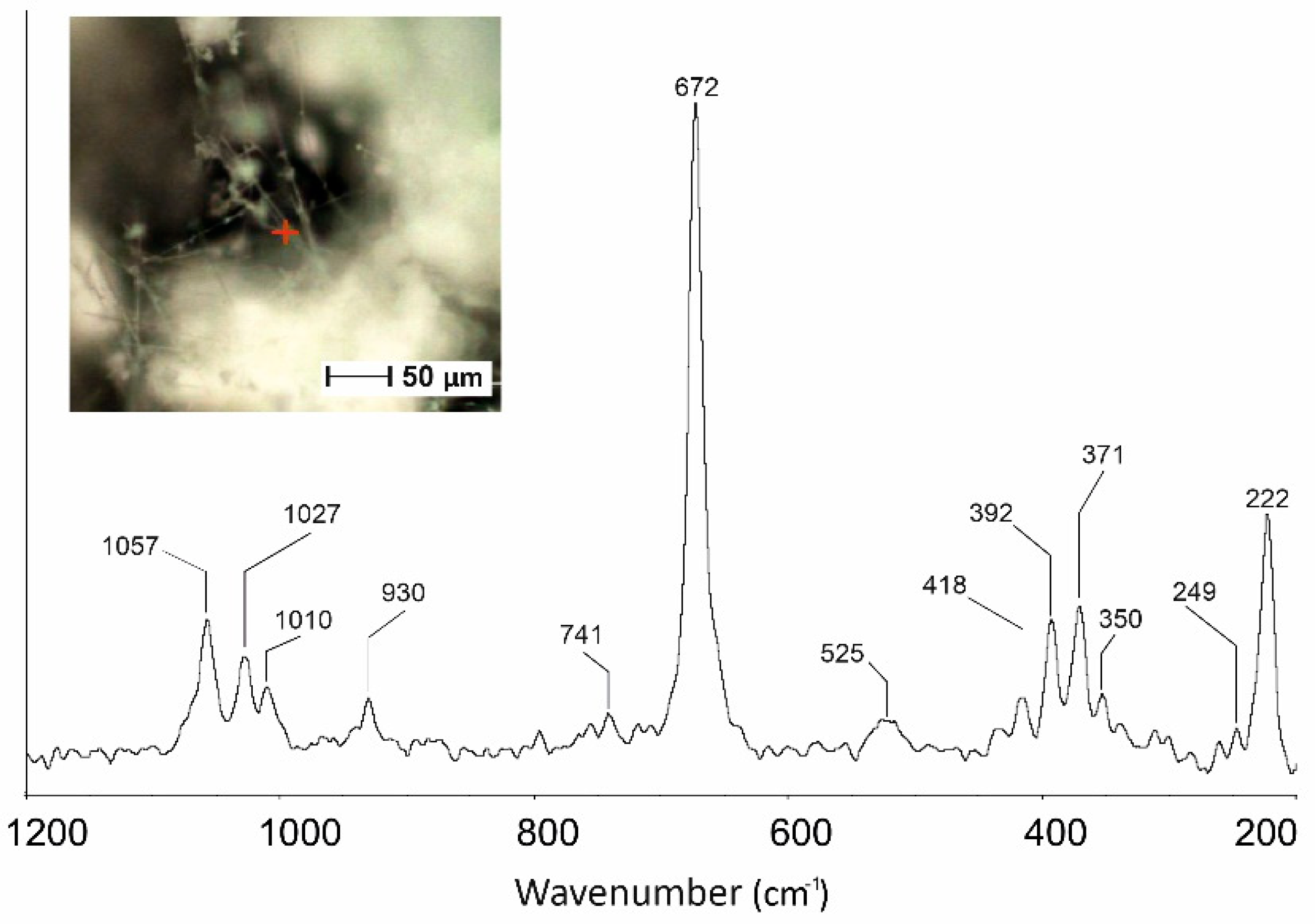
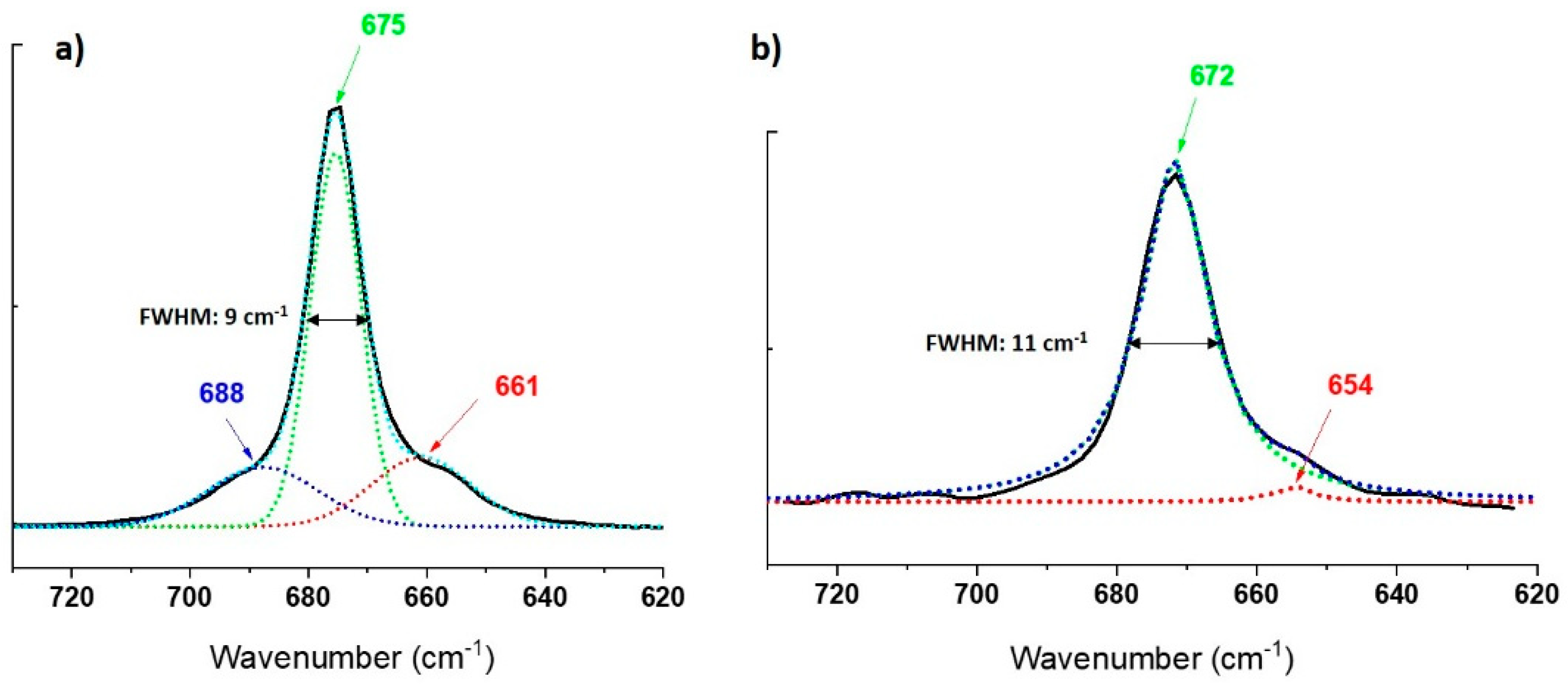
3.4. µ-Raman Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloise, A.; Kusiorowski, R.; Lassinantti Gualtieri, M.; Gualtieri, A.F. Thermal Behaviour of Mineral Fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; Chapter 7; ISBN 9780903056656. [Google Scholar]
- Ross, M.; Nolan, R.P. History of Asbestos Discovery and Use and Asbestos-Related Disease in Context with the Occurrence of Asbestos within Ophiolite Complexes. In Ophiolite Concept and the Evolution of Geological Thought; Dilek, Y., Newcomb, S., Eds.; Geological Society of America: Boulder, CO, USA, 2003; Volume 373, ISBN 9780813723730. [Google Scholar]
- Wylie, A.G.; Bailey, K.F.; Kelse, J.W.; Lee, R.J. The Importance of Width in Asbestos Fiber Carcinogenicity and Its Implications for Public Policy. Am. Ind. Hyg. Assoc. J. 1993, 54, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Korchevskiy, A.A.; Wylie, A.G. Dimensional Characteristics of the Major Types of Amphibole Mineral Particles and the Implications for Carcinogenic Risk Assessment. Inhal. Toxicol. 2022, 34, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.M. The Health Effects of Short Fiber Chrysotile and Amphibole Asbestos. Crit. Rev. Toxicol. 2022, 52, 89–112. [Google Scholar] [CrossRef]
- Kamp, D.W. Asbestos-Induced Lung Diseases: An Update. Transl. Res. 2009, 153, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Fubini, B.; Mollo, L.; Giamello, E. Free Radical Generation at the Solid/Liquid Interface in Iron Containing Minerals. Free Radic. Res. 1995, 23, 593–614. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.F.; Miller, E.; May, M.; Tegeris, A.; Morgan, E. Relation of Particle Dimension to Carcinogenicity in Amphibole Asbestoses and Other Fibrous Minerals. J. Natl. Cancer Inst. 1981, 67, 965–975. [Google Scholar] [CrossRef]
- Turci, F.; Tomatis, M.; Pacella, A. Surface and Bulk Properties of Mineral Fibres Relevant to Toxicity. Eur. Mineral. Union Notes Mineral. 2017, 18, 171–214. [Google Scholar] [CrossRef]
- Bloise, A.; Barca, D.; Gualtieri, A.F.; Pollastri, S.; Belluso, E. Trace Elements in Hazardous Mineral Fibres. Environ. Pollut. 2016, 216, 314–323. [Google Scholar] [CrossRef]
- Liang, F.C.; Kuo, C.C.; Chen, B.Y.; Cho, C.J.; Hung, C.C.; Chen, W.C.; Borsali, R. RGB-switchable porous electrospun nanofiber chemoprobe-filter prepared from multifunctional copolymers for versatile sensing of pH and heavy metals. ACS Appl. Mater. Interfaces 2017, 9, 16381–16396. [Google Scholar] [CrossRef]
- Bloise, A.; Critelli, T.; Catalano, M.; Apollaro, C.; Miriello, D.; Croce, A.; Barrese, E.; Liberi, F.; Piluso, E.; Rinaudo, C.; et al. Asbestos and other fibrous minerals contained in the serpentinites of the Gimigliano-Mount Reventino Unit (Calabria, S-Italy). Environ. Earth Sci. 2014, 71, 3773–3786. [Google Scholar] [CrossRef]
- Kuroda, A. Recent progress and perspectives on the mechanisms underlying Asbestos toxicity. Genes Environ. 2021, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schoonen, M.A.A.; Cohn, C.A.; Roemer, E.; Laffers, R.; Simon, S.R.; O’Riordan, T. Mineral-Induced Formation of Reactive Oxygen Species. Rev. Mineral. Geochem. 2006, 64, 179–221. [Google Scholar] [CrossRef]
- Mossman, B.T.; Gualtieri, A.F. Lung cancer: Mechanisms of carcinogenesis by asbestos. In Occupational Cancers; Springer: Cham, Switzerland, 2020; pp. 239–256. [Google Scholar] [CrossRef]
- Pacella, A.; Tomatis, M.; Viti, C.; Bloise, A.; Arrizza, L.; Ballirano, P.; Turci, F. Thermal Inertization of Amphibole Asbestos Modulates Fe Topochemistry and Surface Reactivity. J. Hazard. Mater. 2020, 398, 123119. [Google Scholar] [CrossRef] [PubMed]
- Bloise, A.; Fornero, E.; Belluso, E.; Barrese, E.; Rinaudo, C. Synthesis and Characterization of Tremolite Asbestos Fibres. Eur. J. Mineral. 2008, 20, 1027–1033. [Google Scholar] [CrossRef]
- Pugnaloni, A.; Giantomassi, F.; Lucarini, G.; Capella, S.; Bloise, A.; Di Primio, R.; Belluso, E. Cytotoxicity Induced by Exposure to Natural and Synthetic Tremolite Asbestos: An in Vitro Pilot Study. Acta Histochem. 2013, 115, 100–112. [Google Scholar] [CrossRef]
- Ballirano, P.; Bloise, A.; Gualtieri, A.F.; Lezzerini, M.; Pacella, A.; Perchiazzi, N.; Dogan, M.; Dogan, A.U. The Crystal Structure of Mineral Fibres. Eur. Mineral. Union Notes Mineral. 2017, 18, 17–64. [Google Scholar] [CrossRef]
- Bowes, D.R.; Farrow, C.M. Major and Trace Element Compositions of the UICC Standard Asbestos Samples. Am. J. Ind. Med. 1997, 32, 592–594. [Google Scholar] [CrossRef]
- Bloise, A.; Catalano, M.; Barrese, E.; Gualtieri, A.F.; Bursi Gandolfi, N.; Capella, S.; Belluso, E. TG/DSC Study of the Thermal Behaviour of Hazardous Mineral Fibres. J. Therm. Anal. Calorim. 2016, 123, 2225–2239. [Google Scholar] [CrossRef]
- Oller, A.R.; Costa, M.; Oberdörster, G. Carcinogenicity Assessment of Selected Nickel Compounds. Toxicol. Appl. Pharmacol. 1997, 143, 152–166. [Google Scholar] [CrossRef]
- Bloise, A.; Belluso, E.; Fornero, E.; Rinaudo, C.; Barrese, E.; Capella, S. Influence of Synthesis Conditions on Growth of Ni-Doped Chrysotile. Microporous Mesoporous Mater. 2010, 132, 239–245. [Google Scholar] [CrossRef]
- Caicedo, M.; Jacobs, J.J.; Reddy, A.; Hallab, N.J. Analysis of Metal Ion-Induced DNA Damage, Apoptosis, and Necrosis in Human (Jurkat) T-Cells Demonstrates Ni2+ and V3+ Are More Toxic than Other Metals: Al3+, Be2+, Co2+, Cr 3+, Cu2+, Fe3+, Mo5+, Nb 5+, Zr2+. J. Biomed. Mater. Res.-Part A 2008, 86, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Hiraku, Y.; Oikawa, S. Mechanism of Guanine-Specific DNA Damage by Oxidative Stress and Its Role in Carcinogenesis and Aging. Mutat. Res.-Rev. Mutat. Res. 2001, 488, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Nishio, K.; Fujita, K.; Kato, H.; Nakamura, A.; Kinugasa, S.; Endoh, S.; Miyauchi, A.; Yamamoto, K.; Murayama, H.; et al. Ultrafine NiO Particles Induce Cytotoxicity in Vitro by Cellular Uptake and Subsequent Ni(II) Release. Chem. Res. Toxicol. 2009, 22, 1415–1426. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Nackerdien, Z.; Chao, B.C.; Gajewski, E.; Rao, G. Chemical Nature of in Vivo DNA Base Damage in Hydrogen Peroxide-Treated Mammalian Cells. Arch. Biochem. Biophys. 1991, 285, 388–390. [Google Scholar] [CrossRef]
- Kelleher, P.; Pacheco, K.; Newman, L.S. Inorganic Dust Pneumonias: The Metal-Related Parenchymal Disorders. Environ. Health Perspect. 2000, 108, 685–696. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt Toxicity in Humans—A Review of the Potential Sources and Systemic Health Effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Perez, J.E.; Contreras, M.F.; Vilanova, E.; Felix, L.P.; Margineanu, M.B.; Luongo, G.; Porter, A.E.; Dunlop, I.E.; Ravasi, T.; Kosel, J. Cytotoxicity and intracellular dissolution of nickel nanowires. Nanotoxicology 2016, 10, 871–880. [Google Scholar] [CrossRef]
- McMillen, C.D.; Kolis, J.W. Hydrothermal Synthesis as a Route to Mineralogically-Inspired Structures. Dalt. Trans. 2016, 45, 2772–2784. [Google Scholar] [CrossRef]
- Modan, E.M.; Plaiasu, A.G. Advantages and Disadvantages of Chemical Methods in the Elaboration of Nanomaterials. Ann. “Dunarea Jos” Univ. Galati. Fascicle IX Metall. Mater. Sci. 2020, 43, 53–60. [Google Scholar] [CrossRef]
- Della Ventura, G.; Hawthorne, F.C.; Robert, J.-L.; Iezzi, G. Synthesis and Infrared Spectroscopy of Amphiboles along the Tremolite-Pargasite Join. Eur. J. Mineral. 2003, 15, 341–347. [Google Scholar] [CrossRef]
- Rinaudo, C.; Belluso, E.; Gastaldi, D. Assessment of the Use of Raman Spectroscopy for the Determination of Amphibole Asbestos. Mineral. Mag. 2004, 68, 455–465. [Google Scholar] [CrossRef]
- Bozhilov, K.N.; Jenkins, D.M.; Veblen, D.R. Pyribole Evolution during Tremolite Synthesis from Oxides. Am. Mineral. 2004, 89, 74–84. [Google Scholar] [CrossRef]
- Jenkins, D.M. Synthesis and Characterization of Tremolite in the System H2O-CaO-MgO-SiO2. Am. Mineral. 1987, 72, 707–715. [Google Scholar]
- Hennessey, J.V.; Chromiak, J.A.; Dellaventura, S.; Guertin, J.; Maclean, D.B. Increase in Percutaneous Muscle Biopsy Yield with a Suction-Enhancement Technique. J. Appl. Physiol. 1997, 82, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Bloise, A.; Pingitore, V.; Miriello, D.; Apollaro, C.; Armentano, D.; Barrese, E.; Oliva, A. Flux Growth and Characterization of Ti- and Ni-Doped Enstatite Single Crystals. J. Cryst. Growth 2011, 329, 86–91. [Google Scholar] [CrossRef]
- Belluso, E.; Cavallo, A.; Halterman, D. Crystal Habit of Mineral Fibres. Eur. Mineral. Union Notes Mineral. 2017, 18, 65–109. [Google Scholar] [CrossRef]
- Maresch, W.V.; Czank, M.; Schreyer, W. Growth Mechanisms, Structural Defects and Composition of Synthetic Tremolite: What Are the Effects on Macroscopic Properties? Contrib. Mineral. Petrol. 1994, 118, 297–313. [Google Scholar] [CrossRef]
- Zimmermann, R.; Heinrich, W.; Rranz, G. Tremolite Synthesis from CaCl2-Bearing Aqueous Solutions. Eur. J. Mineral. 1996, 8, 767–776. [Google Scholar] [CrossRef]
- Müller, W.F.; Schmädicke, E.; Okrusch, M.; Schüssler, U. Intergrowths between Anthophyllite, Gedrite, Calcic Amphibole, Cummingtonite, Talc and Chlorite in a Metamorphosed Ultramafic Rock of the KTB Pilot Hole, Bavaria. Eur. J. Mineral. 2003, 15, 295–307. [Google Scholar] [CrossRef]
- Gottschalk, M.; Andrut, M.; Melzer, S. The Determination of the Cummingtonite Content of Synthetic Tremolite. Eur. J. Mineral. 1999, 11, 967–982. [Google Scholar] [CrossRef]
- Ahn, J.H.; Cho, M.; Jenkins, D.M.; Buseck, P.R. Structural defects in synthetic tremolitic amphiboles. Am. Mineral. 1991, 76, 1811–1823. [Google Scholar]
- Gualtieri, A.F. Journey to the Centre of the Lung. The Perspective of a Mineralogist on the Carcinogenic Effects of Mineral Fibres in the Lungs. J. Hazard. Mater. 2023, 442, 130077. [Google Scholar] [CrossRef] [PubMed]
- IPCS Environmental Health Criteria 53. Asbestos and Other Natural Mineral Fibres; World Health Organization: Long Beach, CA, USA, 1986; pp. 187–196.
- Harper, M.; Lee, E.G.; Slaven, J.E.; Bartley, D.L. An Inter-Laboratory Study to Determine the Effectiveness of Procedures for Discriminating Amphibole Asbestos Fibers from Amphibole Cleavage Fragments in Fiber Counting by Phase-Contrast Microscopy. Ann. Occup. Hyg. 2012, 56, 645–659. [Google Scholar] [CrossRef]
- Harper, M.; Lee, E.G.; Doorn, S.S.; Hammond, O. Differentiating Non-Asbestiform Amphibole and Amphibole Asbestos by Size Characteristics. J. Occup. Environ. Hyg. 2008, 5, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Tindle, A.G.; Webb, P.C. Probe-AMPH—A Spreadsheet Program to Classify Microprobe-Derived Amphibole Analyses. Comput. Geosci. 1994, 20, 1201–1228. [Google Scholar] [CrossRef]
- Bozhilov, K.N.; Brownstein, D.; Jenkins, D.M. Biopyribole Evolution during Tremolite Synthesis from Dolomite and Quartz in CO2-H2O Fluid. Am. Mineral. 2007, 92, 898–908. [Google Scholar] [CrossRef]
- Pawley, A.R.; Graham, C.M.; Navrotsky, A. Tremolite-Richterite Amphiboles: Synthesis, Compositional and Structural Characterization, and Thermochemistry. Am. Mineral. 1993, 78, 23–35. [Google Scholar]
- Bloise, A.; Miriello, D. Multi-Analytical Approach for Identifying Asbestos Minerals in Situ. Geosciences 2018, 8, 133. [Google Scholar] [CrossRef]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T. Comparison of Raman Spectra in Characterizing Gibbsite, Bayerite, Diaspore and Boehmite. J. Raman Spectrosc. 2001, 32, 745–750. [Google Scholar] [CrossRef]
- Apopei, A.I.; Buzgar, N. The Raman study of amphiboles. Geol. Tomul. LVI 2010, 1, 91–95. [Google Scholar]


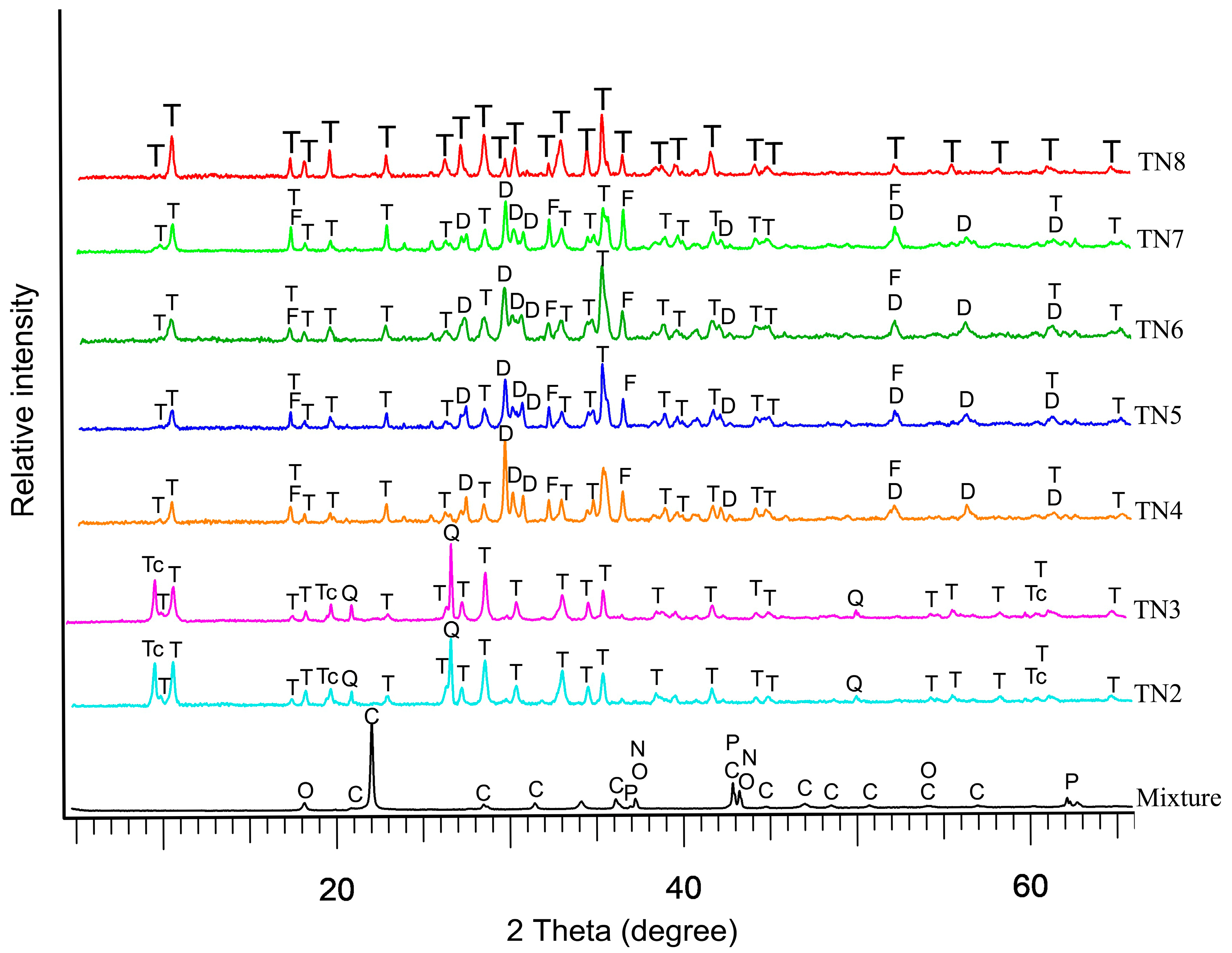
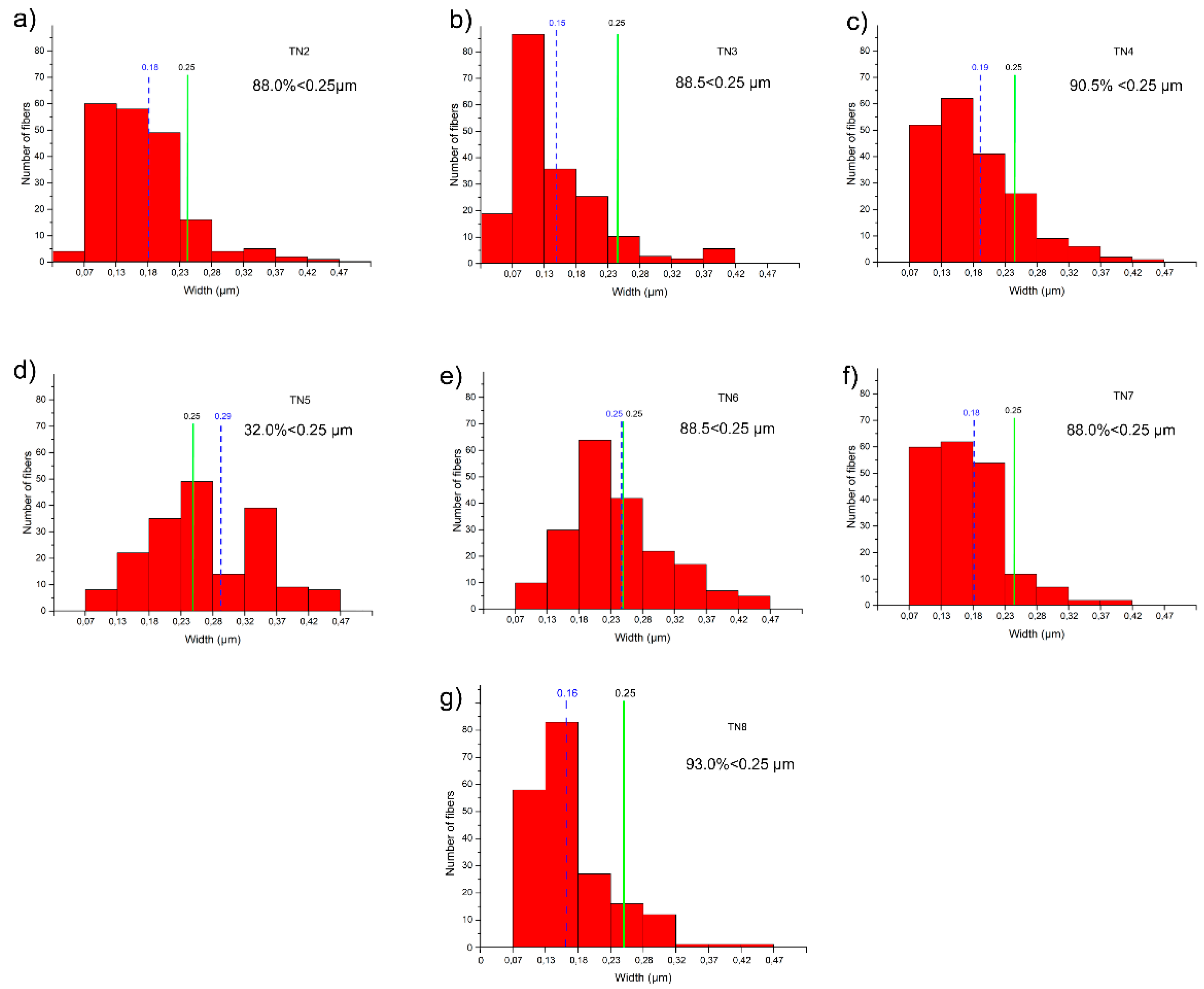
| Run | T (°C) | P (kbar) | t (Day) | H2O (wt.%) | Mineral Phases | Ni-Tr FWHM (110) (Δ°2θ) | Ni-Tr Peak Intensity (110) (cps) | Maximum * Length (µm) | Average ** Width (µm) |
|---|---|---|---|---|---|---|---|---|---|
| TN2 | 800 | 1.5 | 4 | 15 | Ni-Tlc > Ni-Tr > Qtz | 0.230 | 845 | 20 | 0.16 |
| TN3 | 800 | 1.5 | 8 | 15 | Ni-Tlc > Ni-Tr > Qtz | 0.229 | 751 | 30 | 0.15 |
| TN4 | 830 | 1.1 | 7 | 2 | Ni-Di > Ni-Tr >> Ni-Fo | 0.236 | 395 | 50 | 0.19 |
| TN5 | 780 | 1.3 | 10 | 8 | Ni-Tr > Ni-Di > Ni-Fo | 0.260 | 336 | 200 | 0.30 |
| TN6 | 780 | 1.3 | 10 | 4 | Ni-Tr > Ni-Di > Ni-Fo | 0.344 | 289 | 2500 | 0.25 |
| TN7 | 800 | 1.7 | 7 | 10 | Ni-Tr > Ni-Di > Ni-Fo | 0.227 | 447 | 1500 | 0.18 |
| TN8 | 800 | 2.4 | 16 | 8 | Ni-Tr | 0.267 | 717 | 5000 | 0.16 |
| Oxides | TN2 | TN3 | TN4 | TN5 | TN6 | TN7 | TN8 |
|---|---|---|---|---|---|---|---|
| SiO2 | 58.2 (0.5) | 56.2 (0.8) | 55.7 (1.1) | 56.9 (0.4) | 55.2 (0.3) | 56.3 (0.4) | 58.8 (0.4) |
| MgO | 22.1 (0.4) | 18.4 (1.3) | 19.9 (1.4) | 21.3 (0.9) | 21.9 (0.1) | 21.4 (0.9) | 21.5 (0.4) |
| CaO | 14.9 (0.5) | 14.3 (1.5) | 15.1 (0.6) | 17.6 (1.2) | 17.1 (0.1) | 18.7 (1.5) | 10.4 (0.6) |
| NiO | 4.8 (0.1) | 11.0 (0.9) | 9.4 (2.0) | 4.2 (0.2) | 5.9 (0.5) | 3.6 (0.4) | 9.1 (0.8) |
| CATIONS calculated on the basis of 23 oxygens | |||||||
| Si | 7.9 | 7.9 | 7.8 | 7.8 | 7.7 | 7.7 | 8.0 |
| Mg | 4.5 | 3.8 | 4.1 | 4.4 | 4.5 | 4.4 | 4.4 |
| Ca | 2.2 | 2.2 | 2.2 | 2.5 | 2.5 | 2.8 | 1.6 |
| Ni | 0.5 | 1.2 | 1.1 | 0.5 | 0.6 | 0.4 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloise, A.; Giorno, E.; Miriello, D.; Godbert, N. Synthesis of Ni-Doped Tremolite Fibers to Help Clarify the Aetiology of the Cytotoxic Outcome of Asbestos. Nanomaterials 2023, 13, 1303. https://doi.org/10.3390/nano13081303
Bloise A, Giorno E, Miriello D, Godbert N. Synthesis of Ni-Doped Tremolite Fibers to Help Clarify the Aetiology of the Cytotoxic Outcome of Asbestos. Nanomaterials. 2023; 13(8):1303. https://doi.org/10.3390/nano13081303
Chicago/Turabian StyleBloise, Andrea, Eugenia Giorno, Domenico Miriello, and Nicolas Godbert. 2023. "Synthesis of Ni-Doped Tremolite Fibers to Help Clarify the Aetiology of the Cytotoxic Outcome of Asbestos" Nanomaterials 13, no. 8: 1303. https://doi.org/10.3390/nano13081303
APA StyleBloise, A., Giorno, E., Miriello, D., & Godbert, N. (2023). Synthesis of Ni-Doped Tremolite Fibers to Help Clarify the Aetiology of the Cytotoxic Outcome of Asbestos. Nanomaterials, 13(8), 1303. https://doi.org/10.3390/nano13081303









