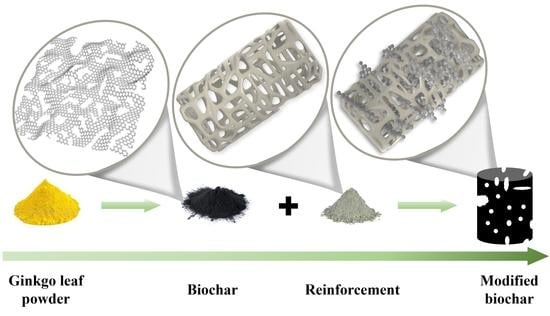
Inorganic Skeleton Reinforcement—A Generic Approach to Improve the Mechanical Properties of Biochar
Abstract
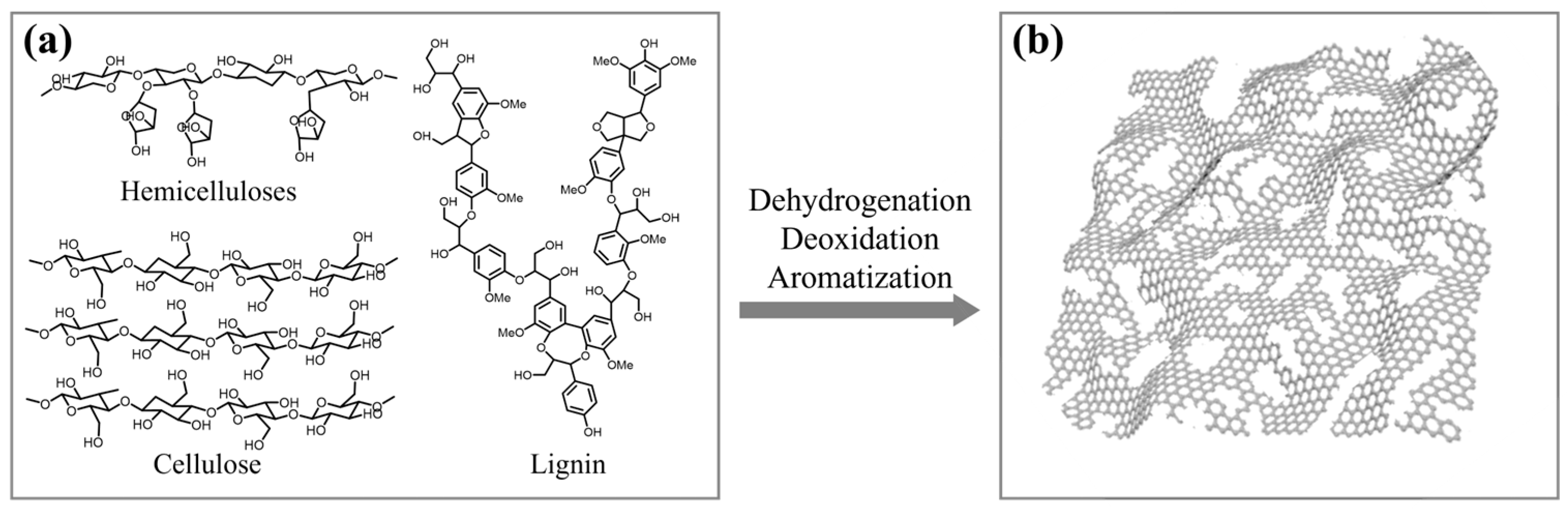
1. Introduction
2. Materials and Methods
2.1. Materials
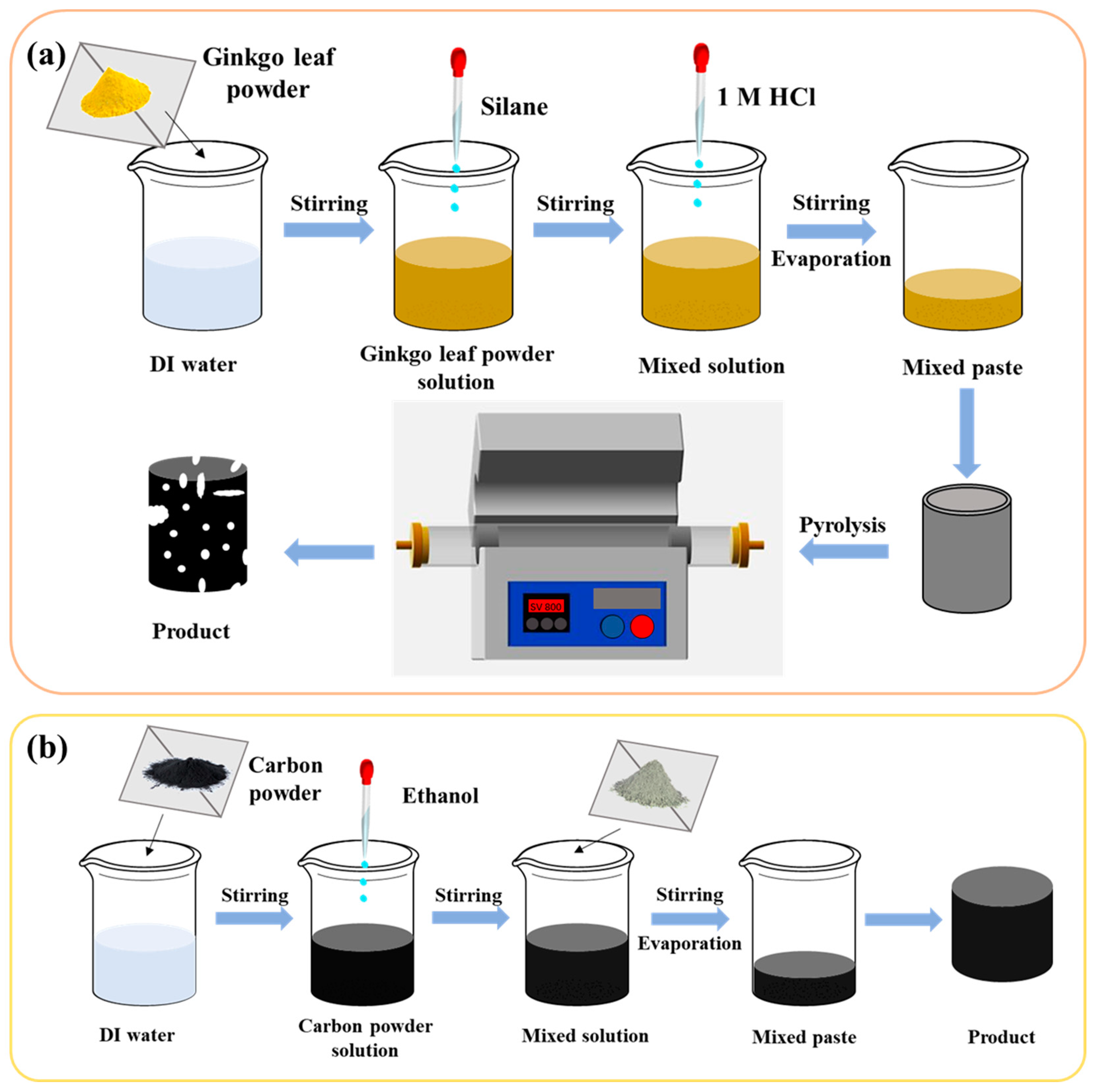
2.2. Synthesis of Ginkgo Leaf Biochar Modified by TEOS
2.3. Synthesis of Inorganic-Skeleton-Reinforced Bio-Carbon with Cement and Fly Ash
2.4. Materials’ Characterization
2.5. Pollutants’ Removal
2.6. Compressive Strength Test
3. Results and Discussions
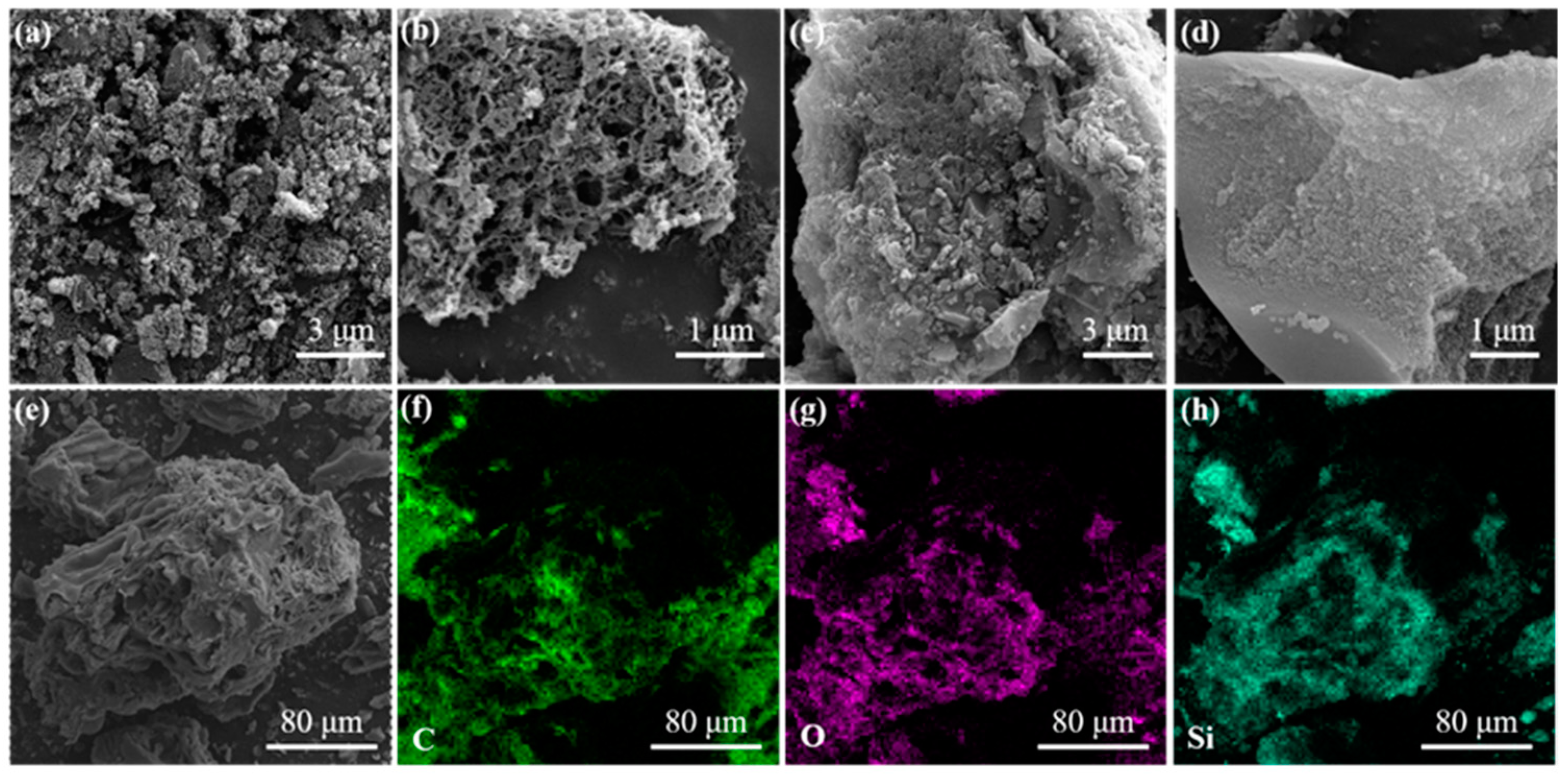
3.1. Characterization of GL-TEOS
3.2. Mechanical Properties of Modified Carbon Materials
3.3. Adsorption Performance of Modified Carbon Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Premarathna, K.S.D.; Rajapaksha, A.U.; Sarkar, B.; Kwon, E.E.; Bhatnagar, A.; Ok, Y.S.; Vithanage, M. Biochar-based engineered composites for sorptive decontamination of water: A review. Chem. Eng. J. 2019, 372, 536–550. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Chen, L.; Fang, W.; Liang, J.; Nabi, M.; Cai, Y.; Wang, Q.; Zhang, P.; Zhang, G. Biochar application in anaerobic digestion: Performances, mechanisms, environmental assessment and circular economy. Resour. Conserv. Recycl. 2023, 188, 106720–106733. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.W.; Kwon, E.E.; Lee, J.; Wang, C.H. A critical review on sustainable biochar system through gasification: Energy and environmental applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Mashhadimoslem, H.; Safarzadeh, M.; Ghaemi, A.; Banna Motejadded Emrooz, H.; Barzegar, M. Biomass derived hierarchical porous carbon for high-performance O2/N2 adsorption; a new green self-activation approach. RSC Adv. 2021, 11, 36125–36142. [Google Scholar] [CrossRef]
- Xie, Z.; Shang, X.; Xu, K.; Yang, J.; Hu, B.; Nie, P.; Jiang, W.; Liu, J. Facile Synthesis of In Situ Graphitic-N Doped Porous Carbon Derived from Ginkgo Leaf for Fast Capacitive Deionization. J. Electrochem. Soc. 2019, 166, E240–E247. [Google Scholar] [CrossRef]
- Waqas, M.; Aburiazaiza, A.S.; Miandad, R.; Rehan, M.; Barakat, M.A.; Nizami, A.S. Development of biochar as fuel and catalyst in energy recovery technologies. J. Clean. Prod. 2018, 188, 477–488. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Luo, D.; Wang, L.; Nan, H.; Cao, Y.; Wang, H.; Kumar, T.V.; Wang, C. Phosphorus adsorption by functionalized biochar: A review. Environ. Chem. Lett. 2022, 21, 497–524. [Google Scholar] [CrossRef]
- Senthil, C.; Lee, C.W. Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew. Sust. Energ. Rev. 2021, 137, 110464–110498. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Liu, H.; Liu, H.; Tao, J.; Cui, M.H.; Zheng, Z.; Wen, D.; Zhan, X. FexN produced in pharmaceutical sludge biochar by endogenous Fe and exogenous N doping to enhance peroxymonosulfate activation for levofloxacin degradation. Water Res. 2022, 224, 119022–119035. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tang, L.; Pang, Y.; Zhou, Y.; Feng, H.; Ren, X.; Tang, J.; Wang, J.; Deng, L.; Shao, B. Non-radical oxidation by N,S,P co-doped biochar for persulfate activation: Different roles of exogenous P/S doping, and electron transfer path. J. Clean. Prod. 2022, 374, 133995–134006. [Google Scholar] [CrossRef]
- Qu, S.; Yuan, Y.; Yang, X.; Xu, H.; Mohamed, A.K.; Zhang, J.; Zhao, C.; Liu, L.; Wang, B.; Wang, X.; et al. Carbon defects in biochar facilitated nitrogen doping: The significant role of pyridinic nitrogen in peroxymonosulfate activation and ciprofloxacin degradation. Chem. Eng. J. 2022, 441, 135864–135875. [Google Scholar] [CrossRef]
- Wan, Z.; Xu, Z.; Sun, Y.; Zhang, Q.; Hou, D.; Gao, B.; Khan, E.; Graham, N.J.D.; Tsang, D.C.W. Stoichiometric carbocatalysis via epoxide-like C-S-O configuration on sulfur-doped biochar for environmental remediation. J. Hazard. Mater. 2022, 428, 128223–128234. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Zhang, X.; Cao, C.; Quan, G.; Wang, M.; Zimmerman, A.R.; Gao, B. Microwave-assisted pyrolysis derived biochar for volatile organic compounds treatment: Characteristics and adsorption performance. Bioresour. Technol. 2022, 355, 127274–127282. [Google Scholar] [CrossRef]
- González-Hourcade, M.; Simões dos Reis, G.; Grimm, A.; Dinh, V.M.; Lima, E.C.; Larsson, S.H.; Gentili, F.G. Microalgae biomass as a sustainable precursor to produce nitrogen-doped biochar for efficient removal of emerging pollutants from aqueous media. J. Clean. Prod. 2022, 348, 131280–131295. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Zhang, Y.; Tao, L.; Wang, T.; Zou, Y.; Wang, Y.; Chen, R.; Wang, S. Defect Engineering for Fuel-Cell Electrocatalysts. Adv. Mater. 2020, 32, e1907879. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Al-Meer, S.; Al-Saad, K.A. Eco-structured Adsorptive Removal of Tigecycline from Wastewater: Date Pits’ Biochar versus the Magnetic Biochar. Nanomaterials 2020, 11, 30. [Google Scholar] [CrossRef]
- Ma, R.; Xue, Y.; Ma, Q.; Chen, Y.; Yuan, S.; Fan, J. Recent Advances in Carbon-Based Materials for Adsorptive and Photocatalytic Antibiotic Removal. Nanomaterials 2022, 12, 4045. [Google Scholar] [CrossRef]
- Li, W.; Zhao, L.; Jiang, X.; Chen, Z.; Zhang, Y.; Wang, S. Confinement Engineering of Electrocatalyst Surfaces and Interfaces. Adv. Funct. Mater. 2022, 32, 2207727. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Zhang, Y.; Lichtfouse, E. Efficient phosphate recycling by adsorption on alkaline sludge biochar. Environ. Chem. Lett. 2022, 21, 21–30. [Google Scholar] [CrossRef]
- Han, L.; Wu, W.; Chen, X.; Chen, M. Co-sorption/co-desorption mechanism of the mixed chlorobenzenes by fresh bulk and aged residual biochar. J. Hazard. Mater. 2022, 429, 128349–128360. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, D.; Liu, T.; Tao, L.; Zhang, Y.; Huang, Y.C.; Du, S.; Dong, C.L.; Kong, Z.; Li, Y.f.; et al. Doping-Modulated Strain Enhancing the Phosphate Tolerance on PtFe Alloys for High-Temperature Proton Exchange Membrane Fuel Cells. Adv. Funct. Mater. 2021, 32, 2109244. [Google Scholar] [CrossRef]
- Gupta, M.; Savla, N.; Pandit, C.; Pandit, S.; Gupta, P.K.; Pant, M.; Khilari, S.; Kumar, Y.; Agarwal, D.; Nair, R.R.; et al. Use of biomass-derived biochar in wastewater treatment and power production: A promising solution for a sustainable environment. Sci. Total. Environ. 2022, 825, 153892–153917. [Google Scholar] [CrossRef] [PubMed]
- Abakari, G.; Luo, G.; Meng, H.; Yang, Z.; Owusu-Afriyie, G.; Kombat, E.O.; Alhassan, E.H. The use of biochar in the production of tilapia (Oreochromis niloticus) in a biofloc technology system—BFT. Aquac. Eng. 2020, 91, 102123–102131. [Google Scholar] [CrossRef]
- Zhang, W.; Zou, Y.; Yu, C.; Zhong, W. Nitrogen-enriched compact biochar-based electrode materials for supercapacitors with ultrahigh volumetric performance. J. Power Sources. 2019, 439, 227067–227077. [Google Scholar] [CrossRef]
- Jiang, W.; Li, L.; Pan, J.; Senthil, R.A.; Jin, X.; Cai, J.; Wang, J.; Liu, X. Hollow-tubular porous carbon derived from cotton with high productivity for enhanced performance supercapacitor. J. Power Sources. 2019, 438, 226936–226943. [Google Scholar] [CrossRef]
- Sun, L.; Chen, D.; Wan, S.; Yu, Z. Performance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. Bioresour. Technol. 2015, 198, 300–308. [Google Scholar] [CrossRef]
- Dong, Z.; Rene, E.R.; Zhang, P.; Hu, Q.; Ma, W. Design and preparation of carbon material catalyst modified with metal framework and sulfonate for biochar generation from low-temperature directional pyrolysis of kitchen waste: Mechanism and performance. Bioresour. Technol. 2023, 371, 128616–128624. [Google Scholar] [CrossRef]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New approaches to measuring biochar density and porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Bridgeman, T.G.; Jones, J.M.; Williams, A.; Waldron, D.J. An investigation of the grindability of two torrefied energy crops. Fuel 2010, 89, 3911–3918. [Google Scholar] [CrossRef]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubiera, F.; Pis, J.J. Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Pongrácz, E.; Fabritius, T. The potential of using biomass-based reducing agents in the blast furnace: A review of thermochemical conversion technologies and assessments related to sustainability. Renew. Sust. Energ. Rev. 2013, 25, 511–528. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K. The love-hate relationship of pyrolysis biochar and water: A perspective. Sci. Total. Environ. 2015, 512, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Budarin, V.L.; Clark, J.H.; North, M.; Wu, X. Rapid and efficient adsorption of methylene blue dye from aqueous solution by hierarchically porous, activated starbons(R): Mechanism and porosity dependence. J. Hazard. Mater. 2022, 436, 129174–129190. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhong, J.; Liu, B. Effective removal of methylene blue with zero-valent iron/tea residual biochar composite: Performance and mechanism. Bioresour. Technol. 2023, 371, 128592–128600. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Cao, D.; Hu, S.; Wang, Y.; Zhang, Y.; Huang, X.; Zhao, H.; Wu, C.; Li, J.; Ding, Y.; et al. High-pressure carbon dioxide-hydrothermal enhance yield and methylene blue adsorption performance of banana pseudo-stem activated carbon. Bioresour. Technol. 2022, 354, 127137–127149. [Google Scholar] [CrossRef]
- Saeed, A.A.H.; Harun, N.Y.; Sufian, S.; Siyal, A.A.; Zulfiqar, M.; Bilad, M.R.; Vagananthan, A.; Al-Fakih, A.; Ghaleb, A.A.S.; Almahbashi, N. Eucheuma cottonii Seaweed-Based Biochar for Adsorption of Methylene Blue Dye. Sustainability 2020, 12, 10318. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Lin, Y.-Q.; Zhou, H.-J.; Liu, Z.-L.; Miao, R.-R.; Xu, X.-M.; He, L.; Guan, Q.-Q. Boosting persulfate activation via paper mill sludge-based biochar for efficient degradation of bisphenol A: Inherent multiple active sites. Chem. Eng. J. 2023, 455, 140795–140809. [Google Scholar] [CrossRef]
- Xia, Y.-C.; Hu, H.-P.; Li, C.-H.; Ren, H.; Shi, G.; Xu, G.; Tian, B.; Shi, F.-N. Preparation of biochar microspheres from leaves of several plants by hydrothermal method. IOP Conf. Ser. Earth Environ. Sci. 2019, 267, 022012. [Google Scholar] [CrossRef]
- Wang, D.; Luo, W.; Zhu, J.; Wang, T.; Gong, Z.; Fan, M. Potential of removing Pb, Cd, and Cu from aqueous solutions using a novel modified ginkgo leaves biochar by simply one-step pyrolysis. Biomass Convers. Biorefin. 2021, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, C.; Qiu, S.; Zhu, Y.; Qin, M.; Meng, Y.; Wang, Y.; Chu, H.; Zou, Y.; Xiang, C.; et al. Nitrogen-doped porous carbon derived from ginkgo leaves with remarkable supercapacitance performance. Diam. Relat. Mater. 2019, 98, 107475–107483. [Google Scholar] [CrossRef]
- Tian, H.; Fang, Q.; Cheng, R.; Li, L.; Zhang, W.; Ran, S.; Ma, L.; Lv, Y. Molten salt template-assisted synthesis of N, S-codoped hierarchically porous carbon nanosheets for efficient energy storage. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126172–126180. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, M.; Cheng, R.; Li, L.; Sun, Y.; Ran, S.; Lv, Y.; Ma, L. Molten metal chloride salt template synthesis of N/S co-doped porous carbon nanosheets for supercapacitors. Diam. Relat. Mater. 2021, 113, 108278–108297. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, h.; Cheng, R.; Wang, Z.; Ma, Y.; Ran, S.; Lv, Y.; Ma, L. A composite-hydroxide-activation strategy for the preparation of N/S dual-doped porous carbon materials as advanced supercapacitor electrodes. J. Mater. Sci. Mater. Electron. 2020, 31, 22498–22511. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, M.; Zhu, F.; Song, W.; Zhang, Z.; Dou, M.; Niu, J.; Wang, F. Biomass-Derived Ternary-Doped Porous Carbon Electrodes for Li-Ion Capacitors: Rational Preparation and Energy-Storage Mechanism Study. J. Electrochem Soc. 2021, 168, 040521. [Google Scholar] [CrossRef]
- Zhao, D.; Guo, T.; Fan, X.; Chen, C.; Ma, Y. Effect of pyrolytic carbon interphase on mechanical properties of mini T800-C/SiC composites. J. Adv. Ceram. 2021, 10, 219–226. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, S.; Yang, Y.; Fu, X.; Jiang, T.; Yang, J. Ammonium Nitrate-Assisted Synthesis of Nitrogen/Sulfur-Codoped Hierarchically Porous Carbons Derived from Ginkgo Leaf for Supercapacitors. ACS Omega. 2019, 4, 5904–5914. [Google Scholar] [CrossRef]
- Lee, M.E.; Park, J.H.; Chung, J.W. Adsorption of Pb(II) and Cu(II) by Ginkgo-Leaf-Derived Biochar Produced under Various Carbonization Temperatures and Times. Int. J. Environ. Res. Public. Health. 2017, 14, 1528. [Google Scholar] [CrossRef]
- Zuo, X.H.; Li, W.; Yu, Z.L.; Ma, F.M.; Ruan, M. Tribological Behavior of Phenolic Resin Composites Modified Using Tetraethyl Orthosilicate. Tribol. Trans. 2013, 56, 115–120. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Jia, J.; Hou, C.; Hao, X.; Zhang, H. Preparation of hydroxyl and (3-aminopropyl)triethoxysilane functionalized multiwall carbon nanotubes for use as conductive fillers in the polyurethane composite. Polym. Compos. 2018, 39, 1212–1222. [Google Scholar] [CrossRef]
- Feriancová, A.; Dubec, A.; Pagáčová, J.; Labaj, I.; Pajtášová, M. The influence of silane on the physico-mechanical properties of vulcanizates using bentonite fillers. IOP Conf. Ser. Earth Environ. Sci. 2021, 1199, 012040. [Google Scholar] [CrossRef]


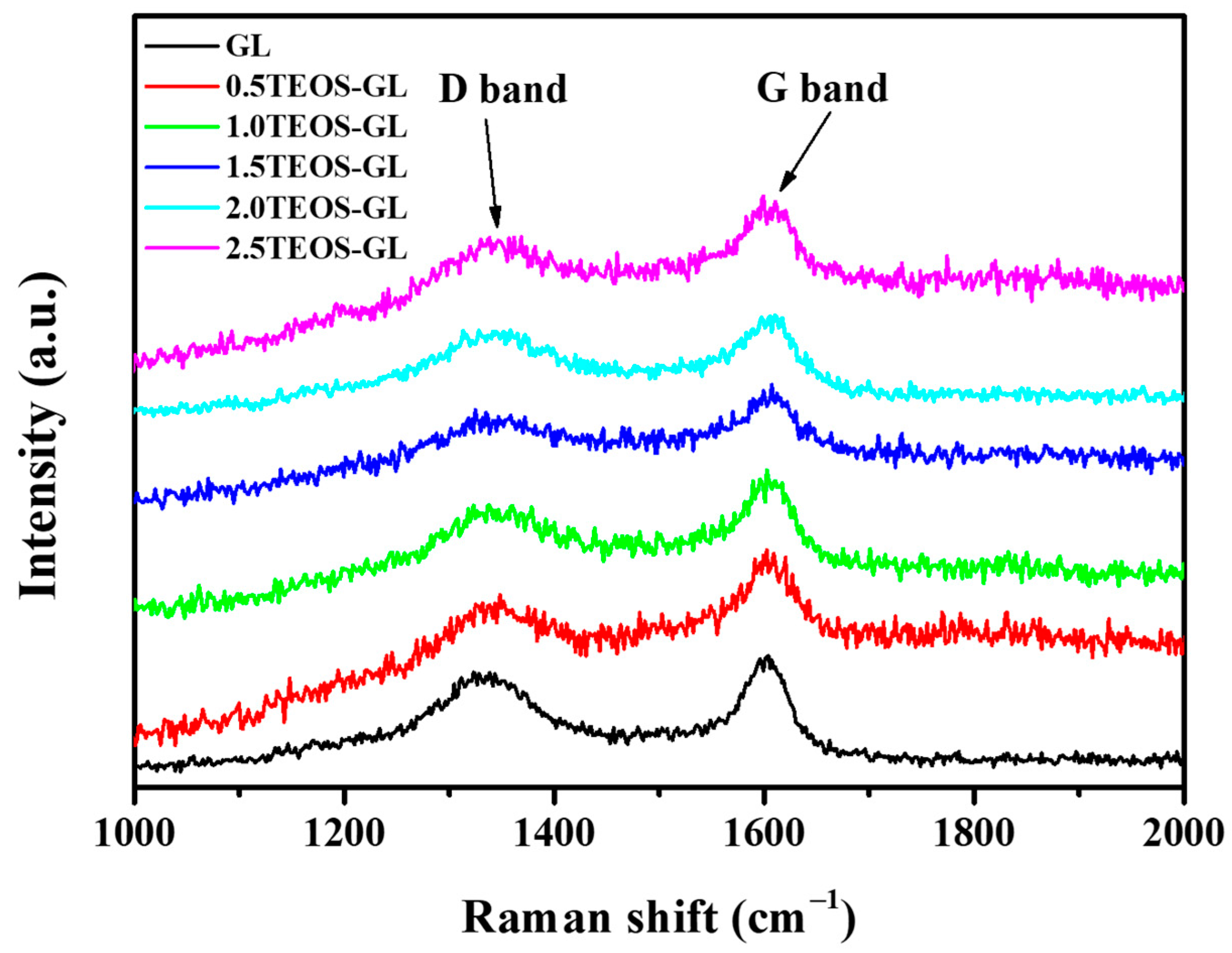
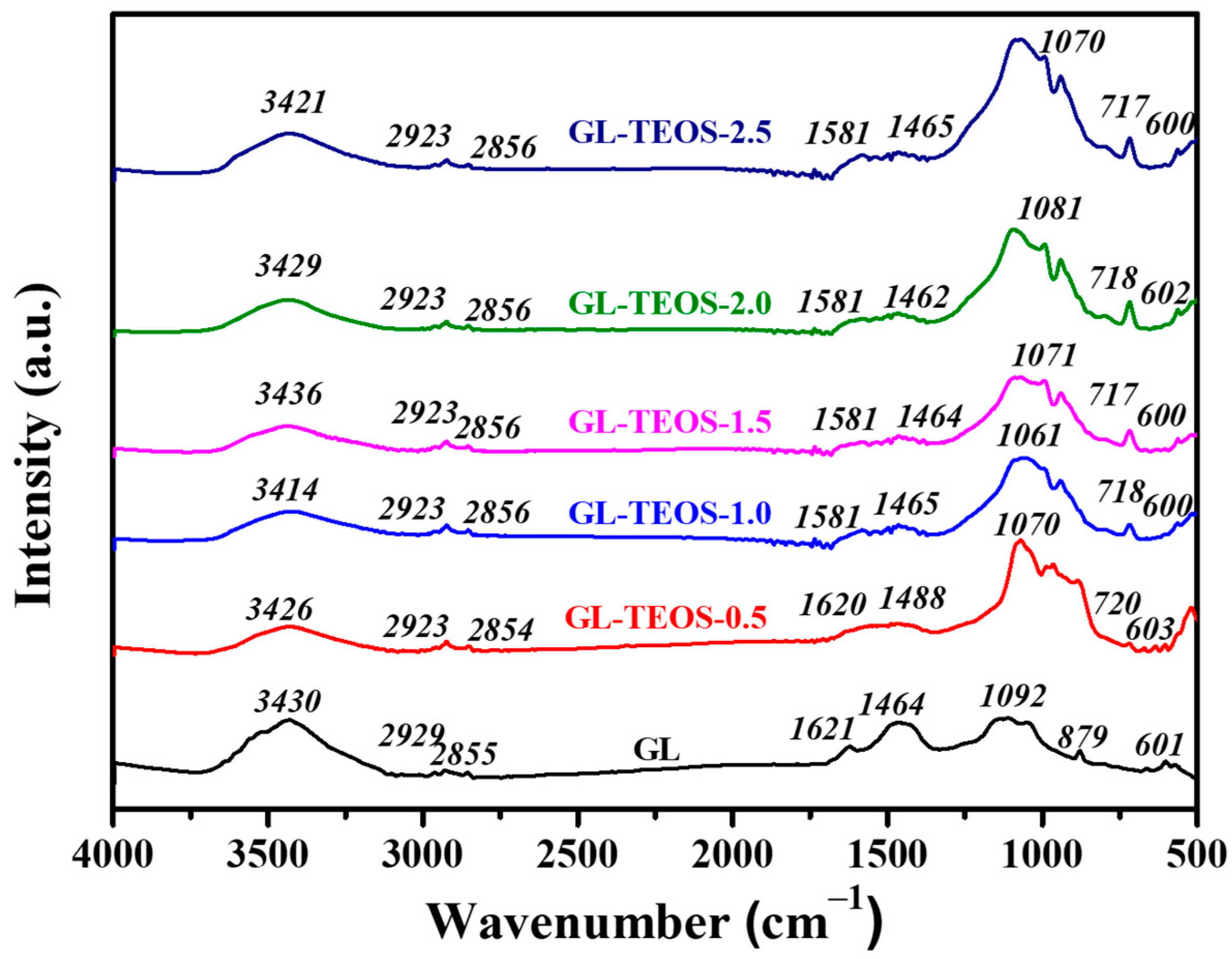
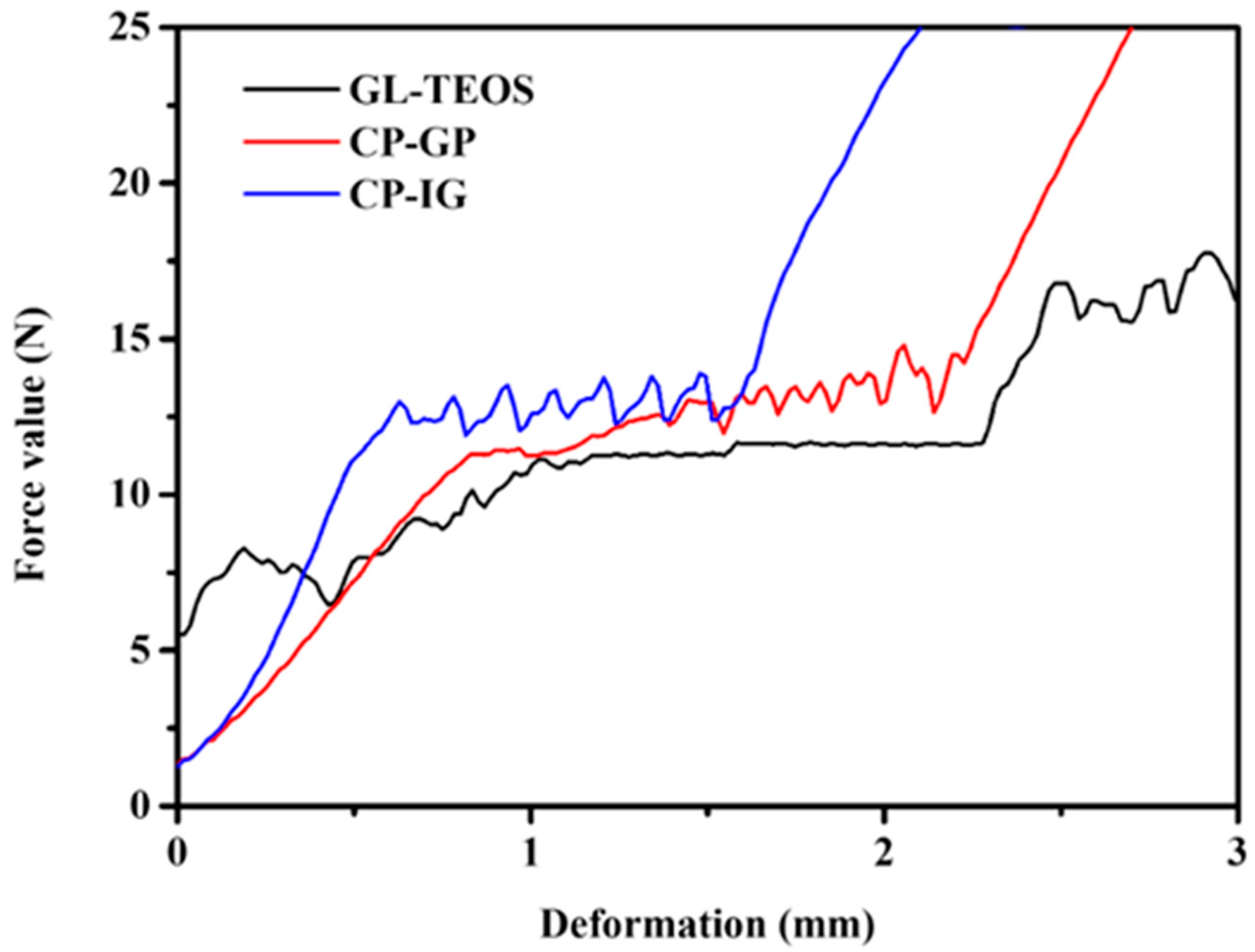
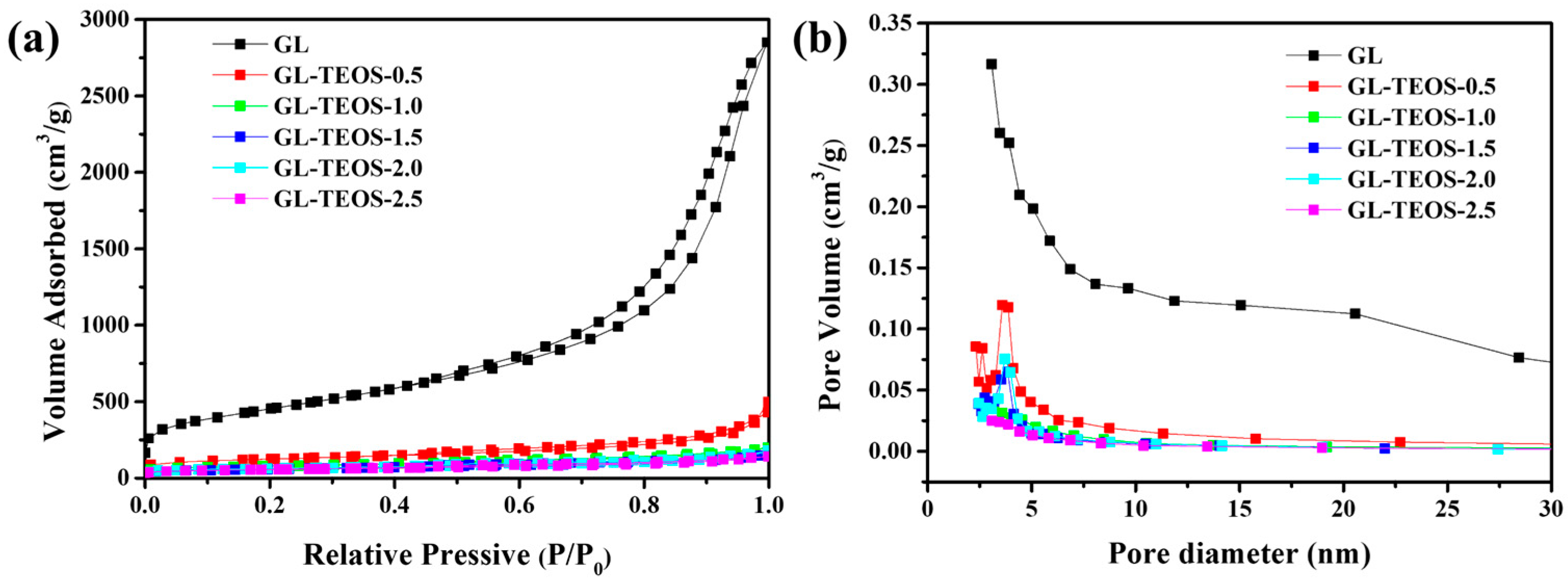

| Material | Product Quantity 1 (g) | Carbon Content (%) | Compressive Strength (kPa) |
|---|---|---|---|
| GL | 0.25 | 100 | 0 |
| GL-TEOS-0.5 | 0.48 | 55.6 | 14.3 |
| GL-TEOS-1.0 | 0.62 | 36.2 | 45.3 |
| GL-TEOS-1.5 | 0.77 | 30.5 | 88.9 |
| GL-TEOS-2.0 | 0.84 | 32.9 | 74.9 |
| GL-TEOS-2.5 | 1.06 | 31.6 | 79.8 |
| Si | 0.58 | 0 | - |
| CP | 2 | 100 | - |
| CP-GP-0.4 | 2.4 | 83.3 | 12.3 |
| CP-GP-0.8 | 2.8 | 71.4 | 18.5 |
| CP-GP-1.2 | 3.2 | 62.5 | 24.3 |
| CP-GP-1.6 | 3.6 | 55.5 | 30.3 |
| CP-GP-2.0 | 4.0 | 50 | 36.8 |
| CP-IG-0.4 | 2.4 | 83.3 | 8.8 |
| CP-IG-0.8 | 2.8 | 71.4 | 13.9 |
| CP-IG-1.2 | 3.2 | 62.5 | 34.9 |
| CP-IG-1.6 | 3.6 | 55.5 | 65.4 |
| CP-IG-2.0 | 4.0 | 50 | 124.6 |
| Material | SBET (m2·g−1) | Pore Volume (cm3·g−1) |
|---|---|---|
| GL | 1614 | 4.41 |
| GL-TEOS-0.5 | 422 | 0.77 |
| GL-TEOS-1.0 | 270 | 0.31 |
| GL-TEOS-1.5 | 197 | 0.25 |
| GL-TEOS-2.0 | 213 | 0.28 |
| GL-TEOS-2.5 | 201 | 0.26 |
| Material | Adsorption Capacity (mg/g) | Material | Adsorption Capacity (mg/g) | Material | Adsorption Capacity (mg/g) |
|---|---|---|---|---|---|
| GL | 434 | CP | 194.4 | CP | 194.4 |
| GL-TEOS-0.5 | 325 | CP-GP-0.4 | 171.7 | CP-IG-0.4 | 109.9 |
| GL-TEOS-1.0 | 251 | CP-GP-0.8 | 155.4 | CP-IG-0.8 | 100.8 |
| GL-TEOS-1.5 | 134 | CP-GP-1.2 | 139.0 | CP-IG-1.2 | 93.4 |
| GL-TEOS-2.0 | 177 | CP-GP-1.6 | 99.1 | CP-IG-1.6 | 85.2 |
| GL-TEOS-2.5 | 136 | CP-GP-2.0 | 93.2 | CP-IG-2.0 | 70.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Jiang, X.; Zhang, Y.; Li, W.; Tang, Z.; Liu, Y.; Zhao, L. Inorganic Skeleton Reinforcement—A Generic Approach to Improve the Mechanical Properties of Biochar. Nanomaterials 2023, 13, 1298. https://doi.org/10.3390/nano13081298
Chen Z, Jiang X, Zhang Y, Li W, Tang Z, Liu Y, Zhao L. Inorganic Skeleton Reinforcement—A Generic Approach to Improve the Mechanical Properties of Biochar. Nanomaterials. 2023; 13(8):1298. https://doi.org/10.3390/nano13081298
Chicago/Turabian StyleChen, Zhikai, Xiaoli Jiang, Yagang Zhang, Wei Li, Zhiqiang Tang, Yanxia Liu, and Lin Zhao. 2023. "Inorganic Skeleton Reinforcement—A Generic Approach to Improve the Mechanical Properties of Biochar" Nanomaterials 13, no. 8: 1298. https://doi.org/10.3390/nano13081298
APA StyleChen, Z., Jiang, X., Zhang, Y., Li, W., Tang, Z., Liu, Y., & Zhao, L. (2023). Inorganic Skeleton Reinforcement—A Generic Approach to Improve the Mechanical Properties of Biochar. Nanomaterials, 13(8), 1298. https://doi.org/10.3390/nano13081298