Effect of Hydrothermal and Vapor Thermal Treatments on Apatite Inductivity of Titanate Nanotubes on Anodized Ti–5Nb–5Mo Surface
Abstract
1. Introduction
2. Materials and Methods
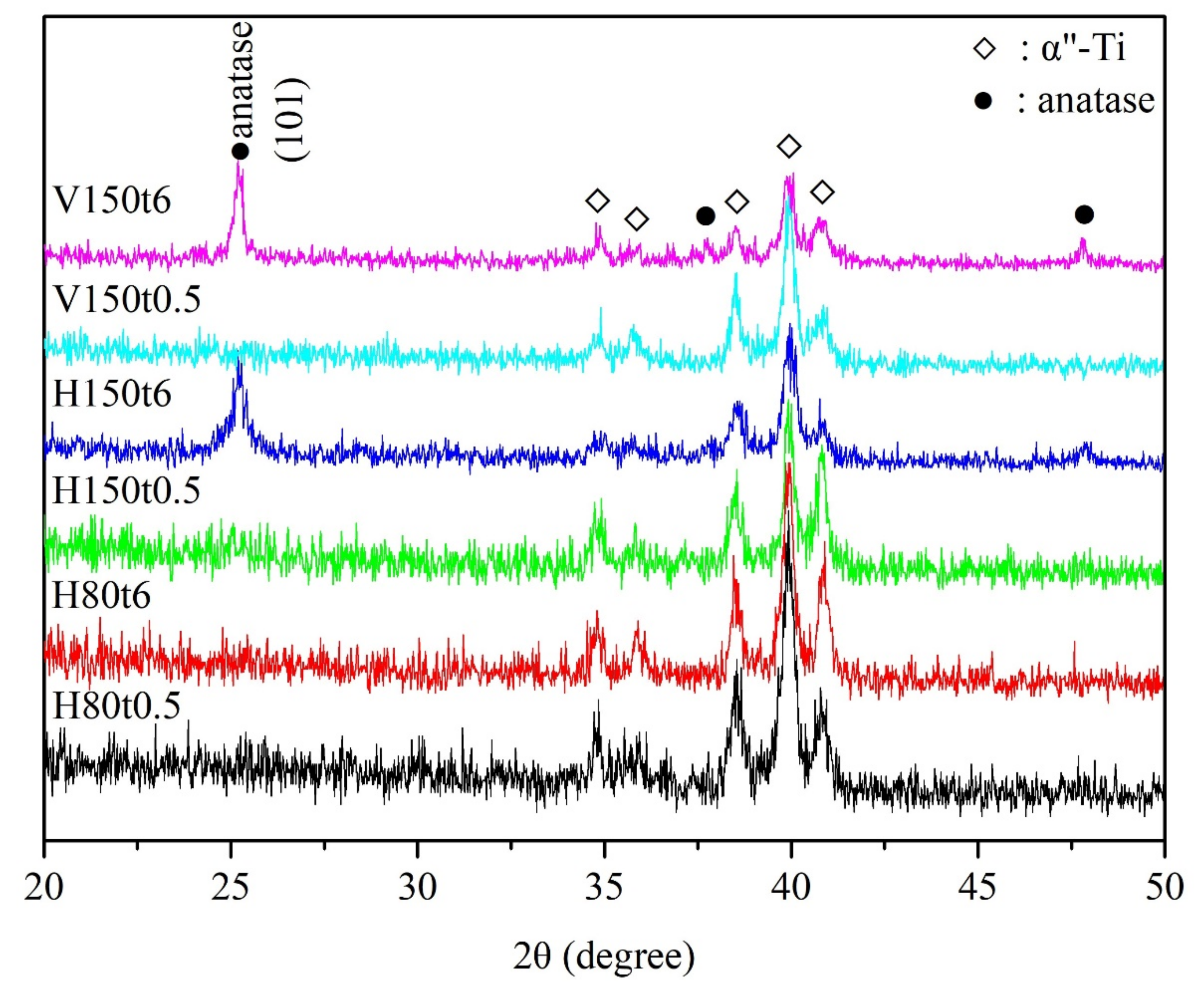
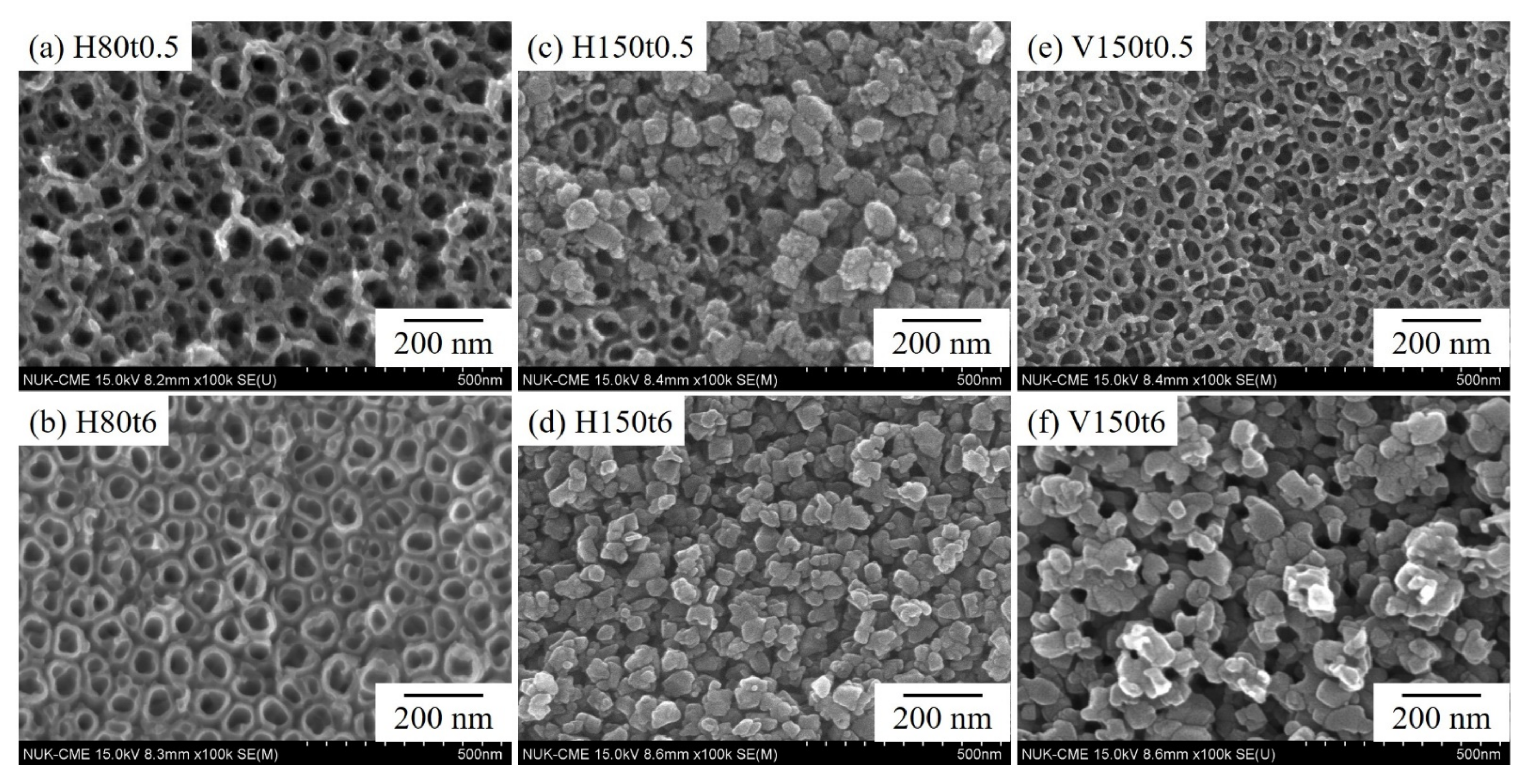
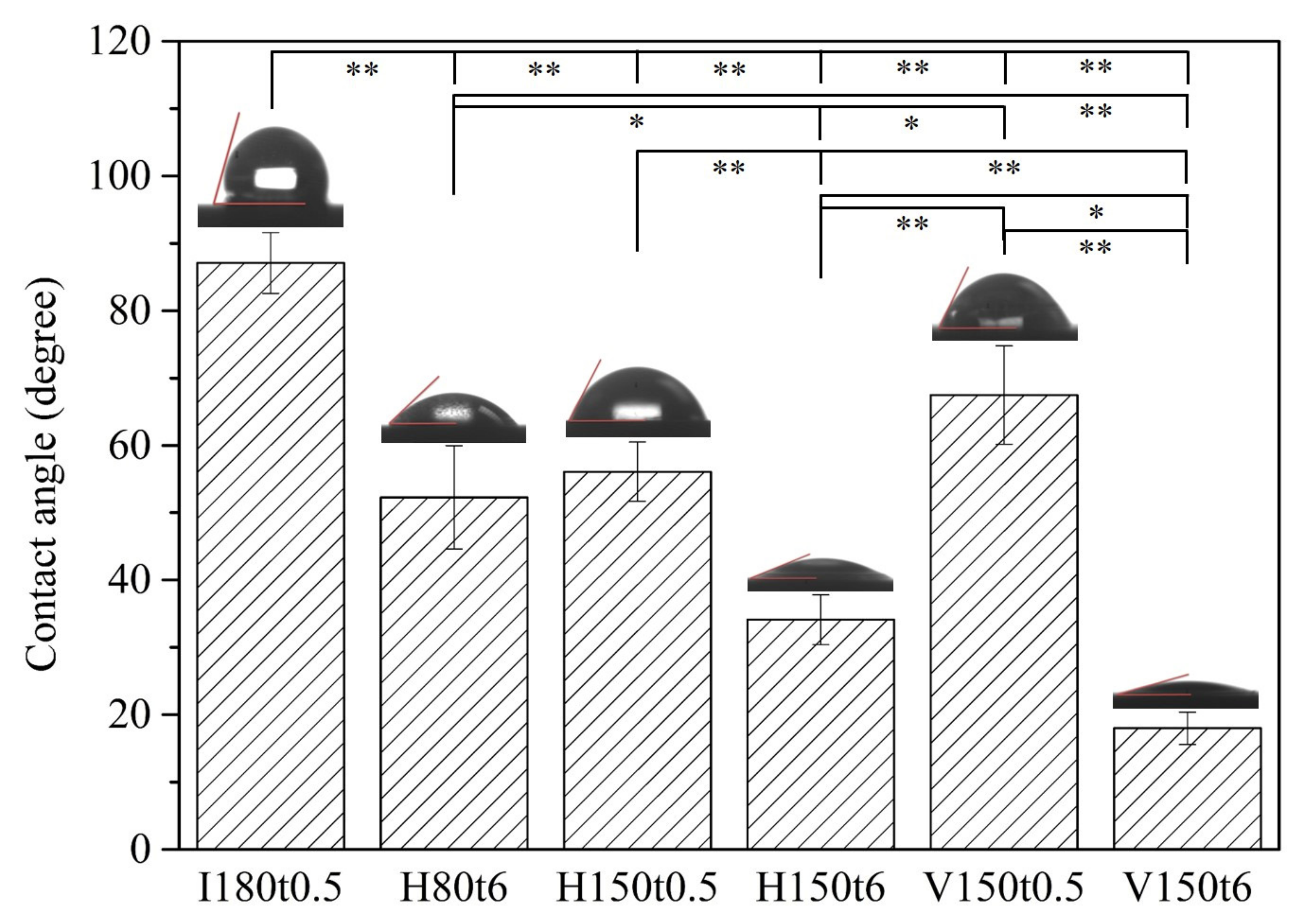
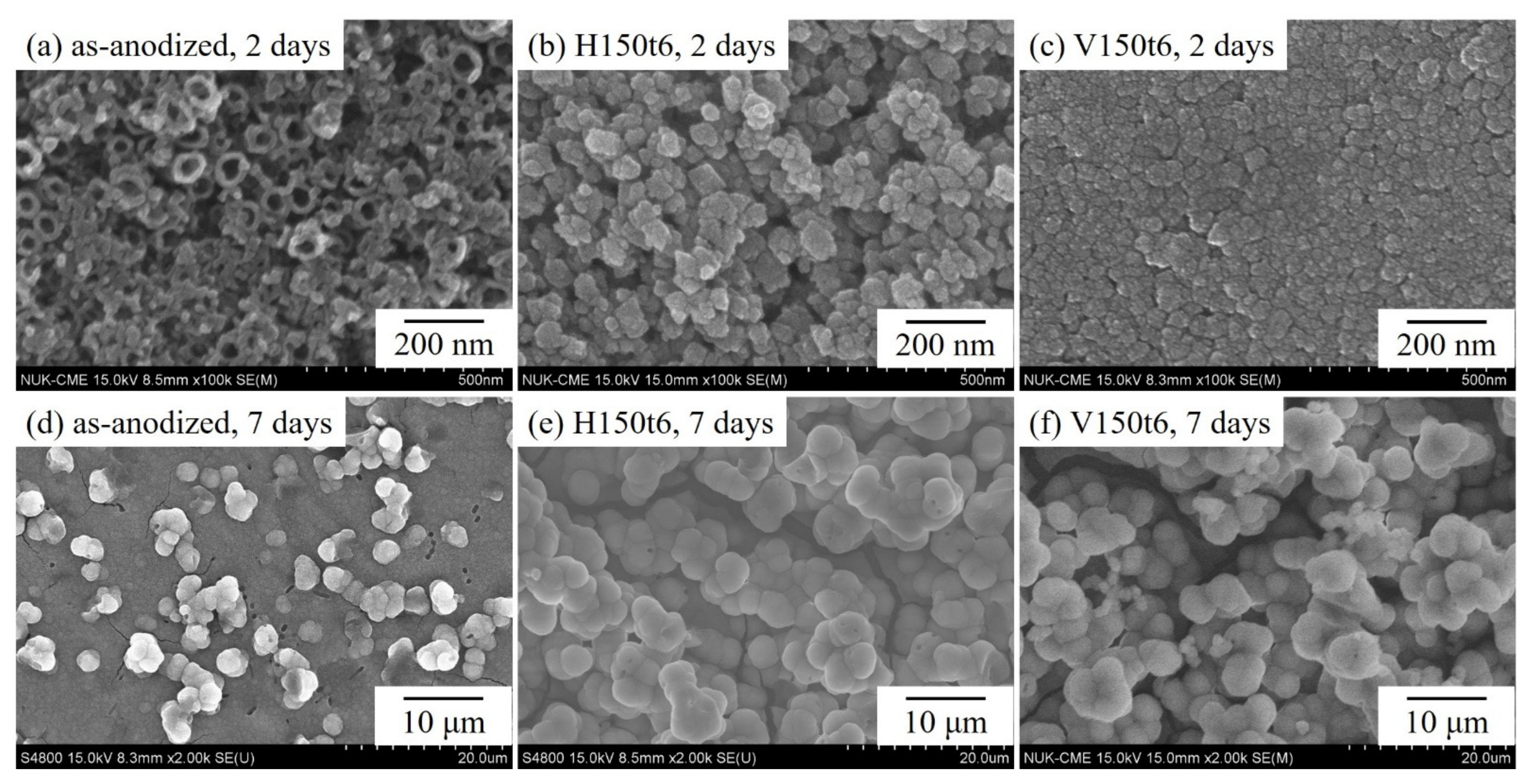
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bittredge, O.; Hassanin, H.; El-Sayed, M.A.; Eldessouky, H.M.; Alsaleh, N.A.; Alrasheedi, N.H.; Essa, K.; Ahmadein, M. Fabrication and Optimisation of Ti-6Al-4V Lattice-Structured Total Shoulder Implants Using Laser Additive Manufacturing. Materials 2022, 15, 3095. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Todai, M.; Nakano, T. Beta titanium single crystal with bone-like elastic modulus and large crystallographic elastic anisotropy. Mater. Sci. Eng. A 2019, 782, 667–671. [Google Scholar] [CrossRef]
- Fouda, N.; Mostafa, R.; Saker, A. Numerical study of stress shielding reduction at fractured bone using metallic and composite bone-plate models. Ain Shams Eng. J. 2019, 10, 481–488. [Google Scholar] [CrossRef]
- Luo, C.; Liu, Y.; Peng, B.; Chen, M.; Liu, Z.; Li, Z.; Kuang, H.; Gong, B.; Li, Z.; Sun, H. PEEK for Oral Applications: Recent Advances in Mechanical and Adhesive Properties. Polymers 2023, 15, 386. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Hariharan, A.; Günther, F.; Zimmermann, M.; Gebert, A. Influence of isothermal omega precipitation aging on deformation mechanisms and mechanical properties of a β-type Ti-Nb alloy. J. Alloys Compd. 2023, 930, 167309. [Google Scholar] [CrossRef]
- Hsu, H.C.; Wu, S.C.; Hsu, S.K.; Kao, W.H.; Ho, W.F. Structure and mechanical properties of as-cast Ti–5Nb-based alloy with Mo addition. Mater. Sci. Eng. A 2013, 579, 86–91. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Luo, D.M. Corrosion behavior of Ti–Mo alloys cold rolled and heat treated. J. Alloys Compd. 2011, 509, 6267–6272. [Google Scholar] [CrossRef]
- Wong, K.K.; Hsu, H.C.; Wu, S.C.; Ho, W.F. Structure and properties of Ti-rich Ti-Zr-Nb-Mo medium-entropy alloys. J. Alloys Compd. 2021, 868, 159137. [Google Scholar] [CrossRef]
- Vishwakarma, V.; Kaliaraj, G.S.; Amirtharaj Mosas, K.K. Multifunctional Coatings on Implant Materials—A Systematic Review of the Current Scenario. Coatings 2023, 13, 69. [Google Scholar] [CrossRef]
- Şimşek, M.; Aldemir, S.D.; Gümüşderelioğlu, M. Anticellular PEO coatings on titanium surfaces by sequential electrospinning and crosslinking processes. Emerg. Mater. 2019, 2, 169–179. [Google Scholar] [CrossRef]
- Shanmugapriya; Sivamaran, V.; Padma Rao, A.; Senthil Kumar, P.; Selvamani, S.T.; Mandal, T.K. Sol–gel derived Al2O3/Gr/HAP nanocomposite coatings on Ti–6Al–4V alloy for enhancing tribo-mech properties and antibacterial activity for bone implants. Appl. Phys. A 2022, 128, 635. [Google Scholar] [CrossRef]
- Huang, H.L.; Tsai, M.T.; Chang, Y.Y.; Lin, Y.J.; Hsu, J.T. Fabrication of a novel Ta(Zn)O thin film on titanium by magnetron sputtering and plasma electrolytic oxidation for cell biocompatibilities and antibacterial applications. Metals 2020, 10, 649. [Google Scholar] [CrossRef]
- Kumari, R.; Yadav, K.B.; Barole, S.; Archana, K.; Besra, L.D. Microstructural characterisation and wettability behaviour of nano-HA coating on Ti-6Al-4V alloy by electrophoretic deposition method (EPD). Adv. Mater. Process. Technol. 2022, 8, 611–618. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Nadaraia, K.V.; Plekhova, N.G.; Imshinetskiy, I.M.; Piatkova, M.A.; Pleshkova, A.I.; Kislova, S.E.; Sinebryukhov, S.L.; Gnedenkov, S.V. Antibacterial Ca/P-coatings formed on Mg alloy using plasma electrolytic oxidation and antibiotic impregnation. Mater. Lett. 2022, 317, 132099. [Google Scholar] [CrossRef]
- Imshinetskiy, I.; Kashepa, V.; Nadaraia, K.; Mashtalyar, D.; Suchkov, S.; Zadorozhny, P.; Ustinov, A.; Sinebryukhov, S.; Gnedenkov, S. PEO Coatings Modified with Halloysite Nanotubes: Composition, Properties, and Release Performance. Int. J. Mol. Sci. 2023, 24, 305. [Google Scholar] [CrossRef]
- Kaseem, M.; Choe, H.C. Synchronized Improvements in the Protective and Bioactive Properties of Plasma-Electrolyzed Layers via Cellulose Microcrystalline. ACS Biomater. Sci. Eng. 2023, 9, 197–210. [Google Scholar] [CrossRef]
- Ocampo, R.A.; Echeverria, F.E. Effect of the anodization parameters on TiO2 nanotubes characteristics produced in aqueous electrolytes with CMC. Appl. Surf. Sci. 2019, 469, 994–1006. [Google Scholar] [CrossRef]
- Pishkar, N.; Ghoranneviss, M.; Ghorannevis, Z.; Akbari, H. Study of the highly ordered TiO2 nanotubes physical properties prepared with two-step anodization. Results Phys. 2018, 9, 1246–1249. [Google Scholar] [CrossRef]
- Khrunyk, Y.Y.; Belikov, S.V.; Tsurkan, M.V.; Vyalykh, I.V.; Markaryan, A.Y.; Karabanalov, M.S.; Popov, A.A.; Wysokowski, M. Surface-Dependent Osteoblasts Response to TiO2 Nanotubes of Different Crystallinity. Nanomaterials 2020, 10, 320. [Google Scholar] [CrossRef]
- Savitha, R.; Raghunathan, R.; Chetty, R. Enhanced visible light sensitized photoreaction by mixed phase titania nanotubes. Appl. Surf. Sci. 2023, 609, 155252. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jin, G.C.; Kim, Y.K.; Kao, W.H.; Park, I.S. Effect of alkali and heat treatments for bioactivity of TiO2 nanotubes. Appl. Surf. Sci. 2014, 321, 412–419. [Google Scholar] [CrossRef]
- Shimagami, K.; Ito, T.; Toda, Y.; Yumoto, A.; Yamabe-Mitarai, Y. Effects of Zr and Si addition on high-temperature mechanical properties and microstructure in Ti–10Al–2Nb-based alloys. Mat. Sci. Eng. A 2019, 756, 46–53. [Google Scholar] [CrossRef]
- Kim, S.P.; Kaseem, M.; Choe, H.C. Plasma electrolytic oxidation of Ti–25Nb–xTa alloys in solution containing Ca and P ions. Surf. Coat. Technol. 2020, 395, 125916. [Google Scholar] [CrossRef]
- Yu, J.; Dai, G.; Cheng, B. Effect of crystallization methods on morphology and photocatalytic activity of anodized TiO2 nanotube array films. J. Phys. Chem. C 2010, 114, 19378–19385. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Yu, J.; Wang, B. Effect of calcination temperature on morphology and photoelectrochemical properties of anodized titanium dioxide nanotube arrays. Appl. Catal. B 2010, 94, 295–302. [Google Scholar] [CrossRef]
- Fischer, K.; Gläser, R.; Schulze, A. Nanoneedle and nanotubular titanium dioxide–PES mixed matrix membrane for photocatalysis. Appl. Catal. B 2014, 160, 456–464. [Google Scholar] [CrossRef]
- Yang, B.; Uchida, M.; Kim, H.M.; Zhang, X.; Kokubo, T. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 2004, 25, 1003–1010. [Google Scholar] [CrossRef]
- Wei, D.; Qing, W.F.; Zhou, R.; Li, B.; Wang, Y.; Zhou, Y.; Jia, D. Titania nanotube/nano-brushite composited bioactive coating with micro/nanotopography on titanium formed by anodic oxidation and hydrothermal treatment. Ceram. Int. 2015, 41, 13115–13125. [Google Scholar] [CrossRef]
- Getz, M.N.; Chatzitakis, A.; Liu, X.; Carvalho, P.A.; Bjorheim, T.S.; Norby, T. Voids in walls of mesoporous TiO2 anatase nanotubes by controlled formation and annihilation of protonated titanium vacancies. Mater. Chem. Phys. 2020, 239, 121953. [Google Scholar] [CrossRef]
- Aeimbhu, A. Effect of calcination temperature on morphology, wettability and anatase/rutile phase ratio of titanium dioxide nanotube arrays. Mater. Today Proc. 2018, 5, 14950–14954. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Kamonsuangkasem, K.; Kidkhunthod, P.; Chirawatkul, P.; Saiyasombat, C.; Chanlek, N.; Wantala, K. Influence of nitrogen content levels on structural properties and photocatalytic activities of nanorice-like N-doped TiO2 with various calcination temperatures. Mater. Res. Bull. 2018, 105, 265–276. [Google Scholar] [CrossRef]
- Ding, K.L.; Miao, Z.J.; Hu, B.J.; An, G.M.; Sun, Z.Y.; Han, B.X.; Liu, Z.M. Study on the anatase to rutile phase transformation and controlled synthesis of rutile nanocrystals with the assistance of ionic liquid. Langmuir 2010, 26, 10294–10302. [Google Scholar] [CrossRef]
- Lima, G.G.; Souza, G.B.; Lepienski, C.M.; Kuromoto, N.K. Mechanical properties of anodic titanium films containing ions of Ca and P submitted to heat and hydrothermal treatment. J. Mech. Behav. Biomed. Mater. 2016, 64, 18–30. [Google Scholar] [CrossRef]
- Roguska, A.; Pisarek, M.; Belcarz, A.; Marconc, L.; Holdynski, M.; Andrzejczuk, M.; Janik-Czachora, M. Improvement of the bio-functional properties of TiO2 nanotubes. Appl. Sur. Sci. 2016, 388, 775–885. [Google Scholar] [CrossRef]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater. Sci. Eng. C 2003, 579, 551–560. [Google Scholar] [CrossRef]
- Xiao, X.; Liang, J.; Tang, H.; Yang, X.; Liu, R.; Chen, Y. Preparation and bioactivity of TiO2 nanotube arrays containing calcium and phosphorus. Appl. Surf. Sci. 2013, 15, 312–319. [Google Scholar] [CrossRef]
- Burant, B.; Robak, J.; Leniart, A.; Piwonski, I.; Batory, D. The effect of concentration and source of calcium ions on anticorrosion properties of Ca-doped TiO2 bioactive sol-gel coatings. Ceram. Int. 2017, 43, 13735–13742. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Müller, L.; Kunze, J.; Müller, F.; Greil, P.; Virtanen, S.; Schmuki, P. Hydroxyapatite growth on anodic TiO2 nanotubes. J. Biomed. Mater. Res. 2006, 77, 534–541. [Google Scholar] [CrossRef]



| Sample Code | Hydrothermal | Vapor Thermal |
|---|---|---|
| H80t0.5 | 80 °C, 0.5 h | |
| H80t6 | 80 °C, 6 h | |
| H150t0.5 | 150 °C, 0.5 h | |
| H150t6 | 150 °C, 6 h | |
| V150t0.5 | 150 °C, 0.5 h | |
| V150t6 | 150 °C, 6 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, K.-H.; Hsu, H.-C.; Wu, S.-C.; Shih, Y.-C.; Yang, H.-W.; Ho, W.-F. Effect of Hydrothermal and Vapor Thermal Treatments on Apatite Inductivity of Titanate Nanotubes on Anodized Ti–5Nb–5Mo Surface. Nanomaterials 2023, 13, 1296. https://doi.org/10.3390/nano13081296
Hsieh K-H, Hsu H-C, Wu S-C, Shih Y-C, Yang H-W, Ho W-F. Effect of Hydrothermal and Vapor Thermal Treatments on Apatite Inductivity of Titanate Nanotubes on Anodized Ti–5Nb–5Mo Surface. Nanomaterials. 2023; 13(8):1296. https://doi.org/10.3390/nano13081296
Chicago/Turabian StyleHsieh, Kuan-Hsiang, Hsueh-Chuan Hsu, Shih-Ching Wu, Yi-Cheng Shih, Hsiang-Wei Yang, and Wen-Fu Ho. 2023. "Effect of Hydrothermal and Vapor Thermal Treatments on Apatite Inductivity of Titanate Nanotubes on Anodized Ti–5Nb–5Mo Surface" Nanomaterials 13, no. 8: 1296. https://doi.org/10.3390/nano13081296
APA StyleHsieh, K.-H., Hsu, H.-C., Wu, S.-C., Shih, Y.-C., Yang, H.-W., & Ho, W.-F. (2023). Effect of Hydrothermal and Vapor Thermal Treatments on Apatite Inductivity of Titanate Nanotubes on Anodized Ti–5Nb–5Mo Surface. Nanomaterials, 13(8), 1296. https://doi.org/10.3390/nano13081296








