Non-Noble FeCrOx Bimetallic Nanoparticles for Efficient NH3 Decomposition
Abstract
1. Introduction
2. Experimental
2.1. Catalyst Synthesis
2.2. Characterizations
2.3. Catalytic Tests
3. Result and Discussion
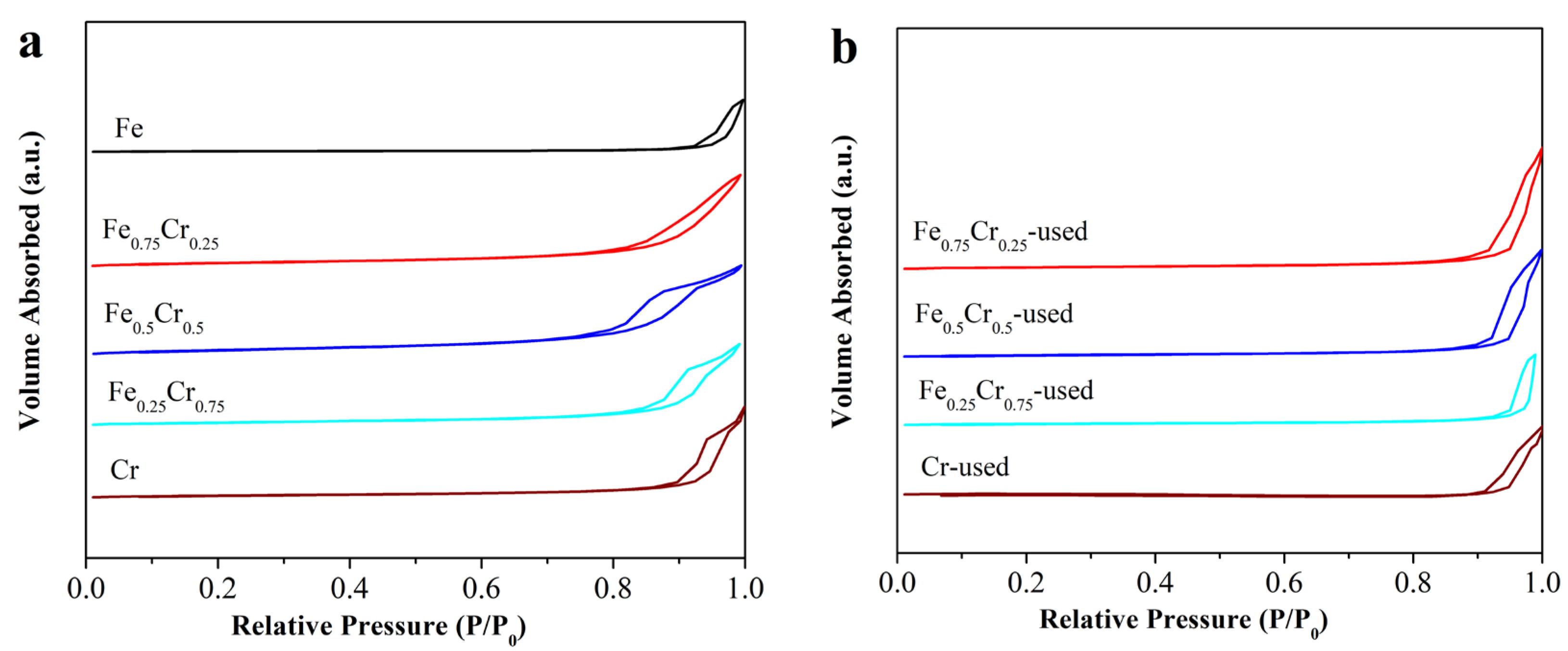
3.1. Catalyst Structure and Ammonia Decomposition Reactivity

| Catalyst | Temperature | GHSV (NH3 cm3 h−1) | Conversion (%) | Reference |
|---|---|---|---|---|
| Fe0.75Cr0.25 | 600 | 22,000 | 88.4 | This work |
| Fe0.75Cr0.25 | 650 | 22,000 | 99.5 | This work |
| Fe-Al2O3 | 600 | 36,000 | 86 | [40] |
| Fe/SiO2 | 600 | 15,000 | 65 | [44] |
| Fe/CMK-5 | 600 | 7500 | 96 | [45] |
| Fe-CNTs | 700 | 5000 | 75 | [46] |
| CoFe5/CNTs | 600 | 36,000 | 50 | [47] |
| Fe/SiO2-Cs | 600 | 30,000 | 90 | [48] |
| Fe-Mo | 550 | 46,000 | 16 | [49] |
| Fe-Co | 550 | 6000 | 77 | [50] |
| Fe-Mg | 550 | 6000 | 86 | [50] |
3.2. The Morphology and Crystal Structure of Used FeCrOx Catalysts
3.3. Identification of Catalytic Active Components
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, S.F.; Xu, B.Q.; Zhou, X.P.; Au, C.T. A mini-review on ammonia decomposition catalysts for on-site generation of hydrogen for fuel cell applications. Appl. Catal. A Gen. 2004, 277, 1–9. [Google Scholar] [CrossRef]
- Schüth, F.; Palkovits, R.; Schlögl, R.; Su, D.S. Ammonia as a possible element in an energy infrastructure: Catalysts for ammonia decomposition. Energy Environ. Sci. 2012, 5, 6278–6289. [Google Scholar] [CrossRef]
- Metkemeijer, R.; Achard, P. Ammonia as a feed stock for a hydrogen fuel cell; reformer and fuel cell behaviour. J. Power Sources 1994, 49, 271–282. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Goodman, D.W. CO-free production of hydrogen via stepwise steam reforming of methane. J. Catal. 2000, 192, 316–321. [Google Scholar] [CrossRef]
- Dongil, A.B. Recent progress on transition metal nitrides nanoparticles as heterogeneous catalysts. Nanomaterials 2019, 9, 1111. [Google Scholar] [CrossRef]
- Mukherjee, S.; Devaguptapu, S.V.; Sviripa, A.; Lund, C.; Wu, G. Low-temperature ammonia decomposition catalysts for hydrogen generation. Appl. Catal. B Environ. 2018, 226, 162–181. [Google Scholar] [CrossRef]
- Lucentini, I.; Garcia, X.; Vendrell, X.; Llorca, J. Review of the decomposition of ammonia to generate hydrogen. Ind. Eng. Chem. Res. 2021, 60, 18560–18611. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, Y.; Kim, H.W.; Lee, S.U.; Kim, J.R.; Kim, T.W.; Lee, Y.G.; Chae, H.J. Ru-supported lanthania-ceria composite as an efficient catalyst for COx-free H2 production from ammonia decomposition. Appl. Catal. B Environ. 2021, 285, 119831. [Google Scholar] [CrossRef]
- Hu, X.C.; Fu, X.P.; Wang, W.W.; Wang, X.; Wu, K.; Si, R.; Ma, C.; Jia, C.J.; Yan, C.H. Ceria-supported ruthenium clusters transforming from isolated single atoms for hydrogen production via decomposition of ammonia. Appl. Catal. B Environ. 2020, 268, 118424. [Google Scholar] [CrossRef]
- Zheng, W. Nanomaterials for Ammonia Decomposition. Ph.D. Thesis, Universitat Berlin, Berlin, Germany, 2011. [Google Scholar]
- Arabczyk, W.; Pelka, R. Studies of the kinetics of two parallel reactions: Ammonia decomposition and nitriding of iron catalys. J. Phys. Chem. A 2008, 113, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Pelka, R.; Moszynska, I.; Arabczyk, W. Catalytic ammonia decomposition over Fe/Fe4N. Catal. Lett. 2009, 128, 72–76. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Xu, C.; Kong, D.; Tsubouchi, N. Decomposition of ammonia with iron and calcium catalysts supported on coal chars. Fuel 2004, 83, 685–692. [Google Scholar] [CrossRef]
- Othman, N.E.F.; Salleh, H.M.; Purwanto, H. Utilization of low-grade iron ore in ammonia decomposition. Procedia Chem. 2016, 19, 119–124. [Google Scholar] [CrossRef]
- Takezawa, N.; Toyoshima, I. The change of the rate-determining step of the ammonia decomposition over an ammonia synthetic iron catalyst. J. Phys. Chem. 1966, 70, 594–595. [Google Scholar] [CrossRef]
- Lanzani, G.; Laasonen, K. NH3 adsorption and dissociation on a nanosized iron cluster. Int. J. Hydrogy Energy 2010, 35, 6571–6577. [Google Scholar] [CrossRef]
- Hu, X.C.; Wang, W.W.; Gu, Y.Q.; Jin, Z.; Song, Q.S.; Jia, C.J. Co-SiO2 nanocomposite catalysts for COx-free hydrogen production by ammonia decomposition. ChemPlusChem 2017, 82, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Yao, Y.; Huang, Z.; Liu, Z.; Zhang, J.; Li, T.; Wang, G.; Shahbazian-Yassar, R.; Hu, L.; Wang, C. Highly efficient decomposition of ammonia using high-entropy alloy catalysts. Nat. Commun. 2019, 10, 4011. [Google Scholar] [CrossRef]
- Srifa, A.; Okura, K.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. COx-free hydrogen production via ammonia decomposition over molybdenum nitride-based catalysts. Catal. Sci. Tech. 2016, 6, 7495–7504. [Google Scholar] [CrossRef]
- Cui, H.Z.; Gu, Y.Q.; He, X.X.; Wei, S.; Jin, Z.; Jia, C.J.; Song, Q.S. Iron-based composite nanostructure catalysts used to produce COx-free hydrogen from ammonia. Sci. Bull. 2016, 61, 220–226. [Google Scholar] [CrossRef]
- Cao, J.L.; Yan, Z.L.; Deng, Q.F.; Yuan, Z.Y.; Wang, Y.; Sun, G.; Wang, X.D.; Hari, B.; Zhang, Z.Y. Homogeneous precipitation method preparation of modified red mud supported Ni mesoporous catalysts for ammonia decomposition. Catal. Sci. Tech. 2014, 4, 361–368. [Google Scholar] [CrossRef]
- Ji, J.; Duan, X.Z.; Qian, G.; Zhou, X.G.; Chen, D.; Yuan, W.K. In situ production of Ni catalysts at the tips of carbon nanofibers and application in catalytic ammonia decomposition. Ind. Eng. Chem. Res. 2013, 52, 1854–1858. [Google Scholar] [CrossRef]
- Muroyama, H.; Saburi, C.; Matsui, T.; Eguchi, K. Ammonia decomposition over Ni/La2O3 catalyst for on-site generation of hydrogen. Appl. Catal. A Gen. 2012, 443, 119–124. [Google Scholar] [CrossRef]
- Do, Q.C.; Kim, Y.; Le, T.A.; Kim, G.J.; Kim, J.-R.; Kim, T.-W.; Lee, Y.-J.; Chae, H.-J. Facile one-pot synthesis of Ni-based catalysts by cation-anion double hydrolysis method as highly active Ru-free catalysts for green H2 production via NH3 decomposition. Appl. Catal. B Environ. 2022, 307, 121167. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, Y.; Long, Z.; Zhao, S.; Wang, Y.; Zhang, W. One-pot synthesis of supported Ni@Al2O3 catalysts with uniform small-sized Ni for hydrogen generation via ammonia decomposition. Int. J. Hydrog. Energy 2021, 46, 4045–4054. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, K.; Huang, H.; Cao, C.-F.; Luo, Y.; Chen, C.-Q.; Lin, L.; Au, C.; Jiang, L. Spatial confinement of electron-rich Ni nanoparticles for efficient ammonia decomposition to hydrogen production. ACS Catal. 2021, 11, 10345–10350. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.; Zhao, Y.; Zhang, R.; Zhang, J.; Guo, H. NH3 decomposition for H2 generation: Effects of cheap metals and supports on plasma-catalyst synergy. ACS Catal. 2015, 5, 4167–4174. [Google Scholar] [CrossRef]
- Duan, X.; Ji, J.; Qian, G.; Fan, C.; Zhu, Y.; Zhou, X.; Chen, D.; Yuan, W. Ammonia decomposition on Fe(110), Co(111), and Ni(111) surfaces: A density functional theory study. J. Mol. Catal. A Chem. 2012, 357, 81–86. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Li, Y. Impact of surface chemistry. Proc. Natl. Acad. Sci. USA 2011, 108, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Strongin, D.R.; Carrazza, J.; Bare, S.R.; Somorjai, G.A. The importance of C7 sites and surface roughness in the ammonia synthesis reaction over iron. J. Catal. 1987, 103, 213–215. [Google Scholar] [CrossRef]
- Boudjemaa, A.; Bouarab, R.; Saadi, S.; Bouguelia, A.; Trari, M. Photoelectrochemical H2-generation over spinel FeCr2O4 in X2- solutions (X2- = S2- and SO32-). Appl. Energy 2009, 86, 1080–1086. [Google Scholar] [CrossRef]
- Maroño, M.; Dufour, J.; Ruiz, A. Synthesis of Fe3O4-based catalysts for the high-temperature water gas shift reaction. Int. J. Hydrog. Energy 2009, 34, 4475–4481. [Google Scholar] [CrossRef]
- Maroño, M.; Ruiz, E.; Sánchez, J.M.; Martos, C.; Dufour, J.; Ruiz, A. Performance of Fe-Cr based WGS catalysts prepared by co-precipitation and oxi-precipitation methods. Int. J. Hydrog. Energy 2009, 34, 8921–8928. [Google Scholar] [CrossRef]
- Doppler, G.; Trautwein, A.X.; Ziethen, H.M. Physical and catalytic properties of high temperature water gas shift catalysts based upon iron-chromium oxides. Appl. Catal. 1988, 40, 119–130. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Z.H.; Wang, S.B.; Yao, X.D.; Yan, Z.F. Chromium oxide catalysts for COx-free hydrogen generation via catalytic ammonia decomposition. J. Mol. Catal. A Chem. 2009, 304, 71–76. [Google Scholar] [CrossRef]
- Langford, J.I. Powder pattern programs. J. Appl. Cryst. 1971, 4, 259–260. [Google Scholar] [CrossRef]
- Langford, J.I. The accuracy of cell dimensions determined by Cohen’s method of least squares and the systematic indexing of powder data. J. Appl. Cryst. 1973, 6, 190–196. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Montero-Cabrera, M.E.; Fuentes-Cobas, L.E.; Macías-Ríos, E.; Fuentes-Montero, M.E. Application of x-ray absorption fine structure(XAFS) to local-order analysis in Fe-Cr maghemite-like materials. AIP Conf. Proc. 2015, 1671, 020008. [Google Scholar]
- Gu, Y.Q.; Jin, Z.; Zhang, H.; Xu, R.J.; Zheng, M.J.; Guo, Y.M.; Song, Q.S.; Jia, C.J. Transition metal nanoparticles dispersed in an alumina matrix as active and stable catalysts for COx-free hydrogen production from ammonia. J. Mater. Chem. A 2015, 3, 17172–17180. [Google Scholar] [CrossRef]
- Bell, T.E.; Torrente-Murciano, L. H2 production via ammonia decomposition using non-noble metal catalysts: A Review. Top. Catal. 2016, 59, 1438–1457. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Liu, C.Y.; Gong, W.M.; Guo, H.C. Plasma driven ammonia decomposition on a Fe-catalyst: Eliminating surface nitrogen poisoning. Chem. Commun. 2013, 49, 3787–3789. [Google Scholar] [CrossRef] [PubMed]
- Opeyemi, O.A.; Zaman, S.F. Ammonia decomposition for hydrogen production: A thermodynamic study. Chem. Pap. 2020. (prepublish). [Google Scholar] [CrossRef]
- Feyen, M.; Weidenthaler, C.; Güttel, R.; Schlichte, K.; Holle, U.; Lu, A.H.; Schüth, F. High-temperature stable, iron-based core-shell catalysts for ammonia decomposition. Chem.-A Eur. J. 2011, 17, 598–605. [Google Scholar] [CrossRef]
- Lu, A.H.; Nitz, J.J.; Comotti, M.; Weidenthaler, C.; Schlichte, K.; Lehmann, C.W.; Terasaki, O.; Schuth, F. Spatially and size selective synthesis of Fe-based nanoparticles on ordered mesoporous supports as highly active and stable catalysts for ammonia decomposition. J. Am. Chem. Soc. 2010, 132, 14152–14162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Comotti, M.; Schüth, F.; Schlögl, R.; Su, D.S. Commercial Fe- or Co- containing carbon nanotubes as catalysts for NH3 decomposition. Chem. Commun. 2007, 19, 1916–1918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Muller, J.O.; Zheng, W.; Wang, D.; Su, D.; Schlgl, R. Individual Fe-Co alloy nanoparticles on carbon nanotubes: Structural and catalyticproperties. Nano Lett. 2008, 8, 2738–2743. [Google Scholar] [CrossRef]
- Li, Y.; Yao, L.; Liu, S.; Zhao, J.; Ji, W.; Au, C.-T. Cs-modified ironnanoparticles encapsulated in microporous and mesoporous SiO, for CO-free Hproduction via ammonia decomposition. Catal. Today 2011, 160, 79–86. [Google Scholar] [CrossRef]
- Lorenzut, B.; Montini, T.; Bevilacqua, M.; Fornasiero, P. FeMo-Based Catalysts for H2 Production by NH3 Decomposition. Appl. Catal. B 2012, 125, 409–417. [Google Scholar] [CrossRef]
- Podila, S.; Driss, H.; Zaman, S.F.; Ali, A.M.; Al-Zahrani, A.A.; Daous, M.A.; Petrov, L.A. MgFe and Mg-Co-Fe Mixed OxidesDerived from Hydrotalcites: Highly Efficient Catalysts for CO FreeHydrogen Production from NH3. Int. Hydrog. Energy 2020, 45, 873–890. [Google Scholar] [CrossRef]
- Khabouri, S.A.; Harthi, S.A.; Maekawa, T.; Yousif, A.A. Composition, Electronic and MagneticInvestigation of the Encapsulated ZnFe,O4 Nanoparticles in Multiwall Carbon NanotubesContaining Ni Residuals. Nanoscale Res. Lett. 2015, 10, 262. [Google Scholar] [CrossRef]
- Abidov, A.; Allabergenov, B.; Lee, J.; Jeon, H.W.; Jeong, S.W.; Kim, S. X-ray photoelectron spectroscopycharacterization of Fe doped TiO2 photocatalyst. Int. J. Mach. Mach. Mater. 2013, 1, 294–296. [Google Scholar]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.; Gerson, A.R.; Smart, R. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Watts, J.F.; Wolstenholme, J. An Introduction to Surface Analysis by XPS and AES[M]; John Wiley &sons Ltd.: Hoboken, NJ, USA, 2003; pp. 6–7. [Google Scholar]
- Yugo, K.; Yuichi, S.; Shigeaki, U. Changes in the chemical state of metallic Cr during deposition on a polyimide substrate: Full soft XPS and ToF-SIMS depth profiles. Appl. Surf. Sci. 2021, 553, 149437. [Google Scholar]
- Rignanese, G.M.; Pasquarello, A.; Charlier, J.C.; Gonze, X.; Car, R. Nitrogen incorporation at Si(001)-SiO2 interfaces: Relation between n 1s core-level shifts and microscopic structure. Phys. Rev. Lett. 1997, 79, 5174–5177. [Google Scholar] [CrossRef]
- Li, Q.Q.; Zhang, S.; Dai, L.M.; Li, L.S. Nitrogen-doped colloidal graphene quantum dots andtheir size-dependent electrocatalytic activity for the oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 18932–18935. [Google Scholar] [CrossRef]
- Galvita, V.; Sundmacher, K. Redox behavior and reduction mechanism of Fe2O3–CeZrO2 as oxygen storage material. J Mater Sci. 2007, 42, 9300–9307. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chen, Y.W.; Li, C.P. The mechanism of reduction of iron oxide by hydrogen. Thermochim. Acta 2003, 400, 61–67. [Google Scholar] [CrossRef]
- Kovalenko, V.V.; Rumyantseva, M.N.; Gaskov, A.M.; Makshina, E.V.; Yushchenko, V.V.; Ivanova, I.I.; Ponzoni, A.; Faglia, G.; Comini, E. SnO2/Fe2O3 nanocomposites: Ethanol-sensing performance and catalytic activity for oxidation of ethanol. Inorg. Mater. 2006, 42, 1088–1093. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Kusrowski, P.; Chmielarz, L. Chromium oxide supported on MCM-41 as a highly active and selective catalyst for dehydrogenation of propane with CO2. Appl. Catal. A Gen. 2008, 349, 62–69. [Google Scholar] [CrossRef]
- Ayari, F.; Mhamdi, M.; Debecker, D.P.; Gaigneaux, E.M.; Álvarez-Rodríguez, J.; Guerrero-Ruiz, A.; Delahay, G.; Ghorbel, A. Effect of the chromium precursor nature on the physicochemical and catalytic properties of Cr-ZSM-5 catalysts: Application to the ammoxidation of ethylene. J. Mol. Catal. A Chem. 2011, 339, 8–16. [Google Scholar] [CrossRef]
- Ilieva, L.I.; Andreeva, D.H. Investigation of the chromium oxide system by means of temperature-programmed reduction. Thermochim. Acta 1995, 265, 223–231. [Google Scholar] [CrossRef]
- Arabczyk, W.; Zamlynny, J. Study of the ammonia decomposition over iron catalysts. Catal. Lett. 1999, 60, 167–171. [Google Scholar] [CrossRef]
- Lendzion-Bieluń, Z.; Pelka, R.; Arabczyk, W. Study of the kinetics of ammonia synthesis and decomposition on iron and cobalt catalysts. Catal. Lett. 2009, 129, 119–123. [Google Scholar] [CrossRef]
- Tsubouchi, N.; Hashimoto, H.; Ohtsuka, Y. High catalytic performance of fine particles of metallic iron formed from limonite in the decomposition of a low concentration of ammonia. Catal. Lett. 2005, 105, 203–208. [Google Scholar] [CrossRef]








| Sample | Surface Atomic Ratio (Fe/Cr) a | Lattice Constants (Å) b | D (nm) c | SBET (m2/g) d Fresh/Uesd |
|---|---|---|---|---|
| Fe | 100/0 | a = 5.0329(1) c = 13.7462(2) | 56 | 13/- |
| Fe0.75Cr0.25 | 67.2/32.8 | a = 5.0192(4) c = 13.708(1) | 25 | 62/34 |
| Fe0.5Cr0.5 | 45.0/55.0 | a = 4.9863(3) c = 13.6357(7) | 16 | 84/34 |
| Fe0.25Cr0.75 | 30.2/69.8 | a = 4.9607(6) c = 13.587(1) | 16 | 51/23 |
| Cr | 0/100 | a = 4.9393(1) c =13.5549(1) | 27 | 39/17 |
| Sample | Phase Composition | (Fe,Cr)2O3 | (Fe,Cr)3O4 | Fe | Fe4N | CrN |
|---|---|---|---|---|---|---|
| Fe-used | lattice constants a(Å) a and crystal grain size D (nm) b | - | - | 2.8667(1) D = 46 | - | - |
| Fe0.75Cr0.25-used | - | 8.3778(1) | 2.8667(1) D = 53 | 3.7967(1) D = 47 | 4.1476(1) | |
| Fe0.5Cr0.5-used | a = 4.9671(3) c=13.5875(7) | 8.3776(2) | 2.8670(1) | 3.7984(1) D = 49 | 4.1469(2) D = 37 | |
| Fe0.25 Cr0.75-used | a = 4.9622(2) c=13.5762(5) D = 29 | - | 2.8668(2) D = 47 | - | 4.1449(1) D = 38 | |
| Cr-used | a =4.9638(1) c=13.5581(4) D = 32 | - | - | - | 4.1438(1) D = 38 |
| Sample | Fe-N | Fe-Fe | D. W. | ∆E0 (eV) | ||
|---|---|---|---|---|---|---|
| R (Å) | CN | R (Å) | CN | |||
| Fe0.75Cr0.25-used α | 1.94 ± 0.01 | 2.8 ± 1.1 | 2.74 ± 0.03 | 6.5 ± 1.1 | 0.006(Fe) 0.003(N) | 9.5 ± 3.2 |
| Fe0.5Cr0.5-used β | 1.71 ± 0.03 | −0.9 ± 0.6 | 2.55 ± 0.03 2.70 ± 0.01 | 2.7 ± 1.7 5.3 ± 1.9 | 7.6 ± 6.2 | |
| Fe0.25Cr0.75-used γ | _ | _ | 2.46 ± 0.01 2.83 ± 0.01 | 6.5 ± 0.8 5.1 ± 1.3 | 2.9 ± 3.8 | |
| Sample | Cr-N/O | Cr-Cr | D. W. | ∆E0 (eV) | ||
|---|---|---|---|---|---|---|
| R (Å) | CN | R (Å) | CN | |||
| Fe0.75Cr0.25-used | 2.02 ± 0.04 | 6.4 ± 0.6 | 2.95 ± 0.02 | 9.7 ± 0.5 | 0.005(Cr) | 9.9 ± 0.5 |
| Fe0.5Cr0.5-used | 2.02 ± 0.05 | 6.3 ± 0.5 | 2.94 ± 0.01 | 9.0 ± 0.5 | ||
| Fe0.25Cr0.75-used | 2.02 ± 0.05 | 6.2 ± 0.5 | 2.94 ± 0.01 | 7.6 ± 0.4 | ||
| Cr-used | 2.00 ± 0.07 | 6.6 ± 0.6 | 2.93 ± 0.00 | 4.2 ± 0.6 | ||
| Sample | Chemical State | ||||
|---|---|---|---|---|---|
| Fe2O3 | Fe Metal | Cr3+ | Metal Nitrides | C-N | |
| (%) | (%) | (%) | (%) | (%) | |
| Fe-used | 89.86 | 10.14 | - | - | - |
| Fe0.75Cr0.25-used | 100 | 0 | 100 | 97.22 | 2.78 |
| Fe0.5Cr0.5-used | 100 | 0 | 100 | 94.39 | 5.61 |
| Fe0.25Cr0.75-used | 100 | 0 | 100 | 85.38 | 14.62 |
| Cr-used | - | - | 100 | 92.42 | 7.58 |
| Sample | TR (°C) a | T50 (°C) b | H2 Consumption (μmol/g) c |
|---|---|---|---|
| Fe | 362, 592 | 532 | 21,328 |
| Fe0.75Cr0.25 | 271, 377, 438 | 490 | 7498 |
| Fe0.5Cr0.5 | 275, 377, 447 | 513 | 6552 |
| Fe0.25Cr0.75 | 263, 355, 491 | 520 | 4241 |
| Cr | 264, 321 | 608 | 1563 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, M.; Guo, L.; Ren, H.; Tao, X.; Li, Y.; Nan, B.; Si, R.; Chen, C.; Li, L. Non-Noble FeCrOx Bimetallic Nanoparticles for Efficient NH3 Decomposition. Nanomaterials 2023, 13, 1280. https://doi.org/10.3390/nano13071280
Du M, Guo L, Ren H, Tao X, Li Y, Nan B, Si R, Chen C, Li L. Non-Noble FeCrOx Bimetallic Nanoparticles for Efficient NH3 Decomposition. Nanomaterials. 2023; 13(7):1280. https://doi.org/10.3390/nano13071280
Chicago/Turabian StyleDu, Meng, Lingling Guo, Hongju Ren, Xin Tao, Yunan Li, Bing Nan, Rui Si, Chongqi Chen, and Lina Li. 2023. "Non-Noble FeCrOx Bimetallic Nanoparticles for Efficient NH3 Decomposition" Nanomaterials 13, no. 7: 1280. https://doi.org/10.3390/nano13071280
APA StyleDu, M., Guo, L., Ren, H., Tao, X., Li, Y., Nan, B., Si, R., Chen, C., & Li, L. (2023). Non-Noble FeCrOx Bimetallic Nanoparticles for Efficient NH3 Decomposition. Nanomaterials, 13(7), 1280. https://doi.org/10.3390/nano13071280







