Biogenic Synthesis of Cu-Mn Bimetallic Nanoparticles Using Pumpkin Seeds Extract and Their Characterization and Anticancer Efficacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Seed Extract
2.3. Biogenic Synthesis of CMBNPs
2.4. Characterization of CMBNPs
2.5. Cell Culture Maintenance
2.6. Neutral Red (NR) Uptake Assay
2.7. In Vitro Scratch Assay
2.8. Comet Assay
3. Results and Discussion
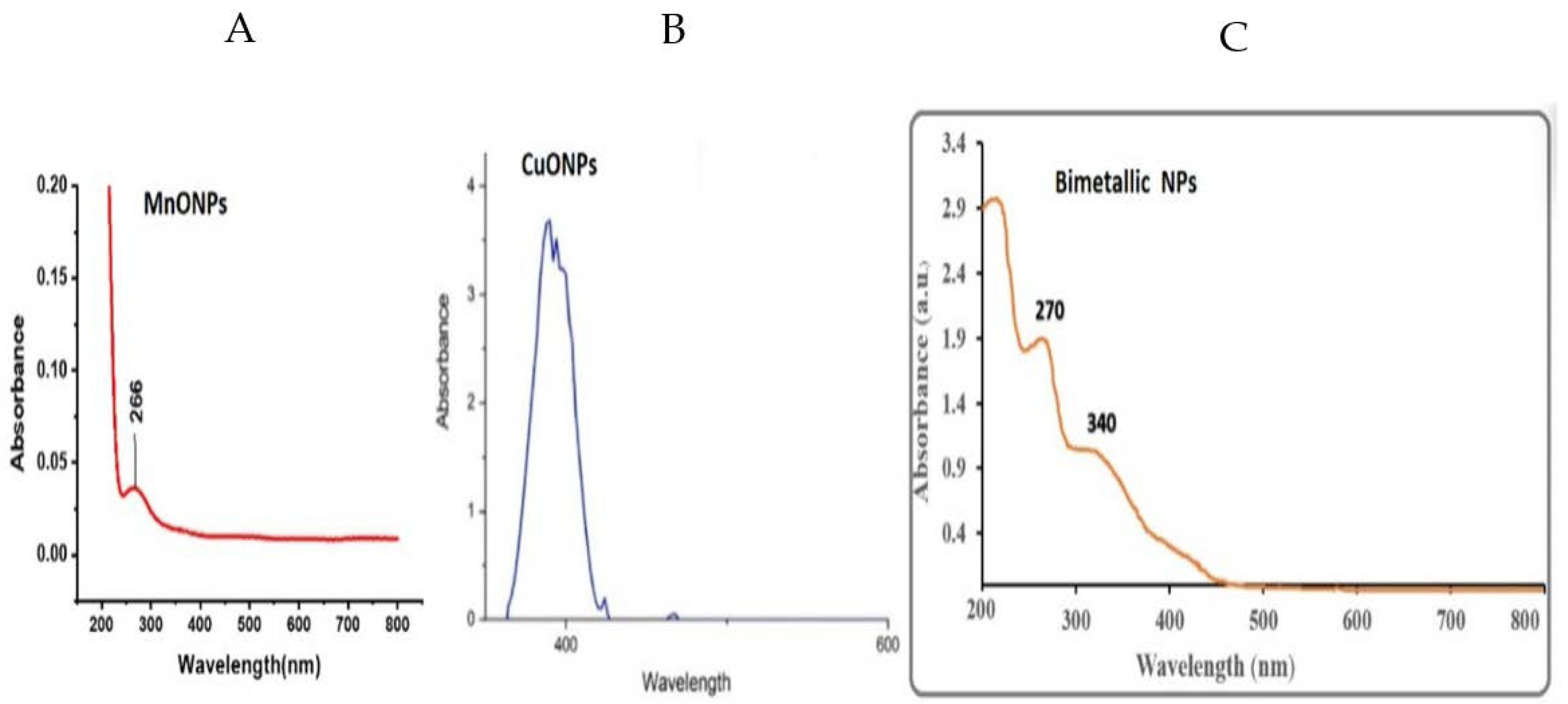
3.1. UV-Vis Spectroscopy
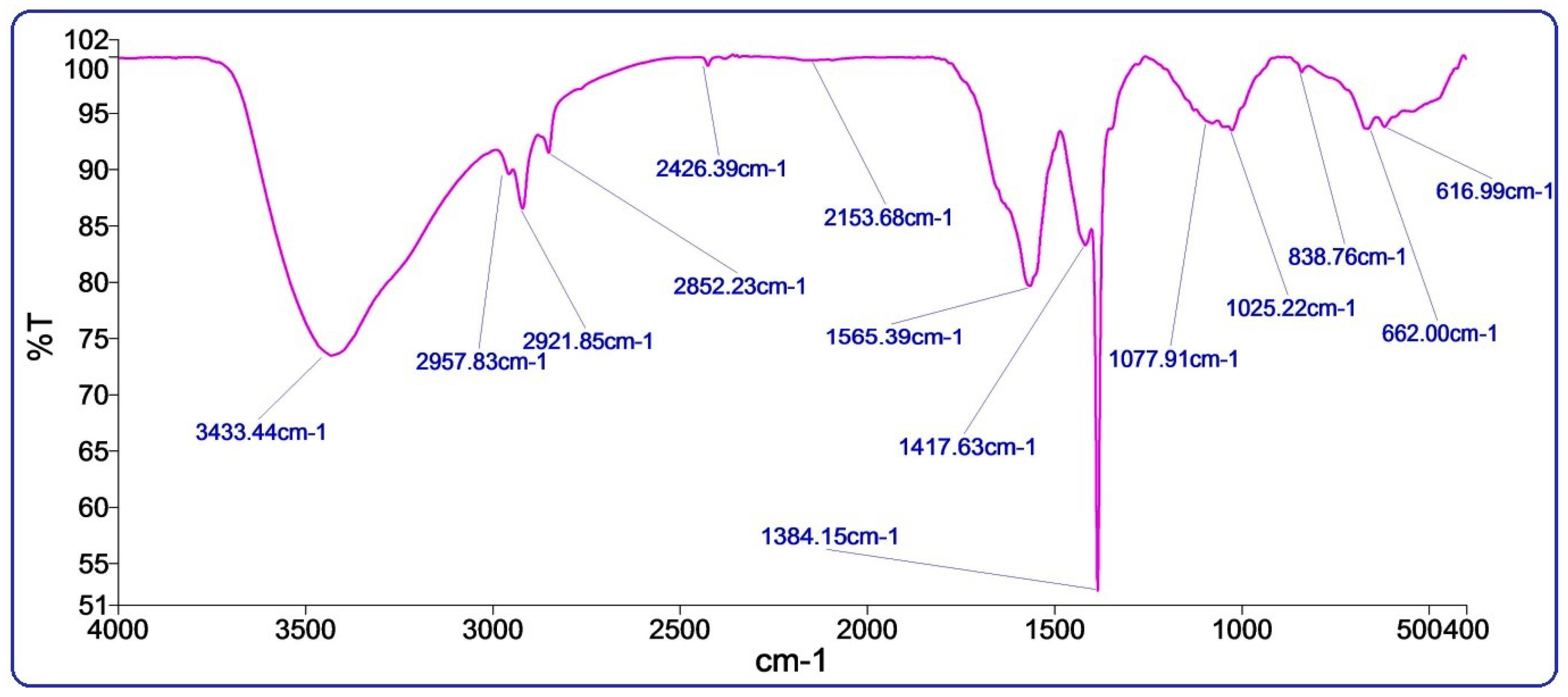
3.2. FTIR Spectroscopy
3.3. X-ray Diffraction (XRD) Pattern of Cu-Mn Bimetallic Nanoparticles
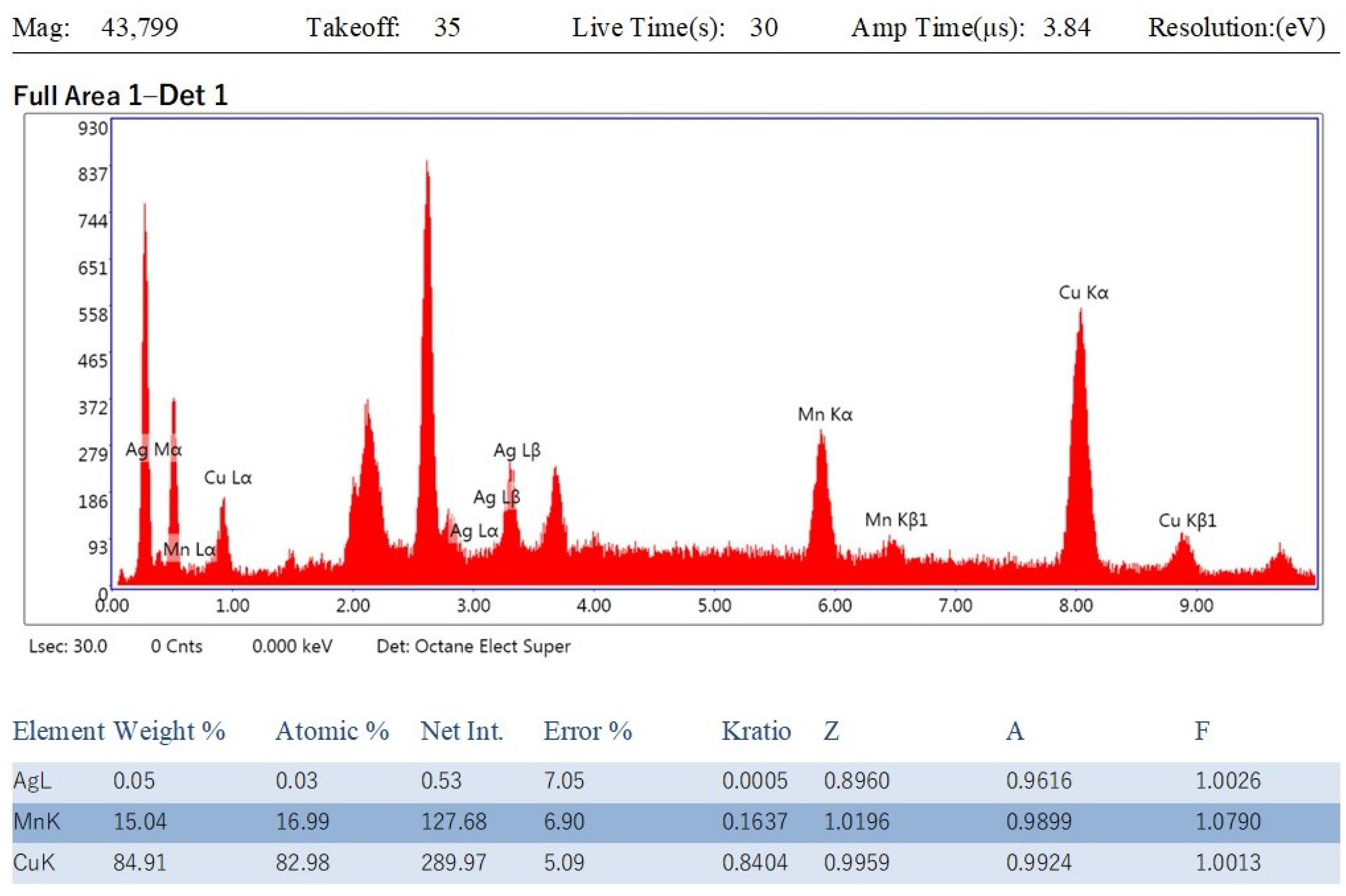
3.4. Energy-Dispersive X-ray (EDX) Analysis of Cu-Mn Bimetallic Nanoparticles
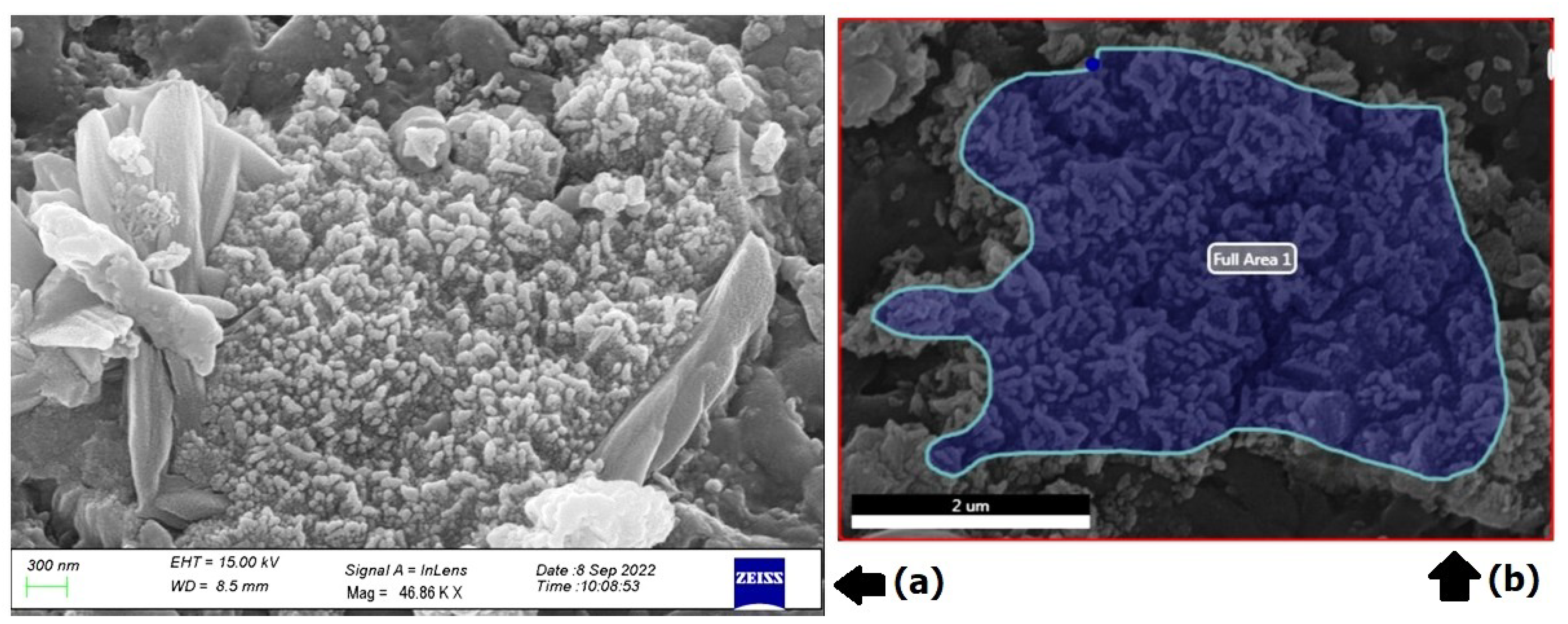
3.5. Scanning Electron Microscopy (SEM)
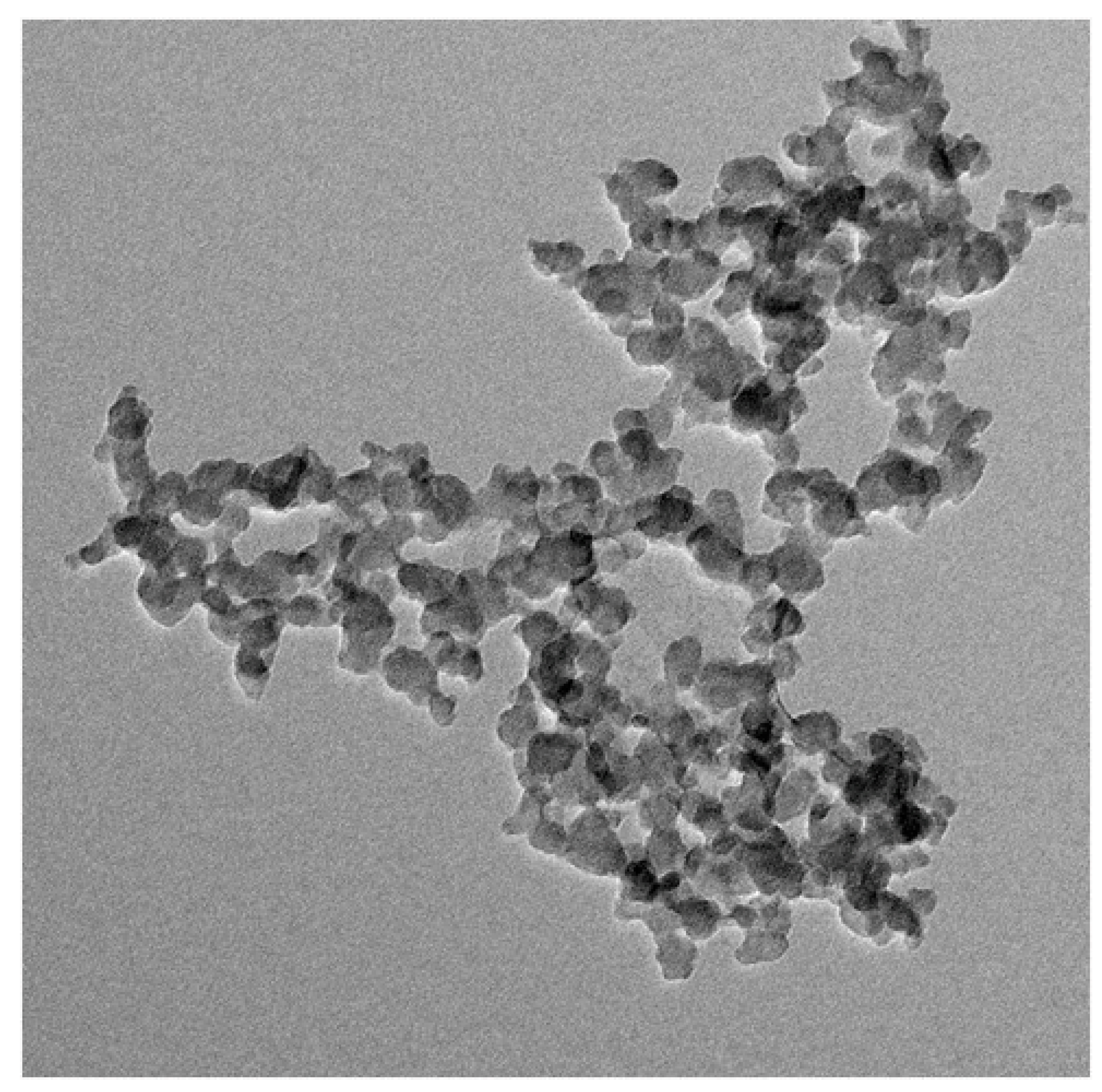
3.6. TEM Analysis
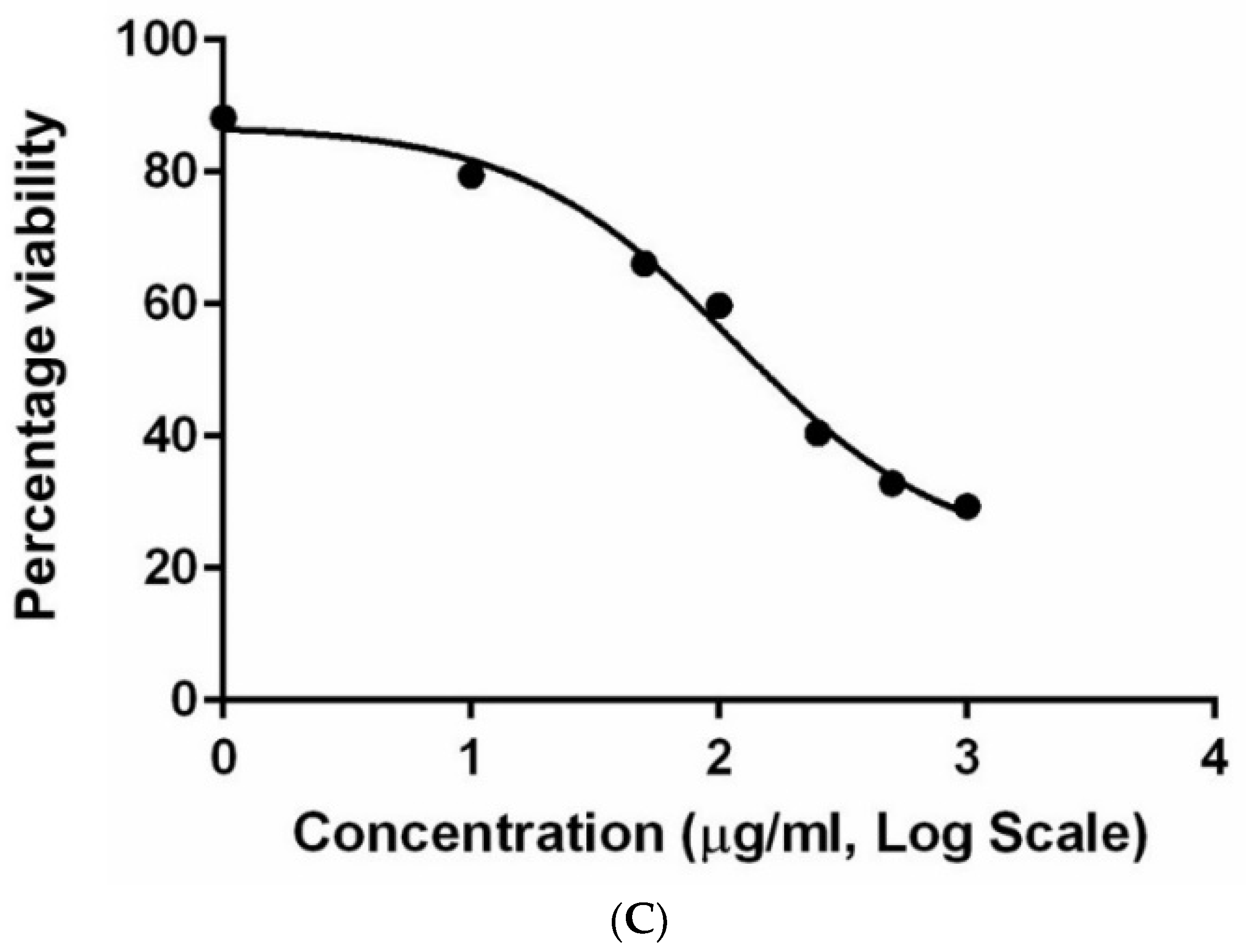
3.7. Cytotoxicity Analysis
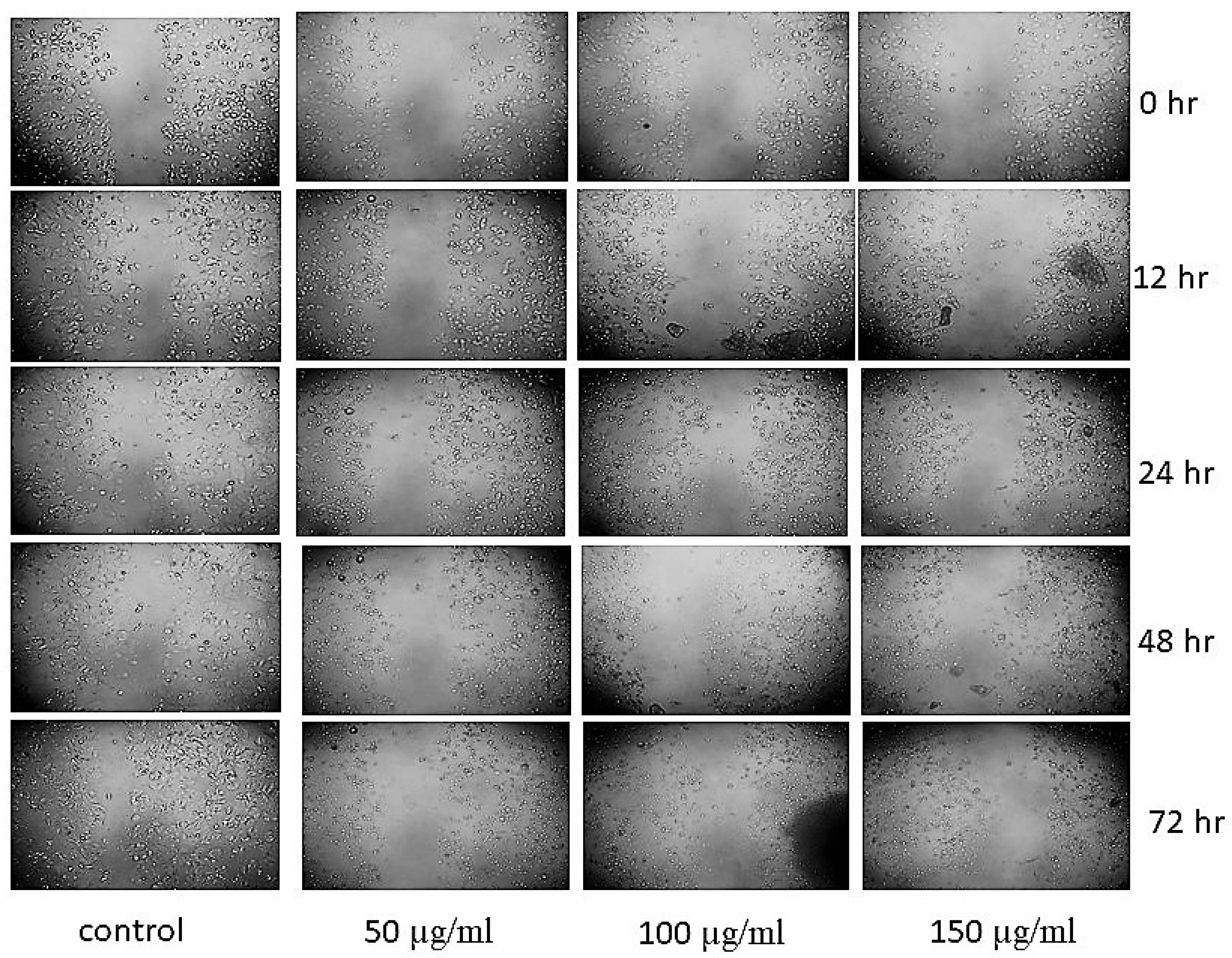
3.8. Scratch Assay
3.9. CMBNP-Induced DNA Damage in HT-29 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Tudor, C. A Novel Approach to Modeling and Forecasting Cancer Incidence and Mortality Rates through Web Queries and Automated Forecasting Algorithms: Evidence from Romania. Biology 2022, 11, 857. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Sig. Transduct. Target Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, C.; Zeng, L.; Zhou, Q.-X.; Fan, Y.-F. Editorial: Novel Small-Molecule Agents in Overcoming Multidrug Resistance in Cancers. Front. Chem. 2022, 10, 921985. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Yadav, P.; Ambudkar, S.V.; Rajendra Prasad, N. Emerging nanotechnology-based therapeutics to combat multidrug-resistant cancer. J. Nanobiotechnol. 2022, 20, 423. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Li, S.; Chen, C.; Xu, L.; Huang, P.; Liu, F.; Su, Y.; Qi, M.; Yu, C.; et al. In situ supramolecular polymerization-enhanced self-assembly of polymer vesicles for highly efficient photothermal therapy. Nat. Commun. 2020, 11, 1724. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, F.; Peng, Y.; Xie, T.; Wang, Y.; Lan, Y. Current Progress in Cancer Treatment Using Nanomaterials. Front. Oncol. 2022, 12, 930125. [Google Scholar] [CrossRef]
- Mujahid, M.H.; Upadhyay, T.K.; Khan, F.; Pandey, P.; Park, M.N.; Sharangi, A.B.; Saeed, M.; Upadhye, V.J.; Kim, B. Metallic and metal oxide-derived nanohybrid as a tool for biomedical applications. Biomed. Pharmacother. 2022, 155, 113791. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, W.; Luo, N.; Xue, Z.; Hu, Q.; Zeng, W.; Xu, J. Bimetallic Nanocrystals: Structure, Controllable Synthesis and Applications in Catalysis, Energy and Sensing. Nanomaterials 2021, 11, 1926. [Google Scholar] [CrossRef]
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic Nanoparticles for Antimicrobial Applications. Front. Chem. 2020, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Berta, L.; Coman, N.-A.; Rusu, A.; Tanase, C. A Review on Plant-Mediated Synthesis of Bimetallic Nanoparticles, Characterisation and Their Biological Applications. Materials 2021, 14, 7677. [Google Scholar] [CrossRef]
- Malik, M.A.; Alshehri, A.A.; Patel, R. Facile one-pot green synthesis of Ag–Fe bimetallic nanoparticles and their catalytic capability for 4-nitrophenol reduction. J. Mater. Res. Technol. 2021, 12, 455–470. [Google Scholar] [CrossRef]
- Katifelis, H.; Mukha, I.; Bouziotis, P.; Vityuk, N.; Tsoukalas, C.; Lazaris, A.C.; Lyberopoulou, A.; Theodoropoulos, G.E.; Efstathopoulos, E.P.; Gazouli, M. Ag/Au Bimetallic Nanoparticles Inhibit Tumor Growth and Prevent Metastasis in a Mouse Model. IJN 2020, 15, 6019–6032. [Google Scholar] [CrossRef]
- Cao, Y.; Dhahad, H.A.; El-Shorbagy, M.A.; Alijani, H.Q.; Zakeri, M.; Heydari, A.; Bahonar, E.; Slouf, M.; Khatami, M.; Naderifar, M.; et al. Green synthesis of bimetallic ZnO–CuO nanoparticles and their cytotoxicity properties. Sci. Rep. 2021, 11, 23479. [Google Scholar] [CrossRef]
- Elsayed, K.A.; Alomari, M.; Drmosh, Q.A.; Alheshibri, M.; Al Baroot, A.; Kayed, T.S.; Manda, A.A.; Al-Alotaibi, A.L. Fabrication of ZnO-Ag bimetallic nanoparticles by laser ablation for anticancer activity. Alex. Eng. J. 2022, 61, 1449–1457. [Google Scholar] [CrossRef]
- Al Tamimi, S.; Ashraf, S.; Abdulrehman, T.; Parray, A.; Mansour, S.A.; Haik, Y.; Qadri, S. Synthesis and analysis of silver–copper alloy nanoparticles of different ratios manifest anticancer activity in breast cancer cells. Cancer Nanotechnol. 2020, 11, 13. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Khan, M.S.; Alomari, A.; Tabrez, S.; Hassan, I.; Wahab, R.; Bhat, S.A.; Alafaleq, N.O.; Altwaijry, N.; Shaik, G.M.; Zaidi, S.K.; et al. Anticancer Potential of Biogenic Silver Nanoparticles: A Mechanistic Study. Pharmaceutics 2021, 13, 707. [Google Scholar] [CrossRef] [PubMed]
- Alafaleq, N.O.; Alomari, A.; Khan, M.S.; Shaik, G.M.; Hussain, A.; Ahmed, F.; Hassan, I.; Alhazza, I.M.; Alokail, M.S.; Alenad, A.M.H.; et al. Anticancer potential of gold nanoparticles (AuNPs) using a battery of in vitro tests. Nanotechnol. Rev. 2022, 11, 3292–3304. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell. Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Alam, M.W.; Al Qahtani, H.S.; Souayeh, B.; Ahmed, W.; Albalawi, H.; Farhan, M.; Abuzir, A.; Naeem, S. Novel Copper-Zinc-Manganese Ternary Metal Oxide Nanocomposite as Heterogeneous Catalyst for Glucose Sensor and Antibacterial Activity. Antioxidants 2022, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Jayandran, M.; Haneefa, M.M.; Balasubramanian, V. Green synthesis and characterization of Manganese nanoparticles using natural plant extracts and its evaluation of antimicrobial activity. J. App. Pharm. Sci. 2015, 5, 105–110. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Kotb, H.M.; Mushtaq, S.; Waheed-Ur-Rehman, M.; Maghanga, C.M.; Alam, M.W. Green Synthesis of Mn + Cu Bimetallic Nanoparticles Using Vinca rosea Extract and Their Antioxidant, Antibacterial, and Catalytic Activities. Crystals 2022, 12, 72. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Sajadi, S.M. Biosynthesis of copper nanoparticles supported on manganese dioxide nanoparticles using Centella asiatica L. leaf extract for the efficient catalytic reduction of organic dyes and nitroarenes. Chin. J. Catal. 2018, 39, 109–117. [Google Scholar] [CrossRef]
- Abd-Elhady, H.M.; Ashor, M.A.; Hazem, A.; Saleh, F.M.; Selim, S.; El Nahhas, N.; Abdel-Hafez, S.H.; Sayed, S.; Hassan, E.A. Biosynthesis and Characterization of Extracellular Silver Nanoparticles from Streptomyces aizuneusis: Antimicrobial, Anti Larval, and Anticancer Activities. Molecules 2022, 27, 212. [Google Scholar] [CrossRef]
- Zadeh, F.A.; Bokov, D.O.; Salahdin, O.D.; Abdelbasset, W.K.; Jawad, M.A.; Kadhim, M.M.; Qasim, M.T.; Kzar, H.H.; Al-Gazally, M.E.; Mustafa, Y.F.; et al. Cytotoxicity evaluation of environmentally friendly synthesis Copper/Zinc bimetallic nanoparticles on MCF-7 cancer cells. Rend. Fis. Acc. Lincei 2022, 33, 441–447. [Google Scholar] [CrossRef]
- Li Petri, G.; Cascioferro, S.; El Hassouni, B.; Carbone, D.; Parrino, B.; Cirrincione, G.; Peters, G.J.; Diana, P.; Giovannetti, E. Biological Evaluation of the Antiproliferative and Anti-migratory Activity of a Series of 3-(6-Phenylimidazo[2,1-b][1,3,4]thiadiazol-2-yl)-1H-indole Derivatives Against Pancreatic Cancer Cells. Anticancer Res. 2019, 39, 3615–3620. [Google Scholar] [CrossRef]
- Armistead, F.J.; Gala De Pablo, J.; Gadêlha, H.; Peyman, S.A.; Evans, S.D. Physical Biomarkers of Disease Progression: On-Chip Monitoring of Changes in Mechanobiology of Colorectal Cancer Cells. Sci. Rep. 2020, 10, 3254. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.H.; Zhang, H.T.; Wang, Y.T.; Liu, S.; Zhou, W.L.; Yuan, X.Z.; Li, T.Y.; Wu, C.F.; Yang, J.Y. Disulfiram combined with copper inhibits metastasis and epithelial–mesenchymal transition in hepatocellular carcinoma through the NF-κB and TGF-β pathways. J. Cell. Mol. Med. 2018, 22, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.B.; Manguinhas, R.; Costa, J.G.; Saraiva, N.; Gil, N.; Rosell, R.; Camões, S.P.; Batinic-Haberle, I.; Spasojevic, I.; Castro, M.; et al. MnTnHex-2-PyP5+ Displays Anticancer Properties and Enhances Cisplatin Effects in Non-Small Cell Lung Cancer Cells. Antioxidants 2022, 11, 2198. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Vighetto, V.; Di Marzio, N.; Ferraro, F.; Hirsch, M.; Ferrante, N.; Mitra, S.; Grattoni, A.; Filgueira, C.S. Gold Nanoparticles Radio-Sensitize and Reduce Cell Survival in Lewis Lung Carcinoma. Nanomaterials 2020, 10, 1717. [Google Scholar] [CrossRef]
- Liu, X.; Kifle, M.T.; Xie, H.; Xu, L.; Luo, M.; Li, Y.; Huang, Z.; Gong, Y.; Wu, Y.; Xie, C. Biomineralized Manganese Oxide Nanoparticles Synergistically Relieve Tumor Hypoxia and Activate Immune Response with Radiotherapy in Non-Small Cell Lung Cancer. Nanomaterials 2022, 12, 3138. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zou, Z.; Wang, B.; Xu, G.; Chen, C.; Qin, X.; Yu, C.; Zhang, J. Copper Oxide Nanoparticles Induce Oxidative DNA Damage and Cell Death via Copper Ion-Mediated P38 MAPK Activation in Vascular Endothelial Cells. IJN 2020, 15, 3291–3302. [Google Scholar] [CrossRef]
- Mukha, I.; Vityuk, N.; Grodzyuk, G.; Shcherbakov, S.; Lyberopoulou, A.; Efstathopoulos, E.P.; Gazouli, M. Anticancer Effect of Ag, Au, and Ag/Au Bimetallic Nanoparticles Prepared in the Presence of Tryptophan. J. Nanosci. Nanotechnol. 2017, 17, 8987–8994. [Google Scholar] [CrossRef]
- Shmarakov, I.; Mukha, I.; Vityuk, N.; Borschovetska, V.; Zhyshchynska, N.; Grodzyuk, G.; Eremenko, A. Antitumor Activity of Alloy and Core-Shell-Type Bimetallic AgAu Nanoparticles. Nanoscale Res. Lett. 2017, 12, 333. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, X.; Velu, P.; Liu, X.; Vijayalakshmi, A. Enhanced green mediated synthesis of optimized Ag-Cu bimetallic nanoparticles using Leucas aspera and its application in Anti-cancer activity against alveolar cancer. Mater. Lett. 2022, 313, 131645. [Google Scholar] [CrossRef]
- Merugu, R.; Gothalwal, R.; Kaushik Deshpande, P.; De Mandal, S.; Padala, G.; Latha Chitturi, K. Synthesis of Ag/Cu and Cu/Zn bimetallic nanoparticles using toddy palm: Investigations of their antitumor, antioxidant and antibacterial activities. Mater. Today Proc. 2021, 44, 99–105. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Nundkumar, N.; Singh, M.; Iyekowa, O. Green synthesis of Ag, Au and Ag-Au bimetallic nanoparticles using Stigmaphyllon ovatum leaf extract and their in vitro anticancer potential. Mater. Lett. 2019, 243, 148–152. [Google Scholar] [CrossRef]


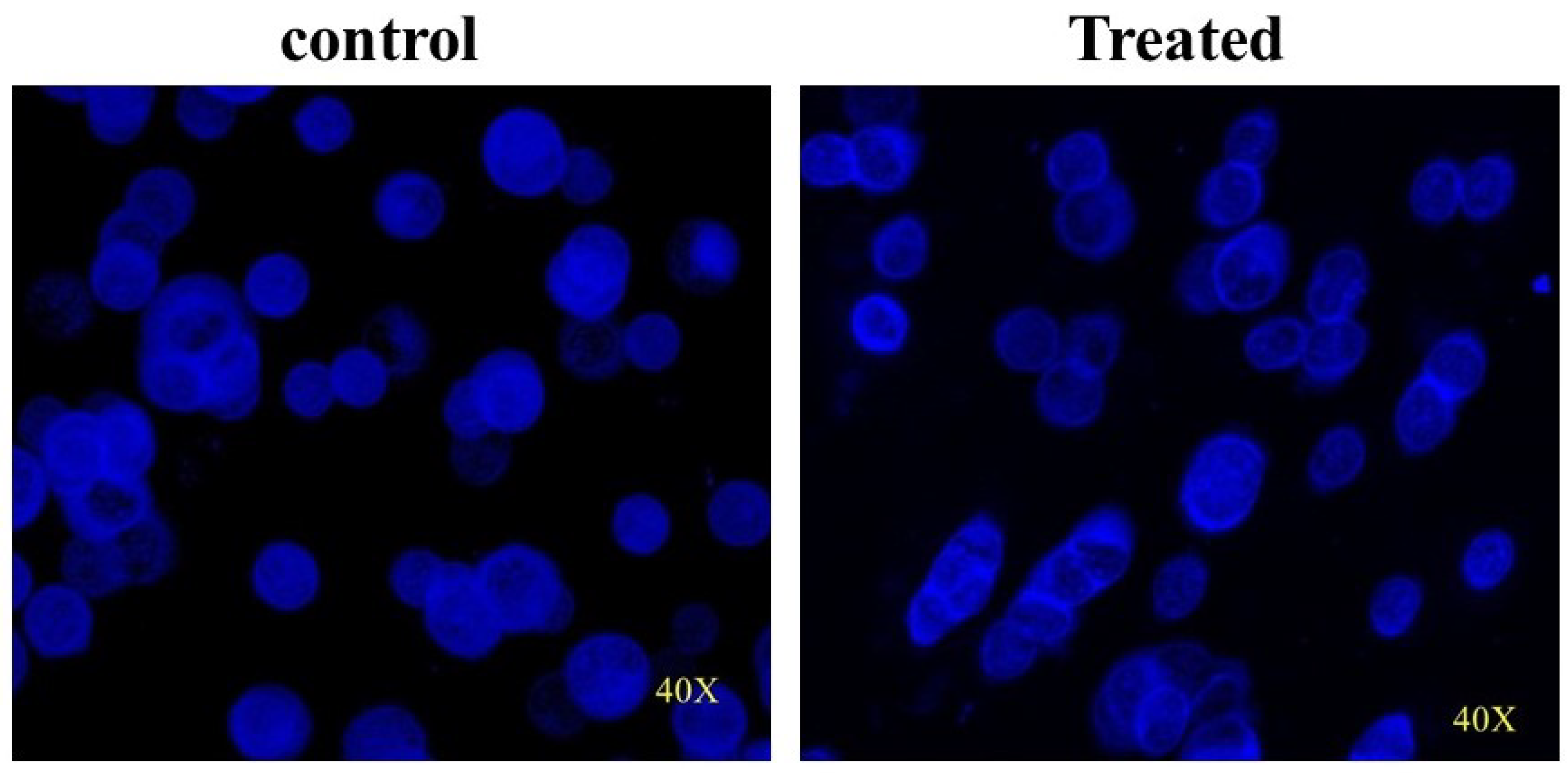
| Control (µm) | Treated (µm) | |
|---|---|---|
| Comet area | 6101.28 ± 814.99 | 8843.92 ± 569.07 |
| Comet length | 70.5 ± 5.97 | 103.51 ± 4.18 |
| Head length | 56.87 ± 6.57 | 58.51 ± 4.71 |
| Head DNA percentage | 61.83 ± 5.18 | 43.98 ± 5.12 |
| Tail length | 13.63 ± 2.47 | 45 ± 5.18 |
| Tail DNA percentage | 38.18 ± 5.18 | 56.03 ± 5.12 |
| Tail moment | 11.97 ± 2.36 | 38.7 ± 4.93 |
| Olive moment | 7.05 ± 1.26 | 23.31 ± 2.69 |
| Bimetallic Nanoparticles | Species | Application | Shape/Morphology | Size | References |
|---|---|---|---|---|---|
| Ag/Au | Amino acid tryptophan | Antitumor effect/cytotoxicity | Cubic/smaller spherical | 50–100 nm | [38] |
| Ag/Au | Alloy and core–shell | Anti-cancerous prototype | Spherical | 25–50 nm | [39] |
| Ag/Au | Amino acid tryptophan | Tumor growth and prevent metastasis in a mouse model | - | - | [16] |
| Ag-Cu | Leucas aspera | Anticancer activity against alveolar cancer | Tetragonal, smooth-surfaced spherical structures | 20 nm | [40] |
| Ag/Cu and Cu/Zn | Toddy palm | Antitumor, antioxidant, and antibacterial activity | 80 nm, 100 nm | [41] | |
| Ag-Au and Ag-Au | Stigmaphyllon ovatum | In vitro anticancer potential | Triangular | 23.5 nm, 78 nm 14.9 nm | [42] |
| Zno-Ag | Laser ablation | Anticancer activity | Hexagonal | 30–130 nm | [18] |
| Cu-Mn | Pumpkin seeds extract | Anticancer activity | Spherical | 50 nm | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alafaleq, N.O.; Zughaibi, T.A.; Jabir, N.R.; Khan, A.U.; Khan, M.S.; Tabrez, S. Biogenic Synthesis of Cu-Mn Bimetallic Nanoparticles Using Pumpkin Seeds Extract and Their Characterization and Anticancer Efficacy. Nanomaterials 2023, 13, 1201. https://doi.org/10.3390/nano13071201
Alafaleq NO, Zughaibi TA, Jabir NR, Khan AU, Khan MS, Tabrez S. Biogenic Synthesis of Cu-Mn Bimetallic Nanoparticles Using Pumpkin Seeds Extract and Their Characterization and Anticancer Efficacy. Nanomaterials. 2023; 13(7):1201. https://doi.org/10.3390/nano13071201
Chicago/Turabian StyleAlafaleq, Nouf Omar, Torki A. Zughaibi, Nasimudeen R. Jabir, Azhar U. Khan, Mohd Shahnawaz Khan, and Shams Tabrez. 2023. "Biogenic Synthesis of Cu-Mn Bimetallic Nanoparticles Using Pumpkin Seeds Extract and Their Characterization and Anticancer Efficacy" Nanomaterials 13, no. 7: 1201. https://doi.org/10.3390/nano13071201
APA StyleAlafaleq, N. O., Zughaibi, T. A., Jabir, N. R., Khan, A. U., Khan, M. S., & Tabrez, S. (2023). Biogenic Synthesis of Cu-Mn Bimetallic Nanoparticles Using Pumpkin Seeds Extract and Their Characterization and Anticancer Efficacy. Nanomaterials, 13(7), 1201. https://doi.org/10.3390/nano13071201





