Co-Carbonized Waste Polythene/Sugarcane Bagasse Nanocomposite for Aqueous Environmental Remediation Applications
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Characterization
2.3. Development of SBPE and SBPEAC Composites
2.4. Adsorption Studies
3. Results and Discussion
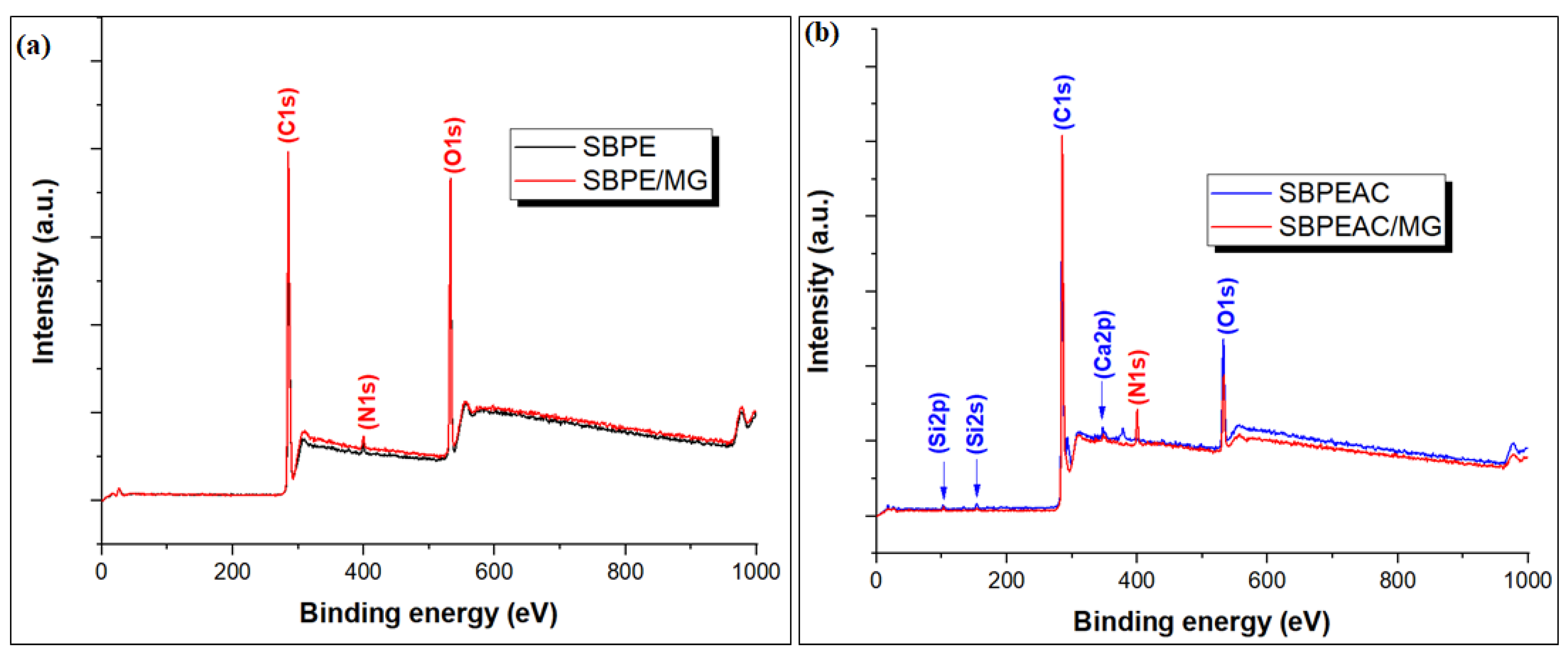
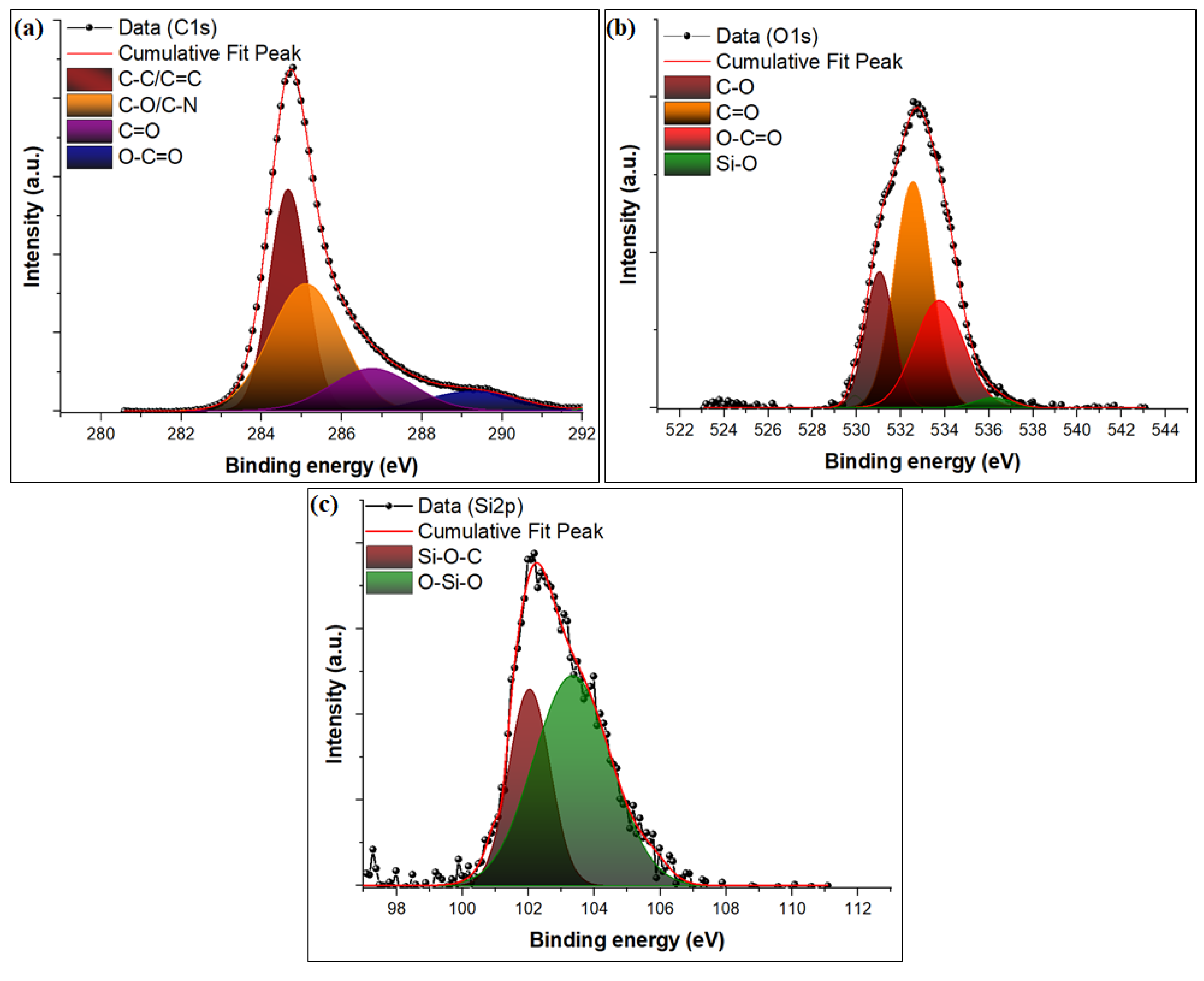
3.1. Characterization of Composites
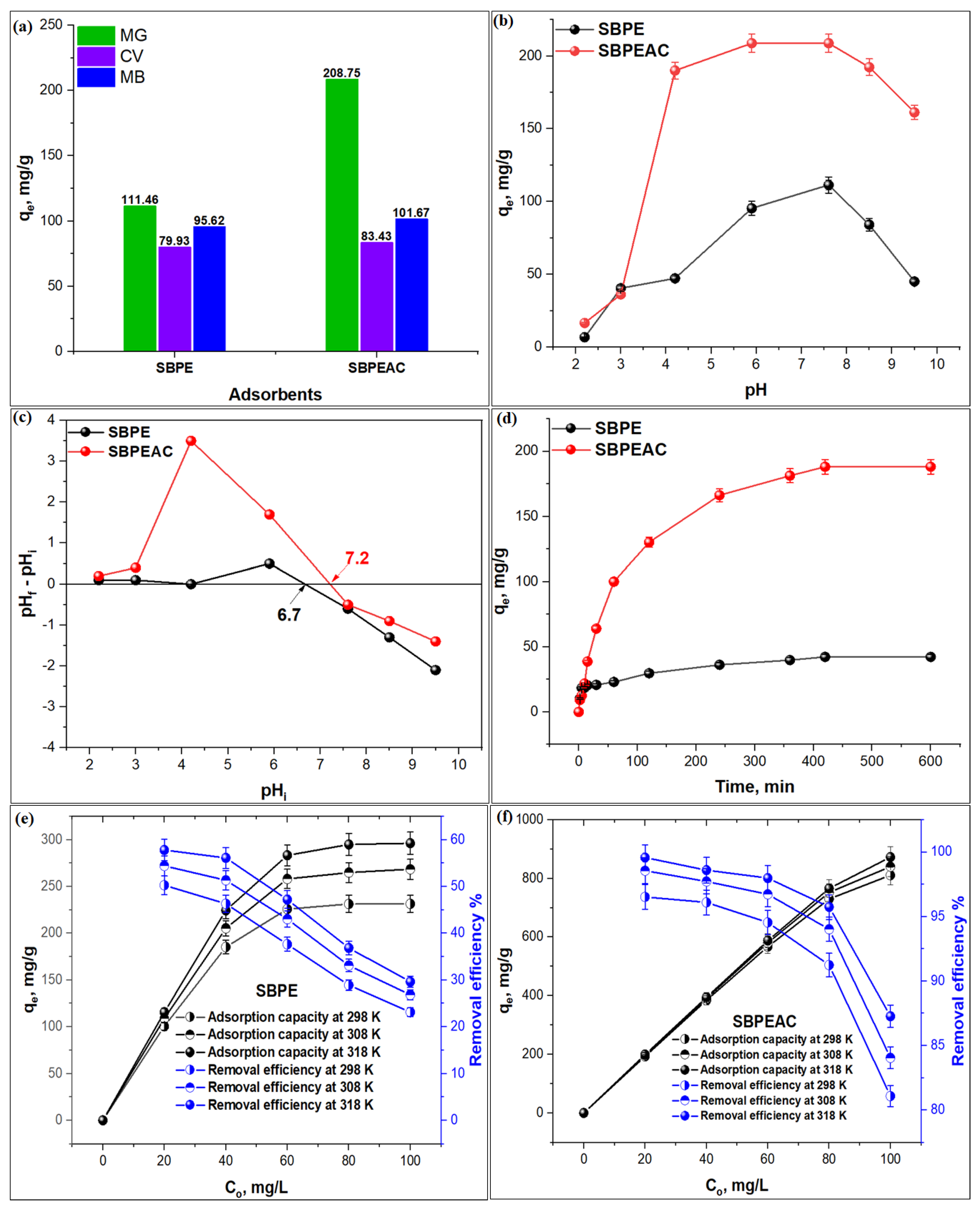
3.2. Adsorption Studies
3.2.1. Selectivity Study
3.2.2. Effect of pH
3.2.3. Effect of Contact Time
3.2.4. Effect of Initial Concentration and Temperature
- The solution pH significantly influences MG adsorption on both SBPE and SBPEAC composites with maximum uptake at pH 7.6.
- The MG adsorption on both composites increases with time, attaining equilibrium at 420 min. The observed adsorption capacities at the equilibrium on SBPE and SBPEAC were 42.3 and 181.3 mg/g, respectively.
- The adsorption process was endothermic, with maximum adsorption efficiencies of 57.8 and 99.6% on SBPE and SBPEAC at 318 K, respectively.
- The results reveal that the SBPEAC composite has an excellent ability to remove MG at 80 and 100 mg/L with a 95.7 and 87.3% removal efficiency, respectively.
3.3. Adsorption Modeling
3.3.1. Adsorption Isotherm
3.3.2. Adsorption Kinetic
3.3.3. Adsorption Thermodynamics
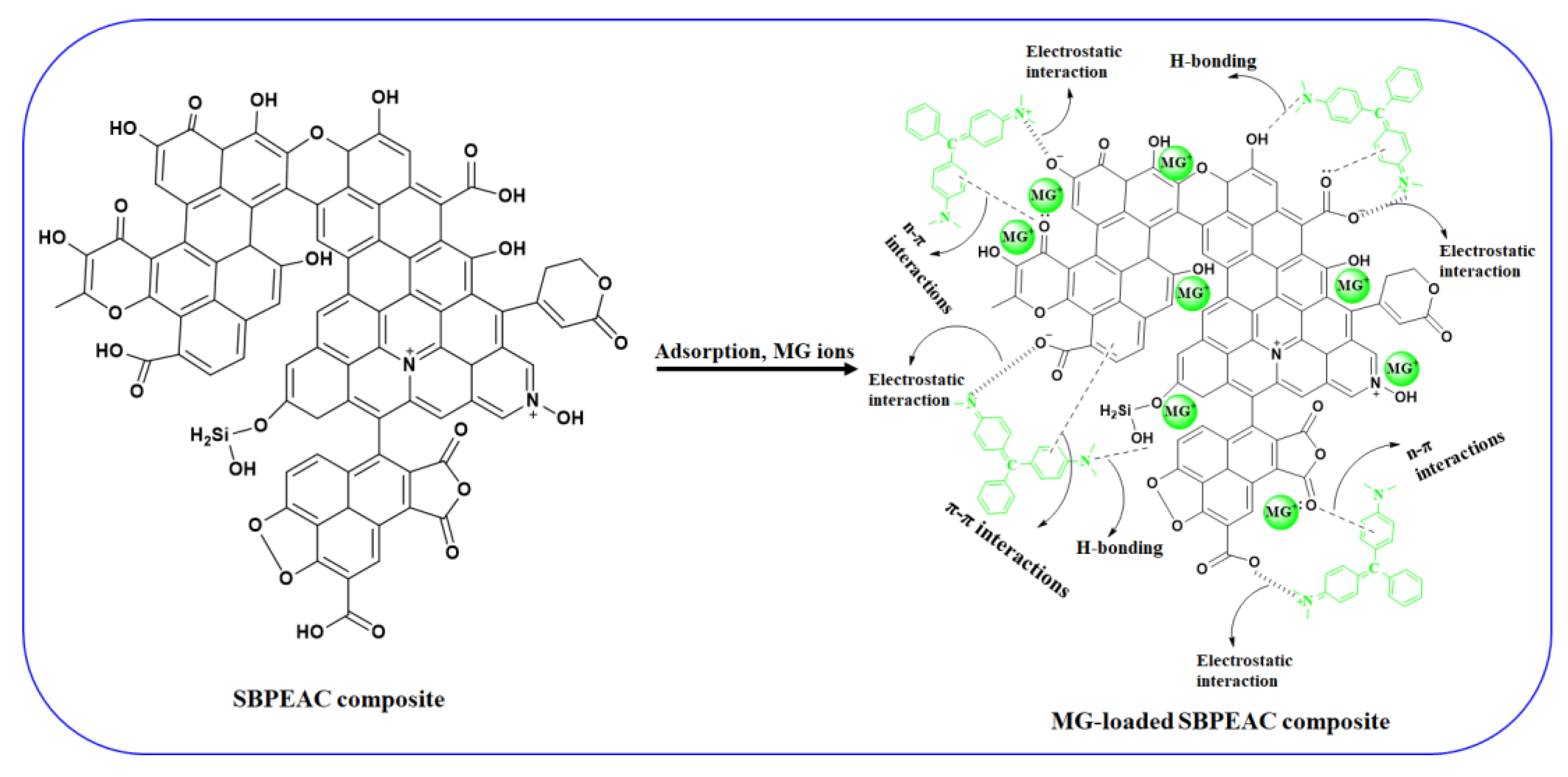
3.4. MG Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aruna; Bagotia, N.; Sharma, A.K.; Kumar, S. A review on modified sugarcane bagasse biosorbent for removal of dyes. Chemosphere 2021, 268, 129309. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jellali, S.; Akrout, H.; Assadi, A.A.; Bousselmi, L. Dynamic investigations on cationic dye desorption from chemically modified lignocellulosic material using a low-cost eluent: Dye recovery and anodic oxidation efficiencies of the desorbed solutions. J. Clean. Prod. 2018, 201, 28–38. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mukherjee, A.; Das, S.; Maddela, N.R.; Iram, S.; Das, P. Study on isotherm, kinetics, and thermodynamics of adsorption of crystal violet dye by calcium oxide modified fly ash. Environ. Eng. Res. 2021, 26, 190372. [Google Scholar] [CrossRef]
- Du, F.; Sun, L.; Huang, Z.; Chen, Z.; Xu, Z.; Ruan, G.; Zhao, C. Electrospun reduced graphene oxide/TiO2/poly(acrylonitrile-co-maleic acid) composite nanofibers for efficient adsorption and photocatalytic removal of malachite green and leucomalachite green. Chemosphere 2020, 239, 124764. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Ahmad, M.A.; Yahaya, N.K.E.; Karim, J. Adsorption of malachite green by activated carbon derived from gasified Hevea brasiliensis root. Arab. J. Chem. 2021, 14, 103104. [Google Scholar] [CrossRef]
- Gopinathan, R.; Kanhere, J.; Banerjee, J. Effect of malachite green toxicity on non target soil organisms. Chemosphere 2015, 120, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Manyatshe, A.; Cele, Z.E.; Balogun, M.O.; Nkambule, T.T.; Msagati, T.A. Chitosan modified sugarcane bagasse biochar for the adsorption of inorganic phosphate ions from aqueous solution. J. Environ. Chem. Eng. 2022, 10, 108243. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Pyrolysis of waste high density polyethylene (HDPE) and low density polyethylene (LDPE) plastics and production of epoxy composites with their pyrolysis chars. J. Clean. Prod. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Renita, A.A.; Vardhan, K.H.; Kumar, P.S.; Ngueagni, P.T.; Abilarasu, A.; Nath, S.; Kumari, P.; Saravanan, R. Effective removal of malachite green dye from aqueous solution in hybrid system utilizing agricultural waste as particle electrodes. Chemosphere 2021, 273, 129634. [Google Scholar] [CrossRef]
- Sonawane, G.; Shrivastava, V. Kinetics of decolourization of malachite green from aqueous medium by maize cob (Zea maize): An agricultural solid waste. Desalination 2009, 247, 430–441. [Google Scholar] [CrossRef]
- Mittal, A.; Krishnan, L.; Gupta, V. Removal and recovery of malachite green from wastewater using an agricultural waste material, de-oiled soya. Sep. Purif. Technol. 2005, 43, 125–133. [Google Scholar] [CrossRef]
- Deniz, F.; Kepekci, R.A. Bioremoval of Malachite green from water sample by forestry waste mixture as potential biosorbent. Microchem. J. 2017, 132, 172–178. [Google Scholar] [CrossRef]
- Hijab, M.; Parthasarathy, P.; Mackey, H.R.; Al-Ansari, T.; McKay, G. Minimizing adsorbent requirements using multi-stage batch adsorption for malachite green removal using microwave date-stone activated carbons. Chem. Eng. Process.-Process. Intensif. 2021, 167, 108318. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, V.; Biriș, S. Sustainable Valorization of Waste and By-Products from Sugarcane Processing. Sustainability 2022, 14, 11089. [Google Scholar] [CrossRef]
- Rocha, G.J.D.M.; Nascimento, V.M.; Gonçalves, A.R.; Silva, V.F.N.; Martín, C. Influence of mixed sugarcane bagasse samples evaluated by elemental and physical–chemical composition. Ind. Crop. Prod. 2015, 64, 52–58. [Google Scholar] [CrossRef]
- Di Bella, C.; Traina, A.; Giosuè, C.; Carpintieri, D.; Dico, G.M.L.; Bellante, A.; Del Core, M.; Falco, F.; Gherardi, S.; Uccello, M.M.; et al. Heavy Metals and PAHs in Meat, Milk, and Seafood from Augusta Area (Southern Italy): Contamination Levels, Dietary Intake, and Human Exposure Assessment. Front. Public Health 2020, 8, 273. [Google Scholar] [CrossRef]
- Praipipat, P.; Ngamsurach, P.; Sanghuayprai, A. Modification of sugarcane bagasse with iron(III) oxide-hydroxide to improve its adsorption property for removing lead(II) ions. Sci. Rep. 2023, 13, 1467. [Google Scholar] [CrossRef]
- Tahir, H.; Sultan, M.; Akhtar, N.; Hameed, U.; Abid, T. Application of natural and modified sugar cane bagasse for the removal of dye from aqueous solution. J. Saudi Chem. Soc. 2016, 20, S115–S121. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N. Conversion of Plastic Waste to Carbon-Based Compounds and Application in Energy Storage Devices. ACS Omega 2022, 7, 13403–13435. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K.; Kassim, M.A.; Ali, U.F.M. Transforming Plastic Waste into Porous Carbon for Capturing Carbon Dioxide: A Review. Energies 2021, 14, 8421. [Google Scholar] [CrossRef]
- Zein, S.H.; Grogan, C.T.; Yansaneh, O.Y.; Putranto, A. Pyrolysis of High-Density Polyethylene Waste Plastic to Liquid Fuels—Modelling and Economic Analysis. Processes 2022, 10, 1503. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D. On Feedstock Recycling of Waste Plastic; RSC Clean Technology, Monographs; Clark, J.H., Ed.; Royal Society of Chemistry: Cambridge, UK, 1999. [Google Scholar]
- Lian, Y.-M.; Utetiwabo, W.; Zhou, Y.; Huang, Z.-H.; Zhou, L.; Faheem, M.; Chen, R.-J.; Yang, W. From upcycled waste polyethylene plastic to graphene/mesoporous carbon for high-voltage supercapacitors. J. Colloid Interface Sci. 2019, 557, 55–64. [Google Scholar] [CrossRef]
- Li, Z.; Chen, K.; Chen, Z.; Li, W.; Biney, B.W.; Guo, A.; Liu, D. Removal of malachite green dye from aqueous solution by adsorbents derived from polyurethane plastic waste. J. Environ. Chem. Eng. 2021, 9, 104704. [Google Scholar] [CrossRef]
- Wei, S.; Kamali, A.R. Waste plastic derived Co3Fe7/CoFe2O4@carbon magnetic nanostructures for efficient dye adsorption. J. Alloys Compd. 2021, 886, 161201. [Google Scholar] [CrossRef]
- Parra-Marfíl, A.; Ocampo-Pérez, R.; Collins-Martínez, V.H.; Flores-Vélez, L.M.; Gonzalez-Garcia, R.; Medellín-Castillo, N.A.; Labrada-Delgado, G.J. Synthesis and characterization of hydrochar from industrial Capsicum annuum seeds and its application for the adsorptive removal of methylene blue from water. Environ. Res. 2020, 184, 109334. [Google Scholar] [CrossRef]
- Noraini, M.; Abdullah, E.; Othman, R.; Mubarak, N. Single-route synthesis of magnetic biochar from sugarcane bagasse by microwave-assisted pyrolysis. Mater. Lett. 2016, 184, 315–319. [Google Scholar] [CrossRef]
- Vyavahare, G.D.; Gurav, R.G.; Jadhav, P.P.; Patil, R.R.; Aware, C.B.; Jadhav, J.P. Response surface methodology optimization for sorption of malachite green dye on sugarcane bagasse biochar and evaluating the residual dye for phyto and cytogenotoxicity. Chemosphere 2018, 194, 306–315. [Google Scholar] [CrossRef]
- Jonathan, M.K.; Osamong, G.A.; Butembu, S.; Kamweru, P.K.; Gichumbi, J.M.; Ndiritu, F.G. Structural properties of high density polyethylene matrix composites reinforced with open air and furnace rice husks ash. J. Chem. Eng. Mater. Sci. 2021, 12, 73–84. [Google Scholar]
- Dogan, O.M.; Kayacan, I. Pyrolysis of Low and High Density Polyethylene. Part II: Analysis of Liquid Products Using FTIR and NMR Spectroscopy. Energy Sources Part A Recover. Util. Environ. Eff. 2008, 30, 392–400. [Google Scholar] [CrossRef]
- Li, Y.; Meas, A.; Shan, S.; Yang, R.; Gai, X. Production and optimization of bamboo hydrochars for adsorption of Congo red and 2-naphthol. Bioresour. Technol. 2016, 207, 379–386. [Google Scholar] [CrossRef]
- Islam, A.; Ahmed, M.; Khanday, W.; Asif, M.; Hameed, B. Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol. Environ. Saf. 2017, 138, 279–285. [Google Scholar] [CrossRef]
- Li, F.; Zimmerman, A.R.; Hu, X.; Yu, Z.; Huang, J.; Gao, B. One-pot synthesis and characterization of engineered hydrochar by hydrothermal carbonization of biomass with ZnCl2. Chemosphere 2020, 254, 126866. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, C.U.; Reddy, K.O.; Muzenda, E.; Shukla, M.; Rajulu, A.V. A Comparative Study of Modified and Unmodified High-Density Polyethylene/Borassus Fiber Composites. Int. J. Polym. Anal. Charact. 2013, 18, 439–450. [Google Scholar] [CrossRef]
- Murthy, T.K.; Gowrishankar, B.; Krishna, R.H.; Chandraprabha, M.; Mathew, B.B. Magnetic modification of coffee husk hydrochar for adsorptive removal of methylene blue: Isotherms, kinetics and thermodynamic studies. Environ. Chem. Ecotoxicol. 2020, 2, 205–212. [Google Scholar] [CrossRef]
- Liu, J.-L.; Qian, W.-C.; Guo, J.-Z.; Shen, Y.; Li, B. Selective removal of anionic and cationic dyes by magnetic Fe3O4-loaded amine-modified hydrochar. Bioresour. Technol. 2021, 320, 124374. [Google Scholar] [CrossRef]
- Saqib, N.U.; Baroutian, S.; Sarmah, A.K. Physicochemical, structural and combustion characterization of food waste hydrochar obtained by hydrothermal carbonization. Bioresour. Technol. 2018, 266, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Rehman, M.; Yousaf, M.; Salam, A.; Gulshan, A.B.; Iqbal, J.; Aziz, I.; Azeem, M.; Rukh, S.; Asghar, R.M.A. Comparative efficiency of wheat straw and sugarcane bagasse biochar reduces the cadmium bioavailability to spinach and enhances the microbial activity in contaminated soil. Int. J. Phytoremediation 2019, 21, 1098–1103. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Li, J.; Ma, D.; Han, R. Characterization of bio-char from pyrolysis of wheat straw and its evaluation on methylene blue adsorption. Desalination Water Treat. 2012, 46, 115–123. [Google Scholar] [CrossRef]
- Alateyah, A.I. High-Density Polyethylene Based on Exfoliated Graphite Nanoplatelets/Nano-Magnesium Oxide: An Investigation of Thermal Properties and Morphology. Mater. Sci. Appl. 2019, 10, 159–169. [Google Scholar] [CrossRef]
- Irawan, C.; Putra, M.D.; Wijayanti, H.; Juwita, R.; Meliana, Y.; Nata, I.F. The Amine Functionalized Sugarcane Bagasse Biocomposites as Magnetically Adsorbent for Contaminants Removal in Aqueous Solution. Molecules 2021, 26, 5867. [Google Scholar] [CrossRef]
- Alsaygh, A.A.; Al-Hamidi, J.; Alsewailem, F.D.; Al-Najjar, I.M.; Kuznetsov, V.L. Characterization of polyethylene synthesized by zirconium single site catalysts. Appl. Petrochem. Res. 2014, 4, 79–84. [Google Scholar] [CrossRef]
- Saafie, N.; Samsudin, M.F.R.; Sufian, S.; Ramli, R.M. Enhancement of the activated carbon over methylene blue removal efficiency via alkali-acid treatment. AIP Conf. Proc. 2019, 2124, 020046. [Google Scholar] [CrossRef]
- Behazin, E.; Ogunsona, E.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M.; Anyia, A.O. Mechanical, Chemical, and Physical Properties of Wood and Perennial Grass Biochars for Possible Composite Application. Bioresources 2016, 11, 1334–1348. [Google Scholar] [CrossRef]
- Song, X.; Xu, R.; Wang, K. High capacity adsorption of malachite green in a mesoporous tyre-derived activated carbon. Asia-Pacific J. Chem. Eng. 2013, 8, 172–177. [Google Scholar] [CrossRef]
- Kaur, J.; Sarma, A.K.; Jha, M.K.; Gera, P. Rib shaped carbon catalyst derived from Zea mays L. cob for ketalization of glycerol. RSC Adv. 2020, 10, 43334–43342. [Google Scholar] [CrossRef]
- Hossain, Z.; Wu, W.; Xu, W.Z.; Chowdhury, M.B.I.; Jhawar, A.K.; Machin, D.; Charpentier, P.A. High-Surface-Area Mesoporous Activated Carbon from Hemp Bast Fiber Using Hydrothermal Processing. C 2018, 4, 38. [Google Scholar] [CrossRef]
- Li, Y.; Zimmerman, A.R.; He, F.; Chen, J.; Han, L.; Chen, H.; Hu, X.; Gao, B. Solvent-free synthesis of magnetic biochar and activated carbon through ball-mill extrusion with Fe3O4 nanoparticles for enhancing adsorption of methylene blue. Sci. Total. Environ. 2020, 722, 137972. [Google Scholar] [CrossRef]
- Liu, X.-J.; Li, M.-F.; Singh, S.K. Manganese-modified lignin biochar as adsorbent for removal of methylene blue. J. Mater. Res. Technol. 2021, 12, 1434–1445. [Google Scholar] [CrossRef]
- Cai, T.; Liu, X.; Zhang, J.; Tie, B.; Lei, M.; Wei, X.; Peng, O.; Du, H. Silicate-modified oiltea camellia shell-derived biochar: A novel and cost-effective sorbent for cadmium removal. J. Clean. Prod. 2021, 281, 125390. [Google Scholar] [CrossRef]
- Zeng, S.; Feng, W.; Peng, S.; Teng, Z.; Chen, C.; Zhang, H.; Peng, S. Dual-functional SiOC ceramics coating modified carbon fibers with enhanced microwave absorption performance. RSC Adv. 2019, 9, 30685–30692. [Google Scholar] [CrossRef] [PubMed]
- Meškinis, Š.; Vasiliauskas, A.; Andrulevičius, M.; Peckus, D.; Tamulevičius, S.; Viskontas, K. Diamond Like Carbon Films Containing Si: Structure and Nonlinear Optical Properties. Materials 2020, 13, 1003. [Google Scholar] [CrossRef] [PubMed]
- Ekmen, E.; Bilici, M.; Turan, E.; Tamer, U.; Zengin, A. Surface molecularly-imprinted magnetic nanoparticles coupled with SERS sensing platform for selective detection of malachite green. Sens. Actuators B Chem. 2020, 325, 128787. [Google Scholar] [CrossRef]
- Geçgel, Ü.; Üner, O.; Gökara, G.; Bayrak, Y. Adsorption of cationic dyes on activated carbon obtained from waste Elaeagnus stone. Adsorpt. Sci. Technol. 2016, 34, 512–525. [Google Scholar] [CrossRef]
- Chinthalapudi, N.; Kommaraju, V.V.D.; Kannan, M.K.; Nalluri, C.B.; Varanasi, S. Composites of cellulose nanofibers and silver nanoparticles for malachite green dye removal from water. Carbohydr. Polym. Technol. Appl. 2021, 2, 100098. [Google Scholar] [CrossRef]
- Sarkar, S.; Tiwari, N.; Basu, A.; Behera, M.; Das, B.; Chakrabortty, S.; Sanjay, K.; Suar, M.; Adhya, T.K.; Banerjee, S.; et al. Sorptive removal of malachite green from aqueous solution by magnetite/coir pith supported sodium alginate beads: Kinetics, isotherms, thermodynamics and parametric optimization. Environ. Technol. Innov. 2021, 24, 101818. [Google Scholar] [CrossRef]
- Sahraei, R.; Hemmati, K.; Ghaemy, M. Adsorptive removal of toxic metals and cationic dyes by magnetic adsorbent based on functionalized graphene oxide from water. RSC Adv. 2016, 6, 72487–72499. [Google Scholar] [CrossRef]
- Sudhakaran, A.; Rajan, R.; Ravindranath, A. Characterization and Application of Biochar from spent fermentation sludge of coir wastes in removing Malachite green from effluent water. Pollution 2022, 8, 1026–1037. [Google Scholar] [CrossRef]
- Nath, J.; Ray, L. Biosorption of Malachite green from aqueous solution by dry cells of Bacillus cereus M116 (MTCC 5521). J. Environ. Chem. Eng. 2015, 3, 386–394. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Sirajudheen, P.; Karthikeyan, P.; Meenakshi, S. Fabrication of sulfur-doped biochar derived from tapioca peel waste with superior adsorption performance for the removal of Malachite green and Rhodamine B dyes. Surf. Interfaces 2021, 23, 100920. [Google Scholar] [CrossRef]
- Ganguly, P.; Sarkhel, R.; Das, P. Synthesis of pyrolyzed biochar and its application for dye removal: Batch, kinetic and isotherm with linear and non-linear mathematical analysis. Surf. Interfaces 2020, 20, 100616. [Google Scholar] [CrossRef]
- Rout, D.R.; Jena, H.M. Removal of malachite green dye from aqueous solution using reduced graphene oxide as an adsorbent. Mater. Today Proc. 2021, 47, 1173–1182. [Google Scholar] [CrossRef]
- Wallis, A.; Dollard, M.F. Local and global factors in work stress—The Australian dairy farming exemplar. Scand. J. Work. Environ. Health Suppl. 2008, 34, 66–74. [Google Scholar]
- Freundlich, H. Über die Adsorption in Lösungen. Zeitschrift Phys. Chemie 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Dubinin, M.M. The Equation of the Characteristic Curve of Activated Charcoal. Proc. Acad. Sci. Phys. Chem. Sect. 1947, 55, 331. [Google Scholar]
- Mahmoud, M.E.; Fekry, N.A.; Abdelfattah, A.M. Novel supramolecular network of graphene quantum dots-vitamin B9-iron (III)-tannic acid complex for removal of chromium (VI) and malachite green. J. Mol. Liq. 2021, 341, 117312. [Google Scholar] [CrossRef]
- Jain, S.N.; Shaikh, Z.; Mane, V.S.; Vishnoi, S.; Mawal, V.N.; Patel, O.R.; Bhandari, P.S.; Gaikwad, M.S. Nonlinear regression approach for acid dye remediation using activated adsorbent: Kinetic, isotherm, thermodynamic and reusability studies. Microchem. J. 2019, 148, 605–615. [Google Scholar] [CrossRef]
- Ma, P.; Ma, M.; Wu, J.; Qian, Y.; Wu, D.; Zhang, X. The effect of plastic on performance of activated carbon and study on adsorption of methylene blue. J. Mater. Res. 2019, 34, 3040–3049. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Handlingar 1898, 24, 1–39. [Google Scholar]
- Chien, S.H.; Clayton, W.R. The catalytic oxidation of carbon monoxide on manganese dioxide. Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Ayad, M.M.; Amer, W.A.; Zaghlol, S.; Minisy, I.M.; Bober, P.; Stejskal, J. Polypyrrole-coated cotton textile as adsorbent of methylene blue dye. Chem. Pap. 2018, 72, 1605–1618. [Google Scholar] [CrossRef]
- Dinh, V.-P.; Huynh, T.-D.; Le, H.M.; Nguyen, V.-D.; Dao, V.-A.; Hung, N.Q.; Tuyen, L.A.; Lee, S.; Yi, J.; Nguyen, T.D.; et al. Insight into the adsorption mechanisms of methylene blue and chromium(iii) from aqueous solution onto pomelo fruit peel. RSC Adv. 2019, 9, 25847–25860. [Google Scholar] [CrossRef] [PubMed]

| Sample | Atomic % | ||||||
|---|---|---|---|---|---|---|---|
| C1s | N1s | O1s | S2p | K2p | Ca2p | Si2p | |
| SBPE | 72.09 | 1.49 | 26.42 | 0.15 | 0 | 0 | 0 |
| SBPEAC | 82.33 | 0.39 | 14.23 | 0.21 | 1.22 | 0.73 | 1.11 |
| SBPE/MG | 74.35 | 1.77 | 23.88 | 0.09 | - | - | - |
| SBPEAC/MG | 85.24 | 4.98 | 9.34 | 0.07 | - | 0.44 | - |
| Sample | Temp. (K) | qe,exp. (mg/g) | Isotherm Models | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Langmuir | Freundlich | DR | |||||||||||
| qm (mg/g) | KL (L/mg) | RL | R2 | KF (mg/g)(L/mg)1/n | n | R2 | qs (mmol/g) | KDR (mol2/kJ2) | E (kJ/mol) | R2 | |||
| SBPE | 298 | 231.5 | 291.1 | 0.0688 | 0.420 | 0.9748 | 62.07 | 3.10 | 0.9343 | 0.647 | 39.74 | 0.113 | 0.9970 |
| 308 | 268.4 | 339.6 | 0.0681 | 0.423 | 0.9741 | 68.31 | 2.96 | 0.9312 | 0.7442 | 34.54 | 0.120 | 0.9956 | |
| 318 | 296.3 | 375.6 | 0.0718 | 0.411 | 0.9706 | 76.66 | 2.96 | 0.9249 | 0.8251 | 30.15 | 0.129 | 0.9969 | |
| SBPEAC | 298 | 810.8 | 905.6 | 0.4527 | 0.099 | 0.9949 | 340.67 | 3.11 | 0.9246 | 2.063 | 0.947 | 0.727 | 0.9591 |
| 308 | 840.5 | 909.7 | 0.8898 | 0.053 | 0.9983 | 424.24 | 3.64 | 0.9272 | 2.112 | 0.387 | 1.137 | 0.9380 | |
| 318 | 872.7 | 928.6 | 1.5920 | 0.030 | 0.9791 | 500.66 | 4.08 | 0.9488 | 2.257 | 0.270 | 1.360 | 0.9072 | |
| Sample | qe,exp. (mg/g) | Kinetic Models | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pseudo-First Order | Pseudo-Second-Order | Elovich | ||||||||
| qe1, cal. (mg/g) | K1 (1/min) | R2 | qe2, cal. (mg/g) | K2 (g/mg-min) | R2 | α (mg/g-min) | β (mg/g) | R2 | ||
| SBPE | 42.31 | 43.17 | 0.0107 | 0.5594 | 41.95 | 0.00106 | 0.8347 | 12.33 | 0.1732 | 0.9584 |
| SBPEAC | 188.17 | 185.39 | 0.0116 | 0.9933 | 215.76 | 0.00006 | 0.9983 | 4.45 | 0.0196 | 0.9905 |
| Sample | Co (mg/L) | ΔH° (kJ/mol) | ΔS° (J/mol-K) | ΔG° (kJ/mol) | ||
|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | ||||
| SBPE | 20 | 11.94 | 59.34 | −5.73 | −6.35 | −6.92 |
| 40 | 15.49 | 69.91 | −5.34 | −6.03 | −6.74 | |
| 60 | 15.51 | 67.03 | −4.45 | −5.18 | −5.79 | |
| SBPEAC | 20 | 83.51 | 326.48 | −13.94 | −16.70 | −20.49 |
| 40 | 41.29 | 184.40 | −13.64 | −15.52 | −17.33 | |
| 60 | 40.65 | 179.29 | −12.77 | −14.57 | −16.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.A.; Alqadami, A.A.; Wabaidur, S.M.; Jeon, B.-H. Co-Carbonized Waste Polythene/Sugarcane Bagasse Nanocomposite for Aqueous Environmental Remediation Applications. Nanomaterials 2023, 13, 1193. https://doi.org/10.3390/nano13071193
Khan MA, Alqadami AA, Wabaidur SM, Jeon B-H. Co-Carbonized Waste Polythene/Sugarcane Bagasse Nanocomposite for Aqueous Environmental Remediation Applications. Nanomaterials. 2023; 13(7):1193. https://doi.org/10.3390/nano13071193
Chicago/Turabian StyleKhan, Moonis Ali, Ayoub Abdullah Alqadami, Saikh Mohammad Wabaidur, and Byong-Hun Jeon. 2023. "Co-Carbonized Waste Polythene/Sugarcane Bagasse Nanocomposite for Aqueous Environmental Remediation Applications" Nanomaterials 13, no. 7: 1193. https://doi.org/10.3390/nano13071193
APA StyleKhan, M. A., Alqadami, A. A., Wabaidur, S. M., & Jeon, B.-H. (2023). Co-Carbonized Waste Polythene/Sugarcane Bagasse Nanocomposite for Aqueous Environmental Remediation Applications. Nanomaterials, 13(7), 1193. https://doi.org/10.3390/nano13071193










