Model of Chronoamperometric Response towards Glucose Sensing by Arrays of Gold Nanostructures Obtained by Laser, Thermal and Wet Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Electrode Fabrication
2.2. Instrumental Characterization
3. Results
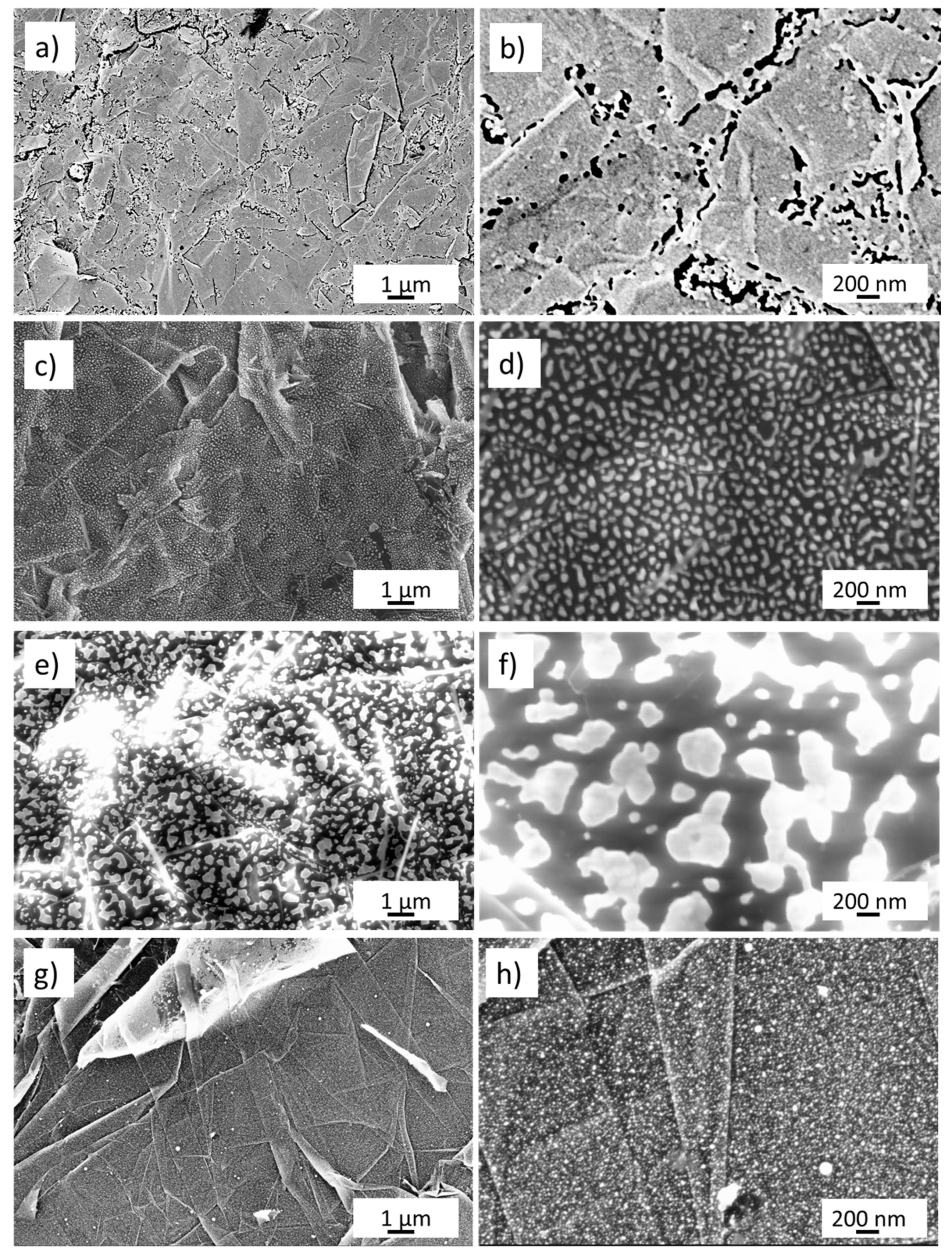
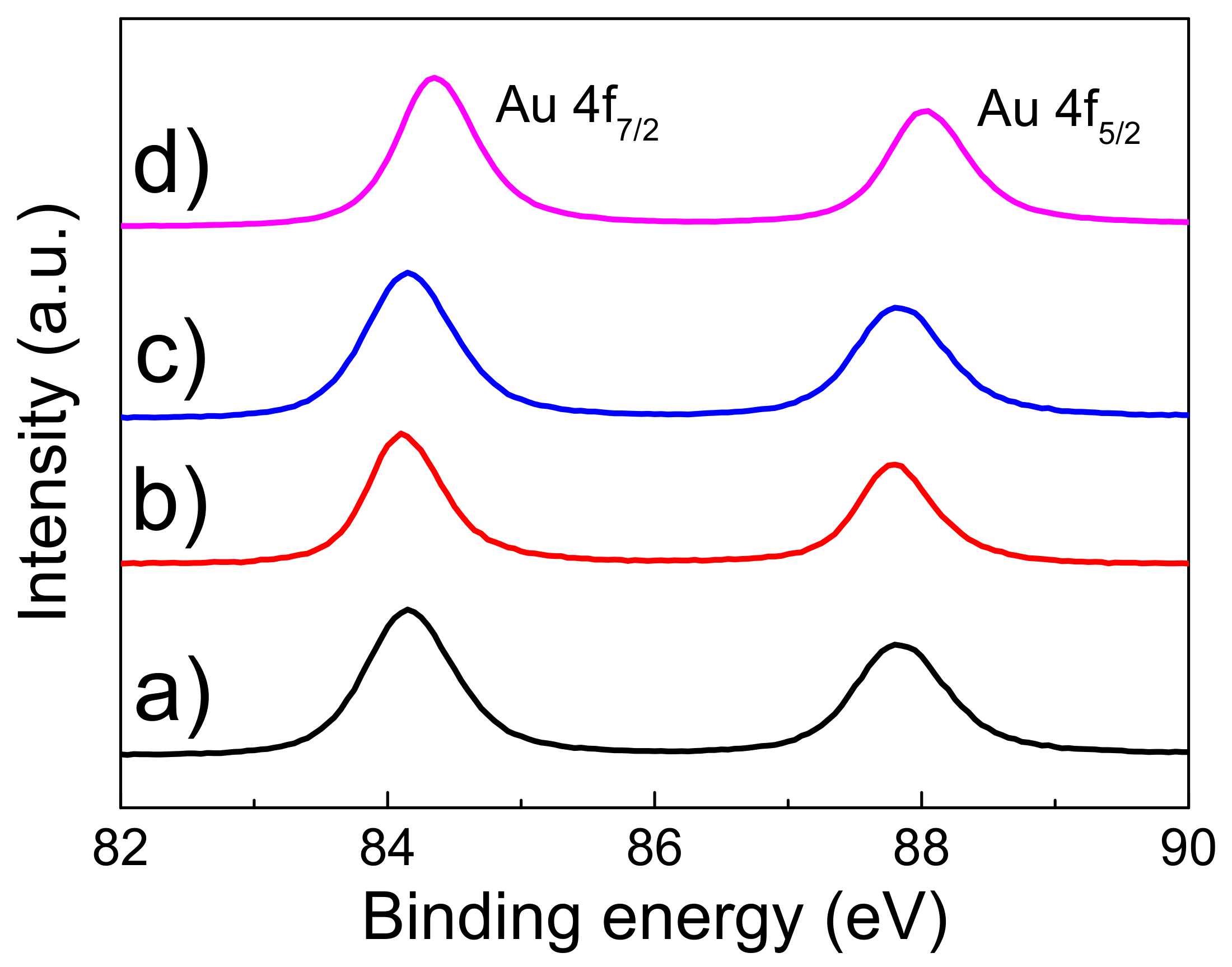
3.1. Morphology and Electronic Structure
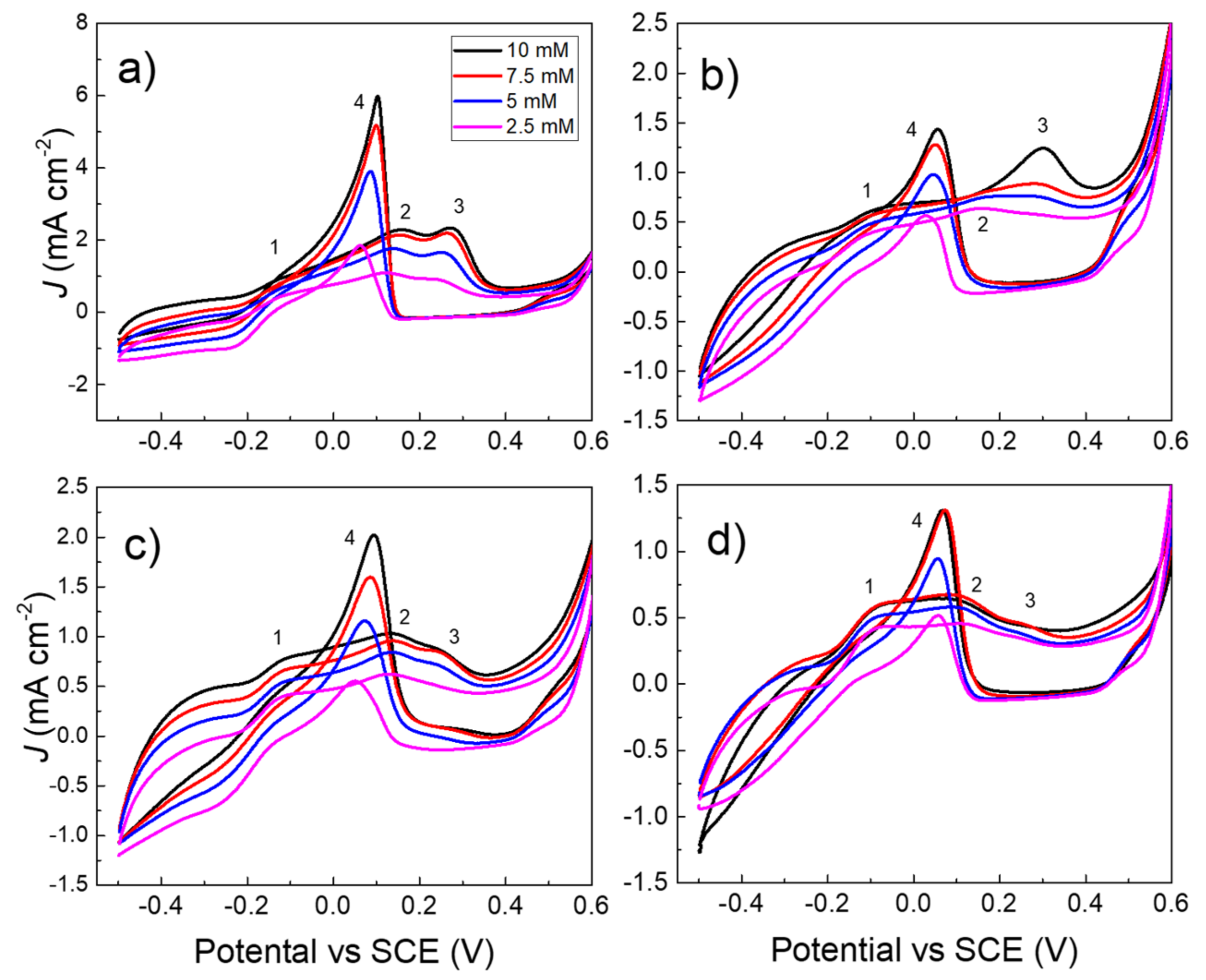
3.2. Electrochemical Characterization
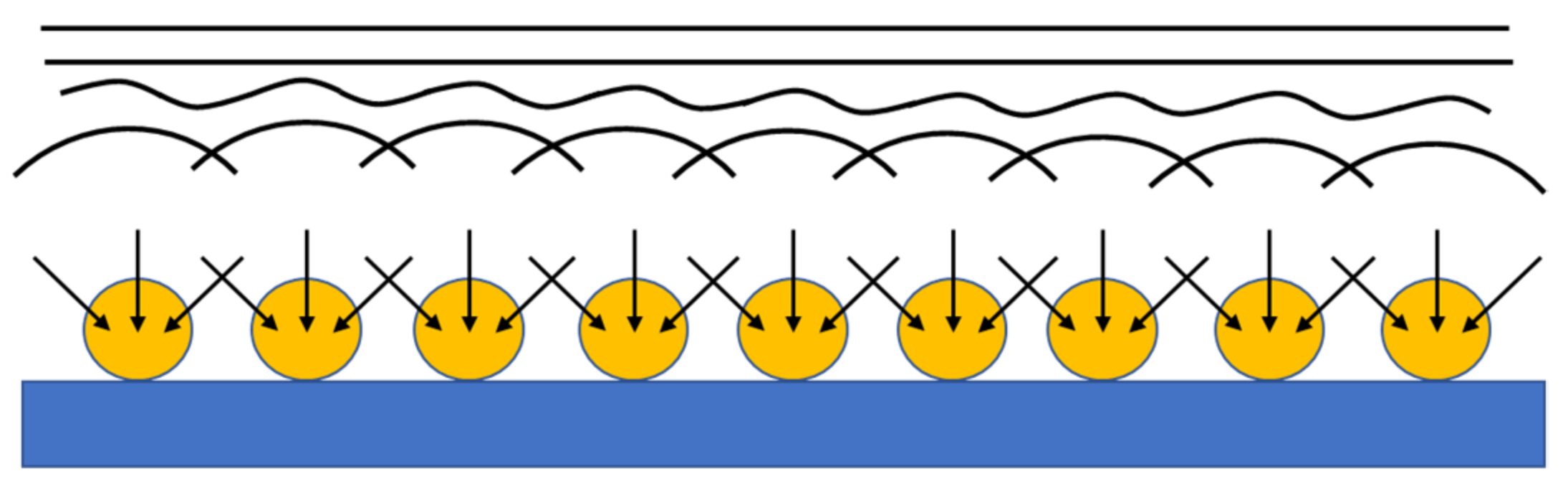
3.3. Modeling of the Current-Time Curves
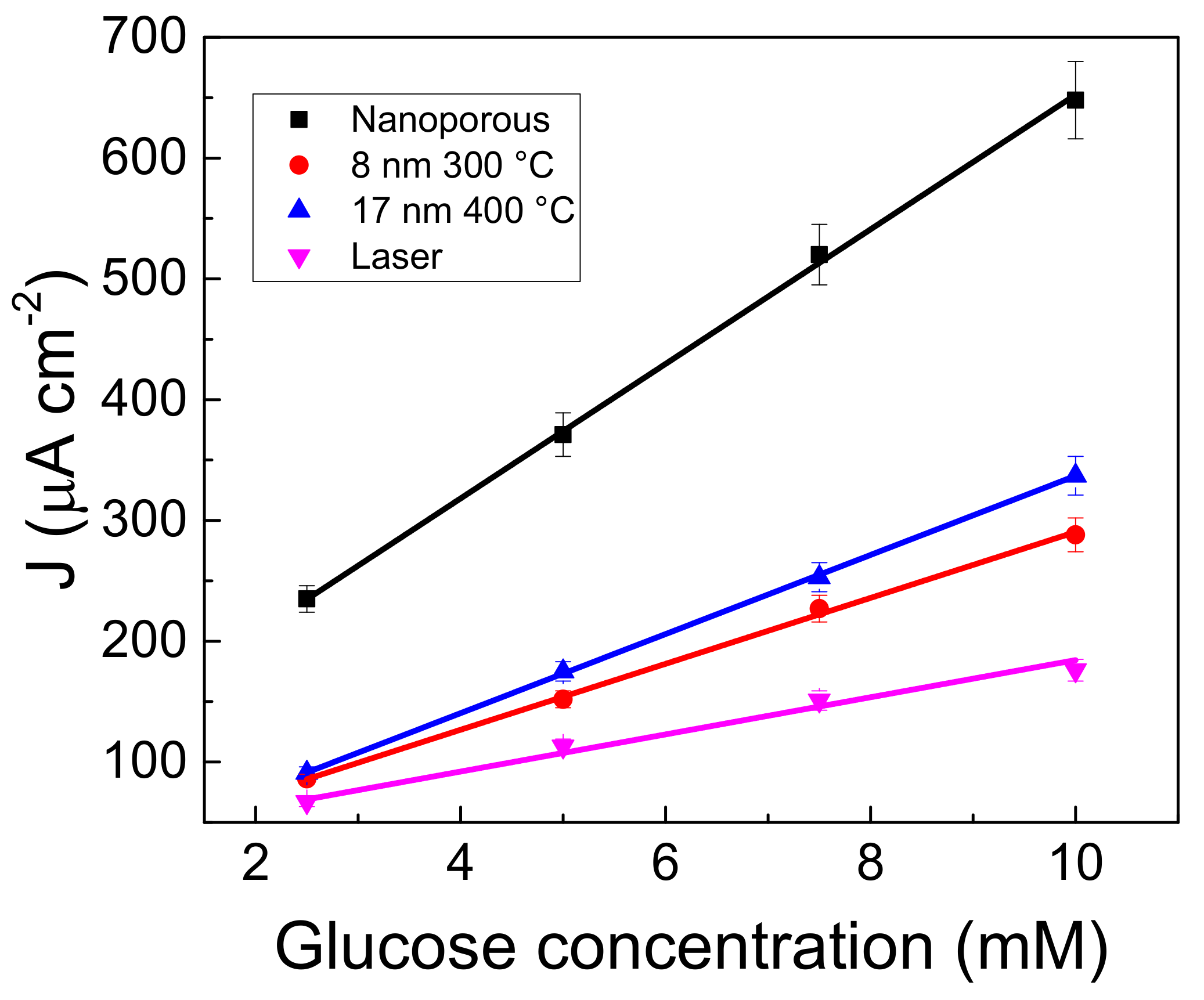
3.4. Glucose Determination in Amperometric Mode
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Idrees, K. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Tsuji, T. Preparation of NPs using laser ablation in liquids: Fundamental aspects and efficient utilization. In Laser Ablation in Liquid: Principles and Applications in the Preparation of Nanomaterials; Yang, G., Ed.; Jenny Stanford Publishing: Singapore, 2012; pp. 2027–2257. ISBN 9789814310956. [Google Scholar]
- Marzun, G.; Streich, C.; Jendrzej, S.; Barcikowski, S.; Wagener, P. Adsorption of colloidal platinum nanoparticles to supports: Charge transfer and effects of electrostatic and steric interactions. Langmuir 2014, 30, 11928–11936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Oko, D.N.; Garbarino, S.; Imbeault, R.; Chaker, M.; Tavares, A.C.; Guay, D.; Ma, D. Preparation of PtAu alloy colloids by laser ablation in solution and their characterization. J. Phys. Chem. C 2012, 116, 13413–13420. [Google Scholar] [CrossRef]
- Reichenberger, S.; Marzun, G.; Muhler, M.; Barcikowski, S. Perspective of Surfactant-Free Colloidal Nanoparticles in Heterogeneous Catalysis. ChemCatChem 2019, 11, 4489–4518. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar]
- Welch, C.M.; Compton, R.G. The use of nanoparticles in electroanalysis: A review. Anal. Bioanal. Chem. 2006, 384, 601–619. [Google Scholar] [CrossRef]
- Simm, A.O.; Ward-Jones, S.; Banks, C.E.; Compton, R.G. Novel Methods for the Production of Silver Microelectrode-Array: Their Characterisation by Atomic Force Microscopy and Application to the Electro-reduction of Halothane. Anal. Sci. 2005, 21, 667–671. [Google Scholar]
- Kang, M.; Park, S.; Jeong, K. Repeated Solid-state Dewetting of Thin Gold Films for Nanogap-rich Plasmonic Nanoislands. Sci. Rep. 2015, 5, 14790. [Google Scholar] [CrossRef]
- Oh, Y.J.; Jeong, K.H. Glass Nanopillar Arrays with Nanogap-Rich Silver Nanoislands for Highly Intense Surface Enhanced Raman Scattering. Adv. Mater. 2012, 24, 2234–2237. [Google Scholar]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, L.; Reichenberger, S.; Barcikowski, S. Design and perspective of amorphous metal nanoparticles from laser synthesis and processing. Phys. Chem. Chem. Phys. 2021, 23, 11121–11154. [Google Scholar]
- Semaltianos, N.G. Nanoparticles by laser ablation of bulk target materials in liquids. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer: Cham, Switzerland, 2016; pp. 67–92. [Google Scholar] [CrossRef]
- Khorasani, M.; Gibson, I.; Ghasemi, A.H.; Hadavi, E.; Rolfe, B. Laser subtractive and laser powder bed fusion of metals: Review of process and production features. Rapid Prototyp. J. 2023. [Google Scholar] [CrossRef]
- Scandurra, A.; Censabella, M.; Gulino, A.; Grimaldi, M.G.; Ruffino, F. Gold nanoelectrode arrays dewetted onto graphene paper for selective and direct electrochemical determination of glyphosate in drinking water. Sens. Bio-Sens. Res. 2022, 36, 100496. [Google Scholar] [CrossRef]
- Scandurra, A.; Ruffino, F.; Censabella, M.; Terrasi, A.; Grimaldi, M.G. Dewetted Gold Nanostructures onto Exfoliated Graphene Paper as High Efficient Glucose Sensor. Nanomaterials 2019, 9, 1794. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, J.; Qu, X. Nano-Gold as Artificial Enzymes: Hidden Talents. Adv. Mater. 2014, 26, 4200–4217. [Google Scholar]
- Scandurra, A.; Ruffino, F.; Sanzaro, S.; Grimaldi, M.G. Laser and Thermal Dewetting of Gold Layer onto Graphene Paper for non-Enzymatic Electrochemical Detection of Glucose and Fructose. Sens. Actuators B Chem. 2019, 301, 127113. [Google Scholar] [CrossRef]
- Müsse, A.; La Malfa, F.; Brunetti, V.; Rizzi, F.; De Vittorio, M. Flexible Enzymatic Glucose Electrochemical Sensor Based on Polystyrene-Gold Electrodes. Micromachines 2021, 12, 805. [Google Scholar] [CrossRef]
- Funtanilla, V.D.; Candidate, P.; Caliendo, T.; Hilas, O. Continuous Glucose Monitoring: A Review of Available Systems. Pharm. Ther. 2019, 44, 550–553. [Google Scholar]
- Brett, C.; Brett, A.M.O. Electrochemistry: Principles, Methods, and Applications; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Hood, S.J.; Kampouris, D.K.; Kadara, R.O.; Jenkinson, N.; Javier del Campo, F.; Muñoz, F.X.; Banks, C.E. Why ‘the bigger the better’ is not always the case when utilizing microelectrode arrays: High density vs. low density arrays for the electroanalytical sensing of chromium(VI). Analyst 2009, 134, 2301–2305. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, F.G. Der Reststrom bei galvanischer Polarisation, betrachtet als ein Diffusionsproblem. Z. Für Phys. Chem. 1903, 42U, 385. [Google Scholar] [CrossRef]
- Aoki, K.; Osteryoung, J. Diffusion-controlled current at the stationary finite disk electrode: Theory. J. Electroanal. Chem. 1981, 122, 19–35. [Google Scholar] [CrossRef]
- Shoup, D.; Szabo, A. Chronoamperometric Current at Finite Disk Electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1982, 140, 237–245. [Google Scholar] [CrossRef]
- Coen, S.; Cope, D.K.; Tallman, D.E. Diffusion current at a band electrode by an integral equation method. J. Electroanal. Chem. Interfacial Electrochem. 1986, 215, 29–48. [Google Scholar] [CrossRef]
- Elliott, J.R.; Le, H.; Yang, M.; Compton, R.G. Using Simulations to Guide the Design of Amperometric Electrochemical Sensors Based on Mediated Electron Transfer. ChemElectroChem 2020, 7, 2797–2815. [Google Scholar] [CrossRef]
- Thompson, M. Xrump. Available online: www.genplot.com (accessed on 7 November 2022).
- Xiong, Z.; Zhang, L.L.; Ma, J.; Zhao, X.S. Photocatalytic degradation of dyes over graphene–gold nanocomposites under visible light irradiation. Chem. Commun. 2010, 46, 6099–6101. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M.P. (Eds.) . Practical Surface Analysis, 2nd ed.; John Wiley & Sons: Chichester, UK, 1990; Volume 1. [Google Scholar]
- Yong, V.; Hahn, H.T. Synergistic, effect of fullerene-capped gold nanoparticles on graphene electrochemical supercapacitors. Adv. Nanopart. 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Viet, N.X.; Takamura, Y. Electrodeposited Gold Nanoparticles Modified Screen Printed Carbon Electrode for Enzyme-Free Glucose Sensor Application, VNU J. Sci. Nat. Sci. Technol. 2016, 32, 83–89. [Google Scholar]
- Juſík, T.; Podešva, P.; Farka, Z.; Kováſ, D.; Skládal, P.; Foret, F. Nanostructured gold deposited in gelatin template applied for electrochemical assay of glucose in serum. Electrochim. Acta 2016, 188, 277–285. [Google Scholar] [CrossRef]
- Leontiev, A.P.; Napolskii, K.S. Numerical Simulation of Chronoamperograms and Voltammograms for Electrode Modified with Nanoporous Film. Russ. J. Electrochem. 2022, 58, 741–750. [Google Scholar] [CrossRef]
- Kakihana, M.; Ikeuchi, H.; Satô, G.P.; Tokuda, K. Diffusion current at microdisk electrodes—Application to accurate measurement of diffusion coefficients. J. Electroanal. Chem. 1981, 117, 201–211. [Google Scholar] [CrossRef]
- Heinze. J. Diffusion processes at finite (micro) disk electrodes solved by digital simulation. J. Electroanal. Chem. 1981, 124, 73–86. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) . Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Xiong, L. Leigh Aldous, Martin, C. Henstridge and Richard, G. Compton, Investigation of the optimal transient times for chronoamperometric analysis of diffusion coefficients and concentrations in non-aqueous solvents and ionic liquids. Anal. Methods 2012, 4, 371–376. [Google Scholar] [CrossRef]
- Hernández, W.T. Electrochemical Characterization of Mass Transport at Microelectrode Arrays. Rev. Cienc. 2014, 18, 101–110. [Google Scholar] [CrossRef]
- Kiss, V.; Ősz, K. Double Exponential Evaluation under Non-Pseudo–First-Order Conditions: A Mixed Second-Order Process Followed by a First-Order Reaction. Int. J. Chem. Kinet. 2017, 49, 602–610. [Google Scholar] [CrossRef]

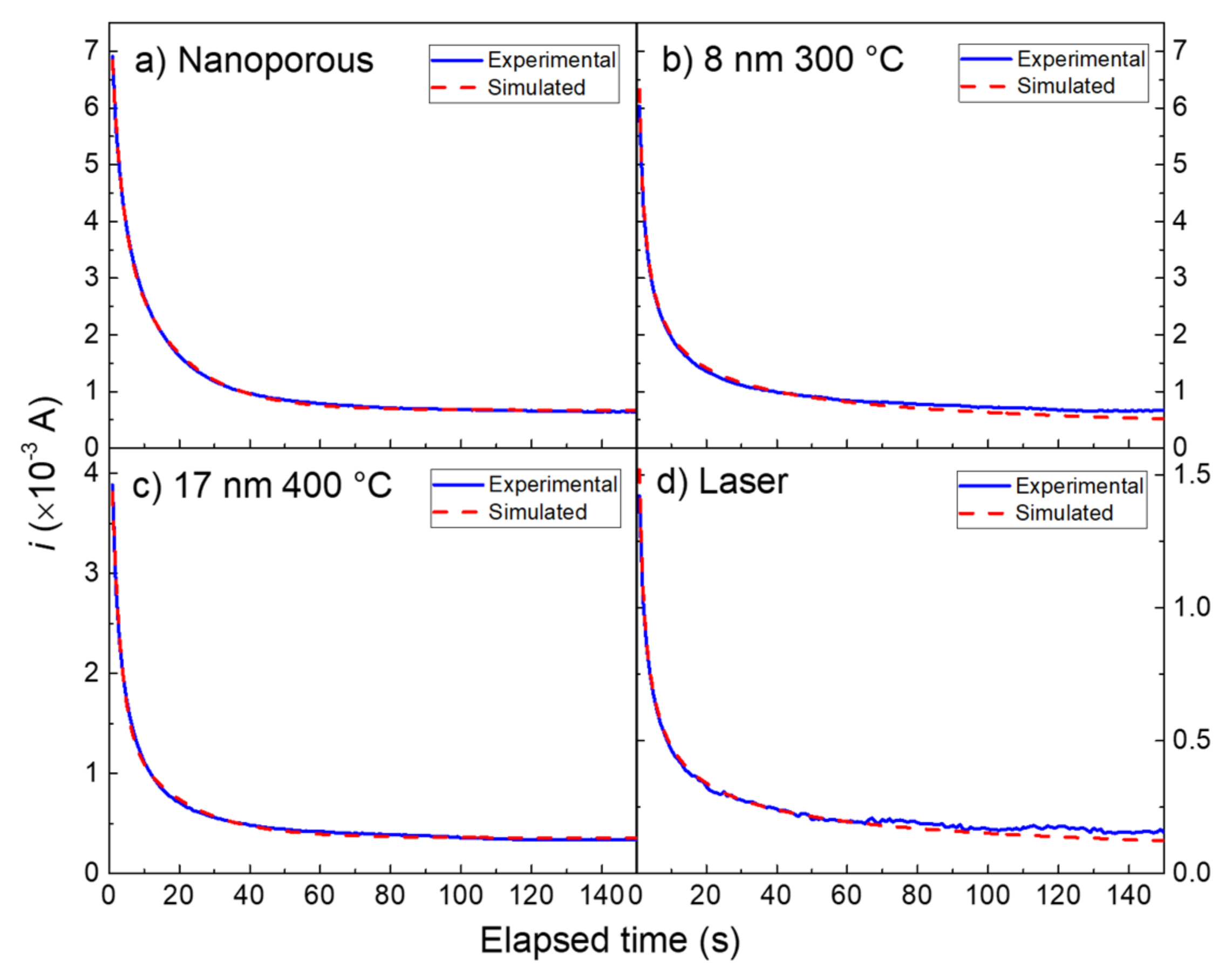
| Cottrel | Two-Phase Exponential Decay | Adj. R2 | |||||
|---|---|---|---|---|---|---|---|
| B0 (A) | k1 (s−1) | k2 (s−1) | |||||
| Nanostructure type | - | - | - | - | - | - | - |
| Nano- porous | - | 6.74 × 10−4 ± 3.02 × 10−6 | 0.00339 | 0.00333 | 0.363 | 0.0625 | 0.99924 |
| 8 nm 300 °C | 0.00633 ± 3.5 × 10−5 | - | - | - | - | - | 0.98530 |
| 17 nm 400 °C | - | 3.58 × 10−4 ± 2.83 × 10−6 | 0.00265 | 0.00119 | 0.425 | 0.0586 | 0.99715 |
| Laser | 0.00152 ± 9.3 × 10−6 | - | - | - | - | - | 0.98172 |
| Sample | Adj R2 | ||
|---|---|---|---|
| a (μAcm−2) | b (μAcm−2mM−1) | ||
| Nano-porous | 95.49 ± 4.25 | 55.69 ± 0.89 | 0.9992 |
| 8 nm 300 °C | 9.48 ± 1.65 | 32.74 ± 0.36 | 0.9996 |
| 17 nm 400 °C | 17.54 ± 2.58 | 27.29 ± 0.58 | 0.9986 |
| Laser | 30.60 ± 6.45 | 15.38 ± 1.24 | 0.9807 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scandurra, A.; Iacono, V.; Boscarino, S.; Scalese, S.; Grimaldi, M.G.; Ruffino, F. Model of Chronoamperometric Response towards Glucose Sensing by Arrays of Gold Nanostructures Obtained by Laser, Thermal and Wet Processes. Nanomaterials 2023, 13, 1163. https://doi.org/10.3390/nano13071163
Scandurra A, Iacono V, Boscarino S, Scalese S, Grimaldi MG, Ruffino F. Model of Chronoamperometric Response towards Glucose Sensing by Arrays of Gold Nanostructures Obtained by Laser, Thermal and Wet Processes. Nanomaterials. 2023; 13(7):1163. https://doi.org/10.3390/nano13071163
Chicago/Turabian StyleScandurra, Antonino, Valentina Iacono, Stefano Boscarino, Silvia Scalese, Maria Grazia Grimaldi, and Francesco Ruffino. 2023. "Model of Chronoamperometric Response towards Glucose Sensing by Arrays of Gold Nanostructures Obtained by Laser, Thermal and Wet Processes" Nanomaterials 13, no. 7: 1163. https://doi.org/10.3390/nano13071163
APA StyleScandurra, A., Iacono, V., Boscarino, S., Scalese, S., Grimaldi, M. G., & Ruffino, F. (2023). Model of Chronoamperometric Response towards Glucose Sensing by Arrays of Gold Nanostructures Obtained by Laser, Thermal and Wet Processes. Nanomaterials, 13(7), 1163. https://doi.org/10.3390/nano13071163











