Sodium Alginate–Aldehyde Cellulose Nanocrystal Composite Hydrogel for Doxycycline and Other Tetracycline Removal
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of Alginate–Cellulose Nanocrystal Composite Hydrogel Beads
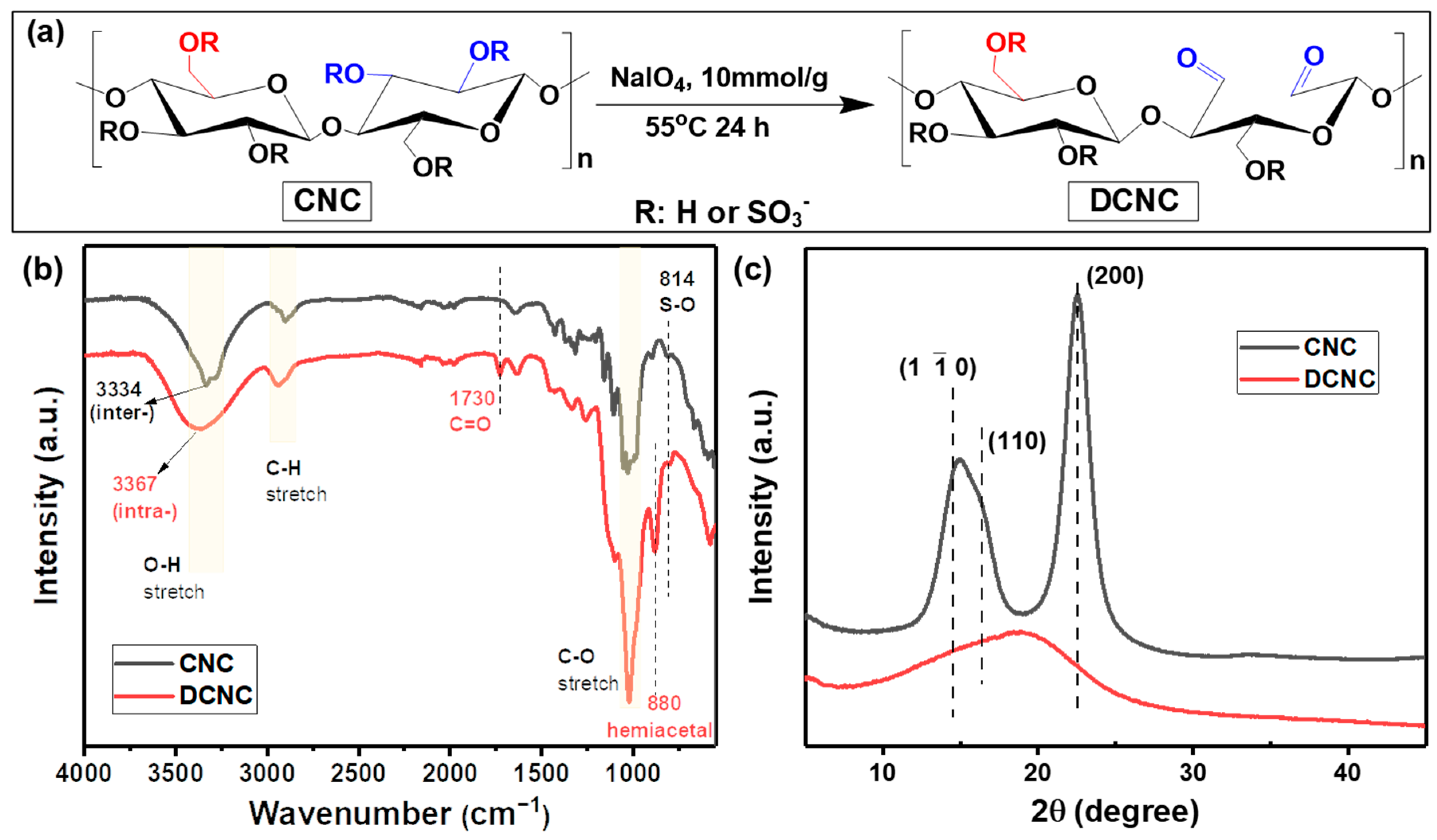
2.2.1. Preparation of Dialdehyde Cellulose Nanocrystal (DCNC)
2.2.2. Preparation of SA–DCNC Hydrogel Beads
2.3. Characterization
2.3.1. Titration to Determine Aldehyde Content in DCNC
2.3.2. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.3.3. X-ray Diffraction (XRD)
2.3.4. Transmission Electron Microscopy (TEM)
2.3.5. Dynamic Light Scattering (DLS)
2.3.6. Thermogravimetric Analysis (TGA)
2.3.7. Property of SA–DCNC Composite Hydrogel
2.4. Antibiotic Adsorption Studies
2.4.1. Effects of Hydrogel Composition on Doxycycline Adsorption
2.4.2. Adsorption Kinetics
2.4.3. Adsorption Isotherm
2.4.4. Effect of pH
2.4.5. Single-Drug Adsorption
2.4.6. Multidrug (Four Tetracyclines) Competitive Adsorption
2.4.7. Reusability Test
3. Results and Discussion
3.1. Characterizations of DCNC
3.2. Formulation and Characterization of SA–DCNC Hydrogel
3.3. Adsorption of Tetracycline Antibiotics on SA–DCNC-40 Hydrogel Bead
3.3.1. Kinetic Study
3.3.2. Isotherm Study
3.3.3. Effect of pH
3.3.4. Competitive Adsorption Test
3.3.5. Reusability Test
3.4. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Giri, B.S.; Shukla, P.; Gupta, P. Recent advancement in remediation of synthetic organic antibiotics from environmental matrices: Challenges and perspective. Bioresour. Technol. 2021, 319, 124161. [Google Scholar] [CrossRef]
- Burgmann, H.; Frigon, D.; Gaze, W.H.; Manaia, C.M.; Pruden, A.; Singer, A.C.; B, F.S.; Zhang, T. Water and sanitation: An essential battlefront in the war on antimicrobial resistance. FEMS Microbiol. Ecol. 2018, 94, 94. [Google Scholar] [CrossRef]
- Kargin, I.D.; Sokolova, L.S.; Pirogov, A.V.; Shpigun, O.A. HPLC determination of tetracycline antibiotics in milk with post-column derivatization and fluorescence detection. Inorg. Mater. 2017, 52, 1365–1369. [Google Scholar] [CrossRef]
- Croitoru, C.; Roata, I.C.; Pascu, A.; Stanciu, E.M. Diffusion and Controlled Release in Physically Crosslinked Poly (Vinyl Alcohol)/Iota-Carrageenan Hydrogel Blends. Polymers 2020, 12, 1544. [Google Scholar] [CrossRef] [PubMed]
- Abrão, L.C.C.; Maia, P.P.; Figueiredo, E.C. Determination of Tetracyclines by Solid-Phase Extraction with a Molecularly Imprinted Polymer and High-Performance Liquid Chromatography. Anal. Lett. 2014, 47, 2183–2194. [Google Scholar] [CrossRef]
- Li, Z.-J.; Qi, W.-N.; Feng, Y.; Liu, Y.-W.; Ebrahim, S.; Long, J. Degradation mechanisms of oxytetracycline in the environment. J. Integr. Agric. 2019, 18, 1953–1960. [Google Scholar] [CrossRef]
- Tang, R.; Wang, Z.; Muhammad, Y.; Shi, H.; Liu, K.; Ji, J.; Zhu, Y.; Tong, Z.; Zhang, H. Fabrication of carboxymethyl cellulose and chitosan modified Magnetic alkaline Ca-bentonite for the adsorption of hazardous doxycycline. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125730. [Google Scholar] [CrossRef]
- Korkut, F.; Saloglu, D. Synthesis, characterization, and tetracycline adsorption behaviour of activated carbon dopped alginate beads: Isotherms, kinetics, thermodynamic, and adsorption mechanism. Desalination Water Treat. 2020, 206, 315–330. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, T.; Wang, M.; Jin, R.; Song, Y.; Zhou, Y.; Qi, Z.; Chen, W. Inhibitory role of citric acid in the adsorption of tetracycline onto biochars: Effects of solution pH and Cu2+. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124731. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.; Li, J.; Zhu, Y.; Yu, H.; Peng, Y. Adsorption and co-adsorption of tetracycline and doxycycline by one-step synthesized iron loaded sludge biochar. Chemosphere 2019, 236, 124254. [Google Scholar] [CrossRef]
- Jin, J.; Liu, M.; Feng, L.; Wang, H.; Wang, Y.; Nguyen, T.A.H.; Wang, Y.; Lu, J.; Li, Y.; Bao, M. 3D Bombax-structured carbon nanotube sponge coupling with Ag3PO4 for tetracycline degradation under ultrasound and visible light irradiation. Sci. Total Environ. 2019, 695, 133694. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zeng, Z.; Li, X.; Zeng, G.; Xiao, R.; Yang, Z.; Zhou, Y.; Zhang, C.; Cheng, M.; Hu, L.; et al. Multi-walled carbon nanotube/amino-functionalized MIL-53(Fe) composites: Remarkable adsorptive removal of antibiotics from aqueous solutions. Chemosphere 2018, 210, 1061–1069. [Google Scholar] [CrossRef]
- Bao, J.; Zhu, Y.; Yuan, S.; Wang, F.; Tang, H.; Bao, Z.; Zhou, H.; Chen, Y. Adsorption of Tetracycline with Reduced Graphene Oxide Decorated with MnFe2O4 Nanoparticles. Nanoscale Res. Lett. 2018, 13, 396. [Google Scholar] [CrossRef]
- Ahamad, T.; Ruksana; Chaudhary, A.A.; Naushad, M.; Alshehri, S.M. Fabrication of MnFe2O4 nanoparticles embedded chitosan-diphenylureaformaldehyde resin for the removal of tetracycline from aqueous solution. Int. J. Biol. Macromol. 2019, 134, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.; Grishkewich, N.; Waeijen, H.A.; Berry, R.M.; Tam, K.C. Continuous flow adsorption of methylene blue by cellulose nanocrystal-alginate hydrogel beads in fixed bed columns. Carbohydr. Polym. 2016, 136, 1194–1202. [Google Scholar] [CrossRef]
- Hu, Z.H.; Omer, A.M.; Ouyang, X.K.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. A Review on Synthesis Methods of Phyllosilicate-and Graphene-Filled Composite Hydrogels. J. Compos. Sci. 2022, 6, 15. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Berry, R.M.; Tam, K.C. Cellulose nanocrystal–alginate hydrogel beads as novel adsorbents for organic dyes in aqueous solutions. Cellulose 2015, 22, 3725–3738. [Google Scholar] [CrossRef]
- Dalei, G.; Das, S.; Pradhan, M. Dialdehyde cellulose as a niche material for versatile applications: An overview. Cellulose 2022, 29, 5429–5461. [Google Scholar] [CrossRef]
- Huang, X.; Hadi, P.; Joshi, R.; Alhamzani, A.G.; Hsiao, B.S. A Comparative Study of Mechanism and Performance of Anionic and Cationic Dialdehyde Nanocelluloses for Dye Adsorption and Separation. ACS Omega 2023, 8, 8634–8649. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Dognani, G.; Hadi, P.; Yang, M.; Job, A.E.; Hsiao, B.S. Cationic Dialdehyde Nanocellulose from Sugarcane Bagasse for Efficient Chromium(VI) Removal. ACS Sustain. Chem. Eng. 2020, 8, 4734–4744. [Google Scholar] [CrossRef]
- Gaspar, D.; Fernandes, S.N.; de Oliveira, A.G.; Fernandes, J.G.; Grey, P.; Pontes, R.V.; Pereira, L.; Martins, R.; Godinho, M.H.; Fortunato, E. Nanocrystalline cellulose applied simultaneously as the gate dielectric and the substrate in flexible field effect transistors. Nanotechnology 2014, 25, 094008. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef]
- Sun, B.; Hou, Q.; Liu, Z.; Ni, Y. Sodium periodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose 2015, 22, 1135–1146. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Fu, S.Y.; Zheng, L.M.; Zhan, H.Y. Effect of nanocellulose isolation techniques on the formation of reinforced poly(vinyl alcohol) nanocomposite films. Express Polym. Lett. 2012, 6, 794–804. [Google Scholar] [CrossRef]
- Lin, N.; Bruzzese, C.; Dufresne, A. TEMPO-oxidized nanocellulose participating as crosslinking aid for alginate-based sponges. ACS Appl. Mater. Interfaces 2012, 4, 4948–4959. [Google Scholar] [CrossRef]
- Bu, X.; Wang, L.; Huang, Y. Effect of pore size on the performance of composite adsorbent. Adsorption 2013, 19, 929–935. [Google Scholar] [CrossRef]
- Rocher, V.; Bee, A.; Siaugue, J.M.; Cabuil, V. Dye removal from aqueous solution by magnetic alginate beads crosslinked with epichlorohydrin. J. Hazard. Mater. 2010, 178, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.A.; Dávila, J.L.; d’Ávila, M.A. Rheological studies on nanocrystalline cellulose/alginate suspensions. J. Mol. Liq. 2019, 277, 418–423. [Google Scholar] [CrossRef]
- Geng, L.; Peng, X.; Zhan, C.; Naderi, A.; Sharma, P.R.; Mao, Y.; Hsiao, B.S. Structure characterization of cellulose nanofiber hydrogel as functions of concentration and ionic strength. Cellulose 2017, 24, 5417–5429. [Google Scholar] [CrossRef]
- Siqueira, P.; Siqueira, E.; de Lima, A.E.; Siqueira, G.; Pinzon-Garcia, A.D.; Lopes, A.P.; Segura, M.E.C.; Isaac, A.; Pereira, F.V.; Botaro, V.R. Three-Dimensional Stable Alginate-Nanocellulose Gels for Biomedical Applications: Towards Tunable Mechanical Properties and Cell Growing. Nanomaterials 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and Characterization of Films Based on Alginate and Aloe Vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Rantuch, P.; Chrebet, T. Thermal decomposition of cellulose insulation. Cellul. Chem. Technol. 2014, 48, 461–467. [Google Scholar]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclética Química 2004, 29, 57–64. [Google Scholar] [CrossRef]
- Kumar, A.; Lee, Y.; Kim, D.; Rao, K.M.; Kim, J.; Park, S.; Haider, A.; Lee, D.H.; Han, S.S. Effect of crosslinking functionality on microstructure, mechanical properties, and in vitro cytocompatibility of cellulose nanocrystals reinforced poly (vinyl alcohol)/sodium alginate hybrid scaffolds. Int. J. Biol. Macromol. 2017, 95, 962–973. [Google Scholar] [CrossRef]
- Mladenovska, K.; Cruaud, O.; Richomme, P.; Belamie, E.; Raicki, R.S.; Venier-Julienne, M.C.; Popovski, E.; Benoit, J.P.; Goracinova, K. 5-ASA loaded chitosan-Ca-alginate microparticles: Preparation and physicochemical characterization. Int. J. Pharm. 2007, 345, 59–69. [Google Scholar] [CrossRef]
- Tanzifi, M.; Yaraki, M.T.; Kiadehi, A.D.; Hosseini, S.H.; Olazar, M.; Bharti, A.K.; Agarwal, S.; Gupta, V.K.; Kazemi, A. Adsorption of Amido Black 10B from aqueous solution using polyaniline/SiO2 nanocomposite: Experimental investigation and artificial neural network modeling. J. Colloid Interface Sci. 2018, 510, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, K.; Deb, K.; Bera, A.; Debnath, A.; Saha, B. Interaction of anionic dyes with polyaniline implanted cellulose: Organic π-conjugated macromolecules in environmental applications. J. Mol. Liq. 2018, 261, 189–198. [Google Scholar] [CrossRef]
- Liu, M.; Zou, D.; Ma, T.; Liu, Z.; Yu, H.; Li, Y.; Qiao, Z.-A. Stable cellulose-based porous binary metal–organic gels as highly efficient adsorbents and their application in an adsorption bed for chlortetracycline hydrochloride decontamination. J. Mater. Chem. A 2020, 8, 6670–6681. [Google Scholar] [CrossRef]
- Olusegun, S.J.; Mohallem, N.D.S. Comparative adsorption mechanism of doxycycline and Congo red using synthesized kaolinite supported CoFe2O4 nanoparticles. Environ. Pollut. 2020, 260, 114019. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Xu, X.; Yang, L.; Chen, C.; Qian, J.; Chen, X.; Sun, D. Soft foam-like UiO-66/Polydopamine/Bacterial cellulose composite for the removal of aspirin and tetracycline hydrochloride. Chem. Eng. J. 2020, 395, 125174. [Google Scholar] [CrossRef]
- Liu, S.; Xu, W.H.; Liu, Y.G.; Tan, X.F.; Zeng, G.M.; Li, X.; Liang, J.; Zhou, Z.; Yan, Z.L.; Cai, X.X. Facile synthesis of Cu(II) impregnated biochar with enhanced adsorption activity for the removal of doxycycline hydrochloride from water. Sci. Total Environ. 2017, 592, 546–553. [Google Scholar] [CrossRef]
- Zeng, Z.W.; Tan, X.F.; Liu, Y.G.; Tian, S.R.; Zeng, G.M.; Jiang, L.H.; Liu, S.B.; Li, J.; Liu, N.; Yin, Z.H. Comprehensive Adsorption Studies of Doxycycline and Ciprofloxacin Antibiotics by Biochars Prepared at Different Temperatures. Front. Chem. 2018, 6, 80. [Google Scholar] [CrossRef]
- Chao, Y.; Zhu, W.; Wu, X.; Hou, F.; Xun, S.; Wu, P.; Ji, H.; Xu, H.; Li, H. Application of graphene-like layered molybdenum disulfide and its excellent adsorption behavior for doxycycline antibiotic. Chem. Eng. J. 2014, 243, 60–67. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Coseri, S.; Biliuta, G.; Zemljič, L.F.; Srndovic, J.S.; Larsson, P.T.; Strnad, S.; Kreže, T.; Naderi, A.; Lindström, T. One-shot carboxylation of microcrystalline cellulose in the presence of nitroxyl radicals and sodium periodate. RSC Adv. 2015, 5, 85889–85897. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.; Huo, P.; Gu, J. Exploration of zwitterionic cellulose acetate antifouling ultrafiltration membrane for bovine serum albumin (BSA) separation. Carbohydr. Polym. 2017, 165, 266–275. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Souza, L.P.; Martins, T.M.M.; Lopes, J.H.; Mattos, B.D.; Mariano, M.; Pinheiro, I.F.; Valverde, T.M.; Livi, S.; Camilli, J.A.; et al. Nanocellulose/bioactive glass cryogels as scaffolds for bone regeneration. Nanoscale 2019, 11, 19842–19849. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Chu, S.-W.; Xue, C.; Zhao, D.; Ha, C.-S. Facile synthesis of mesoporous carbon nitrides using the incipient wetness method and the application as hydrogen adsorbent. J. Mater. Chem. 2011, 21, 10801–10807. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, Y.-F.; Liu, Y.; Li, Y.; Hu, H. Efficient ion-enhanced adsorption of congo red on polyacrolein from aqueous solution: Experiments, characterization and mechanism studies. Sep. Purif. Technol. 2020, 252, 117445. [Google Scholar] [CrossRef]
- O’Leary, J.; Wallis, J.D.; Wood, M.L. 1, 6-Interactions between dimethylamino and aldehyde groups in two biphenyl derivatives. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2001, 57, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Jannat Abadi, M.H.; Nouri, S.M.M.; Zhiani, R.; Heydarzadeh, H.D.; Motavalizadehkakhky, A. Removal of tetracycline from aqueous solution using Fe-doped zeolite. Int. J. Ind. Chem. 2019, 10, 291–300. [Google Scholar] [CrossRef]












| Compound | Structural Formula | pKa1 | pKa2 | pKa3 |
|---|---|---|---|---|
| Tetracycline (TC) |  | 3.3 | 7.7 | 9.7 |
| Oxytetracycline (OTC) |  | 3.3 | 7.3 | 9.1 |
| Chlortetracycline (CTC) |  | 3.3 | 7.4 | 9.3 |
| Doxycycline (DOXY) |  | 3.4 | 7.7 | 9.3 |
| Sample | SA–DCNC Weight Ratio | Component Weight | DB (mm) | SSA (m2 g−1) | VP (m2 g−1) | rP (nm) | ||
|---|---|---|---|---|---|---|---|---|
| SA (g) | DCNC (g) | Water (g) | ||||||
| SA | 100/0 | 0.75 | 0 | 29.25 | 2.8 ± 0.1 | 23.44 | 0.03 | 2.13 |
| SA–DCNC-20 | 80/20 | 0.6 | 0.15 | 29.25 | 2.7 ± 0.1 | 35.43 | 0.05 | 1.90 |
| SA–DCNC-40 | 60/40 | 0.45 | 0.3 | 29.25 | 2.6 ± 0.1 | 51.04 | 0.07 | 1.70 |
| SA–DCNC-80 | 20/80 | 0.15 | 0.6 | 29.25 | 2.5 ± 0.2 | 46.47 | 0.04 | 1.70 |
| Kinetic Model | Parameters | ||
|---|---|---|---|
| Experimental | Qe,exp (mg g−1) | - | |
| 329.9 | - | ||
| Pseudo-first-order | Qe,1 (mg g−1) | K1 | R2 |
| 310.7 | 0.29 | 0.863 | |
| Pseudo-second-order | Qe,2 (mg g−1) | K2 | R2 |
| 363.6 | 0.0013 | 0.999 | |
| Intraparticle diffusion | Ci | Kid | R2 |
| Step 1 | −13.17 | 128.30 | 0.984 |
| Step 2 | 121.21 | 62.43 | 1.000 |
| Step 3 | 275.81 | 10.99 | 0.961 |
| Isotherm Model | Parameters | Temperature | ||
|---|---|---|---|---|
| 20 °C | 35 °C | 45 °C | ||
| Freundlich | KF | 0.24 | 0.36 | 0.59 |
| 1/n | 1.00 | 0.98 | 0.93 | |
| R2 | 0.995 | 0.997 | 0.996 | |
| Adsorbents | Qm (mg g−1) | Condition | Refs |
|---|---|---|---|
| Cu(II)-impregnated biochar | 52.4 | 25 °C, pH 8 | [46] |
| Iron-loaded sludge biochar | 129.0 | 20 °C, pH 6 | [12] |
| Rice straw biochar | 170.4 | 25 °C, pH 6 | [47] |
| Kaolinite–CoFe2O4 nanoparticles | 240 | 25 °C, pH 6 | [44] |
| Graphene-like layered molybdenum disulfide | 319 | 30 °C, pH 7 | [48] |
| Graphene oxide | 398.4 | 25 °C, pH 3.6 | [49] |
| Carboxymethyl cellulose- and chitosan modified-magnetic alkaline Ca–bentonite | 599 | 25 °C, pH 7 | [9] |
| SA–DCNC composite hydrogel | 421.5 | 25 °C, pH 7 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Lee, C.-S.; Zhang, K.; Alhamzani, A.G.; Hsiao, B.S. Sodium Alginate–Aldehyde Cellulose Nanocrystal Composite Hydrogel for Doxycycline and Other Tetracycline Removal. Nanomaterials 2023, 13, 1161. https://doi.org/10.3390/nano13071161
Huang X, Lee C-S, Zhang K, Alhamzani AG, Hsiao BS. Sodium Alginate–Aldehyde Cellulose Nanocrystal Composite Hydrogel for Doxycycline and Other Tetracycline Removal. Nanomaterials. 2023; 13(7):1161. https://doi.org/10.3390/nano13071161
Chicago/Turabian StyleHuang, Xiangyu, Cheng-Shiuan Lee, Katherine Zhang, Abdulrahman G. Alhamzani, and Benjamin S. Hsiao. 2023. "Sodium Alginate–Aldehyde Cellulose Nanocrystal Composite Hydrogel for Doxycycline and Other Tetracycline Removal" Nanomaterials 13, no. 7: 1161. https://doi.org/10.3390/nano13071161
APA StyleHuang, X., Lee, C.-S., Zhang, K., Alhamzani, A. G., & Hsiao, B. S. (2023). Sodium Alginate–Aldehyde Cellulose Nanocrystal Composite Hydrogel for Doxycycline and Other Tetracycline Removal. Nanomaterials, 13(7), 1161. https://doi.org/10.3390/nano13071161









