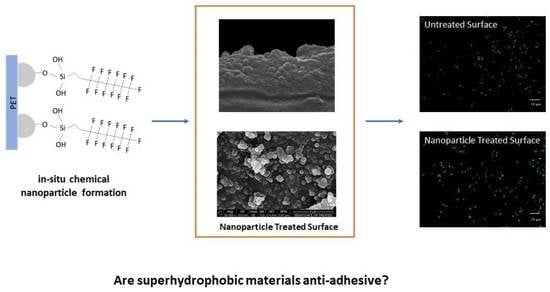
Can Superhydrophobic PET Surfaces Prevent Bacterial Adhesion?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pre-Treatment of the Surfaces
2.3. In Situ Nanoparticle (NP) Modification of PET Surfaces
2.4. Contact Angle
2.5. Atomic Force Microscopy (AFM)
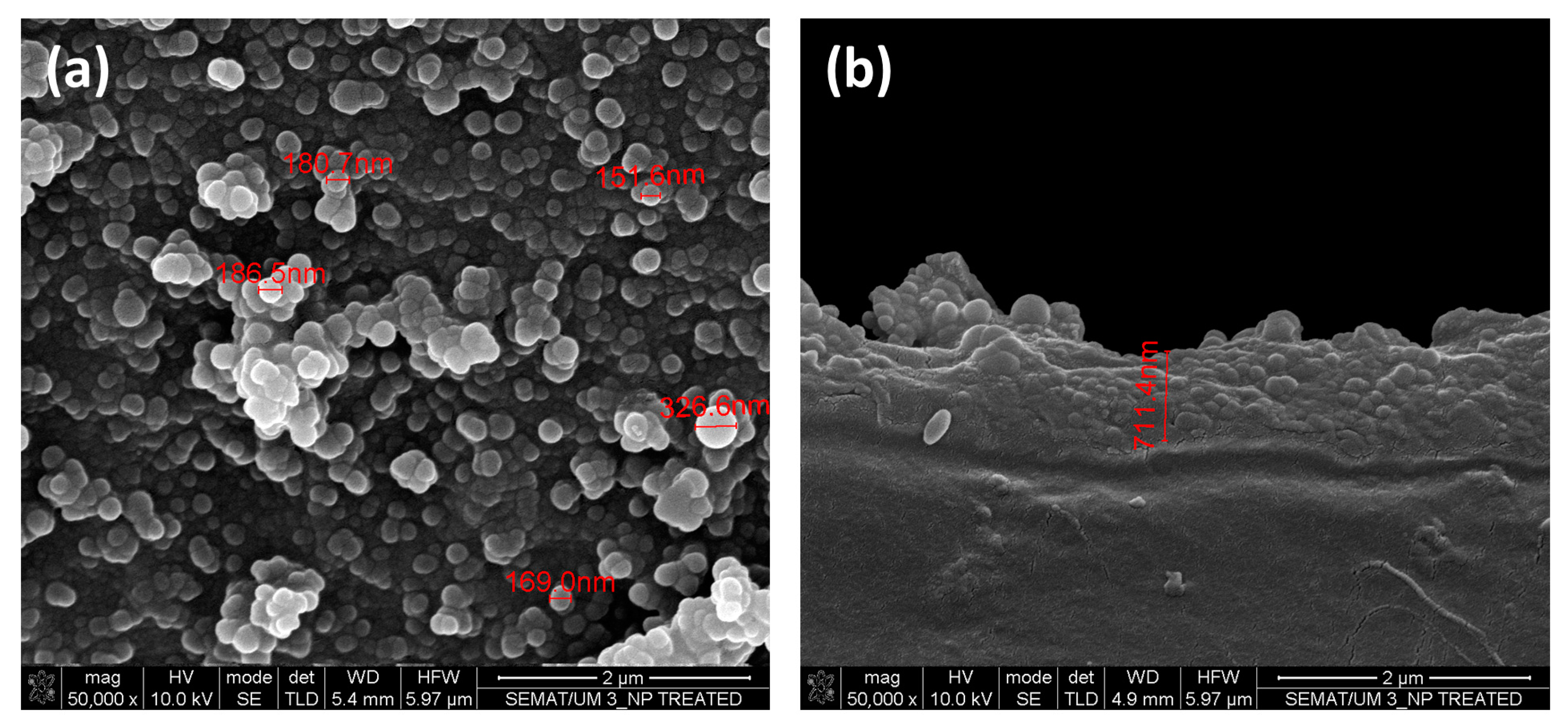
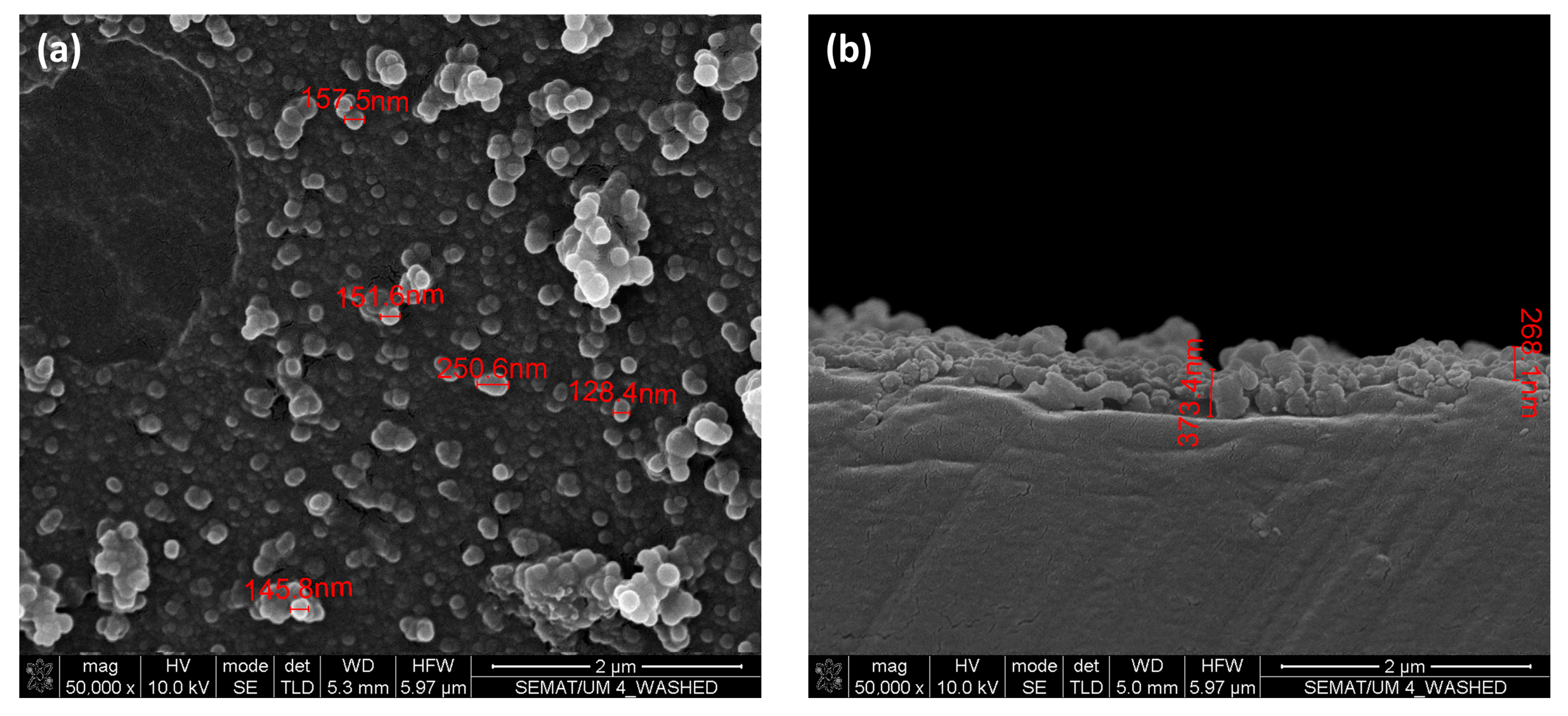
2.6. Scanning Electron Microscopy (SEM)/Energy Dispersive Spectroscopy (EDS)
2.7. Dynamic Light Scattering (DLS)
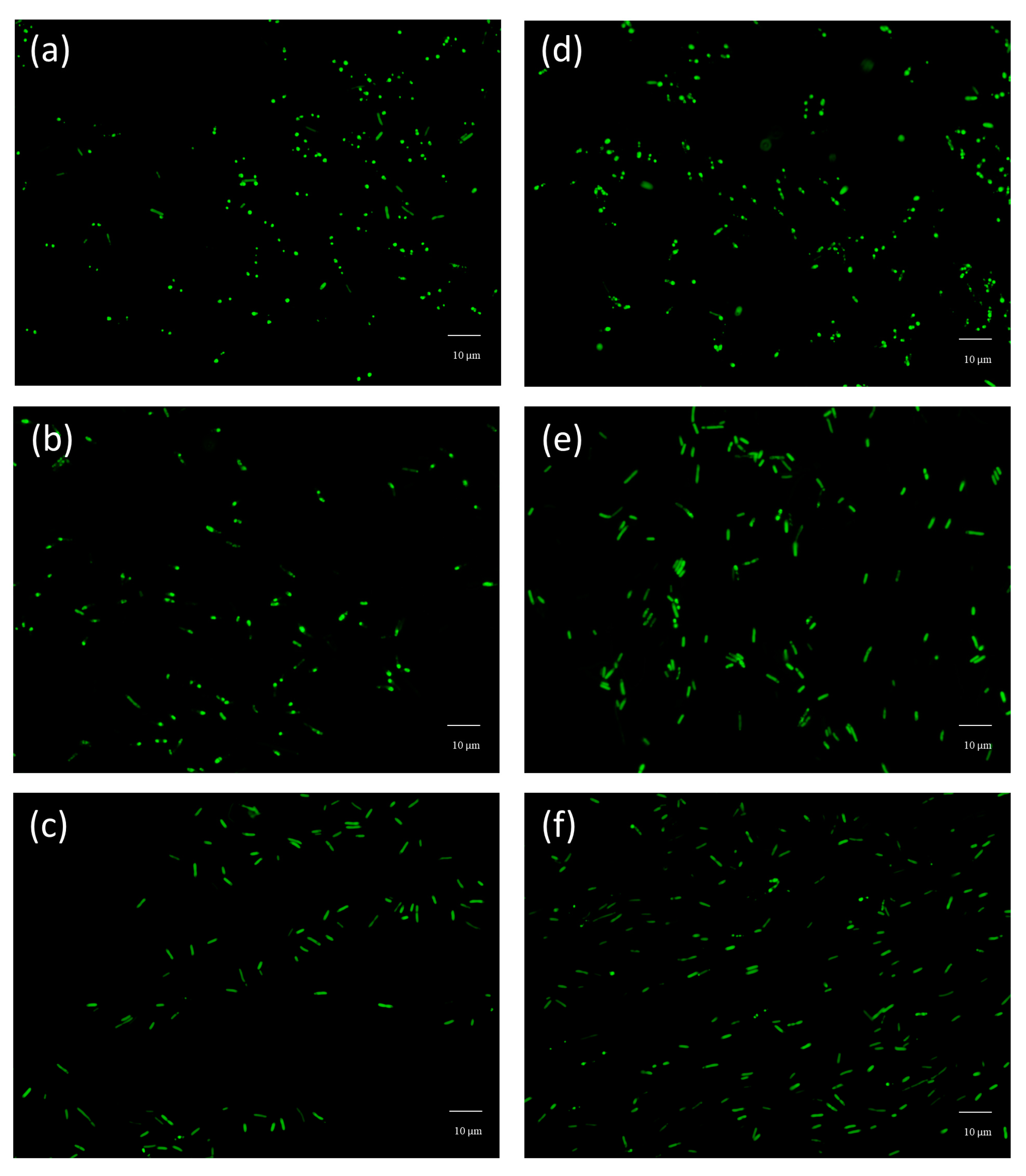
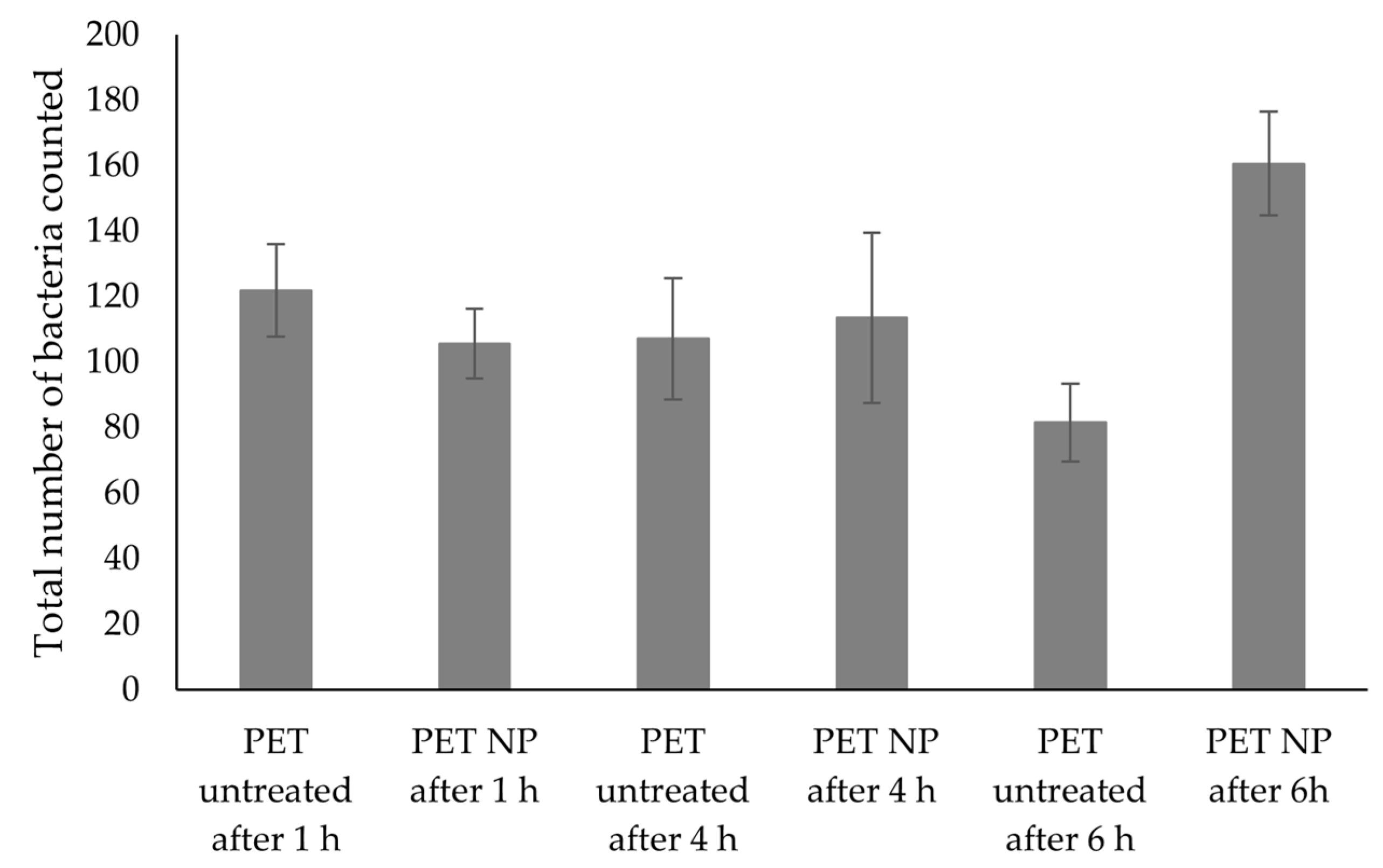
2.8. Bacterial Adhesion
3. Results and Discussion
3.1. Surface Modification and Reaction Mechanism
3.2. Contact Angle and Surface Energy
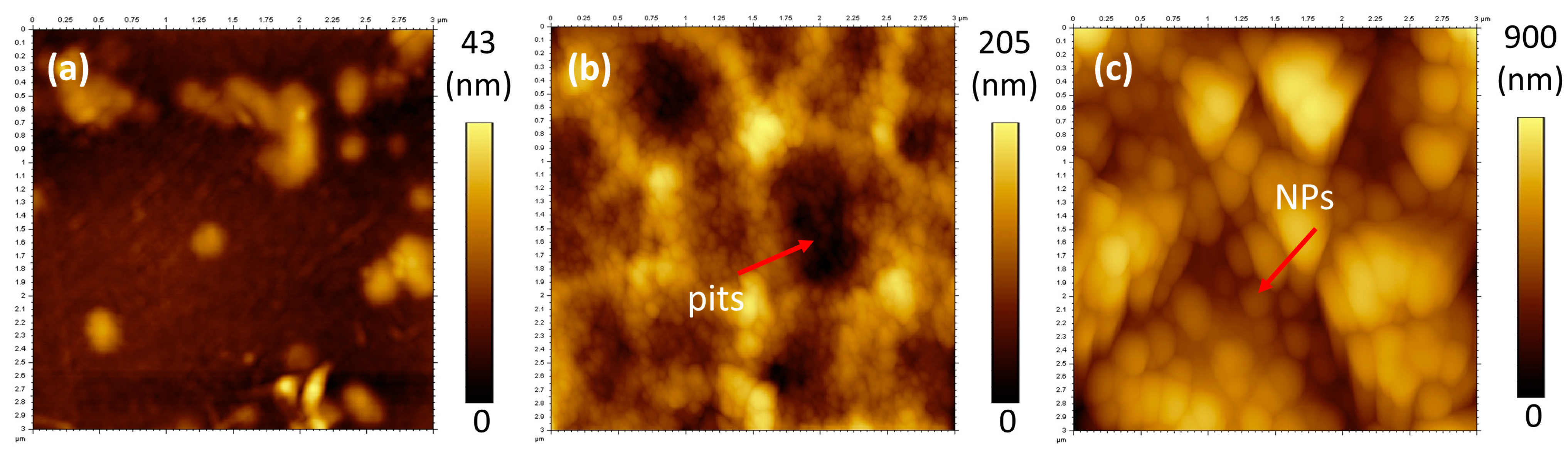
3.3. Atomic Force Microscopy (AFM) and Surface Roughness
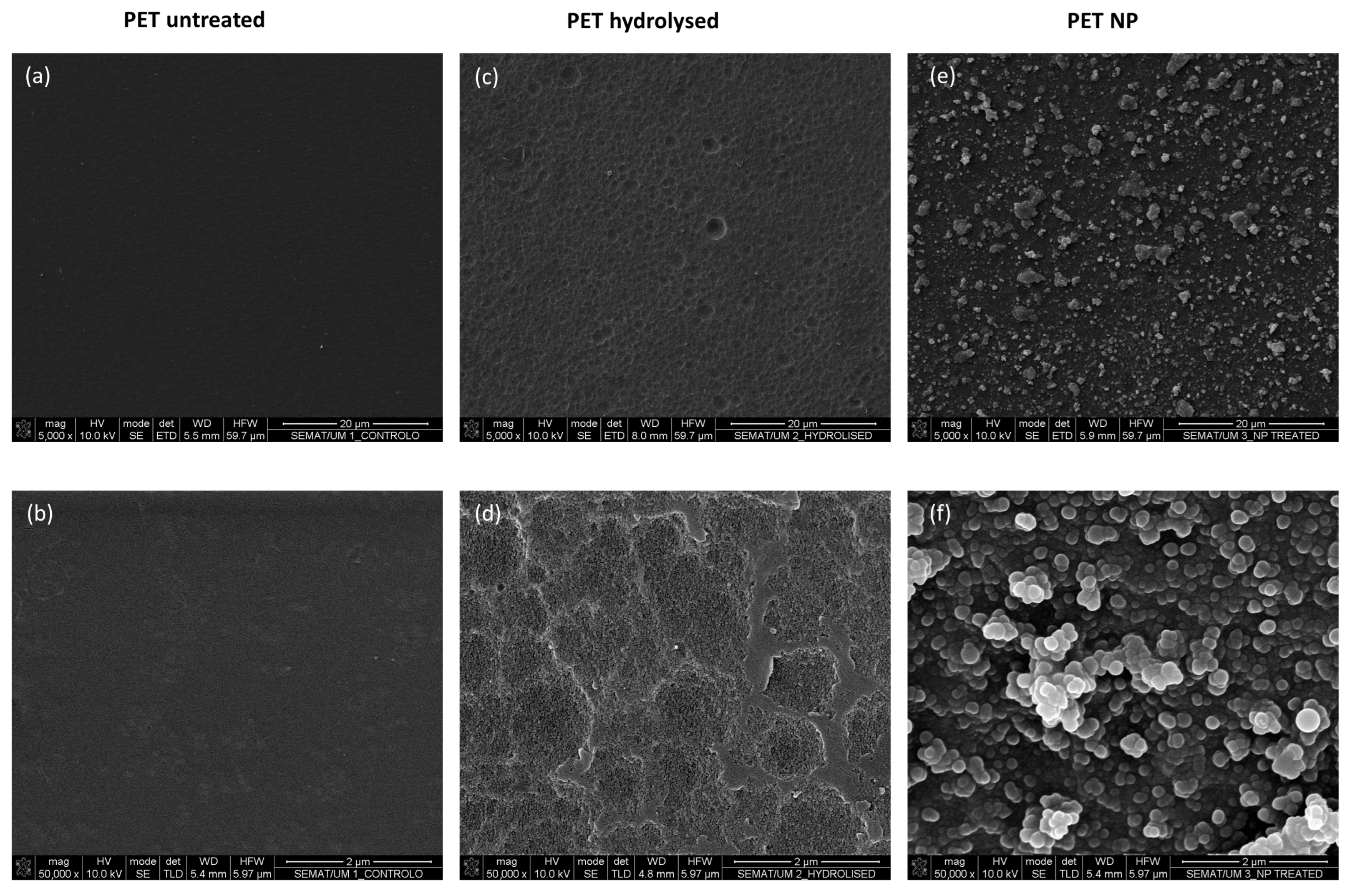
3.4. Scanning Electron Microscopy (SEM)/Energy Dispersive Spectroscopy (EDS) and Dynamic Light Scattering (DLS) Measurements
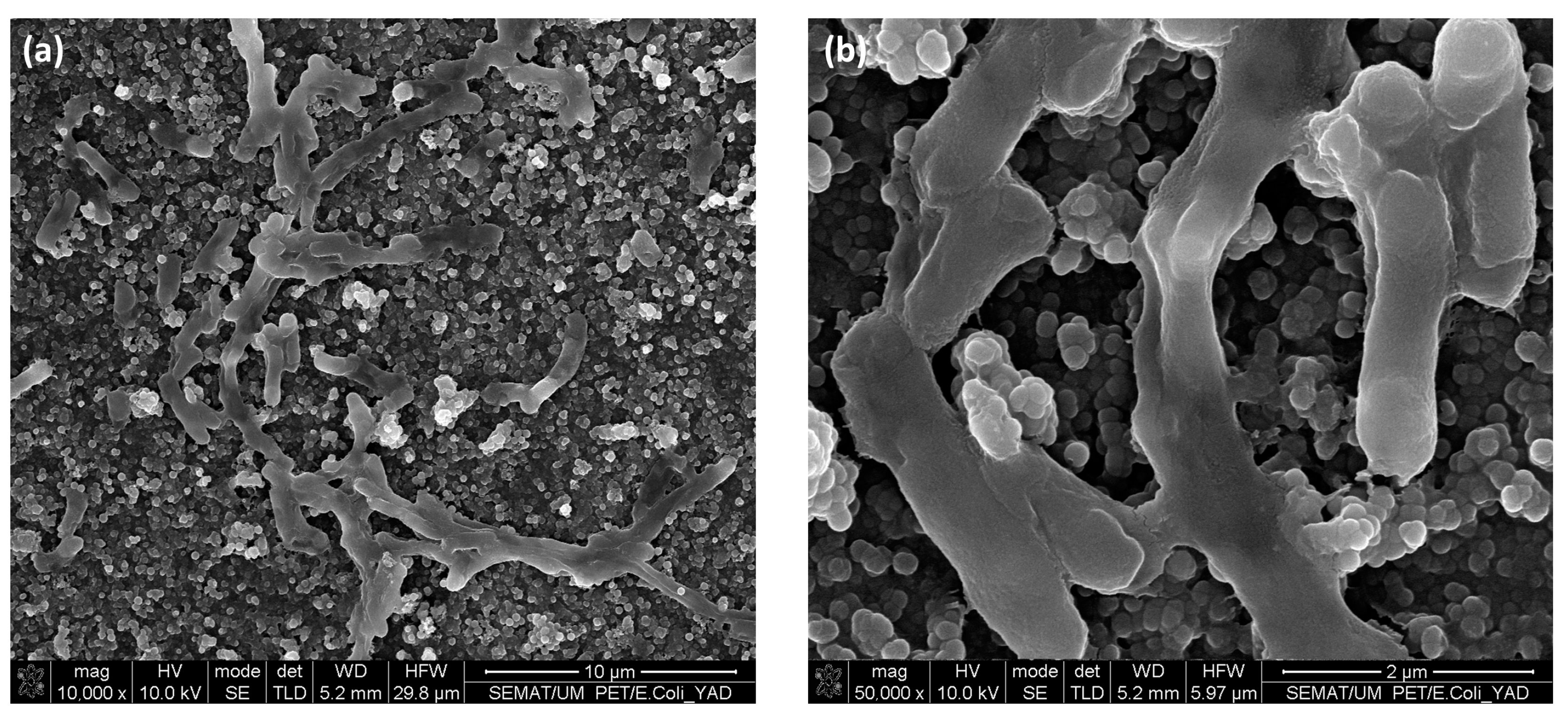
3.5. Bacterial Adhesion Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Wang, L.; Levänen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Çaykara, T.; Sande, M.G.; Azoia, N.; Rodrigues, L.R.; Silva, C.J. Exploring the potential of polyethylene terephthalate in the design of antibacterial surfaces. Med. Microbiol. Immunol. 2020, 209, 363–372. [Google Scholar] [CrossRef]
- Liu, L.; Shi, H.; Yu, H.; Yan, S.; Luan, S. The recent advances in surface antibacterial strategies for biomedical catheters. Biomater. Sci. 2020, 8, 4095–4108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, S.; Müller-Steinhagen, H. Tailored surface free energy of membrane diffusers to minimize microbial adhesion. Appl. Surf. Sci. 2004, 230, 371–378. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Wen, G.; Guo, Z.; Liu, W. Biomimetic polymeric superhydrophobic surfaces and nanostructures: From fabrication to applications. Nanoscale 2017, 9, 3338–3366. [Google Scholar] [CrossRef]
- Liravi, M.; Pakzad, H.; Moosavi, A.; Nouri-Borujerdi, A. A comprehensive review on recent advances in superhydrophobic surfaces and their applications for drag reduction. Prog. Org. Coat. 2020, 140, 105537. [Google Scholar] [CrossRef]
- Czyzyk, S.; Dotan, A.; Dodiuk, H.; Kenig, S. Processing effects on the kinetics morphology and properties of hybrid sol- gel superhydrophobic coatings. Prog. Org. Coat. 2020, 140, 105501. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Lin, Z.; Ding, L.; Zhang, X.; Dai, R.; Yan, Q.; Wang, X. Highly Sensitive Dissolved Oxygen Sensor with a Sustainable Antifouling, Antiabrasion, and Self-Cleaning Superhydrophobic Surface. ACS Omega 2019, 4, 1715–1721. [Google Scholar] [CrossRef]
- Gayani, B.; Dilhari, A.; Kottegoda, N.; Ratnaweera, D.R.; Weerasekera, M.M. Reduced Crystalline Biofilm Formation on Superhydrophobic Silicone Urinary Catheter Materials. ACS Omega 2021, 6, 11488–11496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, L.; Liang, X.; Vorstius, J.; Keatch, R.; Corner, G.; Nabi, G.; Davidson, F.; Gadd, G.M.; Zhao, Q. Enhanced Antibacterial and Antiadhesive Activities of Silver-PTFE Nanocomposite Coating for Urinary Catheters. ACS Biomater. Sci. Eng. 2019, 5, 2804–2814. [Google Scholar] [CrossRef]
- Qian, H.; Li, M.; Li, Z.; Lou, Y.; Huang, L.; Zhang, D.; Xu, D.; Du, C.; Lu, L.; Gao, J. Mussel-inspired superhydrophobic surfaces with enhanced corrosion resistance and dual-action antibacterial properties. Mater. Sci. Eng. C 2017, 80, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; John, J.; Kolewe, K.W.; Schiffman, J.D.; Carter, K.R. Scaling Up Nature: Large Area Flexible Biomimetic Surfaces. ACS Appl. Mater. Interfaces 2015, 7, 23439–23444. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, T.; Hu, J.-M. A robust, room-temperature curable and molecular-level superhydrophobic coating with excellent antibacterial and antifouling properties. Chem. Eng. J. 2022, 450, 136557. [Google Scholar] [CrossRef]
- Han, K.; Park, T.Y.; Yong, K.; Cha, H.J. Combinational Biomimicking of Lotus Leaf, Mussel, and Sandcastle Worm for Robust Superhydrophobic Surfaces with Biomedical Multifunctionality: Antithrombotic, Antibiofouling, and Tissue Closure Capabilities. ACS Appl. Mater. Interfaces 2019, 11, 9777–9785. [Google Scholar] [CrossRef]
- Li, J.; Ding, H.; Zhang, H.; Guo, C.; Hong, X.; Sun, L.; Ding, F. Superhydrophobic Methylated Silica Sol for Effective Oil-Water Separation. Materials 2020, 13, 842. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Jin, T.; He, L.; Zeng, Y.; Ma, Q.; Li, N. Fabrication of superhydrophobic coating from non-fluorine siloxanes via a one-pot sol–gel method. J. Mater. Sci. 2018, 53, 11253–11264. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, Y.; Zhang, D.; Zhang, Z.; Fu, F.; Zhang, X.; Yang, Y.; Lin, H.; Chen, Y. Fabrication of robust and self-healing superhydrophobic PET fabrics based on profiled fiber structure. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 609, 125686. [Google Scholar] [CrossRef]
- Sparks, B.J.; Hoff, E.F.T.; Xiong, L.; Goetz, J.T.; Patton, D.L. Superhydrophobic hybrid inorganic-organic thiol-ene surfaces fabricated via spray-deposition and photopolymerization. ACS Appl. Mater. Interfaces 2013, 5, 1811–1817. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, X.; Bi, H.; Sun, J. Robust superhydrophobic surfaces fabricated by self-growth of TiO2 particles on cured silicone rubber. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 603, 125227. [Google Scholar] [CrossRef]
- Liu, Y.; He, T.; Gao, C. Surface modification of poly (ethylene terephthalate) via hydrolysis and layer-by-layer assembly of chitosan and chondroitin sulfate to construct cytocompatible layer for human endothelial cells. Colloids Surf. B Biointerfaces 2005, 46, 117–126. [Google Scholar] [CrossRef]
- Pérez-Álvarez, L.; Lizundia, E.; del Hoyo, S.; Sagasti, A.; Rubio, L.R.; Vilas, J.L. Polysaccharide polyelectrolyte multilayer coating on poly (ethylene terephthalate). Polym. Int. 2016, 65, 915–920. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Lu, H.; Yang, H.; Zhou, X.; Xin, J.H. In-situ growth of silica nanoparticles on cellulose and application of hierarchical structure in biomimetic hydrophobicity. Cellulose 2010, 17, 1103–1113. [Google Scholar] [CrossRef]
- Kalin, M.; Polajnar, M. The wetting of steel, DLC coatings, ceramics and polymers with oils and water: The importance and correlations of surface energy, surface tension, contact angle and spreading. Appl. Surf. Sci. 2014, 293, 97–108. [Google Scholar] [CrossRef]
- Caykara, T.; Silva, J.; Fernandes, S.; Braga, A.; Rodrigues, J.; Rodrigues, L.R.; Silva, C. Modification of PET surfaces with gum Arabic towards its bacterial anti-adhesiveness using an experimental factorial design approach. Mater. Today Commun. 2021, 28, 102684. [Google Scholar]
- Wollmann, P.; Zeth, K.; Lupas, A.N.; Linke, D. Purification of the YadA membrane anchor for secondary structure analysis and crystallization. Int. J. Biol. Macromol. 2006, 39, 3–9. [Google Scholar] [CrossRef]
- Hetemi, D.; Pinson, J. Surface functionalisation of polymers. Chem. Soc. Rev. 2017, 46, 5701–5713. [Google Scholar] [CrossRef]
- Swar, S.; Zajícová, V.; Rysová, M.; Lovětinská-Šlamborová, I.; Voleský, L.; Stibor, I. Biocompatible surface modification of poly (ethylene terephthalate) focused on pathogenic bacteria: Promising prospects in biomedical applications. J. Appl. Polym. Sci. 2017, 134, 1–11. [Google Scholar] [CrossRef]
- Atallah, C.; Mortazavi, S.; Tremblay, A.Y. Thermal stability of hydrophilic PEO-silane modified ceramic membranes. Colloids Surf. A 2019, 561, 254–266. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review. Adv. Colloid Interface Sci. 2020, 286, 102298. [Google Scholar] [CrossRef]
- Söz, C.K.; Yilgör, E.; Yilgör, I. Influence of the average surface roughness on the formation of superhydrophobic polymer surfaces through spin-coating with hydrophobic fumed silica. Polymer 2015, 62, 118–128. [Google Scholar] [CrossRef]
- Rigon, R.B.; Gonçalez, M.L.; Severino, P.; Alves, D.A.; Santana, M.H.A.; Souto, E.B.; Chorilli, M. Solid lipid nanoparticles optimized by 22 factorial design for skin administration: Cytotoxicity in NIH3T3 fibroblasts. Colloids Surf. B Biointerfaces 2018, 171, 501–505. [Google Scholar] [CrossRef]
- Zambaux, M.F.; Bonneaux, F.; Gref, R.; Maincent, P.; Dellacherie, E.; Alonso, M.J.; Labrude, P.; Vigneron, C. Influence of experimental parameters on the characteristics of poly (lactic acid) nanoparticles prepared by a double emulsion method. J. Control. Release 1998, 50, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.; Khurana, D.; Kumar, A.; Subudhi, S. Stability analysis of Al2O3/ water nanofluids. J. Exp. Nanosci. 2017, 12, 140–151. [Google Scholar] [CrossRef]
- Hwang, G.B.; Page, K.; Patir, A.; Nair, S.P.; Allan, E.; Parkin, I.P. The Anti-Biofouling Properties of Superhydrophobic Surfaces are Short-Lived. ACS Nano 2018, 12, 6050–6058. [Google Scholar] [CrossRef] [PubMed]
- Kayes, I.; Galante, A.J.; Stella, N.A.; Haghanifar, S.; Shanks, R.M.Q.; Leu, P.W. Stable lotus leaf-inspired hierarchical, fluorinated polypropylene surfaces for reduced bacterial adhesion. React. Funct. Polym. 2018, 128, 40–46. [Google Scholar] [CrossRef]
- Ozkan, E.; Mondal, A.; Singha, P.; Douglass, M.; Hopkins, S.P.; Devine, R.; Garren, M.; Manuel, J.; Warnock, J.; Handa, H. Fabrication of Bacteria- and Blood-Repellent Superhydrophobic Polyurethane Sponge Materials. Appl. Mater. Interfaces 2020, 12, 51160–51173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, X.; Zhao, Q. Superhydrophobic Coatings for Urinary Catheters to Delay Bacterial Biofilm Formation and Catheter-Associated Urinary Tract Infection. Appl. Bio Mater. 2020, 3, 282–291. [Google Scholar] [CrossRef]
- Sousa, C.; Rodrigues, D.; Oliveira, R.; Song, W.; Mano, J.F.; Azeredo, J. Superhydrophobic poly (L-lactic acid ) surface as potential bacterial colonization substrate. AMB Express 2011, 1, 34. [Google Scholar] [CrossRef]
- Ferraris, S.; Cochis, A.; Cazzola, M.; Tortello, M.; Scalia, A.; Spriano, S.; Rimondini, L. Cytocompatible and Anti-bacterial Adhesion Nanotextured Titanium Oxide Layer on Titanium Surfaces for Dental and Orthopedic Implants. Front. Bioeng. Biotechnol. 2019, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins Involved in Attachment to Abiotic Surfaces by Gram-Negative Bacteria. Microb. Spectr. 2015, 3, 163–199. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, R.S.; Vlamakis, H.; Kim, P.; Khan, M.; Kolter, R.; Aizenberg, J. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc. Natl. Acad. Sci. USA 2013, 110, 5624–5629. [Google Scholar] [CrossRef] [PubMed]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef]
- Truong, V.K.; Webb, H.K.; Fadeeva, E.; Chichkov, B.N.; Wu, A.H.F.; Lamb, R.; Wang, J.Y.; Crawford, R.J.; Ivanova, E.P. Air-directed attachment of coccoid bacteria to the surface of superhydrophobic lotus-like titanium. Biofouling 2012, 28, 539–550. [Google Scholar] [CrossRef]

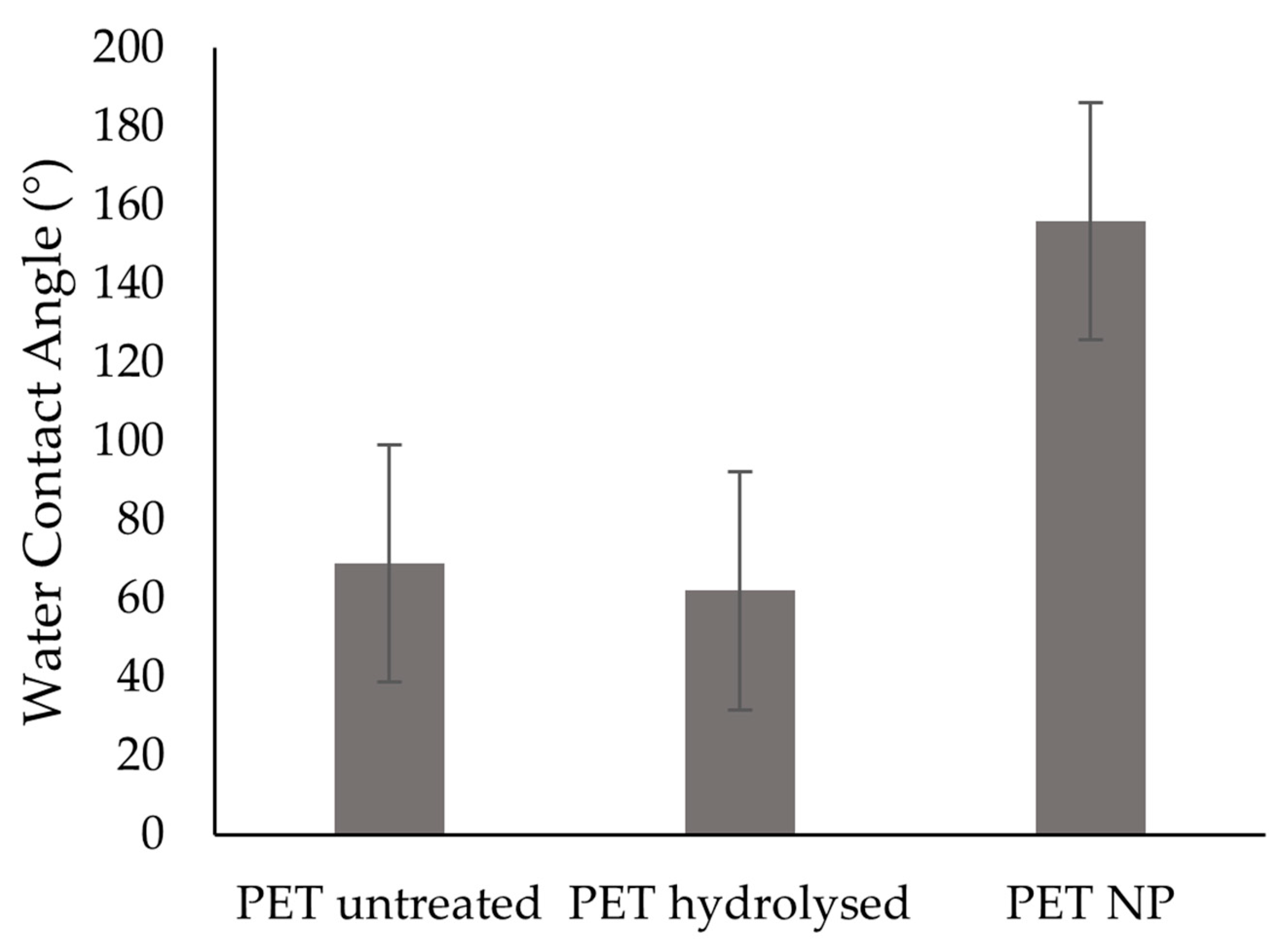
| Samples | Water Contact Angle (°) | Diiodomethane Contact Angle (°) | SFE Disperse mJ/m2 | SFE Polar mJ/m2 | SFE Total mJ/m2 |
|---|---|---|---|---|---|
| PET untreated | 69 ± 10 | 35 ± 4 | 42.0 ± 1.8 | 7.2 ± 4.0 | 49.2 ± 4.2 |
| PET hydrolysed | 62 ± 6 | 35 ± 3 | 41.9 ± 1.5 | 10.8 ± 3.1 | 52.7 ± 3.7 |
| PET NP | 156 ± 12 | 114 ± 14 | 1.3 ± 1.2 | 3.3 ± 5.7 | 6.7 ± 4.3 |
| Samples | Roughness, Ra (nm) |
|---|---|
| PET untreated | 5 ± 1 |
| PET hydrolysed | 28 ± 2 |
| PET NP | 104 ± 20 |
| Samples/Elements (% Atomic) | C | O | Si | F |
|---|---|---|---|---|
| PET untreated | 86 | 14 | n.d. | n.d. |
| PET hydrolysed | 85 | 15 | n.d. | n.d. |
| PET NP | 62 | 28 | 8 | 2 |
| PET NP after 10 washing cycles with water and ethanol | 62 | 27 | 7 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caykara, T.; Fernandes, S.; Braga, A.; Rodrigues, J.; Rodrigues, L.R.; Silva, C.J. Can Superhydrophobic PET Surfaces Prevent Bacterial Adhesion? Nanomaterials 2023, 13, 1117. https://doi.org/10.3390/nano13061117
Caykara T, Fernandes S, Braga A, Rodrigues J, Rodrigues LR, Silva CJ. Can Superhydrophobic PET Surfaces Prevent Bacterial Adhesion? Nanomaterials. 2023; 13(6):1117. https://doi.org/10.3390/nano13061117
Chicago/Turabian StyleCaykara, Tugce, Sara Fernandes, Adelaide Braga, Joana Rodrigues, Ligia R. Rodrigues, and Carla Joana Silva. 2023. "Can Superhydrophobic PET Surfaces Prevent Bacterial Adhesion?" Nanomaterials 13, no. 6: 1117. https://doi.org/10.3390/nano13061117
APA StyleCaykara, T., Fernandes, S., Braga, A., Rodrigues, J., Rodrigues, L. R., & Silva, C. J. (2023). Can Superhydrophobic PET Surfaces Prevent Bacterial Adhesion? Nanomaterials, 13(6), 1117. https://doi.org/10.3390/nano13061117