Green Synthesis of Silver Nanoparticles and Its Combination with Pyropia columbina (Rhodophyta) Extracts for a Cosmeceutical Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instrumentation
2.2. Preparation of Aqueous Extract (WEOP) of the Different Plant Species
2.3. Green Synthesis of AgNPs Using the WEOP
2.4. Estimation of Total Phenolic Content (TPC) in Plant Species
2.5. Obtention and Analysis of PCE
2.6. Encapsulation of PCE in βCD
2.7. Encapsulation in pNIPAM
2.8. Cosmetic Formulation
2.9. Photoprotection Factor
3. Results and Discussion
3.1. Preparation of WEOP and AgNPs
3.2. Estimation of Total Phenolic Content (TPC) in Plant Species
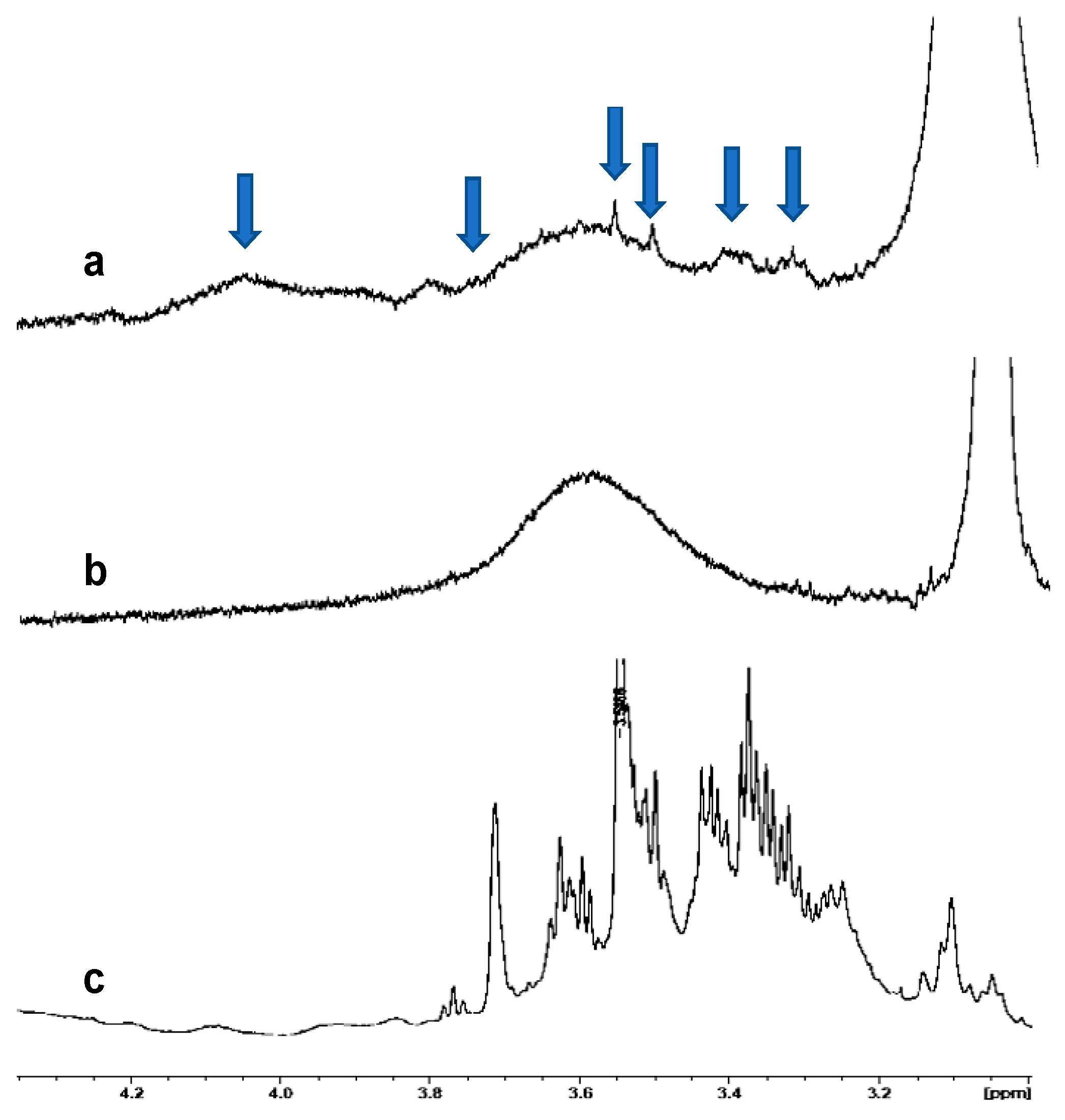
3.3. Analysis of PCE
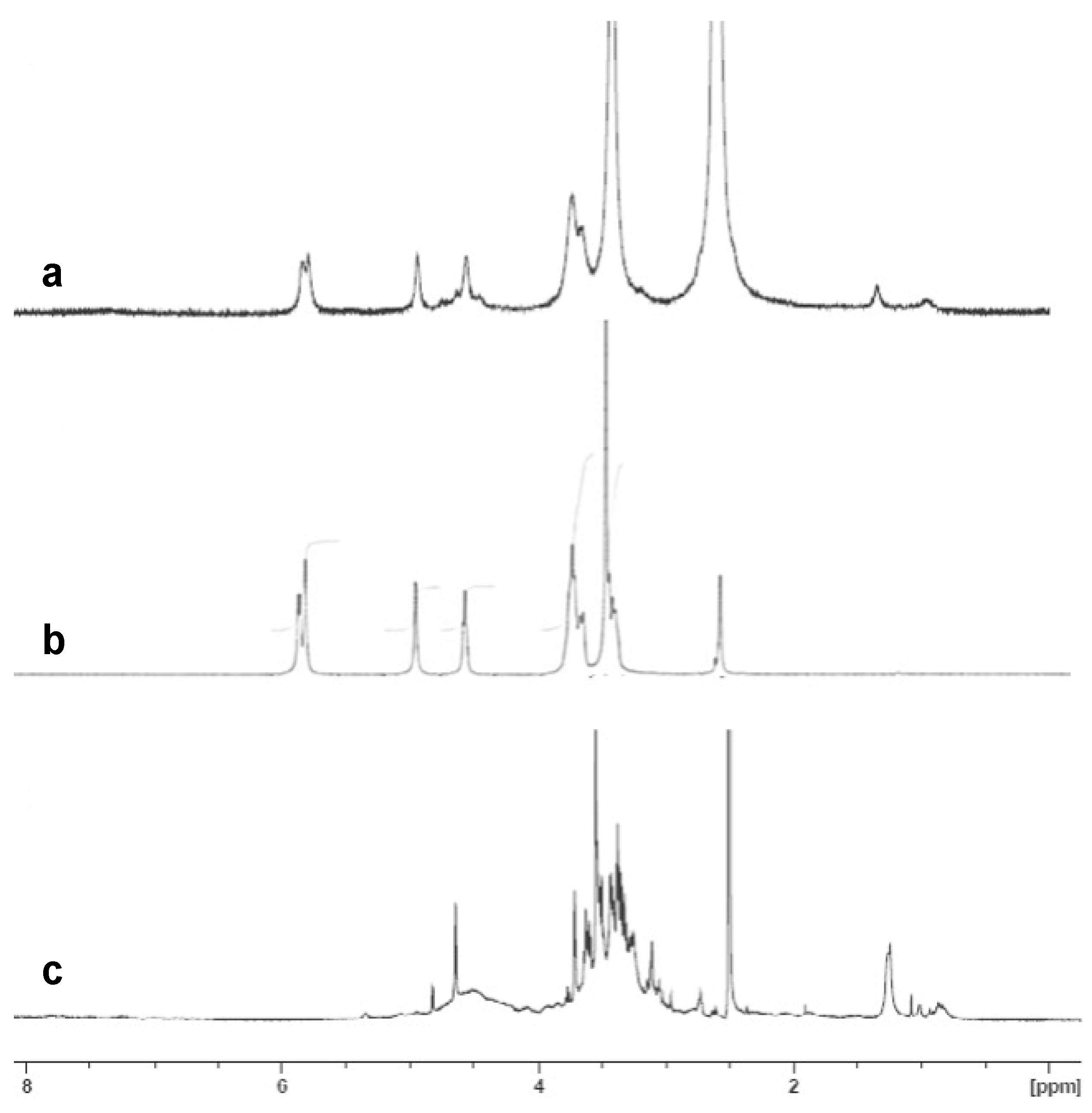
3.4. Encapsulation of PCE and Release Analyses
3.5. Incorporation of AgNPs and PCE into Cosmetic Formulations
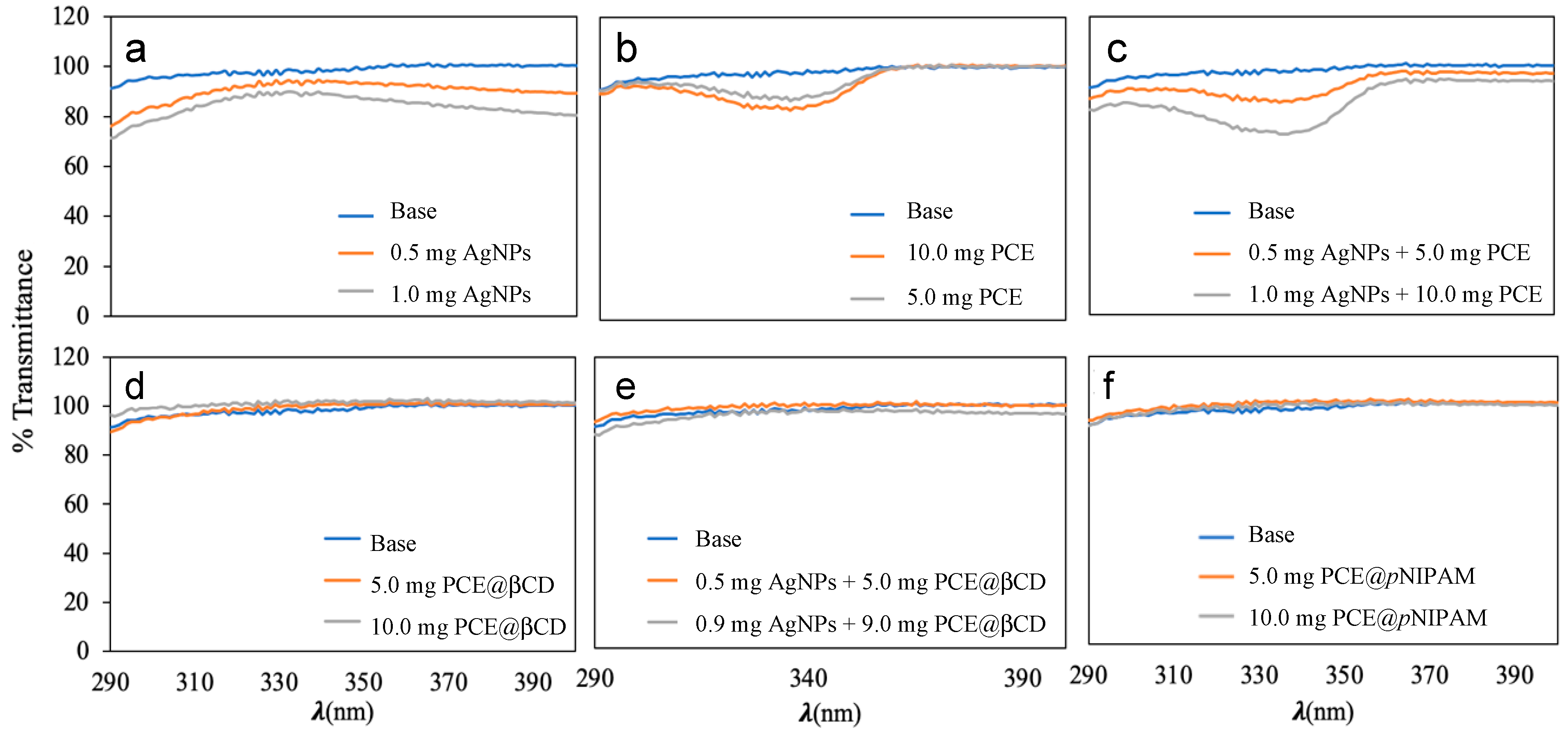
3.6. Photoprotective Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammadlou, M.; Maghsoudi, H.; Jafarizadeh-Malmiri, A. A review on green silver nanoparticles based on plants: Synthesis, potential applications, and eco-friendly approach. Int. Food Res. J. 2016, 23, 446–463. [Google Scholar]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef]
- Melchert, W.R.; Reis, B.F.; Rocha, F.R.P. Green chemistry and the evolution of flow analysis. A review. Anal. Chim. Acta 2012, 714, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, B.; Monisha, N.; Shalini, H.R.; Prathap, G.K.; Poyya, J.; Shantaram, M.; Hampapura, J.S.; Karigar, C.S.; Joshi, C.G. Antibacterial activity of silver nanoparticles synthesized using endophytic fungus—Penicillium cinnamopurpureum. Spectrosc. Lett. 2022, 55, 20–34. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Herdade, A.M.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Paranhos, A.; Veiga, F. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. 2021, 597, 120311. [Google Scholar] [CrossRef]
- Demissie, A.G.; Lele, S.S. Phytosynthesis and characterization of silver nanoparticles using callus of Jatropha curcas: A biotechnological approach. Int. J. Nanosci. 2013, 12, 1350012. [Google Scholar] [CrossRef]
- Hegazy, H.S.; Rabie, G.H.; Shaaban, L.D.; Raie, S.D. Extracellular synthesis of silver nanoparticles by callus of Medicago sativa. Life Sci. J. 2014, 11, 1211–1214. [Google Scholar]
- Nabikhan, A.; Kandasamy, K.; Raj, A.; Alikunhi, N.M. Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf. B-Biointerfaces 2010, 79, 488–493. [Google Scholar] [CrossRef]
- Iyer, R.I.; Panda, T. Biosynthesis of gold and silver nanoparticles using extracts of callus cultures of pumpkin (Cucurbita maxima). J. Nanosci. Nanotechnol. 2018, 18, 5341–5353. [Google Scholar] [CrossRef]
- Asmathunisha, N.; Kathiresan, K. Rapid biosynthesis of antimicrobial silver and gold nanoparticles by in vitro callus and leaf extracts from Lycopersicon esculentum Mill. Int. J. Pharma. Bio Sci. 2013, 4, 334–344. [Google Scholar]
- Osibe, D.A.; Chiejina, N.V.; Ogawa, K.; Aoyagi, H. Stable antibacterial silver nanoparticles produced with seed-derived callus extract of Catharanthus roseus. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Deeksha, B.; Sadanand, V.; Hariram, N.; Rajulu, A.V. Preparation and properties of cellulose nanocomposite fabrics with in situ generated silver nanoparticles by bioreduction method. J. Bioresour. Bioprod. 2021, 6, 75–81. [Google Scholar] [CrossRef]
- Bernabé-Antonio, A.; Martínez-Ceja, A.; Romero-Estrada, A.; Sánchez-Carranza, J.N.; Columba-Palomares, M.C.; Rodríguez-López, V.; Meza-Contreras, J.C.; Silva-Guzmán, J.A.; Gutiérrez-Hernández, J.M. Green synthesis of silver nanoparticles using Randia aculeata L. cell culture extracts, characterization, and evaluation of antibacterial and antiproliferative Activity. Nanomaterials 2022, 12, 4184. [Google Scholar] [CrossRef] [PubMed]
- Manan, S.; Ullah, M.V.; Ul-Islam, M.; Atta, O.M.; Yang, G. Synthesis and applications of fungal mycelium-based advanced functional materials. J. Bioresour. Bioprod. 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Role of green silver nanoparticles synthesized from Symphytum officinale leaf extract in protection against UVB-induced photoaging. J. Nanostruct. Chem. 2018, 8, 359–368. [Google Scholar] [CrossRef]
- Dewez, D.; Oukarroum, A. Silver nanoparticles toxicity effect on photosystem II photochemistry of the green alga Chlamydomonas reinhardtii treated in light and dark conditions. Toxicol. Environm. Chem. 2012, 94, 1536–1546. [Google Scholar] [CrossRef]
- Guinea, M.; Franco, V.; Araujo-Bazán, L.; Rodríguez-Martín, I.; González, S. In vivo UVB-photoprotective activity of extracts from commercial marine macroalgae. Food Chem. Toxicol. 2012, 50, 1109–1117. [Google Scholar] [CrossRef]
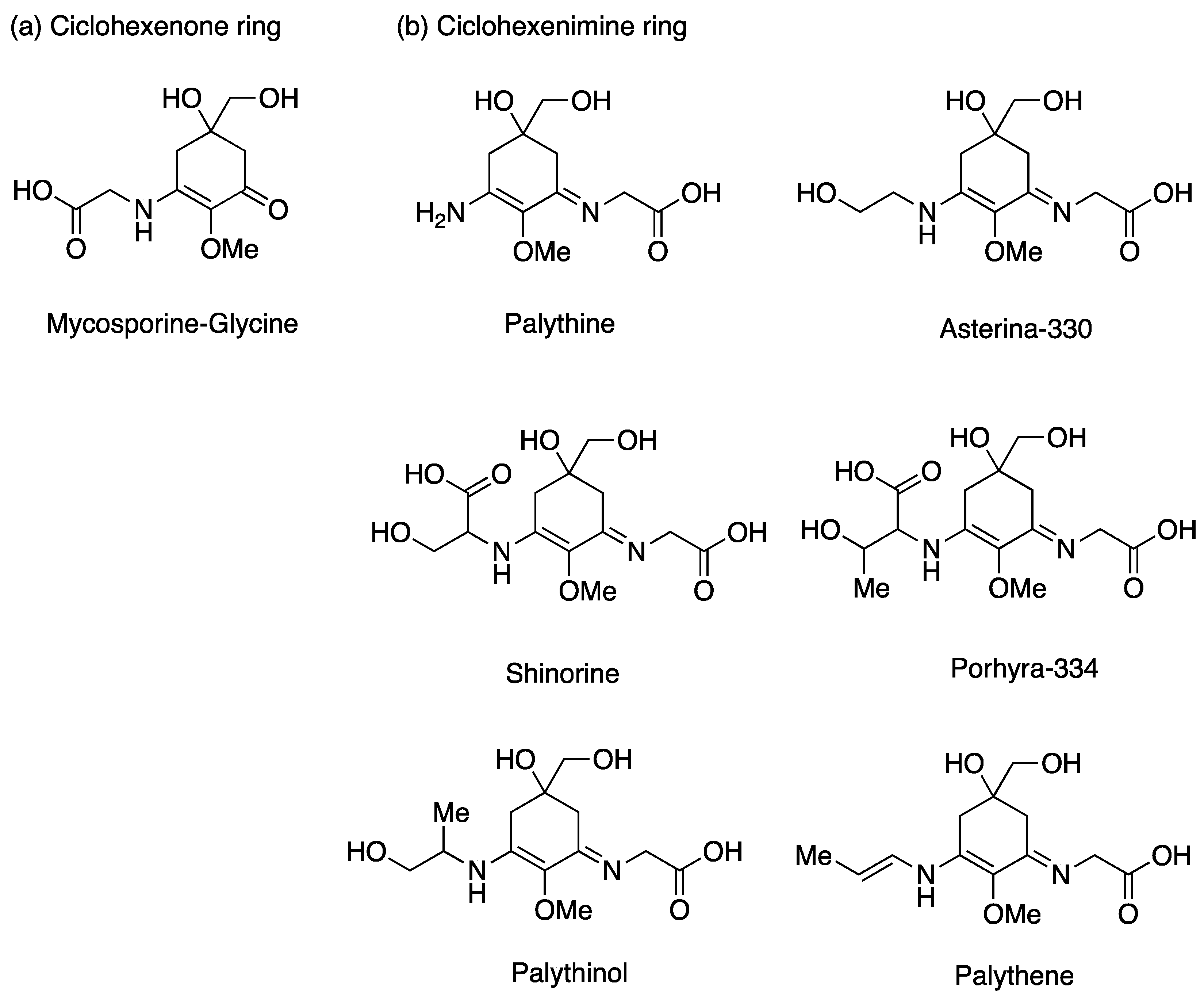
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, C.; Figueroa, F.L. Mycosporine-like amino acids from red macroalgae: UV-photoprotectors with potential cosmeceutical applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Álvarez-Gómez, F.; Bonomi-Barufi, J.; Vega, J.; Massocato, T.F.; Gómez-Pinchetti, J.L.; Korbee, N. Interactive effects of solar radiation and inorganic nutrients on biofiltration, biomass production, photosynthetic activity, and the accumulation of bioactive compounds in Gracilaria cornea (Rhodophyta). Algal Res. 2022, 68, 102890. [Google Scholar] [CrossRef]
- Korbee, N.; Díaz, R.T.A.; Figueroa, F.L.; Helbling, W. Ammonium and UV radiation stimulate the accumulation of mycosporine-like amino acids in Porphyra columbina (Rhodophyta) from Patagonia, Argentina. J. Phycol. 2004, 40, 248–259. [Google Scholar]
- Bonomi-Barufi, J.; Figueroa, F.L.; Korbee, N.; Momoli, M.M.; Martins, A.P.; Colepicolo, P.; Van Sluys, M.A.; Oliveira, M.C. How macroalgae can deal with radiation variability and photoacclimation capacity: The example of Gracilaria tenuistipitata (Rhodophyta) in laboratory. Algal Res. 2020, 50, 102007. [Google Scholar] [CrossRef]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and Red Macroalgae as Potential Sources of Antioxidants and UV Radiation-Absorbing Compounds for Cosmeceutical Applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Figueroa, F.L.; Vega, J.; Chaves, P.; Álvarez-Gómez, F.; Korbee, N.; Bonomi-Barufi, J. Photoprotection properties of marine photosynthetic organisms grown in high ultraviolet exposure areas: Cosmeceutical applications. Algal Res. 2020, 49, 101956. [Google Scholar] [CrossRef]
- Moreira, B.R.; Vega, J.; Sisa, A.D.A.; Bernal, J.S.B.; Abdala-Díaz, R.T.; Maraschin, M.; Bonomi-Barufi, J. Antioxidant and anti-photoaging properties of red marine macroalgae: Screening of bioactive molecules for cosmeceutical applications. Algal Res. 2022, 68, 102893. [Google Scholar] [CrossRef]
- Rodríguez-Santana, P.; Jiménez-Abizanda, A.I.; Hernández-Creus, A.; Jiménez-Moreno, F. Synthesis and physical characterization of Ag nanoparticles and their interaction with Fe. J. Lumin. 2017, 190, 207–214. [Google Scholar] [CrossRef]
- Yap, Y.H.; Azmi1, A.A.; Mohd, N.K.; Yong, F.S.J.; Kan, S.-Y.; Thirmizir, M.Z.A.; Chia, P.W. Green synthesis of silver nanoparticles using water extract of onion peel and application in the acetylation reaction. Arab. J. Sci. Eng. 2020, 45, 4797–4807. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biolol. Chem. 1927, 3, 627–649. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Amer. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Madaan, R.; Bansal, G.; Kumar, S.; Sharma, A. Estimation of total phenols and flavonoids in extracts of Actaea spicata roots and antioxidant activity studies. Indian J. Pharm. Sci. 2011, 73, 666–669. [Google Scholar] [CrossRef]
- Chaves-Peña, P.; de la Coba, F.; Figueroa, F.L.; Korbee, N. Quantitative and qualitative HPLC analysis of mycosporine-like amino acids extracted in distilled water for cosmetical uses in four Rhodophyta. Mar. Drugs 2019, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Sawall, T.; Hanelt, D.; Bischof, K.; Figueroa, F.L.; Flores-Moya, A.; Wiencke, C. An inventory of UV-absorbing mycosporine-like amino acids in macroalgae from polar to warm-temperate regions. Bot. Mar. 1998, 41, 443–453. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, K. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Pissavini, M.; Tricaud, C.; Wiener, G.; Lauer, A.; Contier, M.; Kolbe, L.; Matts, P.J. Validation of an in vitro sun protection factor (SPF) method in blinded ring-testing. Int. J. Cosmet. Sci. 2018, 40, 263–268. [Google Scholar] [CrossRef] [PubMed]
- ISO 24443:2012; Determination of Sunscreen UVA Photoprotection In Vitro. International Organization for Standarization: London, UK. Available online: www.iso.org (accessed on 10 February 2023).
- De la Coba, F.; Aguilera, J.; Korbee, N.; de Gálvez, M.V.; Herrera-Ceballos, E.; Álvarez-Gómez, F.; Figueroa, F.L. UVA and UVB photoprotective capabilities of topical formulations containing mycosporine-like amino acids (MAAs) through different biological effective protection factors (BEPFs). Mar. Drugs 2019, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, A.F. Reference action spectrum for ultraviolet induced erythema in human skin. CIE J. 1987, 6, 17–22. [Google Scholar]
- Wulf, H.C.; Poulsen, T.; Davies, R.E.; Urbach, F. Narrow-band UV radiation and induction of dermal elastosis and skin cancer. Photodermatology 1989, 6, 44–51. [Google Scholar]
- Bissett, D.L.; Hannon, D.P.; Orr, T.V. Wavelength dependence of histological, physical and visible changes in chronically UV-irradiated hairless mouse skin. Photochem. Photobiol. 1989, 50, 763–769. [Google Scholar] [CrossRef]
- Ijaz, I.; Bukhari, A.; Gilani, E.; Nazir, A.; Zain, H.; Saeed, R.; Hussain, S.; Hussain, T.; Bukhari, A.; Naseer, Y.; et al. Green synthesis of silver nanoparticles using different plants parts and biological organisms, characterization and antibacterial activity. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100704. [Google Scholar] [CrossRef]
- Zhao, L.; Du, C.; Zhang, Q.; Sun, C.; Wang, S.; Luo, S. The ultraviolet-visible absorbance and fluorescence characterization of dissolved organic matter derived from the leaf litter of Populus simonii, Artemisia desertorum, Salix cheilophila, and Populus tomentosa. Environ. Sci. Pollut. Res. 2020, 27, 36439–36449. [Google Scholar] [CrossRef]
- Bernaert, N.; De Paepe, D.; Bouten, C.; De Clercq, H.; Stewart, D.; Van Bockstaele, E.; De Loose, M.; Van Droogenbroeck, B. Antioxidant capacity, total phenolic and ascorbate content as a function of the genetic diversity of leek (Allium ampeloprasum var. porrum). Food Chem. 2012, 134, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Briani, B.; Sissini, M.N.; Lucena, L.A.; Batista, M.B.; Costa, I.A.; Nunes, J.M.C.; Schmitz, C.; Ramlov, F.; Maraschin, M.; Korbee, N.; et al. The influence of environmental features in the content of mycosporine-like amino acids in red marine algae along the Brazilian coast. J. Phycol. 2018, 54, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pinel, B.; Ortega-Rodríguez, A.; Porras-Alcalá, C.; Contreras-Cáceres, R.; Ortiz, R.; Díaz, A.; Moscoso, A.; Sarabia, F.; Prados, J.; López-Romero, J.M.; et al. Magnetically active pNIPAM nanosystems as temperature-sensitive biocompatible structures for controlled drug delivery. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Benavente, J.; García, M.E.; Urbano, N.; López-Romero, J.M.; Contreras-Cáceres, R.C.; Casado-Rodríguez, M.A.; Moscoso, A.; Hierrezuelo, J. Inclusion of silver nanoparticles for improving regenerated cellulose membrane performance and reduction of biofouling. Int. J. Biol. Macromol. 2017, 103, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Moncho-Jordá, A.; Jódar-Reyes, A.B.; Kanduc, M.; Germán-Bellod, A.; López-Romero, J.M.; Contreras-Caceres, R.; Sarabia, F.; García-Castro, M.; Pérez-Ramírez, H.A.; Odriozola, G. Scaling laws in diffusive release of neutral cargo from hollow hydrogel nanoparticles. Application to paclitaxel loaded poly(4-vinylpyridine) systems. ACS Nano 2020, 14, 15227–15240. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cáceres, R.; Leiva, M.C.; Ortiz, R.; Díaz, A.; Perazzoli, G.; Casado-Rodríguez, M.A.; Melguizo, C.; Baeyens, J.M.; López-Romero, J.M.; Prados, J. Hollow-p4VP Nanoparticles Loading Paclitaxel to Enhance Drug Chemotherapeutic Efficacy in Lung and Breast Cancer Cell Lines. Nano Res. 2017, 10, 856–875. [Google Scholar] [CrossRef]
- Marto, J.; Ascenso, A.; Simoes, S.; Almeida, A.J.; Ribeiro, H.M. Pickering emulsions: Challenges and opportunities in topical delivery. Expert Opin. Drug Deliv. 2016, 13, 1093–1107. [Google Scholar] [CrossRef]
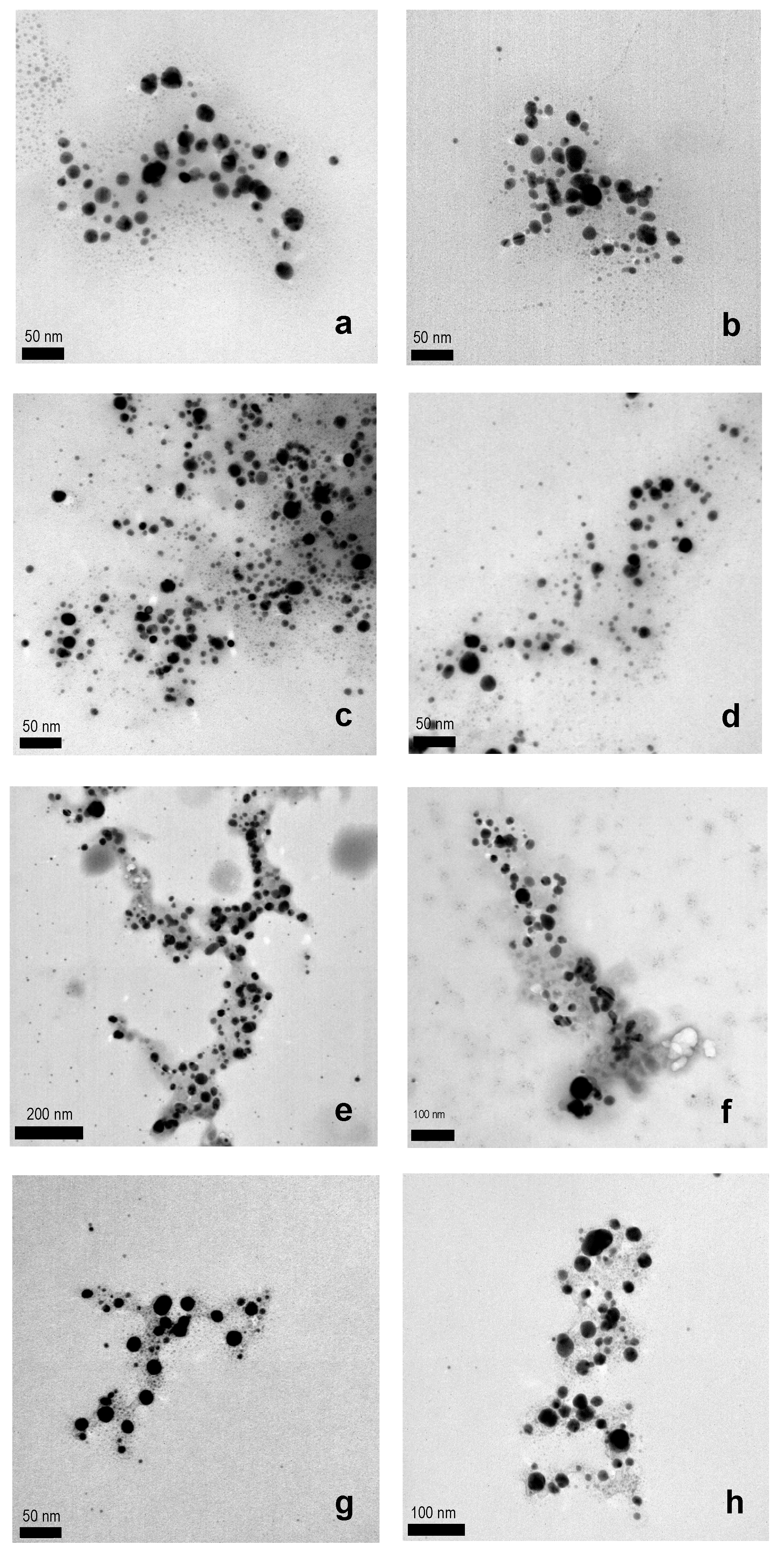
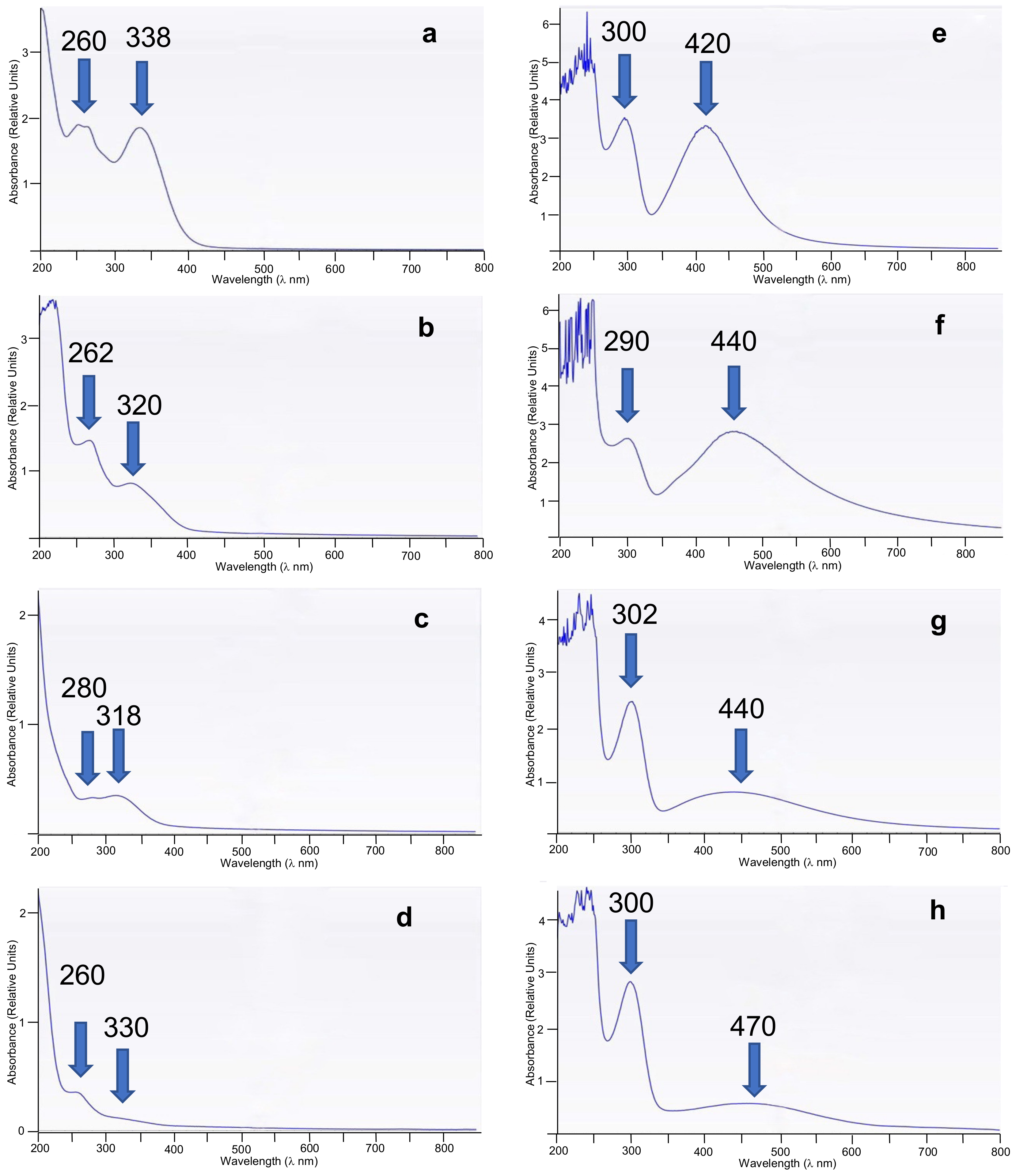

| Entry | WEOP | UV WEOP (λmax, log ε) | AgNPs (mg) | UV AgNPs (λmax, log ε) | Yield (%) | Shape & Size (nm Range & Average) |
|---|---|---|---|---|---|---|
| 1 | Daisy | 264 (3.20), 338 (3.25) | 783.271 | 300 (3.75), 420 (3.80) | 84 | Spherical (5–23, 16.1 ± 1.1) |
| 2 | Garlic | 262 (3.10), 320 (2.70) | 593.758 | 290 (3.70), 440 (3.79) | 64 | Spherical (5–25, 17.4 ± 1.7) |
| 3 | Leek green zone | 280 (2.35), 318 (2.45) | 348.437 | 302 (3.87), 440 (2.45) | 38 | Spherical (9–35, 18.3 ± 2.1) |
| 4 | Leek white zone | 260 (2.25), 330 (2.01) | 336.597 | 300 (3.97), 470 (2.40) | 36 | Spherical (5–25, 17.0 ± 2.0) |
| Entry | Sample | C (mg/L Gallic Acid) | V (mL WEOP) | W (mg Plant) | TPC (mg Gallic Acid/g Dry Extract) |
|---|---|---|---|---|---|
| 1 | Daisy | 36.30 ± 0.50 | 89 | 0.425 | 7.60 ± 0.10 |
| 2 | Garlic | 7.76 ± 0.07 | 91 | 0.514 | 1.37 ± 0.10 |
| 3 | Leek green zone | 4.20 ± 0.40 | 93 | 1.018 | 0.38 ± 0.04 |
| 4 | Leek white zone | 3.53 ± 0.08 | 92 | 1.028 | 0.316 ± 0.007 |
| MAAs (mg/g DE) | Phenols (mg/g DE) | Antioxidant Capacity (μmol TE/g DE) |
|---|---|---|
| 30.3 ± 1.8 | 53.5 ± 3.5 | 32.5 ± 2.3 |
| Entry | SPF | UVAPF | Elastosis | Photoaging | |
|---|---|---|---|---|---|
| 1 | Base cream | 1.03 ± 0.00 | 1.01 ± 0.00 | 1.01 ± 0.00 | 1.01 ± 0.00 |
| 2 | 0.5 mg AgNPs | 1.13 ± 0.02 | 1.08 ± 0.01 | 1.09 ± 0.01 | 1.08 ± 0.01 |
| 3 | 1.0 mg AgNPs | 1.20 ± 0.01 | 1.16 ± 0.01 | 1.17 ± 0.01 | 1.15 ± 0.01 |
| 4 | 5.0 mg PCE | 1.07 ± 0.00 | 1.06 ± 0.00 | 1.05 ± 0.00 | 1.06 ± 0.00 |
| 5 | 10.0 mg PCE | 1.10 ± 0.03 | 1.08 ± 0.02 | 1.07 ± 0.02 | 1.09 ± 0.03 |
| 6 | 0.5 mg AgNPs + 5.0 mg PCE | 1.11 ± 0.01 | 1.09 ± 0.00 | 1.08 ± 0.00 | 1.09 ± 0.01 |
| 7 | 1.0 mg AgNPs + 10.0 mg PCE | 1.21 ± 0.01 | 1.19 ± 0.01 | 1.17 ± 0.01 | 1.20 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Conde, M.; Vega, J.; López-Figueroa, F.; García-Castro, M.; Moscoso, A.; Sarabia, F.; López-Romero, J.M. Green Synthesis of Silver Nanoparticles and Its Combination with Pyropia columbina (Rhodophyta) Extracts for a Cosmeceutical Application. Nanomaterials 2023, 13, 1010. https://doi.org/10.3390/nano13061010
González-Conde M, Vega J, López-Figueroa F, García-Castro M, Moscoso A, Sarabia F, López-Romero JM. Green Synthesis of Silver Nanoparticles and Its Combination with Pyropia columbina (Rhodophyta) Extracts for a Cosmeceutical Application. Nanomaterials. 2023; 13(6):1010. https://doi.org/10.3390/nano13061010
Chicago/Turabian StyleGonzález-Conde, Mercedes, Julia Vega, Félix López-Figueroa, Miguel García-Castro, Ana Moscoso, Francisco Sarabia, and J. Manuel López-Romero. 2023. "Green Synthesis of Silver Nanoparticles and Its Combination with Pyropia columbina (Rhodophyta) Extracts for a Cosmeceutical Application" Nanomaterials 13, no. 6: 1010. https://doi.org/10.3390/nano13061010
APA StyleGonzález-Conde, M., Vega, J., López-Figueroa, F., García-Castro, M., Moscoso, A., Sarabia, F., & López-Romero, J. M. (2023). Green Synthesis of Silver Nanoparticles and Its Combination with Pyropia columbina (Rhodophyta) Extracts for a Cosmeceutical Application. Nanomaterials, 13(6), 1010. https://doi.org/10.3390/nano13061010








